Abstract
There are no approved drugs for the treatment of heart failure with preserved ejection fraction (HFpEF), which is characterized by left ventricular (LV) diastolic dysfunction. We demonstrate that ITF2357 (givinostat), a clinical-stage inhibitor of histone deacetylase (HDAC) catalytic activity, is efficacious in two distinct murine models of diastolic dysfunction with preserved EF. ITF2357 blocked LV diastolic dysfunction due to hypertension in Dahl salt-sensitive (DSS) rats and suppressed aging-induced diastolic dysfunction in normotensive mice. HDAC inhibitor–mediated efficacy was not due to lowering blood pressure or inhibiting cellular and molecular events commonly associated with diastolic dysfunction, including cardiac fibrosis, cardiac hypertrophy, or changes in cardiac titin and myosin isoform expression. Instead, ex vivo studies revealed impairment of cardiac myofibril relaxation as a previously unrecognized, myocyte-autonomous mechanism for diastolic dysfunction, which can be ameliorated by HDAC inhibition. Translating these findings to humans, cardiac myofibrils from patients with diastolic dysfunction and preserved EF also exhibited compromised relaxation. These data suggest that agents such as HDAC inhibitors, which potentiate cardiac myofibril relaxation, hold promise for the treatment of HFpEF in humans.
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is characterized by increased left ventricular (LV) filling pressure, increased LV stiffness, and prolonged relaxation in the presence of normal systolic function (1). This relaxation abnormality is the final common end point of many different types of pathologic stress, including hypertension and aging. Over the past two decades, patients with HF with reduced EF (HFrEF; systolic HF) have seen clinical benefits through pharmacological management (2). Unfortunately, large clinical trials using standard-of-care HFrEF medications have failed to demonstrate efficacy in patients with HFpEF (3–5), and thus, clinicians have no guidance on which biochemical pathways to target for the treatment of patients with HFpEF, whose prognosis is as poor as those with systolic HF. Patients with HFpEF report similar symptom burden and reduction in quality of life as patients with HFrEF, suffering from fluid retention, dyspnea, fatigue, and exercise intolerance. All-cause mortality is equivalent for patients with HFrEF and HFpEF, and the 5-year mortality rate after first admission for HF is more than 40% (3). Hence, it is crucial to investigate the molecular mechanisms governing HFpEF, with the goal of discovering novel therapeutic targets.
Diastolic dysfunction in HFpEF is often attributed to increased interstitial fibrosis (6). Collagen deposition is thought to be responsible for elevated ventricular stiffness, because measures of fibrosis correlate positively with reduced chamber compliance (7). In a recent autopsy study, HFpEF patients were shown to have more severe cardiac fibrosis than control subjects, and cardiac fibrosis was inversely related to microvascular density in the heart (8). However, in a separate study of human cardiac biopsies from HFpEF patients with severe diastolic dysfunction (9), about one-third of the samples examined did not have significant fibrosis. When biopsies from HFpEF and HFrEF patients were compared (10), no significant differences were found in interstitial fibrosis between the groups (12 ± 1.4% versus 14.4 ± 1.5%, respectively), despite the fact that measures of LV relaxation were highly dissimilar. These data suggest that fibrosis is not the sole mechanism responsible for ventricular dysfunction in HFpEF.
Diastolic dysfunction may also involve cardiomyocyte-autonomous defects in relaxation. The interaction between the thin and the thick filaments of cardiomyocyte myofibrils has been shown to play a critical role in regulating cardiomyocyte relaxation, and alterations of myofibrillar proteins, such as troponin I (TnI), myosin-binding protein C (MyBP-C), tropomyosin, and myosin heavy chain (MyHC), can affect cardiac relaxation in model systems and humans (11–15). The giant myofibrillar protein titin has been implicated in the regulation of cardiomyocyte relaxation (16), and the abundance of specific titin isoforms, as well as the phosphorylation state of titin, affects the stiffness of cardiomyocytes independently of fibrosis, both in experimental models and in hearts of patients with HFpEF (9, 17–19).
Histone deacetylases (HDACs) are a family of enzymes that catalyze removal of acetyl groups from lysine residues. Small-molecule inhibitors of HDAC catalytic activity have previously been shown to be efficacious in preclinical models of systolic HF, in part due to their ability to block cardiac hypertrophy and fibrosis (20, 21). Given that four HDAC inhibitors are approved for the treatment of cancer, there is considerable interest in translating these preclinical findings to test HDAC inhibitors in the HF population. Despite extensive use of HDAC inhibitors in models of systolic cardiac disease, nothing is known about the impact of this class of compounds on diastolic function of the heart.
Here, we demonstrate that the HDAC inhibitor ITF2357 (givinostat), currently in a phase 3 trial in patients with Duchenne muscular dystrophy (22), improves relaxation of the heart in rodent models of diastolic dysfunction with preserved EF. ITF2357 did not elicit discernable toxicity, and efficacy was independent of alterations in blood pressure, cardiac hypertrophy, cardiac fibrosis, or cardiac titin and MyHC isoform expression. We define impaired myofibril relaxation as an HDAC-dependent mechanism for diastolic dysfunction and demonstrate that patients with restrictive cardiomyopathy (RCM) characterized by diastolic dysfunction with preserved EF also exhibit diminished myofibril relaxation properties. The data suggest that stress stimuli impinge on myofibrils to promote diastolic dysfunction, and HDAC inhibitors or other agents that target myofibrils could be used to improve diastolic function of the heart in the context of HFpEF.
RESULTS
HDAC inhibition attenuates diastolic dysfunction in Dahl salt-sensitive rats
Dahl salt-sensitive (DSS) rats have mutations in genes that regulate sodium and renin homeostasis, leading to hypertension when maintained on a high-salt (HS) diet (23, 24). Traditionally, DSS rats have been fed 8% NaCl, which leads to severe hypertension, progressive LV dilation, and combined systolic and diastolic dysfunction (25). To more closely model human chronic hypertensive heart disease and diastolic dysfunction with preserved EF, here DSS rats were fed chow containing 4% NaCl (referred to as an HS diet). Doppler echo-cardiography was used to quantify diastolic function by measuring the ratio of the early filling (E) phase of the LV during diastole, which is due to relaxation of the LV, and the late filling (A) phase, which is mediated by contraction of the atrium. Septal mitral annulus velocity (E′/A′) and isovolumic relaxation time (IVRT) were also determined. Compared to DSS rats fed a normal-salt (NS) diet, 10 weeks of HS feeding led to diastolic dysfunction as evidenced by reduced E/A, E′/A′, and increased IVRT (fig. S1, A to C). Animals on 4% NaCl had preserved EF (fig. S1D).
