Abstract
Wnt proteins form a family of highly conserved secreted molecules that are critical mediators of cell-cell signaling during embryogenesis. Partial data on Wnt activity in different tissues and at different stages have been reported in frog embryos. Our objective here is to provide a coherent and detailed description of Wnt activity throughout embryo development. Using a transgenic Xenopus tropicalis line carrying a Wnt-responsive reporter sequence, we depict the spatial and temporal dynamics of canonical Wnt activity during embryogenesis. We provide a comprehensive series of in situ hybridization in whole-mount embryos and in cross-sections, from gastrula to tadpole stages, with special focus on neural tube, retina and neural crest cell development. This collection of patterns will thus constitute a valuable resource for developmental biologists to picture the dynamics of Wnt activity during development.
Introduction
The Wnt/β-catenin pathway plays a crucial role in cell proliferation, cell polarity and cell fate determination during vertebrate development [1]. Its early deregulation in the mouse is embryonic lethal. At later development stages, abnormal Wnt/β-catenin signaling results in birth defects. In adults, Wnt/β-catenin signaling deregulation leads to cancer and other diseases [2]. Intense research seeks to better understand Wnt signaling and to develop therapies for the treatment of tumors.
Wnt proteins are secreted by the signaling cells, diffuse over short or long range [3] and act on target cells through either β-catenin-dependent or -independent Wnt pathways (reviewed in [4]). In the former case, when a given Wnt ligand binds to its cognate Frizzled receptor(s) and LRP5/6 co-receptors, this results in a complex intracellular cascade, leading to β-catenin stabilization. β-catenin then translocates into the nucleus, associates with LEF/TCF family transcription factors and induces the transcriptional activation of Wnt target genes such as CyclinD1 and Axin2 [5,6].
The developmental expression of Wnt/β-catenin pathway components has been extensively described in vertebrate animal models, including expression of various Wnt ligands and Wnt receptors (reviewed in [4]). However, multiple extracellular, cytoplasmic, and nuclear inputs are integrated and modulate Wnt signaling. For example, receptor-ligand specificity and multiple feedback loops control Wnt signaling efficiency (reviewed in [4]). Additionally, it was recently described in zebrafish that Wnt8a can be transported over long distances within the signaling cell through filopodia, increasing Wnt signaling range [7]. This makes it difficult to infer the Wnt responsive tissue from the site of ligand synthesis. Another way to describe Wnt activity is to study the expression of known direct target genes. However, CyclinD1, for instance, is transcriptionally regulated by many other inducers and repressors (reviewed in [8,9]) and thus is not a strict readout for Wnt activity. Axin2, also widely used as reporter, is not fully reliable either: for example it is found not to be expressed in mouse lung cells while Wnt/β-catenin pathway is active in these cells [10].
Finally, it is possible to follow Wnt/β-catenin activity by using transgenic lines allowing monitoring the spatial and temporal activity through the expression of a reporter gene (gfp or lacZ). The majority of these lines are generated in mice (Table 1). These lines often rely on the expression of a reporter gene driven by a Wnt target gene promoter. However, these lines may be questionable since the reporter gene expression differs significantly between different reporter mice [10] probably due to specific regulation of each promoter.
Table 1. Wnt reporter transgenic lines in vertebrates.
| Transgenic line names | Species | References |
|---|---|---|
| TOP-GALC | mouse | [11] |
| ins-TOPEGFP and ins-TOPGAL | mouse | [12] |
| Lgr5-EGFP-IRES-creERT2 | mouse | [13] |
| LEF-EGFP | mouse | [14] |
| TCF/Lef:H2B-GFP reporter TCF/Lef-LacZ | mouse | [15] |
| Axin2-CreERT2 | mouse | [16] |
| Tcf3-CreER | mouse | [17] |
| p-LEF7-fos-GFP | Xenopus | [18] |
| TOP/FOPTK-iGFP | Xenopus | [19] |
| pbin7Lef-dGFP | Xenopus | [20] |
| TOPdGFP | zebrafish | [21] |
| Tcf/Lef-miniP:dGFP | zebrafish | [22] |
| Tg(7xTCF-Xla.Siam:GFP)ia4 and Tg(7xTCF-Xla.Siam:nlsmCherry)ia5 | zebrafish | [23] |
To obtain a more direct and reliable readout for Wnt/β-catenin signaling, we here use a synthetic promoter harboring seven optimal binding sequences for LEF-1/TCF [24]. A transgenic reporter line, in which gfp gene expression is driven by this synthetic promoter, was generated in the frog Xenopus tropicalis, allowing visualization of Wnt/β-catenin activity in vivo. The line was validated previously as a reliable tool to monitor Wnt activity in tadpoles treated with compounds known to modulate Wnt activity: activation with 6-bromoindirubin-3-oxime (BIO), a selective GSK-3 inhibitor [25], or inhibition with IWR-1, a small molecule that prevents Axin protein degradation [26], [27]. This transgenic line, was previously used to study Wnt activity during eye or brain development [27–29], allows generating many transgenic embryos with reproducible in vivo expression patterns [20].
Here, we provide a detailed atlas illustrating Wnt/β-catenin spatio-temporal activity during Xenopus tropicalis embryogenesis, using whole-mount in situ hybridization and serial transverse sections at various developmental stages, from gastrula (stage 11) to tadpole (stage 40) stages. We provide a complete collection of pictures in supplementary data (Figs A-Q in S1 File). Moreover, we provide in-depth analysis of Wnt activity during three selected developmental processes: neural tube patterning, neural crest specification and migration and retinal development. We take advantage of this study to compare our observations with the data scattered in various previous articles.
Materials and methods
Ethics statement
Animal care and experimentation were conducted in accordance with institutional and national guidelines, under the institutional licenses (number B 91-471-102 up to 2012 and C 91-471-102 since 2013). Protocols were approved by the “Comité d’éthique en experimentation animale n°118” and received an authorization by the “Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche” under the reference APFIS#7043.
Embryos
Xenopus tropicalis transgenic embryos were obtained by conventional methods of hormone-induced egg laying and in vitro fertilization [30] between a wild type female and a transgenic male Tg(pbin7Lef-dGFP), carrying the Wnt reporter previously described (Image A in S1 Fig; [20,24]). Beforehand, the male was selected as having a single insertion site of the transgene (as inferred by mendelian ratios in its progeny) in order to insure homogeneous levels of gfp expression in the offspring [27]. Embryos were grown, collected and fixed in 4% paraformaldehyde (PFA) from embryonic stage 11 to stage 40 according to Nieuwkoop and Faber’s staging table of development [31]. The embryos were then washed in 1x PBS, dehydrated in 100% methanol, and stored at –20 °C.
In situ hybridization and sectioning
Digoxigenin-labeled antisense RNA probes were generated according to the manufacturer’s instructions (DIG RNA Labeling Mix, Roche) from the following plasmids: enr2 [32], fezf2 [33], krox20 [34], pax3 [35], otx2 [36], snai2 [37], sox2 [38], twist [39] and wnt1 [37]. A digoxigenin-labeled antisense RNA probe and a fluorescein-labeled antisense RNA probe (fluorescein-12-UTP, Roche) were generated from the plasmid pCS2-MT-eGFP (a gift from David Turner, University of Michigan, Ann Arbor, USA). For embryos under NF stage 20, single whole-mount in situ hybridization (WISH) was carried out as previously described [40]. For later stages, WISH was carried out following a protocol described by Parain et al. [41], except for the bleaching treatment that we performed after embryo staining. Briefly, following overnight incubation with the probe and then with alkaline phosphatase-conjugated anti-DIG antibody, enzymatic activity was revealed using NBT/BCIP substrate. Of note, the described patterns were observed in all the examined embryos (n≥8 for each probe). For double in situ hybridization, DIG-labeled probes were revealed with NBT/BCIP substrates and the fluorescein-labeled gfp probe was revealed with Fast Red substrate (Roche). After the first revelation, the embryos were treated by 10mM EDTA in PBS for 30 minutes at 60°C, then in 0.1M Glycine-HCl, at pH 2.2 for 10 minutes at RT, then processed for the second revelation. Sections (40μm thick) were cut using a Leica VT1000 vibratome after gelatin-albumin embedding. Sections were mounted in glycerol. The same embryo has been used to generate all of the pictures provided at a given stage (whole mount and sections).
Microscopy
Whole-mount images were captured using a stereomicroscope Lumar V12 equipped with bright field and color camera (Zeiss). Pictures of sections were captured using a digital Axiocam MRc camera on a Leica microscope and processed with AxioVision REL 7.8 and Adobe Photoshop CS4 softwares.
