Abstract
BACKGROUND
Both major depressive disorder (MDD) and posttraumatic stress disorder (PTSD) are characterized by depressive symptoms, abnormalities in brain regions important for cognitive control, and response to cognitive behavioral therapy (CBT). However, whether a common neural mechanism underlies CBT response across diagnoses is unknown.
METHODS
Brain activity during a cognitive control task was measured using functional magnetic resonance imaging in 104 participants: 28 patients with MDD, 53 patients with PTSD, and 23 healthy control subjects; depression and anxiety symptoms were determined on the same day. A patient subset (n = 31) entered manualized CBT and, along with controls (n = 19), was rescanned at 12 weeks. Linear mixed effects models assessed the relationship between depression and anxiety symptoms and brain activity before and after CBT.
RESULTS
At baseline, activation of the left dorsolateral prefrontal cortex was negatively correlated with Montgomery–Åsberg Depression Rating Scale scores across all participants; this brain–symptom association did not differ between MDD and PTSD. Following CBT treatment of patients, regions within the cognitive control network, including ventrolateral prefrontal cortex and dorsolateral prefrontal cortex, showed a significant increase in activity.
CONCLUSIONS
Our results suggest that dimensional abnormalities in the activation of cognitive control regions were associated primarily with symptoms of depression (with or without controlling for anxious arousal). Furthermore, following treatment with CBT, activation of cognitive control regions was similarly increased in both MDD and PTSD. These results accord with the Research Domain Criteria conceptualization of mental disorders and implicate improved cognitive control activation as a transdiagnostic mechanism for CBT treatment outcome.
Keywords: Affective disorders, Cognitive behavioral therapy, Cognitive control, Dimensionality, MDD, PTSD, RDoC
Posttraumatic stress disorder (PTSD) and major depressive disorder (MDD) are common disorders causing significant morbidity and mortality (1,2). Both are chronic and enduring conditions (3–6), with significant impairment in social and occupational functioning, significant incidence of suicide (7–11), and high rates of recurrence (12). PTSD and MDD frequently coexist and share phenomenological, behavioral, and neural circuitry abnormalities. Prior research has shown that MDD occurs in 48% to 69% of individuals with PTSD (3,13–15). Likewise, PTSD in the context of MDD often goes unrecognized and might not be appropriately assessed and treated. In addition, patients treated for PTSD may have residual ongoing unrecognized symptoms of depression, both subthreshold and meeting criteria for MDD. Although there remain construct questions with regard to symptom overlap with PTSD and MDD, recent research has shown that these high rates of comorbidity continued to occur even when overlapping symptoms were removed from the diagnoses (15,16). Therefore, understanding the underlying neural dysfunction across these disorders has the potential to improve identification of occult or residual symptoms. Cognitive problems are especially linked to illness chronicity in PTSD (17) and have been shown to be particularly difficult to treat in MDD using pharmacotherapy (18). It has thus been suggested that cognitive problems may be worth tracking across disorders and may be a sensitive marker for early intervention to prevent the onset of illness (19).
Traditionally, MDD and PTSD have been considered distinct disorders, each with its own psychopathology (20). Given that many symptoms and biological phenomena overlap between apparently distinct psychiatric disorders and extend between psychiatric patients and the general population, the Research Domain Criteria (RDoC) initiative (21) has emphasized dimensions of psychopathology, including genes, behaviors, and brain circuits (such as the cognitive control circuit), that are likely to extend across diagnostic categories. Cognitive control has been suggested to “modulate the operation of other cognitive and emotional systems, in the service of goal-directed behavior” (22), and comprises components including monitoring and implementing control (23). Cognitive control systems are likely to be a key circuit cutting across diagnostic categories. Depressive symptoms (overlapping with the RDoC “loss” and “frustrated nonreward” dimensions) are a fundamental symptom construct common across multiple psychiatric disorders that in both MDD (24–27) and PTSD (24) are associated with dysfunction within cognitive control regions such as the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex, anterior cingulate cortex, and anterior insula (28–30). However, neural substrates of cognitive control have not been investigated in a unified study examining task-induced brain activity across MDD and PTSD, both of which have a high prevalence of depressive symptoms (13) and cognitive problems (17,18) but are considered distinct disorders due to distinguishing features (20).
Cognitive behavioral therapy (CBT) is an effective treatment for both MDD and PTSD, with equally efficacious but more enduring effects than antidepressants for MDD (31–33) and with efficacy in PTSD for a variety of trauma types (34,35). The fact that various mental disorders endorsing elevated depressive symptoms can be alleviated by CBT suggests that common neural mechanisms may be engaged in treatment response. Studies examining neural substrates of CBT have demonstrated changes in cognitive control regions in MDD following treatment (36–39) and in PTSD (24,40–42). However, there are no published studies investigating neural substrates underlying treatment response to CBT across both MDD and PTSD participants conducted in the same study. For this reason, we studied a sample of both MDD and PTSD participants, all of whom received CBT.