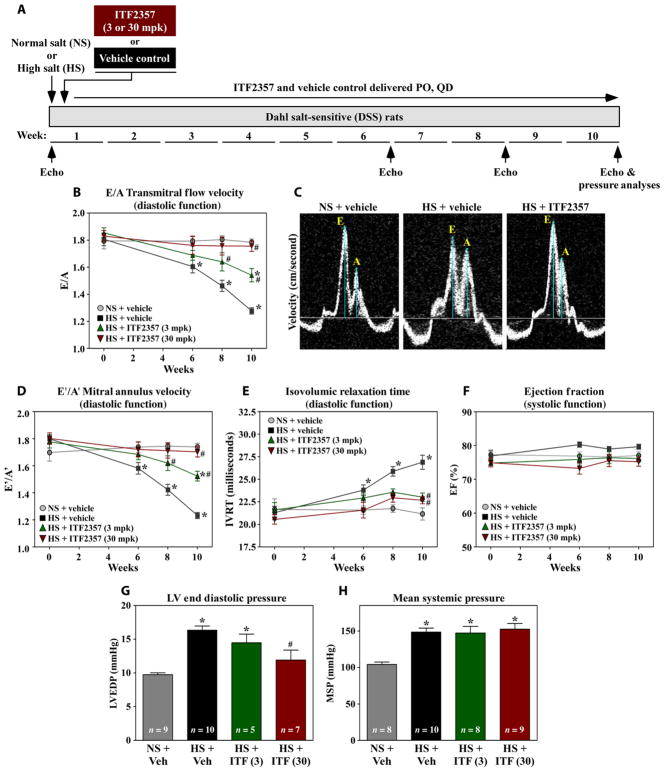
To begin to test whether HDACs contribute to hypertension-induced diastolic dysfunction, DSS rats were fed HS diet and gavaged daily with the HDAC inhibitor ITF2357 (givinostat). Serial echocardiography assessed the impact of HS and ITF2357 on diastolic function over the course of 10 weeks (Fig. 1A). ITF2357 did not elicit discernable hematological toxicity (table S1), which is notable because a major dose-limiting toxicity of HDAC inhibitors in humans is thrombocytopenia (26). Compared to vehicle-treated controls, ITF2357 dose-dependently prevented diastolic dysfunction due to HS feeding, as illustrated by the ability of the compound to normalize E/A, E′/A′, and IVRT (Fig. 1, B to E). Consistent with the pilot study, LV systolic function was preserved during the course of the 10-week study (Fig. 1F). Invasive hemodynamic measurements obtained at study end point revealed that ITF2357 also normalized LV end diastolic pressure while having no effect on the hypertensive response of the rats to HS feeding (Fig. 1, G and H). A repeat study, which included more exhaustive echocardiographic and pressure-volume analyses, confirmed the ability of ITF2357 to normalize diastolic function in the face of sustained hypertension in DSS rats (table S2). These findings suggest that HDAC inhibition in DSS rats directly affects the LV to improve relaxation, as opposed to indirectly ameliorating diastolic dysfunction by normalizing blood pressure.
Fig. 1. HDAC inhibition attenuates diastolic dysfunction in DSS rats.
(A) Study overview. DSS rats were fed NS diet or a 4% NaCl–containing diet (HS) for 10 weeks. Beginning at the time of HS feeding, animals were treated with ITF2357 (pan-HDAC inhibitor) (3 or 30 mg/kg) or vehicle control by oral gavage (PO) once a day (QD). Serial echocardiography was performed before HS feeding and after 6, 8, and 10 weeks. (B) Doppler measurement of mitral inflow velocity (E/A) in DSS rats treated with vehicle or HDAC inhibitor. (C) Representative E/A Doppler echocardiography images. The ratio of the early filling (E) phase of the LV during diastole and the late filling (A) phase is a common echocardiographic measurement of diastolic function. Measurements of septal mitral annulus velocities (E′/A′) (D) and isovolumic relaxation time (IVRT) (E). (F) EF throughout the experiment. (G) LV end diastolic pressure (LVEDP). (H) Mean systemic pressure (MSP). For (G) and (H), ITF (3) and ITF (30) represent the dose of ITF in mg/kg. For all graphs, mean ± (or +) SEM values are shown and were compared by one-way analysis of variance (ANOVA) with Newman-Keuls post-test. *P < 0.05 versus NS + vehicle, #P < 0.05 versus HS + vehicle. For (B) and (D) to (F), rat numbers for each condition and time are provided in table S7. Each figure includes the number of animals used in the analysis. When such format was not practical, table S7 summarizes the number of animals used.
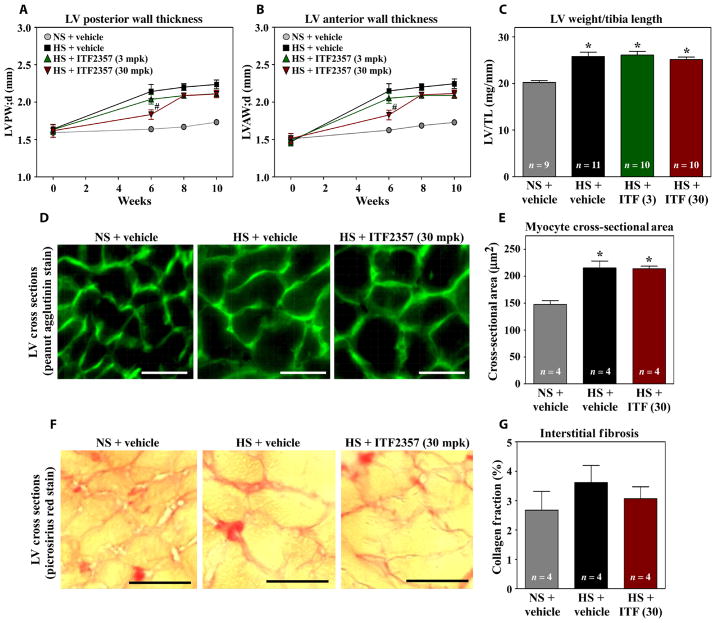
Diastolic dysfunction is often attributed to cardiac hypertrophy and fibrosis (8, 19). Echocardiography revealed that HS-mediated cardiac hypertrophy was unaffected by ITF2357 treatment in DSS rats (Fig. 2, A and B). Consistent with these findings, ITF2357 also failed to reduce LV-to-tibia length ratio and cardiomyocyte cross-sectional area (Fig. 2, C to E, and table S3). In contrast to previous observations in DSS rats fed 8% NaCl, animals fed 4% NaCl chow for 10 weeks did not exhibit significant LV interstitial fibrosis (Fig. 2, F and G, and table S3; P = 0.51). Together, these findings suggest that HDAC inhibition improves diastolic function in DSS rats independently of effects on cardiac hypertrophy and fibrosis.
Fig. 2. HDAC inhibition improves diastolic function in DSS rats independently of effects on cardiac hypertrophy or fibrosis.
Echocardiographic assessment of LV posterior (LVPW) (A) and anterior (LVAW) (B) wall thickness in NS- and HS-fed rats treated with vehicle or ITF2357. LV-to-tibia length (C) and images and quantification of myocyte cross-sectional area (D and E) in 10-week samples. Picrosirius red staining (F) and collagen quantification (G) of 10-week LV sections from DSS rats. For (C), (E), and (G), ITF (3) and ITF (30) represent the dose of ITF in mg/kg. For all graphs, mean ± (or +) SEM values are shown and were compared by one-way ANOVA with Newman-Keuls post-test. *P < 0.05 versus NS + vehicle, #P < 0.05 versus HS + vehicle. For (A) and (B), rat numbers for each end point and each time point are provided in table S7; at least eight animals were used per analysis. Scale bars, 20 μm.
