Abstract
Purpose of review
Myocarditis is an inflammatory disease of the cardiac muscle mainly caused by viral infection. Due to the diverse clinical presentation of myocarditis, accurate diagnosis demands simultaneous histologic, immunohistochemical and molecular biological workup of endomyocardial biopsies (EMBs) as defined by the position statement of the Working Group on Myocardial and Pericardial Diseases of the European Society of Cardiology on myocarditis.
Recent findings
Endomyocardial biopsy-based analysis of viral transcriptional activity, mRNA expression, epigenetics and region-specific protein expression analysis via imaging mass spectrometry have led to the identification of novel potential diagnostic criteria, markers with prognostic value and therapeutic targets for the treatment of viral myocarditis, opening new avenues for novel therapies, including cell therapies, as well as the use of established treatment options, be it from other indications.
Summary
Under certain clinical scenarios EMB-based analysis is required to come to a tailored individualized therapy that improves symptoms and prognosis of patients with acute and chronic viral-driven cardiac inflammation.
Keywords: coxsackievirus, endomyocardial biopsy, inflammation, parvovirus, viral myocarditis
INTRODUCTION
Myocarditis is an inflammatory disease of the cardiac muscle tissue caused by myocardial infiltration of immunocompetent cells following any kind of cardiac injury. Infectious causes include a vast number of viruses, bacteria, protozoa or fungi, but most frequently the myocardial inflammatory process is directed against viral pathogens. During the past decades, a shift is observed from adenoviruses and enteroviruses, including coxsackievirus B3 (CVB3) to parvovirus B19 (B19V) and human herpes virus 6 (HHV6) as the most frequently found cardiotropic viruses in endomyocardial biopsies (EMBs) [1–3]. Myocarditis leads to cardiac dysfunction and can progress to dilated cardiomyopathy (DCM). Patients with DCM have only a 5-year survival rate of 55% under current heart failure treatment, indicating the need for target-specific strategies [4]. A plethora of reasons speaks for the difficulty to diagnose and treat viral myocarditis: the diversity of the clinical presentation of myocarditis, the heterogeneity of the underlying cause, the difference in virus-specific target cell and pathogenesis, and the different disease stages following infection, indicating the need to combine clinical evaluation with in depth analysis of EMB, allowing characterization of the specific virus, and the amount and type of infiltrated immune cells [5]. The present review briefly outlines the relevance of the EMB for the diagnosis of viral myocarditis. In this context, the use of imaging techniques as diagnostic tool for viral myocarditis is critically discussed. Furthermore, the review summarizes how ‘nonstandard’ EMB-based analysis [viral transcriptional activity, mRNA expression, epigenetics, and region-specific protein expression analysis via imaging mass spectrometry (MS)] has led and may further lead to the detection of novel potential diagnostic, prognostic markers and therapeutic targets for the treatment of viral myocarditis. Novel treatments options are discussed including established therapeutic interventions, which are currently used for other indications, and cell therapies including mesenchymal stromal cells (MSCs) [6,7] and the EMB-based cell product: CardAP cells [8–10].
Endomyocardial biopsy as diagnostic tool
Despite well known limitations giving rise to false-negative results (sampling error) if only low numbers (<8–10 samples) are taken, EMB is a safe diagnostic tool and up to date the gold standard for the diagnosis of (viral) myocarditis, as via histology, immunohistochemistry and viral diagnostics, it allows the quantification and identification of immune cell infiltrates, the proof of viral RNA and DNA presence, quantification of viral loads and confirmation of virus subtypes via sequencing [11–16] via imaging MS on EMB, region-dependent analysis of protein regulation is possible, which enables the differentiation between patients cohorts as already shown for the discrimination between patients with or without cardiac inflammation [5].
The landmark study from Kuhl et al.[1] revealed that genomes from cardiotropic viruses can be found in EMBs of 75% of patients with suspected myocarditis. B19V and HHV6 are detected in about 70% and 14–18% of EMB of patients with persisting symptoms of unexplained heart failure, respectively [1]. Furthermore, a chromosomally integrated form of HHV6 infection (ciHHV6) is detected in 0.8% of patients with myocarditis or DCM [17], whereas adenoviruses/enteroviruses are found in about 10% of the cases and associated with a reduced outcome if no spontaneous viral clearance occurs [18]. The prognosis of HHV6 and B19V persistence seems to be better or even not significantly impaired compared with this of adenoviral/enteroviral presence and mainly depends on the degree of the inflammatory response [19]. For the single-stranded DNA virus, B19V, the analysis of DNA copy number and VP1/VP2 RNA expression, representing transcriptional activity, is discussed to be important. The additional measurement of VP1/VP2 RNA expression seems to be required as B19V DNA can also be found in the heart of healthy patients [20,21] and in many other nonerythroid tissues including the liver, lung, skin and brain [22]. Therefore, the etiological role of B19V in the development of myocarditis and DCM still remains unclear, and it is considered that B19V might rather be a bystander than a cause of myocarditis. Only high copy numbers of B19V DNA are currently thought to be myocarditis-related [23], but these are rarely found in EMBs. Recent findings from a collective of 415 consecutive cardiac B19V-positive patients with clinically suspected cardiomyopathy indicate that a subgroup of myocarditis patients characterized by transcriptionally active cardiotropic B19V have an altered cardiac gene expression compared with control patients and myocarditis patients with latent B19V. Imaging MS from a single-patient use study illustrated a different protein muster between EMB of B19V mRNA-positive and B19V mRNA-negative patients (Fig. 1), further supporting the hypothesis that probably mRNA B19V is related to myocarditis [15].
FIGURE 1.
