Abstract
Recombinant adeno-associated viral (rAAV) vectors have been used in more than 150 clinical trials with a good safety profile and significant clinical benefit in many genetic diseases. In addition, due to their ability to infect non-dividing and dividing cells and to serve as efficient substrate for homologous recombination, rAAVs are being used as a tool for gene-editing approaches. However, manufacturing of these vectors at high quantities and fulfilling current good manufacturing practices (GMP) is still a challenge, and several technological platforms are competing for this niche. Herein, we will describe the most commonly used upstream methods to produce rAAVs, paying particular attention to the starting materials (input) used in each platform and which related impurities can be expected in final products (output). The most commonly found impurities in rAAV stocks include defective particles (i.e., AAV capsids that do contain the therapeutic gene or are not infectious), residual proteins from host cells and helper viruses (adenovirus, herpes simplex virus, or baculoviruses), and illegitimate DNA from plasmids, cells, or helper viruses that may be encapsidated into rAAV particles. Given the role that impurities may play in immunotoxicity, this article reviews the impurities inherently associated with each manufacturing platform.
Keywords: gene therapy, viral vectors, AAV, manufacturing, quality controls, impurities
Main Text
Adeno-associated virus (AAV) is a very small (20–26 nm), icosahedral, and nonenveloped virus (Figure 1). AAV particles contain a single-stranded DNA genome consisting of approximately 4.7 kb. The genome contains three open reading frames (ORFs) encoding for replication proteins (Rep), capsid proteins (Caps), and the assembly activating protein (AAP), and is flanked by two inverted terminal repeats (ITRs). These 145-nt ITRs are partially paired, and they fold upon themselves to maximize base pairing and form a T-shaped hairpin structure. The AAV genome contains two viral promoters known as p5 and p19, which regulate the transcription of the four Rep proteins with apparent molecular masses of 78, 68, 52, and 40 kDa (Rep68 and Rep40 being the splice variants of Rep78 and Rep52, respectively). Expression of the cap gene is driven by the P40 promoter and regulated by alternative splicing and different translation initiation sites, resulting in three Caps (VP1, VP2, and VP3) that form an icosahedral capsid of ∼3.9 kDa. The molecular ratio of these proteins (VP1:VP2:VP3) is approximately 1:1:10. The AAV genome also encodes for the AAP in an alternative ORF of the cap gene that plays a major role for capsid assembly.
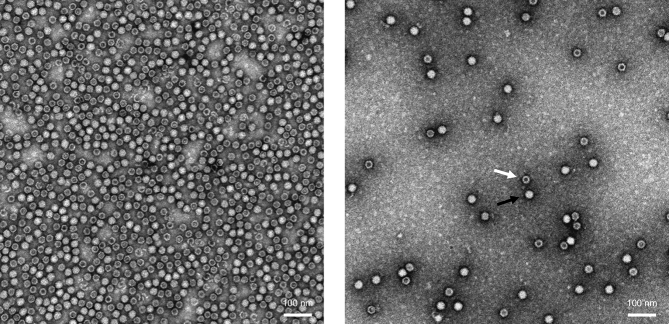
Figure 1.
Electron Microscopy Images of rAAV Particles
Original magnification ×75,000. Electron-dense particles observed by electron microscopy (EM) after negative staining with uranyl acetate correspond to empty particles (white arrow, right panel). A full rAAV particle is indicated by a black arrow.
The life cycle of AAV is dependent on the presence or absence of a helper virus, hence its name. In the absence of helper viruses, the wtAAV genome is able to latently persist as episomes or be integrated into the host genome. This site-specific integration occurs preferentially into a 4-kb region on chromosome 19 (q13.4), named AAVS1, and requires the Rep proteins.1 These latent forms may be activated and “rescued” upon subsequent helper virus infection by inducing active replication of the viral genome, Caps synthesis, and DNA packaging, with these steps taking place inside the nucleus.2 In 1984, Hermonat and Muzyczka3 published the first paper of a recombinant AAV (rAAV) vector that was capable of expressing foreign genes in mammalian cells. Since then, AAV vectors from serotype 2 (AAV2) have been used as a prototype for gene therapy, and the subsequent identification of more than 12 AAV serotypes and more than a hundred variants in human and nonhuman primate (NHP) populations extended its applications.4 For most of the serotypes, the ITR and rep gene from AAV2 can be kept constant, while the cap gene from different serotypes or isolates is used to derive “pseudotyped” vectors that only differ by the nature of their capsid.5 To date, the following methods have been established for the generation of rAAV vectors at large scale: (1) plasmid DNA transfection in mammalian cells, (2) Ad infection of stable mammalian cell lines, (3) infection of mammalian cells with recombinant herpes simplex viruses (rHSVs), and (4) infection of insect cells with recombinant baculoviruses. With the same objective to offer a cost-effective, safe, and scalable manufacturing process, other alternatives have been explored. For example, taking advantage that vaccinia virus replicates in the cytoplasm, another method based on dual infection of HeLa-S3 cells with one vaccinia vector and one Ad-AAV hybrid vector has been used for rAAV production.6, 7 The advantage of this system is that rep-cap sequence and rAAV genome are sequestered in different sub-cellular compartments, which eliminates the contamination with replication-competent AAV (rcAAV). Following seminal works of A. Galli’s8, 9 and R.O. Snyder’s groups,10, 11 Barajas et al.12 have reported recently that rAAV2 infectious particles can be generated in Saccharomyces cerevisiae after transformation with four plasmids allowing the expression of rep78/52, the three AAV Caps, and AAP under the control of galactose-inducible promoters. Although vector genome packaging efficiency remains less efficient than in mammalian and insect cell-based platforms, yeast offers better possibilities for scale-up at reduced cost. Among the different available methods, this review will focus on those that have already been used for rAAV preclinical and clinical lots manufacturing.