Diastolic dysfunction in DSS rats correlates with HDAC-dependent impairment of myofibril relaxation
To begin to address whether HDAC inhibition directly affects cardiomyocyte relaxation, cultured adult rat ventricular myocytes (ARVMs) were treated with ITF2357 for 24 hours. The cells were subsequently electrically paced, and mechanical parameters were measured. ARVMs exposed to ITF2357 had markedly increased rates of cellular relaxation compared to vehicle-treated controls (fig. S2, A and B). In contrast, the contractility of ARVMs was unaffected by the HDAC inhibitor (fig. S2, C and D). Representative contraction/relaxation traces from individual cells treated with ITF2357 and vehicle control are shown (fig. S2, E and F).
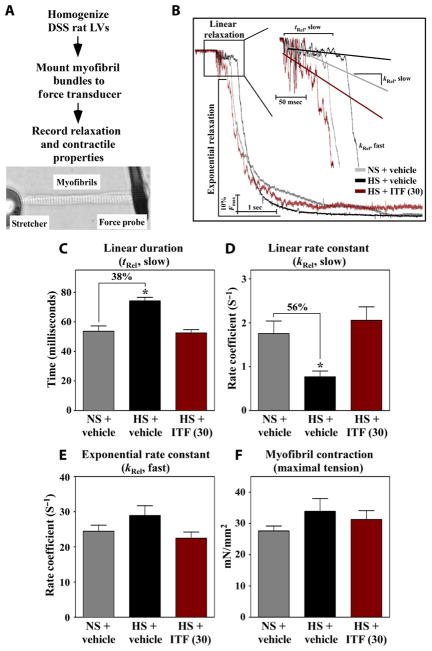
We hypothesized that HDAC-dependent alteration in intrinsic relaxation properties of cardiomyocytes contributes to diastolic dysfunction in DSS rats. To test this, myofibrils from homogenized LVs were mounted on a force transducer in a tissue bath, rapidly shifted from a solution with a high calcium concentration to a solution without calcium, and relaxation kinetics were quantified (Fig. 3A). Myofibrils from DSS rats fed HS diet for 10 weeks exhibited severe decrements in the duration and rate constant of the linear phase of relaxation, which were normalized by the high dose of ITF2357 (Fig. 3, B to D). The linear phase of relaxation represents inactivation of thin filament regulatory proteins, which halts additional cross-bridge association. The exponential phase of myofibril relaxation was not altered by HS feeding or ITF2357 (Fig. 3E); the exponential phase of relaxation is governed by the rate of actin and myosin dissociation. Unlike the observed impairment of myofibril relaxation, calcium-activated maximal tension generation (force of contraction) was unaffected by HS feeding or HDAC inhibition (Fig. 3F). These findings reveal a myofibril-autonomous defect in HS-fed DSS rats that correlates with diastolic dysfunction at the whole-organ level and demonstrate that inhibition of HDAC activity prevents this decrement in myofibril relaxation.
Fig. 3. Diastolic dysfunction in DSS rats is associated with prolongation of myo-fibril relaxation that is normalized with HDAC inhibition.
(A) Schematic representation of the ex vivo myofibril mechanics quantification assay. (B) Representative relaxation traces of myofibrils obtained from LVs of DSS rats fed NS or HS for 10 weeks and gavaged daily with ITF2357 (30 mg/kg) or vehicle control. Duration (tRel, slow) (C) and rate constant (kRel, slow) (D) of the linear relaxation phase of myofibrils from rats at 10 weeks. Exponential relaxation phase of myofibrils (kRel, fast) (E) and myofibril maximal tension generation of myofibrils (F) from rats at 10 weeks. For all graphs, mean + SEM values are shown and were compared by one-way ANOVA with Newman-Keuls post-test. *P < 0.05 versus NS + vehicle. n = 6 rats for NS + vehicle and HS + ITF2357 and n = 5 for HS + vehicle; 7 to 12 myofibrils per animal were analyzed.
HDAC inhibitor–mediated improvement of cardiac relaxation is not due to changes in myofibril myosin composition, calcium sensitivity, or titin function
The relative abundance of the thick filament proteins α-MyHC and β-MyHC in the LV is an important determinant of sarcomere cross-bridge activation and relaxation (27). Because stress signals increase the expression of cardiac β-MyHC, this isoform switch could contribute to the myofibril relaxation impairment observed in HS-fed DSS rats. However, ITF2357 failed to attenuate up-regulation of β-MyHC in LVs of DSS rats fed a HS diet for 10 weeks and did not affect the reduced kinetics of myofibril contraction, which is governed by the slower adenosine triphosphatase (ATPase) activity of β-MyHC (fig. S3, A to C). The ability of ITF2357 to improve myofibril relaxation without altering β-MyHC expression or function suggests that HDAC inhibition targets a distinct mechanism that controls the process.
Increased myofibril calcium sensitivity contributes to diastolic dysfunction (28). Thus, force-calcium concentration (pCa) curves were constructed to examine whether the dynamics and/or affinity of myofibril calcium binding and force production were altered in DSS rats. HS feeding resulted in a nonsignificant right shift of pCa50 (P = 0.85), which was unaffected by ITF2357 treatment (fig. S4). The Hill coefficient, a measurement of cooperativity of myofibrillar protein interaction, was not altered by HS feeding or ITF2357 treatment (fig. S4). Thus, altered relaxation in DSS rats does not correlate with changes in myofibril calcium sensitivity.
Increased resting tension of myofibrils, which is controlled by the giant protein titin (17, 18), contributes to diastolic dysfunction of the heart (19). Consistent with this, myofibrils from DSS rats fed HS exhibited increased resting tension (fig. S5A). However, ITF2357 treatment did not attenuate resting tension, and titin isoform expression was unaffected by HS or ITF2357 (fig. S5, A to E). Together, these data suggest that HDAC inhibition increases the rate of myofibril relaxation through a mechanism that is independent of changes in MyHC, myofibril calcium sensitivity, or titin isoform switching.
Acetylation/deacetylation of cardiac myofibrils alters relaxation
ITF2357-mediated enhancement of myofibril relaxation in DSS rats could be due to direct effects of HDAC inhibition on myofibrillar protein function or through distal actions that indirectly affect myofibril mechanical properties, such as alteration of kinase activity and downstream myofibrillar protein phosphorylation. DSS rats treated with ITF2357 did not exhibit overt alterations in myofibrillar protein composition or general phosphorylation state (fig. S6). Evaluation of site-specific phosphorylation also failed to reveal HS or ITF2357-mediated differences in titin or MyBP-C, although an increase in phosphorylation of the protein kinase A (PKA) target sites of TnI was observed in hearts of rats fed HS and treated with ITF2357 (fig. S7, A to G). Conversely, phosphorylation of the PKA target site of the calcium-handling protein phospholamban (PLN) was unaffected by either treatment (fig. S7H).