Results and discussion
An atlas of Wnt activity during development
To provide an atlas depicting canonical Wnt/β-catenin activity during embryogenesis, we took advantage of the transgenic Xenopus tropicalis Wnt reporter line, Tg(pbin7Lef-dGFP), described in [20] (Image A in S1 Fig). Briefly, the transgene, flanked by chromosomal insulator sequences derived from the chicken ß-globin locus, contains a synthetic promoter harboring seven copies of an optimal binding sequence for LEF-1/TCF upstream of eGFP coding sequence. Because a weak GFP fluorescence signal can be difficult to distinguish from the natural auto-fluorescence of the embryos, and to obtain a clear staining in tissue with low levels of expression, we used whole-mount in situ hybridization with a gfp antisense probe to detect the Wnt/β-catenin activity. From gastrula (stage 11) to tadpole (stage 40) stages, whole-mount-stained embryos were pictured from different views (anterior, dorsal, posterior and lateral) and serial transverse vibratome sections were then cut (Figs 1–4, Figs A-Q in S1 File).
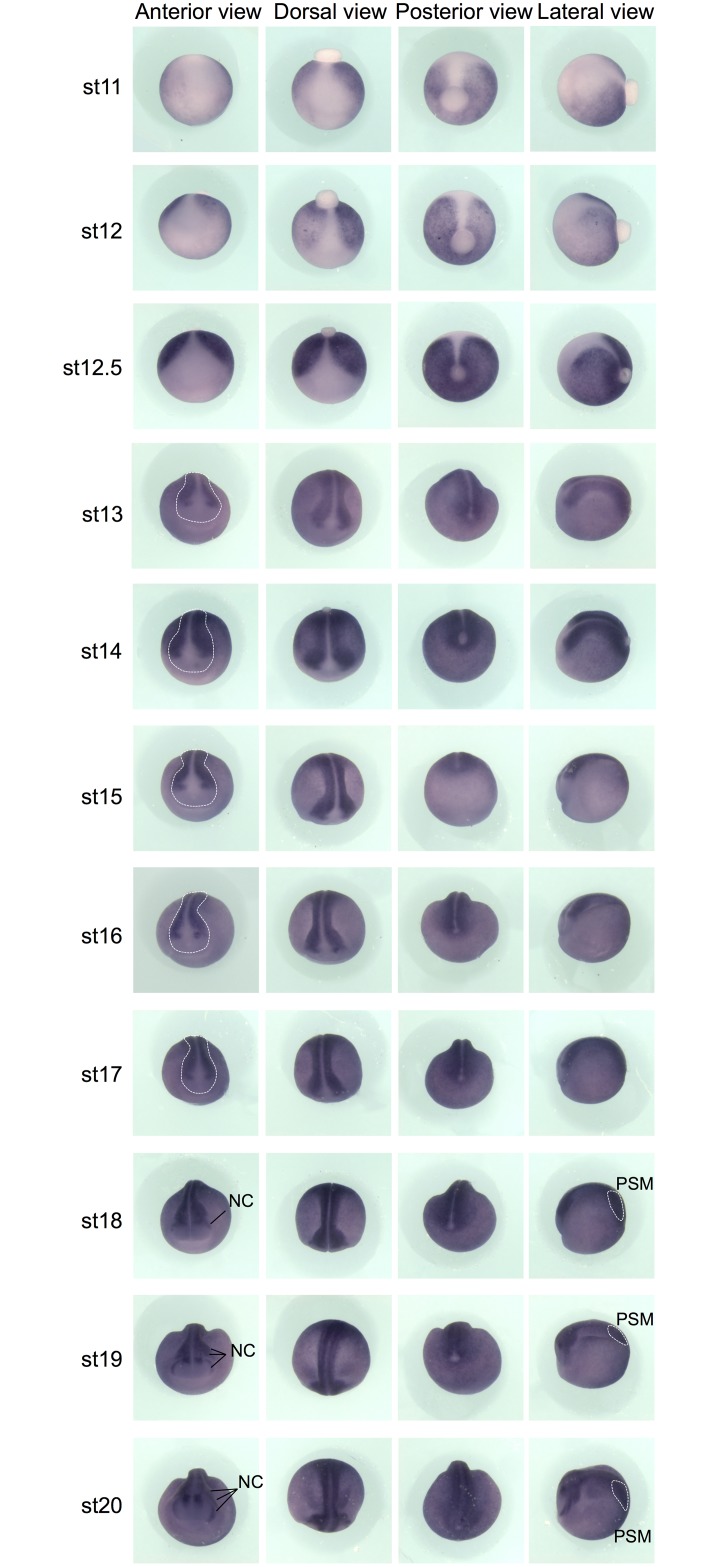
Fig 1. Wnt activity during gastrulation and neurulation in whole embryos.
Whole-mount gfp in situ hybridization on Tg(pbin7Lef-dGFP) embryos from stage 11 to stage 20. For each stage, anterior, dorsal, posterior and lateral views are shown. White dotted lines on anterior views delineate the prospective central nervous system during neurulation. NC: migrating neural crest cells, PSM: posterior presomitic mesoderm.
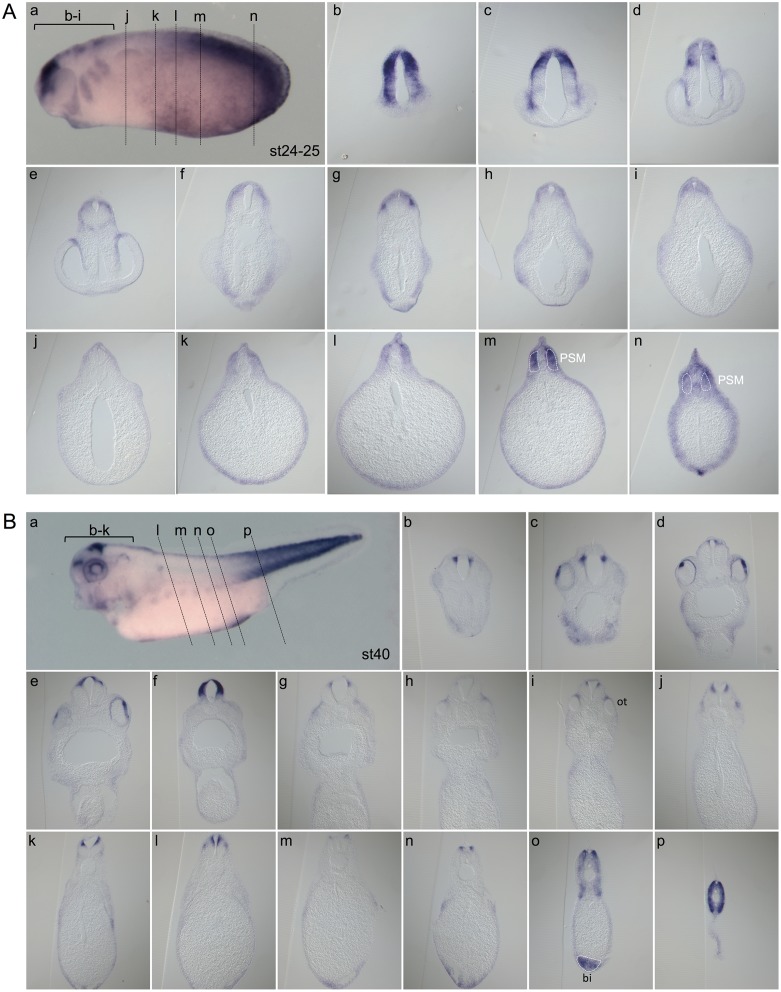
Fig 4. Wnt activity at stages 24–25 and 40.
Whole-mount gfp in situ hybridization (Aa, and Ba, lateral views) of Tg(pbin7Lef-dGFP) embryos at stage 24–25 (A) and stage 40 (B). For each stage, transverse sections are shown (Ab-n and Bb-p). The different levels of sections are indicated in panels a. bi: blood islands; ot: otic vesicle. PSM: posterior presomitic mesoderm.
During gastrulation (stage 11 to 12.5), gfp transcripts are detected around the whole embryo except in the anterior and dorsal region (Fig 1). Transverse sections at stage 12.5 show a staining restricted to the inner ectodermal cell layer called “sensory" (or basal) layer of the non-neural ectoderm (Image B in S1 Fig). This is consistent with Xenopus experiments illustrating that Wnt/β-catenin signaling regulates specification and differentiation of cells in Xenopus mucociliary epidermis ([42], reviewed in [43]).
