Abstract
Alveolar epithelial cells are critical to the pathogenesis of pulmonary inflammation and fibrosis, which are associated with overexpression of type 2 cytokine IL-13. IL-13 is known to induce the production of profibrotic (e.g., periostin) and pro-inflammatory (e.g., eotaxin-3) mediators in human airway epithelial cells, but it remains unclear if human primary alveolar epithelial cells increase periostin and eotaxin expression following IL-13 stimulation. The goals of this study are to determine if alveolar epithelial cells increase periostin and eotaxin expression upon IL-13 stimulation, and if alveolar and airway epithelial cells from the same subjects have similar responses to IL-13. Paired alveolar and airway epithelial cells were isolated from donors without any lung disease, and cultured under submerged or air-liquid interface conditions with or without IL-13. Up-regulation of periostin protein and mRNA was observed in IL-13-stimulated alveolar epithelial cells, which was comparable to that in IL-13-stimulated paired airway epithelial cells. IL-13 also increased eotaxin-3 expression in alveolar epithelial cells, but the level of eotaxin mRNA was lower in alveolar epithelial cells than in airway epithelial cells. Our findings demonstrate that human alveolar epithelial cells are able to produce periostin and eotaxin in responses to IL-13 stimulation. This study suggests the need to further determine the contribution of alveolar epithelial cell-derived mediators to pulmonary fibrosis.
Introduction
Fibrotic lung diseases such as idiopathic pulmonary fibrosis (IPF) remain a significant challenge in clinical care, and highlight the need of more rigorous basic and translational research to define their pathogenesis and effective therapy [1–3]. Previous studies suggest that type 2 cytokines, particularly IL-13, are involved in the inflammatory and fibrotic processes [4, 5]. In airways diseases such as asthma, IL-13 has been shown to increase the expression of periostin and eotaxins (e.g., eotaxin-3) that are the key mediators in airway remodeling and eosinophilic inflammation [6, 7]. Interestingly, eosinophilic inflammation also exists in pulmonary fibrosis, and has been used as a marker of disease progression [8]. Periostin is a matricellular protein that is expressed by many types of cells, particularly in diseased conditions [9, 10]. It has multiple functions, including tissue repair and remodeling (e.g., fibrosis). Various cytokines such as IL-13 and TGF-beta act on structural and immune cells to promote periostin production [11, 12].
Among the tissue structural cells involved in IPF, alveolar epithelial cells play a critical role [13, 14]. During lung injury, type I alveolar (ATI) cells are injured, which are then repaired and replaced by type II alveolar (ATII) cells, leading to restoration of alveolar structure [15]. However, under pathological conditions, ATI cells may not be repaired, which in turn promotes persistent injury and remodeling with fibrosis as the most common but deleterious outcome [16].
The regulation of periostin and eotaxins has been extensively studied in human large airway epithelial cells exposed to IL-13 [17–19]. Interestingly, eotaxins promote fibroblast migration, a key process of fibrosis [20]. However, whether periostin is up-regulated in alveolar epithelial cells by IL-13 is unclear. Moreover, whether alveolar and airway epithelial cells respond similarly to IL-13 stimulation regarding periostin and eotaxin expression has not been addressed. By leveraging a cell bank of paired primary human alveolar and airway epithelial cells at our institution, we performed a cell culture study to determine IL-13-mediated periostin and eotaxin-3 expression by alveolar epithelial cells. The use of paired samples overcame the concern of genetic determinants in comparing the results obtained from alveolar and airway epithelial cells. Unraveling the role of alveolar epithelial cells in terms of periostin and eotaxin expression likely improves our understanding of periostin and/or eotaxin as biomarkers of lung injury/fibrosis predicting disease activity/severity and therapeutic responses.
Materials and methods
Materials
Recombinant human IL-13 protein from the R&D Systems, Minneapolis, MN was reconstituted in 0.1% bovine serum albumin (BSA) and stored at -80°C. Bronchial epithelial cell growth medium (BEGM) with antibiotics was purchased from Lonza, Walkersville, MD, USA. Air-liquid interface culture media (F6 media) consisted of 1:1 ratio of bronchial epithelial basal medium (BEBM) and Dulbecco's modified eagle medium (DMEM) plus insulin, transferrin, epinephrine, bovine pituitary extract (BPE), gentamicin and amphotericin, bovine albumin (0.5 μg/ml, ethanolamine (80 μM), MgCl2 (0.3 mM), MgSO4 (0.4 mM), CaCl2 (1 mM), retinoic acid (30 ng/ml), hEGF (10 ng/ml). Bovine collagen I (3 mg/ml) was obtained from Advanced BioMatrix (San Diego, CA). RNA lysis buffer (RLT) was from Qiagen (Hilden, Germany). RIPA Western lysis buffer was purchased from Thermo-Fisher Scientific (Waltham, MA).
Human donor information
To isolate human primary ATII and airway epithelial cells, we obtained human lungs from de-identified organ donors whose lungs were not suitable for transplantation and donated for medical research through the National Disease Research Interchange (Philadelphia, PA), the International Institute for the Advancement of Medicine (Edison, NJ) or Donor Alliance of Colorado. The Institutional Research Board (IRB) at National Jewish Health approved this study (approval number: HS-2598). Donors were chosen based on lung function with a PaO2/FiO2 ratio of >225, no history of clinical lung diseases, a chest radiograph indicating no infection, and a time on the ventilator of <5 days. Table 1 shows the clinical information of five donors included for our current study who did not have any lung disease.