In this study, we tested the overarching hypothesis that symptoms of depression are dimensionally related to circuit-level abnormalities within cognitive control regions across a patient sample including both patients with MDD and those with PTSD and that CBT improves these abnormalities through a common mechanism in both disorders. Specifically, we hypothesized that hypoactivity in cognitive control regions would be remediated by CBT. To test this hypothesis, we used an emotional conflict task (43) that engages cognitive control to focus attention on either houses or faces and inhibit the response to the other stimulus type. We applied a dimensional cross-disorder analysis on task-induced functional magnetic resonance imaging (fMRI) brain responses to probe neural circuits related to depressive and anxiety symptoms across MDD and PTSD. We predicted that higher levels of depressive symptoms would be associated with decreased brain activation in areas involved in cognitive control in MDD and PTSD at baseline. In secondary analyses, we also evaluated the neural circuitry related to anxiety due to its importance in both MDD and PTSD. Furthermore, we probed the brain mechanisms of CBT in ameliorating cognitive control dysregulation by evaluating task-induced fMRI response before and after treatment. We predicted that CBT would correct such abnormalities similarly across both disorders.
METHODS AND MATERIALS
Participants
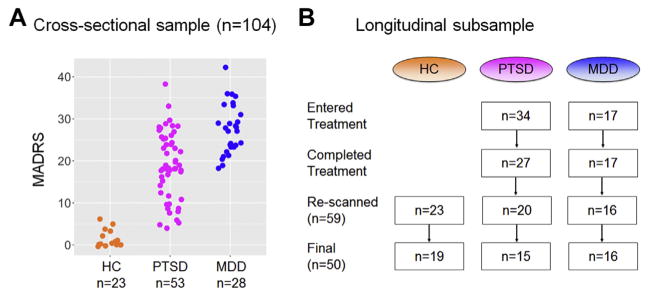
Our baseline cross-sectional sample consisted of 104 participants: 28 patients diagnosed with MDD, 53 patients diagnosed with PTSD, and 23 healthy control subjects included for comparison (Figure 1A). This full dataset was used to associate the spectrum of depressive symptoms with a continuum of deficits within the cognitive control circuit at time 1. A subset of patients (17 MDD and 34 PTSD) also volunteered for a cognitive behavior therapy treatment trial. Of these, 17 MDD and 27 PTSD participants completed treatment, and 16 MDD and 20 PTSD participants returned for follow-up imaging. Following data exclusions (see Supplement for details), a longitudinal imaging sample of 16 patients with MDD, 15 patients with PTSD, and 19 control subjects with usable MRI scans at two time points was included (Figure 1B). (See Supplement for the ethnic description.) All participants received their MRI scans at Washington University.
Figure 1.
Participant characteristics. (A) Distribution of Montgomery–Åsberg Depression Rating Scale (MADRS) scores for the cross-sectional sample by diagnostic group. (B) Flowchart of the longitudinal subsample. HC, healthy control subjects; MDD, patients with major depressive disorder; PTSD, patients with posttraumatic stress disorder.
All participants were right-handed, English speaking, and aged 18 to 56 years (Table 1). Inclusion diagnosis for MDD and PTSD was established according to DSM-IV-TR Structured Clinical Interview for DSM criteria (44). All MDD participants had MDD as the primary diagnosis. All PTSD participants had PTSD as the primary diagnosis, and 31.03% had significant depression severity [defined by Hamilton Depression Rating Scale scores ≥ 17, a standard cutoff for inclusion in trials of MDD (45)]. PTSD participants had a lifetime mean total score on the Clinician-Administered PTSD Scale of 78.16 ± 18.51. All PTSD participants reported interpersonal violence-based trauma (rape, domestic violence, etc.), usually multiple episodes of long-standing duration. Exclusionary diagnoses by Structured Clinical Interview for DSM criteria included 1) comorbid neurological disorders; 2) current alcohol or substance abuse disorder; 3) history of psychotic disorder, bipolar disorder, anxiety disorder predating MDD or PTSD onset, or obsessive-compulsive disorder; 4) current suicide risk; 5) treatment with any psychotropic or central nervous system–active drug within the previous 3 weeks (5 weeks for fluoxetine); and 6) inadequate MRI scan quality. The initial inclusionary/exclusionary interviews were conducted by Dr. Bruce at the University of Missouri–St. Louis and by Dr. Sheline at Washington University.
Table 1.
Participant Demographic, Clinical, and Behavioral Information
| Cross-sectional Sample (n = 104) | Longitudinal Subsample at 12 Weeks (n = 50) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HC | Patients | HC | Patients | |||
|
|
|
|||||
| PTSD | MDD | PTSD | MDD | |||
| Sample Size, n | 23 | 53 | 28 | 19 | 15 | 16 |
|
| ||||||
| Gender, Male/Female, n | 4/19 | 0/53 | 6/22 | 4/15 | 0/15 | 2/14 |
|
| ||||||
| Age, Years (SD) | 32.6 (9.55) | 32.5 (9.72) | 33.9 (8.27) | 33.4 (10.1) | 32.9 (11.7) | 34.6 (8.29) |
|
| ||||||
| Education, Years (SD) | 17.2 (2.82) | 14.9 (3.02) | 15.3 (2.15) | 17.3 (2.94) | 15.2 (1.47) | 15.1 (2.31) |
|
| ||||||
| % on Psychotropics | N/A | 0 | 0 | N/A | 0 | 0 |
|
| ||||||
| MADRS, Mean (SD) | 1.38 (2.03) [n = 16] | 19.0 (7.93) [n = 51] | 27.4 (5.95) [n = 28] | 1.11 (2.42) [n = 13] | 6.80 (6.71) [n = 15] | 7.00 (7.47) [n = 16] |
|
| ||||||
| CAPS_m, Mean (SD) | N/A | 67.8 (16.1) [n = 52] | N/A | N/A | 21.2 (11.6) [n = 14] | N/A |
|
| ||||||
| MASQ-AA, Mean (SD) | 20.2 (3.57) [n = 20] | 32.8 (10.8) [n = 51] | 34.9 (12.3) [n = 25] | 19.9 (3.03) [n = 16] | 23.2 (5.44) [n = 14] | 25.1 (9.54) [n = 13] |
|
| ||||||
| Accuracy, % (SD) | 78.6 (11.6) | 77.4 (14.1) | 81.6 (9.92) | 73.9 (26.7) | 83.1 (8.26) | 75.6 (24.3) |
|
| ||||||
| Reaction Time, ms (SD) | 811.14 (208.63) | 831.86 (177.63) | 851.80 (202.03) | 778.90 (190.22) | 789.00 (166.59) | 759.63 (169.65) |
CAPS_m, Clinician-Administered PTSD Scale past-month total; HC, healthy control subjects; MADRS, Montgomery–Åsberg Depression Rating Scale; MASQ-AA, Anxious Arousal subscale of Mood and Anxiety Symptoms Questionnaire; MDD, patients with major depressive disorder; N/A, not applicable; PTSD, patients with posttraumatic stress disorder.