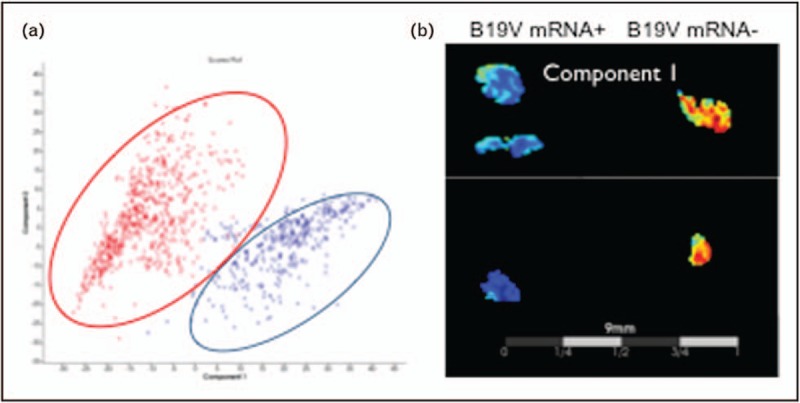
Imaging mass spectrometry enables the differentiation between parvovirus B19 mRNA-positive and mRNA-negative patients. In-situ tissue typing of endomyocardial biopsies in parvovirus B19-positive cardiomyopathy patients with low cardiac inflammation before (mRNA+) and after telbivudine treatment (mRNA−) via imaging mass spectrometry. (a) Principal component analysis distinguishes the protein signatures of the parvovirus B19 mRNA+ (red) and parvovirus B19 mRNA− patient group (blue). (b) Principal component analysis component I illustrates an increased intensity in distribution in parvovirus B19 mRNA+ versus parvovirus B19 mRNA− patients.
In addition to the extent of the viral load [24,25] and transcriptional activity of B19V [15], other studies further suggest that the relevance of B19V in myocarditis and DCM depends on the presence of other cardiotropic viruses [24]. For all these measurements, EMB-based analysis is required.
The screening of microRNA (miRNA) in EMB may further help to diagnose viral myocarditis. Twenty-nine differentially regulated miRNAs were detected between patients with latent and reactivated B19V infection [26]. Furthermore, transcriptome mapping of CVB3 cardiomyopathy patients revealed distinctive cardiac miRNA patterns associated with spontaneous virus clearance and recovery versus virus persistence and progressive clinical deterioration, indicating the prognostic value of cardiac miRNA profiling to assess the risk of virus persistence and progressive clinical deterioration in CVB3 cardiomyopathy [27]. miRNAs reflecting cardiomyocyte injury, including miRNA-208 and miRNA-499, are increased in the blood of acute myocarditis [28]. However, these circulating markers are also upregulated in hypertensive cardiac disease and myocardial infarction (MI), indicating their unspecificity and inability to be used as diagnostic marker for (viral) myocarditis.
In general, the EMB cannot be replaced by viral serology, which often can lead to a false positive diagnosis. However, blood diagnostics allow to discriminate an acute viral infection from endogenous B19V or HHV6 reactivation, especially in cases with high virus loads, as occasionally detected in patients with HHV6 reactivation [29].
The measurement of laboratory parameters [high-sensitivity troponin T, N-terminal B-type natriuretic peptide, C-reactive protein (CRP)] alone are not sufficient for risk assessment in myocarditis. With the knowledge that persistent late gadolinium enhancement (LGE) is a risk marker of myocarditis [30], Berg et al.[31] recently conducted a study with the aim to evaluate whether routine laboratory parameters at diagnosis predict the dynamic of LGE by cardiac MRI. They found that cardiac enzymes and inflammatory parameters did not sufficiently reflect LGE in myocarditis and that by part of the patients with normalizing laboratory parameters, the LGE worsened. These findings stress that laboratory levels are too few for risk assessment in myocarditis and indicate that MRI might add value here too. Recently, LGE was found to be a risk marker even in patients with myocarditis and preserved ejection fraction [32,33].
Although imaging techniques including MRI can provide noninvasive tissue characterization and may localize large areas of inflammation, local and diffuse fibrosis, they cannot replace the EMB for the diagnosis of myocarditis due to the still high negative predictive value in acute as well as chronic settings [34], their inability to quantify inflammatory cell numbers and characterize the infiltrated immune cell subtypes and their incapacity to detect and quantify different virus types and loads [35]. In fact, a recent direct comparison of the EMB versus cardiac magnetic resonance in the MyoRacer-Trial [34] illustrated that in patients with acute symptoms, mapping techniques were a useful tool for confirming the diagnosis of myocarditis and superior to the Lake Louis Criteria, whereas T2-mapping had only acceptable diagnostic performance in patients with chronic symptoms, further supporting the EMB as gold standard for the diagnosis of viral myocarditis. Nevertheless, the indication to perform EMB remains controversial due to its invasiveness and false-negative results, especially in cases of focal pathological substrates [36]. Three-dimensional electroanatomical voltage mapping (EVM; e.g. CARTO system; Biosense Webster, Inc., Diamond Bar, CA) offers the potential to identify low-voltage areas that correspond to regions with structural and functional changes [37]. Cardiac MRI including parametric-mapping can noninvasively characterize areas of inflammation, diffuse and focal fibrosis and wall motion abnormalities, identifying areas different from EVM [34]. EVM-guidance or MRI-guidance could therefore increase the sensitivity and specificity of the conventional EMB approach by reducing sampling errors and allowing a deeper insight of different (local) pathologies [38].
Box 1.
no caption available
Endomyocardial biopsy for treatment decisions
The major benefit to perform EMBs in patients with suspected viral myocarditis and cardiogenic shock (class I; C recommendation) or no recovery of cardiac function over 3 months despite conservative therapy (class 2A; C recommendation) is to exclude a severe cardiac viral persistence [39]. This cannot be done by MRI. Exclusion of viral persistence allows the use of immunosuppressive therapy, known to improve cardiac function [40,41]. Immunosuppression is contraindicated in enterovirus and adenovirus-positive patients. These patients might profit from antiviral IFN-ß, which induces viral clearance [42,43]. With respect to HHV6, only HHV6 symptomatic patients with reactivated viruses may need antiviral treatment with, for example, ganciclovir. Such treatments may improve cardiac complaints and heart failure by suppressing transcriptional virus activities, but in most cases, they do not clear the virus from the myocardium [17]. In comparison with B19V infections, latent HHV6 infections are less frequently associated with an inflammatory process of the myocardium [44]. If inflammation is severe, experienced centres also use anti-inflammatory drugs in HHV6-positive patients, as the virus can anyway not be cleared, even by antiviral medications, and ongoing inflammation is the most important driver for a worse outcome [19]. A similar discussion started with respect to B19V DNA-positive patients, suggesting that B19V DNA presence represents a more innocent bystander finding. However, antiviral therapies in myocarditis patients are not established yet and should only be offered by experienced centres and/or on the basis of studies.
