Abstract
Neurofibromatosis type 1 (NF1) is a common cancer predisposition syndrome caused by mutations in the NF1 gene. The NF1-encoded protein (neurofibromin) functions as an inhibitor of RAS to control cell growth and survival. Individuals with NF1 are prone to developing low-grade tumors of the optic nerves, chiasm, tracts, and radiations, termed optic pathway gliomas (OPGs), which can cause vision loss. A paucity of surgical tumor specimens and patient-derived xenografts for investigative studies has limited our understanding of human NF1-associated OPG (NF1-OPG). However, mice genetically engineered to harbor Nf1 gene mutations develop optic gliomas that share many features of their human counterparts. These genetically engineered mouse (GEM) strains have provided important insights into the cellular and molecular determinants that underlie mouse Nf1 optic glioma development, maintenance, and associated vision loss, with relevance to human NF1-OPG disease. Herein, we review our current understanding of NF1-OPG pathobiology and describe the mechanisms responsible for tumor initiation, growth, and associated vision loss in Nf1 GEM models. We also discuss how Nf1 GEM strains and other preclinical models can be deployed to identify and evaluate molecularly targeted therapies for OPG, particularly as they pertain to future strategies aimed at preventing or improving tumor-associated vision loss in children with NF1.
Introduction
Tumors of the visual system occur in a number of inherited disorders, including retinoblastoma, caused by germline mutations in the RB1 gene (Vogel 1979), retinal astrocytic hamartoma in tuberous sclerosis (Rowley et al. 2001), retinal hemangioblastoma in von Hippel-Lindau disease (Lonser et al. 2003), optic nerve sheath meningioma in neurofibromatosis type 2 (Bosch et al. 2006), and optic pathway glioma (OPG) in neurofibromatosis type 1 (NF1) (Listernick et al. 2007). Of these disorders, NF1 is the most common, affecting 1 in 3,000 individuals worldwide (Crowe 1956; Evans et al. 2010; Friedman 1999; Huson et al. 1989).
NF1 is an autosomal dominant syndrome caused by loss-of-function mutations in the NF1 tumor suppressor gene (Cawthon et al. 1990; Viskochil et al. 1990; Wallace et al. 1990), which encodes the protein, neurofibromin. Comprising more than 2800 amino acids (~220 kDa), neurofibromin contains a small domain (280–300 amino acids) that is structurally and functionally similar to a family of proteins that function as negative RAS regulators (Basu et al. 1992; Bollag and McCormick 1991; Xu et al. 1990a; Xu et al. 1990b). Since increased RAS activation is associated with numerous human cancers (Imperial et al. 2017; Simanshu et al. 2017), individuals with NF1 are predisposed to a range of tumors affecting the central and peripheral nervous systems, including OPGs, which are a source of significant morbidity in this population (Listernick et al. 2007).
Nearly all NF1-associated OPGs (NF1-OPGs) are benign pilocytic astrocytomas (PAs; World Health Organization grade I astrocytomas), which can arise anywhere along the optic pathway, including the optic nerves, optic chiasm, optic tracts, and optic radiations (Guillamo et al. 2003; Listernick et al. 2007; Liu et al. 2004) (Figure 1A–C). However, in individuals with NF1, the majority (75–85%) of OPGs are located within the optic nerve and chiasm (pre-chiasmal or anterior optic pathway), with a smaller proportion of tumors located in the optic tracts and radiations (post-chiasmal or posterior optic pathway). NF1-OPGs occur most frequently in young children (median age at diagnosis = 4.5 years) (Listernick et al. 1994; Listernick et al. 1989; Prada et al. 2015), with rare cases described in older adolescents (Chong et al. 2013; Listernick et al. 2004). As such, OPG is a manifestation of NF1 that predominates in young children, who are often preverbal with co-morbid attention deficits, further complicating diagnosis and accurate visual assessment in an at-risk population (Listernick et al. 2007).
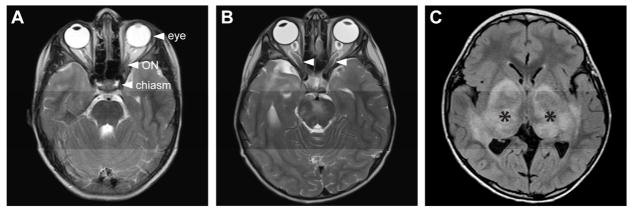
Figure 1. Location and MRI characteristics of NF1-optic pathway gliomas (OPGs).
(A) Axial T2-weighted MRI scan depicts a normal optic chiasm and optic nerves (ONs) in a child with NF1 lacking an OPG.
(B) Axial T2-weighted MRI scan shows an OPG in a child with NF1 involving the optic chiasm and nerves. The chiasm is enlarged and diffusely hyperintense. The optic nerves show fusiform enlargement and tortuosity bilaterally (arrowheads).
(C) Axial T1-weighted non-contrast MRI scan in a different child with NF1 with an OPG involving the optic radiations (asterisks).
NF1-OPGs demonstrate significant clinical heterogeneity with respect to location, age at initial detection, and vision loss (Listernick et al. 1994). Risk factors for vision loss from NF1-OPG are age less than 2 years (Fisher et al. 2012), female sex (Diggs-Andrews et al. 2014b), and tumor involvement of the post-chiasmal optic pathway (Balcer et al. 2001; Fisher et al. 2012). Treatment is usually reserved for patients with progressive symptoms (e.g., vision loss) and frequently involves chemotherapy. While most symptomatic children are treated with carboplatin/vincristine therapy (Mahoney et al. 2000; Packer et al. 1997; Packer et al. 1993), newer molecularly targeted treatments have recently emerged. Chemotherapy often successfully attenuates tumor growth (60–70% response rates), however few patients have improved visual acuity following treatment (Dalla Via et al. 2007; Dodgshun et al. 2015; Fisher et al. 2012; Listernick et al. 2007; Shofty et al. 2011).
Most patients are treated with chemotherapy without a prior tissue diagnosis (tumor biopsy) and few patients undergo surgical resection of their tumors. The lack of human tumor specimens for study and the absence of patient-derived xenograft models have hindered efforts to understand the molecular and cellular determinants of human NF1-OPG and to discover new therapeutic leads. To this end, much of our understanding of NF1-OPG draws from studies of mice engineered to harbor mutations in the Nf1 gene (Gutmann et al. 2012). In this review, we summarize our current understanding of NF1-OPG pathobiology, including the mechanisms underlying vision loss in mice engineered to harbor Nf1 optic gliomas. We further discuss promising leads in the development of molecularly targeted and neuroprotective therapies relevant to preventing or limiting progressive vision loss.
Tumorigenesis in NF1 requires biallelic inactivation of the NF1 gene
Children without NF1 are born with two functional copies (alleles) of the NF1 gene. In contrast, individuals with NF1 are born with one functional NF1 allele and another allele harboring a loss-of-function (LOF) NF1 gene mutation (referred to as the germline mutation). For tumorigenesis to occur in people with NF1, a vulnerable cell type (i.e., glioma cell of origin) must undergo somatic inactivation of the remaining functional NF1 allele to render both NF1 alleles nonfunctional (Brems et al. 2009; Laycock-van Spyk et al. 2011; Maertens et al. 2006). This “two-hit” model of tumorigenesis was first proposed for retinoblastoma (Knudson 1971), but has proven correct for many other inherited cancer predisposition syndromes. Commensurate with this “two-hit” model, examination of human NF1-PAs revealed simultaneous presence of a germline NF1 gene mutation and somatic inactivation of the functional NF1 allele through several mechanisms. As such, somatic NF1 gene inactivation in tumors can result from loss of heterozygosity (LOH), genetic mutation, or epigenetic modification (e.g., methylation) of the NF1 locus (Gutmann et al. 2003; Gutmann et al. 2013; Kluwe et al. 2001), all leading to undetectable levels of neurofibromin (the protein encoded by NF1) in tumor cells (Gutmann et al. 2000). Loss of neurofibromin expression and function in these cells predisposes to inappropriately controlled cell proliferation or survival, thus facilitating tumor formation.
Modeling optic pathway gliomas in genetically engineered mice
The first mouse genetically engineered mouse (GEM) models of NF1 harbored a germline inactivating mutation resulting in a loss-of-function (null or knockout) Nf1 allele (Brannan et al. 1994; Jacks et al. 1994). While mice homozygous for a germline null Nf1 allele (Nf1null) die in utero as a result of a severe cardiac malformation (double outlet right ventricle), those heterozygous for a null allele survive into adulthood and were largely phenotypically indistinguishable from wild-type mice. To circumvent the embryonic lethality associated with complete Nf1 loss in all cells, subsequent Nf1 GEM models leveraged Cre-loxP technology to generate mice harboring a conditional Nf1 gene mutation. In this approach, loxP sites were inserted into intronic (non-protein coding) sequences within the Nf1 gene (Nf1flox), such that Cre-mediated recombination produced a nonfunctional (null) allele (Zhu et al. 2001). In the absence of Cre recombinase, the Nf1flox allele behaves as a functional (wild-type) allele. To model the “two-hit” genetics observed in patients with NF1-OPG, mice were generated harboring one germline null (Nf1null) allele, one conditional null (Nf1flox) allele, and a third allele in which expression of a cre transgene was restricted to neuroglial progenitor cells (e.g., Nf1flox/null, GFAP-cre mice) (Bajenaru et al. 2003; Zhu et al. 2005; Zhu et al. 2001). Approximately 90–95% of these genetically engineered Nf1flox/null, GFAP-cre mice developed low-grade gliomas of the optic nerves and chiasm by 3 months of age. Histologically, these mouse gliomas share many features with their human counterparts, including increased numbers of GFAP-immunopositive astrocytes, low proliferative indices, microglial infiltration, and nuclear atypia. In addition, mice harboring Nf1 optic gliomas develop retinal ganglion cell (RGC) axonal dysfunction and death, accompanied by retinal nerve fiber layer (RNFL) thinning, similar to humans (Avery et al. 2011). However, mouse optic gliomas lack other classical features of human NF1-OPGs, including Rosenthal fibers and eosinophilic granular bodies. Moreover, none of the current mouse Nf1 models forms gliomas involving the optic tracts or radiations.
