Abstract
Acute ischemic stroke causes a high rate of deaths and permanent neurological deficits in survivors. Current interventional treatment, in the form of enzymatic thrombolysis, benefits only a small percentage of patients. Brain ischemia triggers mobilization of innate immunity, specifically the complement system and Toll-like receptors (TLRs), ultimately leading to an exaggerated inflammatory response. Here we demonstrate that intravenous immunoglobulin (IVIG), a scavenger of potentially harmful complement fragments, and C1-esterase inhibitor (C1-INH), an inhibitor of complement activation, exert a beneficial effect on the outcome of experimental brain ischemia (I) and reperfusion (R) injury induced by transient occlusion of middle cerebral artery in mice. Both IVIG and C1-INH significantly and in a dose–responsive manner reduced brain infarction size, neurological deficit and mortality when administered to male mice 30 min before ischemia or up to 6 h after the onset of reperfusion. When combined, suboptimal doses of IVIG and C1-INH potentiated each other’s neuro-protective therapeutic effects. Complement C3 and TLR2 signals were colocalized and significantly greater in brain cells adjacent to infracted brain lesions when compared to the corresponding regions of the contralateral hemisphere and to control (sham) mice. Treatment with IVIG and C1-INH effectively reduced deposition of C3b and downregulated excessive TLR2 and p-JNK1 expression at the site of I/R injury. Taken together, these results provide a rationale for potential use of IVIG and C1-INH, alone or in combination with ischemic stroke and other neurological conditions that involve inappropriately activated components of the innate immune system.
Keywords: Murine stroke, Neuroprotection, IVIG, Complement, TLR2
Introduction
Acute ischemic stroke (AIS) represents a major health problem of global proportions. It is responsible for almost 10% of all deaths reported annually worldwide and is the most frequent cause of permanent neurological disabilities (Lloyd-Jones et al. 2009). In about 85% of stroke incidents, there is evidence of thromboembolic occlusion of a brain artery (most commonly middle cerebral) and resulting ischemia (Adams 2003). Brain ischemia is a potent trigger of the complement activation cascades which, in turn, initiate and augment the inflammatory response (D’Ambrosio et al. 2001). Active complement fragments mediate both direct brain cell damage as well as activation of other pro-inflammatory circuits. We previously reported that mice lacking the C5 gene and subjected to brain ischemia/reperfusion (I/R) injury exhibited improved functional outcome and less damage to brain cells compared to their wild-type littermates (Arumugam et al. 2007). Furthermore, brain I/R in normal mice led to an increase in C3b fragment deposition that was confined to the site of injury. Strong complement activation, determined by high levels of C5b-9 and C4d, is associated with unfavorable outcome of ischemic stroke in humans (Széplaki et al. 2009). Patients with ischemic stroke who had elevated C3 and C4 complement levels at discharge had a higher degree of permanent neurological disability (Cojocaru et al. 2008). Considering its important role in neuronal cell damage after I/R injury, complement appears to be an attractive target for specific interventional therapy.
Less than 6% of all AIS patients in the USA are eligible for current standard of care treatment in the form of enzymatic thrombolysis by recombinant tissue plasminogen activator rtPA (Adeoye et al. 2011). We have previously demonstrated that high-dose intravenous immunoglobulin (IVIG), a scavenger of activated complement fragments, effectively attenuates brain damage after occlusion of middle cerebral artery in mice (Arumugam et al. 2007). Here we show that another IVIG preparation, as well as C1 esterase inhibitor (C1-INH), which blocks complement activation cascades, reduces brain damage following experimental stroke. When suboptimal doses of IVIG and C1-INH were given sequentially as a combination treatment, the neuroprotective effects exceeded those achieved with the individual therapeutic agents. Furthermore, both IVIG and C1-INH inhibited expression of C3, TLR2 and p-JNK1 in brain cells in close proximity to infarction, suggesting that TLRs could be a joint innate immune target of complement-attenuating therapy for stroke.
Materials and Methods
Focal Cerebral Ischemia/Reperfusion Model
All experimental protocols and procedures were approved by the Animal Care and Use Committee of the National Aging Institute and were conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals. Three-month-old male and female C57BL/6 mice and 13–14 month-old C57BL/6 mice were anesthetized with isoflurane (induction 5.0%, maintenance 1.5–2.0%). The animals were then subjected to transient middle cerebral artery occlusion (TMCAO), using the previously described intraluminal filament method (Longa et al. 1989). Briefly, the left common carotid artery, external carotid artery (ECA) and internal carotid artery (ICA) were exposed via a midline neck incision. Then, a silicon-coated monofilament (diameter with coating 0.23 ± 0.02 mm) was introduced into the ECA lumen through an incision and advanced from the ECA to the ICA lumen until light resistance was felt. The monofilament was removed after a period of 1 h to initiate reperfusion for up to 72 h. Sham-operated mice were treated identically with the exception of insertion of the filament to produce an occlusion. Animals were subjected to cerebral blood flow measurements using a laser Doppler perfusion monitor (Moor Lab) placed perpendicular to the surface of the right parietal skull (1 mm posterior and 5 mm lateral to the bregma) to monitor blood flow in the MCAO territory. In addition, operators were blinded to treatment group and randomization was based on predesigned lists using color-coded cages and reagents. Exclusion criteria were excessive bleeding or death within 24 h after tMCAO. All experimental procedures followed the “Australian code of practice for the care and use of animals for scientific purposes,” approved by The University of Queensland. We have strictly followed ARRIVE guidelines.