Endomyocardial biopsy for the search of therapeutical targets
Screening of EMBs from patients with acute myocarditis, dilated inflammatory cardiomyopathy and DCM showed that the expression of nucleotide-binding oligomerization domain-containing protein 2 (NOD2), a pattern recognition receptor which recognizes single-stranded RNA-like CVB3 [45], was upregulated in EMBs of cardiac CVB3-positive versus CVB3-negative acute myocarditis, dilated inflammatory cardiomyopathy, DCM and control patients. Furthermore, NOD2 expression was increased in CVB3-positive patients but not in patients with a persistence of other cardiotropic viruses like the double-stranded DNA virus HHV6 or the single-stranded DNA virus B19V [46], suggesting that coinfection with HHV6 or B19V found in some CVB3-positive patients was irrelevant for NOD2 signalling. The regulation of NOD2 expression was independent of the grade and modus of inflammation or cardiac function/remodeling. The importance of NOD2 in CVB3-induced myocarditis was in addition deducted from NOD2−/− mice, which were protected from the detrimental effects of CVB3 [47▪▪]. The potential clinical relevance of NOD2 further followed from the consistent pairwise drop of NOD2 mRNA expression between time point 1 and 2 in CVB3-positive patients who eliminated CVB3 and improved cardiac function over time, an effect which was only found with respect to IL-1ß in four out of six patients. Further studies have to show whether the analysis of NOD2 in EMB could be useful as an additional differential diagnostic marker for CVB3-induced myocarditis under clinical conditions.
Evaluation of EMBs from CVB3-positive versus CVB3-negative patients further showed that the danger associated molecular patterns (DAMPs)/alarmins S100A8 and S100A9 are higher expressed in CVB3-positive versus CVB3-negative patients. In addition, S100A8 and S100A9 expression dropped in CVB3-positive patients, who eliminated the CVB3 virus over time, which was associated with an improved clinical course [48▪▪]. The pathophysiological role of the DAMPs S100A8 and S100A9 in cardiac CVB3-induced myocarditis further followed from the observations that S100A9−/− mice exhibited an improved left ventricular (LV) function, associated with less cardiac infiltrates of neutrophils and monocytes, a reduced LV oxidative stress and CVB3 copy number compared with WT CVB3 mice. In contrast, intraperitoneal application of S100A8 in S100A9−/− CVB3 mice induced the CVB3 copy number and cardiac inflammation versus S100A9−/− CVB3 mice and resulted in an impaired cardiac function resembling the wild-type phenotype again [48▪▪]. The abovementioned data together with the existence of specific anti-S100A8/S100A9 compounds [49] indicate that these alarmins may represent a new potential avenue for the treatment of (CVB3)-induced myocarditis, and that S100A8 and S100A9 might be diagnostic biomarkers [50]. A previous study in experimental autoimmune myocarditis demonstrated that administration of S100A8/S100A9 was protective [51], emphasising that the fate of S100A8/S100A9, depends on the disease and/or the stage of the disease, further stressing the need for personalized and EMB-guided evaluation.
Novel treatment options
Based on the abovementioned findings from EMB indicating the relevance of S100A8 and S100A9 [48▪▪] and NOD2 [47▪▪] in CVB3 myocarditis, the finding that S100A8 and S100A9 activate the Nod-like receptor (NLR) Family Pyrin Domain Containing 3 (NLRP3) inflammasome [52] and that NOD2 downstream signalling [45] involves the NLRP3 inflammasome and IL-1ß, the S100A9 inhibitor paquinimod and several drugs counteracting the NLRP3 inflammasome and/or IL-1ß signalling are potential promising therapeutics for the treatment of CVB3 myocarditis. The relevance of the NLRP3 inflammasome also beyond CVB3 myocarditis [53] indicates the potential of NLRP3-targeting therapies for myocarditis with other (viral) causes. Nevertheless, the EMB data [47▪▪] and the inverse correlation between IL-1ß and the antiviral IFN-ß [45] collaborate that particularly CVB3 myocarditis patients may profit from these pharmaca as antiviral strategy. Indeed, with respect to IFN-ß, it has been shown that solely patients with CVB3 profit from IFN-ß therapy [42] and not B19V patients, by which IFN-ß is associated with B19V DNA persistence [42,54]. However, it should be noted that IFN-ß was only evaluated in patients with B19V DNA and not with transcriptional active B19V. Therefore, the efficacy of IFN-ß in patients with transcriptional active B19V cannot be excluded.
Paquinimod
Paquinimod, an immunomodulatory compound preventing S100A9 binding to Toll-like receptor-4 and receptor for advanced glycation end products [55], has been shown to improve experimental osteoarthritis [56], systemic sclerosis [57] and atherogenesis in diabetes [58]. Its potential to treat viral myocarditis has not been evaluated so far.
Colchicine
Colchicine is an anti-inflammatory agent, traditionally used to treat gout [59]. Its main working mechanisms are the inhibition of neutrophil chemotaxis, adhesion and mobilization, the reduction in superoxide production and the inhibition of NLRP3 inflammasome activity and IL-1β production. In addition, colchicine has antifibrotic and endothelial-protective features [60]. Colchicine has recently been shown to be successful for the treatment of different inflammatory cardiac disorders, including stable coronary artery disease [61] and postpericarditomy syndrome [62]. The latter was successfully investigated in a placebo-controlled study. Most importantly, idiopathic (viral) pericarditis, triggered in 80% by typical cardiotropic viruses [63], inducing thereby often a peri/myocarditic response, was successfully treated with colchicine [64–66]. Colchicine is now a new European Society of Cardiology (ESC) recommended treatment option for different scenarios of pericarditis [67]. Therefore, colchicine is most likely effective in inflammatory cardiomyopathy too, as in the most cases, inflammatory processes as well as the role of cardiotropic viruses do not differ between pericarditis and myocarditis [63]. Indeed, a recent case report without controls indicated the efficacy of colchicine to treat patients with suggested myocarditis [68].