Insights from genetically engineered mouse models of NF1-OPG
The cell of origin dictates the timing of mouse Nf1 optic glioma formation
There is a growing consensus that for many pediatric and adult brain tumors, the cells of origin—i.e., those cells that initiate a tumor—are neural progenitor cells (NPCs) (Hemmati et al. 2003; Schuller et al. 2008; Yang et al. 2008). Studies in mice have iteratively applied different Cre driver lines using the genetic strategy described above to target somatic Nf1 loss to specific cell types. In these experiments, either the endogenous promoter (gene expression regulatory sequences) or a fragment thereof was employed to direct Cre recombinase expression (and Nf1 gene inactivation) to Gfap-, Blbp-, and Prom1 (CD133)-expressing neural stem/progenitor cells (Bajenaru et al., 2003(Hegedus et al. 2007; Solga et al. 2017). In each of these three Nf1 GEM models, mice develop optic gliomas by 3 months of age, collectively showing that these are the likely cells of origin for mouse Nf1 optic gliomas. In contrast, targeting somatic Nf1 loss to Ng2-expressing glial cells or to postnatal Gfap-expressing astrocytes does not result in glioma formation, excluding these cell types as the potential cells of origin for mouse Nf1 optic glioma.
Moreover, the neuroglial stem/progenitor cells capable of initiating optic gliomagenesis reside in the subventricular zone of the third ventricle (tv-SVZ), rather than in a comparable germinal zone of the lateral ventricles, which sources the progenitor cells of origin for some high-grade gliomas (Hegedus et al. 2007; Lee et al. 2012; Lee et al. 2010). The restricted anatomical location of the putative mouse Nf1 optic glioma cell of origin correlates with its role in normal optic nerve development. Specifically, tv-SVZ NPCs actively divide during a restricted window in early childhood (Dahiya et al. 2011; Fuentealba et al. 2012) to give rise to Olig2-expressing oligodendrocyte precursor cells (OPCs) that migrate into the optic nerves. These more lineage-restricted OPCs (as compared to neural stem/progenitor cells) then divide to give rise to differentiated glial cells in the optic nerve (Gao and Miller 2006; Ono et al. 1997). As such, OPCs may also serve as a potential cell of origin for optic glioma. Commensurately, somatic Nf1 loss in Olig2-expressing cells in an Nf1 GEM model (i.e., Nf1flox/null, Olig2-cre mice) leads to optic gliomagenesis, but with a prolonged latency (six months versus three months) relative to neuroglial stem/progenitor cells expressing Gfap, Prom1, or Blbp (Solga et al. 2017).
Lastly, using the Nf1flox/null, Prom1-cre mouse strain, in which Cre recombinase activity can be regulated (Zhu et al. 2009), the consequence of somatic Nf1 inactivation at different times was assessed. In these studies, Nf1 loss in Prom1-expression cells was required prior to postnatal day 1 for optic gliomas to form, arguing that there is a developmental window during which somatic Nf1 gene inactivation must occur in order to facilitate gliomagenesis (Solga et al. 2017). Taken together, these findings argue that NF1-OPG formation is heavily influenced by the cell of origin and the requirement for somatic Nf1 gene inactivation to occur in specific susceptible cell types during brain development, consistent with the clinical observation that NF1-OPGs are largely a tumor of young children.
Deregulated RAS effector pathway activity contributes to NF1-OPG pathogenesis
Individuals with NF1 harbor germline mutations in the NF1 gene located on chromosome 17q11.2. The NF1 gene encodes neurofibromin, which functions primarily as a GTPase activating protein (GAP) for the oncoprotein p21 Ras. In this regard, neurofibromin contains a 300-amino acid residue GAP-related domain (GRD), which inhibits RAS activity by accelerating the conversion of active GTP-bound RAS to its inactive GDP-bound form ((Ballester et al. 1990; Ohba et al. 2000; Xu et al. 1990a), see (Bos et al. 2007) and (Cox and Der 2010) for reviews). RAS is a small GTPase important for promoting cell growth and survival in numerous cell types, including the developing and mature mammalian brain, such that gain-of-function mutations in the RAS proto-oncogene are frequently found in human cancers (Fernandez-Medarde and Santos 2011; Simanshu et al. 2017). NF1-associated tumor cells with biallelic NF1 gene inactivation (i.e., lacking neurofibromin protein) exhibit elevated RAS activity ((Basu et al. 1992; Bollag et al. 1996; DeClue et al. 1991), for review see (Dasgupta and Gutmann 2003)), and studies in Nf1 GEM models similarly support a critical role for aberrant RAS pathway activation in tumor pathogenesis.
As a critical growth regulator, RAS may transmit its signal through at least three downstream effector pathways that each contribute to Nf1 optic glioma pathogenesis in mice. These pathways include: (1) the MEK (mitogen-activated protein kinase [MAPK] kinase)–ERK (extracellular signal-regulated kinase) pathway (Donovan et al. 2002; Lau et al. 2000; See et al. 2012), (2) the PI3K (phosphoinositide 3-kinase)–AKT (protein kinase B)–mTOR (mechanistic target of rapamycin) pathway (Dasgupta et al. 2005; Johannessen et al. 2005; Lau et al. 2000), and (3) the adenylyl cyclase-mediated cyclic AMP (cAMP) signaling pathway (Anastasaki and Gutmann 2014; Dasgupta and Gutmann 2005; Warrington et al. 2010).
In tumor cells lacking neurofibromin (termed NF1-deficient), the first two pathways (i.e., the MEK-ERK and PI3K-AKT-mTOR pathways) are upregulated (Figure 2A). These two pathways can converge to activate mTOR (Kaul et al., 2015), a serine/threonine kinase that positively regulates cell growth, survival, and proliferation (Laplante and Sabatini 2012). In addition, findings reveal mTOR-independent growth regulatory functions of the MEK-ERK pathway (Chen et al. 2015). In the third RAS effector pathway, elevated RAS activity leads to reduced cAMP production, through signaling intermediates that likely converge on the enzyme (adenylyl cyclase) that ultimately generates cAMP (Figure 2A). Pharmacologic modulation of any of the three RAS effector pathways slows tumor growth in mice with Nf1 optic gliomas (Hegedus et al. 2008; Kaul et al. 2015). Inhibition of the MEK-ERK pathway using the MEK inhibitor PD0325901, the PI3K-AKT-mTOR pathway using the PI3K inhibitor NPV-BKM120, or mTOR using rapamycin decreases tumor cell proliferation and tumor volumes in mice (Hegedus et al. 2008; Kaul et al. 2015). In a similar manner, increasing intracellular cAMP levels with the phosphodiesterase-4 inhibitor rolipram reduces tumor proliferative indices and size (Warrington et al. 2010). Unfortunately, all of these clinical-grade inhibitors attenuate tumor growth only during the period of treatment, and tumors increase their proliferation to pretreatment levels following the cessation of therapy (Hegedus et al., 2008). The lack of a durable response is potentially problematic for the treatment of these tumors in children, possibly necessitating prolonged treatment periods.
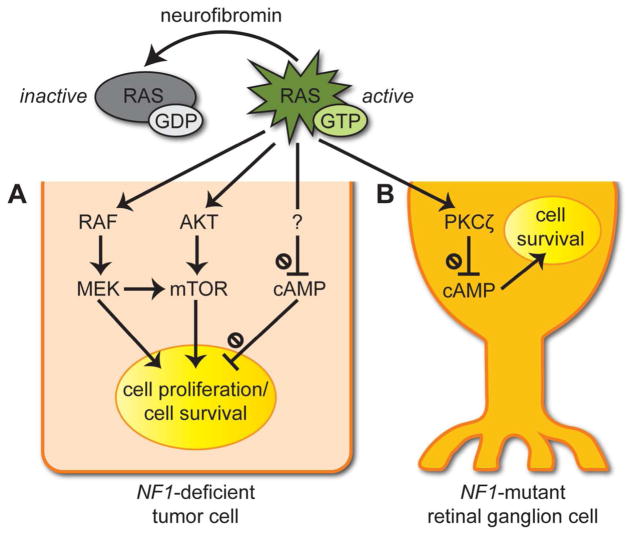
Figure 2. RAS effector pathway de-regulation underlies optic pathway glioma (OPG) growth and tumor-associated retinal ganglion cell (RGC) dysfunction.
Neurofibromin is a negative regulator of the RAS proto-oncogene product. It accelerates the conversion of active RAS-GTP to inactive GDP-bound RAS.
(A) In tumor cells with biallelic NF1 inactivation (NF1-deficient cells), there is increased activation of the MEK-ERK and PI3K-AKT-mTOR pathways, resulting in greater cell proliferation and survival. RAS hyperactivation also leads to decreased cyclic AMP (cAMP) levels to promote tumor cell survival.
