Abstract
Purpose
Little is known about the extent to which genetic counseling is integrated into community practices for patients newly diagnosed with breast cancer. We examined the receipt of clinically indicated genetic counseling in these patients.
Patients and Methods
We surveyed 5,080 patients between the ages of 20 and 79 years, diagnosed from July 2013 to August 2015 with early-stage breast cancer and reported to the SEER registries of Georgia and Los Angeles County. Surveys were linked to SEER clinical data and genetic test results. The study sample (N = 1,711) comprised patients with indications for formal genetic risk evaluation.
Results
Overall, 47.4% did not get tested, 40.7% tested negative, 7.4% had a variant of uncertain significance only, and 4.5% had a pathogenic mutation. Three quarters (74.6%) received some form of genetic counseling (43.5%, formal counseling and 31.1%, physician-directed discussion). Virtually all tested patients (96.1%) reported some form of genetic discussion (62.2%, formal counseling and 33.9%, physician-directed discussion). However, only one half (50.6%) of those not tested received any discussion about genetics. Younger women were more likely to report some type of counseling, controlling for other factors: odds ratio, 4.5 (95% CI, 2.6 to 8.0); 1.9 (95% CI, 1.1 to 3.3); and 1.5 (95% CI, 1.0 to 2.3) for women younger than 50 years of age, 50 to 59 years of age, and 60 to 69 years of age versus those 70 years of age and older. Patients’ assessments of the amount of information they received about whether to get tested were similarly high whether they were counseled by a genetics expert or by a physician only (80.8% v 79.4% stated information was just right, P = .59).
Conclusion
Less than one half (43.5%) of patients with clinical indications received formal genetic counseling. There is a large gap between mandates for timely pretest formal genetic counseling in higher-risk patients and the reality of practice today.
INTRODUCTION
Genetic counseling for patients with breast cancer is indicated in those with an elevated pretest risk of pathogenic mutation carriage.1 Approximately one third of patients newly diagnosed with breast cancer have an elevated risk of mutation carriage on the basis of a family history of cancer, ancestry, and/or tumor characteristics.2,3 Importantly, counseling should occur ideally before final surgery decisions because bilateral mastectomy is one of the options for breast cancer treatment and risk reduction for pathogenic mutation carriers.
However, putting guidelines about genetic counseling into practice is challenging.4-6 Multigene panel testing, which seems to be replacing BRCA1/2-only testing, requires more counseling expertise, from initial consideration of test type, to discussion of results and formulation of a management plan. Integrating genetic counseling into the treatment decision workflow after a diagnosis of breast cancer is problematic because treatments focus on the diagnosed cancer and the pace of decision making is brisk. Addressing the risk and prevention of secondary cancers and the potential risk of hereditary cancers in relatives may be perceived by patients and their physicians as lower priorities than treating the diagnosed cancer. Furthermore, cancer physicians’ knowledge and attitudes about genetic testing are evolving. The growth of more extensive germline multigene panel testing seems to have already outstripped the timely availability of genetic counselor expertise in practice, despite requirements by insurers and recommendations by guidelines for formal pretest counseling.7
However, we know little about the extent to which formal genetic counseling for patients with newly diagnosed breast cancer has been integrated into community practices. We have shown that only approximately one half of women with an elevated pretest risk of pathogenic mutation are tested, and only 40% of these women discuss testing with a genetic counselor.2,3 Other published studies have been limited by small patient samples accrued before the advent of widespread multigene panel testing and have been drawn largely from a few academic centers or clinical practices.
To address these gaps, we examined the receipt of genetic discussion, directed either by an expert in genetic counseling (formal counseling) or by a cancer physician (physician-directed discussion), for patients newly diagnosed with breast cancer in a large population-based contemporary sample who had indications for formal genetic risk evaluation. We examined the patterns and correlates of discussion modalities, as well as patient assessment of the amount of information they received about whether to undergo testing.
PATIENTS AND METHODS
Study Population and Data Collection
The iCanCare study identified a large, diverse population of women with favorable-prognosis breast cancer from the SEER registries of Georgia and Los Angeles County who were 20 to 79 years of age and newly diagnosed with ductal carcinoma in situ or invasive breast cancer. Approximately 3 months after surgery, surveys were sent on a monthly basis from July 2013 to August 2015. Exclusions included prior breast cancer, stage III or IV disease, tumors > 5 cm, more than three involved lymph nodes, a residence at diagnosis outside of the SEER region, or the inability to speak English or Spanish. Patients were mailed materials with a $20 cash gift, and a modified Dillman method was used to encourage response.8 Overall, 7,810 patients were mailed surveys (507 were later deemed ineligible), and 5,080 surveys were completed by eligible patients (response rate = 70%).