Anakinra
The relevance of the NLRP3 inflammasome in CVB3 myocarditis [69], its association with a worse prognosis on the long term [53] and the fact that IL-1ß is proteolytically activated upon NLRP3 activity [45,70] make the IL-1 receptor antagonist Anakinra an attractive candidate to treat viral myocarditis. Patients with fulminant myocarditis were successfully treated with Anakinra [71,72]. However, the potential of Anakinra to treat viral myocarditis has not been tested in clinical studies yet.
Canakinumab
Anti-inflammatory therapy targeting the IL-1β pathway with the fully human monoclonal antibody against IL-1β canakinumab led to a significantly lower rate of recurrent cardiovascular events than placebo, independent of lipid-level lowering in patients with previous MI and high high-sensitivity CRP levels (≥2 mg/l) [73]. This successful effect of Canakinumab in preselected high-risk patients with high CRP levels supports the need to differentiate patient cohorts and to come to individualized therapies and collaborate its use for myocarditis patients with high CRP levels.
Mesenchymal stromal cells
MSCs are well known for their cardioprotective [74] and immunomodulatory properties [75]. In experimental settings of CVB3, we previously demonstrated that MSC also exert antiviral effects. Upon coculture with CVB3-infected HL-1 cells, MSC reduced the CVB3-induced apoptosis and consequent viral progeny release [6]. Furthermore, MSC decreased CVB3-induced CD4-T-cell and CD8-T-cell activation upon coculture with carboxyfluorescein succinimidyl ester-labelled mononuclear cells. MSC exerted these antiviral and immunomodulatory effects in a nitric-oxide-dependent manner and required priming via IFN-γ. In vivo, intravenous MSC application improved the contractility and relaxation parameters in CVB3-induced myocarditis, which was paralleled with a reduction in cardiac apoptosis, cardiomyocyte damage, cardiac mononuclear cell activation [6], and cardiac fibrosis, and a moderate, but NS drop in CVB3 load [7]. In depth evaluation of the immunomodulatory properties of MSC under CVB3 conditions further revealed that heart and blood proinflammatory Ly6Chigh and Ly6Cmiddle monocytes were reduced in CVB3 MSC versus CVB3 mice, whereas anti-inflammatory Ly6Clow monocytes increased in the blood, heart and spleen of MSC-treated CVB3 versus untreated CVB3 mice. In frame with the MSC-mediated modulation in monocyte migration towards the heart in CVB3 mice, LV expression of the chemokines CCL2/MCP-1 and MCP-2 attracting proinflammatory cells was reduced in CVB3 MSC mice, whereas LV stromal cell-derived factor-1α mRNA expression and systemic levels of fractalkine, known to attract anti-inflammatory cells [76], were increased in CVB3 MSC mice [77▪]. In a separate study, we additionally demonstrated that MSC limit cardiac and systemic NLRP3 inflammasome activation in CVB3 mice [78].
Regulatory T cells
Regulatory T cells (Tregs), a subpopulation of CD4+ cells, constituting 5–10% of the peripheral T cells, and proclaimed as masters and regulators of the immune response [79], play a pivotal role in the induction and maintenance of immune homeostasis and tolerance in the setting of viral myocarditis [80]. Studies in myocarditis [81] and DCM [82] have shown that Tregs are quantitatively and/or qualitatively impaired under these conditions and consequently ineffective to balance the immune system. Therefore, direct Tregs application might be an attractive strategy to treat myocarditis, a hypothesis which is supported by experimental studies showing that prophylactic [81,83] and therapeutic [84] adoptive transfer of Tregs improves CVB3 myocarditis, and a strategy, which is feasible thanks to new technologies [85].
Telbivudine
Telbivudine is an antiviral nucleoside analogue reverse transcriptase inhibitor, which is especially effective for retroviral and pararetroviral (hepatitis B viruses) infections and has pleiotropic immunomodulatory/anti-inflammatory properties [86–89]. The single-stranded B19V DNA genome replicates through a specific rolling-hairpin-mechanism to generate a double-stranded DNA molecule mimicking DNA-synthesis during the reverse transcription process comparable with retroviruses and hepatitis B viruses [90]. As telbivudine preferentially inhibits the DNA-dependent single-stranded DNA synthesis, it is theoretically able to interfere with the unique replication mode of B19V, too. In a single-patient use, we could show an improvement of clinical symptoms, clearance of mRNA levels and changes in cardiac protein pattern after treatment with telbivudine in two B19V mRNA-positive patients with low cardiac inflammation (Fig. 1). This specific antiviral working mechanism of telbivudine together with its anti-inflammatory effects [86–89] have been the rationale to evaluate the efficacy of telbivudine in myocarditis associated with B19V transcriptional activity: the PreTopic Study (EudraCT-Number: 2016-004825-17).
Endomyocardial biopsy as cell source
Significantly, EMBs can be used for the generation of so called Cardiac-derived Adherent Proliferating cells, CardAP cells [8]. Similar to MSC, CardAP have immunomodulatory [91], antiviral [9] and cardioprotective properties [10] and are able to reduce CVB3 viral progeny release in a nitric-oxide-dependent and IL-10-dependent manner. They also require IFN-γ to exert their antiviral effects. Intravenous application of CardAP cells in CVB3 mice led to an improvement in LV function, which was associated with a decrease in cardiac apoptosis, cardiac mononuclear cell activity, an increase in Tregs and T-cell apoptosis, and importantly, a reduction in cardiac CVB3 viral load [9]. In-vitro stimulation of CardAP cells with antiviral IFN-ß leads to the secretion of higher levels of antiviral IL-10 (Fig. 2), hereby further boosting their antiviral potential.
FIGURE 2.