In mammalian cell-based production systems, the assembly of rAAV vectors requires: (1) the recombinant vector genome composed of the gene of interest (GOI) and the regulation elements for the GOI expression in target cells (promoter, poly A, introns, etc.) flanked by AAV ITRs, (2) the AAV rep and cap genes provided in trans, and (3) helper functions from Ad or HSV for efficient replication and rescue of the recombinant genome.
In 2002, Kotin and collaborators13 demonstrated that the replication and assembly of rAAV vectors can also occur in insect cells by expressing rep and cap genes via baculovirus vectors. Interestingly, when rAAV is assembled in insect cells there is no need to add auxiliary HSV or Ad genes, very likely because baculovirus is also providing helper functions, although the genes involved in this process have not been identified yet. Each aforementioned system is capable of generating AAV particles. However, the overall yields and quality of the vectors still greatly vary based on the method utilized. Depending on the biological raw materials used upstream (input), i.e., plasmids, recombinant viruses, and cells, the final product of each manufacturing platform will differ in particular in terms of impurities (output). Likewise, the quantity of rAAV vectors and product-related contaminants (non-infectious vectors, particles that do not contain the transgene, degraded capsids) may vary from one method to another.
Upstream Methods for AAV Vector Assembly (Input)
Transient Transfection of HEK293 Cells
The most established method for the production of rAAV vectors is the plasmid transfection of human embryonic HEK293 cells. Typically, HEK293 cells are simultaneously transfected by a vector plasmid (containing the GOI) and one or two helper plasmids, using calcium phosphate or polyethylenimine (PEI), a cationic polymer. The helper plasmid(s) allow the expression of the four Rep proteins, the three AAV structural proteins VP1, VP2, and VP3, the AAP, and the adenoviral auxiliary functions E2A, E4, and VA RNA. The additional adenoviral E1A/E1B co-factors necessary for rAAV replication are expressed in HEK293 producer cells (Table 1). Rep-cap and adenoviral helper sequences are either cloned on two separate plasmids or combined on one plasmid, hence evolving from a triple plasmid system to transfection with only two plasmids (Figure 2, input).14 Nowadays, the dual and triple plasmid protocols coexist because the latter method lends versatility with a cap gene that can easily be switched from one serotype to another. The plasmids are usually produced by conventional techniques in E. coli using bacterial origin and antibiotic-resistance gene or by minicircle (MC) technology.15 Although transient transfection in adherent HEK293 cells has been used for large-scale manufacturing of rAAV vectors, it often required multiple production batches to fulfill the needs of clinical trials, resulting in lengthy and often costly production campaigns. Recently, HEK293 cells have been adapted to suspension conditions to be economically viable in the long term.16
Table 1.
Characteristics of rAAV Producer Cell Lines Related to Safety
| HEK293 | human origin |
| left arm of adenovirus 5 genome (∼4.3-kbp fragment of Ad5 including the 3′ terminal repeat, the E1A/B gene, and the partial IX and IVa2 genes sequences)133 | |
| HEK293T | human origin |
| left arm of adenovirus 5 genome | |
| SV40 T antigen (temperature-sensitive allele [tsA1609] of the SV40 T antigen allele and neomycin/geneticin-resistance gene)134 | |
| HeLa | human origin |
| human papillomavirus type 18 partial genome (no expression of E4, E5, and L2 genes)135 | |
| A549 | human origin |
| non-transformed cell line136 | |
| BHK21 | hamster origin |
| non-transformed cell line137 | |
| Sf9 | insect origin |
| non-transformed cell line21 |
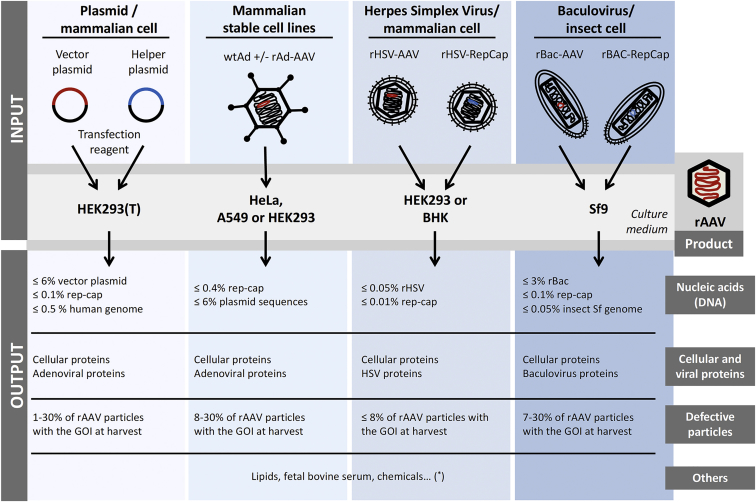
Figure 2.
Schematic Representation of Raw Materials (Input) and Product-Related Impurities (Output) in Each System Used for the Production of rAAV Vectors
Asterisk (*) indicates depending on culture medium composition and on the lysis and clarification steps. The percentages of DNA contaminants were published in Lecomte et al.,64 Ye et al.,67 and Penaud-Budloo et al.68
HEK293 lines are usually propagated in DMEM completed with L-glutamine, 5%–10% of fetal bovine serum (FBS), and 1% penicillin-streptomycin, except for suspension HEK293 cells that are maintained in serum-free suspension F17, Expi293, or other manufacturer-specific media.16 For adherent cells, the percentage of FBS can be reduced during rAAV production in order to limit contamination by animal-derived components. Generally, the rAAV vectors are recovered 48–72 hr after plasmid transfection from the cell pellet and/or supernatant, depending on the serotype. The cell lysis step has a great impact on the amount and type of impurities found in the crude bulk, as well as on particles recovery, and thus will be described hereafter.