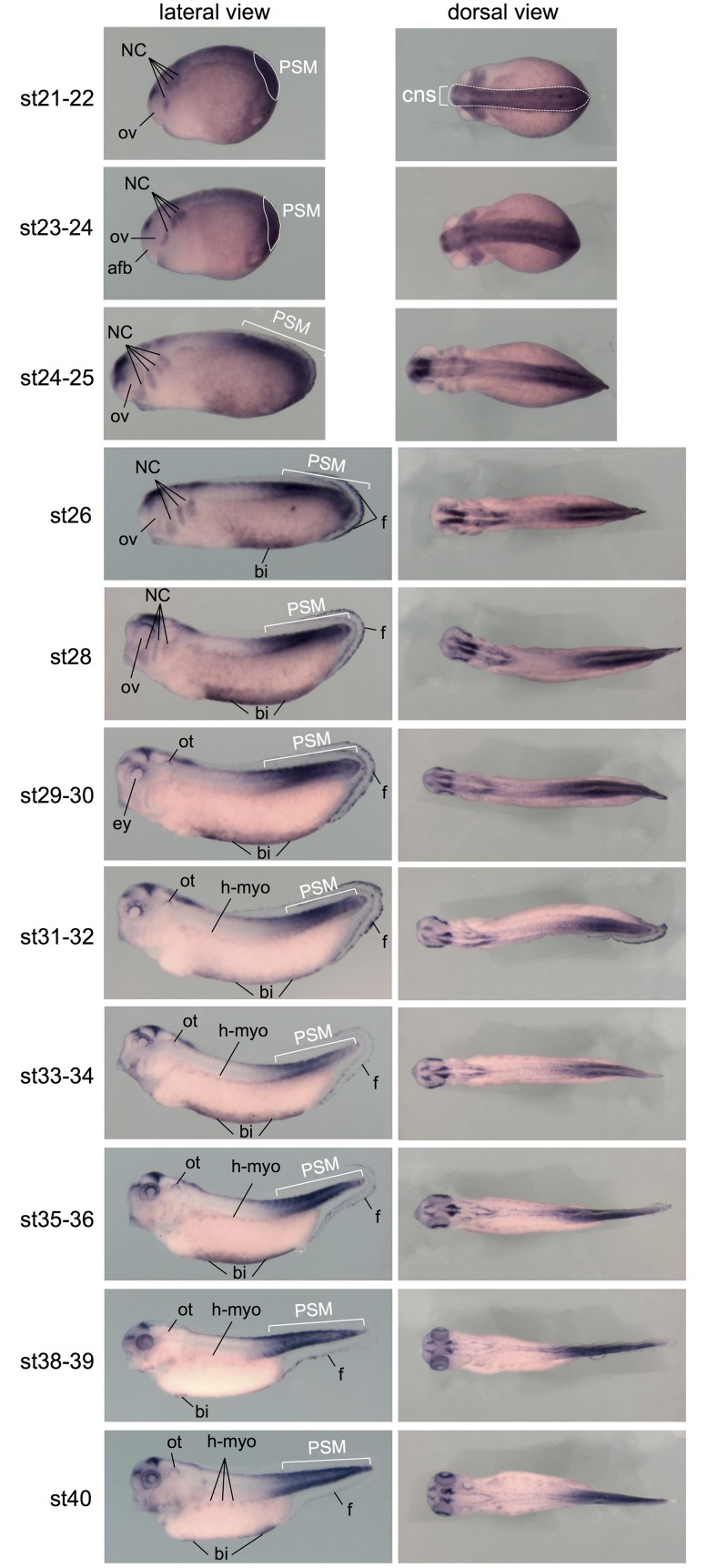
From the end of gastrulation (stage 13) onwards, Wnt activity is detected in the developing central nervous system, except in its anterior-most region (Fig 1, dotted lines). From late neurula stage (stage 18) onwards, gfp transcripts become particularly abundant in the migrating neural crest cells. The canonical Wnt/β-catenin pathway is also strongly active in the posterior presomitic mesoderm (Fig 1). This is consistent with data showing nuclear β-catenin translocation during maturation of this structure in mouse [44]. On sections, we observe a faint staining in the somites at stage 14 (Fig 2, white dotted lines). The somite staining increases to reach a strong level at stage 20. Previous studies in chick and mouse have shown that Wnt signaling promotes the dermomyotome fate and not the sclerotome fate during somite patterning [45,46]. In Xenopus, the majority of somite cells express myoD indicating that they are almost all myotome cells [47]. Consistently, we show here that somites are entirely Wnt-responsive (Fig 2 and Image C in S1 Fig). From stage 21, gfp mRNAs are detected in the central nervous system, in the neural crest cells migrating towards the branchial arches, and in the periphery of the optic vesicle (Fig 3). These patterns will be described in the following paragraphs. Until the tadpole stage, we observe that the canonical Wnt/β-catenin pathway is still strongly active in the posterior presomitic mesoderm (Figs 3, 4Am and 4An). From stage 31–32, a weak Wnt activity is detected in myotome cells that have emigrated from the somite to form hypaxial muscles (Fig 3). From stage 29–30, expression in the otic vesicles is also apparent, and persists until tadpole stage (Fig 3). The staining is restricted to the dorsal part of the otic vesicle, as confirmed on sections (Fig 4Bi). We can also note that, whereas ventral and dorsal Xenopus fins have independent induction and formation processes [48], they are both Wnt-responsive from stage 26 to stage 31–32 (Fig 3 and Image D in S1 Fig). Finally, Wnt/β-catenin activity is also detected in the ventral blood islands from stage 26 (Figs 3 and 4Bo), where its role on specification and maintenance of the primitive blood cells has been demonstrated [24].
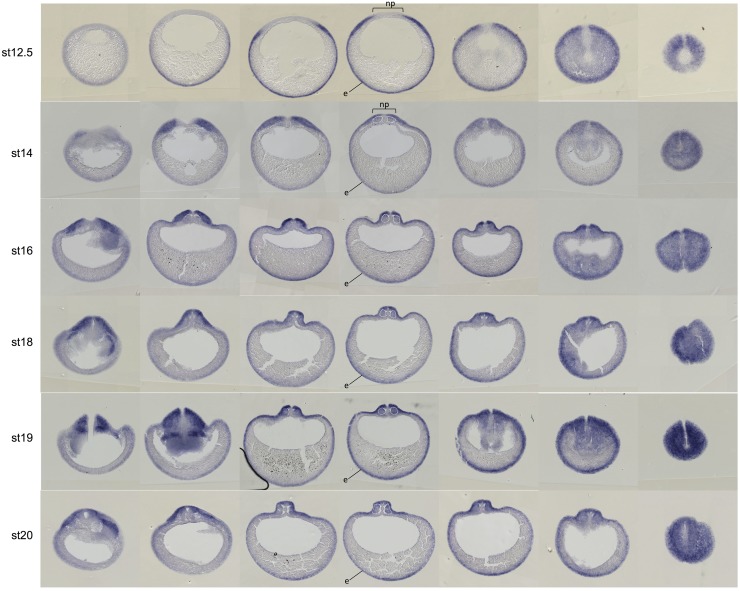
Fig 2. Wnt activity during gastrulation and neurulation in cross-sections.
Embryo transverse (or cross-)sections following whole-mount gfp in situ hybridization on Tg(pbin7Lef-dGFP) embryos, from stage 12.5 to stage 20. For each stage, 7 serial sections are shown. White dotted lines delineate the somites. e: nonneural ectoderm; np: neural plate: brackets indicate the approximate width of the neural plate.
Fig 3. Wnt activity during organogenesis in whole embryos.
Whole-mount gfp in situ hybridization on Tg(pbin7Lef-dGFP) embryos from stage 21 to stage 40. For each stage, lateral (side) and dorsal views are shown afb: anterior part of the forebrain; bi: blood islands; cns: central nervous system; ey: eye; f: fins; h-myo: hypaxial myoblast; NC: migrating neural crest cells; ot: otic vesicle; ov: optic vesicle; PSM: posterior presomitic mesoderm.