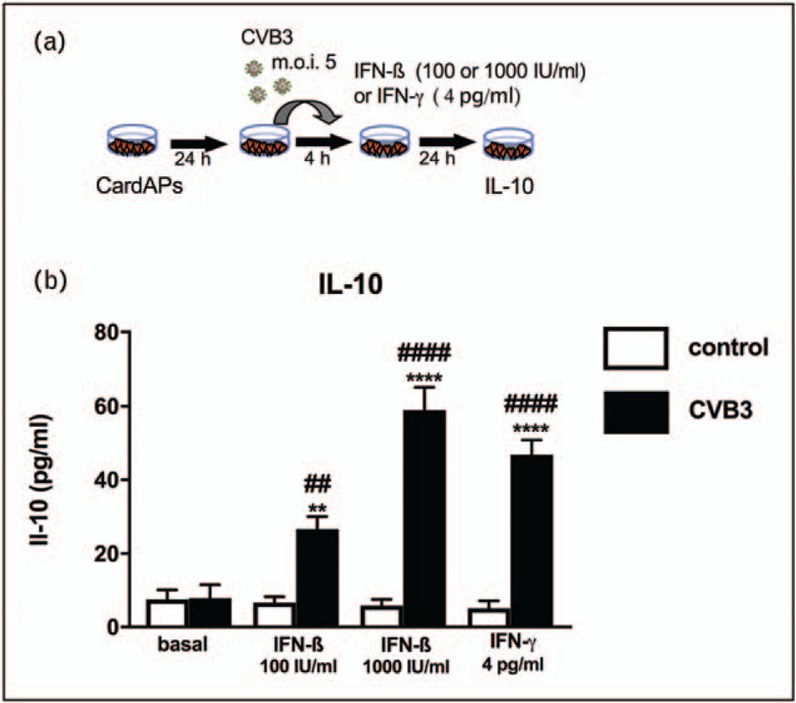
Impact of IFN-ß and IFN-γ on antiviral IL-10 expression of coxsackievirus B3-infected CardAPs. (a) Experimental design illustrating how CardAPs 24 h after plating were infected with coxsackievirus B3 at a multiplication of infection of 5 and 4 h after infection supplemented with/out 100 or 1000 IU/ml of IFN-ß or 4 pg/ml of IFN-γ. Twenty-four hours later, supernatant was collected for subsequent IL-10 analysis via ELISA. (b) Bar graphs represent the mean ± SEM of IL-10 in the supernatant of control CardAPs (open bars) or coxsackievirus B3-infected CardAPs (closed bars) supplemented with/out IFN-ß or IFN-γ, as indicated, with n = 4–6/group and ∗∗P < 0.01 and ∗∗∗∗P < 0.0001 versus respective control group and ##P < 0.01 and ####P < 0.0001 versus the basal coxsackievirus B3 group.
CONCLUSION
Viral myocarditis remains a major challenge in modern cardiology and underscores the need to explore innovative therapeutic options, which allow a sufficient antiviral defence with a balanced immune response preventing hyperactive inflammatory toxicity. The most recent position paper of the ESC working group on myocardial and pericardial disease stresses the need to search for novel biomarkers to improve diagnosis, prognosis and therapy of (viral) myocarditis [13]. EMB-based histological, immunohistological and molecular biological informations are prerequisites to establish an accurate diagnosis of viral myocarditis and successful management of patients and cannot be substituted by any noninvasive clinical analysis. Detailed EMB-based analysis has led to the identification of novel diagnostic, prognostic markers and therapeutics targets (Fig. 3), allowing differentiation of patients with viral myocarditis in smaller cohorts and mechanistically based individualized interventions.
FIGURE 3.
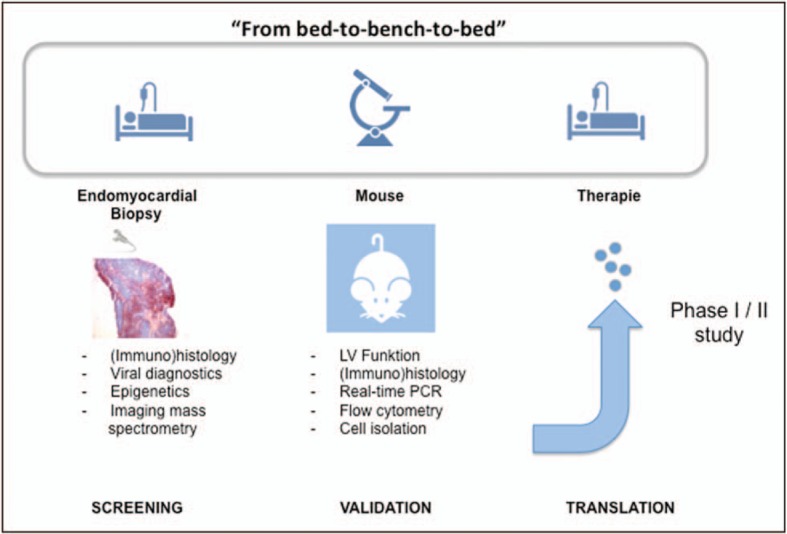
Bed-to-bench-to-bed strategy. Analysis of endomyocardial biopsies allows the identification of potential novel biomarkers and therapeutical targets, which are next validated in experimental mouse models. The efficacy of novel drugs will subsequently be tested in clinical Phase I/II trials (translation).
Acknowledgements
We thank Oliver Klein from the BCRT Cardioproteomics lab for the imaging mass spectrometry data. This study was supported by the Berlin-Brandenburg Center for Regenerative Therapies – BCRT (Bundesministerium für Bildung und Forschung – 0313911) to C.T., by the DZHK to S.V.L. and C.T. and by the Friede Springer Herz Stiftung and Dr Marija Orlovic Stiftung to S.V.L.