(B) In RGCs heterozygous for a germline NF1 gene mutation (NF1-mutant cells), impaired neurofibromin function leads to reduced adenylyl cyclase-mediated cAMP production and increased RGC death. Based on mechanistic studies in other CNS neurons, the reduced cAMP in RGCs likely results from increased RAS-dependent activation of protein kinase C-ζ (PKCζ).
In addition to its role in promoting the survival and proliferation in NF1-deficient tumor cells, aberrant RAS pathway activity also contributes to axonal dysfunction and death of NF1-mutant retinal ganglion cells (RGCs), with relevance to visual dysfunction (Figure 2B). RGCs are the neurons that integrate visual information (light) from retinal photoreceptors; their cell bodies reside in the innermost layer of the retina (termed the ganglion cell layer), and their axons course through the RNFL before forming the optic nerves (Figure 3A,B). In individuals with NF1, RGCs are heterozygous for a germline NF1 gene mutation (termed NF1-mutant). In these NF1-mutant RGCs, impaired neurofibromin function lowers cAMP levels and decreases cell survival (Brown et al. 2010), an effect of cAMP opposite to that observed in Nf1-deficient tumor cells. Studies in other CNS neuron types harboring NF1 gene mutations (e.g., hippocampal neurons, human induced pluripotent cell-derived neurons) implicate neurofibromin/RAS-mediated activation of the atypical protein kinase C (PKC)-zeta (PKCζ) as the likely mechanism for this RGC-specific reduction in cAMP (Anastasaki and Gutmann, 2014). Increased PKCζ activity leads to inhibition of G protein-coupled receptor (GPCR) stimulation of G protein-mediated adenylyl cyclase activity (Anastasaki and Gutmann, 2014). Importantly, pharmacologic inhibition of RAS activity (lovastatin) or elevation of cAMP levels (rolipram) attenuates RGC apoptosis in Nf1 optic glioma-bearing mice (Brown et al. 2010; Toonen et al. 2017a). Recently, proof-of-principle preclinical studies showed that RAS inhibition (and cAMP elevation) during a period after RGC death and RNFL thinning begins, but before more than 50% RGC loss occurs, protects against continued RGC loss and RNFL thinning for up to 2 months following the cessation of treatment (Toonen et al. 2017a). These findings suggest that a vulnerable window exists during which treatment may produce more durable responses.
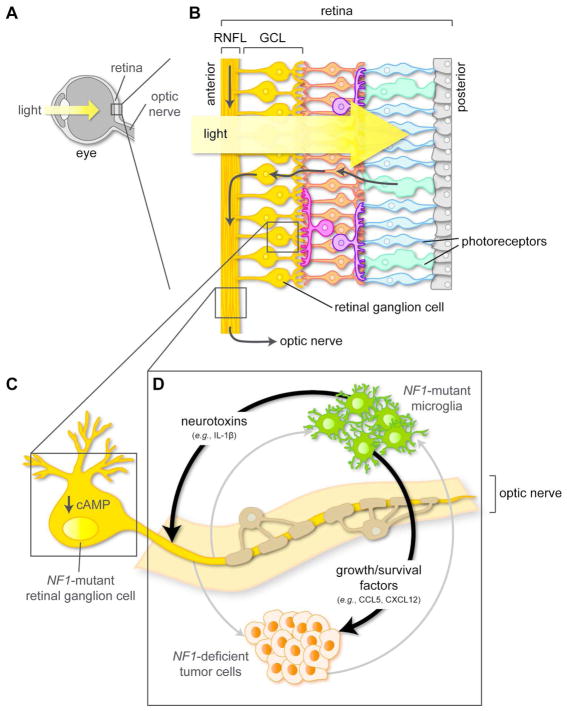
Figure 3. Cell-autonomous and cell-non-autonomous mechanisms underlying optic pathway glioma pathogenesis and associated vision loss.
(A, B) Sagittal sections through the mammalian eye (A) and retina (B). Photoreceptor (cone and rod) afferent pathways converge on retinal ganglion cells (RGCs). RGC bodies reside in the ganglion cell layer (GCL) of the retina, and their axons course through the retinal nerve fiber layer (RNFL) into the optic nerve.
(C) In NF1-mutant RGCs, impaired neurofibromin function reduces intracellular cAMP levels, thereby lowering the threshold for RGC death and subsequent vision loss.
(D) NF1-mutant microglia secrete chemokines (e.g., CCL5, CXCL12), which promote the proliferation and survival of NF1-deficient tumor cells. In addition, estrogen receptor β (ERβ)-mediated microglial priming leads to the production of neurotoxins (e.g., IL-1β) that increase NF1-mutant RGC axonal dysfunction and death, causing vision loss in a sex-dependent manner.
Tan-colored cells denote NF1-mutant oligodendrocytes that ensheath the optic nerve axons. The gray arrows indicate potential intercellular interactions in NF1-OPG, which are described in the main text.
Microglia positively regulate Nf1 optic glioma initiation and growth
Both human and mouse optic gliomas contain numerous distinct cell types, both neoplastic and non-neoplastic, the latter of which includes astrocytes, oligodendrocytes, neurons (i.e., RGCs), and microglia (Bajenaru et al. 2003; Louis et al. 2007; Marquardt and Zimmerman 1982; Otero et al. 2011; Stern et al. 1980; Zhu et al. 2005). Some of the most abundant of these non-neoplastic cell types are microglia (resident macrophages of the central nervous system), accounting for 30–50% of the cells in low-grade human gliomas (Gutmann et al. 2013; Morantz et al. 1979; Rossi et al. 1987; Simmons et al. 2011). Studies employing genetic and pharmacologic inhibitors have demonstrated that microglia contribute to Nf1 mouse optic glioma formation. In these studies, genetic reduction of microglial recruitment delays optic glioma formation (Pong et al. 2013), while pharmacologically inhibiting microglial function attenuates optic glioma growth and tumor cell proliferation (Daginakatte et al. 2008; Daginakatte and Gutmann 2007; Simmons et al. 2011). Microglia promote glioma formation and growth through the elaboration of growth factors and chemokines, including chemokine ligand 5 (CCL5) (Solga et al. 2015) and stromal cell-derived factor 1 (CXCL12) (Warrington et al. 2007) (Figure 3D). As such, inhibition of these chemokine/receptor axes (for CXCL12 and its cognate receptor CXCR4) with pharmacological inhibitors or neutralizing antibodies (against CCL5) reduces glioma growth. Collectively, these preclinical proof-of-concept studies reveal an obligate role for microglia in glioma pathogenesis, as well as therapeutic targets for future stromal-directed treatment approaches.
Sexually dimorphic vision loss in Nf1 optic glioma
In both humans and mice, vision loss from optic gliomas is caused by loss of RGCs, which are the visual pathway neurons that convey light information from the retina to the brain (Figure 3A,B). In mice harboring Nf1 optic gliomas, there is a stereotyped pattern of visual system pathology, beginning with RGC axonal damage, followed by increased RGC apoptosis and RNFL thinning, and culminating in decreased visual acuity (Hegedus et al. 2009; Kim et al. 2010; Toonen et al. 2017a).
Studies in mice have revealed two etiologies—one cell autonomous and one cell non-autonomous—that underlie optic glioma-associated death of Nf1-mutant RGCs and vision loss. First, Nf1-mutant RGCs, by virtue of their impaired neurofibromin function, have baseline reduced levels of intracellular cAMP, which lowers the threshold for RGC death in the setting of neuroinflammatory or neurotoxic stimuli (Brown et al. 2010) (Figure 3C). Second, specifically in female mice, gonadal estradiol acts through the estrogen receptor β (ERβ) to stimulate Nf1-mutant microglia, thereby causing death of Nf1-mutant RGCs, thinning of the RNFL (which comprises RGC axons), and decreased visual acuity (Diggs-Andrews et al. 2014b; Toonen et al. 2017b). Sexually dimorphic retinal pathology in Nf1 optic glioma mice is independent of tumor size and can be corrected by pharmacologic inhibition of microglial activation, ERβ blockade, and by chemical or surgical ovariectomy (Toonen et al. 2017b). In this cell non-autonomous mechanism, microglia are hypothesized to secrete neurotoxic cytokines (e.g., IL-1β) that either directly or indirectly damage RGC axons (Figure 3D), perhaps through disruption of normal axo-glial contacts as seen in other forms of axonal injury (Howell et al. 2010). Taken together, reduced cAMP levels and microglial production of neurotoxic cytokines likely synergize to culminate in RGC death and subsequent vision loss in the setting of NF1-OPG. While investigations have illuminated some of the mechanisms underlying sexually dimorphic vision loss in mice, the etiology of the increased risk for vision loss in girls with NF1-OPG remains to be identified (Diggs-Andrews et al. 2014a; Diggs-Andrews et al. 2014b; Fisher et al. 2014).