Wnt activation during neural tube development
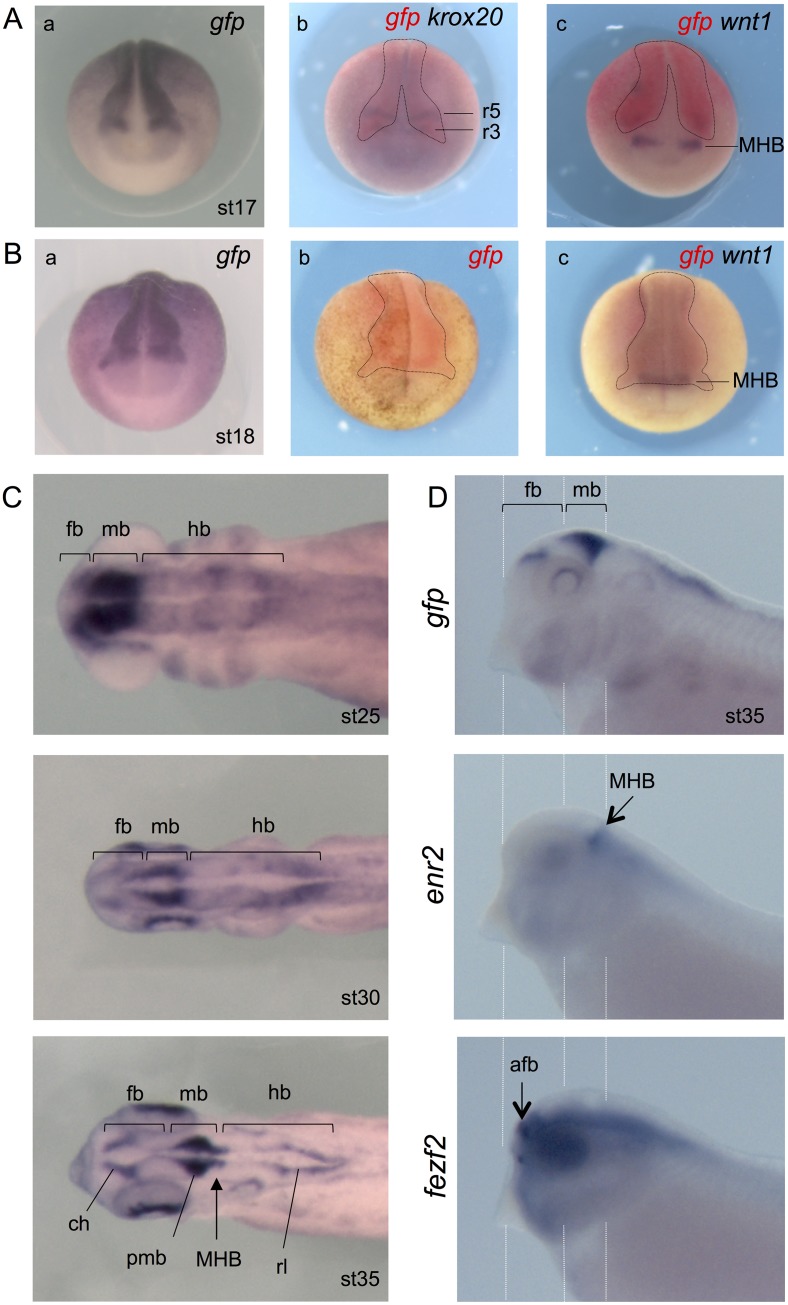
In situ hybridization at stage 12.5 and 13 shows an exclusion of gfp transcripts from most of the anterior neural plate (Fig 2), as confirmed by double in situ hybridization with the neural plate marker sox2 (Fig 1 and Image E in S1 Fig). This is consistent with the well-known role of Wnt signaling on posteriorization of the neuroectoderm [49]. On sections at stage 14, we observe the absence of Wnt activity in the medial region of the neural plate (Fig 2). From the end of neurulation (stage 18), Wnt activity is detected in the dorsal and dorsal-lateral part of the neural tube and is excluded from its ventral-lateral part and from the floorplate (Image F in S1 Fig). During neurulation, we observe a shift of Wnt activity towards the anterior region. At stage 17, the anterior boundary of the staining co-localizes with krox20 at the level of the rhombomere 3 and is posterior to the expression pattern of wnt1, a marker of the midbrain/hindbrain boundary (MHB) (Fig 5A). At stage 18, the anterior limit of Wnt activity corresponds to the wnt1 domain (Fig 5B).
Fig 5. Wnt activity during neural tube development.
(A) Anterior views of Tg(pbin7Lef-dGFP) embryos at stage 17 hybridized with probes against gfp alone (a) or gfp and krox20 or gfp (b) or gfp and wnt1(c). Dotted lines delineated the gfp staining. (B) Anterior views of Tg(pbin7Lef-dGFP) embryos at stage 18 hybridized with probes against gfp alone revealed with NBT/BCIP (a) or Fast Red (b) substrates or gfp and wnt1 (c). The same embryo is shown in b and c. Dotted lines delineate the gfp staining. (C) Dorsal views of embryos hybridized with probe against gfp at stage 25, 30 and 35. (D) Lateral views of the anterior part of embryos hybridized with probes against gfp, fezf2 or enr2 at stage 35. afb: anterior forebrain; ch: cortical hem; fb: forebrain; hb: hindbrain; mb: midbrain; MHB: Midbrain Hindbrain Boundary; pmb: posterior midbrain; r3 and r5: rhombomeres 3 and 5; rl: rhombic lip.
At stage 21–24, Wnt signaling is active all along the developing central nervous system, except in the more anterior region, i.e. located anterior to the optic vesicles and corresponding to the anterior part of the forebrain (Fig 3). From stage 25, discontinuities in the staining appear in the central nervous system, some areas being less labeled than others (Fig 5C). We observe a very high gfp expression in the midbrain and at least a part of the forebrain. In the hindbrain, domains with high or low gfp expression alternate, consistent with the role of the Wnt signaling in the zebrafish hindbrain metamerization [50]. Later, from stage 30, the staining in the brain is more restricted. We observe Wnt activity in the posterior region of the midbrain, which seems to be just anterior to the MHB as suggested by the comparison with the engrailed (enr2) mRNA hybridization, a MHB marker (Fig 5D). Moreover, gfp transcripts become detectable in a brain region derived from the forebrain. This gfp positive region seems to partially co-localize with fezf2 expression pattern, a marker of the anterior part of the diencephalon (prosomere p3) and of the telencephalon [51] (Fig 5D). This region is probably the cortical hem, an organizing center in the telencephalon known to present enriched expression of multiple members of the Wnt morphogens [52–54]. The cortical hem gives rise to the sub-ependymal zone where reside adult neural stem cells in rodents and human. At the level of the hindbrain, Wnt activity is detected dorsally, in the lower rhombic lip (Fig 5C). Rhombic lip produces the granular neuron progenitors of the cerebellum. By using Wnt reporter mice, it has been shown that Wnt/β-catenin activity is present transiently at the embryonic rhombic lip during development of the mouse cerebellum [55]. Both the rhombic lip and cortical hem are germinal zones where neurogenesis takes place and neurons are distributed tangentially.
Wnt activity during neural crest specification/migration
Neural crest is a migratory cell population, which gives rise to many cell types such as neurons and glia of the peripheral nervous system, pigment cells, and progenitors of craniofacial mesenchyme and skeleton. These cells are specified at the border between the neural and nonneural ectoderm, an area named the neural (plate) border.
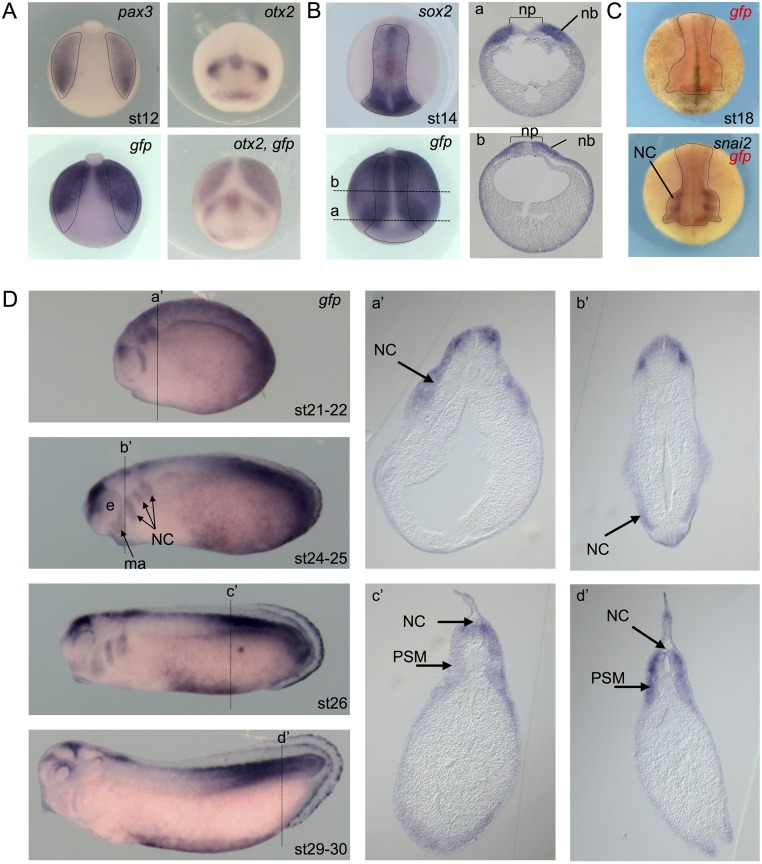
At stage 12, by using pax3 expression pattern (dotted lines on Fig 6A) to define the neural border, we observe that Wnt activity is present in the neural border except in its most anterior part [56]. This observation is confirmed by double in situ hybridization (Image G in S1 Fig). These data are consistent with the described involvement of Wnt signaling in posterior neural border specification [57–59]. In parallel, Wnt activity is excluded from the otx2-expressing domain, which labels the anterior part of the brain (Fig 6A and Image G in S1 Fig).