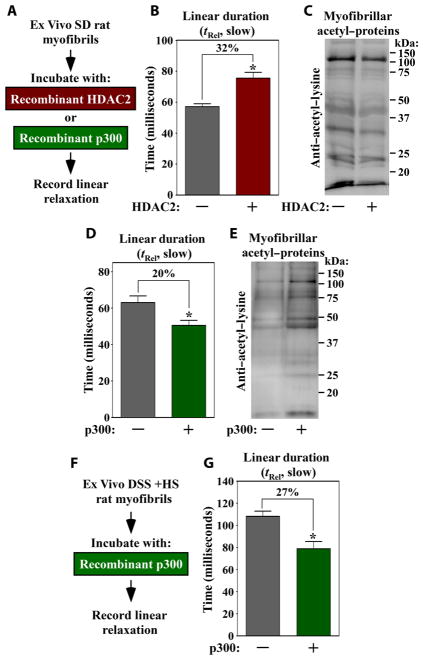
Consistent with the possibility that an HDAC directly regulates myofibril function independently of effects on myofibrillar protein phosphorylation, HDAC2 co-purified with myofibrils from DSS rat LVs (fig. S8). HDAC3 and HDAC4 were not found in association with myofibrils (fig. S8), suggesting a specific role for HDAC2 in the control of myofibrils. Deacetylation of myofibrils with recombinant HDAC2 ex vivo caused a 32% increase in myofibril relaxation duration (Fig. 4, A to C). In contrast, acetylation of myofibrils with recombinant p300 had the converse effect of augmenting the rate of myofibril relaxation (Fig. 4, D and E). Ex vivo acetylation by p300 also ameliorated relaxation of severely impaired DSS myofibrils (Fig. 4, F and G). Ex vivo acetylation/deacetylation did not alter myofibril contraction (fig. S9, A to C), consistent with the data obtained with in vivo samples (Fig. 3F). These findings demonstrate that myofibril relaxation can be controlled by myofibrillar protein acetylation.
Fig. 4. Ex vivo deacetylation of cardiac myofibrils prolongs relaxation kinetics.
(A) Experimental design. Myofibrils from normal Sprague-Dawley rat LVs were incubated with recombinant HDAC2 or p300 before performing mechanics studies. Myo-fibril relaxation (B) and protein acetylation (C) with and without HDAC2. Myofibril relaxation (D) and protein acetylation (E) with and without p300. (F) Experimental design. Myofibrils isolated from DSS + HS–fed rats were treated with recombinant p300 and acetyl-CoA (coenzyme A). (G) Myofibril relaxation with and without p300. Data are mean + SEM and were compared by Student’s t test. *P < 0.05 versus untreated controls. Data from three separate ARVM preparations were combined; five to nine myofibril bundles were analyzed per ARVM preparation.
DSS rat myofibrils analyzed with general anti–acetyl-lysine antibodies revealed subtle changes associated with diastolic dysfunction (fig. S10). Definitive assessment of stress-dependent alterations in myofibril protein acetylation will require use of site-specific anti-acetyl antibodies and quantitative mass spectrometry.
HDAC inhibition blunts age-dependent diastolic dysfunction and myofibril relaxation impairment in mice
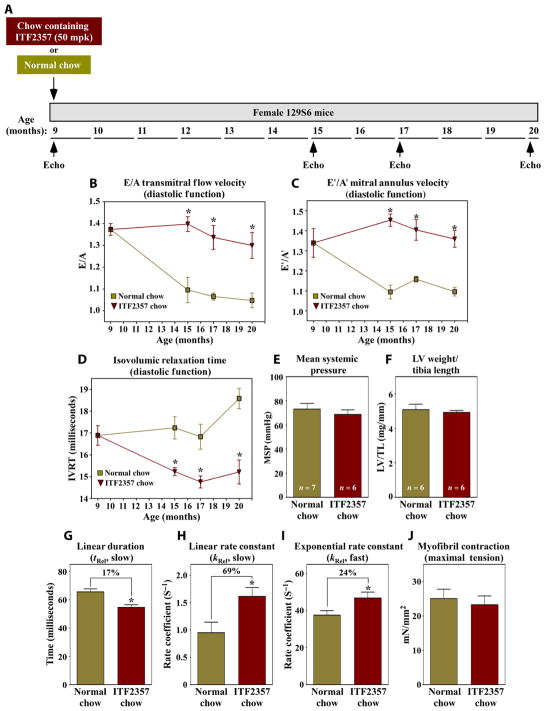
We next addressed whether HDAC-dependent myofibril relaxation impairment contributes to diastolic dysfunction caused by distinct etiology. Advanced age is an independent risk factor for the development of HFpEF: More than 90% of HFpEF patients are over the age of 60 years at the time of diagnosis (3, 5), and LV systolic function is preserved during aging (29). To begin to address whether HDAC inhibition also blocks age-dependent diastolic dysfunction, 9-month-old female 129S6 mice were fed normal chow or ITF2357-containing chow, and diastolic function was assessed serially by echocardiography (Fig. 5A). Female mice were used because age-related HFpEF disproportionately affects women (3). Diastolic function (E/A, E′/A′, and IVRT) progressively declined in mice fed normal chow but was preserved out to 20 months of age (time of study completion) in mice fed ITF2357 (Fig. 5, B to D, and tables S4 and S5). Consistent with findings in DSS rats, ITF2357 did not alter blood pressure or LV hypertrophy in aged female mice (Fig. 5, E and F).
Fig. 5. HDAC inhibition blocks age-dependent diastolic dysfunction.
(A) Study overview. Nine-month-old female 129S6/SvEvTac mice were fed normal chow or chow containing ITF2357 (50 mg/kg) for 11 months. Serial echocardiog-raphy was performed at 9, 15, 17, and 20 months. (B) Doppler measurement of mitral inflow velocity (E/A) in aged mice treated with vehicle or HDAC inhibitor. Measurements of septal mitral annulus velocities (E′/A′) (C) and IVRT (D). Mouse numbers for each time point are provided in table S7. Mean systemic blood pressure (MSP) (E) and LV-to-tibia length (F) in 20-month-old mice treated with or without ITF2357. (G to J) Ex vivo mechanics analyses of myofibrils from 20-month-old mice fed normal chow or ITF2357-containing chow. Data are mean + SEM and were compared by Student’s t test. *P < 0.05 versus untreated controls. n = 4 mice per group; 9 to 10 myofibril bundles were analyzed per animal.
Myofibril mechanical studies revealed that linear and exponential phase relaxation was more rapid in myofibrils from aged mice fed ITF2357 compared to myofibrils from control mice fed normal chow (Fig. 5, G to I), whereas myofibril contraction was equivalent in both groups (Fig. 5J). Thus, in two models of diastolic dysfunction with preserved EF, HDAC inhibition improved LV diastolic function in a manner that correlates with enhanced kinetics of myofibril relaxation.