Treatment Regimen
The IVIG preparation (Privigen, CSL Behring AG, Bern, Switzerland) used in this work is a 10% protein solution containing > 98% IgG, and < 0.025 mg/ml IgA. IgG molecules in Privigen are stabilized with 250 mmol/L l-proline (vehicle). Lyophilized, plasma-derived human C1 s esterase inhibitor, C1-INH, (Berinert, CSL Behring GmbH, Marburg, Germany) was reconstituted with 10 ml of sterile water to achieve a final C1-INH dose of 500 U in vehicle solution containing 10 mg/ml of glycine, 8.5 mg/ml of NaCl and 3 mg/ml of sodium citrate. Reconstituted C1-INH was kept at +4 °C and used within 14 days. Both IVIG and C1-INH were administered by slow infusion into the femoral vein either 30 min before (pre-treatment) or at designated time points (1 h and 3 h) after MCAO (post-treatment). Vehicle solutions for both therapeutics (as described above) were infused into control animals at the volume that equaled the volume in which optimal doses of IVIG and C1-INH were delivered.
Neurological Deficit Evaluation
At the designed post-stroke time points, the functional consequences of focal cerebral I/R injury were evaluated using a six-point neurological deficit score (0, no deficit; 1, failure to extend right paw; 2, circling to the right; 3, falling to the right; 4, unable to walk spontaneously and 5, dead) and each mouse was assessed in a blinded fashion by at least two well-trained investigators.
Quantification of Cerebral Infarction
Mice were anesthetized, decapitated and their brains removed. Two-millimeter coronal slices were made using a rodent brain matrix (Kent Scientific, Torrington, CT) and then stained for 20 min at 37 °C with 2% 2,3,5-triph-enyltetrazolium chloride monohydrate (TTC, Sigma, St Louis, MO) in phosphate buffer. The sections were scanned, and the infarction size was evaluated using NIH image analysis software (ImageJ; National Institutes of Health, Bethesda, MD).
Gel Electrophoresis and Western Blotting
Brain cortical and striatal samples from IVIG- and C1-INH-treated mice and appropriate vehicle control mice were placed in lysis buffer containing 300 mM NaCl, 1.5 mM MgCl2, 25 mM HEPES, 20 mM β-glycerophosphate, 0.2 mM EDTA, 0.5 mM dithiothreitol (DTT) and 0.1%Triton-X with protease inhibitors. Tissues were lysed by 30-s sonication at 4 °C using an ultrasonic processor (VC 130 PB, Sonic & Materials Inc., Newtown, CT) and the cellular extracts were centrifuged for 5 min at 18,000×g. Protein content was then determined by a bicinchoninic acid (BCA) assay kit from Bio-Rad (Hercules, CA). Supernatant samples were separated on 10% SDS gels and the resolved proteins electrophoretically transferred to a 0.45 μm polyvinylidene fluoride (PVDF) membrane (Millipore Corporation, Bedford, MA). The blots were incubated with a primary anti-C3b antibody (Cell Sciences, Canton, MA), rabbit polyclonal anti-TLR2 (Cell Signaling, Beverly, MA), rabbit polyclonal anti-p-JNK (Cell Signaling, Beverly, MA) and anti-βactin monoclonal antibody (Sigma Chemical Co., St. Louis, MO) overnight at 4 °C. Following incubation with horseradish peroxidase-conjugated secondary IgG for 1 h, reaction products were visualized using the enhanced chemiluminescence assay (Amersham Corporation, Piscataway, NJ). Relative changes in protein expression were estimated from the mean pixel density of each protein band using the Scion Image program (Orem, UT).
Immunohistochemical Evaluation
Mice designated for immunohistochemical studies were deeply anaesthetized after 24 h of reperfusion, and transcardially perfused with PBS until the outflow ran clear. Fixation was achieved with 4% paraformaldehyde in PBS. Brains were dissected out and placed in the same fixative overnight at 4 °C. The frontal brain was processed for paraffin sectioning. Serial coronal sections were cut (5 μm thick) and mounted on glass slides. Sections were deparaffinized, rehydrated and subjected to antigen retrieval using boiling high-pH TRIS–EDTA buffer. All cell counts were carried out in defined areas maintained across all sections. Following blockade of nonspecific antibody binding, sections were incubated overnight with primary rabbit polyclonal anti-C3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and rat monoclonal anti-TLR2 (Biolegend, CA) at 4 °C. After being incubated with appropriate Alexa Fluor 568 (red fluorescence) and Alexa Fluor 488 (green fluorescence) conjugated secondary antibodies (Invitrogen, Carlsbad, CA) for 45 min and counterstained with Hoechst 33258, images were acquired with an Olympus fluorescence microscope. Images were processed with Photoshop 7.0 (Adobe Systems, San Jose, CA); with the input levels adjusted to span the range of acquired signal intensities exactly. Quantitation of pixel intensity was done using IP labs Software (BD biosciences, San Diego, CA).
Statistical Analysis
Statistical comparisons were made by using Student’s t test, Chi-square and ANOVA followed by Newman–Keuls post hoc tests for pairwise comparisons.