Table 1. Characteristics of donors included in the study.
| Subjects | Age (yrs) | Gender | Smoking status | Cause of death |
|---|---|---|---|---|
| 1 | 30 | Female | Smoker (1/2 pack for 15 yrs) |
Anoxia |
| 2 | 52 | Male | Smoker | Cerebrovascular accident (CVA) |
| 3 | 44 | Male | Smoker (1/2 pack for 30 yrs) |
CVA |
| 4 | 54 | Male | Smoker (1/2 pack for 30 yrs) |
Anoxia |
| 5 | 18 | Female | Non-smoker | Anoxia |
Isolation and culture alveolar epithelial cells
We modified the human primary ATII cell isolation method published by Fang et al. [21]. Briefly, the right middle lobe of the lung was perfused, lavaged, and then instilled with elastase (Worthington, Lakewood, NJ) for 40 min at 37°C. The lung was minced, and the cells were isolated by filtration and partially purified by centrifugation on a discontinuous density gradient made of Optiprep (Accurate Chemical Scientific, Westbury, NY) with densities of 1.080 and 1.040, and by negative selection with binding to IgG-coated Petri dishes (Sigma, St. Louis, MO). The isolated cells were resuspended in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 2.5 μg/ml amphotericin B, 100 μg/ml streptomycin, 100 μg/ml penicillin G (GIBCO, Life Technologies, Rockville, MD), and 10 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO).
For submerged culture, human primary ATII cells (2.0x106 cells/well) were plated on 12-well cell culture plates (Costar, Corning, NY) coated with rat-tail collagen (Thermo Fisher Scientific, Waltham, MA) in DMEM with 10% FBS (D10). On day 2, the culture medium was changed to DMEM with 5% FBS (D5) with recombinant human IL-13 protein (rhIL-13,10 ng/ml) (R&D systems, Minneapolis, MN) or 1% BSA (vehicle for rhIL-13). Cell lysates and culture medium were harvested at 24 or 48 hrs after stimulation.
For air-liquid interface (ALI) culture, human primary ATII cells (1.5x106 cells/well) were plated on Millicell hanging inserts (pore size 1.0 μm, membrane surface area 1.1 cm2) (EMD Millipore, Darmstadt, Germany) in 12 well plates coated with a mixture of 20% Matrigel (Corning Inc., Corning, NY) and 80% rat-tail collagen in D10 (apical 0.5 ml, basal 1.5 ml). On day 2 and day 4, the culture medium was changed to DMEM with 1% charcoal-stripped (CS) FBS (Thermo Fisher Scientific), 2 mM L-glutamine, 2.5 μg/ml amphotericin B, 100 μg/ml streptomycin, 100 μg/ml penicillin G, 10 μg/ml gentamicin, 10 ng/ml recombinant human keratinocyte growth factor (R&D systems) (KGF) and 10−8 M dexamethasone (Dex) designated as 1%CS with KGF and Dex (apical 50 μl, basal 1.2 ml). On day 6 the culture medium was changed to 1%CS with KGF and Dex with 10 ng/ml rhIL-13 or 1% BSA. Then, cell lysates and culture medium were harvested at 24 or 48 hrs after stimulation.
Because the data from 24 and 48 hrs were similar, we have presented the data from the 48 hour time point in the Results section.
Isolation and culture of human tracheobronchial airway epithelial cells
To isolate tracheobronchial epithelial (TBE) cells, tracheal and main bronchial tissue was digested with 0.1% protease in DMEM (GE Life Sciences, Logan, Utah) overnight at 4°C, and processed as previously described [22]. TBE at passage one were expanded in collagen-coated 60-mm tissue culture dishes in BEGM media at 37 °C, 5% CO2. When the cells were 80% confluent, they were trypsinized and seeded onto collagen-coated 12-well plates for submerged culture and transwell inserts for ALI cultures.
For submerged culture, TBE cells in BEGM media were seeded into 12-well plates (2x105 cells/well) coated with collagen. After 48 hours of seeding, the medium was changed, followed by IL-13 stimulation (10 ng/ml) or 0.1% BSA treatment. The cells were harvested after 24 and 48 hrs in RLT or in RIPA buffer with protease and phosphatase inhibitors. The supernatants were collected for ELISA.
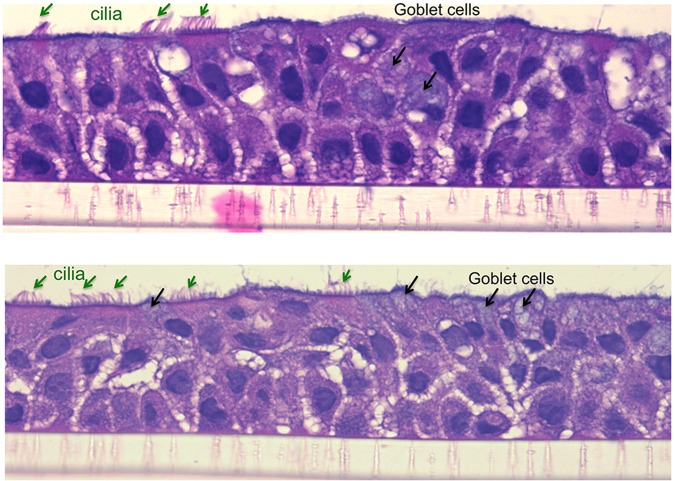
For ALI culture, TBE cells were seeded at 4x104 cells/insert onto collagen coated transwell inserts in 12-well plates with 300 μL of F6 media at apical surface, and 1.2 mL of F6 media at the basolateral side. After a week in the immersed culture condition, the cells reached 100% confluence and were shifted to ALI condition by reducing the apical medium volume to 50 μL. ALI conditions were maintained for 14 days when cells demonstrated mucociliary differentiation (Fig 1), and treatment with IL-13 (10 ng/ml) or 0.1% BSA started on day 15. The cells and the apical and basolateral media were harvested at 24 and 48 hrs post IL-13 treatment.