We used two scales to measure depression severity. The Hamilton Depression Rating Scale, the most widely used instrument to ascertain depression severity, was used for study inclusion for MDD and, in parallel, to assess depressive symptoms in PTSD at baseline as having significant depression severity (45). The clinician-administered Montgomery–Åsberg Depression Rating Scale (MADRS), shown to be sensitive to change in symptoms (46), was used to assess baseline severity and treatment response across diagnoses and over time. Mean baseline MADRS scores were significantly higher in MDD participants (27.4 ± 6.0) than in PTSD participants (19.0 ± 7.9), t70 = 5.30, p < .001. The Anxious Arousal subscale of the self-report Mood and Anxiety Symptoms Questionnaire (MASQ-AA) (47) was administered to examine the potential effect of anxiety as compared with depression. Mean baseline MASQ-AA scores did not differ between MDD participants (34.9 ± 12.3) and PTSD participants (32.8 ± 10.8), t43 = 0.71, p = .48. (See Supplement for discussion of MASQ-AA choice as the anxiety measure.) Symptom and brain imaging data were always collected on the same day.
For the longitudinal treatment study, patients received 12 weeks of manualized psychotherapy, either CBT for MDD or cognitive processing therapy (CPT) for PTSD, delivered or supervised by the same clinical psychologist (SEB), a highly trained CBT therapist. (See Supplement for CBT quality assurance procedures and similarity between CBT and CPT.) Control subjects underwent the same imaging and assessment procedures separated by 12 weeks. All participants provided written informed consent; the Human Subjects Committees of both Washington University and the University of Missouri–St. Louis approved all study procedures.
Emotional Conflict Task
In this task, subjects were asked to pay attention to either houses or fearful/neutral faces presented in a target axis (horizontal or vertical, randomized) while ignoring the stimuli presented in the other axis (distractors) (Supplemental Figure S1A). This task was designed to probe cognitive control and emotional circuits (43). (See Supplement for further details.)
Image Acquisition
See Supplement for details of image acquisition and preprocessing.
Statistical Analyses
To study the association between the severity of depressive symptoms and the level of brain dysfunction, we fit voxelwise linear mixed-effects (LME) models (using the nlme package in R) (48) on task activation data across all participants (control, MDD, and PTSD). We included all participants instead of patients only because MADRS scores were continuous across healthy and patient populations (Figure 1A), although for depressed participants there were no MADRS scores < 18. This dimensional approach allowed us to characterize a more complete spectrum of depressive symptoms. Thus, our LME model included fixed-effect terms for MADRS, task condition (a categorical variable with four levels), MADRS by condition interaction, age, gender, education, and head motion (mean relative displacement) to estimate their associations with brain activation. A random intercept term was included in the model to account for within-subject correlations among the repeated measures. (See Supplement for anxiety analyses.)
For the analysis of the longitudinal impact of CBT, we used voxelwise LME modeling and conducted voxelwise analyses within the whole brain. We first tested for a group by time interaction and then assessed activation in patients to identify brain regions that changed significantly after treatment. Specifically, fixed effects included treatment (as an indicator for pre-CBT vs. post-CBT), task condition, task condition by treatment interaction, age, gender, and head motion; a random effect for subject was also included. For clusters identified in this voxelwise analysis, we extracted the region of interest (ROI) means in patients and control subjects at baseline and at 12 weeks. For patients, a post hoc comparison was performed to illustrate the treatment effect divided by diagnosis (MDD vs. PTSD). For control subjects, paired-sample t tests were performed within these a priori defined areas to test for time and practice effects. (See Supplement for within-subject analyses in control subjects.) For brain regions showing significant changes in patients following CBT (but not in control subjects), we computed partial correlations (controlling for age, sex, and motion) between change scores of activation and behavioral measures, either depressive symptoms or task reaction time (RT). Both change in symptoms and task RT were quantified using a normalized score defined as 100 × (postCBT − preCBT)/preCBT.
For voxelwise analyses, multiple comparisons were corrected using Gaussian random field theory (49) (easythresh command implemented in FSL). We used a cluster-defining threshold of Z > 3.09 because recent work has emphasized that lower thresholds are subject to a higher risk of type I error. Ecklund et al. (50) showed that a cluster threshold of Z > 3.09 produced type I error rates that were close to the nominal familywise error rate of alpha = .05, whereas a more liberal threshold of Z > 2.33 resulted in familywise error rates of 10% to 50%.