The impact of the germline Nf1 gene mutations on optic glioma formation
Converging evidence from population-based clinical studies, human induced pluripotent stem cells (iPSCs), and genetically engineered mice has revealed intriguing genotype-phenotype correlations in NF1. For example, children with NF1 who harbor mutations in the 5′-end of the NF1 gene coding sequence are more likely to develop OPGs than patients with mutations elsewhere in the coding sequence (Anastasaki et al. 2017; Sharif et al. 2011). In addition, human iPSCs from patients with NF1 (i.e., cells heterozygous for a germline patient-specific NF1 gene mutation) have variable neurofibromin protein levels and function. Lastly, isogenic mouse strains engineered to harbor distinct germline Nf1 gene mutations found in patients with NF1 exhibit markedly different tumor phenotypes: Mice with the Gly848Arg patient mutation do not form optic gliomas, whereas those harboring an Arg681X mutation develop optic gliomas of greater volumes and proliferative indices that those arising in mice harboring the artificial knockout allele (Toonen et al. 2016). Coupled with other emerging genotype-phenotype correlations involving neurofibromas (Koczkowska et al. 2018; Pinna et al. 2015; Upadhyaya et al. 2007) and autism spectrum symptomatology (Morris and Gutmann 2018), these findings argue against the notion that all NF1 gene mutations are functionally equivalent (Anastasaki et al. 2015), and support the idea that the germline NF1 gene mutation may be another critical factor in determining disease penetrance and clinical heterogeneity. Future studies are currently being executed to identify the cell types that are differentially impacted by the germline NF1 gene mutation (i.e., microglia, RGCs), and what mechanisms underlie these effects.
Therapeutic insights
Molecularly targeted and ecological therapies
Traditional cancer therapeutic strategies typically target the cancer cells, through either surgical removal or the induction of cell death using cytotoxic chemotherapy and/or radiation therapy. In NF1-OPG, treatment options have largely been limited to chemotherapy due to the diffusely infiltrative nature of the tumors, which precludes surgical resection (Alvord and Lofton 1988), and the heightened risk of radiation therapy-induced secondary malignancy in this patient population (Evans et al. 2006; Sharif et al. 2006). While frequently effective at attenuating tumor growth, chemotherapies used to treat NF1-OPG are less clearly effective at preventing or reversing tumor-associated vision loss (Moreno et al. 2010). Furthermore, their use is complicated by both short- and long-term side effects, ranging from fatigue and nausea to bone marrow suppression, hypersensitivity reactions, and permanent cognitive impairment (Packer et al. 1993; Verstappen et al. 2003). More recently, molecularly targeted therapies, including inhibitors of the RAS effectors MEK and mTOR have been applied to the treatment of patients with NF1-OPG in early-phase clinical trials (Banerjee et al. 2017; Yalon et al. 2013). However, results from pilot studies have been mixed, underscoring the need for alternative therapeutic strategies, specifically those that aim to prevent or reduce vision loss from NF1-OPG.
With a growing appreciation of the key role of the low-grade glioma ecosystem in disease pathogenesis, new therapies that target cells and signals in the tumor microenvironment have begun to emerge. In the case of NF1-OPGs, these “ecological therapies” (see (Hoelzinger et al. 2007; Pienta et al. 2008) for reviews), might target microglia/macrophages (Figure 3D). Attenuating microglia recruitment and/or priming has been proposed for other tumor types, including glioblastoma and metastatic brain cancer (Andreou et al. 2017; Frazier et al. 2003; Hoelzinger et al. 2007). For example, an ongoing Phase I clinical trial is exploring the use of the antibiotic minocycline, which reduces microglial activation, in combination with temozolamide in individuals with newly diagnosed glioblastoma (NCT02272270). Based on encouraging preclinical results using microglia-targeted agents in mice engineered to harbor Nf1 optic gliomas, future therapies might integrate a similar approach for treating children with NF1-OPG.
In addition to this strategy, other ecological therapeutic opportunities may emerge from the continued study of microglia recruitment and activation. In this regard, the use of more selective agents could be envisioned, which silence microglial function in the setting of glioma or inhibit tumor cell or RGC receptor activation by microglia-produced growth factors and cytokines. Understanding the mechanisms that underlie microglia attraction to the tumor bed, their activation by other immune system cells, and their reprogramming to create a supportive microenvironment may yield additional therapeutic targets. As one example, neuronal signaling to microglia (e.g., through the chemokine fractalkine, which binds the CX3CR1 receptor on microglia) is implicated in modulation of microglia behavior in other neurodegenerative diseases (Paolicelli et al. 2014). Whether Nf1-mutant RGCs similarly signal to Nf1-mutant microglia in the context of Nf1 optic glioma remains to be determined; however, if relevant, would suggest additional targetable pathways for therapeutic exploration (Figure 3D).
Finally, based on pioneering studies by Michelle Monje and colleagues, it is possible that neurons or neuronal activity influences tumor cell behavior through the elaboration of growth factors. Proof-of-principle experiments using a high-grade glioma (HGG) mouse xenograft model showed that cortical neurons secrete neuroligin-3 (NLGN3) in an activity-dependent manner to increase HGG cell proliferation and tumor growth (Venkatesh et al. 2015). In NF1-OPG, the close anatomical proximity of NF1-deficient tumor cells to NF1-mutant RGC axons suggests the possibility that analogous neuron-to-tumor cell relationships exist in optic gliomas (Figure 3D). Further studies may determine whether RGC-to-tumor cell paracrine interactions are operative, which might additionally influence NF1-OPG pathogenesis.
Future strategies for visual recovery in NF1-OPG
The currently available chemotherapeutic agents (e.g., carboplatin/vincristine), targeted RAS pathway inhibitors, and potential ecological therapies described above all represent strategies for limiting further tumor growth. However, one limitation of these therapeutic strategies is that few are specifically designed to prevent continued vision loss in the setting of NF1-OPG. Future treatments could focus on promoting RGC survival and/or reducing axonal damage from tumor-associated microglia. These approaches might entail the use of therapies that elevate RGC cAMP levels (Brown et al., 2010) or reduce microglia-induced axonal damage. Specifically, the latter approach may interfere with ERβ-mediated microglia reprogramming or disrupt the paracrine signaling pathways that culminate in axonal damage and RGC apoptosis (Hambardzumyan et al. 2016; McCarty 2006).
Second, neuroprotective strategies (e.g., activating intrinsic RGC survival pathways, inhibiting RGC apoptotic pathways, or altering mitochondrial function in RGCs) might delay or arrest NF1-OPG-related NF1-mutant RGC loss. These neuroprotective approaches have been explored in preclinical models of glaucoma, a disease in which RGC axonal dysfunction leads to neuronal death and vision loss. In studies of mouse models of glaucoma, both brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) increase RGC numbers and retinal function (Domenici et al. 2014; Lambiase et al. 2009). As such, a recent Phase II trial randomized eighteen individuals (of whom thirteen carried a diagnosis of NF1) with stable OPG disease and severe visual impairment to receive NGF or placebo eye drops (Chiaretti et al. 2011; Falsini et al. 2016). Those patients treated with NGF exhibited improved RGC function based on electrophysiological properties and visual field enlargement, compared to placebo-treated individuals who demonstrated visual field worsening. Although small, this study highlights a promising neuroprotective strategy for vision loss in NF1-OPG.
Finally, other neuroprotective or neurorestorative approaches that employ viral or non-viral vectors to deliver genes that modify RGC gene expression and increase cell survival could be considered. Such gene therapy approaches have shown promise in preclinical models of optic nerve injury (Caprioli et al. 2009; Ishikawa et al. 2005; Mo et al. 2002) and glaucoma (Wilson and Di Polo 2012). Additionally, several groups are exploring the possibility of reprogramming Müller glia into RGCs to improve retinal function or transplanting autologous human iPSC-derived RGCs into the retina (Jorstad et al. 2017; Sanges et al. 2016; Venugopalan et al. 2016).
Conclusions
Herein, we describe the current state of understanding of the pathobiology of NF1-OPG, and review insights from Nf1 GEM models relevant to the molecular and cellular determinants that underlie tumor formation, progression, and associated vision loss. The inherent limitations of mouse models underscore the need for additional small-animal models and other experimental platforms that capture the spectrum of clinical heterogeneity that characterizes these tumors in children with NF1.
First, future mouse modeling experiments should aim to include tumors that arise in the optic tract and radiations, which represent the more clinically aggressive subtype in children with NF1. In addition, studies should incorporate mice harboring different germline Nf1 gene mutations, as seen in patients with NF1, as well as tumors harboring less common secondary genomic alterations (e.g., PTEN mutation, KIAA1549:BRAF duplication, fibroblast growth factor receptor-1 mutation) (Forshew et al. 2009; Jacques et al. 2010; Jones et al. 2008; Pfister et al. 2008; Zuckermann et al. 2015).
Second, next-generation mouse preclinical trials should consider incorporating clinical endpoints used in human clinical trials, such as MRI and visual assessments. With the advent of high-resolution small-animal MRI (enabling measurement of tumor size) and ocular coherence tomography (enabling evaluation of RNFL thickness) (Avery et al. 2015; Gu et al. 2014), future preclinical trials that include drug levels and clinically relevant outcomes could be designed.
Lastly, complementary preclinical models should be considered. Mice rely less on vision for survival, and their visual systems may not accurately represent the human condition. In this regard, efforts to develop genetically engineered models of NF1 in pigs have been initiated (Meyerholz et al. 2017). The visual system and brains of pigs more closely approximate those of humans and have already been used to model glaucoma (Ruiz-Ederra et al. 2005). Swine might represent a more large-animal model of NF1-OPG because of the greater anatomical similarity of the porcine retina to the human retina (compared to other large non-primate mammals (Prince 1960)) and the greater affordability and availability for study (relative to primates).
Taken together, there has been encouraging and exciting progress in our understanding of the pathophysiology of NF1-OPG since the discovery of the NF1 gene in 1990. With further refinements and deeper study, it is possible that the future care of this unique population of children will include presymptomatic risk assessments for OPG formation, more optimized screening for early retinal impairment, and the application of vision restoration therapies.
Significance.