Fig 1. Mucociliary differentiation of human tracheobronchial epithelial (TBE) cells grown at the air-liquid-interface culture for 14 days.
H&E staining, original magnification x400.
Because the data from 24 and 48 hrs were similar, we have presented the data from the 48 hour time point in the Results section.
ELISA of human periostin and eotaxin-3
Periostin and eotaxin-3 protein levels were measured in cell supernatants using their specific DuoSet ELISA kits (R&D Systems, Minneapolis, MN) as per manufacturer’s instruction.
RT-PCR for human periostin and eotaxin-3
Real-time PCR was performed on the CFX96 (Bio-Rad) using TaqMan gene expression assays purchased from the Applied Biosystems (Life Technologies, Foster City, CA, USA). An identical threshold was applied to each gene of interest. mRNA relative expression levels were calculated using the delta cycle threshold (Ct) method. Target gene (e.g., periostin) expression was normalized to GAPDH.
Western blot analysis
Equal amounts of total proteins from samples with different treatments were separated on 15% SDS-PAGE, transferred onto polyvinylidene difluoride (PVDF) membranes, and probed with an anti-proSP-C antibody (Seven Hills Bioreagents, Cincinnati, OH), an anti-NKX2.1 antibody (R&D Systems, Minneapolis, MN), an anti-AQP5 antibody (Sigma-Aldrich, St. Louis, MO) or a mouse anti-ß-actin (Santa Cruz Biotechnology, Inc.). Blots were then incubated with appropriate HRP-linked secondary antibodies and developed with ECL Western blot substrate.
Immunoflurorescent staining of ATII cells
To confirm ATII differentiation of alveolar epithelial cells cultured at air-liquid interface, cells on Transwell membranes were fixed in 10% formalin, embedded in paraffin, and cut at 5 μm thickness for immunoflurescent staining. Briefly, cell sections were incubated with a mouse anti-human AT II cell primary antibody (provided by Dr. L. G. Dobbs, University of California, San Francisco), followed by incubation with a secondary antibody (goat anti-mouse IgM) conjugated with Texas red. After the cells were washed, DAPI was added to counterstain the nuclei. The cells were coverslipped and viewed under a fluorescence microscope.
Statistical analysis
The Mann-Whitney test was used for data comparisons of the two groups. A p value < 0.05 was considered significant.
Results
Alveolar epithelial cells increase periostin and eotaxin-3 expression upon IL-13 stimulation