Survey data were linked to SEER data, and genetic testing information was obtained from four laboratories (Ambry Genetics, Aliso Viejo, CA; GeneDx, Gaithersburg, MD; Invitae, San Francisco, CA; and Myriad Genetics, Salt Lake City, UT). Using a probabilistic matching strategy performed by Information Management Services (Rockville, MD), test results were merged to 5,026 of the 5,080 patient respondents with complete information on all SEER variables used for test linkage. A de-identified data set was sent to the University of Michigan. Institutional review boards of the University of Michigan, Emory University, the University of Southern California; the Georgia Department of Public Health; the California State Committee for the Protection of Human Subjects; and the California Cancer Registry approved the study, and data use agreements were established among the University of Michigan, the genetic laboratories, and Information Management Services.
Measures
Genetic Counseling Measures.
We asked all respondents (1) whether they “had discussed having a genetic test for breast cancer risk with a doctor or other health professional” and (2) whether they “had genetic counseling with a genetic counseling expert—that is, an appointment where the whole discussion is about genetic risk of breast cancer.” These questions were used to construct two binary measures indicating (1) receipt of any discussion with a physician or other health professional about genetic testing and (2) receipt of formal counseling by a genetic counseling expert. Those who indicated that they had an expert genetic counseling session (regardless of how they responded to the general discussion question) were coded as having had formal counseling; those who answered “yes” to the question on discussion about genetic testing with a physician, but did not indicate expert formal counseling, were coded as having had a physician-directed discussion. In addition, we used a separate question about whether the patient consulted with a genetic counselor before or after surgery (added in the latter half of the survey period) in the analysis.
Satisfaction Measure.
Patients were asked to rate the amount of information they received when making the decision about whether to have genetic testing (5-point response categories ranged from “Not enough” to “Too much” information, with the middle designating “Just right”).
Clinical Need Measures.
We categorized respondents into three need priority groups: (1) patients for whom testing identified a pathogenic mutation or VUS-only result, (2) those who tested negative, and (3) those who did not receive a genetic test. The rationale for these priority groups is that higher-risk patients with pathogenic mutations or VUS are a critically important target for formal genetic counseling. In addition, those with negative results in the context of their residual elevated risk of cancer are a higher priority for formal counseling than are untested patients.