The novel treatment options are all products, which are not labelled for the use under discussion and still investigational.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Kuhl U, Pauschinger M, Noutsias M, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with ‘idiopathic’ left ventricular dysfunction. Circulation 2005; 111:887–893. [DOI] [PubMed] [Google Scholar]
- 2.Kuhl U, Pauschinger M, Seeberg B, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 2005; 112:1965–1970. [DOI] [PubMed] [Google Scholar]
- 3.Verdonschot J, Hazebroek M, Merken J, et al. Relevance of cardiac parvovirus B19 in myocarditis and dilated cardiomyopathy: review of the literature. Eur J Heart Fail 2016; 18:1430–1441. [DOI] [PubMed] [Google Scholar]
- 4.Van Linthout S, Tschope C, Schultheiss HP. Lack in treatment options for virus-induced inflammatory cardiomyopathy: can iPS-derived cardiomyocytes close the gap? Circ Res 2014; 115:540–541. [DOI] [PubMed] [Google Scholar]
- 5.Van Linthout S, Tschope C. Lost in markers? Time for phenomics and phenomapping in dilated cardiomyopathy. Eur J Heart Fail 2017; 19:499–501. [DOI] [PubMed] [Google Scholar]
- 6.Van Linthout S, Savvatis K, Miteva K, et al. Mesenchymal stem cells improve murine acute coxsackievirus B3-induced myocarditis. Eur Heart J 2011; 32:2168–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savvatis K, van Linthout S, Miteva K, et al. Mesenchymal stromal cells but not cardiac fibroblasts exert beneficial systemic immunomodulatory effects in experimental myocarditis. PLoS One 2012; 7:e41047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haag M, Van Linthout S, Schroder SE, et al. Endomyocardial biopsy derived adherent proliferating cells – a potential cell source for cardiac tissue engineering. J Cell Biochem 2010; 109:564–575. [DOI] [PubMed] [Google Scholar]
- 9.Miteva K, Haag M, Peng J, et al. Human cardiac-derived adherent proliferating cells reduce murine acute Coxsackievirus B3-induced myocarditis. PLOS One 2011; 6:e28513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miteva K, Van Linthout S, Pappritz K, et al. Human endomyocardial biopsy specimen-derived stromal cells modulate angiotensin II-induced cardiac remodeling. Stem Cells Transl Med 2016; 5:1707–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzmann M, Nicko A, Kuhl U, et al. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation 2008; 118:1722–1728. [DOI] [PubMed] [Google Scholar]
- 12.Chimenti C, Frustaci A. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies: a retrospective study over a 28-year period. Circulation 2013; 128:1531–1541. [DOI] [PubMed] [Google Scholar]
- 13.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34:2636–2648. 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 14.Kuhl U, Schultheiss HP. Myocarditis in children. Heart Fail Clin 2010; 6:483–496. viii–ix. [DOI] [PubMed] [Google Scholar]
- 15.Kuhl U, Lassner D, Dorner A, et al. A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression. Basic Res Cardiol 2013; 108:372. [DOI] [PubMed] [Google Scholar]
- 16.Tschope C, Kherad B, Schultheiss HP. How to perform an endomyocardial biopsy? Turk Kardiyol Dern Ars 2015; 43:572–575. [DOI] [PubMed] [Google Scholar]
- 17.Kuhl U, Lassner D, Wallaschek N, et al. Chromosomally integrated human herpesvirus 6 in heart failure: prevalence and treatment. Eur J Heart Fail 2015; 17:9–19. [DOI] [PubMed] [Google Scholar]
- 18.Kuhl U, Pauschinger M, Schwimmbeck PL, et al. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation 2003; 107:2793–2798. [DOI] [PubMed] [Google Scholar]
- 19.Kindermann I, Kindermann M, Kandolf R, et al. Predictors of outcome in patients with suspected myocarditis. Circulation 2008; 118:639–648. [DOI] [PubMed] [Google Scholar]
- 20.Schenk T, Enders M, Pollak S, et al. High prevalence of human parvovirus B19 DNA in myocardial autopsy samples from subjects without myocarditis or dilative cardiomyopathy. J Clin Microbiol 2009; 47:106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lotze U, Egerer R, Gluck B, et al. Low level myocardial parvovirus B19 persistence is a frequent finding in patients with heart disease but unrelated to ongoing myocardial injury. J Med Virol 2010; 82:1449–1457. [DOI] [PubMed] [Google Scholar]
- 22.Adamson-Small LA, Ignatovich IV, Laemmerhirt MG, Hobbs JA. Persistent parvovirus B19 infection in nonerythroid tissues: possible role in the inflammatory and disease process. Virus Res 2014; 190:8–16. [DOI] [PubMed] [Google Scholar]
- 23.Bock CT, Klingel K, Kandolf R. Human parvovirus B19-associated myocarditis. N Engl J Med 2010; 362:1248–1249. [DOI] [PubMed] [Google Scholar]
- 24.Bock CT, Duchting A, Utta F, et al. Molecular phenotypes of human parvovirus B19 in patients with myocarditis. World J Cardiol 2014; 6:183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennert R, van Paassen P, Wolffs P, et al. Differences in virus prevalence and load in the hearts of patients with idiopathic dilated cardiomyopathy with and without immune-mediated inflammatory diseases. Clin Vaccine Immunol 2012; 19:1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhl U, Rohde M, Lassner D, et al. miRNA as activity markers in Parvo B19 associated heart disease. Herz 2012; 37:637–643. [DOI] [PubMed] [Google Scholar]
- 27.Kuehl U, Lassner D, Gast M, et al. Differential cardiac microRNA expression predicts the clinical course in human enterovirus cardiomyopathy. Circ Heart Fail 2015; 8:605–618. [DOI] [PubMed] [Google Scholar]
- 28.Corsten MF, Dennert R, Jochems S, et al. Circulating microRNA-208b and microRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet 2010; 3:499–506. [DOI] [PubMed] [Google Scholar]
- 29.Kuhl U, Schultheiss HP. Viral myocarditis. Swiss Med Wkly 2014; 144:w14010. [DOI] [PubMed] [Google Scholar]
- 30.Barone-Rochette G, Augier C, Rodiere M, et al. Potentially simple score of late gadolinium enhancement cardiac MR in acute myocarditis outcome. J Magn Reson Imaging 2014; 40:1347–1354. [DOI] [PubMed] [Google Scholar]
- 31.Berg J, Kottwitz J, Baltensperger N, et al. Cardiac magnetic resonance imaging in myocarditis reveals persistent disease activity despite normalization of cardiac enzymes and inflammatory parameters at 3-month follow-up. Circ Heart Fail 2017; 10: pii: e004262. [DOI] [PubMed] [Google Scholar]