We discuss our current understanding of the pathobiology of optic pathway glioma (OPG) in individuals with Neurofibromatosis type 1 (NF1), including possible mechanisms of NF1-OPG-related vision loss. We emphasize the value of genetically engineered mouse (GEM) models of NF1-associated OPG (NF1-OPG) in elucidating the cellular and molecular determinants that underlie tumor formation, growth, and associated vision loss in mice. By extension, these findings may inform our understanding of NF1-OPG in humans and enable the identification of novel therapeutic leads to prevent or ameliorate vision loss in children with NF1.
Acknowledgments
Support: National Cancer Institute 1-R01-CA195692-01 (D.H.G.), National Institutes of Health 1-R35-NS07211-01 (D.H.G.)
Footnotes
Conflict of interest: The authors declare no conflicts of interest
References
- Alvord EC, Jr, Lofton S. Gliomas of the optic nerve or chiasm. Outcome by patients’ age, tumor site, and treatment. J Neurosurg. 1988;68(1):85–98. doi: 10.3171/jns.1988.68.1.0085. [DOI] [PubMed] [Google Scholar]
- Anastasaki C, Gutmann DH. Neuronal NF1/RAS regulation of cyclic AMP requires atypical PKC activation. Hum Mol Genet. 2014;23(25):6712–6721. doi: 10.1093/hmg/ddu389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasaki C, Le LQ, Kesterson RA, Gutmann DH. Updated nomenclature for human and mouse neurofibromatosis type 1 genes. Neurol Genet. 2017;3(4):e169. doi: 10.1212/NXG.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasaki C, Woo AS, Messiaen LM, Gutmann DH. Elucidating the impact of neurofibromatosis-1 germline mutations on neurofibromin function and dopamine-based learning. Hum Mol Genet. 2015;24(12):3518–3528. doi: 10.1093/hmg/ddv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou KE, Soto MS, Allen D, Economopoulos V, de Bernardi A, Larkin JR, Sibson NR. Anti-inflammatory Microglia/Macrophages As a Potential Therapeutic Target in Brain Metastasis. Front Oncol. 2017;7:251. doi: 10.3389/fonc.2017.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery RA, Cnaan A, Schuman JS, Trimboli-Heidler C, Chen CL, Packer RJ, Ishikawa H. Longitudinal Change of Circumpapillary Retinal Nerve Fiber Layer Thickness in Children With Optic Pathway Gliomas. Am J Ophthalmol. 2015;160(5):944–952. e941. doi: 10.1016/j.ajo.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery RA, Liu GT, Fisher MJ, Quinn GE, Belasco JB, Phillips PC, Maguire MG, Balcer LJ. Retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2011;151(3):542–549. e542. doi: 10.1016/j.ajo.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenaru ML, Hernandez MR, Perry A, Zhu Y, Parada LF, Garbow JR, Gutmann DH. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63(24):8573–8577. [PubMed] [Google Scholar]
- Balcer LJ, Liu GT, Heller G, Bilaniuk L, Volpe NJ, Galetta SL, Molloy PT, Phillips PC, Janss AJ, Vaughn S, Maguire MG. Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol. 2001;131(4):442–445. doi: 10.1016/s0002-9394(00)00852-7. [DOI] [PubMed] [Google Scholar]
- Ballester R, Marchuk D, Boguski M, Saulino A, Letcher R, Wigler M, Collins F. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990;63(4):851–859. doi: 10.1016/0092-8674(90)90151-4. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Jakacki RI, Onar-Thomas A, Wu S, Nicolaides T, Young Poussaint T, Fangusaro J, Phillips J, Perry A, Turner D, Prados M, Packer RJ, Qaddoumi I, Gururangan S, Pollack IF, Goldman S, Doyle LA, Stewart CF, Boyett JM, Kun LE, Fouladi M. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol. 2017;19(8):1135–1144. doi: 10.1093/neuonc/now282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356(6371):713–715. doi: 10.1038/356713a0. [DOI] [PubMed] [Google Scholar]
- Bollag G, Clapp DW, Shih S, Adler F, Zhang YY, Thompson P, Lange BJ, Freedman MH, McCormick F, Jacks T, Shannon K. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat Genet. 1996;12(2):144–148. doi: 10.1038/ng0296-144. [DOI] [PubMed] [Google Scholar]
- Bollag G, McCormick F. Differential regulation of rasGAP and neurofibromatosis gene product activities. Nature. 1991;351(6327):576–579. doi: 10.1038/351576a0. [DOI] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129(5):865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Bosch MM, Wichmann WW, Boltshauser E, Landau K. Optic nerve sheath meningiomas in patients with neurofibromatosis type 2. Arch Ophthalmol. 2006;124(3):379–385. doi: 10.1001/archopht.124.3.379. [DOI] [PubMed] [Google Scholar]
- Brannan CI, Perkins AS, Vogel KS, Ratner N, Nordlund ML, Reid SW, Buchberg AM, Jenkins NA, Parada LF, Copeland NG. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8(9):1019–1029. doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- Brems H, Park C, Maertens O, Pemov A, Messiaen L, Upadhyaya M, Claes K, Beert E, Peeters K, Mautner V, Sloan JL, Yao L, Lee CC, Sciot R, De Smet L, Legius E, Stewart DR. Glomus tumors in neurofibromatosis type 1: genetic, functional, and clinical evidence of a novel association. Cancer Res. 2009;69(18):7393–7401. doi: 10.1158/0008-5472.CAN-09-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Gianino SM, Gutmann DH. Defective cAMP generation underlies the sensitivity of CNS neurons to neurofibromatosis-1 heterozygosity. J Neurosci. 2010;30(16):5579–5589. doi: 10.1523/JNEUROSCI.3994-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli J, Munemasa Y, Kwong JM, Piri N. Overexpression of thioredoxins 1 and 2 increases retinal ganglion cell survival after pharmacologically induced oxidative stress, optic nerve transection, and in experimental glaucoma. Trans Am Ophthalmol Soc. 2009;107:161–165. [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Weiss R, Xu GF, Viskochil D, Culver M, Stevens J, Robertson M, Dunn D, Gesteland R, O’Connell P, et al. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell. 1990;62(1):193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- Chen YH, McGowan LD, Cimino PJ, Dahiya S, Leonard JR, Lee DY, Gutmann DH. Mouse low-grade gliomas contain cancer stem cells with unique molecular and functional properties. Cell Rep. 2015;10(11):1899–1912. doi: 10.1016/j.celrep.2015.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaretti A, Falsini B, Servidei S, Marangoni D, Pierri F, Riccardi R. Nerve growth factor eye drop administration improves visual function in a patient with optic glioma. Neurorehabil Neural Repair. 2011;25(4):386–390. doi: 10.1177/1545968310395601. [DOI] [PubMed] [Google Scholar]
- Chong AL, Pole JD, Scheinemann K, Hukin J, Tabori U, Huang A, Bouffet E, Bartels U. Optic pathway gliomas in adolescence--time to challenge treatment choices? Neuro Oncol. 2013;15(3):391–400. doi: 10.1093/neuonc/nos312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AD, Der CJ. Ras history: The saga continues. Small GTPases. 2010;1(1):2–27. doi: 10.4161/sgtp.1.1.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe FW. A clinical, pathological, and genetic study of multiple neurofibromatosis. Springfield, Ill: Thomas; 1956. p. 181. [Google Scholar]
- Daginakatte GC, Gianino SM, Zhao NW, Parsadanian AS, Gutmann DH. Increased c-Jun-NH2-kinase signaling in neurofibromatosis-1 heterozygous microglia drives microglia activation and promotes optic glioma proliferation. Cancer Res. 2008;68(24):10358–10366. doi: 10.1158/0008-5472.CAN-08-2506. [DOI] [PubMed] [Google Scholar]
- Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16(9):1098–1112. doi: 10.1093/hmg/ddm059. [DOI] [PubMed] [Google Scholar]
- Dahiya S, Lee DY, Gutmann DH. Comparative characterization of the human and mouse third ventricle germinal zones. J Neuropathol Exp Neurol. 2011;70(7):622–633. doi: 10.1097/NEN.0b013e31822200aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Via P, Opocher E, Pinello ML, Calderone M, Viscardi E, Clementi M, Battistella PA, Laverda AM, Da Dalt L, Perilongo G. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neuro Oncol. 2007;9(4):430–437. doi: 10.1215/15228517-2007-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Gutmann DH. Neurofibromatosis 1: closing the GAP between mice and men. Curr Opin Genet Dev. 2003;13(1):20–27. doi: 10.1016/s0959-437x(02)00015-1. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Gutmann DH. Neurofibromin regulates neural stem cell proliferation, survival, and astroglial differentiation in vitro and in vivo. J Neurosci. 2005;25(23):5584–5594. doi: 10.1523/JNEUROSCI.4693-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65(7):2755–2760. doi: 10.1158/0008-5472.CAN-04-4058. [DOI] [PubMed] [Google Scholar]
- DeClue JE, Cohen BD, Lowy DR. Identification and characterization of the neurofibromatosis type 1 protein product. Proc Natl Acad Sci U S A. 1991;88(22):9914–9918. doi: 10.1073/pnas.88.22.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggs-Andrews KA, Brown JA, Gianino SM, D’Agostino McGowan L, Rubin JB, Wozniak DF, Gutmann DH. Reply: To PMID 24375753. Ann Neurol. 2014a;75(5):800–801. doi: 10.1002/ana.24156. [DOI] [PubMed] [Google Scholar]