Other Covariates.
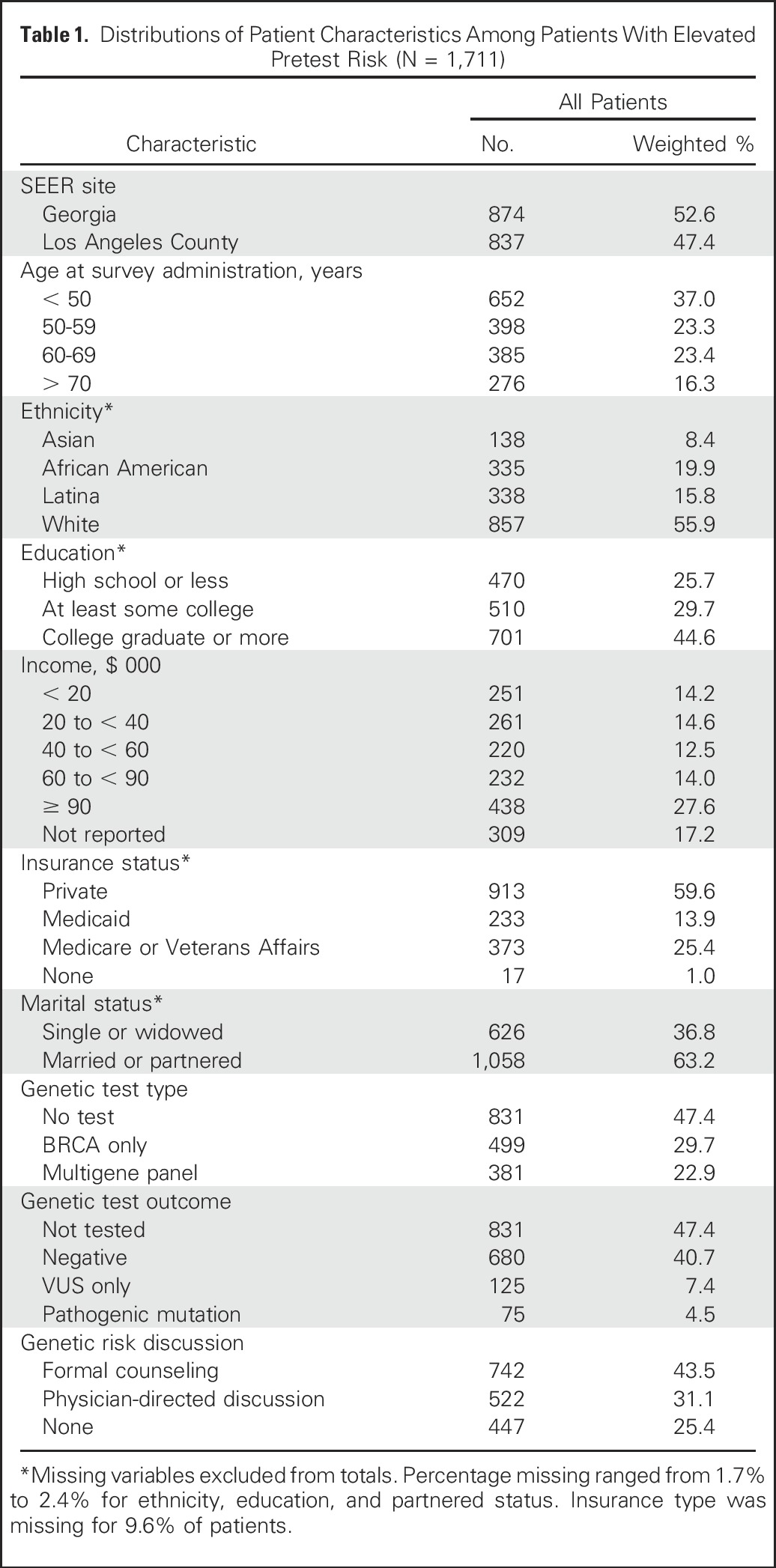
Other covariates considered included age groups, ethnicity, education, insurance status, income, marital status, and SEER site (Table 1). We also adjusted for time differences in the date of diagnosis and the survey completion interval (interval from diagnosis to receipt of the completed survey) to account for time period and potential recall bias.
Table 1.
Distributions of Patient Characteristics Among Patients With Elevated Pretest Risk (N = 1,711)
Statistical Analysis
We first examined the distribution of genetic discussion modalities by clinical need group. We then performed a multivariable logistic regression to examine correlates of genetic discussion and selected covariates using a two-part approach. We next performed a similar logistic model that regressed receipt of formal counseling versus physician-directed discussion among those who received any form of genetic discussion. In a secondary analysis, we included patients missing insurance information (9.0% of the sample) using a dummy variable, and we found no relevant differences in results. Variables included in all models were SEER site, age group, ethnicity, education level, insurance type, marital status, date of diagnosis, time interval from diagnosis to receipt of the completed survey (survey completion interval), and stratified need group. Diagnosis date and survey completion interval were continuous variables, whereas all others were grouped (Table 1). Finally, we examined patient appraisal of the amount of testing information by discussion modalities among those who received any counseling. All results were weighted to account for differential sampling and survey nonresponse. Analyses were performed using SAS version 9.4 (Cary, NC). Rao-Scott and Wald χ2 tests were used to assess significance for differences in proportions and logistic regression factors.
RESULTS
The analytic sample comprised 1,711 of the 5,080 women (34.0%) who had clinical indications for formal genetic risk evaluation according to National Comprehensive Cancer Network (NCCN) guidelines contemporaneous with the study period, as described previously.9 Table 1 lists the distribution of patient characteristics for the full analytic sample. Patients were distributed evenly between the two SEER regions. The sample was diverse with regard to age, ethnicity, education, and income. In this higher pretest risk sample, 47.4% were not tested, 29.7% received a BRCA1/2-only test, and 22.9% received a multigene panel test (representing 43.5% of testers). Among those tested, 14.0% had VUS only, 8.6% had a pathogenic mutation, and the remainder (77.4%) had a negative (normal) test. Overall, 74.6% of patients received some counseling (43.5%, formal counseling v 31.1%, physician-directed discussion).