Infection of Insect Cells with Recombinant Baculovirus
As an alternative to the rAAV production in mammalian cells, the baculovirus-Sf9 platform has been notably established as a GMP-compatible and scalable system,17, 18 generating as many vector genomes per cell as the mammalian cell-based methods, i.e., up to 2 × 105 vector genomes (vg) per cell in crude harvests.19 The production of rAAV vectors by infection of insect cells with three recombinant baculoviruses was first described in 2002 by Kotin’s group.13 Subsequent improvements were then introduced, reducing the number of baculoviruses to two: a baculovirus expression vector (BEV) allowing the synthesis of Rep78/52 and Caps, and a recombinant baculovirus carrying the GOI flanked by the AAV ITRs (Figure 2, input).20 Sf9 cell line, a subclone of the Sf21AE cells stemming from spontaneous immortalization of Spodoptera frugiperda ovarian cells,21 has been commonly adopted for the generation of recombinant baculovirus and the production of rAAV vectors (Table 1). Several serum-free media are adapted for Sf9 cell growth in suspension.22 The dual-baculovirus-Sf9 production system has many advantages over other production platforms regarding these safety issues: (1) the use of serum-free media; (2) despite the discovery of adventitious virus transcripts in Sf cell lines, most of the viruses infecting insects do not replicate actively in mammalian cells; and (3) no helper virus is required for rAAV production in insect cells besides baculovirus. More recently, stable Sf9 insect cell lines expressing Rep and Cap proteins have been developed requiring the infection of only one recombinant baculovirus for the production of infectious rAAV vectors at high yield.23, 24 However, baculovirus-related impurities should not be considered as a trivial concern because this issue has been raised in the assessment report of Glybera,25 an AAV-based product for the treatment of lipoprotein lipase deficiency and the first gene therapy drug approved by the European Medicines Agency (EMA). Indeed, in this report, the EMA committee asked for a more comprehensive characterization of contaminants of baculovirus origin (residual DNA, proteins, infectious baculovirus particles).
Infection of Mammalian Cells with rHSV Vectors
Although less common than the other two methods described above, the AAV production using co-infection with rHSV is a very efficient method to generate a large amount of rAAV. In addition to high overall yields (up to 1.5 × 105 vg/cell), the apparent increased quality of the rAAV stocks, as measured by an improved viral potency, makes this method very attractive.26, 27 The principle of the method relies on the role of the HSV in AAV life cycle as a helper virus for replication in permissive cells. Therefore, the HSV virus can serve both as a helper and as a shuttle to deliver the necessary AAV functions that support AAV genome replication and packaging to the producing cells. Cells, typically the hamster BHK21 cell line27 (Table 1) or HEK293 and derivatives,26, 28 are infected with two rHSVs, one carrying the GOI bracketed by AAV ITR (rHSV-AAV) and the second with the AAV rep and cap ORFs of the desired serotype (rHSV-repcap) (Figure 2, input). After 2–3 days, the cells and/or the media are collected and rAAV is purified over multiple purification steps to remove cellular impurities, HSV-derived contaminants, and unpackaged AAV DNA.26, 28, 29 Processes utilized to date to remove process-derived impurities from rAAV stocks will be described briefly below. To date, three clinical trials have been initiated for one metabolic disorder, alpha-1 antitrypsin (AAT), and two retinal diseases, achromatopsia and X-linked retinoschisin (XLRS), supporting the suitability of the method for human use.
Mammalian Stable Cell Lines
The establishment of stable mammalian cells for the production of rAAV vectors was first described by Clark et al.,30 who created stable HeLa-derived producer cell lines transformed with a plasmid containing both rep-cap genes of AAV2 and an rAAV vector genome along with a drug selection marker. Several groups have also established packaging cell lines through transformation with the rep-cap genes of AAV2 in HeLa31, 32, 33 and A549 cells34, 35 (Table 1). The high-level expression of the rep and cap genes induced by Ad infection and subsequent rAAV assembly was correlated with gene amplification36, 37 and associated with the presence of a replication element within the AAV2 p5 promoter.38, 39 Infectious rAAV vectors can be generated upon infection of these packaging cells lines with wild-type Ad type 540 and providing the rAAV genome by either plasmid transfection or after infection with a recombinant Ad/AAV hybrid virus (Figure 2, input).41 Possible replacement of Ad with HSV-1 as the helper virus was also demonstrated.42
Establishment of producer cell lines was also reported in HEK293 cells. In this case, expression of the Rep proteins had to be tightly controlled by a Cre recombinase-inducible system where the p5 promoter is separated from the rep and cap coding sequences by transcription termination signals flanked by loxP sites, because Rep is known to inhibit cell proliferation by inducing cell-cycle arrest43, 44, 45, 46 or apoptosis.47 Indeed, HEK293 cells constitutively express the Ad5 E1 gene that activates the AAV p5 promoter.48 Production of rAAV from these cells is then triggered by infection with a recombinant, E1-deleted, Ad5 virus expressing Cre recombinase.49, 50, 51
Mammalian stable cell lines infected with Ad were actually the first system to provide a scalable upstream process for manufacturing of purified rAAV particles.34, 35, 52, 53 Moreover, a HeLa-based stable cell line was used for production of the first AAV product administrated in humans in a clinical trial for the treatment of cystic fibrosis.54 The selection of producer cells for manufacturing of clinical-grade AAV vectors has been optimized using HeLaS3 cells, a suspension adapted HeLa subclone,55, 56 and a full manufacturing process including production in animal components-free medium, and purification was developed at the 250-L scale for a rAAV1 vector.55 The process was recently implemented to a 2,000-L commercial scale for rAAV1-SERCA2a, a gene therapy product intended for the treatment of heart failure, as reported in a press release (Celladon Corporation Conducts Initial Scale Up of Manufacturing Process for MYDICAR up to Commercial Scale, January 5, 2015).