- 32.Kasner M, Aleksandrov A, Escher F, et al. Multimodality imaging approach in the diagnosis of chronic myocarditis with preserved left ventricular ejection fraction (MCpEF): the role of 2D speckle-tracking echocardiography. Int J Cardiol 2017; 243:374–378. [DOI] [PubMed] [Google Scholar]
- 33.Aquaro GD, Perfetti M, Camastra G, et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J Am Coll Cardiol 2017; 70:1977–1987. [DOI] [PubMed] [Google Scholar]
- 34.Lurz P, Luecke C, Eitel I, et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer-trial. J Am Coll Cardiol 2016; 67:1800–1811. [DOI] [PubMed] [Google Scholar]
- 35.Friedrich MG. Tissue characterization of acute myocardial infarction and myocarditis by cardiac magnetic resonance. JACC Cardiovasc Imaging 2008; 1:652–662. [DOI] [PubMed] [Google Scholar]
- 36.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18:891–975. [DOI] [PubMed] [Google Scholar]
- 37.Corrado D, Basso C, Leoni L, et al. Three-dimensional electroanatomical voltage mapping and histologic evaluation of myocardial substrate in right ventricular outflow tract tachycardia. J Am Coll Cardiol 2008; 51:731–739. [DOI] [PubMed] [Google Scholar]
- 38.Pieroni M, Dello Russo A, Marzo F, et al. High prevalence of myocarditis mimicking arrhythmogenic right ventricular cardiomyopathy differential diagnosis by electroanatomic mapping-guided endomyocardial biopsy. J Am Coll Cardiol 2009; 53:681–689. [DOI] [PubMed] [Google Scholar]
- 39.Francis R, Lewis C. Myocardial biopsy: techniques and indications. Heart 2017; pii: heartjnl-2017-311382. [DOI] [PubMed] [Google Scholar]
- 40.Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J 2009; 30:1995–2002. [DOI] [PubMed] [Google Scholar]
- 41.Dominguez F, Kuhl U, Pieske B, et al. Update on myocarditis and inflammatory cardiomyopathy: reemergence of endomyocardial biopsy. Rev Esp Cardiol 2016; 69:178–187. [DOI] [PubMed] [Google Scholar]
- 42.Schultheiss HP, Piper C, Sowade O, et al. Betaferon in chronic viral cardiomyopathy (BICC) trial: effects of interferon-beta treatment in patients with chronic viral cardiomyopathy. Clin Res Cardiol 2016; 105:763–773. [DOI] [PubMed] [Google Scholar]
- 43.Kuhl U, Lassner D, von Schlippenbach J, et al. Interferon-Beta improves survival in enterovirus-associated cardiomyopathy. J Am Coll Cardiol 2012; 60:1295–1296. [DOI] [PubMed] [Google Scholar]
- 44.Escher F, Kuhl U, Gross U, et al. Aggravation of left ventricular dysfunction in patients with biopsy-proven cardiac human herpesvirus A and B infection. J Clin Virol 2015; 63:1–5. [DOI] [PubMed] [Google Scholar]
- 45.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci 2014; 1319:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tschope C, Bock CT, Kasner M, et al. High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation 2005; 111:879–886. [DOI] [PubMed] [Google Scholar]
- 47▪▪.Tschope C, Muller I, Xia Y, et al. NOD2 (nucleotide-binding oligomerization domain 2) is a major pathogenic mediator of coxsackievirus B3-induced myocarditis. Circ Heart Fail 2017; 10: [DOI] [PubMed] [Google Scholar]; The study illustrates the relevance of nucleotide-binding oligomerization domain-containing protein 2 (NOD2) in the pathogenesis of coxsackievirus B3 (CVB3)-induced myocarditis and opens new avenues to explore whether the analysis of NOD2 in endomyocardial biopsies could be useful as an additional differential diagnostic marker for CVB3-induced myocarditis under clinical conditions and whether the NOD2 signalling pathway could serve as a new important interventional target to treat CVB3-induced myocarditis.
- 48▪▪.Müller I, Vogl T, Pappritz K, et al. Pathogenic role of the damage-associated molecular patterns S100A8 and S100A9 in coxsackievirus B3-induced myocarditis. Circ Heart Fail 2017; 10: [DOI] [PubMed] [Google Scholar]; The study demonstrates the importance of the damage-associated molecular patterns S100A8 and S100A9 in the pathogenesis of CVB3-induced myocarditis and addresses the potential use of S100A8/A9 inhibitors to treat CVB3) myocarditis.
- 49.Bengtsson AA, Sturfelt G, Lood C, et al. Pharmacokinetics, tolerability, and preliminary efficacy of paquinimod (ABR-215757), a new quinoline-3-carboxamide derivative: studies in lupus-prone mice and a multicenter, randomized, double-blind, placebo-controlled, repeat-dose, dose-ranging study in patients with systemic lupus erythematosus. Arthritis Rheum 2012; 64:1579–1588. [DOI] [PubMed] [Google Scholar]
- 50.Cooper LT., Jr The changing face of cardiac inflammation: new opportunities in the management of myocarditis. Circ Heart Fail 2017; 10: [DOI] [PubMed] [Google Scholar]
- 51.Otsuka K, Terasaki F, Ikemoto M, et al. Suppression of inflammation in rat autoimmune myocarditis by S100A8/A9 through modulation of the proinflammatory cytokine network. Eur J Heart Fail 2009; 11:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simard JC, Cesaro A, Chapeton-Montes J, et al. S100A8 and S100A9 induce cytokine expression and regulate the NLRP3 inflammasome via ROS-dependent activation of NF-κB. PLoS One 2013; 8:e72138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toldo S, Kannan H, Bussani R, et al. Formation of the inflammasome in acute myocarditis. Int J Cardiol 2014; 171:e119–e121. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt-Lucke C, Spillmann F, Bock T, et al. Interferon beta modulates endothelial damage in patients with cardiac persistence of human parvovirus b19 infection. J Infect Dis 2010; 201:936–945. [DOI] [PubMed] [Google Scholar]
- 55.Bjork P, Bjork A, Vogl T, et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol 2009; 7:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schelbergen RF, Geven EJ, van den Bosch MH, et al. Prophylactic treatment with S100A9 inhibitor paquinimod reduces pathology in experimental collagenase-induced osteoarthritis. Ann Rheum Dis 2015; 74:2254–2258. [DOI] [PubMed] [Google Scholar]
- 57.Stenstrom M, Nyhlen HC, Torngren M, et al. Paquinimod reduces skin fibrosis in tight skin 1 mice, an experimental model of systemic sclerosis. J Dermatol Sci 2016; 83:52–59. [DOI] [PubMed] [Google Scholar]