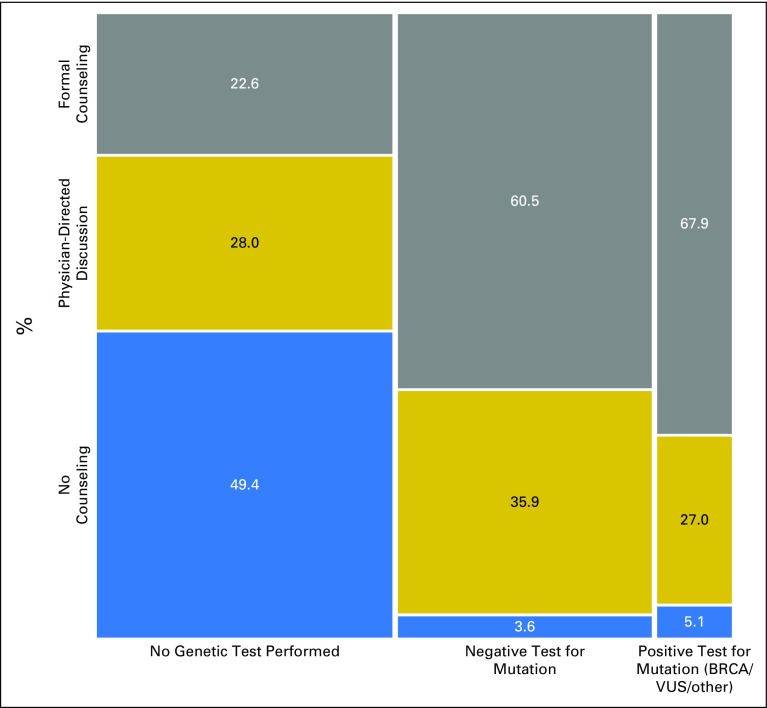
Figure 1 shows the distribution of genetic counseling modalities by clinical need group. One half of women (50.6%) who were not tested received some form of counseling (22.6%, formal counseling and 28.0%, physician-directed discussion). By contrast, virtually all tested patients reported some form of counseling (96.4% and 94.9% of those with negative tests and those with a pathogenic mutation or VUS, respectively), and two thirds reported formal counseling (60.5% and 67.9% of those with negative tests and pathogenic mutations or VUS, respectively). Appendix Table A1 (online only) lists sample sizes for subgroups and CIs for the point estimates described in Figure 1.
Fig 1.
Distribution of genetic discussion modality by clinical need group for our study sample of individuals having indication for genetic testing (N = 1,711). The tile width is proportional to the number of cases in each group: not tested (n = 831), tested negative (n = 680), tested pathogenic (n = 200).
Approximately one half of the patient sample (n = 894) was administered a question about the timing of a visit with a genetic counselor relative to surgery: 62.9% of the 327 patients who reported that they had a formal genetic counseling session met with a counselor before surgery.
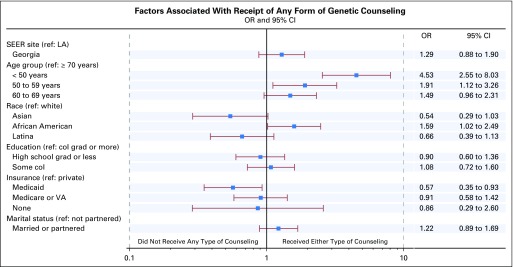
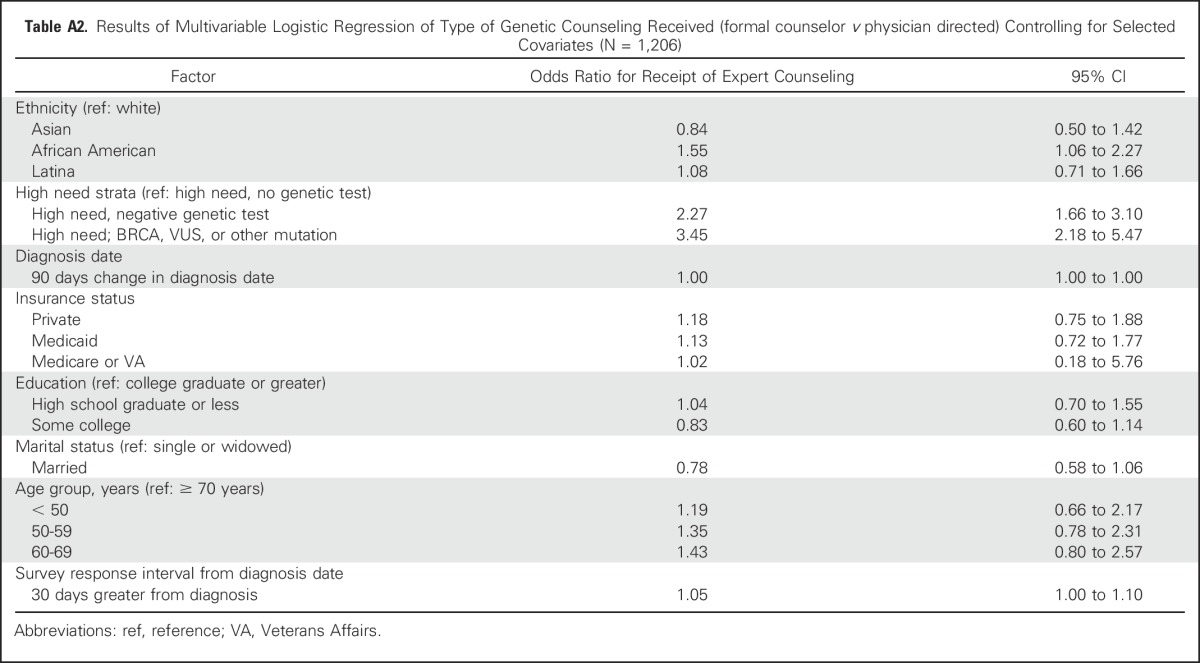
Figure 2 shows correlates of receipt of any genetic discussion, controlling for clinical need, diagnosis date, and survey completion interval. Younger women were more likely to report any discussion versus no discussion (odds ratio [OR], 4.5 [95% CI, 2.6 to 8.0]; 1.9 [95% CI, 1.1 to 3.3]; and 1.5 [95% CI, 1.0 to 2.3] for women younger than 50 years of age, 50 to 59 years of age, and 60 to 69 years of age, respectively, relative to those older than 70 years of age. Patients receiving Medicaid reported less counseling than did those privately insured (OR, 0.57 [95% CI, 0.35 to 0.93]). African Americans reported more counseling than did whites (OR, 1.6 [95% CI, 1.0 to 2.5]). Income had no effect on whether patients had any genetic discussion, nor was it a confounding factor in the analysis. Thus, this variable was omitted from the model. There was no association between survey completion interval and patient report of counseling (OR, 1.02 [95% CI, 0.96 to 1.1]) or confounding with other covariates. In a second model that regressed the type of discussion (formal genetic counseling v physician-directed discussion only) on selected covariates among those who received any discussion (Appendix Table A2, online only), ethnicity was only marginally significant (Wald χ2 P = .10), with African Americans receiving more formal than physician-directed counseling relative to whites (OR, 1.6 [95% CI, 1.1 to 2.3).