Myofibril relaxation is impaired in human diastolic dysfunction
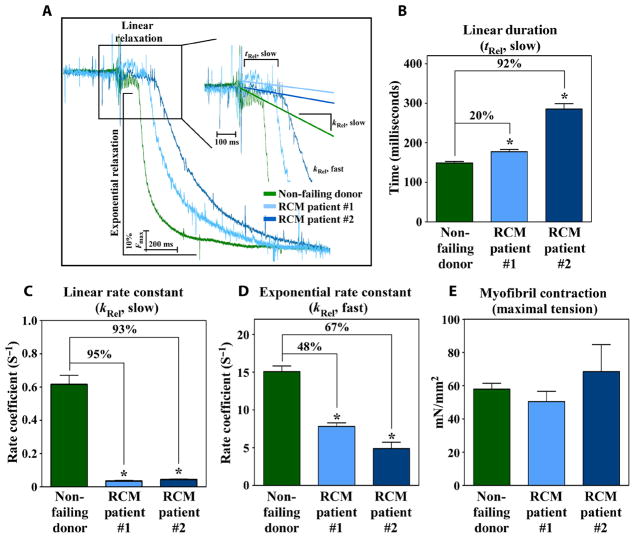
To address whether myofibril relaxation impairment contributes to diastolic dysfunction in patients, mechanics studies were performed with myofibrils obtained from explanted LV tissue from two individuals diagnosed with idiopathic RCM who underwent cardiac transplantation. HFpEF can arise from diverse predisposing causes, such as hypertension, aging, and metabolic disease. Furthermore, by excluding risk factors, such as infiltrative cardiomyopathies, a diagnosis of RCM can be made. RCM represents a pure form of severe diastolic dysfunction with preserved EF that is inherent to the heart. Age-matched nonfailing donor hearts were used as controls (table S6). Isolated myofibrils from both RCM patients exhibited markedly prolonged kinetics of the linear phase of relaxation compared to control myofibrils (Fig. 6, A to C). Myofibrils from the HF patients also had reduced kinetics of the exponential phase of relaxation (Fig. 6D). Maximum force generation was unchanged between myofibrils obtained from the patients and controls, consistent with the clinical presentation of preserved systolic function (Fig. 6E). These findings suggest that myofibril relaxation impairment contributes to diastolic dysfunction in humans. A model for HDAC inhibitor–mediated control of diastolic cardiac function is shown (fig. S11).
Fig. 6. Human HFpEF is associated with impaired cardiac myofibril relaxation.
(A) Representative relaxation traces of myofibrils from nonfailing donor heart (n = 1) and hearts from two individuals with HFpEF superimposed on identical time scale. Linear phase duration (tRel, slow) (B) and slope of the linear phase relaxation (kRel, slow) (C) from myofibrils from RCM patients and control nonfailing donor heart. Exponential phase relaxation (kRel, fast) (D) and maximal tension generation (E) from RCM patients and nonfailing donor heart. Data are mean + SEM and were compared by one-way ANOVA with Newman-Keuls post-test. *P < 0.05 versus nonfailing controls. n = 5 nonfailing donors (7 to 16 myofibril bundles per donor). For RCM patient #1, 16 myofibril bundles were analyzed, and for RCM patient #2, 12 myofibril bundles were analyzed.
DISCUSSION
Pathophysiological processes governing HFpEF remain poorly defined, which could account for the numerous failed clinical trials in this patient population. Here, we provide evidence of a role for impaired myofibril relaxation in the control of diastolic dysfunction in preclinical models and in human patients with HFpEF. A clinical-stage HDAC inhibitor, ITF2357 (givinostat), prevented hypertension- and aging-induced diastolic dysfunction in rodents in a manner associated with amelioration of myofibril relaxation impairment ex vivo. Furthermore, acetylation and deacetylation of myofibrils ex vivo led to enhanced and impeded relaxation, respectively, suggesting a direct role for this posttranslational modification in the control of diastolic function of the heart. We propose that attenuation of myofibril relaxation kinetics by HDACs is a previously unrecognized mechanism for the development of diastolic dysfunction, which could be therapeutically exploited for the treatment of HFpEF.
The three largest phase 3 clinical trials for HFpEF targeted the aldosterone receptor (TOPCAT) or the angiotensin II (Ang II) receptor (I-PRESERVE and CHARM-Preserved). The rationale for these approaches was largely based on the role of the aldosterone/Ang II axis in promoting adverse cardiac fibrosis and hypertrophy, and underlying hypertension (30). In this regard, it is noteworthy that ITF2357 improved diastolic function without significantly affecting blood pressure or fibrosis. Although ITF2357 transiently blunted hypertension-induced increases in LV wall thickness, it failed to exert prolonged anti-hypertrophic action, which is in contrast to previous reports of HDAC inhibitors blocking cardiac hypertrophy (20). The reason for this discrepancy remains unknown but could be related to ITF2357 dose, route of administration, and/or pharmacokinetic properties. We propose that combined use of HDAC inhibitors with current goal-directed therapy, such as aldosterone and/or Ang II receptor blockers, will provide superior efficacy in HFpEF patients by simultaneously targeting distinct mechanisms that control relaxation and compliance of the heart.
Concerns have been raised regarding the use of pan-HDAC inhibitors for chronic indications such as HF (20). The major dose-limiting effect associated with pan-HDAC inhibition is thrombocytopenia (26). However, in our study of DSS rats, ITF2357 did not reduce platelet counts or cause other hematological toxicities. ITF2357 was found to improve skeletal muscle parameters at doses that did not significantly affect platelets in a phase 2 trial in boys with Duchenne muscular dystrophy (22); ITF2357 is currently being evaluated in a phase 3 trial in this patient population. It is important to define the HDAC isoforms that control diastolic dysfunction with the goal of developing the least toxic isoform-selective HDAC inhibitors for HFpEF, but it is also possible that an existing pan-HDAC inhibitor, such as ITF2357, could be sufficiently tolerated and efficacious to be “repurposed” for this cardiac indication.
The substrates of acetylation/deacetylation that control myofibril relaxation remain unknown. We hypothesize that deacetylation of lysine residues in specific myofibrillar proteins leads to impaired relaxation and diastolic dysfunction of the heart. Acetyl-proteomics studies have demonstrated extensive acetylation of proteins that comprise cardiac myofibrils (31). In vitro experiments showed that acetylation of α- and β-MyHC increased the actin-sliding velocity of both myosin isoforms, which would be predicted to speed the exponential phase of relaxation, and HDAC3 was implicated as the MyHC deacetylase (32). However, HDAC3-mediated deacetylation of MyHC is unlikely to account for our observations, because we failed to see a significant amount of HDAC3 co-purifying with myofibrils. HDAC inhibition had a profound effect on linear phase relaxation, which involves inactivation of the thin filament of myofibrils, as opposed to the MyHC-containing thick filament. Our results are also unlikely to be related to the recent demonstration that skeletal muscle actin in Drosophila melanogaster is regulated by acetylation, because this posttranslational modification was shown to augment flight muscle contraction and we did not observe a change in myofibril force generation upon HDAC inhibitor treatment (33). Nonetheless, the actin findings further illustrate the emerging role of lysine acetylation in the control of muscle mechanics.
The current data suggest noncanonical, nongenomic roles for acetyltransferases and deacetylases in the direct control of myofibrils and cardiac relaxation. Consistent with this, HDAC2 co-purified with myofibrils from DSS rat hearts, and treatment of myofibrils with recombinant HDAC2 or p300 ex vivo decreased and increased myofibril relaxation, respectively. We provide evidence to suggest that HDACs control myofibril relaxation through a mechanism distinct from those which have been previously described to control this process. For example, phosphorylation of cardiac TnI has been shown to increase the kinetics of myofibril relaxation while reducing calcium sensitivity (34). Although we observed a modest increase in TnI phosphorylation in hearts of DSS rats fed HS and treated with ITF2357, calcium sensitivity was unchanged in HS-fed and HS-fed ITF2357-treated DSS rats, indicating a divergent mechanism of thin filament regulation upon HDAC inhibition. Increased myofibril calcium sensitivity due to glutathionylation of MyBP-C has also been previously linked to diastolic dysfunction (35). However, the lack of a change in calcium sensitivity upon ITF2357 treatment suggests that the ability of the HDAC inhibitor to improve relaxation of the heart is independent of effects on glutathionylation of MyBP-C. Finally, titin isoform switching and phosphorylation are associated with diastolic dysfunction (17, 18); yet, we failed to observe changes in the large titin isoform (N2BA), the short titin isoform (N2B), or phosphorylation of PKC, PKA, or PKG target sites on titin in DSS rats fed HS with or without ITF2357 (figs. S5 and S7). Future structure-function studies should pinpoint the precise roles of myofibrillar protein acetylation in the control of diastolic function.