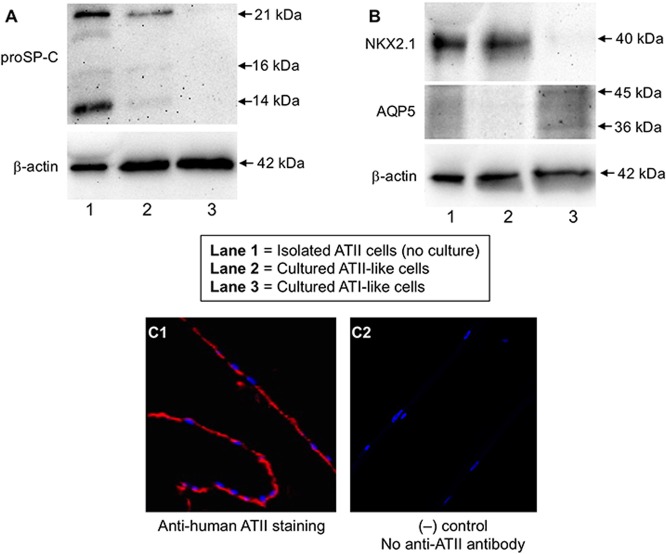
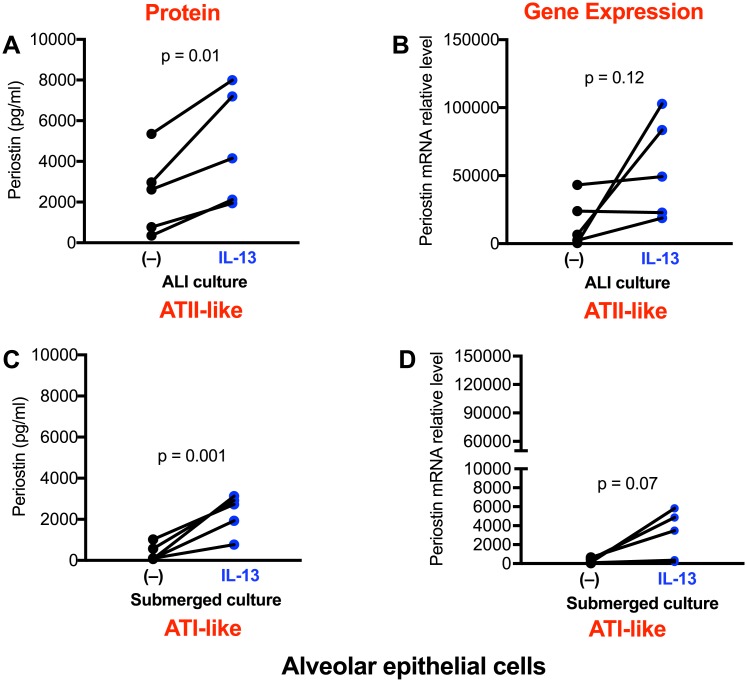
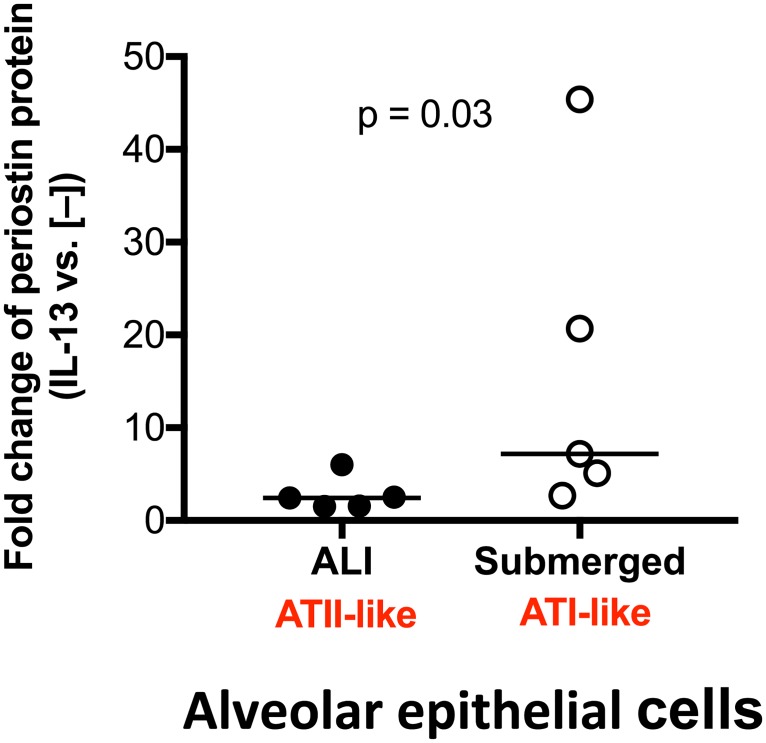
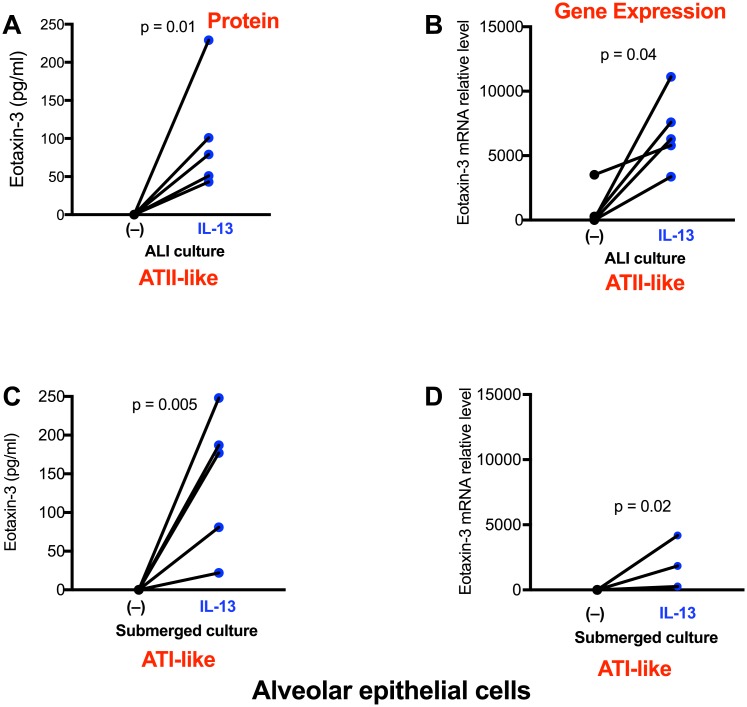
Under ALI culture, alveolar epithelial cells underwent ATII-like differentiation as confirmed by Western blot of proSP-C and NKX2.1, and immunofluorescent staining for ATII cells (Fig 2A, 2B and 2C). As shown in Fig 3A, alveolar epithelial cells cultured in ALI produced modest levels of periostin in basolateral supernatants. Periostin increased significantly following IL-13 stimulation. Periostin mRNA was also increased by IL-13, but was not statistically significant (p = 0.12) (Fig 3B). Under submerged culture, alveolar epithelial cells present an ATI-like phenotype as they expressed AQP5, but not proSP-C and NKX2.1 (Fig 2A and 2B). Like the ALI culture data, IL-13 increased periostin protein secretion (Fig 3C) and levels of periostin mRNA (Fig 3D), however the mRNA increase was not statistically significant. Of note, ATI-like cells expressed lower levels (14.2-fold, p = 0.008) of periostin mRNA than the ATII-like cells in the presence of IL-13 stimulation. However, the fold changes of periostin protein induction by IL-13 stimulation were greater (about 3-fold, p = 0.03) in ATI-like cells than ATII-like cells (Fig 4).
Fig 2. Confirmation of alveolar type II (ATII)-like cells cultured at the air-liquid interface.
Alveolar epithelial cells isolated from a donor were cultured for 7 days to differentiate into ATII-like cells. The cultured ATII-like cells, isolated (non-cultured) ATII cells (positive control) and cultured AT1-like cells from the same donor were processed for Western blot of proSP-C (A), NKX2.1 and AQP5 (B). Additional bands were observed for pro-SP-C (predicted size: 21 kDa) and AQP5 (predicted size: 36 kDa), which is likely due to post-translational modifications, post-translational cleavages, and/or other experimental factors. Cells on the transwell membrane were embedded in paraffin and cut into a 5 μm thickness section for immunofluorescent staining of ATII-like cells (C1) using an anti-ATII cell antibody as described in the Methods section. C2: the negative control—no addition of an anti-ATII cell antibody during immunofluorescent staining. The red and blue colors indicate the cytoplasm and nuclei of ATII-like cells, respectively.