Fig 2.
Forest plot showing results of multivariable logistic regression for receipt of any form of genetic discussion (complete case [n = 1,429]). The model is also adjusted for clinical need group, date of diagnosis, and survey completion interval, which are not shown: negative test odds ratio (OR), 21.6 (95% CI, 12.5 to 37.4); positive mutation OR, 13.2 (95% CI, 6.2 to 28.0), and survey response after diagnosis interval per 90 days greater OR, 1.02 (95% CI, 0.96 to 1.1). col, college; grad, graduate; LA, Los Angeles; ref, reference; VA, Veterans Affairs.
Patients’ assessment of the amount of information they received about whether to have testing was similarly high whether they received formal genetic counseling or a physician-directed discussion only (80.8% v 79.4% stated information was “just right”; P = .58). Importantly, among those who had any mode of genetic discussion, Latinas were much less likely to be satisfied with the information about genetic testing. Only 63.5% considered the information “just right” (compared with 86.5% of whites, 75.6% of African Americans, and 80.3% of Asians; P < .001), with most Latinas perceiving too much information during the encounters (16.5% v 1.4%, 6.5%, and 4.1% for white, African American, and Asian patients, respectively; P < .001).
DISCUSSION
In this large, diverse, contemporary sample of patients with newly diagnosed breast cancer who had clinical indications for formal genetic risk evaluation, three quarters received some form of genetic discussion, but less than one half (43.5%) received formal counseling by a genetics expert. Reassuringly, virtually all tested patients (approximately one half of the sample) received some form of genetic discussion. Approximately two thirds of tested patients received formal counseling, but only two thirds of those received it before surgery. In particular, older women and those with Medicaid insurance were less likely to receive any type of genetic discussion, suggesting lingering access barriers. However, we did not observe ethnic group disparities in the receipt of any genetic discussion or formal counseling. Finally, satisfaction with the amount of information about whether to undergo genetic testing did not vary by modality of genetic discussion (formal counseling v physician-directed discussion), but more Latinas reported being overwhelmed by the information they received.