We cannot rule out the possibility that HDAC inhibition improves diastolic heart function through additional mechanisms, besides altering myofibrillar protein acetylation. Although fibrosis did not appear to contribute significantly to diastolic dysfunction in the models presented here, extracellular matrix remodeling has been implicated in cardiac relaxation impairment in HFpEF (8) and can be attenuated by HDAC inhibitors in other settings (36). Furthermore, it has been suggested that oxidative stress promotes diastolic dysfunction (35, 37), and HDAC inhibitors exhibit antioxidant properties (38). Elucidation of the full constellation of mechanisms by which HDAC inhibitors improve relaxation of the heart awaits further investigation.
We acknowledge that a limitation of this study is the reliance on rodent models of diastolic dysfunction, which do not fully recapitulate HFpEF in humans (39). Future translational studies will need to use large animal models of HFpEF, such as feline, canine, or porcine models (40–42). In addition, echocardiographic assessment of diastolic function in rodents has previously been hindered by the lack of color Doppler technology. During the course of our investigation, we implemented a Vevo2100 instrument, thereby increasing the precision of early-to-late mitral annular velocity measurements. Finally, HFpEF patients have not historically been referred for cardiac transplantation, and thus, we were limited to evaluating a small number of human hearts. With the heightened awareness of HFpEF as a clinical syndrome, we anticipate that an increased number of cardiac tissue samples from this patient population will become available for research purposes in the future. Evaluation of myofibril mechanics with these samples will enable a determination of the generalizability of relaxation impairment as a pathogenic mechanism in HFpEF.
In summary, we define impaired myofibril relaxation as an HDAC-dependent mechanism for diastolic dysfunction of the heart. The findings suggest that agents, such as HDAC inhibitors, which target myofibrils to improve relaxation, should be evaluated for clinical efficacy in patients with HFpEF, a condition for which no approved drugs currently exist.
MATERIALS AND METHODS
Study design
The objectives of this study were to determine whether HDAC inhibition could improve cardiac relaxation in two rodent models of diastolic dysfunction and to determine whether diastolic dysfunction can be traced to the level of myofibril relaxation abnormalities. Functional measurements of whole-heart, single-cardiomyocyte, and myofibril mechanics were used to assess cardiac function. Morphological features were also used to assess response to chronic hypertension and aging. The specificity of HDAC inhibition in ameliorating diastolic dysfunction was confirmed with ex vivo manipulation of lysine modification with recombinant HDAC and HAT (histone acetyltransferase) enzymes. For rodent models, all animals were randomly assigned to treatment groups. Sample size was guided by our previous experience with these animals and was based on development of cardiac hypertrophy. The number of animals per group was determined by power calculation to assess cardiac hypertrophy, with α = 0.05 and β = 0.8. Human hearts with known HFpEF were also used to show clinical applicability of the concept that cardiac relaxation localizes to myofibril relaxation abnormalities.
Animal models
Animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver (UCD). Sprague-Dawley rats were obtained from Charles River Laboratories. DSS rats were obtained from Envigo (formerly Harlan Laboratories) and fed NS diet (Teklad; 2020X; 0.4% NaCl) or the same diet but containing 4% NaCl. ITF2357 was delivered daily by gavage (3 or 30 mg/kg body weight) in a vehicle of hydroxypropyl cellulose (0.5%) and Tween 80 (0.5%) in water. ITF2357-containing chow was prepared by Dyets Inc. by mixing enough ITF2357 to reach 555 mg/kg of food, equivalent to a daily dose of 50 mg/kg body weight. Female 129S6/SvEvTac mice (Taconic) were aged to 9 months at UCD before initiating feeding with ITF2357-containing chow.
Hemodynamic analyses
Serial transthoracic echocardiography and Doppler analyses were performed using Vevo770 (DSS rat studies) and Vevo2100 (aging mouse study) instruments (VisualSonics). Animals were anesthetized with 2% isoflurane, their chests were shaved, and their body temperatures were maintained at 37°C. Long and short parasternal axis views of the LV were obtained. Short-axis two-dimensional views of the LV at the papillary muscle level were used to obtain M-mode targeted recordings. Anterior and posterior end-diastolic and end-systolic LV wall thickness and internal diameter were measured using the leading-edge method to calculate EF. Doppler signals of mitral inflow and myocardial tissue movement at the level of the mitral annulus were obtained to calculate the ratio of early and active filling wave peak of diastolic flow velocity (E/A) and the ratio of peak diastolic tissue velocity (E′/A′) to assess diastolic cardiac function. All measurements were averaged from three consecutive cardiac cycles on the exhale phase. Echocardiography was performed in a blinded manner. Subsequently, a battery of LV hemodynamic indices was assessed using a pressure-volume system (Scisense Inc.). For DSS rats, systemic blood pressure was measured using a fluid-filled catheter placed in the femoral artery. For the aging mice, systemic blood pressure was assessed in conscious animals using a noninvasive tail-cuff system (CODA, Kent Scientific).
Human heart samples
Human hearts were obtained from a tissue bank maintained by the Division of Cardiology at the UCD (COMIRB 01-568). Characteristics of the patients and nonfailing donor controls are provided in table S6. All patients were followed by the University of Colorado Heart Failure Program and offered participation in the research protocol. Hearts were collected at the time of orthotopic cardiac transplantation. For the HFpEF cohort, two patients were selected with the following criteria: (i) preserved LV systolic function (>50%), (ii) presence of diastolic dysfunction, and (iii) no known genetic causes of HFpEF. Control hearts were obtained from unused donor hearts that could not be used for transplantation. Six control hearts were age-and gender-matched to the two HFpEF hearts.
Statistical analyses
Mean ± SEM values are shown and were compared by Student’s t test (two unpaired groups) or one-way ANOVA (more than two groups) with Newman-Keuls post-test. Probability values of P < 0.05 were considered significant. For the aging study, a linear mixed model was implemented in SAS (version 9.4) to estimate outcome measurement for treated and control groups across time for each outcome variable. To allow for nonlinear trends over time, time was treated as a class variable and an interaction effect between time and group was included in the model. A random effect of subject was included in the model to account for in-subject variance. Pairwise comparisons between treatment and control groups at each time point were performed post hoc. The number of animals analyzed for each end point is listed in table S7.
Supplementary Material
Fig. S1. DSS rats fed a 4% NaCl–containing diet develop diastolic dysfunction with preserved EF.
Fig. S2. Treatment of ARVMs in culture with ITF2357 led to acceleration of myocyte relaxation kinetics without affecting contractility.
Fig. S3. HDAC inhibition does not affect HS diet–induced increases in β-MyHC expression or function.
Fig. S4. Myofibril calcium sensitivity is unaffected by HS feeding or ITF2357 treatment of DSS rats.
Fig. S5. Titin expression and function are unaffected by HS feeding or ITF2357 treatment of DSS rats.