Fig 6. Wnt activity during neural crest formation.
(A) Dorsal view of a stage 12 Tg(pbin7Lef-dGFP) embryo hybridized with pax3 or gfp probes (posterior side is up). Dotted shapes delineate the presumptive neural border on both sides. Anterior view of a stage 12 Tg(pbin7Lef-dGFP) embryo hybridized with otx2 probe alone or otx2 and gfp probes together (dorsal side is up). (B) Dorsal view of a stage 14 Tg(pbin7Lef-dGFP) embryo hybridized with sox2 or gfp or both probes. Dotted shapes delineate the neural plate. The a and b dotted lines indicate the level of shown transverses sections. (C) Anterior views of a stage 18 Tg(pbin7Lef-dGFP) embryo first hybridized with probe against gfp and secondarily with probe against snai2. Dotted lines delineate gfp staining. (D) In situ hybridization against gfp on stage 21–22, 24–25, 26 and 29–30 Tg(pbin7Lef-dGFP) embryos (lateral views). For each stage, the dotted line indicates the level of the shown transverse section (a’-d’). ma: mandibular arch; nb: neural border; NC: migrating neural crest cells; np: neural plate; PSM: posterior presomitic mesoderm.
In the early neurula (stage 14), Wnt activity is detected in the entire neural border and lateral part of the neural plate (expressing sox2), excluding only the ventral neural plate, as confirmed on sections (Fig 6B). This is consistent with the Wnt role in neural crest specification [60–63].
From late neurula stage (stage 18), gfp transcripts are abundant in the migrating neural crest cells, as observed by comparing gfp expression pattern with that of snai2, a neural crest marker (Fig 6C). This location of the Wnt/β-catenin activity was recently described also in the chick [64]. Interestingly, the anterior boundary of the gfp staining corresponds to the more anterior migrating neural crests cells. At stage 24–25, Wnt activity is clearly detected in neural crest cells surrounding the eye, in the mandibular arch, and in the 3 following branchial arches (Fig 6D). This staining in the branchial arches disappears at stage 31–32, as neural crest cells reach their destination. A number of reports from different laboratories, using frog, zebrafish and chick embryos, have demonstrated that distinct elements of a non-canonical Wnt signaling, the PCP signaling, are essential for neural crest migration (reviewed in [65]). The activation of PCP signaling occurs at the cell–cell contact where it leads to the localized regulation of Rho and Rac proteins mediating directional migration of neural crest cells by controlling the formation of protrusions. Our observations suggest that Wnt signaling plays a role during neural crest cell migration not only through its PCP pathway but also through its β-catenin pathway. This is consistent with a recent paper demonstrating, through a combination of in vitro and in vivo approaches, that canonical Wnt activity is involved in neural crest migration and needs to be tightly controlled to enable it [66]. In the mouse head and branchial arch region, canonical Wnt activity is detected thanks to reporter transgenic lines in cranial neural crest cells at the neural folds, as well as in cells migrating into the face and branchial arches [14,15]. In zebrafish, interfering with LRP5 function, a co-receptor in canonical Wnt signaling, leads to a migration defects of neural crest cells [67]. Interestingly, it has been recently demonstrated in chick that the neural crest delamination requires cell-autonomously transient inhibition of Wnt signaling which needs to be reversible [64].
Wnt signaling and retinogenesis
Wnt signaling pathway is known to regulate many aspects of retinogenesis, including patterning, specification, proliferation, regeneration, but some of these functions appear to be species specific (reviewed in [68] and [69]). We previously examined Wnt activity in the Xenopus retina using both X. laevis and X. tropicalis transgenic reporter lines, in different contexts such as the determination of optimal concentrations and exposure conditions of pharmacological compounds or gene expression comparison [27,29,70]. Scattered data reporting retinal Wnt activity in Xenopus can thus be found in these studies at different stages of eye development. In order to provide a global view of Wnt activity during retinal development in a single set of data easily explorable by the community, we decided to describe in this atlas Wnt activity at all key stages of retinogenesis in both whole-mount embryos and in retinal sections.
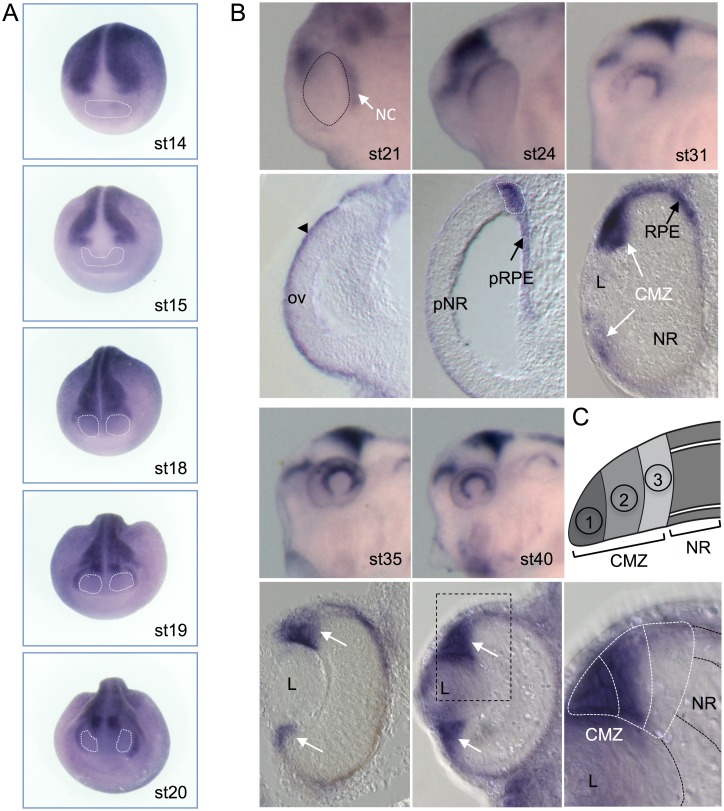
We do not detect any Wnt activity during gastrulation in the eye field (doted lines in the Fig 7A). This is consistent with data demonstrating that the eye field specification requires Wnt/ β-catenin signaling inhibition [71]. Nevertheless, several evidences indicate involvement of the non-canonical Wnt signaling, resulting in the expression of different Wnt pathway components in the eye field (Wnt11, fzd5) [72]. During neurulation, the eye field splits and evaginates laterally to form the optic vesicles. Again, Wnt reporter activity is not detectable in the forming optic vesicles (Fig 7B, stage 21). However, a signal can be detected in these optic vesicles from stage 24 onwards. It is located in the presumptive retinal pigmented epithelium (pRPE) and in the most dorsal part of the optic vesicle. Active Wnt/β-catenin signaling is detected in the pRPE not only in frogs but also in chickens, fishes, and mice [21,73–75]. The spatial and temporal regulation of Wnt/ β-catenin signaling has been shown to be essential for development of the RPE in mice [76–78] and chick [79]. The restricted activation of canonical Wnt signaling in the dorsal part of the optic vesicle has also been reported in other species [73–75,79]. Our observation is consistent with the hypothesis that Wnt/β-catenin activity plays a crucial role in the maintenance of dorsal retinal identity [75,78]. It was further suggested in mouse that this dorsal Wnt activity is involved in the establishment of the proper boundary between neural versus non-neural territories in the retina [80]. This region in Xenopus has been proposed to give rise to adult retinal stem cells [29].
Fig 7. Wnt activity during retinogenesis.
(A) Whole-mount gfp in situ hybridization on Tg(pbin7Lef-dGFP) embryos from stage 14 to stage 20. Anterior views with the eye fields (dotted lines) are shown. (B) In situ hybridization against gfp on stage 21 to stage 40 Tg(pbin7Lef-dGFP) embryos. Whole-mount (lateral views of the head, anterior to the left) and transverse retinal sections are shown. Arrowhead points to surface ectoderm, black arrows to the Retinal Pigmented Epithelium (RPE) and presumptive RPE (pRPE), white arrows to the Ciliary Marginal Zone (CMZ). Black dotted lines delineate the optic vesicle. White dotted lines delineate the boundary between neural versus non-neural territories in the retina. (C) Schematic of a CMZ showing its spatial organisation with stem cells closest to the periphery (region 1), proliferative retinoblasts in the middle (region 2) and postmitotic cells at the central edge (region 3). On the bottom, an enlargement of the region delineated with black dotted lines in the stage 40 retinal section image shows the gfp signal in the peripheral half of the CMZ. White dotted lines delineate the 3 zones depicted in the schema. A strong staining is observed in zone 1, a fainter staining is detected in zone 2 and barely no staining is observed in zone 3. CMZ: Ciliary Marginal Zone; L: lens; NC: migrating neural crest cells; ov: optic vesicle; pNR: presumptive Neural Retina, pRPE: presumptive Retinal Pigmented Epithelium.