Few studies have addressed genetic counseling after breast cancer diagnosis in the modern era of multigene panel testing. Two previous articles2,3 that used the iCanCare data set showed that formal genetic counseling (including discussion about test results) was more prevalent among higher-risk patients than among those at an average pretest risk for mutation carriage. Virtually all other studies that have examined the use of genetic counseling for breast and gynecologic cancer risk were performed before the advent of widespread multigene panel testing.2,3,10-12 Childers et al13 evaluated discussions about genetic testing and testing rates from three waves of the National Health Interview Survey through 2015. They found that patient report and receipt of testing was low in higher-risk patients as defined by the guidelines. Wood et al used medical record information to examine receipt of genetic counseling in patients diagnosed with breast and colorectal cancer from 2009 to 2011 in clinical practices participating in the Quality Oncology Practice Initiative.14 They showed that approximately one half of patients with an increased risk of hereditary cancer received counseling.
Integrating germline genetic discussions and testing after a diagnosis of breast cancer has become increasingly important.15 Although the need for prompt genetic assessment and counseling after breast cancer diagnosis in patients with an elevated pretest risk of a pathogenic mutation is broadly accepted, the question of who should do the counseling is less settled. Indeed, ASCO and the American Society of Breast Surgeons do not specify who should direct counseling in current genetic testing recommendations,7 whereas other guidelines explicitly advise formal counseling by a genetics expert.1 Those who favor formal genetic counseling in higher-risk patients argue that trained counselors are required to ensure an accurate interpretation of findings and their implications for patients and families. Furthermore, some cancer physicians may lack confidence in discussing genetic test results.3,16 Some insurance companies currently mandate pretest formal genetic counseling by experts before approving payment. A counterargument to this mandate is that counseling can be delivered more efficiently and effectively if directed by cancer physicians, and that training physicians to do this would not be difficult. Some view insurance mandates for formal pretest counseling as a barrier to achieving the goal of testing patients with breast cancer at higher genetic risk.4
Our findings underscore the challenges of delivering genetic counseling and testing to patients with breast cancer who meet the criteria for formal genetic risk evaluation. First, it seems that a substantial minority of these patients (approximately one quarter) do not receive any form of genetic discussion. This highlights the substantial unmet need to identify and counsel patients with clinical indications, especially those who are older or have limited insurance. Second, our results suggest that only a minority of patients receive formal genetic counseling before surgery. This underscores a large gap between mandates for timely pretest formal genetic counseling of higher-risk patients and the reality of practice today. Furthermore, this gap may widen if genetic testing becomes more extensive and widespread as clinical indications broaden and simultaneous tumor-germline testing spreads.17,18 As observed in our study and others, only a small proportion of patients diagnosed with curable breast cancer will have a pathogenic mutation identified, a finding that may motivate surgeons faced with many patients desiring prompt surgical treatment to defer counseling until later in the management process.
Finally, most tested patients rated the amount of testing information they received as “just right,” with no differences in patient appraisal by counseling modality (formal v physician directed). This finding reinforces the need for more research about patient perspectives regarding the counseling and testing experience and their subsequent decisions in terms of managing results (eg, enhanced screening, prophylactic surgery, notification of relatives, and so forth). Importantly, Latinas were much more likely to feel overwhelmed by the information they received, a finding we observed for other treatment decisions after diagnosis of breast cancer, particularly in Latinas with low acculturation.19 This emphasizes the need to tailor information and feedback to individual patients as high-throughput molecular testing becomes increasingly complex and essential to clinical oncology practice.
Aspects of the study merit comment. Strengths include a large, diverse, contemporary population-based sample accrued shortly after the advent of multigene panel testing; granular information about testing indications and patient experiences with counseling and testing; and detailed information about test use and results from linkage to testing laboratories. Although the test linkage process was highly successful, we may have missed some tests. Patient report of expert counseling may be prone to recall bias. However, we did not observe an association between survey completion interval and counseling outcomes. Patient recall of the timing of visits with a genetic counselor relative to surgery may have been particularly prone to missing information. This bias could have resulted in an overestimate of the proportion of patients who received presurgery genetic counseling that we observed in this study. In addition, we had limited information about patient assessment of experiences with counseling by professionals and communication with relatives because the iCanCare study had a broad set of specific aims. Generalizability of the results is limited to two large regions of the United States and to patients with early-stage breast cancer.