Fig. S6. Myofibrillar protein phosphorylation and expression are unaffected by HS feeding or ITF2357 treatment of DSS rats.
Fig. S7. Site-specific phosphorylation of myofibrillar protein and myofibrillar protein expression are unaffected by HS feeding or ITF2357 treatment of DSS rats.
Fig. S8. HDAC2 co-purifies with cardiac myofibrils.
Fig. S9. Ex vivo acetylation/deacetylation does not alter myofibril contraction.
Fig. S10. Anti–acetyl-lysine immunoblotting of cardiac myofibrils from DSS rats.
Fig. S11. A model for HDAC inhibitor–mediated improvement in diastolic function and treatment of HFpEF.
Table S1. Hematological profiles of DSS rats.
Table S2. LV hemodynamic and echocardiographic parameters of DSS rats.
Table S3. Quantification of myocyte cross-sectional area and interstitial fibrosis in DSS rats.
Table S4. Echocardiographic parameters of aging mice.
Table S6. Characteristics of the RCM patients and nonfailing donor controls.
Table S7. Group sizes of animal models.
Table S5. Echocardiographic, hemodynamic, and hypertrophy parameters of aging mice.
Acknowledgments
We thank L. Walker (UCD) for advice regarding myofibrillar protein analyses, A. Moses (University of Denver) for assistance with electrical engineering of the myofibril force recording apparatus, L. Vanderlinden for assistance with statistical analyses, and C. Poggesi (University of Florence) for invaluable guidance on myofibril mechanics analyses. We are grateful to J. Nyborg (Colorado State University) for recombinant p300 and P. Buttrick (UCD) for critical reading of the manuscript. We wish to acknowledge P. Buttrick, M. Bristow, and the UCD Division of Cardiology for ongoing maintenance of the human cardiac tissue biobank.
Funding: M.Y.J. was supported by the NIH and UCD Colorado Clinical and Translational Sciences Institute (5KL2TR001080-02), a Hartford/Jahnigen Center of Excellence in Geriatrics Pilot Grant, and a Sarnoff Endowment Fellow-to-Faculty Transition Award. Y.H.L. was supported by an American Heart Association postdoctoral fellowship (16POST30960017). A.V.A. was supported by a Scientist Development Grant from the American Heart Association and by the Boettcher Foundation’s Webb-Waring Biomedical Research Program. H.L.G. and C.S. were supported by the NIH (R01HL115988). T.A.M. was supported by the NIH (HL116848, HL127240, and AG043822) and the American Heart Association (16SFRN31400013). The cardiac tissue bank used REDCap, which is provided by NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Author contributions: M.Y.J., M.A.C., and T.A.M. designed and supervised the study. M.Y.J. and T.A.M. wrote the manuscript. M.Y.J. constructed the myofibril mechanical machine, and M.Y.J., Y.H.L., and J.H.M. completed the myofibril and intact cardiomyocyte experiments. S.A.W. and K.M.D.-D. performed echocardiographic analyses. M.A.C. completed the invasive hemodynamic measurements. C.S. executed all titin measurements. T.B.R. and A.V.A. provided human cardiac samples. A.V.A., H.L.G., V.M., P.M., and C.A.D. discussed and analyzed data and gave conceptual advice. All authors discussed the results and commented on the manuscript.
Competing interests: V.M. and P.M. are employees of Italfarmaco. The other authors declare that they have no competing financial interests.
Data and materials availability: Human heart tissue is available from the University of Colorado Division of Cardiology under a material transfer agreement with the University. ITF2357 is available from Italfarmaco under a material transfer agreement with the Company.
REFERENCES AND NOTES
- 1.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2.Nair AP, Timoh T, Fuster V. Contemporary medical management of systolic heart failure. Circ J. 2012;76:268–277. doi: 10.1253/circj.cj-11-1424. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 4.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 5.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: Treat now by treating comorbidities. JAMA. 2008;300:431–433. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- 6.Lamberts RR, Willemsen MJJMF, Pérez NG, Sipkema P, Westerhof N. Acute and specific collagen type I degradation increases diastolic and developed tension in perfused rat papillary muscle. Am J Physiol Heart Circ Physiol. 2004;286:H889–H894. doi: 10.1152/ajpheart.00967.2001. [DOI] [PubMed] [Google Scholar]
- 7.Diez J, Querejeta R, López B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borbély A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 10.van Heerebeek L, Borbely A, Niessen HWM, Bronzwaer JGF, van der Velden J, Stienen GJM, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson C, Palmer BM, Zile M, Maughan DW, Ikonomidis JS, Granzier H, Meyer M, VanBuren P, LeWinter MM. Myosin cross-bridge dynamics in patients with hypertension and concentric left ventricular remodeling. Circ Heart Fail. 2012;5:803–811. doi: 10.1161/CIRCHEARTFAILURE.112.968925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James J, Zhang Y, Osinska H, Sanbe A, Klevitsky R, Hewett TE, Robbins J. Transgenic modeling of a cardiac troponin I mutation linked to familial hypertrophic cardiomyopathy. Circ Res. 2000;87:805–811. doi: 10.1161/01.res.87.9.805. [DOI] [PubMed] [Google Scholar]
- 13.Muthuchamy M, Grupp IL, Grupp G, O’Toole BA, Kier AB, Boivin GP, Neumann J, Wieczorek DF. Molecular and physiological effects of overexpressing striated muscle β-tropomyosin in the adult murine heart. J Biol Chem. 1995;270:30593–30603. doi: 10.1074/jbc.270.51.30593. [DOI] [PubMed] [Google Scholar]
- 14.Poggesi C, Tesi C, Stehle R. Sarcomeric determinants of striated muscle relaxation kinetics. Pflugers Arch. 2005;449:505–517. doi: 10.1007/s00424-004-1363-5. [DOI] [PubMed] [Google Scholar]
- 15.Rosas PC, Liu Y, Abdalla MI, Thomas CM, Kidwell DT, Dusio GF, Mukhopadhyay D, Kumar R, Baker KM, Mitchell BM, Powers PA, Fitzsimons DP, Patel BG, Warren CM, Solaro RJ, Moss RL, Tong CW. Phosphorylation of cardiac myosin-binding protein-C is a critical mediator of diastolic function. Circ Heart Fail. 2015;8:582–594. doi: 10.1161/CIRCHEARTFAILURE.114.001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeWinter MM, Zile MR. Could modification of titin contribute to an answer for heart failure with preserved ejection fraction? Circulation. 2016;134:1100–1104. doi: 10.1161/CIRCULATIONAHA.116.023648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borbély A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJM, Paulus WJ. Hypophosphorylation of the stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 18.Chung CS, Hutchinson KR, Methawasin M, Saripalli C, Smith JE, III, Hidalgo CG, Luo X, Labeit S, Guo C, Granzier HL. Shortening of the elastic tandem immunoglobulin segment of titin leads to diastolic dysfunction. Circulation. 2013;128:19–28. doi: 10.1161/CIRCULATIONAHA.112.001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL, LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: Contributions of collagen and titin. Circulation. 2015;131:1247–1259. doi: 10.1161/CIRCULATIONAHA.114.013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinsey TA. Therapeutic potential for HDAC inhibitors in the heart. Annu Rev Pharmacol Toxicol. 2012;52:303–319. doi: 10.1146/annurev-pharmtox-010611-134712. [DOI] [PubMed] [Google Scholar]