RESULTS
Task Performance and Clinical Symptom Scales
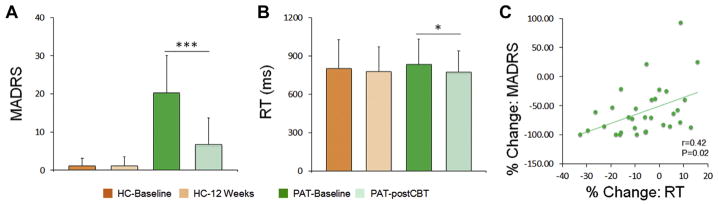
At baseline, patients (78.84 ± 12.89%) and healthy controls (78.58 ± 11.57%) did not differ significantly in average performance accuracy (p > .20). (See Supplement for further details.) A repeated-measures analysis of variance revealed that there was no significant group effect (patients vs. controls) or group by task condition interaction on RT for correct trials (p > .20). After 12 weeks, RT was significantly decreased in patients following CBT (t30 = 2.75, p = .01, d = 0.51) but not in healthy control subjects (p > .20). After treatment, patients’ depressive symptoms assessed by MADRS were also significantly improved (t30 = −6.15, p < .001, d = 1.12; mean reduction relative to baseline: 66.31%). Following treatment, mean MADRS scores did not differ between MDD participants (7.0 ± 7.5) and PTSD participants (6.8 ± 6.7), t29 = 0.08, p = .94. In addition, mean MASQ scores did not differ between MDD participants (25.1 ± 9.5) and PTSD participants (23.2 ± 5.4), t21 = 0.60, p = .52. Importantly, the improvement in depressive symptoms was significantly correlated with improvement in RT (partial r = .42, p = .02, n = 30, controlling for age and gender), with greater symptom improvement associated with greater reductions in RT (Figure 2).
Figure 2.
Change in depression severity and reaction time (RT) with cognitive behavioral therapy (CBT). (A, B) Montgomery–Åsberg Depression Rating Scale (MADRS) scores (A) and task RT (B) were significantly decreased at time 2 following CBT in patients (PAT). Healthy control subjects (HC) did not change. The percentage change of RT is plotted against the percentage change of the MADRS in (C). % Change is defined as (PostCBT − Baseline)/Baseline × 100. *p < .05; ***p < .005.
Correlations Between Task Activity and Depressive and Anxiety Symptoms in MDD and PTSD
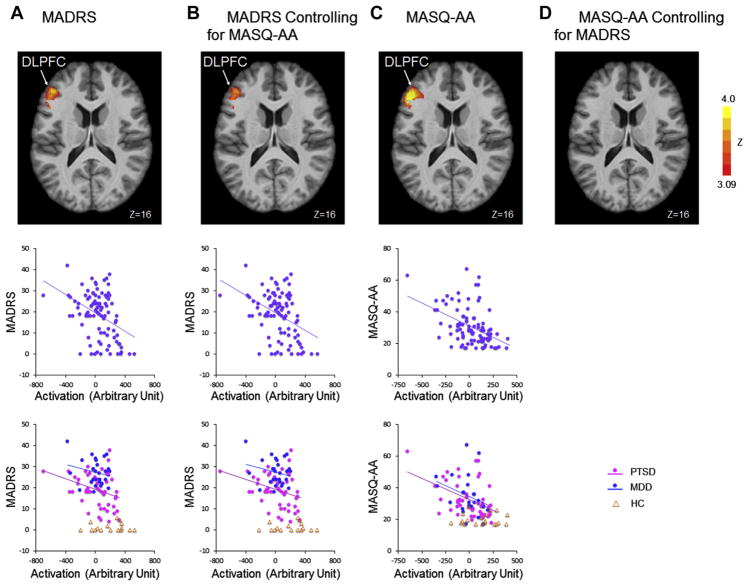
Correlations between task activity and depressive symptoms were assessed using baseline data. Because no interaction effect was found to be significant (symptom by task condition), the interaction term was dropped from the model. Thus, symptom score and task condition were kept in the model as main effects only. We found that the activation of the left DLPFC (center of mass in Montreal Neurological Institute coordinates: x = −45, y = 34, z = 17) was negatively correlated with MADRS scores across all participants (r = −0.40, p < 0.001, n = 95) (Figure 3A), and this brain–symptom association did not differ between patients with MDD and those with PTSD (p > .20). The similarity in brain–symptom association between MDD and PTSD was not driven by comorbid depression status among patients with PTSD (see Supplemental Figure S2) given that similar results were obtained after excluding the patients with PTSD with higher depressive symptom scores (Hamilton Depression Rating Scale ≥ 17) (p > .20). This similarity was also not dependent on including the control subjects (see Supplemental Figure S2). Because depression and anxiety symptoms were correlated (r = .55, p < .001), we also examined the correlation between depressive symptoms and brain activation within clusters identified in the main analyses after controlling for MASQ-AA scores. A strongly overlapping cluster located within the left DLPFC (center of mass in Montreal Neurological Institute coordinates: x = −46, y = 35, z = 17) was negatively correlated with MADRS scores (r = −.40, p < .001, n = 95) (Figure 3B). This specificity analysis allowed us to examine the unique variance explained by depression after controlling for anxiety. We also examined the effect of anxiety without (Figure 3C) and with (Figure 3D) controlling for depression. (See Supplemental Results for the analysis of anxiety symptom correlation with brain activity.)