At stages 31 and 35, Wnt activity labeling remains visible in the developing RPE. Signal is also detected in the most peripheral part of the neural retina, the forming ciliary marginal zone (CMZ) (Fig 7B and 7C). This region contains retinal stem and progenitor cells allowing for retinal growth throughout the animal life [81]. In the mature retina (stage 40), Wnt activity is confined to the peripheral half of the CMZ where stem cells and young progenitors reside. Of note, the staining is often stronger in the dorsal side of the CMZ, consistent with the dorso-ventral gradient observed in whole-mount embryos, with no or very low staining in the most ventral side at the position of the optic fissure. We previously showed that Wnt activity in the post-embryonic CMZ is essential for retinal stem cell proliferative maintenance [27,70]. Other LEF/TCF reporters revealed activation of the Wnt/β-catenin pathway in the ciliary margin of other species [73,74]. Moreover, in mouse and chick, Wnt signaling has been described to be involved in development of this region [82,83].
To summarize, here we present a detailed atlas illustrating Wnt/β-catenin activity in Xenopus tropicalis from gastrula to tadpole stages. This library of serial pictures allows to analyse spatial and temporal activity of Wnt pathway and thereby to predict new roles of this signaling pathway. We observe that Wnt/β-catenin pathway is active in many structures during development and especially in proliferative zones of the central nervous system such as the rhombic lip, the cortical hem and the retinal ciliary marginal zone. It is also active in non-neural tissues and interestingly in the sensory layer of the epidermis, which will give rise to the stratum germinativum where stem cells will reside.
Supporting information
(Fig A) stage 12.5, (Fig B) stage 14, (Fig C) stage 16, (Fig D) stage 18, (Fig E) stage 19, (Fig F) stage 20, (Fig G) stage 21–22, (Fig H) stage 23–24, (Fig I) stage 24–25, (Fig J) stage 26, (Fig K) stage 28, (Fig L) stage 29–30, (Fig M) stage 31–32, (Fig N) stage 33–34, (Fig O) stage 35–36, (Fig P) stage 38–39, (Fig Q) stage 40. The same embryo has been used to generate all of the images provided at a given stage.
(ZIP)
(A) Schematic of the Wnt reporter construct containing chicken β-globin insulators [20]. (B, C) Embryo transverse sections following whole-mount gfp in situ hybridization on Tg(pbin7Lef-dGFP) embryos, at stage 12.5 (B) and 22 (C). Right panels are enlargement of blue squares. Black dotted lines delineate the somites. (D) Whole-mount gfp in situ hybridization of Tg(pbin7Lef-dGFP) embryos at stages 29–30 and transversal sections at indicated level showing gfp staining in the dorsal and ventral fins. (E) Dorsal views of a stage 13 Tg(pbin7Lef-dGFP) embryo first hybridized with probe against gfp and secondarily with probe against sox2. Black dotted lines delineate the gfp-expressing domain. (F) Embryo transverse sections following whole-mount gfp in situ hybridization on Tg(pbin7Lef-dGFP) embryos, at stage 18 and 24–25. The squared regions delineated with the dotted line were enlarged. White dotted lines delineate the neural tube. (G) Dorsal view of a stage 12 Tg(pbin7Lef-dGFP) embryo hybridized with gfp and pax3 probes or gfp and otx2 probes (posterior side is up). Dotted lines delineate gfp staining. d-f: dorsal fins; ectod.: ectoderm; ins: fp: floorplate; insulator; mesod.: mesoderm; n: notochord; np: neural plate; NT: neural tube; v-f: ventral fins.
(TIF)
Acknowledgments
We thank B. Durand for providing fezf2 and enr2 probes; P. Pla for critical reading of the manuscript; A. Chesneau for animal care.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Fondation pour la Recherche Medicale [https://www.frm.org/] (grant number DEQ20150331733), Funding to AHMB; Agence Nationale de la Recherche [www.agence-nationale-recherche.fr] (grant number ANR-Blanc e SVSE2 -2011-CRESTNET, ANR-15-CE13-0012-01-CRESTNETMETABO), Funding to AHMB; Fondation pour la Recherche Medicale [https://www.frm.org/] (grant number DEQ20150331739), Funding to MP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Logan CY, Nusse R. The Wnt Signaling Pathway in Development and Disease. Annu Rev Cell Dev Biol. 2004;20: 781–810. doi: 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- 2.Clevers H, Nusse R. Wnt/β-Catenin Signaling and Disease. Cell. 2012;149: 1192–1205. doi: 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 3.Boutros M, Niehrs C. Sticking Around: Short-Range Activity of Wnt Ligands. Dev Cell. Elsevier Inc.; 2016;36: 485–486. doi: 10.1016/j.devcel.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 4.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13: 767–779. doi: 10.1038/nrm3470 [DOI] [PubMed] [Google Scholar]
- 5.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96: 5522–5527. doi: 10.1073/pnas.96.10.5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jho E, Zhang T, Domon C, Joo C-K, Freund J-N, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22: 1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanganello E, Hagemann AIH, Mattes B, Sinner C, Meyen D, Weber S, et al. Filopodia-based Wnt transport during vertebrate tissue patterning. Nat Commun. Nature Publishing Group; 2015;6: 5846 doi: 10.1038/ncomms6846 [DOI] [PubMed] [Google Scholar]
- 8.Klein EA, Assoian RK. Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci. 2008;121: 3853–3857. doi: 10.1242/jcs.039131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen B, Shimizu M, Izrailit J, Ng NFL, Buchman Y, Pan JG, et al. Cyclin D1 is a direct target of JAG1-mediated Notch signaling in breast cancer. Breast Cancer Res Treat. 2009;123: 113–124. doi: 10.1007/s10549-009-0621-9 [DOI] [PubMed] [Google Scholar]
- 10.Al Alam D, Green M, Irani RT, Parsa S, Danopoulos S, Sala FG, et al. Contrasting Expression of Canonical Wnt Signaling Reporters TOPGAL, BATGAL and Axin2 LacZ during Murine Lung Development and Repair. PLoS One. 2011;6: e23139 doi: 10.1371/journal.pone.0023139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boras-Granic K, Chang H, Grosschedl R, Hamel PA. Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev Biol. 2006;295: 219–231. doi: 10.1016/j.ydbio.2006.03.030 [DOI] [PubMed] [Google Scholar]
- 12.Moriyama A, Kii I, Sunabori T, Kurihara S, Takayama I, Shimazaki M, et al. GFP transgenic mice reveal active canonical Wnt signal in neonatal brain and in adult liver and spleen. Genesis. 2007;45: 90–100. doi: 10.1002/dvg.20268 [DOI] [PubMed] [Google Scholar]
- 13.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449: 1003–1007. doi: 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- 14.Currier N, Chea K, Hlavacova M, Sussman DJ, Seldin DC, Dominguez I. Dynamic Expression of a LEF-EGFP WNT Reporter in Mouse Development and Cancer. 2010;48: 183–194. doi: 10.1002/dvg.20604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis A-K. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ß-catenin signaling in the mouse. BMC Dev Biol. 2010;10: 121 doi: 10.1186/1471-213X-10-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Amerongen R, Bowman AN, Nusse R. Developmental Stage and Time Dictate the Fate of Wnt/β-Catenin-Responsive Stem Cells in the Mammary Gland. Cell Stem Cell. 2012;11: 387–400. doi: 10.1016/j.stem.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 17.Howard JM, Nuguid JM, Ngole D, Nguyen H. Tcf3 expression marks both stem and progenitor cells in multiple epithelia. Development. 2014;141: 3143–3152. doi: 10.1242/dev.106989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng X, Xiao L, Lin GF, Hu R, Wang JH, Rupp RAW, et al. Lef/Tcf-dependent Wnt/β-catenin signaling during Xenopus axis specification. FEBS Lett. 2003;547: 1–6. doi: 10.1016/S0014-5793(03)00639-2 [DOI] [PubMed] [Google Scholar]
- 19.Denayer T, Van Roy F, Vleminckx K. In vivo tracing of canonical Wnt signaling in Xenopus tadpoles by means of an inducible transgenic reporter tool. FEBS Lett. 2006;580: 393–8. doi: 10.1016/j.febslet.2005.11.084 [DOI] [PubMed] [Google Scholar]