Fig 3. Alveolar epithelial cells increase periostin expression after IL-13 treatment.
Cells under the air-liquid interface (A, B) and submerged (C, D) cultures were stimulated with IL-13 for 48 hrs. Periostin protein and mRNA were measured by ELISA and real-time RT-PCR, respectively. N = 5 donors.
Fig 4. Comparison of the fold changes of periostin protein induction by IL-13 stimulation in alveolar epithelial cells cultured under air-liquid interface (ALI) versus submerged conditions.
The horizontal bars indicate the medians. N = 5 donors.
Alveolar epithelial cells also demonstrated an increase in eotaxin-3 protein and mRNA expression (p<0.05) following the IL-13 treatment under both ALI and submerged conditions (Fig 5A to 5D). Upon IL-13 stimulation, eotaxin-3 mRNA levels in AT1-like cells were lower (3.4-fold, p = 0.02) than those in the ATII-like cells.
Fig 5. Alveolar epithelial cells increase eotaxin-3 expression after IL-13 treatment.
Cells under the air-liquid interface (A, B) and submerged (C, D) cultures were stimulated with IL-13 for 48 hrs. Eotaxin-3 protein and mRNA were measured by ELISA and real-time RT-PCR, respectively. N = 5 donors.
Comparison of IL-13-induced periostin and eotaxin-3 expression between alveolar epithelial cells and tracheobronchial epithelial (TBE) cells
IL-13 has been shown to induce periostin and eotaxin-3 in airway epithelial cells. However, the relative abundance of periostin and eotaxin expression in paired airway and alveolar epithelial cells from the same subjects has not been compared.
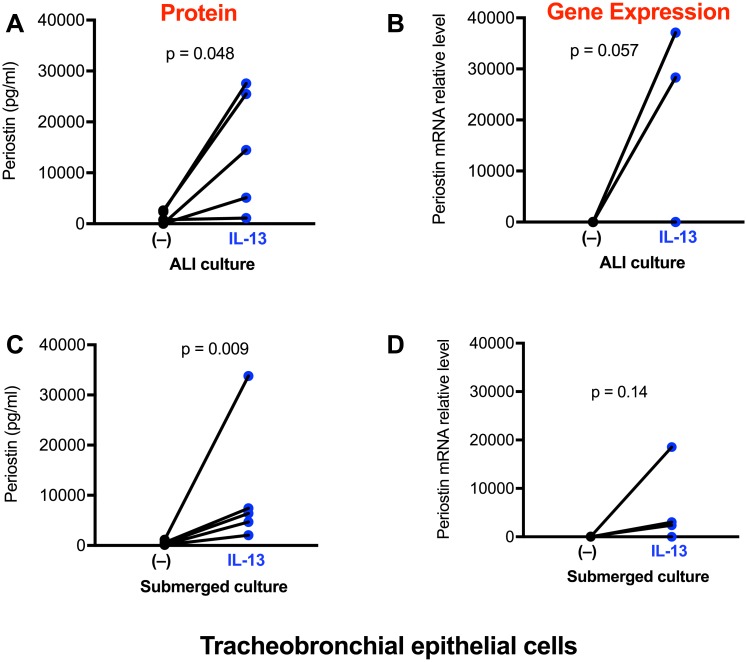
Under ALI condition, IL-13 consistently increased periostin protein production in TBE cells and the induced increase was statistically significant (Fig 6A). Although IL-13 in TBE cells increased periostin mRNA, there was no significant difference between the controls and IL-13-stimulated cells (Fig 6B). Notably, in the presence or absence of IL-13 stimulation, periostin mRNA levels trended to be lower (about 3-fold, p = 0.09) in well-differentiated TBE cells than ATII-like cells. Due to the differences of cell density and components of cell culture media between alveolar and airway epithelial cells, comparison of periostin proteins levels was not performed.
Fig 6. Tracheobronchial epithelial (TBE) cells increase periostin expression after IL-13 treatment.
Cells under the air-liquid interface (A, B) and submerged (C, D) cultures were stimulated with IL-13 for 48 hrs. Periostin protein and mRNA were measured by ELISA and real-time RT-PCR, respectively. N = 5 donors.
Under submerged culture, IL-13 also increased periostin protein expression in TBE cells (Fig 6C). However, the increase in periostin mRNA levels by IL-13 was not statistically significant (Fig 6D). Both TBE and alveolar epithelial cells demonstrated similar levels of periostin mRNA.