Figure 3.
Activation in cognitive control regions correlates with depression severity across major depressive disorder (MDD) and posttraumatic stress disorder (PTSD) groups at baseline. (A) Brain regions showing significant correlation with Montgomery–Åsberg Depression Rating Scale (MADRS) scores are shown in axial slice view in Montreal Neurological Institute coordinates (Z > 3.09, p < .05, Gaussian random field theory corrected). The cluster mean activations were plotted against the baseline MADRS scores across all subjects. (B) Brain regions showing significant correlation with MADRS scores after regressing out Anxious Arousal subscale of Mood and Anxiety Symptoms Questionnaire (MASQ-AA) scores are shown in axial slice view in Montreal Neurological Institute coordinates (Z > 3.09, p < .05). The residual activation and MADRS scores computed after regressing out MASQ-AA scores were plotted against each other. (C) Brain regions showing significant correlation with MASQ-AA scores are shown in axial slice view in Montreal Neurological Institute coordinates (Z > 3.09, p < .05, Gaussian random field theory corrected). The cluster mean activations were plotted against the baseline MASQ-AA scores across all subjects. (D) Brain regions showing significant correlation with MASQ-AA scores after regressing out MADRS scores. DLPFC, dorsolateral prefrontal cortex. HC, healthy control subjects.
Increased Activation Within the Cognitive Control System Following CBT
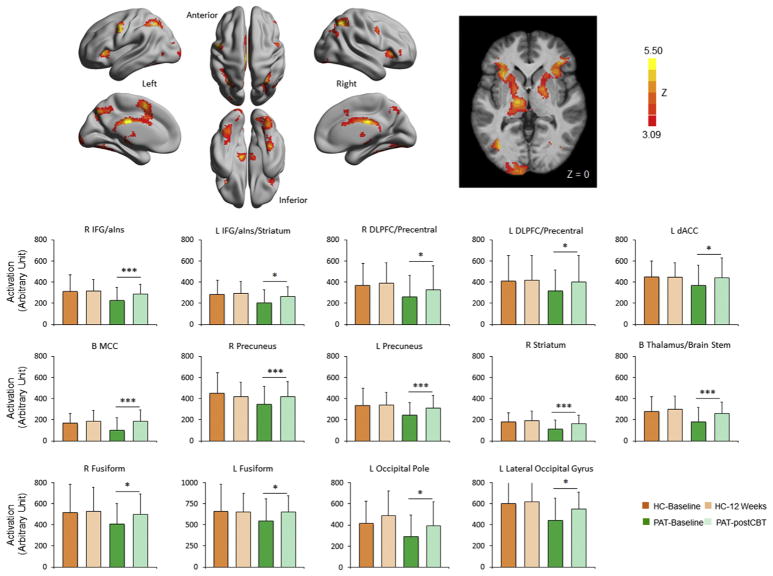
Although trending toward greater increase in patients (see Supplemental Figure S4), no time by group interactions survived correction for multiple comparisons in the longitudinal data analysis. We did, however, identify 14 clusters showing significant changes in patients following CBT in the within-group analyses. These clusters included the bilateral inferior frontal gyrus/anterior insula, right middle frontal/precentral gyrus, left precentral gyrus, dorsal anterior cingulate, middle cingulate, precuneus, thalamus/brain stem, striatum, and various visual areas (Figure 4 and Supplemental Table S1). Supplemental Figure S5 shows the fMRI activity of patients split by MDD versus PTSD diagnosis. ROI-based analyses on these clusters in healthy control subjects revealed that none showed significant changes from time 1 to time 2. Although CBT was associated with improved symptoms and enhanced activity in cognitive control areas, no significant correlations were found between these changes in brain activation and changes in depressive symptoms (p > .20). (See Supplement for longitudinal brain activity changes in controls and comparison of patients who were vs. were not included in the longitudinal study.)
Figure 4.
Longitudinal change in activation following cognitive behavioral therapy in patients. Brain regions where the activation during the conflict task exhibited significant increases (Z > 3.09, p < .05, Gaussian random field theory corrected) following cognitive behavioral therapy (CBT) in patients (PAT) with major depressive disorder or posttraumatic stress disorder are shown on a surface map using BrainNet Viewer (63) and in slice view in Montreal Neurological Institute coordinates. Region of interest mean activation of the 14 clusters showing significant increase in patients following cognitive behavioral therapy treatment are plotted for patients and healthy control subjects (HC) at baseline and at 12 weeks (see Supplemental Table S1 for spatial location of these clusters). None of these clusters showed significant change at 12 weeks in HC. *p < .05; ***p < .005. B, bilateral; dACC, dorsal anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; IFG/aIns, inferior frontal gyrus/anterior insula; L, left; MCC, middle cingulate cortex; R, right.
DISCUSSION
In this study, we extended the investigation of depressive and anxiety symptoms to a transdiagnostic task-based study in MDD and PTSD in the context of CBT, a standardized and highly evidence-supported therapy. We found that DLPFC activity at baseline was related to depressive symptoms, even after controlling for anxiety. We also found that anxiety symptoms at baseline were related to DLPFC activity; however, this relationship did not survive after controlling for depressive symptoms. Furthermore, following CBT, patients had statistically significant increases in activation in multiple cognitive control regions, including bilateral ventrolateral prefrontal cortex/anterior insula, middle frontal gyrus, DLPFC, dorsal anterior cingulate, striatum, and thalamus. These results support the need for additional RDoC (21) style analyses across disorders and treatment modalities to better inform diagnostic nosology and to understand common mechanisms of treatment action.