Results
IVIG is Neuroprotective Both Before and After Ischemia in Experimental Stroke
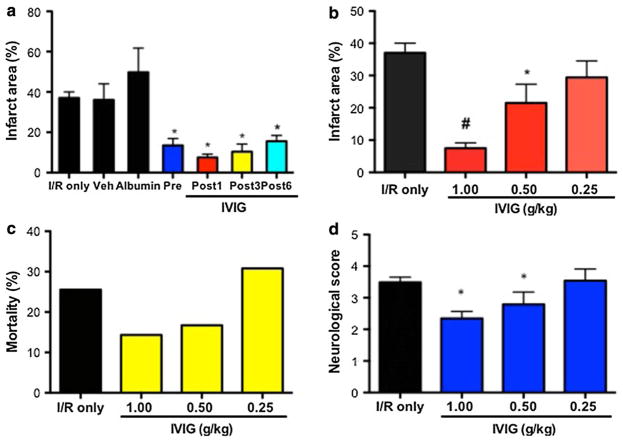
The effect of IVIG (Privigen, CSL Behring) on the outcome of brain I/R injury was evaluated in the murine stroke induced by 1 h MCAO and 72 h reperfusion. In non-treated (I/R only) mice, this procedure resulted in an average brain infarction of 36% of the affected hemisphere. IVIG treatment at 1 g/kg 30 min before and 1 h after MCAO as well as 3 h and 6 h post-ischemia at 2 g/kg reduced the infarction size by 56, 80, 58 and 59%, respectively. Treatment with control reagents, 250 mM l-proline (vehicle or stabilizer for immunoglobulin molecules in the Privigen IVIG preparation) or human albumin for IV use at 1 g/kg in vehicle solution had no significant effect on the size of infarction when compared to non-treated mice (Fig. 1a).
Fig. 1.
IVIG exerts dose–responsive neuroprotective effect in the mouse model of stroke when administered before or after ischemia. a Brain infarction size 72 h post-ischemia in albumin treated (N = 19), IVIG (1 g/kg 30 min before ischemia) pretreated (N = 12), IVIG treated 1 h (Post 1 h, N = 30) and 3 h (Post 3 h, N = 11) after ischemia compared with non-treated (I/R only, N = 35) and vehicle (N = 18) treated mice; values are mean ± SEM; *P < 0.001 b Brain infarct area in mice treated with decreasing doses of IVIG at 1 g/kg (N = 30), 0.5 g/kg (N = 10) and 0.25 g/kg (N = 9); #P < 0.001, *P < 0.05. c Mortality in mice subjected to decreasing doses of IVIG at 1 g/kg (N = 30), 0.5 g/kg (N = 10) and 0.25 g/kg (N = 9); d neurological deficit score evaluated at 72 h post-ischemia in mice treated with IVIG 1 g/kg (N = 35), 0.5 g/kg (N = 12) and 0.25 g/kg (N = 13)
To determine if the marked reduction of ischemic brain injury observed in IVIG-treated mice resulted in a reduced functional impairment, neurological deficits were evaluated daily at 24, 48 and 72 h in mice that were eventually killed for the brain infarction measurements. IVIG treatments significantly reduced the neurological deficits at 48 and 72 h after the ischemia (Table 1). IVIG also exerted a dose-dependent effect on the consequences of ischemic brain injury. With half-decreasing doses of Privigen (1.0 g/kg, 0.5 g/kg and 0.25 g/kg) stepwise increases in brain infarction size (Fig. 1b), mortality (Fig. 1c) and neurological deficit at 72 h (Fig. 1d) were observed.
Table 1.
Kinetics of neurological dysfunction in IVIG-treated, C1-INH-treated and control mice following MCAO
| Treatment | Neurological deficit score at | ||
|---|---|---|---|
|
| |||
| 24 h | 48 h | 72 h | |
| I/R only | 3.10 ± 0.18 | 2.95 ± 0.16 | 3.49 ± 0.16 |
| IVIG vehicle | 3.08 ± 0.11 | 3.37 ± 0.20 | 3.63 ± 0.25 |
| IVIG 1 g/kg | 2.94 ± 0.06 | 2.57 ± 0.18* | 2.35 ± 0.22* |
| IVIG 0.5 g/kg | 2.54 ± 0.14 | 2.58 ± 0.22* | 2.62 ± 0.25* |
| C1-INH vehicle | 2.77 ± 0.09 | 3.34 ± 0.15 | 3.66 ± 0.24 |
| C1-INH 300 U/kg | 2.64 ± 0.09 | 2.46 ± 0.20** | 2.84 ± 0.30** |
| C1-INH 75 U/Kg | 2.66 ± 0.08 | 2.58 ± 0.22** | 2.62 ± 0.25** |
| IVIG + C1-INH | 2.22 ± 0.12# | 1.96 ± 0.15# | 2.17 ± 0.29# |
P < 0.05 compared to IVIG vehicle
P < 0.05 compared to C1-INH vehicle
P < 0.05 compared to I/R only
C1-INH Decreases Infarction Size and Neurological Deficit in Mice Subjected to Brain I/R Injury
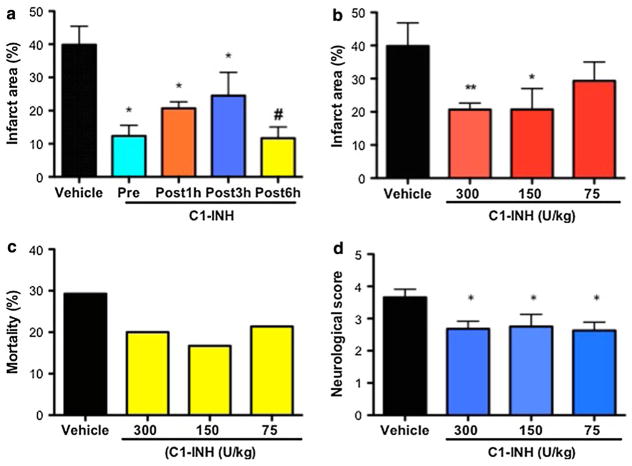
A single dose of C1-inhibitor (Berinert, CSL Behring) at 300 U/kg administered 30 min before ischemia and during reperfusion at 1 h, 3 h and 6 h post-ischemia, significantly reduced brain infarction size relative to vehicle-treated mice by 69, 48, 50 and 67%, respectively (Fig. 2a). C1-INH treatment also significantly reduced functional impairment, as the neurological deficit scores (at 48 h and 72 h after MCAO) were significantly reduced in comparison with mice that received vehicle alone (Table 1). Reduction of the C1-INH dose to 150 U/kg and 75 U/kg did not result in corresponding incremental worsening of the experimental stroke endpoints as seen in the IVIG dose–response experiment. Instead, there was no significant difference in the degree of inhibition of brain infarction, neurological deficit at 72 h post-ischemia and mortality among the three doses of C1-INH. (Fig. 2b–d).