- 20.Tran HT, Vleminckx K. Design and use of transgenic reporter strains for detecting activity of signaling pathways in Xenopus. Methods. 2014;66: 422–432. doi: 10.1016/j.ymeth.2013.06.028 [DOI] [PubMed] [Google Scholar]
- 21.Dorsky RI, Sheldahl LC, Moon RT. A transgenic Lef1/beta-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev Biol. 2002;241: 229–37. doi: 10.1006/dbio.2001.0515 [DOI] [PubMed] [Google Scholar]
- 22.Shimizu N, Kawakami K, Ishitani T. Visualization and exploration of Tcf/Lef function using a highly responsive Wnt/Β-catenin signaling-reporter transgenic zebrafish. Dev Biol. 2012;370: 71–85. doi: 10.1016/j.ydbio.2012.07.016 [DOI] [PubMed] [Google Scholar]
- 23.Moro E, Ozhan-Kizil G, Mongera A, Beis D, Wierzbicki C, Young RM, et al. In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Dev Biol. 2012;366: 327–340. doi: 10.1016/j.ydbio.2012.03.023 [DOI] [PubMed] [Google Scholar]
- 24.Tran HT, Sekkali B, Van Imschoot G, Janssens S, Vleminckx K. Wnt/β-catenin signaling is involved in the induction and maintenance of primitive hematopoiesis in the vertebrate embryo. Proc Natl Acad Sci USA. 2010;107: 16160–16165. doi: 10.1073/pnas.1007725107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meijer L, Skaltsounis A-L, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, et al. GSK-3-Selective Inhibitors Derived from Tyrian Purple Indirubins. Chem Biol. 2003;10: 1255–1266. doi: 10.1016/j.chembiol.2003.11.010 [DOI] [PubMed] [Google Scholar]
- 26.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan C-W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5: 100–7. doi: 10.1038/nchembio.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borday C, Cabochette P, Parain K, Mazurier N, Janssens S, Tran HT, et al. Antagonistic cross-regulation between Wnt and Hedgehog signalling pathways controls post-embryonic retinal proliferation. Development. 2012;139: 3499–3509. doi: 10.1242/dev.079582 [DOI] [PubMed] [Google Scholar]
- 28.Pourebrahim R, Houtmeyers R, Ghogomu S, Janssens S, Thelie A, Tran HT, et al. Transcription factor Zic2 inhibits Wnt/β-catenin protein signaling. J Biol Chem. 2011;286: 37732–37740. doi: 10.1074/jbc.M111.242826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Yakoubi W, Borday C, Hamdache J, Parain K, Tran HT, Vleminckx K, et al. Hes4 controls proliferative properties of neural stem cells during retinal ontogenesis. Stem Cells. 2012;30: 2784–2795. doi: 10.1002/stem.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sive H.L., Grainger R.M., Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Press, Cold Spring Harbor N, editor. 2000. [Google Scholar]
- 31.Nieuwkoop N, Faber J. Normal Table of Xenopus laevis. 3rd Ed Garland NY, editor. 1994. [Google Scholar]
- 32.Hemmati-Brivanlou a, de la Torre JR, Holt C, Harland RM. Cephalic expression and molecular characterization of Xenopus En-2. Development. 1991;111: 715–24. Available: http://www.ncbi.nlm.nih.gov/pubmed/1679005 [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Seguel E, Alarcón P, Gómez-Skarmeta JL. The Xenopus Irx genes are essential for neural patterning and define the border between prethalamus and thalamus through mutual antagonism with the anterior repressors Fezf and Arx. Dev Biol. 2009;329: 258–268. doi: 10.1016/j.ydbio.2009.02.028 [DOI] [PubMed] [Google Scholar]
- 34.Nieto MA, Bradley LC, Wilkinson DG. Conserved segmental expression of Krox-20 in the vertebrate hindbrain and its relationship to lineage restriction. Development. 1991;Suppl 2: 59–62. Available: http://www.ncbi.nlm.nih.gov/pubmed/1688180 [PubMed] [Google Scholar]
- 35.Monsoro-Burq AH, Wang E, Harland R. Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev Cell. 2005;8: 167–178. doi: 10.1016/j.devcel.2004.12.017 [DOI] [PubMed] [Google Scholar]
- 36.Lamb TM, Knecht aK, Smith WC, Stachel SE, Economides aN, Stahl N, et al. Neural induction by the secreted polypeptide noggin. Science. 1993;262: 713–718. doi: 10.1126/science.8235591 [DOI] [PubMed] [Google Scholar]
- 37.Grammer TC, Liu KJ, Mariani FV., Harland RM. Use of large-scale expression cloning screens in the Xenopus laevis tadpole to identify gene function. Dev Biol. 2000;228: 197–210. doi: 10.1006/dbio.2000.9945 [DOI] [PubMed] [Google Scholar]
- 38.Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125: 579–587. [DOI] [PubMed] [Google Scholar]
- 39.Hopwood ND, Pluck A, Gurdon JB. A Xenopus mRNA related to Drosophila twist is expressed in response to induction in the mesoderm and the neural crest. Cell. 1989;59: 893–903. doi: 10.1016/0092-8674(89)90612-0 [DOI] [PubMed] [Google Scholar]
- 40.Monsoro-Burq AH. A rapid protocol for whole-mount in situ hybridization on Xenopus embryos. CSH Protoc. 2007;2007: pdb.prot4809. doi: 10.1101/pdb.prot4809 [DOI] [PubMed] [Google Scholar]
- 41.Parain K, Mazurier N, Bronchain O, Borday C, Cabochette P, Chesneau A, et al. A large scale screen for neural stem cell markers in Xenopus retina. Dev Neurobiol. 2012;72: 491–506. doi: 10.1002/dneu.20973 [DOI] [PubMed] [Google Scholar]
- 42.Walentek P, Beyer T, Hagenlocher C, Müller C, Feistel K, Schweickert A, et al. ATP4a is required for development and function of the Xenopus mucociliary epidermis—a potential model to study proton pump inhibitor-associated pneumonia. Dev Biol. 2015;408: 292–304. doi: 10.1016/j.ydbio.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walentek P, Quigley IK. What we can learn from a tadpole about ciliopathies and airway diseases: Using systems biology in Xenopus to study cilia and mucociliary epithelia. Genesis. 2017;55: 1–12. doi: 10.1002/dvg.23001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aulehla A, Wiegraebe W, Baubet V, Wahl MB, Deng C, Taketo M, et al. A β-catenin gradient links the clock and wavefront systems in mouse embryo segmentation. Nat Cell Biol. 2008;10: 186–193. doi: 10.1038/ncb1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan C-M, Lee CS, Tessier-Lavigne M. A Role for WNT Proteins in Induction of Dermomyotome. Dev Biol. 1997;191: 160–165. doi: 10.1006/dbio.1997.8713 [DOI] [PubMed] [Google Scholar]
- 46.Wagner J, Schmidt C, Nikowits W, Christ B. Compartmentalization of the Somite and Myogenesis in Chick Embryos Are Influenced by Wnt Expression. Dev Biol. 2000;228: 86–94. doi: 10.1006/dbio.2000.9921 [DOI] [PubMed] [Google Scholar]
- 47.Pegoraro C, Pollet N, Monsoro-Burq AH. Tissue-specific expression of Sarcoplasmic/Endoplasmic Reticulum Calcium ATPases (ATP2A/SERCA) 1, 2, 3 during Xenopus laevis development. Gene Expr Patterns. Elsevier B.V.; 2011;11: 122–128. doi: 10.1016/j.gep.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 48.Tucker A s., Slack J m. w. Independent induction and formation of the dorsal and ventral fins in Xenopus laevis. Dev Dyn. 2004;230: 461–467. doi: 10.1002/dvdy.20071 [DOI] [PubMed] [Google Scholar]
- 49.Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128: 4189–4201. [DOI] [PubMed] [Google Scholar]
- 50.Riley BB, Chiang M-Y, Storch EM, Heck R, Buckles GR, Lekven AC. Rhombomere boundaries are Wnt signaling centers that regulate metameric patterning in the zebrafish hindbrain. Dev Dyn. 2004;231: 278–291. doi: 10.1002/dvdy.20133 [DOI] [PubMed] [Google Scholar]
- 51.Zhang S, Li J, Lea R, Vleminckx K, Amaya E. Fezf2 promotes neuronal differentiation through localised activation of Wnt/β-catenin signalling during forebrain development. Development. Oxford University Press for The Company of Biologists Limited; 2014;141: 4794–805. doi: 10.1242/dev.115691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125: 2315–2325. Available: http://dev.biologists.org/content/125/12/2315 [DOI] [PubMed] [Google Scholar]
- 53.Grove EA. NEUROSCIENCE: Organizing the Source of Memory. Sci (New York, NY). 2008;319: 288–289. doi: 10.1126/science.1153743 [DOI] [PubMed] [Google Scholar]