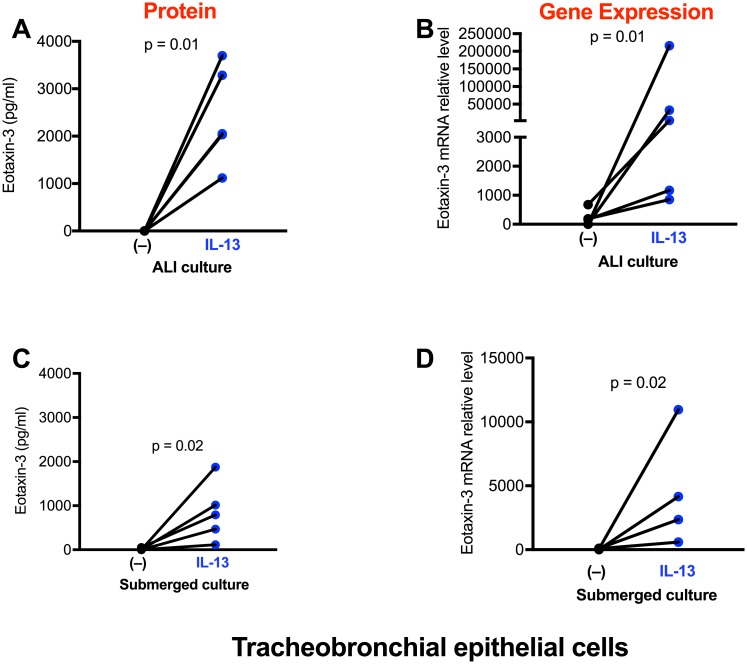
As expected, TBE cells expressed very low levels of eotaxin-3 mRNA and protein in the absence of IL-13 stimulation (Fig 7A to 7D). After IL-13 treatment, TBE cells significantly increased eotaxin-3 expression. TBE cells under ALI culture expressed higher levels of eotaxin-3 mRNA than cells under the submerged condition. Interestingly, unlike the periostin mRNA data, eotaxin-3 mRNA expression induced by IL-13 under ALI culture was similar (p = 0.84) between TBE cells and alveolar epithelial cells
Fig 7. Tracheobronchial epithelial (TBE) cells increase eotaxin-3 expression after IL-13 treatment.
Cells under the air-liquid interface (A, B) and submerged (C, D) cultures were stimulated with IL-13 for 48 hrs. Eotaxin-3 protein and mRNA were measured by ELISA and real-time RT-PCR, respectively. N = 5 donors.
Discussion
This is the first cell culture study to examine the expression of periostin and eotaxin in alveolar epithelial cells isolated from humans. We found that like the airway epithelial cells, alveolar epithelial cells increase periostin protein expression following IL-13 treatment. Notably, ATII-like cells expressed higher levels of periostin mRNA than ATI-like cells. Since periostin may contribute to fibrosis, our study may provide a rationale to inhibit periostin expression in alveolar epithelium under diseased conditions such as pulmonary fibrosis.
Examining the effects of IL-13 on alveolar epithelial periostin expression is significant. Alveolar epithelial cell injury and repair are critical to the pathogenesis of interstitial lung diseases such as pulmonary fibrosis. In this regard, ATII cell dysfunction is a common feature of fibrotic lungs [23]. Periostin expression is elevated in lung tissue and blood of patients with IPF [24, 25], however it remains unclear if alveolar epithelial cells contribute. Although ATII cells produce various profibrotic cytokines such as TGF-beta [26, 27], it is unknown whether they produce periostin at the normal and diseased conditions. We discovered that even at the baseline, ATII-like cells do express a detectable level of periostin mRNA and protein. The physiological relevance of periostin expression by “normal” ATII cells has not been investigated. Given the beneficial effect of periostin on wound repair [28], a “normal or physiological” level of periostin would be critical to maintain the alveolar structure and function. However, a pathological level of periostin, such as that significantly induced by IL-13, would be detrimental to the lung injury and repair. As periostin is able to induce fibroblast differentiation into myofibroblasts, and bind to other extracellular matrix components [9], targeting its aberrant up-expression would be pivotal to more effectively control the fibrotic process. Our results suggest the need to target alveolar epithelial cell-derived periostin in lungs exposed to type 2 cytokine IL-13.
The results from study on submerged culture of alveolar epithelial cells allow us to determine how normal ATI-like cells respond to IL-13 stimulation. Interestingly, ATI-like cells express much lower levels of periostin mRNA than ATII-like cells. These findings suggest that the ATI cells are likely less involved in the fibrotic process.
It is well established that IL-13 induces periostin in human primary airway epithelial cells [29], which may in part explain the profibrotic role of IL-13 in the airways. It is unclear whether IL-13-mediated periostin production is similar between alveolar and airway epithelial cells. By using the paired alveolar and airway epithelial cells from the same donors, we demonstrated that both types of epithelial cells robustly increase periostin expression following IL-13 treatment. Moreover, airway basal cells (submerged culture) and mucociliary cells (air-liquid interface culture) demonstrated a similar increase of periostin mRNA upon IL-13 stimulation. Together, when IL-13 is increased in the lung, the entire lung epithelial system, whether intact or injured, would respond by increasing periostin expression. As periostin has multiple functions involved in repair, fibrosis and inflammation [9], it remains to be determined if up-regulation of periostin by IL-13 in airway vs. alveolar epithelial cells would result in similar or different outcomes.
An additional finding in this study is to demonstrate the ability of alveolar epithelial cells to increase eotaxin expression upon IL-13 treatment. Although eosinophilia is a common feature in asthmatic airways, it also exists in the alveolar tissue of patients with pulmonary fibrosis [30–32]. Our results suggest that alveolar epithelial cells may contribute to eosinophil recruitment into fibrotic lungs under a type 2 cytokine (e.g., IL-13) milieu. Comparison of eotaxin-3 mRNA expression in paired alveolar and airway epithelial cells indicate that airway and alveolar epithelial cells respond similarly to IL-13. This finding further suggests the ability of entire lung epithelium to induce eosinophilic inflammation. Future studies are warranted to determine if a subset of pulmonary fibrosis patients with elevated type 2 cytokines such as IL-13 may increase eotaxin production coupled with worse fibrosis and clinical outcomes.