Thus, stable producer cells allow efficient and scalable rAAV vector production. In addition, wild-type Ad5 helper virus is genetically stable and can be easily produced at high titers. However, a time-consuming step is the establishment and screening of stable and high-producer clones. Indeed, this step requires selecting cells with (but not limited to) the following characteristics: (1) the integrated rep-cap and rAAV vector sequences remain stable over time without maintaining drug selection; (2) the AAV promoters remain silenced during cells sub-culturing and expansion; and (3) infection with Ad5 induces a high level of rep and cap gene expression and amplification, as well as rAAV genome rescue and replication. Thus, screening of tens to thousands of clones may be required to get a high producer cell line for a specific vector,35, 39, 56, 57 which has probably limited the wide adoption of this system. Another critical aspect of this technology is the requirement for a downstream process allowing efficient clearance of the Ad5 helper virus, which will be discussed below.
Characterization of rAAV Stocks and Related Impurities (Output)
In vivo data have validated the safety of all of the above-mentioned methods for the production of pre-clinical and clinical-grade rAAV. However, the safety and efficiency profiles of the AAV-based drugs depend on the upstream and downstream steps that ensure complete removal of process-derived impurities and on the development of robust and precise assays for the detection and quantification of these impurities (Table 2). Here, we will mostly focus on impurities that are inherently associated with each of the production methods and steps utilized to reduce, eliminate, and quantify them (Figure 2, output). In addition, we will try to compare data across the manufacturing platforms. However, because very limited data exist with direct side-by-side comparisons, the overall interpretation should be concluded with caution. Finally, specifications are not always established for rAAV quality tests, and the determination of acceptable levels of these impurities has to be done based on risk assessment studies.58
Table 2.
Current Testing and Specifications for AAV-Based Products Purity and Safety
| Test | Method | Specification |
|---|---|---|
| Safety | ||
| endotoxin | LAL assay (EP 2.6.14, USP85) | <2 EU/injected dosea |
| rabbit pyrogen assays (EP 2.6.8) | negative | |
| sterility/bacterio- and fungi-static activity | sterility tests (EP 2.6.1, USP71) | negativea |
| replication-competent AAV | serial infection on permissive cells/rep or cap qPCR | <1 rcAAV in 1 × 108 vg |
| Purity | ||
| general purity | SDS-PAGE/silver staining | identify proteins other than VP (or VP degradation products) |
| protein | SDS-PAGE/Coomassie blue | ≥80% |
| HPLC | ||
| residual BSAb | ELISA | ≤50 ng/mLa |
| residual production reagentc | HPLC, MS, ELISA… | report results |
| host cell proteins | ELISA, MS | reports results |
| residual cell DNA | qPCR, HTS | ≤10 ng/injected dosea |
| residual DNA from raw materiald | qPCR, HTS | report results |
| empty/intermediate particles |
ratio vg (qPCR)/vp (ELISA) | report results |
| electron microscopy, AUC | ||
| Quality | ||
| total protein | Bradford protein assay, BCA, NanoOrange | report results |
| appearance | visual inspection | clear to slightly hazy, free of foreign materials |
| pH | potentiometry | pH 6.5–8 |
| osmolality | osmometry | product specific, report results (250–350 mOsm/kg) |
| aggregation | DLS | report results |
AUC, analytical ultracentrifugation; BCA, bicinchoninic acid protein assay; DLS, dynamic light scattering; EP, endotoxin units; HPLC, high-performance liquid chromatography; HTS, high-throughput sequencing; LAL, limulus amebocyte lysate; MS, mass spectrometry; USP, U.S. Pharmacopoeia.
US Food and Drug Administration or European Pharmacopoeia specifications; other specifications are recommended.
Only for processes using fetal bovine serum.
Depending on the purification process (Benzonase, detergent, BSA, cesium, PEG, affinity ligands).
Plasmid, baculovirus, adenovirus, or HSV DNA.
Nucleic Acids
Recombinant rAAV capsids are known to internalize not only the recombinant genomes, but also illegitimate DNA during their production in mammalian and insect cells. To limit the genotoxic risk of residual DNA in rAAV stocks, the US Food and Drug Administration (FDA) recommends a level of residual cellular DNA below 10 ng per parenteral dose and a median DNA fragment size of less than 200 bp.59 Supplementary quality controls regarding DNA contaminants are also asked for gene therapy product, in particular qPCR quantification of potential hazardous sequences, such as antibiotic-resistance genes and E1 and E4 adenoviral genes. For the transient transfection method, less than 100 ng of residual plasmid DNA, as determined by antibiotic-resistance gene-targeted qPCR, is advised per 1 × 1012 vg in the purified bulk product.60 However, without associated risk assessment studies, it would be recommended to inquire about the proportion of each potential DNA contaminant in rAAV stocks taking advantage of novel sequencing technologies.