Dimensional Representations of Depression
Using the same task paradigm, previous work has demonstrated abnormal cross-sectional fMRI blood oxygen level–dependent responses for either patients with MDD (51) or patients with PTSD (52) within cognitive control circuitries. Here, we built on these prior results by examining the relationship between task activation and dimensional severity of depression across diagnostic categories. The observed associations between depressive symptoms and DLPFC activation during this task are consistent with meta-analyses that have reliably implicated hypoactivity of the DLPFC in depression (24,27) and have found that various forms of successful treatments remediate this hypoactivity (26,33). The relationship between depressive symptoms and DLPFC hypoactivation was present in the PTSD group, which is particularly relevant given that it is common for patients with PTSD to endorse elevated depressive symptoms and frequently to have a comorbid clinical MDD diagnosis (13). Notably, this dimensional relationship between activation and depression severity was present regardless of whether patients with PTSD had high depression severity, suggesting that even lower levels of depression affect this circuitry in patients. Thus, our data support a common neural signature associated with depression across MDD and PTSD.
Psychotherapy Processes Across Disorders
It is now well established that CBT reduces depressive symptom severity with large effect sizes for MDD and PTSD examined separately (53). The neural mechanisms of CBT have been extensively examined in MDD [for reviews, see (33,38)] and also in PTSD [for reviews, see (24,42)]. For example, increased activity in lateral prefrontal cortex following CBT has been seen in MDD (33) but has not been similarly evaluated in a dimensional study examining both MDD and PTSD together. Among patients with PTSD, DLPFC task activation extending into the inferior/ventrolateral prefrontal cortex has been shown to predict reductions in PTSD symptoms following selective serotonin reuptake inhibitors treatment (54). Given that CBT is an effective treatment for both disorders, the disorders themselves may have a common underlying abnormality that responds to CBT. Our data suggest that there may be a neural representation of this common element of clinical response. Consistent with an RDoC approach emphasizing dimensions that cut across clinical disorders, we found symptom overlaps across disorders and similar brain changes (Supplemental Figure S5) in response to CBT treatment across disorders. This is further supported by our finding that amelioration in symptoms was correlated with improvement in task performance across diagnoses.
Reciprocally connected prefrontal cortical regions together with the dorsal anterior cingulate cortex, posterior parietal lobe, thalamus, and striatum have long been described as forming a cognitive control network. This network has been associated with active goal planning and maintenance (55), behavioral inhibition, task flexibility/cognitive control (56), and reorienting attention (57). Prominent studies have identified dissociable neural components of cognitive control in monitoring cognitive control (more associated with anterior cingulate activity) and implementing cognitive control (more associated with DLPFC activity) (23). Thus, given the primary association between decreased DLPFC activity and depressive symptoms at baseline, it may be that an inability to exert cognitive control could be implicated in the association of DLPFC hypoactivity with depression. A growing number of studies report alterations of this control system across a striking range of mental disorders, suggesting a critical role for the control system in promoting and maintaining mental health (58,59). An influential theory of the neural mechanisms of psychotherapy suggests that successful treatment of depression is associated with increased activity in control system regions and decreased activity in amygdala and other emotional processing regions (33,60). Consistent with this theory, the flexible hub theory also suggests that CBT may enhance the control system by augmenting its feedback control mechanism and promoting the cognitive flexibility necessary for psychotherapy to be effective (58). Using the same task, we previously found (51) that subjects with depression had amygdala and subgenual anterior cingulate cortex hyperactivity in addition to DLPFC hypoactivity. However, that study defined a priori ROIs. In the current study, we used a data-driven approach that identified DLPFC hypoactivity across a spectrum of MDD and PTSD participants. In addition, we previously (61) found a treatment effect of medication that was associated with increased DLPFC hypoactivity and decreased amygdala hyperactivity in a priori defined ROI. In contrast, the current work focused on the treatment effect of CBT in a data-driven approach that is transdiagnostic.
Our prediction that anxiety symptoms would be independently associated with brain activity abnormalities was not borne out by the data. Whereas regressing out anxiety symptoms did not change the correlation of MADRS with DLPFC activity, the converse was not true; regressing out depression scores removed the association of MASQ-AA with DLPFC activity. In part, this may have been due to a restricted range of MASQ-AA scores. (See Supplement for further discussion of anxiety symptoms in PTSD survivors with multiple traumas.)
Limitations
Our PTSD population mainly included female interpersonal violence survivors, and thus further studies of PTSD resulting from other traumatic events and including men would be necessary to determine the generalizability of our findings. While there was a correlation between improvement in symptoms and task performance, we did not see an association between symptom change and changes in brain activity after treatment, suggesting that the degree of cognitive control function enhancement following CBT might not be related to depressive symptoms in a simple linear fashion. It is possible that our sample size was not sufficient to capture these associations or that the relationship between symptom improvement and brain alteration was too complex to be captured by the current regression model. Therefore, understanding mechanisms of CBT will also require further nuanced approaches to dismantle constituent processes of the treatment and to link them more tightly to task probes of specific cognitive/affective functioning in the scanner. In this regard, lack of a placebo patient control group limits our interpretation of brain changes as purely CBT induced. We did not find any significant interaction effects between time and group (patients vs. control subjects) in the longitudinal analysis. However, we believe that the changes observed in patients in cognitive control brain regions are likely attributable to more than just time or practice effects because the control subjects studied across the same time span did not show significant brain activity changes in these regions. Another possibility that must be considered is that the effects of repeat testing could be baseline dependent (e.g., easier to see an increase when the initial activity was reduced). The significance of the increase in task-related cognitive control region activation is intriguing. It could be that these changes are a consequence of CBT/CPT, signifying broadly enhanced top-down control that may influence psychiatric health indirectly through improved coping behaviors but having no direct relationship with degree of symptom changes.