- Diggs-Andrews KA, Brown JA, Gianino SM, Rubin JB, Wozniak DF, Gutmann DH. Sex Is a major determinant of neuronal dysfunction in neurofibromatosis type 1. Ann Neurol. 2014b;75(2):309–316. doi: 10.1002/ana.24093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgshun AJ, Elder JE, Hansford JR, Sullivan MJ. Long-term visual outcome after chemotherapy for optic pathway glioma in children: Site and age are strongly predictive. Cancer. 2015;121(23):4190–4196. doi: 10.1002/cncr.29649. [DOI] [PubMed] [Google Scholar]
- Domenici L, Origlia N, Falsini B, Cerri E, Barloscio D, Fabiani C, Sanso M, Giovannini L. Rescue of retinal function by BDNF in a mouse model of glaucoma. PLoS One. 2014;9(12):e115579. doi: 10.1371/journal.pone.0115579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan S, See W, Bonifas J, Stokoe D, Shannon KM. Hyperactivation of protein kinase B and ERK have discrete effects on survival, proliferation, and cytokine expression in Nf1-deficient myeloid cells. Cancer Cell. 2002;2(6):507–514. doi: 10.1016/s1535-6108(02)00214-3. [DOI] [PubMed] [Google Scholar]
- Evans DG, Birch JM, Ramsden RT, Sharif S, Baser ME. Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumour prone syndromes. J Med Genet. 2006;43(4):289–294. doi: 10.1136/jmg.2005.036319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, Lalloo F. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A(2):327–332. doi: 10.1002/ajmg.a.33139. [DOI] [PubMed] [Google Scholar]
- Falsini B, Chiaretti A, Rizzo D, Piccardi M, Ruggiero A, Manni L, Soligo M, Dickmann A, Federici M, Salerni A, Timelli L, Guglielmi G, Lazzareschi I, Caldarelli M, Galli-Resta L, Colosimo C, Riccardi R. Nerve growth factor improves visual loss in childhood optic gliomas: a randomized, double-blind, phase II clinical trial. Brain. 2016;139(Pt 2):404–414. doi: 10.1093/brain/awv366. [DOI] [PubMed] [Google Scholar]
- Fernandez-Medarde A, Santos E. Ras in cancer and developmental diseases. Genes Cancer. 2011;2(3):344–358. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MJ, Loguidice M, Gutmann DH, Listernick R, Ferner RE, Ullrich NJ, Packer RJ, Tabori U, Hoffman RO, Ardern-Holmes SL, Hummel TR, Hargrave DR, Bouffet E, Charrow J, Bilaniuk LT, Balcer LJ, D’Agostino McGowan L, Liu GT. Gender as a disease modifier in neurofibromatosis type 1 optic pathway glioma. Ann Neurol. 2014;75(5):799–800. doi: 10.1002/ana.24157. [DOI] [PubMed] [Google Scholar]
- Fisher MJ, Loguidice M, Gutmann DH, Listernick R, Ferner RE, Ullrich NJ, Packer RJ, Tabori U, Hoffman RO, Ardern-Holmes SL, Hummel TR, Hargrave DR, Bouffet E, Charrow J, Bilaniuk LT, Balcer LJ, Liu GT. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6):790–797. doi: 10.1093/neuonc/nos076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forshew T, Tatevossian RG, Lawson AR, Ma J, Neale G, Ogunkolade BW, Jones TA, Aarum J, Dalton J, Bailey S, Chaplin T, Carter RL, Gajjar A, Broniscer A, Young BD, Ellison DW, Sheer D. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218(2):172–181. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- Frazier JL, Wang PP, Case D, Tyler BM, Pradilla G, Weingart JD, Brem H. Local delivery of minocycline and systemic BCNU have synergistic activity in the treatment of intracranial glioma. J Neurooncol. 2003;64(3):203–209. doi: 10.1023/a:1025695423097. [DOI] [PubMed] [Google Scholar]
- Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet. 1999;89(1):1–6. [PubMed] [Google Scholar]
- Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10(6):698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Miller RH. Specification of optic nerve oligodendrocyte precursors by retinal ganglion cell axons. J Neurosci. 2006;26(29):7619–7628. doi: 10.1523/JNEUROSCI.0855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Glaug N, Cnaan A, Packer RJ, Avery RA. Ganglion cell layer-inner plexiform layer thickness and vision loss in young children with optic pathway gliomas. Invest Ophthalmol Vis Sci. 2014;55(3):1402–1408. doi: 10.1167/iovs.13-13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamo JS, Creange A, Kalifa C, Grill J, Rodriguez D, Doz F, Barbarot S, Zerah M, Sanson M, Bastuji-Garin S, Wolkenstein P. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain. 2003;126(Pt 1):152–160. doi: 10.1093/brain/awg016. [DOI] [PubMed] [Google Scholar]
- Gutmann DH, Donahoe J, Brown T, James CD, Perry A. Loss of neurofibromatosis 1 (NF1) gene expression in NF1-associated pilocytic astrocytomas. Neuropathol Appl Neurobiol. 2000;26(4):361–367. doi: 10.1046/j.1365-2990.2000.00258.x. [DOI] [PubMed] [Google Scholar]
- Gutmann DH, James CD, Poyhonen M, Louis DN, Ferner R, Guha A, Hariharan S, Viskochil D, Perry A. Molecular analysis of astrocytomas presenting after age 10 in individuals with NF1. Neurology. 2003;61(10):1397–1400. doi: 10.1212/wnl.61.10.1397. [DOI] [PubMed] [Google Scholar]
- Gutmann DH, McLellan MD, Hussain I, Wallis JW, Fulton LL, Fulton RS, Magrini V, Demeter R, Wylie T, Kandoth C, Leonard JR, Guha A, Miller CA, Ding L, Mardis ER. Somatic neurofibromatosis type 1 (NF1) inactivation characterizes NF1-associated pilocytic astrocytoma. Genome Res. 2013;23(3):431–439. doi: 10.1101/gr.142604.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann DH, Parada LF, Silva AJ, Ratner N. Neurofibromatosis type 1: modeling CNS dysfunction. J Neurosci. 2012;32(41):14087–14093. doi: 10.1523/JNEUROSCI.3242-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–27. doi: 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus B, Banerjee D, Yeh TH, Rothermich S, Perry A, Rubin JB, Garbow JR, Gutmann DH. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68(5):1520–1528. doi: 10.1158/0008-5472.CAN-07-5916. [DOI] [PubMed] [Google Scholar]
- Hegedus B, Dasgupta B, Shin JE, Emnett RJ, Hart-Mahon EK, Elghazi L, Bernal-Mizrachi E, Gutmann DH. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1(4):443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Hegedus B, Hughes FW, Garbow JR, Gianino S, Banerjee D, Kim K, Ellisman MH, Brantley MA, Jr, Gutmann DH. Optic nerve dysfunction in a mouse model of neurofibromatosis-1 optic glioma. J Neuropathol Exp Neurol. 2009;68(5):542–551. doi: 10.1097/NEN.0b013e3181a3240b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100(25):15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007;99(21):1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- Howell OW, Rundle JL, Garg A, Komada M, Brophy PJ, Reynolds R. Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J Neuropathol Exp Neurol. 2010;69(10):1017–1033. doi: 10.1097/NEN.0b013e3181f3a5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson SM, Compston DA, Clark P, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet. 1989;26(11):704–711. doi: 10.1136/jmg.26.11.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial R, Toor OM, Hussain A, Subramanian J, Masood A. Comprehensive pancancer genomic analysis reveals (RTK)-RAS-RAF-MEK as a key dysregulated pathway in cancer: Its clinical implications. Semin Cancer Biol. 2017 doi: 10.1016/j.semcancer.2017.11.016. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Takano M, Matsumoto N, Sawada H, Ide C, Mimura O, Dezawa M. Effect of GDNF gene transfer into axotomized retinal ganglion cells using in vivo electroporation with a contact lens-type electrode. Gene Ther. 2005;12(4):289–298. doi: 10.1038/sj.gt.3302277. [DOI] [PubMed] [Google Scholar]
- Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat Genet. 1994;7(3):353–361. doi: 10.1038/ng0794-353. [DOI] [PubMed] [Google Scholar]
- Jacques TS, Swales A, Brzozowski MJ, Henriquez NV, Linehan JM, Mirzadeh Z, COM, Naumann H, Alvarez-Buylla A, Brandner S. Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour phenotypes. EMBO J. 2010;29(1):222–235. doi: 10.1038/emboj.2009.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102(24):8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, Collins VP. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorstad NL, Wilken MS, Grimes WN, Wohl SG, VandenBosch LS, Yoshimatsu T, Wong RO, Rieke F, Reh TA. Stimulation of functional neuronal regeneration from Muller glia in adult mice. Nature. 2017;548(7665):103–107. doi: 10.1038/nature23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul A, Toonen JA, Cimino PJ, Gianino SM, Gutmann DH. Akt- or MEK-mediated mTOR inhibition suppresses Nf1 optic glioma growth. Neuro Oncol. 2015;17(6):843–853. doi: 10.1093/neuonc/nou329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Ju WK, Hegedus B, Gutmann DH, Ellisman MH. Ultrastructural characterization of the optic pathway in a mouse model of neurofibromatosis-1 optic glioma. Neuroscience. 2010;170(1):178–188. doi: 10.1016/j.neuroscience.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluwe L, Hagel C, Tatagiba M, Thomas S, Stavrou D, Ostertag H, von Deimling A, Mautner VF. Loss of NF1 alleles distinguish sporadic from NF1-associated pilocytic astrocytomas. J Neuropathol Exp Neurol. 2001;60(9):917–920. doi: 10.1093/jnen/60.9.917. [DOI] [PubMed] [Google Scholar]
- Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczkowska M, Chen Y, Callens T, Gomes A, Sharp A, Johnson S, Hsiao MC, Chen Z, Balasubramanian M, Barnett CP, Becker TA, Ben-Shachar S, Bertola DR, Blakeley JO, Burkitt-Wright EMM, Callaway A, Crenshaw M, Cunha KS, Cunningham M, D’Agostino MD, Dahan K, De Luca A, Destree A, Dhamija R, Eoli M, Evans DGR, Galvin-Parton P, George-Abraham JK, Gripp KW, Guevara-Campos J, Hanchard NA, Hernandez-Chico C, Immken L, Janssens S, Jones KJ, Keena BA, Kochhar A, Liebelt J, Martir-Negron A, Mahoney MJ, Maystadt I, McDougall C, McEntagart M, Mendelsohn N, Miller DT, Mortier G, Morton J, Pappas J, Plotkin SR, Pond D, Rosenbaum K, Rubin K, Russell L, Rutledge LS, Saletti V, Schonberg R, Schreiber A, Seidel M, Siqveland E, Stockton DW, Trevisson E, Ullrich NJ, Upadhyaya M, van Minkelen R, Verhelst H, Wallace MR, Yap YS, Zackai E, Zonana J, Zurcher V, Claes K, Martin Y, Korf BR, Legius E, Messiaen LM. Genotype-Phenotype Correlation in NF1: Evidence for a More Severe Phenotype Associated with Missense Mutations Affecting NF1 Codons 844–848. Am J Hum Genet. 2018;102(1):69–87. doi: 10.1016/j.ajhg.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Aloe L, Centofanti M, Parisi V, Bao SN, Mantelli F, Colafrancesco V, Manni GL, Bucci MG, Bonini S, Levi-Montalcini R. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc Natl Acad Sci U S A. 2009;106(32):13469–13474. doi: 10.1073/pnas.0906678106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau N, Feldkamp MM, Roncari L, Loehr AH, Shannon P, Gutmann DH, Guha A. Loss of neurofibromin is associated with activation of RAS/MAPK and PI3-K/AKT signaling in a neurofibromatosis 1 astrocytoma. J Neuropathol Exp Neurol. 2000;59(9):759–767. doi: 10.1093/jnen/59.9.759. [DOI] [PubMed] [Google Scholar]
- Laycock-van Spyk S, Thomas N, Cooper DN, Upadhyaya M. Neurofibromatosis type 1-associated tumours: their somatic mutational spectrum and pathogenesis. Hum Genomics. 2011;5(6):623–690. doi: 10.1186/1479-7364-5-6-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Gianino SM, Gutmann DH. Innate neural stem cell heterogeneity determines the patterning of glioma formation in children. Cancer Cell. 2012;22(1):131–138. doi: 10.1016/j.ccr.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Yeh TH, Emnett RJ, White CR, Gutmann DH. Neurofibromatosis-1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region-specific manner. Genes Dev. 2010;24(20):2317–2329. doi: 10.1101/gad.1957110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125(1):63–66. doi: 10.1016/s0022-3476(94)70122-9. [DOI] [PubMed] [Google Scholar]
- Listernick R, Charrow J, Greenwald MJ, Esterly NB. Optic gliomas in children with neurofibromatosis type 1. J Pediatr. 1989;114(5):788–792. doi: 10.1016/s0022-3476(89)80137-4. [DOI] [PubMed] [Google Scholar]
- Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61(3):189–198. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listernick R, Ferner RE, Piersall L, Sharif S, Gutmann DH, Charrow J. Late-onset optic pathway tumors in children with neurofibromatosis 1. Neurology. 2004;63(10):1944–1946. doi: 10.1212/01.wnl.0000144341.16830.01. [DOI] [PubMed] [Google Scholar]
- Liu GT, Brodsky MC, Phillips PC, Belasco J, Janss A, Golden JC, Bilaniuk LL, Burson GT, Duhaime AC, Sutton LN. Optic radiation involvement in optic pathway gliomas in neurofibromatosis. Am J Ophthalmol. 2004;137(3):407–414. doi: 10.1016/j.ajo.2003.09.055. [DOI] [PubMed] [Google Scholar]
- Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH. von Hippel-Lindau disease. Lancet. 2003;361(9374):2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens O, Prenen H, Debiec-Rychter M, Wozniak A, Sciot R, Pauwels P, De Wever I, Vermeesch JR, de Raedt T, De Paepe A, Speleman F, van Oosterom A, Messiaen L, Legius E. Molecular pathogenesis of multiple gastrointestinal stromal tumors in NF1 patients. Hum Mol Genet. 2006;15(6):1015–1023. doi: 10.1093/hmg/ddl016. [DOI] [PubMed] [Google Scholar]
- Mahoney DH, Jr, Cohen ME, Friedman HS, Kepner JL, Gemer L, Langston JW, James HE, Duffner PK, Kun LE. Carboplatin is effective therapy for young children with progressive optic pathway tumors: a Pediatric Oncology Group phase II study. Neuro Oncol. 2000;2(4):213–220. doi: 10.1093/neuonc/2.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt MD, Zimmerman LE. Histopathology of meningiomas and gliomas of the optic nerve. Hum Pathol. 1982;13(3):226–235. doi: 10.1016/s0046-8177(82)80181-0. [DOI] [PubMed] [Google Scholar]
- McCarty MF. Down-regulation of microglial activation may represent a practical strategy for combating neurodegenerative disorders. Med Hypotheses. 2006;67(2):251–269. doi: 10.1016/j.mehy.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Ofori-Amanfo GK, Leidinger MR, Goeken JA, Khanna R, Sieren JC, Darbro BW, Quelle DE, Weimer JM. Immunohistochemical Markers for Prospective Studies in Neurofibromatosis-1 Porcine Models. J Histochem Cytochem. 2017;65(10):607–618. doi: 10.1369/0022155417729357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo X, Yokoyama A, Oshitari T, Negishi H, Dezawa M, Mizota A, Adachi-Usami E. Rescue of axotomized retinal ganglion cells by BDNF gene electroporation in adult rats. Invest Ophthalmol Vis Sci. 2002;43(7):2401–2405. [PubMed] [Google Scholar]
- Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg. 1979;50(3):305–311. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- Moreno L, Bautista F, Ashley S, Duncan C, Zacharoulis S. Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer. 2010;46(12):2253–2259. doi: 10.1016/j.ejca.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Morris SM, Gutmann DH. A genotype-phenotype correlation for quantitative autistic trait burden in neurofibromatosis 1. Neurology. 2018;90(8):377–379. doi: 10.1212/WNL.0000000000005000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba Y, Mochizuki N, Yamashita S, Chan AM, Schrader JW, Hattori S, Nagashima K, Matsuda M. Regulatory proteins of R-Ras, TC21/R-Ras2, and M-Ras/R-Ras3. J Biol Chem. 2000;275(26):20020–20026. doi: 10.1074/jbc.M000981200. [DOI] [PubMed] [Google Scholar]
- Ono K, Yasui Y, Rutishauser U, Miller RH. Focal ventricular origin and migration of oligodendrocyte precursors into the chick optic nerve. Neuron. 1997;19(2):283–292. doi: 10.1016/s0896-6273(00)80939-3. [DOI] [PubMed] [Google Scholar]
- Otero JJ, Rowitch D, Vandenberg S. OLIG2 is differentially expressed in pediatric astrocytic and in ependymal neoplasms. J Neurooncol. 2011;104(2):423–438. doi: 10.1007/s11060-010-0509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer RJ, Ater J, Allen J, Phillips P, Geyer R, Nicholson HS, Jakacki R, Kurczynski E, Needle M, Finlay J, Reaman G, Boyett JM. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- Packer RJ, Lange B, Ater J, Nicholson HS, Allen J, Walker R, Prados M, Jakacki R, Reaman G, Needles MN, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11(5):850–856. doi: 10.1200/JCO.1993.11.5.850. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bisht K, Tremblay ME. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci. 2014;8:129. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister S, Janzarik WG, Remke M, Ernst A, Werft W, Becker N, Toedt G, Wittmann A, Kratz C, Olbrich H, Ahmadi R, Thieme B, Joos S, Radlwimmer B, Kulozik A, Pietsch T, Herold-Mende C, Gnekow A, Reifenberger G, Korshunov A, Scheurlen W, Omran H, Lichter P. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118(5):1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienta KJ, McGregor N, Axelrod R, Axelrod DE. Ecological therapy for cancer: defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl Oncol. 2008;1(4):158–164. doi: 10.1593/tlo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna V, Lanari V, Daniele P, Consoli F, Agolini E, Margiotti K, Bottillo I, Torrente I, Bruselles A, Fusilli C, Ficcadenti A, Bargiacchi S, Trevisson E, Forzan M, Giustini S, Leoni C, Zampino G, Digilio MC, Dallapiccola B, Clementi M, Tartaglia M, De Luca A. p.Arg1809Cys substitution in neurofibromin is associated with a distinctive NF1 phenotype without neurofibromas. Eur J Hum Genet. 2015;23(8):1068–1071. doi: 10.1038/ejhg.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pong WW, Higer SB, Gianino SM, Emnett RJ, Gutmann DH. Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann Neurol. 2013;73(2):303–308. doi: 10.1002/ana.23813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada CE, Hufnagel RB, Hummel TR, Lovell AM, Hopkin RJ, Saal HM, Schorry EK. The Use of Magnetic Resonance Imaging Screening for Optic Pathway Gliomas in Children with Neurofibromatosis Type 1. J Pediatr. 2015;167(4):851–856. e851. doi: 10.1016/j.jpeds.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince JH. Anatomy and histology of the eye and orbit in domestic animals. Springfield, Ill: C.C. Thomas; 1960. p. 307. [Google Scholar]