rep-cap Sequences and Generation of rcAAV
Early after the generation of the first rAAV vector in 1984, many groups had looked for rep-cap-derived sequences in rAAV stocks because of the risk of generating replication-competent or pseudo-wild-type AAV particles. rcAAV particles contain all of the AAV genetic elements required for their propagation in the presence of a helper virus, whereas pseudo-wtAAV particles harbor only a portion of the wild-type genome. Although AAV is considered as nonpathogenic, these types of particles are undesirable, in particular because Rep proteins may induce nicks in DNA, and cap expression could trigger immune response. The main cause of rcAAV formation was attributed to recombination events that could arise between homologous sequences in ITR and adjacent sequences and the rep/cap cassette. Fortunately, these were avoided by replacing the p5 promoter with heterologous promoters14, 61 or removing RBE adjacent to rep-cap sequences.23 Splitting of the rep and cap sequences in two separate cassettes and engineering of an oversized cap sequence by addition of an intronic sequence were also proposed to limit rep-cap encapsidation.62 Using optimized protocols, rcAAVs were generally reduced to an undetectable level in single-stranded AAV (ssAAV) stocks (<1 copy in 1 × 108 vg). However, in HEK293-derived rAAV vector batches, sequences derived from rep-cap plasmid were still present at a level of 0.02%–1% of vector genomes.56, 63 More recently, we reported by high-throughput sequencing (HTS) analysis that the number of reads that aligned to the helper plasmid (including rep-cap and adenoviral genes) corresponds to 0.01%–0.11% of the total reads amplified from rAAV8 vectors64 (Table 3). To compare our results with previous studies, we performed qPCR side by side with our SSV-seq (single-stranded virus sequencing) protocol. The percentage of helper plasmid sequences was calculated from the copy number obtained by a qPCR targeted to a sequence in the helper plasmid, normalized to each reference size and divided by gfp copy number. The qPCR data correlated with SSV-seq results with 0.004%–0.4% of helper sequences. Our results were consistent with the qPCR data obtained from rAAV-FIX clinical lots used for hemophilia B treatment. Indeed, 0.018% and 0.02% of cap copies were detected for the ssAAV265 and the scAAV8 vectors,66 respectively. The first vector was produced using three plasmids and purified by cesium chloride (CsCl) gradient ultracentrifugation, whereas the second one was generated after HEK293 cells transfection with two plasmids and purified by size exclusion and ion-exchange chromatography (IEX) chromatography. However, a great variability has been observed between studies because of: (1) the technique used for the quantification of DNA contaminants (slot blot, qPCR, or HTS); (2) the partial and selective nature of the slot blot and qPCR, which depend on the choice of the probe and the target, respectively; (3) the intrinsic intra- and inter-laboratory variability of each method; (4) the percentage calculation (normalized to the target size or not); and (5) the disparate production and purification parameters.
Table 3.
Percentages of DNA Contaminants in rAAV8-gfp Particles Produced in Mammalian and Insect Cells
| Method | Reference Sequence | HEK293 | Sf9 |
|---|---|---|---|
| SSV-seq | rAAV genome | 99.48 | 98.90 |
| baculovirus or vector plasmid backbone | 0.38 | 1.03 | |
| rep-cap | 0.11 | 0.05 | |
| producer cell genome | 0.04 | 0.02 | |
| qPCR | rAAV genome | 99.04 | 99.40 |
| baculovirus backbone or vector plasmid backbonea | 0.95 | 0.55 | |
| rep-capb | 0.01 | 0.06 | |
| producer cell genome | < LOQ | < LOQ |
The percentages were calculated based on next generation sequencing or qPCR data, relative to each DNA species length. The 3.3-kb rAAV genome is composed of the human cytomegalovirus promoter, the EGFP reporter gene, and the 3′ UTR of the human hemoglobin b gene, flanked by AAV ITR2 from the pSub-201 plasmid.141 The plasmid and baculovirus backbones are 4.8 and 144.4 kb in length, respectively. Recombinant AAV8-gfp vectors were produced in HEK293 mammalian cells by co-transfection of the pDP8 and pFB-gfp plasmids or in Sf9 insect cells by dual-baculovirus infection. After purification by CsCl gradients ultracentrifugation, the rAAV stocks were pretreated with a DNases cocktail before SSV-seq and qPCR analyses.
LOQ, limit of quantification.
Quantification of DNA contaminants derived from the baculovirus backbone or the vector plasmid backbone was performed by real-time PCR targeted to the baculoviral DNA polymerase or the amp gene, respectively.
For helper sequences quantification, qPCR was targeted to the rep2 sequence.
Few data were published concerning the proportion of rep-cap sequences in rAAV stocks produced in other production platforms. It has been reported that rAAV2-SEAP (secreted alkaline phosphatase) vector batches produced in HeLaS3-derived cell lines and purified by AVB immunoaffinity column contained 0.2%–0.4% copies of rep-cap relative to the vector genomes.56 Besides that, 0.01% of rep copies were detected in an rAAV1 vector stock generated in BHK cells by rHSV infection.67 Moreover, less than 0.04% of rep and cap sequences contaminated the rAAV1, rAAV2, and rAAV8 vectors produced in the OneBac2.0 cell line by baculovirus infection.23 Finally, we recently published that less than 0.1% of Illumina reads corresponded to rep-cap genes in rAAV8 batches produced in the dual-baculovirus/Sf9 cells system68 (Table 3).