- 58.Kraakman MJ, Lee MK, Al-Sharea A, et al. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J Clin Invest 2017; 127:2133–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinon F, Petrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440:237–241. [DOI] [PubMed] [Google Scholar]
- 60.Leung YY, Yao Hui LL, Kraus VB. Colchicine – update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015; 45:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deftereos S, Giannopoulos G, Papoutsidakis N, et al. Colchicine and the heart: pushing the envelope. J Am Coll Cardiol 2013; 62:1817–1825. [DOI] [PubMed] [Google Scholar]
- 62.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. JAMA 2014; 312:1016–1023. [DOI] [PubMed] [Google Scholar]
- 63.Brucato A, Maestroni S, Cumetti D, et al. Recurrent pericarditis: infectious or autoimmune? Autoimmun Rev 2008; 8:44–47. [DOI] [PubMed] [Google Scholar]
- 64.Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005; 112:2012–2016. [DOI] [PubMed] [Google Scholar]
- 65.Imazio M, Brucato A, Cemin R, et al. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann Intern Med 2011; 155:409–414. [DOI] [PubMed] [Google Scholar]
- 66.Imazio M, Brucato A, Cemin R, et al. A randomized trial of colchicine for acute pericarditis. N Engl J Med 2013; 369:1522–1528. [DOI] [PubMed] [Google Scholar]
- 67.Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015; 36:2921–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gultekin N, Kucukates E. Microtubule inhibition therapy by colchicine in severe myocarditis especially caused by Epstein-Barr and cytomegalovirus co-infection during a two-year period: a novel therapeutic approach. J Pak Med Assoc 2014; 64:1420–1423. [PubMed] [Google Scholar]
- 69.Wang Y, Gao B, Xiong S. Involvement of NLRP3 inflammasome in CVB3-induced viral myocarditis. Am J Physiol Heart Circ Physiol 2014; 307:H1438–1447. [DOI] [PubMed] [Google Scholar]
- 70.Bracey NA, Beck PL, Muruve DA, et al. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1beta. Exp Physiol 2013; 98:462–472. [DOI] [PubMed] [Google Scholar]
- 71.Cavalli G, Foppoli M, Cabrini L, et al. Interleukin-1 receptor blockade rescues myocarditis-associated end-stage heart failure. Front Immunol 2017; 8:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cavalli G, Pappalardo F, Mangieri A, et al. Treating life-threatening myocarditis by blocking interleukin-1. Crit Care Med 2016; 44:e751–e754. [DOI] [PubMed] [Google Scholar]
- 73.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 74.Van Linthout S, Hamdani N, Miteva K, et al. Placenta-derived adherent stromal cells improve diabetes mellitus-associated left ventricular diastolic performance. Stem Cells Transl Med 2017; 6:2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miteva K, Van Linthout S, Volk HD, Tschope C. Immunomodulatory effects of mesenchymal stromal cells revisited in the context of inflammatory cardiomyopathy. Stem Cells Int 2013; 2013:353097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muller I, Pappritz K, Savvatis K, et al. CX3CR1 knockout aggravates coxsackievirus B3-induced myocarditis. PLoS One 2017; 12:e0182643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77▪.Miteva K, Pappritz K, El-Shafeey M, et al. Mesenchymal stromal cells modulate monocytes trafficking in coxsackievirus B3-induced myocarditis. Stem Cells Transl Med 2017; 6:1249–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study illustrates the potential of mesenchymal stromal cells to modulate monocyte trafficking in CVB3-induced myocarditis.
- 78.Miteva K, Pappritz K, Sosnowski M, et al. Mesenchymal stromal cells inhibit NLRP3 inflammasome activation in a model of coxsackievirus B3-induced inflammatory cardiomyopathy. Sci Rep 2018; 8:2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008; 8:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marchant DJ, McManus BM. Regulating viral myocarditis: allografted regulatory T cells decrease immune infiltration and viral load. Circulation 2010; 121:2609–2611. [DOI] [PubMed] [Google Scholar]
- 81.Cao Y, Xu W, Xiong S. Adoptive transfer of regulatory T cells protects against coxsackievirus B3-induced cardiac fibrosis. PLoS One 2013; 8:e74955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang H, Zhong Y, Zhu Y, et al. Low responder T cell susceptibility to the suppressive function of regulatory T cells in patients with dilated cardiomyopathy. Heart 2010; 96:765–771. [DOI] [PubMed] [Google Scholar]
- 83.Shi Y, Fukuoka M, Li G, et al. Regulatory T cells protect mice against coxsackievirus-induced myocarditis through the transforming growth factor beta-coxsackie-adenovirus receptor pathway. Circulation 2010; 121:2624–2634. [DOI] [PubMed] [Google Scholar]
- 84.Pappritz K, Savvatis K, Lindner D, et al. Administration of regulatory T cells ameliorates myocardial inflammation in experimental myocarditis. Eur Heart J 2013; 34:279. [Google Scholar]
- 85.Abou-El-Enein M, Volk HD, Reinke P. Clinical development of cell therapies: setting the stage for academic success. Clin Pharmacol Ther 2017; 101:35–38. [DOI] [PubMed] [Google Scholar]
- 86.Meng N, Gao X, Yan W, et al. Efficacy of telbivudine in the treatment of chronic hepatitis b and liver cirrhosis and its effect on immunological responses. J Huazhong Univ Sci Technolog Med Sci 2015; 35:230–234. [DOI] [PubMed] [Google Scholar]
- 87.Pan X, Yao W, Fu J, et al. Telbivudine improves the function of myeloid dendritic cells in patients with chronic hepatitis B. Acta Virol 2012; 56:31–38. [DOI] [PubMed] [Google Scholar]
- 88.Li J, Jia M, Liu Y, et al. Telbivudine therapy may shape CD4(+) T-cell response to prevent liver fibrosis in patients with chronic hepatitis B. Liver Int 2015; 35:834–845. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y, Li X, Ye B, et al. Effect of telbivudine therapy on the cellular immune response in chronic hepatitis B. Antiviral Res 2011; 91:23–31. [DOI] [PubMed] [Google Scholar]
- 90.Ozawa K, Kurtzman G, Young N. Productive infection by B19 parvovirus of human erythroid bone marrow cells in vitro. Blood 1987; 70:384–391. [PubMed] [Google Scholar]
- 91.Haag M, Stolk M, Ringe J, et al. Immune attributes of cardiac-derived adherent proliferating (CAP) cells in cardiac therapy. J Tissue Eng Regen Med 2013; 7:362–370. [DOI] [PubMed] [Google Scholar]