- Rossi ML, Hughes JT, Esiri MM, Coakham HB, Brownell DB. Immunohistological study of mononuclear cell infiltrate in malignant gliomas. Acta Neuropathol. 1987;74(3):269–277. doi: 10.1007/BF00688191. [DOI] [PubMed] [Google Scholar]
- Rowley SA, O’Callaghan FJ, Osborne JP. Ophthalmic manifestations of tuberous sclerosis: a population based study. Br J Ophthalmol. 2001;85(4):420–423. doi: 10.1136/bjo.85.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Garcia M, Hernandez M, Urcola H, Hernandez-Barbachano E, Araiz J, Vecino E. The pig eye as a novel model of glaucoma. Exp Eye Res. 2005;81(5):561–569. doi: 10.1016/j.exer.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Sanges D, Simonte G, Di Vicino U, Romo N, Pinilla I, Nicolas M, Cosma MP. Reprogramming Muller glia via in vivo cell fusion regenerates murine photoreceptors. J Clin Invest. 2016;126(8):3104–3116. doi: 10.1172/JCI85193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, Ma Q, Alvarez-Buylla A, McMahon AP, Rowitch DH, Ligon KL. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14(2):123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See WL, Tan IL, Mukherjee J, Nicolaides T, Pieper RO. Sensitivity of glioblastomas to clinically available MEK inhibitors is defined by neurofibromin 1 deficiency. Cancer Res. 2012;72(13):3350–3359. doi: 10.1158/0008-5472.CAN-12-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif S, Ferner R, Birch JM, Gillespie JE, Gattamaneni HR, Baser ME, Evans DG. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006;24(16):2570–2575. doi: 10.1200/JCO.2005.03.8349. [DOI] [PubMed] [Google Scholar]
- Sharif S, Upadhyaya M, Ferner R, Majounie E, Shenton A, Baser M, Thakker N, Evans DG. A molecular analysis of individuals with neurofibromatosis type 1 (NF1) and optic pathway gliomas (OPGs), and an assessment of genotype-phenotype correlations. J Med Genet. 2011;48(4):256–260. doi: 10.1136/jmg.2010.081760. [DOI] [PubMed] [Google Scholar]
- Shofty B, Ben-Sira L, Freedman S, Yalon M, Dvir R, Weintraub M, Toledano H, Constantini S, Kesler A. Visual outcome following chemotherapy for progressive optic pathway gliomas. Pediatr Blood Cancer. 2011;57(3):481–485. doi: 10.1002/pbc.22967. [DOI] [PubMed] [Google Scholar]
- Simanshu DK, Nissley DV, McCormick F. RAS Proteins and Their Regulators in Human Disease. Cell. 2017;170(1):17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons GW, Pong WW, Emnett RJ, White CR, Gianino SM, Rodriguez FJ, Gutmann DH. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J Neuropathol Exp Neurol. 2011;70(1):51–62. doi: 10.1097/NEN.0b013e3182032d37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solga AC, Pong WW, Kim KY, Cimino PJ, Toonen JA, Walker J, Wylie T, Magrini V, Griffith M, Griffith OL, Ly A, Ellisman MH, Mardis ER, Gutmann DH. RNA Sequencing of Tumor-Associated Microglia Reveals Ccl5 as a Stromal Chemokine Critical for Neurofibromatosis-1 Glioma Growth. Neoplasia. 2015;17(10):776–788. doi: 10.1016/j.neo.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solga AC, Toonen JA, Pan Y, Cimino PJ, Ma Y, Castillon GA, Gianino SM, Ellisman MH, Lee DY, Gutmann DH. The cell of origin dictates the temporal course of neurofibromatosis-1 (Nf1) low-grade glioma formation. Oncotarget. 2017;8(29):47206–47215. doi: 10.18632/oncotarget.17589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J, Jakobiec FA, Housepian EM. The architecture of optic nerve gliomas with and without neurofibromatosis. Arch Ophthalmol. 1980;98(3):505–511. doi: 10.1001/archopht.1980.01020030501014. [DOI] [PubMed] [Google Scholar]
- Toonen JA, Anastasaki C, Smithson LJ, Gianino SM, Li K, Kesterson RA, Gutmann DH. NF1 germline mutation differentially dictates optic glioma formation and growth in neurofibromatosis-1. Hum Mol Genet. 2016;25(9):1703–1713. doi: 10.1093/hmg/ddw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toonen JA, Ma Y, Gutmann DH. Defining the temporal course of murine neurofibromatosis-1 optic gliomagenesis reveals a therapeutic window to attenuate retinal dysfunction. Neuro Oncol. 2017a;19(6):808–819. doi: 10.1093/neuonc/now267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toonen JA, Solga AC, Ma Y, Gutmann DH. Estrogen activation of microglia underlies the sexually dimorphic differences in Nf1 optic glioma-induced retinal pathology. J Exp Med. 2017b;214(1):17–25. doi: 10.1084/jem.20160447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya M, Huson SM, Davies M, Thomas N, Chuzhanova N, Giovannini S, Evans DG, Howard E, Kerr B, Griffiths S, Consoli C, Side L, Adams D, Pierpont M, Hachen R, Barnicoat A, Li H, Wallace P, Van Biervliet JP, Stevenson D, Viskochil D, Baralle D, Haan E, Riccardi V, Turnpenny P, Lazaro C, Messiaen L. An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970–2972 delAAT): evidence of a clinically significant NF1 genotype-phenotype correlation. Am J Hum Genet. 2007;80(1):140–151. doi: 10.1086/510781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS, Woo PJ, Malenka RC, Vogel H, Bredel M, Mallick P, Monje M. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell. 2015;161(4):803–816. doi: 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan P, Wang Y, Nguyen T, Huang A, Muller KJ, Goldberg JL. Transplanted neurons integrate into adult retinas and respond to light. Nat Commun. 2016;7:10472. doi: 10.1038/ncomms10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 2003;63(15):1549–1563. doi: 10.2165/00003495-200363150-00003. [DOI] [PubMed] [Google Scholar]
- Viskochil D, Buchberg AM, Xu G, Cawthon RM, Stevens J, Wolff RK, Culver M, Carey JC, Copeland NG, Jenkins NA, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62(1):187–192. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- Vogel F. Genetics of retinoblastoma. Hum Genet. 1979;52(1):1–54. doi: 10.1007/BF00284597. [DOI] [PubMed] [Google Scholar]
- Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM, Fountain JW, Brereton A, Nicholson J, Mitchell AL, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249(4965):181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- Warrington NM, Gianino SM, Jackson E, Goldhoff P, Garbow JR, Piwnica-Worms D, Gutmann DH, Rubin JB. Cyclic AMP suppression is sufficient to induce gliomagenesis in a mouse model of neurofibromatosis-1. Cancer Res. 2010;70(14):5717–5727. doi: 10.1158/0008-5472.CAN-09-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington NM, Woerner BM, Daginakatte GC, Dasgupta B, Perry A, Gutmann DH, Rubin JB. Spatiotemporal differences in CXCL12 expression and cyclic AMP underlie the unique pattern of optic glioma growth in neurofibromatosis type 1. Cancer Res. 2007;67(18):8588–8595. doi: 10.1158/0008-5472.CAN-06-2220. [DOI] [PubMed] [Google Scholar]
- Wilson AM, Di Polo A. Gene therapy for retinal ganglion cell neuroprotection in glaucoma. Gene Ther. 2012;19(2):127–136. doi: 10.1038/gt.2011.142. [DOI] [PubMed] [Google Scholar]
- Xu GF, Lin B, Tanaka K, Dunn D, Wood D, Gesteland R, White R, Weiss R, Tamanoi F. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell. 1990a;63(4):835–841. doi: 10.1016/0092-8674(90)90149-9. [DOI] [PubMed] [Google Scholar]
- Xu GF, O’Connell P, Viskochil D, Cawthon R, Robertson M, Culver M, Dunn D, Stevens J, Gesteland R, White R, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990b;62(3):599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- Yalon M, Rood B, MacDonald TJ, McCowage G, Kane R, Constantini S, Packer RJ. A feasibility and efficacy study of rapamycin and erlotinib for recurrent pediatric low-grade glioma (LGG) Pediatr Blood Cancer. 2013;60(1):71–76. doi: 10.1002/pbc.24142. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, Schuller U, Machold R, Fishell G, Rowitch DH, Wainwright BJ, Wechsler-Reya RJ. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008;14(2):135–145. doi: 10.1016/j.ccr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457(7229):603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Harada T, Liu L, Lush ME, Guignard F, Harada C, Burns DK, Bajenaru ML, Gutmann DH, Parada LF. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development. 2005;132(24):5577–5588. doi: 10.1242/dev.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15(7):859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann M, Hovestadt V, Knobbe-Thomsen CB, Zapatka M, Northcott PA, Schramm K, Belic J, Jones DT, Tschida B, Moriarity B, Largaespada D, Roussel MF, Korshunov A, Reifenberger G, Pfister SM, Lichter P, Kawauchi D, Gronych J. Somatic CRISPR/Cas9-mediated tumour suppressor disruption enables versatile brain tumour modelling. Nat Commun. 2015;6:7391. doi: 10.1038/ncomms8391. [DOI] [PMC free article] [PubMed] [Google Scholar]