Antibiotic Resistance Sequences
In 2005, a landmark study realized in our laboratory warned for the potential risk of transferring the prokaryotic sequences originated from the vector plasmid in patients.69 This work showed that rAAV2 stocks produced by transfection with the pDG plasmid14 and a pAAV-gfp plasmid and purified by CsCl gradient ultracentrifugation contained 1.5 × 109 copies/mL of kanamycin resistance for 3.8 × 1010 copies of gfp/mL, which represents by extrapolation 3.9% of the vector genomes. However, the percentage calculation based on qPCR data did not take into account the length of each DNA species and the encapsidation heterogeneity of each sequence along the vector plasmid and the rAAV genome. Contamination of rAAV lots with antibiotic-resistance sequences was then quite systematically investigated using real-time PCR. For example, 1.87% of the kanamycin-resistance gene sequence was detected in the scAAV8-FIX clinical lot purified with three-column chromatography and used to treat patients with hemophilia B.66 In more recent studies, 0.5%–3% of ampicillin-resistance gene (amp) sequences were quantified after Benzonase treatment in rAAV2-gfp and rAAVrh8R-FVIII stocks.15 Using an HTS approach, we confirmed that DNA impurities originate in the great majority from the vector plasmid, ranging from 0.4% to 6% of total DNA in single-stranded AAV vector batches produced by dual-plasmid transfection in mammalian HEK293 cells64 (Table 3). However, the illegitimate encapsidation of plasmid (antibiotic-resistance) sequences may vary depending on the plasmid backbone size, the rAAV genome sequence, and the type of AAV vector. Indeed, the level of residual plasmid DNA has been described to be higher for scAAV with up to 26% of the amp sequence detected in scAAV-gfp stocks.15
Antibiotic sequences were also found in rAAV particles produced in mammalian stable cell lines through Ad infection, whether the clone was generated by rAAV infection or by plasmid transfection. Indeed, amp copies represented 3%–6% of the total rAAV2-lacZ vg generated in stable cell lines.69 Consistently, puromycin-resistance DNA was also found in rAAV2, rAAV8, and rAAV-rh8R lots produced in HeLaS3-derived producer cell lines at a level of 0.2%–1%.56, 70 In these cases, antibiotic resistance could come from the rep-cap plasmid or the plasmid harboring rAAV genome that were used to stably integrate the corresponding sequences in the cell genome.69
Helper Virus Sequences
In the dual-baculovirus/Sf9 platform, we demonstrated that the baculoviral DNA can account for up to 2.1% of rAAV8 vector genomes content, with an increased proportion for the sequences adjacent to ITRs to be encapsidated, such as the gentamycin-resistance gene in the Bac-to-Bac backbone.68 Thus, undesirable packaging of vector plasmid sequences is strongly thought to be ITR dependant.
To date, there are no formal data aimed at determining specifically the percentage of viral or genomic DNA encapsidated in AAV vectors when using the HSV system. However, total residual HSV DNA detection in AAV stocks has been described in detail. Because the genome of HSV is very large (∼150 kb), multiple primer sets must be selected across the genome to ensure proper detection of possible variants. They often include a primer set of ICP27 to detect ICP27 revertants (see section below, Residual HSV).67 Values reported ranged between 72 and 124 ng/mL for pre-clinical stocks71, 72, 73, 74 and 76 ng/mL for the AAV1-AAT product,75 which would correspond to 6.5 μg of total DNA at the highest doses. Furthermore, Ye et al.67 showed that HSV DNA impurities account for a very small proportion of the total DNA, i.e., 0.01%–0.026% of the total rAAV vg, independently of the HSV gene targeted by qPCR, the closest target being UL23, which is less than 1 kb away from the transgene insertion site.
In the Ad-infected mammalian producer cells, packaging of Ad5 DNA in AAV capsids has not been reported in the literature. Because the Ad genome is covalently linked to the terminal protein (used as a primer for DNA replication), illegitimate packaging of Ad DNA is not expected to occur at a high level, but next generation sequencing technologies, with higher sensitivity, should be used to further investigate these events. Encapsidation of viral oncogenes integrated in the producer cells genome is also of concern. Thus, the presence of Ad E1 and HPV E6-E7 gene sequences is systematically investigated in AAV produced in HEK293 cells and HeLa-based cell lines, respectively, although they are consistently found below the detection limit.56, 64, 66
How Can We Limit the Risk Related to DNA Impurities?
Different purification strategies have been implemented to minimize residual DNA depending on their origin and their susceptibility to DNase treatment. The addition of a nuclease treatment step after clarification can be active on free DNA species if digestion conditions are met (pH, Mg2+, and salt concentrations). For DNA that is protected by the helper virus capsids, specific removal of Ad, baculovirus, or herpes virus particles is required. However, we have demonstrated that the vast majority of contaminating DNA in purified rAAV stocks is protected by the AAV capsid and is thus resistant to drastic DNase treatment.64, 68
For vector plasmids, the bacterial selection marker should be carefully chosen in order to avoid spread of resistance against antibiotics used in humans. For example, the FDA recommends not using β-lactamase. Another strategy was developed to avoid the transfer of plasmid-derived sequences into the target tissue, namely, MCs. Schnödt and co-workers15 recently took advantage of this technology to produce ssAAV and scAAV in HEK293 cells in comparison with the traditional dual-plasmid transfection. The replacement of the helper plasmid by a helper MC had no effect on the amp gene sequences encapsidation level, confirming that prokaryotic impurities in ssAAV stocks come mostly from the vector plasmid, whereas changing the vector plasmid by an MC reduced the amp copy number by 2 logs. This effect is particularly remarkable for scAAV vectors where the use of MCs diminished the percentage of amp contaminants from 26.1% to 0.2% (vector MC + helper plasmid) or to an undetectable level (vector MC + helper MC). In conclusion, MC technology can be considered as a substantial improvement in rAAV vectors production in HEK293 cells, although it may significantly increase the cost of raw materials.
Another approach to decrease DNA contaminants in rAAV particles is to redesign the vector and helper plasmids. First, it has been suggested that increasing the vector plasmid backbone size over the rAAV packaging capacity (>5 kb) can decrease significantly ITR-mediated reverse packaging and subsequently plasmid-derived contaminants (7.6-fold decrease between a 2.6- and a 7.0-kb backbone for an rAAV genome of 4.3–4.8 kb).65, 76 Furthermore, we and others have demonstrated that sequences close to ITRs are more prone to encapsidation.68, 77 In conclusion, as advised by Gray and Zolotukhin,78 it is more secure to clone the potential hazardous sequences of the vector plasmid, such as antibiotic-resistance genes, far from the ITRs and to insert large fragments of stuffer DNA (>5 kb) in between.