- 21.Morales CR, Li DL, Pedrozo Z, May HI, Jiang N, Kyrychenko V, Cho GW, Kim SY, Wang ZV, Rotter D, Rothermel BA, Schneider JW, Lavandero S, Gillette TG, Hill JA. Inhibition of class I histone deacetylases blunts cardiac hypertrophy through TSC2-dependent mTOR repression. Sci Signal. 2016;9:ra34. doi: 10.1126/scisignal.aad5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettica P, Petrini S, D’Oria V, D’Amico A, Catteruccia M, Pane M, Sivo S, Magri F, Brajkovic S, Messina S, Vita GL, Gatti B, Moggio M, Puri PL, Rocchetti M, De Nicolao G, Vita G, Comi GP, Bertini E, Mercuri E. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26:643–649. doi: 10.1016/j.nmd.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Herrera VL, Ruiz-Opazo N. Alteration of alpha 1 Na+,K(+)-ATPase 86Rb+ influx by a single amino acid substitution. Science. 1990;249:1023–1026. doi: 10.1126/science.1975705. [DOI] [PubMed] [Google Scholar]
- 24.Rapp JP, Wang SM, Dene H. A genetic polymorphism in the renin gene of Dahl rats cosegregates with blood pressure. Science. 1989;243:542–544. doi: 10.1126/science.2563177. [DOI] [PubMed] [Google Scholar]
- 25.Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D. Development of heart failure in chronic hypertensive Dahl rats: Focus on heart failure with preserved ejection fraction. Hypertension. 2006;47:901–911. doi: 10.1161/01.HYP.0000215579.81408.8e. [DOI] [PubMed] [Google Scholar]
- 26.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124:30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzsimons DP, Patel JR, Moss RL. Role of myosin heavy chain composition in kinetics of force development and relaxation in rat myocardium. J Physiol. 1998;513(Pt 1):171–183. doi: 10.1111/j.1469-7793.1998.171by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh RA. Sympathetic control of diastolic function in congestive heart failure. Circulation. 1990;82:I52–I58. [PubMed] [Google Scholar]
- 29.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 30.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O’Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: A randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972.e10. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, Kelstrup CD, Dmytriyev A, Choudhary C, Lundby C, Olsen JV. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012;2:419–431. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samant SA, Pillai VB, Sundaresan NR, Shroff SG, Gupta MP. Histone deacetylase 3 (HDAC3)-dependent reversible lysine acetylation of cardiac myosin heavy chain isoforms modulates their enzymatic and motor activity. J Biol Chem. 2015;290:15559–15569. doi: 10.1074/jbc.M115.653048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viswanathan MC, Blice-Baum AC, Schmidt W, Foster DB, Cammarato A. Pseudo-acetylation of K326 and K328 of actin disrupts Drosophila melanogaster indirect flight muscle structure and performance. Front Physiol. 2015;6:116. doi: 10.3389/fphys.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao V, Cheng Y, Lindert S, Wang D, Oxenford L, McCulloch AD, McCammon JA, Regnier M. PKA phosphorylation of cardiac troponin I modulates activation and relaxation kinetics of ventricular myofibrils. Biophys J. 2014;107:1196–1204. doi: 10.1016/j.bpj.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong E-M, Monasky MM, Gu L, Taglieri DM, Patel BG, Liu H, Wang Q, Greener I, Dudley SC, Jr, Solaro RJ. Tetrahydrobiopterin improves diastolic dysfunction by reversing changes in myofilament properties. J Mol Cell Cardiol. 2013;56:44–54. doi: 10.1016/j.yjmcc.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stratton MS, McKinsey TA. Epigenetic regulation of cardiac fibrosis. J Mol Cell Cardiol. 2016;92:206–213. doi: 10.1016/j.yjmcc.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silberman GA, Fan THM, Liu H, Jiao Z, Xiao HD, Lovelock JD, Boulden BM, Widder J, Fredd S, Bernstein KE, Wolska BM, Dikalov S, Harrison DG, Dudley SC., Jr Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinsey TA. Targeting inflammation in heart failure with histone deacetylase inhibitors. Mol Med. 2011;17:434–441. doi: 10.2119/molmed.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horgan S, Watson C, Glezeva N, Baugh J. Murine models of diastolic dysfunction and heart failure with preserved ejection fraction. J Card Fail. 2014;20:984–995. doi: 10.1016/j.cardfail.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Schwarzl M, Hamdani N, Seiler S, Alogna A, Manninger M, Reilly S, Zirngast B, Kirsch A, Steendijk P, Verderber J, Zweiker D, Eller P, Höfler G, Schauer S, Eller K, Maechler H, Pieske BM, Linke WA, Casadei B, Post H. A porcine model of hypertensive cardiomyopathy: Implications for heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2015;309:H1407–H1418. doi: 10.1152/ajpheart.00542.2015. [DOI] [PubMed] [Google Scholar]
- 41.Wallner M, Eaton DM, Berretta RM, Borghetti G, Wu J, Baker ST, Feldsott EA, Sharp TE, III, Mohsin S, Oyama MA, von Lewinski D, Post H, Wolfson MR, Houser SR. A feline HFpEF model with pulmonary hypertension and compromised pulmonary function. Sci Rep. 2017;7:16587. doi: 10.1038/s41598-017-15851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zakeri R, Moulay G, Chai Q, Ogut O, Hussain S, Takahama H, Lu T, Wang XL, Linke WA, Lee HC, Redfield MM. Left atrial remodeling and atrioventricular coupling in a canine model of early heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. DSS rats fed a 4% NaCl–containing diet develop diastolic dysfunction with preserved EF.
Fig. S2. Treatment of ARVMs in culture with ITF2357 led to acceleration of myocyte relaxation kinetics without affecting contractility.
Fig. S3. HDAC inhibition does not affect HS diet–induced increases in β-MyHC expression or function.
Fig. S4. Myofibril calcium sensitivity is unaffected by HS feeding or ITF2357 treatment of DSS rats.
Fig. S5. Titin expression and function are unaffected by HS feeding or ITF2357 treatment of DSS rats.
Fig. S6. Myofibrillar protein phosphorylation and expression are unaffected by HS feeding or ITF2357 treatment of DSS rats.
Fig. S7. Site-specific phosphorylation of myofibrillar protein and myofibrillar protein expression are unaffected by HS feeding or ITF2357 treatment of DSS rats.
Fig. S8. HDAC2 co-purifies with cardiac myofibrils.
Fig. S9. Ex vivo acetylation/deacetylation does not alter myofibril contraction.
Fig. S10. Anti–acetyl-lysine immunoblotting of cardiac myofibrils from DSS rats.
Fig. S11. A model for HDAC inhibitor–mediated improvement in diastolic function and treatment of HFpEF.
Table S1. Hematological profiles of DSS rats.
Table S2. LV hemodynamic and echocardiographic parameters of DSS rats.
Table S3. Quantification of myocyte cross-sectional area and interstitial fibrosis in DSS rats.
Table S4. Echocardiographic parameters of aging mice.
Table S6. Characteristics of the RCM patients and nonfailing donor controls.
Table S7. Group sizes of animal models.
Table S5. Echocardiographic, hemodynamic, and hypertrophy parameters of aging mice.