Indications for formal genetic risk evaluation are evolving rapidly,1 but our results suggest that many patients with breast cancer with indications do not receive timely counseling by a genetics expert. Thus, there is a need for innovative approaches to improving the integration of genetic counseling into community practice. All cancer specialties have the opportunity and responsibility to incorporate genetic counseling more fully into breast cancer management. Surgeons see patients first, and test results most immediately affect surgical management (eg, a decision for bilateral mastectomy). Thus, surgical practice is a central target for improvement in genetic risk assessment and management strategies. However, genetic test results have become increasingly relevant to systemic therapy with the advent of poly(ADP-ribose) polymerase inhibitors for BRCA1/2-associated breast cancers20 and to radiation therapy with emerging concerns about radiation safety for carriers of certain pathogenic mutations (eg, TP531). Thus, clear communication of current guidelines for testing to all specialties will help ensure that the maximum number of at-risk individuals receive appropriate counseling and testing. Although genetic counselors have traditionally provided direct support to people at risk, our results and others18 suggest that a multipronged strategy will be required: (1) training all cancer specialists to integrate testing into decision making with the aid of interactive clinical decision support tools,21 (2) additional integration of genetic testing results and management into multidisciplinary tumor boards, and (3) a commitment by all cancer specialists to ensure timely testing of patients when there is a substantial likelihood that results could change treatment. The guidance and participation of genetic counselors and other genetics experts will be essential to develop more successful care delivery strategies.
ACKNOWLEDGMENT
We acknowledge the work of our project staff (Mackenzie Crawford and Kiyana Perrino from the Georgia Cancer Registry; Jennifer Zelaya, Pamela Lee, Maria Gaeta, Virginia Parker, BA, and Renee Bickerstaff-Magee from the University of Southern California; and Rebecca Morrison, Alexandra Jeanpierre, Stefanie Goodell, Irina Bondarenko, Paul Abrahamse, MA, and Rose Juhasz from the University of Michigan). We acknowledge with gratitude the patients with breast cancer who responded to our survey.
Appendix
Table A1.
Distribution of Genetic Discussion Modality by Genetic Counseling Need Group
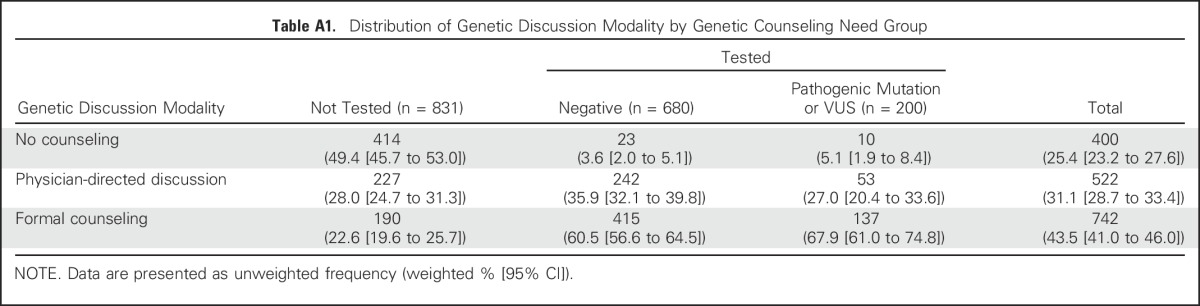
Table A2.
Results of Multivariable Logistic Regression of Type of Genetic Counseling Received (formal counselor v physician directed) Controlling for Selected Covariates (N = 1,206)
Footnotes
Supported by Grant P01 CA163233 to the University of Michigan from the National Cancer Institute. Conducted work was also supported by the University of Michigan Cancer Center Biostatistics, Analytics and Bioinformatics shared resource (P30CA46592). The collection of Los Angeles County cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute’s SEER program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute. The collection of cancer incidence data in Georgia was supported by contract HHSN261201300015I, Task Order HHSN26100006 from the National Cancer Institute and by cooperative agreement 5NU58DP003875-04-00 from the Centers for Disease Control and Prevention.