Proteins
Residual proteins found in purified vector stocks originate from helper viruses, producer cell lines, possible adventitious viruses, culture medium, and purification process. In particular, the clearance of proteins throughout the purification process needs to be documented, which is usually performed by measurement of total proteins concentration. Concentration of host cell proteins in the purified vector is measured using cell-line-specific ELISA kit (Host Cell Protein ELISA kits; Cygnus Technologies). Protein contaminants can also be identified using specific antibodies or mass spectrometry-based methods. Indeed, about 20 cellular proteins were identified by GeLC-MS (gel electrophoresis liquid chromatography-mass spectrometry) in rAAV stocks produced in HEK293 cells.79, 80 Among them, SET proteins were found associated to the full capsids in rAAV2 lots purified by CsCl gradient ultracentrifugation, independently of the serotype (2, 5, 6, 8, 9), but they were not detected or detected at lower level in IEX- or iodixanol-purified rAAV2 batches, respectively. This indicates that protein contamination in rAAV final stocks depends not only on the production system, but also on the purification method. Using liquid chromatography-tandem mass spectrometry (LC-MS/MS), Satkunanathan et al.81 found 44 cellular proteins that were associated to AAV2, AAV5, or AAV8 particles produced in HEK293T cells and purified by AVB immunoaffinity chromatography. Strikingly, nucleolin and nucleophosmin were found in the three above-mentioned studies. These two nucleolar proteins have been previously described to bind specifically AAV2 capsids in vitro82, 83, 84 and may be involved in AAV particles assembly. Actually, most of the identified proteins have already been described as co-factors that restrict or enhance AAV genome replication and capsid assembly or trafficking. However, another study has identified a protein a priori unrelated to AAV life cycle in rAAV6 vectors produced in HEK293 cells and purified through CsCl gradients.85 This protein, galectin-3 binding protein (G3BP), is naturally secreted in body fluids and was previously found to interact with the capsid of some AAV serotypes (1, 5, and 6) incubated in human serum.86 Interestingly, G3BP expressed in HEK293 cells was co-purified with AAV6 particles collected from the culture supernatant, but not with particles purified from the cell pellet.85 Indeed, the hyperglycosylated secreted form of G3BP was found to bind AAV6 capsids, whereas the hypoglycosylated intracellular form was not. As a consequence, gene transfer of a SEAP reporter gene was found less efficient in mice when using rAAV6 vectors purified from the culture supernatant, which highlights the importance of carefully analyzing contaminants when designing a manufacturing process.
In rAAV vectors produced in stable cell lines, contamination with Ad Caps was reported when purification was performed through CsCl gradients.34 Efficient removal of Ad capsid polypeptides is critical because they are highly immunogenic.87 This can be achieved by purification through IEX using appropriate separation conditions,34, 52, 88 and the use of affinity chromatography to specifically capture rAAV would also be a suitable option.
Similarly, residual HSV proteins or peptides present in the final AAV stocks could potentially elicit toxic or immune reactions in the host. Their detection is performed by in-house-developed ELISAs because there is no commercial HSV-1 ELISA currently available29, 89 (N.C., unpublished data). However, the assay accuracy is greatly challenged by the lack of standard for fully quantified HSV-1 preparations that are used to establish the standard curve. The purification methods, the matrices, and the accessibility of various HSV-1 epitopes all can affect the sensitivity and reliability of the assay, and render direct comparison between groups difficult. Typically, in-house-produced and chromatography-purified HSV-1 preparations have been used as protein standards29, 89 (N.C., unpublished data). Pre-clinical stocks tested showed values ranging from 17.2 and 120 ng/mL of HSV proteins or less than 16 ng per dose. No major response was detected in injected animals, but anti-HSV antibodies were detected at the highest doses.71, 72, 73, 74 In the AAT clinical trial, the AAV1 vector lot contained 834 ng/mL and the highest human dose tested contained approximately 72 μg of HSV proteins.75 Overall, published data showed that residual HSV proteins are detectable in final rAAV stocks, but extensive pre-clinical toxicology studies showed that these levels were tolerated in animals and no reactions in humans have been reported so far.
Infectious Helper Viruses
Residual Baculovirus
Baculoviruses can infect mammalian cells,90 and thus are used as viral vectors for gene transfer.91 Furthermore, 12 baculoviral genes, including the immediate early 1 gene,92 have been shown to be transcribed in HeLa and BHK cells after Autographa californica multiple nuclear polyhedrosis virus (AcMNPV) infection by DNA microarray and RT-PCR analysis.93 Although there is no evidence of their functionality, baculoviral DNA and proteins could be immunogenic.94 Indeed, contamination with baculovirus capsids is likely to activate an innate immune response as shown in vaccine preparations,95 which may negatively impact gene transfer efficiency. AAV vectors are generally recovered from insect cells by chemical lysis. Triton X-100, added at a concentration of 0.5% v/v at harvest, has the dual advantage of being efficient for cell membrane and baculovirus envelope disruption.96 This step inactivates most of the baculoviruses, but does not alter the integrity of the nucleocapsids. Of approximatively 50 nm in diameter and 300 nm in length, they are generally subsequently removed during the purification process, in particular at the filtration steps.97 For example, Planova 35-nm cutoff nanofilters have been proven to specifically clear infectious baculovirus spiked in rAAV vector stocks by 6 logs (below the 1.5 log10 limit of quantification of the 50% tissue culture infective dose [TCID50] test).98 Despite the fact that baculovirus contamination was a major concern of the Glybera report assessment,25 no standard assay is available to detect residual infectious baculovirus in rAAV batches. Nonetheless, baculovirus can transduce mammalian cells but are not able to replicate,99 and there is no evidence that baculoviruses are detrimental to human health, unless intravenously injected at very high doses (>109 IU). Furthermore, many recombinant products and vaccines are produced with BEV in insect cells.