Fig. 2.
C1-INH has a therapeutic time window of up to 6 h post-ischemia in experimental stroke. a Brain infarction area in mice treated with 300 U/kg of C1-INH before (N = 23) and 1 h (NB = 28) and 3 h (N = 11) post-ischemia, compared with vehicle-treated mice (N = 22); values are mean ± SEM; *P < 0.05. b Infarction size in animals treated 1 h post-ischemia with C1-INH at 300 U/kg (N = 28), 150 U/kg (N = 10) and 75 U/kg (N = 11); *P < 0.05, **P < 0.01. c Mortality rate among mice treated 1 h post-ischemia with C1-INH at 300 U/kg (N = 35), 150 U/kg (N = 12) and 75 U/kg (N = 14). d Neurological deficit score at 72 h after the initiation of reperfusion in mice injected with C1-INH as 300 U/kg (N = 35), 150 U/kg (N = 12) and 75 U/kg (N = 14)
Combined Treatment with IVIG and C1-INH Provides Superior Neuroprotection in Experimental Stroke
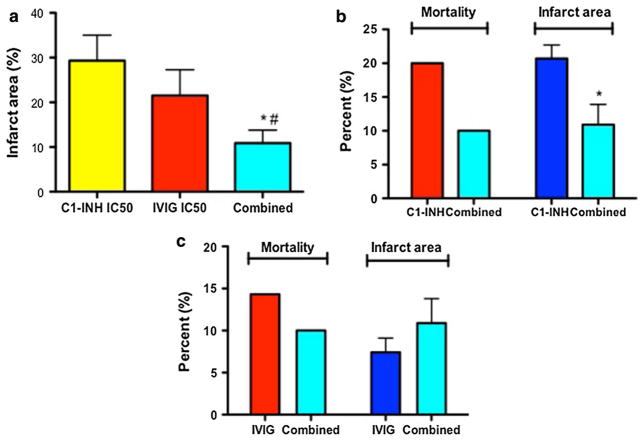
To investigate the neuroprotective potential of combination therapy with IVIG and C1-INH, we administered a single dose of 0.50 mg/g IVIG at 1 h after ischemia, followed by a single IV injection of C1-INH at the dose of 75 U/kg 30 min later. The brain infarction volume was determined following a 72-h reperfusion period. The combination of 0.50 mg/g IVIG and 75 U/kg dose of C1-INH resulted in threefold reduction of brain infarct volume relative to 75 U/kg of C1-INH and two times more reduction than 0.5 g/kg of IVIG alone (Fig. 3a). The combined treatment was more neuroprotective even when compared to the optimal C1-INH dose; it reduced both mortality rate and the infarction size by 50% relative to animals infused with 300 U/kg of C1-INH (Fig. 3b). The combined IVIG/C1-INH therapy was equally effective as the optimal dose of IVIG at 1 g/kg—mortality rates and infarction sizes did not significantly vary between the two groups of animals (Fig. 3b). The neurological deficit was significantly reduced at all time points (24, 48 and 72 h post-ischemia) in mice receiving both IVIG and C1-INH in comparison with I/R only group and 17–24% lower than in mice treated with the suboptimal doses of IVIG or C1-INH alone (Table 1). The combined administration was significantly and equally effective as IVIG and C1-INH in terms of reducing the size of brain infarction (by 67% in comparison with non-treated animals) even when applied 6 h after ischemia (Fig. S1A). Following combined treatment at 6 h post-ischemia, the neurological disabilities score improved by 71%; this effect was significantly more pronounced compared to mice treated with optimal individual doses of IVIG and C1-INH (Fig. SIB).
Fig. 3.