We are aware of several limitations in the current study. First, the cells we used were from donors without any lung disease. As there is no published evidence showing perisotin expression by ATII cells in IPF patients, it would be important to compare periostin and eotaxin expression levels in alveolar and airway epithelial cells isolated from lungs of patients with or without pulmonary fibrosis. Second, we used cells from both smokers and a non-smoker. As direct exposure of cultured lung epithelial cells to cigarette smoke affects eotaxin and periostin expression [33, 34], future studies are warranted to determine if smoking history has a significant impact on IL-13-mediated periostin and eotaxin expression in human airway and alveolar epithelial cells. Third, the varying baseline expression of periostin between ATI-like and ATII-like cells may be explained in part by the use of different media for submerged and air-liquid interface cultures of alveolar epithelial cells. Lastly, the functions of perisotin up-regulation by IL-13 were not determined in the context of epithelial cells and fibroblasts. We plan to utilize gene over-expression or knockdown approaches to reveal the impact of periostin on epithelial or fibroblast proliferation, secretion of surfactant proteins, mucins or collagens.
In summary, our research findings suggest that like the airway epithelial cells, alveolar epithelial cells represent one of the major sources of periostin in a type 2 inflammation setting. This work would set the foundation for future exploration of alveolar epithelial cells-derived periostin in various physiological and pathological processes.
Data Availability
All relevant data are within the paper.
Funding Statement
The research work described in this manuscript has been supported by the National Institutes of Health (NIH) grants R01HL125128, R01AI106287, R01HL122321, and 1U19AI125357 that were awarded to Hong Wei Chu. Among the authors, Yoko Ito, William Janssen, James Finigan and Hong Wei Chu received the salary support from these NIH grants. The funder (NIH) had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sgalla G, Biffi A, Richeldi L. Idiopathic pulmonary fibrosis: Diagnosis, epidemiology and natural history. Respirology. 2016;21(3):427–37. doi: 10.1111/resp.12683 [DOI] [PubMed] [Google Scholar]
- 2.Maher TM, Molina-Molina M, Russell AM, Bonella F, Jouneau S, Ripamonti E, et al. Unmet needs in the treatment of idiopathic pulmonary fibrosis-insights from patient chart review in five European countries. BMC pulmonary medicine. 2017;17(1):124 doi: 10.1186/s12890-017-0468-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenbloom J, Macarak E, Piera-Velazquez S, Jimenez SA. Human Fibrotic Diseases: Current Challenges in Fibrosis Research. Methods Mol Biol. 2017;1627:1–23. doi: 10.1007/978-1-4939-7113-8_1 [DOI] [PubMed] [Google Scholar]
- 4.Passalacqua G, Mincarini M, Colombo D, Troisi G, Ferrari M, Bagnasco D, et al. IL-13 and idiopathic pulmonary fibrosis: Possible links and new therapeutic strategies. Pulmonary pharmacology & therapeutics. 2017;45:95–100. doi: 10.1016/j.pupt.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 5.Murray LA, Zhang H, Oak SR, Coelho AL, Herath A, Flaherty KR, et al. Targeting interleukin-13 with tralokinumab attenuates lung fibrosis and epithelial damage in a humanized SCID idiopathic pulmonary fibrosis model. American journal of respiratory cell and molecular biology. 2014;50(5):985–94. doi: 10.1165/rcmb.2013-0342OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izuhara K, Nunomura S, Nanri Y, Ogawa M, Ono J, Mitamura Y, et al. Periostin in inflammation and allergy. Cell Mol Life Sci. 2017. doi: 10.1007/s00018-017-2648-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James A, Janson C, Malinovschi A, Holweg C, Alving K, Ono J, et al. Serum periostin relates to type-2 inflammation and lung function in asthma: Data from the large population-based cohort Swedish GA(2)LEN. Allergy. 2017. doi: 10.1111/all.13181 [DOI] [PubMed] [Google Scholar]
- 8.Peterson MW, Monick M, Hunninghake GW. Prognostic role of eosinophils in pulmonary fibrosis. Chest. 1987;92(1):51–6. [DOI] [PubMed] [Google Scholar]
- 9.O'Dwyer DN, Moore BB. The role of periostin in lung fibrosis and airway remodeling. Cell Mol Life Sci. 2017. doi: 10.1007/s00018-017-2649-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izuhara K, Conway SJ, Moore BB, Matsumoto H, Holweg CT, Matthews JG, et al. Roles of Periostin in Respiratory Disorders. American journal of respiratory and critical care medicine. 2016;193(9):949–56. doi: 10.1164/rccm.201510-2032PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, et al. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14170–5. doi: 10.1073/pnas.1009426107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohta N, Kurakami K, Ishida A, Furukawa T, Suzuki Y, Aoyagi M, et al. Roles of TGF-beta and periostin in fibrosclerosis in patients with IgG4-related diseases. Acta Otolaryngol. 2013;133(12):1322–7. doi: 10.3109/00016489.2013.831187 [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, Nielsen LD, Lucas JJ, Mason RJ. Transforming growth factor-beta antagonizes alveolar type II cell proliferation induced by keratinocyte growth factor. American journal of respiratory cell and molecular biology. 2004;31(6):679–86. doi: 10.1165/rcmb.2004-0182OC [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni T, de Andrade J, Zhou Y, Luckhardt T, Thannickal VJ. Alveolar epithelial disintegrity in pulmonary fibrosis. American journal of physiology Lung cellular and molecular physiology. 2016;311(2):L185–91. doi: 10.1152/ajplung.00115.