Our investigation of treatment effects was performed in a relatively large number of unmedicated patients, which might not be representative of the usual medicated patient population. However, this could also be a strength of the research study because getting such a sample is unusual and it aids in interpretability of brain imaging findings. Larger samples of patients with PTSD with less depressive symptom endorsements, although less representative of the typical patient with PTSD, might be used in future studies to determine unique PTSD brain features and processes. Finally, the lack of interaction with emotion type (fear/neutral) in our imaging data may be due to the known inconsistencies in the depression literature with eliciting hyperresponse to aversive stimuli in MDD (62). Our results suggest that perhaps the system affected by MDD and PTSD and most responsive to psychotherapeutic treatment in both disorders is the cognitive control system, which (as we discussed above) is not a single unitary construct but rather has several distinct constituents.
Implications
Our results suggest that abnormalities in the activation of cognitive control regions across MDD and PTSD are associated with symptoms of depression. Furthermore, we provided evidence that activation of cognitive control regions is similarly enhanced following treatment with CBT in both MDD and PTSD. These results accord with the RDoC conceptualization of mental disorders and implicate improved cognitive control activation as a transdiagnostic mechanism for CBT treatment outcome.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health (NIMH) Grant Nos. RC MH089704, R01MH064821, and K24MH098260 (to YIS), NIMH Grant No. R01MH107703 (to TDS), and NIMH Grant No. K23 MH090366 (to SEB).
Footnotes
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Shalev A, Liberzon I, Marmar C. Post-traumatic stress disorder. N Engl J Med. 2017;376:2459–2469. doi: 10.1056/NEJMra1612499. [DOI] [PubMed] [Google Scholar]
- 2.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: Course of illness and substance abuse. Am J Psychiatry. 1996;153:369–375. doi: 10.1176/ajp.153.3.369. [DOI] [PubMed] [Google Scholar]
- 5.Zlotnick C, Rodriguez BF, Weisberg RB, Bruce SE, Spencer MA, Culpepper L, et al. Chronicity in posttraumatic stress disorder and predictors of the course of posttraumatic stress disorder among primary care patients. J Nerv Ment Dis. 2004;192:153–159. doi: 10.1097/01.nmd.0000110287.16635.8e. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg PE, Birnbaum HG. The economic burden of depression in the US: Societal and patient perspectives. Expert Opin Pharmacother. 2005;6:369–376. doi: 10.1517/14656566.6.3.369. [DOI] [PubMed] [Google Scholar]
- 7.Bruce SE, Weisberg RB, Dolan RT, Machan JT, Kessler RC, Manchester G, et al. Trauma and posttraumatic stress disorder in primary care patients. Prim Care Companion J Clin Psychiatry. 2001;3:211–217. doi: 10.4088/pcc.v03n0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kessler RC. Posttraumatic stress disorder: The burden to the individual and to society. J Clin Psychiatry. 2000;61(suppl 5):4–12. discussion 13–14. [PubMed] [Google Scholar]
- 9.Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999;56:617–626. doi: 10.1001/archpsyc.56.7.617. [DOI] [PubMed] [Google Scholar]
- 10.Mazza JJ. The relationship between posttraumatic stress symptomatology and suicidal behavior in school-based adolescents. Suicide Life Threat Behav. 2000;30:91–103. [PubMed] [Google Scholar]
- 11.Wunderlich U, Bronisch T, Wittchen HU. Comorbidity patterns in adolescents and young adults with suicide attempts. Eur Arch Psychiatry Clin Neurosci. 1998;248:87–95. doi: 10.1007/s004060050023. [DOI] [PubMed] [Google Scholar]
- 12.Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55:694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- 13.Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110:585–599. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- 14.Keane TM, Kaloupek DG. Comorbid psychiatric disorders in PTSD: Implications for research. Ann N Y Acad Sci. 1997;821:24–34. doi: 10.1111/j.1749-6632.1997.tb48266.x. [DOI] [PubMed] [Google Scholar]
- 15.Elhai JD, Grubaugh AL, Kashdan TB, Frueh BC. Empirical examination of a proposed refinement to DSM-IV posttraumatic stress disorder symptom criteria using the National Comorbidity Survey replication data. J Clin Psychiatry. 2008;69:597–602. doi: 10.4088/jcp.v69n0411. [DOI] [PubMed] [Google Scholar]
- 16.Ford JD, Elhai JD, Ruggiero KJ, Frueh BC. Refining post-traumatic stress disorder diagnosis: Evaluation of symptom criteria with the National Survey of Adolescents. J Clin Psychiatry. 2009;70:748–755. doi: 10.4088/JCP.08m04692. [DOI] [PubMed] [Google Scholar]
- 17.Bryant RA, Creamer M, O’Donnell M, Forbes D, McFarlane AC, Silove D, et al. Acute and chronic posttraumatic stress symptoms in the emergence of posttraumatic stress disorder: A network analysis. JAMA Psychiatry. 2017;74:135–142. doi: 10.1001/jamapsychiatry.2016.3470. [DOI] [PubMed] [Google Scholar]
- 18.Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, Etkin A. Effect of antidepressant treatment on cognitive impairments associated with depression: A randomised longitudinal study. Lancet Psychiatry. 2016;3:425–435. doi: 10.1016/S2215-0366(16)00012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNally RJ. Networks and nosology in posttraumatic stress disorder. JAMA Psychiatry. 2017;74:124–125. doi: 10.1001/jamapsychiatry.2016.3344. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: American Psychiatric Press; 2013. [Google Scholar]