Combined treatment with suboptimal doses of IVIG and C1-INH is superior to the effects of individual doses. a Infarction size in mice subjected to half-maximal inhibitory concentration (IC50) of IVIG treatment 1 h post-ischemia at 0.5 g/kg (N = 10), half-maximal inhibitory concentration (IC50) of C1-INH at 75 U/kg (N = 11) and the combined treatment consisting of 0.5 g/kg of IVIG followed by 75 U/kg 30 min later (N = 19); P < 0.05. b Comparisons between mortality rate (N = 35, red bar) and infarction size (N = 28, blue bar) in mice treated with 300 U/kg of C1-INH and the same endpoints in animals that received combination (combined) treatment (N = 20 and 18, respectively; cyan bars) as described above. c Mortality (N = 35, red bar) and infarction size (N = 30, blue bar) in mice treated with IVIG at 1 g/kg and the combination (combined IVIG + C1-INH treatment; N = 20 and 18, respectively, cyan bars)
IVIG and C1-INH Protect Older Mice and Female Mice from Injury Induced by Brain Ischemia/Reperfusion
Human stroke affects both sexes equally, and its incidence rises with age. For that reason, we tested the effectiveness of IVIG, C1-INH and the combined treatment in female mice and 13–14 month-old male mice 6 h post-ischemia. As shown in Figure S2, both older male mice and female mice exhibited significantly reduced infarction size and the degree of neurological disabilities at 72 h after ischemia compared to non-treated animals, when infused with IVIG at 1 g/kg, C1-INH at 300 U/kg, as well as with half-maximal inhibitory (IC50) concentrations of both treatments administered in succession. In older mice, combined therapy was approximately 50 and 70% more effective in terms of reducing the infarction size and neurological deficit score, respectively, when compared to optimal individual doses of IVIG and C1-INH (Fig. S2).
Both IVIG and C1-INH Reduce In Situ Accumulation of C3b
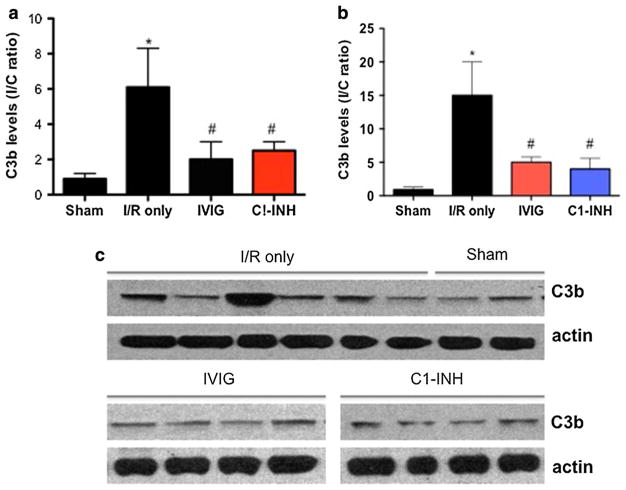
We evaluated the effect of IVIG/C1-INH treatment on the levels of C3b, the active fragment of the third complement component, in the brain samples from mice subjected to I/R injury. We first determined that basal levels of C3b observed in brains of normal and sham-operated animals were similar to the C3b level in the contralateral (non-injured) hemisphere of I/R mice. Following I/R injury, C3b at the site of injury (in the ipsilateral hemisphere) was increased five-fold in the cortex and 15-fold in the striatum. Both IVIG (at 1.0 g/kg) and C1-INH (at 300 U/kg) administered 1 h after the onset of reperfusion, reduced C3b expression by approximately threefold in both the cortex and striatum of the affected hemisphere (Fig. 4a, b). The data suggest that complement is activated in response to brain ischemia, leading to in situ deposition of active fragments. Both complement scavenging and inhibition appear to be equally effective in reducing neuronal damage triggered by C3b deposition. More pronounced deposition of C3b in the striatal portion of the infarction correlates with previous findings of more extensive anatomical damage in that area, providing further cause-and-effect relations between I/R brain injury and complement activation.
Fig. 4.
IVIG and C1-INH attenuate ischemia-induced C3b deposition in both the cortex and striatum of brain areas adjacent to the infarction. a Ipsilateral (I) and contralateral cortical samples from sham-operated (N = 5), non-treated (I/R only, N = 10) and mice treated with IVIG at 1 g/kg (N = 5) as well as C1-INH at 300 U/kg 1 h after ischemia (N = 5) were subjected to immunoblotting and densitometric analysis of the C3b signal. Data are I/C ratios of C3b band intensity and are expressed as mean ± SEM; *P < 0.05 compared to sham, #P < 0.05 compared to I/R only. b Densitometric analysis of ipsilateral (I) and contralateral c striatal samples from the same groups of animals as in (a); c representative immunoblots showing the level of C3b signals in ipsilateral versus contralateral samples in sham and I/R only mice (upper strip); starting from the far left, loaded lanes alternate between ipsilateral and contralateral protein samples. Two lower strips show the effect of IVIG and C1-INH treatment on the expression of C3b in ipsilateral samples (N = 4 each)
Evidence that Complement Activation Upregulates TLR2 in Ischemic Stroke
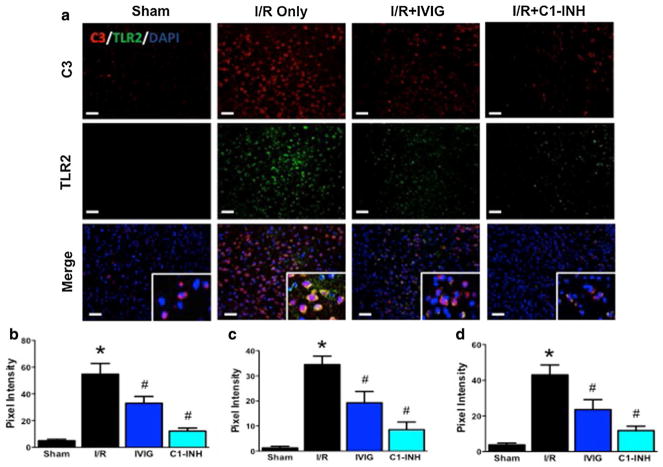
Complement and Toll-like receptors (TLRs) belong to the front line of the host’s innate immune defense system, and react instantly to invading pathogens and altered self-antigens via pattern recognition (Okun et al. 2011). We therefore evaluated the possible coactivation of complement and TLR pathways, and attenuation by IVIG and C1-INH, by performing double-label immunohistochemistry using antibodies against C3b and TLR2. In tissue sections taken from the cerebral cortex penumbra region 24 h after I/R injury, over 90% of the brain cells showed levels of intrinsic C3 and TLR2 expression that were 11(C3) and 28 (TLR2) times higher than in the same sections from sham-operated animals, as determined by quantifying pixel intensity of the signals (Fig. 5a–c). In mice treated with IVIG (1 g/kg) and C1-INH (300 U/kg) 1 h post-ischemia, both C3 and TLR2 signals were significantly suppressed (Fig. 5a–c). Double-label immunofluorescence staining for C3 and TLR2 revealed that in almost all brain cells these two molecules colocalize (Fig. 5a). In sections from mice treated with both IVIG and C1-INH the colocalized signal was suppressed equally effectively as the individual signals. (Fig. 5a, d).