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. American journal of physiology Lung cellular and molecular physiology. 2010;298(6):L715–31. doi: 10.1152/ajplung.00361.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasper M, Barth K. Potential contribution of alveolar epithelial type I cells to pulmonary fibrosis. Biosci Rep. 2017;37(6). doi: 10.1042/BSR20171301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, Fahy JV. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. The Journal of allergy and clinical immunology. 2014;133(2):388–94. doi: 10.1016/j.jaci.2013.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzaki I, Kawano S, Komiya K, Tanabe T, Akaba T, Asano K, et al. Inhibition of IL-13-induced periostin in airway epithelium attenuates cellular protein expression of MUC5AC. Respirology. 2017;22(1):93–100. doi: 10.1111/resp.12873 [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Guisa JM, Powers C, File D, Cochrane E, Jimenez N, Debley JS. Airway epithelial cells from asthmatic children differentially express proremodeling factors. The Journal of allergy and clinical immunology. 2012;129(4):990–7 e6. doi: 10.1016/j.jaci.2011.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohan M, Puxeddu I, Reich R, Levi-Schaffer F, Berkman N. Eotaxin-2/CCL24 and eotaxin-3/CCL26 exert differential profibrogenic effects on human lung fibroblasts. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2010;104(1):66–72. doi: 10.1016/j.anai.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 21.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, et al. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. American journal of physiology Lung cellular and molecular physiology. 2006;290(2):L242–9. Epub 2005/09/07. doi: 10.1152/ajplung.00178.2005 [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Jiang D, Smith S, Thaikoottathil J, Martin RJ, Bowler RP, et al. IL-13 dampens human airway epithelial innate immunity through induction of IL-1 receptor-associated kinase M. The Journal of allergy and clinical immunology. 2012;129(3):825–33 e2. Epub 2011/12/14. doi: 10.1016/j.jaci.2011.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulugeta S, Nureki S, Beers MF. Lost after translation: insights from pulmonary surfactant for understanding the role of alveolar epithelial dysfunction and cellular quality control in fibrotic lung disease. American journal of physiology Lung cellular and molecular physiology. 2015;309(6):L507–25. doi: 10.1152/ajplung.00139.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohta S, Okamoto M, Fujimoto K, Sakamoto N, Takahashi K, Yamamoto H, et al. The usefulness of monomeric periostin as a biomarker for idiopathic pulmonary fibrosis. PloS one. 2017;12(3):e0174547 doi: 10.1371/journal.pone.0174547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tajiri M, Okamoto M, Fujimoto K, Johkoh T, Ono J, Tominaga M, et al. Serum level of periostin can predict long-term outcome of idiopathic pulmonary fibrosis. Respir Investig. 2015;53(2):73–81. doi: 10.1016/j.resinv.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 26.Keating DT, Sadlier DM, Patricelli A, Smith SM, Walls D, Egan JJ, et al. Microarray identifies ADAM family members as key responders to TGF-beta1 in alveolar epithelial cells. Respiratory research. 2006;7:114 doi: 10.1186/1465-9921-7-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Velikoff M, Canalis E, Horowitz JC, Kim KK. Activated alveolar epithelial cells initiate fibrosis through autocrine and paracrine secretion of connective tissue growth factor. American journal of physiology Lung cellular and molecular physiology. 2014;306(8):L786–96. doi: 10.1152/ajplung.00243.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker JT, McLeod K, Kim S, Conway SJ, Hamilton DW. Periostin as a multifunctional modulator of the wound healing response. Cell Tissue Res. 2016;365(3):453–65. doi: 10.1007/s00441-016-2426-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J, Minami Y, Etling E, Coleman JM, Lauder SN, Tyrrell V, et al. Preferential Generation of 15-HETE-PE Induced by IL-13 Regulates Goblet Cell Differentiation in Human Airway Epithelial Cells. American journal of respiratory cell and molecular biology. 2017. doi: 10.1165/rcmb.2017-0031OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birring SS, Parker D, McKenna S, Hargadon B, Brightling CE, Pavord ID, et al. Sputum eosinophilia in idiopathic pulmonary fibrosis. Inflamm Res. 2005;54(2):51–6. doi: 10.1007/s00011-004-1321-x [DOI] [PubMed] [Google Scholar]
- 31.Boomars KA, Schweizer RC, Zanen P, van den Bosch JM, Lammers JW, Koenderman L. Eosinophil chemotactic activity in bronchoalveolar lavage from idiopathic pulmonary fibrosis is dependent on cytokine priming of eosinophils. The European respiratory journal. 1998;11(5):1009–14. [DOI] [PubMed] [Google Scholar]
- 32.Hallgren R, Bjermer L, Lundgren R, Venge P. The eosinophil component of the alveolitis in idiopathic pulmonary fibrosis. Signs of eosinophil activation in the lung are related to impaired lung function. Am Rev Respir Dis. 1989;139(2):373–7. doi: 10.1164/ajrccm/139.2.373 [DOI] [PubMed] [Google Scholar]
- 33.Oltmanns U, Chung KF, Walters M, John M, Mitchell JA. Cigarette smoke induces IL-8, but inhibits eotaxin and RANTES release from airway smooth muscle. Respiratory research. 2005;6:74 doi: 10.1186/1465-9921-6-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu M, Chen D, Zhou H, Zhang W, Xu J, Chen L. The Role of Periostin in the Occurrence and Progression of Eosinophilic Chronic Sinusitis with Nasal Polyps. Scientific reports. 2017;7(1):9479 doi: 10.1038/s41598-017-08375-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.