- 54.Mangale VS, Hirokawa KE, Satyaki PRV, Gokulchandran N, Chikbire S, Subramanian L, et al. Lhx2 Selector Activity Specifies Cortical Identity and Suppresses Hippocampal Organizer Fate. Science (80-). 2008;319: 304–309. doi: 10.1126/science.1151695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selvadurai HJ, Mason JO. Wnt/β-catenin signalling is active in a highly dynamic pattern during development of the mouse cerebellum. PLoS One. 2011;6: 6–13. doi: 10.1371/journal.pone.0023012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plouhinec J, Medina-Ruiz S, Borday C, Bernard E, Vert J, Eisen MB, et al. A molecular atlas of the developing ectoderm defines neural, neural crest, placode, and nonneural progenitor identity in vertebrates. PLoS biology. 2017. doi: 10.1371/journal.pbio.2004045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Crozé N, Maczkowiak F, Monsoro-Burq AH. Reiterative AP2a activity controls sequential steps in the neural crest gene regulatory network. Proc Natl Acad Sci U S A. 2011;108: 155–60. doi: 10.1073/pnas.1010740107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milet C, Monsoro-Burq AH. Neural crest induction at the neural plate border in vertebrates. Dev Biol. Elsevier B.V.; 2012;366: 22–33. doi: 10.1016/j.ydbio.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 59.Steventon B, Mayor R. Early neural crest induction requires an initial inhibition of Wnt signals. Dev Biol. Elsevier Inc.; 2012;365: 196–207. doi: 10.1016/j.ydbio.2012.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998;125: 2403–2414. [DOI] [PubMed] [Google Scholar]
- 61.Lewis JL, Bonner J, Modrell M, Ragland JW, Moon RT, Dorsky RI, et al. Reiterated Wnt signaling during zebrafish neural crest development. Development. 2004;131: 1299–1308. doi: 10.1242/dev.01007 [DOI] [PubMed] [Google Scholar]
- 62.Patthey C, Edlund T, Gunhaga L. Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development. 2009;136: 73–83. doi: 10.1242/dev.025890 [DOI] [PubMed] [Google Scholar]
- 63.Monsoro-Burq A-H, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development. The Company of Biologists Ltd; 2003;130: 3111–24. doi: 10.1242/dev.00531 [DOI] [PubMed] [Google Scholar]
- 64.Rabadán MA, Herrera A, Fanlo L, Usieto S, Carmona-Fontaine C, Barriga EH, et al. Delamination of neural crest cells requires transient and reversible Wnt inhibition mediated by Dact1/2. Development. Oxford University Press for The Company of Biologists Limited; 2016;143: 2194–205. doi: 10.1242/dev.134981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mayor R, Theveneau E. The role of the non-canonical Wnt–planar cell polarity pathway in neural crest migration. Biochem J. 2014;457: 19–26. doi: 10.1042/BJ20131182 [DOI] [PubMed] [Google Scholar]
- 66.Maj E, Künneke L, Loresch E, Grund A, Melchert J, Pieler T, et al. Controlled levels of canonical Wnt signaling are required for neural crest migration. Dev Biol. Elsevier; 2016;417: 77–90. doi: 10.1016/j.ydbio.2016.06.022 [DOI] [PubMed] [Google Scholar]
- 67.Willems B, Tao S, Yu T, Huysseune A, Witten PE, Winkler C. The Wnt co-receptor Lrp5 is required for cranial neural crest cell migration in zebrafish. PLoS One. 2015;10: 1–21. doi: 10.1371/journal.pone.0131768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fuhrmann S. Wnt signaling in eye organogenesis. Organogenesis. 2008;4: 60 Available: https://www-ncbi-nlm-nih-gov.gate1.inist.fr/pmc/articles/PMC2613311/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fujimura N. WNT/β-Catenin Signaling in Vertebrate Eye Development. Front Cell Dev Biol. 2016;4: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Denayer T, Locker M, Borday C, Deroo T, Janssens S, Hecht A, et al. Canonical Wnt signaling controls proliferation of retinal stem/progenitor cells in postembryonic Xenopus eyes. Stem Cells. 2008;26: 2063–74. doi: 10.1634/stemcells.2007-0900 [DOI] [PubMed] [Google Scholar]
- 71.Esteve P, Bovolenta P. Secreted inducers in vertebrate eye development: More functions for old morphogens. Curr Opin Neurobiol. 2006;16: 13–19. doi: 10.1016/j.conb.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 72.Cavodeassi F, Carreira-Barbosa F, Young RM, Concha ML, Allende ML, Houart C, et al. Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/β-catenin pathway. Neuron. 2005;47: 43–56. doi: 10.1016/j.neuron.2005.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho S-H, Cepko CL. Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006;133: 3167–3177. doi: 10.1242/dev.02474 [DOI] [PubMed] [Google Scholar]
- 74.Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA. Mapping canonical Wnt signaling in the developing and adult retina. Investig Ophthalmol Vis Sci. The Association for Research in Vision and Ophthalmology; 2006;47: 5088–5097. doi: 10.1167/iovs.06-0403 [DOI] [PubMed] [Google Scholar]
- 75.Veien ES, Rosenthal JS, Kruse-Bend RC, Chien C-B, Dorsky RI. Canonical Wnt signaling is required for the maintenance of dorsal retinal identity. Development. The Company of Biologists Ltd; 2008;135: 4101–11. doi: 10.1242/dev.027367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fujimura N, Taketo MM, Mori M, Korinek V, Kozmik Z. Spatial and temporal regulation of Wnt/β-catenin signaling is essential for development of the retinal pigment epithelium. Dev Biol. Elsevier Inc.; 2009;334: 31–45. doi: 10.1016/j.ydbio.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 77.Westenskow P, Piccolo S, Fuhrmann S. Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression. Development. 2009;136: 2505–10. doi: 10.1242/dev.032136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hägglund AC, Berghard A, Carlsson L. Canonical Wnt/β-catenin signalling is essential for optic cup formation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Westenskow PD, McKean JB, Kubo F, Nakagawa S, Fuhrmann S. Ectopic mitf in the embryonic chick retina by co-transfection of β-catenin and Otx2. Investig Ophthalmol Vis Sci. 2010;51: 5328–5335. doi: 10.1167/iovs.09-5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heavner WE, Andoniadou CL, Pevny LH. Establishment of the neurogenic boundary of the mouse retina requires cooperation of SOX2 and WNT signaling. Neural Dev. 2014;9: 27 doi: 10.1186/1749-8104-9-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol. 1998;199: 185–200. doi: 10.1006/dbio.1998.8939 [DOI] [PubMed] [Google Scholar]
- 82.Liu H, Xu S, Wang Y, Mazerolle C, Thurig S, Coles BLK, et al. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev Biol. 2007;308: 54–67. doi: 10.1016/j.ydbio.2007.04.052 [DOI] [PubMed] [Google Scholar]
- 83.Trimarchi JM, Cho SH, Cepko CL. Identification of genes expressed preferentially in the developing peripheral margin of the optic cup. Dev Dyn. 2009;238: 2327–2339. doi: 10.1002/dvdy.21973 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(Fig A) stage 12.5, (Fig B) stage 14, (Fig C) stage 16, (Fig D) stage 18, (Fig E) stage 19, (Fig F) stage 20, (Fig G) stage 21–22, (Fig H) stage 23–24, (Fig I) stage 24–25, (Fig J) stage 26, (Fig K) stage 28, (Fig L) stage 29–30, (Fig M) stage 31–32, (Fig N) stage 33–34, (Fig O) stage 35–36, (Fig P) stage 38–39, (Fig Q) stage 40. The same embryo has been used to generate all of the images provided at a given stage.
(ZIP)
(A) Schematic of the Wnt reporter construct containing chicken β-globin insulators [20]. (B, C) Embryo transverse sections following whole-mount gfp in situ hybridization on Tg(pbin7Lef-dGFP) embryos, at stage 12.5 (B) and 22 (C). Right panels are enlargement of blue squares. Black dotted lines delineate the somites. (D) Whole-mount gfp in situ hybridization of Tg(pbin7Lef-dGFP) embryos at stages 29–30 and transversal sections at indicated level showing gfp staining in the dorsal and ventral fins. (E) Dorsal views of a stage 13 Tg(pbin7Lef-dGFP) embryo first hybridized with probe against gfp and secondarily with probe against sox2. Black dotted lines delineate the gfp-expressing domain. (F) Embryo transverse sections following whole-mount gfp in situ hybridization on Tg(pbin7Lef-dGFP) embryos, at stage 18 and 24–25. The squared regions delineated with the dotted line were enlarged. White dotted lines delineate the neural tube. (G) Dorsal view of a stage 12 Tg(pbin7Lef-dGFP) embryo hybridized with gfp and pax3 probes or gfp and otx2 probes (posterior side is up). Dotted lines delineate gfp staining. d-f: dorsal fins; ectod.: ectoderm; ins: fp: floorplate; insulator; mesod.: mesoderm; n: notochord; np: neural plate; NT: neural tube; v-f: ventral fins.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.