Fig. 5.
IVIG and C1-INH downregulate ischemia-induced increase in C3b and TLR2 expression. a Immunostaining for C3b (red fluorescence) and TLR2 (green fluorescence) of sections from the penumbra area of sham-operated mice (N = 4), non-treated mice (N = 4), and mice treated with IVIG at 1 g/kg (N = 6) and 300 U/kg of C1-INH (N = 6) 1 h after ischemia. The inserts in the merged images show colocalization (yellow fluorescence) of C3b and TLR2 signals. Representative images from the region of interest; Scale bar, 50 μm. b–d Pixel intensity quantification of C3-stained sections (b), TLR2-stained sections (c) and merged fields (d). Data are mean ± SEM; *P < 0.001 I/R versus Sham; #P < 0.01 IVIG versus I/R; #P < 0.001 C1-INH versus I/R
Evidence for the Involvement of Suppressed p-JNK1 Activation in the Neuroprotective Actions of IVIG and C1-INH
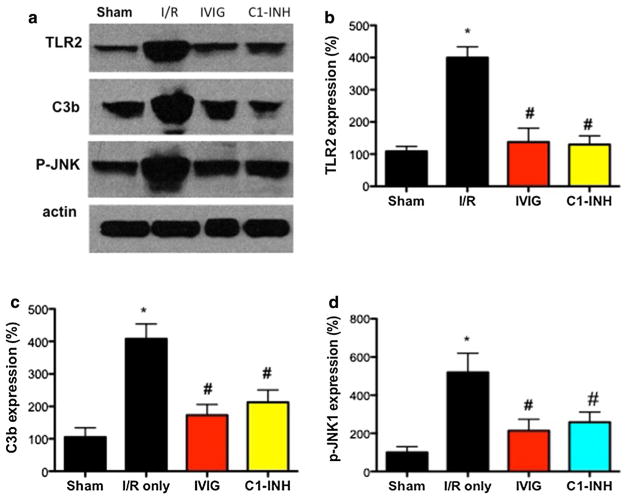
Using Western blot methods, we determined that the levels of activated JNK1 (p-JNK1) in brain cells close to the infarction at 72 h after ischemia rose significantly and simultaneously with an increase in TLR2 and C3b signals, when compared to animals subjected to sham intervention. Further, stroke-induced p-JNK1 was suppressed by both IVIG and C1-INH treatments, along with the reduction of TLR2 and C3b levels (Fig. 6a–d).
Fig. 6.
IVIG and C1-INH suppress JNK1 activation in the ischemic penumbra. a Representative Western blots of TLR2, C3b and p-JNK1 levels in the penumbra region of mice subjected to sham procedure (N = 5), I/R injury (N = 5), IVIG (N = 5) and C1-INH (N = 5) treatments at optimal doses (1 g/kg and 300 U/kg, respectively). b–d Densitometric quantification of TLR2 (b), C3b (c), and p-JNK1 (d) signals normalized to actin and presented as a percentage of the mean value of sham-treated mice. *P < 0.001, I/R versus Sham; #P < 0.01, IVIG/C1-INH versus I/R
Discussion
Our results demonstrate that IVIG and C1-INH, alone or in combination, significantly reduce brain damage and improve functional outcome in a mouse model of ischemic stroke. Both therapeutic modalities significantly suppressed stroke-induced upregulation of the innate immunity components, the complement cascade and TLR signaling, as indicated by reductions in C3b and TLR2 levels, respectively. This effect appears to be achieved by inhibiting the p-JNK1 signaling pathway that is known to mediate both TLR2 (Tang et al. 2007) and complement lytic activities (Ganz et al. 2009).