- 21.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuthbert B, Garvey M, Heinssen R, Kozak M, Morris S, Pine D, et al. Cognitive Systems: Workshop Proceedings. Rockville, MD: National Institute of Mental Health; 2011. [Google Scholar]
- 23.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 24.Frewen PA, Dozois DJ, Lanius RA. Neuroimaging studies of psychological interventions for mood and anxiety disorders: Empirical and methodological review. Clin Psychol Rev. 2008;28:228–246. doi: 10.1016/j.cpr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: A meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- 26.van Waarde JA, Scholte HS, van Oudheusden LJ, Verwey B, Denys D, van Wingen GA. A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment-resistant depression. Mol Psychiatry. 2015;20:609–614. doi: 10.1038/mp.2014.78. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhe HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. NeuroImage. 2012;61:677–685. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Fournier JC, Chase HW, Greenberg T, Etkin A, Almeida JR, Stiffler R, et al. Neuroticism and individual differences in neural function in unmedicated major depression: Findings from the EMBARC Study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:138–148. doi: 10.1016/j.bpsc.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeRubeis RJWC, Tany TZ, Beck AT. Cognitive therapy. In: Dobson KS, editor. Handbook of Cognitive-Behavioral Therapies. New York: Guilford; 2010. pp. 227–316. [Google Scholar]
- 32.McMain S, Newman MG, Segal ZV, DeRubeis RJ. Cognitive behavioral therapy: Current status and future research directions. Psychother Res. 2015;25:321–329. doi: 10.1080/10503307.2014.1002440. [DOI] [PubMed] [Google Scholar]
- 33.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: Treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA. A comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. J Consult Clin Psychol. 2002;70:867–879. doi: 10.1037//0022-006x.70.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz PMRP, Huber LC, Griffin MG. The effectiveness of cognitive processing therapy for PTSD with refugees in a community setting. Cogn Behav Pract. 2006;13:332–331. [Google Scholar]
- 36.Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: Changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res. 2011;45:577–587. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63:1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- 38.Messina I, Sambin M, Palmieri A, Viviani R. Neural correlates of psychotherapy in anxiety and depression: A meta-analysis. PLoS One. 2013;8:e74657. doi: 10.1371/journal.pone.0074657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franklin G, Carson AJ, Welch KA. Cognitive behavioural therapy for depression: systematic review of imaging studies. Acta Neuro-psychiatry. 2016;28:61–74. doi: 10.1017/neu.2015.41. [DOI] [PubMed] [Google Scholar]
- 40.Porto PR, Oliveira L, Mari J, Volchan E, Figueira I, Ventura P. Does cognitive behavioral therapy change the brain? A systematic review of neuroimaging in anxiety disorders. J Neuropsychiatry Clin Neurosci. 2009;21:114–125. doi: 10.1176/jnp.2009.21.2.114. [DOI] [PubMed] [Google Scholar]
- 41.Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends Cogn Sci. 2007;11:413–418. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Brooks SJ, Stein DJ. A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues Clin Neurosci. 2015;17:261–279. doi: 10.31887/DCNS.2015.17.3/sbrooks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 44.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), Version 2. New York: New York State Psychiatric Institute, Biometrics Research; 1996. [Google Scholar]
- 45.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 47.Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 48.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-Plus. New York: Springer; 2000. [Google Scholar]
- 49.Brett M, Penny W, Kiebel S. An introduction to random field theory. In: Frackowiak RSJ, Ashburner JT, Penny WD, Zeki S, Friston KJ, Frith CD, Dolan RJ, Price CJ, editors. Human Brain Function. 2. San Diego: Academic Press; 2004. [Google Scholar]
- 50.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchholz KR, Bruce SE, Koucky EM, Artime TM, Wojtalik JA, Brown WJ, et al. Neural correlates of trait rumination during an emotion interference task in women with PTSD. J Trauma Stress. 2016;29:317–324. doi: 10.1002/jts.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: A review of meta-analyses. Clin Psychol Rev. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 54.MacNamara A, Rabinak CA, Kennedy AE, Fitzgerald DA, Liberzon I, Stein MB, et al. Emotion regulatory brain function and SSRI treatment in PTSD: Neural correlates and predictors of change. Neuropsychopharmacology. 2016;41:611–618. doi: 10.1038/npp.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 56.Cole MW, Ito T, Braver TS. The behavioral relevance of task information in human prefrontal cortex. Cereb Cortex. 2016;26:2497–2505. doi: 10.1093/cercor/bhv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clark DA, Beck AT. Cognitive theory and therapy of anxiety and depression: Convergence with neurobiological findings. Trends Cogn Sci. 2010;14:418–424. doi: 10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 61.Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112:206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rottenberg J. Emotions in depression: What do we really know? Annu Rev Clin Psychol. 2017;13:241–263. doi: 10.1146/annurev-clinpsy-032816-045252. [DOI] [PubMed] [Google Scholar]
- 63.Xia M, Wang J, He Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.