The ideas and opinions expressed herein are those of the authors, and endorsement by the States of California and Georgia, the Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred
AUTHOR CONTRIBUTIONS
Conception and design: Steven J. Katz, Kevin C. Ward, Ann S. Hamilton, Lauren P. Wallner, Monica Morrow, Reshma Jagsi, Sarah T. Hawley, Allison W. Kurian
Financial support: Steven J. Katz, Reshma Jagsi, Sarah T. Hawley
Administrative support: Steven J. Katz
Provision of study materials or patients: Kevin C. Ward, Ann S. Hamilton
Collection and assembly of data: Kevin C. Ward, Ann S. Hamilton, M. Chandler McLeod
Data analysis and interpretation: Steven J. Katz, M. Chandler McLeod, Lauren P. Wallner, Monica Morrow, Reshma Jagsi, Sarah T. Hawley, Allison W. Kurian
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Gaps in Receipt of Clinically Indicated Genetic Counseling After Diagnosis of Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Steven J. Katz
No relationship to disclose
Kevin C. Ward
No relationship to disclose
Ann S. Hamilton
No relationship to disclose
M. Chandler McLeod
No relationship to disclose
Lauren P. Wallner
Research Funding: GlaxoSmithKline (Inst)
Monica Morrow
Honoraria: Genomic Health
Travel, Accommodations, Expenses: Genomic Health
Reshma Jagsi
Employment: University of Michigan
Consulting or Advisory Role: Amgen
Research Funding: AbbVie (Inst)
Sarah T. Hawley
No relationship to disclose
Allison W. Kurian
Research Funding: Myriad Genetics (Inst), Invitae (Inst), Ambry Genetics (Inst), Genomic Health (Inst), GeneDx/BioReference (Inst), Genentech (a member of the Roche Group; Inst)
REFERENCES
- 1.Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: Genetic/familial high-risk assessment: Breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9–20. doi: 10.6004/jnccn.2017.0003. [DOI] [PubMed] [Google Scholar]
- 2.Kurian AW, Griffith KA, Hamilton AS, et al. Genetic testing and counseling among patients with newly diagnosed breast cancer. JAMA. 2017;317:531–534. doi: 10.1001/jama.2016.16918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35:2232–2239. doi: 10.1200/JCO.2016.71.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. doi: 10.1200/JCO.2017.74.7899. Hughes KS: Genetic testing: What problem are we trying to solve? J Clin Oncol 35:3789-3791, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Ahn S, Port ER. Genetic testing in patients with newly diagnosed breast cancer: Room for improvement. J Clin Oncol. 2017;35:2221–2223. doi: 10.1200/JCO.2017.72.8816. [DOI] [PubMed] [Google Scholar]
- 6.Agnese DM, Pollock RE. Breast cancer genetic counseling: A surgeon’s perspective. Front Surg. 2016;3:4. doi: 10.3389/fsurg.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33:3660–3667. doi: 10.1200/JCO.2015.63.0996. [DOI] [PubMed] [Google Scholar]
- 8.Dillman D, Smyth J, Christian L. Internet, Mail, and Mixed-Mode Surveys: The Tailored Design Method. (ed 2). Hoboken, NY: John Wiley & Sons; 2009. [Google Scholar]
- 9.Daly MB, Pilarski R, Axilbund JE, et al. Genetic/familial high-risk assessment: Breast and ovarian, version 2.2015. J Natl Compr Canc Netw. 2016;14:153–162. doi: 10.6004/jnccn.2016.0018. [DOI] [PubMed] [Google Scholar]
- 10.Willis AM, Smith SK, Meiser B, et al. Sociodemographic, psychosocial and clinical factors associated with uptake of genetic counselling for hereditary cancer: A systematic review. Clin Genet. 2017;92:121–133. doi: 10.1111/cge.12868. [DOI] [PubMed] [Google Scholar]
- 11.Wevers MR, Aaronson NK, Verhoef S, et al. Impact of rapid genetic counselling and testing on the decision to undergo immediate or delayed prophylactic mastectomy in newly diagnosed breast cancer patients: Findings from a randomised controlled trial. Br J Cancer. 2014;110:1081–1087. doi: 10.1038/bjc.2013.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wevers MR, Ausems MGEM, Verhoef S, et al. Does rapid genetic counseling and testing in newly diagnosed breast cancer patients cause additional psychosocial distress? Results from a randomized clinical trial. Genet Med. 2016;18:137–144. doi: 10.1038/gim.2015.50. [DOI] [PubMed] [Google Scholar]
- 13.Childers CP, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800–3806. doi: 10.1200/JCO.2017.73.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood ME, Kadlubek P, Pham TH, et al. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: A pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. J Clin Oncol. 2014;32:824–829. doi: 10.1200/JCO.2013.51.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Offit K, Bradbury A, Storm C, et al. Gene patents and personalized cancer care: Impact of the Myriad case on clinical oncology. J Clin Oncol. 2013;31:2743–2748. doi: 10.1200/JCO.2013.49.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray SW, Hicks-Courant K, Cronin A, et al. Physicians’ attitudes about multiplex tumor genomic testing. J Clin Oncol. 2014;32:1317–1323. doi: 10.1200/JCO.2013.52.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandelker D, Zhang L, Kemel Y, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA. 2017;318:825–835. doi: 10.1001/jama.2017.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Allen EM. The potential and challenges of expanded germline testing in clinical oncology. JAMA. 2017;318:801–803. doi: 10.1001/jama.2017.11022. [DOI] [PubMed] [Google Scholar]
- 19.Katz SJ, Wallner LP, Abrahamse PH, et al. Treatment experiences of Latinas after diagnosis of breast cancer. Cancer. 2017;123:3022–3030. doi: 10.1002/cncr.30702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol. 2014;32:618–626. doi: 10.1200/JCO.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]