It was previously reported that a different IVIG preparation (Flebogama, Grifols, Spain) protected mice from experimental stroke by a mechanism involving suppression of complement-mediated neuronal death (Arumugam et al. 2007). Using the same model of brain ischemia/reperfusion (I/R) injury, here we show that another IVIG preparation (Privigen, CSL Behring) reduces brain infarction size and associated functional impairments when given before and up to 6 h after brain ischemia in a dose–responsive manner. As with Flebogama, Privigen significantly reduced C3b deposition in the ischemic penumbra, strongly suggesting that neuroprotection exerted by both of these preparations is related to immunoglobulin molecules that attenuate complement cascade activation, rather than an unrelated attribute of one specific IVIG preparation.
As implied by its name (C1 s esterase inhibitor), the function of Cl-INH is to inhibit the first component of the classical complement cascade by binding to activated C1 s subcomponent (capable of cleaving the next protein in the cascade) and thus preventing its incorporation into the C1qrs complex (Davis et al. 2008). Similarly, C1-INH inactivates the lectin complement pathway, again by interfering with the initial or recognition phase of the cascade; in addition, C1-INH can bind to C3b molecules and thereby intercept the amplification loop of the alternative complement pathway (Davis et al. 2008). Inhibition of complement activation by plasma-derived C1-INH was shown to be beneficial in the mouse (De Simoni et al. 2003, 2004) as well as rat (Akita et al. 2001, 2003) MCAO model of AIS by reducing brain infarction volume and decreasing post-I/R neurological deficits. However, the therapeutic window observed in these studies was less than 1-h post-ischemia (De Simoni et al. 2004; Akita et al. 2001). This is in contrast to a recent find-ing that recombinant C1-INH is capable of reducing the brain infarction size even when given 18 h following MCAO (Gesuete et al. 2009). The general notion until now was that plasma-derived C1-INH, due to its short therapeutic window, is not as promising as recombinant C1-INH in terms of possible use in human stroke. Our data indicate that C1-INH at 150 U/kg (3.8 U per animal) administered 3 h after the completion of ischemia reduced brain infarction size to the same significant extent as the identical dose when administered 1 h after ischemia. This suggests that this plasma-derived C1-INH may have a place in stroke treatment.
Suppression of an exaggerated complement system response presents itself as an attractive therapeutic target, since complement activation plays a prominent role in pathogenesis of stroke. Recent studies emphasize the role of the lectin pathway of complement activation in the neuroinflammation and associated neuronal degeneration in human stroke (Chamorro et al. 2012). It was reported that deficiency of mannose-binding lectin (the recognition component of the lectin complement cascade) correlates with smaller infarction size and favorable outcomes in human acute ischemic stroke (Osthoff et al. 2011). The rationale for using both IVIG and C1-INH in the same model of stroke is that the two preparations could act as a superior complement attenuation therapy. C1-INH inhibits complement activation, but does not neutralize already generated, potentially harmful, complement activation fragments. On the other hand, IVIG, does not inhibit new complement activation but does scavenge activated complement fragments. Since in a complement-mediated disease, such as stroke, new bouts of complement activation occur sporadically with generation of pathogenic complement activation products, a combination of an inhibitor and a scavenger could be an optimal therapeutic strategy. Our data support this notion, since the combination of suboptimal doses of both preparations not only exceeded the effects of these individual concentrations but of optimal C1-INH therapeutic amounts as well and equaled the therapeutic potential of IVIG at 1 g/kg. This potentiation could have a place in therapy of human stroke, since both IVIG and C1-INH are being used in the treatments of other human conditions and have been proven safe. The combination of C1-INH and IVIG may enhance therapeutic outcomes while employing reduced treatment dosages, which, in turn, could lower the cost as well as the risk of adverse reactions.
TLRs recognize conserved structures expressed on the surface of microorganisms, which enables the innate immune system to respond instantly and with a fair degree of specificity to the threat imposed by pathogens (Okun et al. 2011). Similar to TLRs, complement recognizes certain cell-surface molecular arrangements unique to invading microorganisms, such as simple sugar expression and pattern motifs to which mannose-binding lectin (MBL; the recognition molecule of the lectin pathway of complement activation) binds and initiates complement activation. The molecular interplay (collaboration) between TLRs and the complement system serves primarily to initiate and optimally mobilize pro-inflammatory capacity of innate host defenses against invading microorganisms. On the other hand, excessive activation of either compartment of the innate immune system can lead, through the same cross talk, to an exaggerated inflammatory response (and self-damage) that can threaten the host (Hajishengallis and Lambris 2010). TLR2 was shown to be increased in neurons subjected to experimental I/R injury and contribute to cell damage (Tang et al. 2007). In stroke patients, the level of TLR2 expression in neutrophils was found to be correlated with the severity and outcome of stroke (Brea et al. 2011).
Concurrent upregulation of TLR and complement system components, in states that are known to trigger excessive inflammation such as stroke, myocardial infarction and hemorrhagic shock, has not been previously investigated. In brain I/R injury, the expressions of TLR2 and complement protein 3 appear to colocalize in neurons and augment subsequent cell damage. Our findings represent the first body of evidence that brain ischemia induces overexpression of both TLR2 and C3 on the surface of the brain cells and that the two signals colocalize. Furthermore, in addition to C3, treatment with IVIG and/or C1-INH suppressed expression of TLR2, implying that the complement cascade upregulates the other (TLR) arm of the innate immune system in the process of ischemic brain injury. Previous studies established a critical role for JNK1 activation in ischemic neuronal death (Okuno et al. 2004). JNK1 is a likely mediator of ischemic neuronal death downstream of complement and TLR2 because we found that levels of p-JNK1 were increased by I/R injury simultaneously with C3b and TLR2, and JNK1 activation was effectively inhibited by both IVIG and C1-INH.
Taken together, our results show that IVIG and C1-INH exert profound neuroprotection in experimental stroke. When their optimal doses are compared side by side, IVIG exerts more reduction of brain injury than C1-INH. The combination of their IC50 doses appears to surpass neuro-protective effects of the maximal dose of C1-INH and equal those exhibited by the optimal concentrations of IVIG, except in older mice where combined treatment exhibited superior brain protection compared to IVIG alone. Both therapies, in addition to suppressing complement, target TLR2 receptors that are upregulated following I/R injury and colocalize with intrinsic C3 in brain cells, as well as the p-JNK1 signaling pathway. This reveals a novel mechanism of their action and suggests a potential for use of combination therapy with low-dose IVIG and C1-INH in the treatment of ischemic stroke.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Mohamed R. Mughal for animal management and Drs. Ruiquian Wan, Eitan Okun and Dong Liu for their technical assistance. Supported, in part, by the Investigator Initiated Research grant from CSL Behring to Biovisions, Inc, and by the Intramural Research Program of the National Institute on Aging.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s12017-017-8474-6) contains supplementary material, which is available to authorized users.
References
- Adams HP. Stroke: A vascular pathology with inadequate management. Journal of Hypertension. Supplement. 2003;21:S3–S7. doi: 10.1097/00004872-200306005-00002. [DOI] [PubMed] [Google Scholar]
- Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: A doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita N, Nakase H, Kaido T, Kanemoto Y, Sakaki T. Protective effect of C1 esterase inhibitor on reperfusion injury in the rat middle cerebral artery occlusion model. Neurosurgery. 2003;52:395–400. doi: 10.1227/01.neu.0000043710.61233.b4. [DOI] [PubMed] [Google Scholar]
- Akita N, Nakase H, Kanemoto Y, Kaido T, Nishioka T, Sakaki T. The effect of C1 esterase inhibitor on ischemia: Reperfusion injury in the rat brain. No To Shinkei. 2001;53:641–644. [PubMed] [Google Scholar]
- Arumugam TV, Tang SC, Lathia JD, Cheng A, Mughal MR, Chigurupati S, et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14104–14109. doi: 10.1073/pnas.0700506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brea D, Blanco M, Ramos-Cabrer P, Moldes O, Arias S, Pérez-Mato M, et al. Toll-like receptors 2 and 4 in ischemic stroke: Outcome and therapeutic values. Journal of Cerebral Blood Flow and Metabolism. 2011;31:1424–1431. doi: 10.1038/jcbfm.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nature Reviews Neurology. 2012;8:401–410. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- Cojocaru IM, Cojocaru M, Tănăsescu R, Burcin C, Atanasiu AN, Petrescu AM, et al. Changes in plasma levels of complement in patients with acute ischemic stroke. Romanian Journal of Internal Medicine. 2008;46:77–80. [PubMed] [Google Scholar]
- D’Ambrosio AL, Pinsky DJ, Connolly ES. The role of the complement cascade in ischemia/reperfusion injury: Implications for neuroprotection. Molecular Medicine. 2001;7:367–382. [PMC free article] [PubMed] [Google Scholar]
- Davis AE, 3rd, Mejia P, Lu F. Biological activities of C1 inhibitor. Molecular Immunology. 2008;45:4057–4063. doi: 10.1016/j.molimm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni MG, Rossi E, Storini C, Pizzimenti S, Echart C, Bergamaschini L, et al. The powerful neuroprotective action of C1-inhibitor on brain ischemia-reperfusion injury does not require C1q. American Journal of Pathology. 2004;64:1857–1863. doi: 10.1016/S0002-9440(10)63744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni MG, Storini C, Barba M, Catapano L, Arabia AM, Rossi E, et al. Neuroprotection by complement (C1) inhibitor in mouse transient brain ischemia. Journal of Cerebral Blood Flow and Metabolism. 2003;23:232–239. doi: 10.1097/01.WCB.0000046146.31247.A1. [DOI] [PubMed] [Google Scholar]
- Ganz D, Donin N, Fishelson Z. Involvement of the c-jun N-terminal kinases JNK1 and JNK2 in complement-mediated cell death. Molecular Immunology. 2009;47:310–317. doi: 10.1016/j.molimm.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Gesuete R, Storini C, Fantin A, Stravalaci M, Zanier ER, Orsini F, et al. Recombinant C1 inhibitor in brain ischemic injury. Annals of Neurology. 2009;66:332–342. doi: 10.1002/ana.21740. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends in Immunology. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics—2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends in Neurosciences. 2011;34:269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno S, Saito A, Hayashi T, Chan PH. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. Journal of Neuroscience. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osthoff M, Katan M, Fluri F, Schuetz P, Bingisser R, Kappos L, et al. Mannose-binding lectin deficiency is associated with smaller infarction size and favorable outcome in ischemic stroke patients. PLoS ONE. 2011;6:e21338. doi: 10.1371/journal.pone.0021338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Széplaki G, Szegedi R, Hirschberg K, Gombos T, Varga L, Karádi I, et al. Strong complement activation after acute ischemic stroke is associated with unfavorable outcomes. Atherosclerosis. 2009;204:315–320. doi: 10.1016/j.atherosclerosis.2008.07.044. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.