Residual HSV
The major risk associated with the HSV method is the potential presence of residual rHSV from the inoculum used to infect the cells, as well as replication-competent variants that may occur during the rHSV stock production. HSVs are engineered from a replication-deficient variant of HSV type 1 (d27.1) that is lacking the ORF encoding for the viral ICP27 protein (infected cell protein 27). In the absence of ICP27, the rHSV genome cannot replicate unless ICP27 is provided in trans, like in the V27 cells, a Vero cell derivative stably transformed to express ICP27.100 V27 is the only cell line currently used for large-scale manufacturing of rHSV stocks used for AAV production. HSV stocks are prepared by infecting monolayers or V27 in flasks, or alternatively, in suspension using micro-carriers, harvested after 3–4 days and concentrated.28, 101 HSV stocks, used as raw materials in AAV clinical production, contain serum and V27 cell-derived impurities (DNA, proteins). Further, rcHSV or ICP27 revertants may arise during amplification on V27 by homologous recombination.102 There are two assays utilized to determine the presence of rHSV and rcHSV in HSV and ultimately in AAV stocks. HSV stocks are assayed both on V27 cells, to determine the plaque-forming unit (PFU) titer of rHSV, and on Vero cells, which do not contain ICP27, to assess the presence of rcHSV. Upon replication in the cell nucleus, rHSV or rcHSV induces a cytopathic effect and infected cells form plaques.28, 102 Detection of residual HSV should be established to ensure the lowest detection limit possible. Currently, detection limits as low as 10–20 PFUs/mL have been described.67, 89 Processes designed to purify AAV from an HSV-containing matrix typically contain at least one inactivation step. The two main methods described are: (1) use of powerful detergent such Triton X-100 at concentration as high as 1% v/v, added at collection of the harvest; and (2) low pH-induced flocculation of cellular and HSV proteins from denatured viral capsids. The first method has been thoroughly validated by viral clearance studies, demonstrating up to 6 log10 reduction of the rHSV input.102 The second has been tested at pre-clinical stage and has shown to be successful in reducing the rHSV input by several logs with a detection limit of 100 PFUs/mL.28 Virus inactivation by detergent is a widely accepted neutralization step and is likely more suitable for large-scale manufacturing. However, both methods present the challenge that typically the detergent-containing or low-pH matrices can be toxic or inhibit this cell-based assay, affecting the overall assay sensitivity. Ye and colleagues75, 102 demonstrated an overall HSV clearance of up to 14 log10 when treatment with Triton was combined with multi-step chromatography for an AAV1 drug product. It was calculated that the largest human dose administered in the AAT trial would theoretically contain less than 2.74 × 105 PFUs of rHSV, and that rcHSVs were not detectable.75, 102 Low levels of rcHSV have been shown in rHSV stocks, reported less than 1 PFU per 3 × 108 rHSV PFUs. It is worth noting that at the present time, guidance on the acceptable amounts of residual HSV in AAV drug products have yet to be issued by the regulatory agencies. Alternatively, or additionally, a PCR-based assay for ICP27 could be utilized for the detection of rHSV and/or rcHSV, with the major limitation that it will not indicate whether the particle is infectious. Serial passaging may reveal whether the detected signal is amplified over time, to determine whether the particles are replication competent and/or infectious.
Still, several small- to large-scale animal studies have been completed by various groups, using various AAV serotypes and production and purification methods, and a wide range of administered doses ranging from as low as 5 × 1010 vg/kg to an excess of 2 × 1014 vg/kg in mice and non-human primates. Pre-clinical evaluation by Investigational New Drug (IND)-enabling toxicology studies concluded that no life-threatening toxicity or immune reaction were observed, and the doses were all well tolerated.28, 29, 71, 72, 73, 74, 89 In addition, the highest dose administered to a human patient is 6 × 1012 vg/kg, or approximately 4.3 × 1014 vg total dose. No life-threatening reaction or major side effect has been reported, and the trial has been completed.75, 103 However, ongoing studies evaluating higher range doses for systemic disorders will tell more about the safety of AAV vectors produced with rHSVs (N.C., unpublished data).
Residual Ad
In the case of mammalian stable cell lines, the main issue is the use of wild-type adenovirus. As a main difference to HSV, inactivation of Ad is usually achieved by physical treatments. The most commonly used method is heat inactivation at a temperature where Ad capsid is disrupted and AAV capsid is not, usually between 51°C and 56°C, but other methods such as high pressure have been proposed.104 For rAAV1 vector manufacturing in HeLa producer cells, 10-min incubation at 52°C was shown to reduce Ad5 infectious titer by ∼6 log10 with no impact on AAV infectivity.88 For large-scale clinical-grade rAAV1 production, a complete Ad clearance was ensured by a complex process including specific steps such as: (1) IEX to capture Ad in the cleared lysate, (2) AAV capture step followed by Ad heat inactivation, and (3) further AAV purification step followed by nano-filtration and polishing.55
Another approach to reduce contamination with Ad5 is to use mutant Ads. In particular, the use of Ad with mutation in the E2b gene affecting the Ad DNA polymerase (ts149) or the pre-terminal protein (sub100r and del308ΔpTP) resulted in similar rAAV production yields compared with wild-type Ad5.31, 35, 105 However, production of these mutant helper viruses is more laborious because they replicate only at low temperature (i.e., 32°C for ts149 and sub100r) or in trans-complementing cells (HEK293-pTP for del308ΔpTP). The use of single-round replication helper with the Ad protease deleted (AdΔPS) was also investigated but resulted in less efficient rAAV production.106
AAV Defective Particles
The systems described above have been continuously improved, now allowing the production of approximatively 1 × 105 vector genomes per cell. These different platforms coexist in vector core facilities, each with their pro and cons. However, rare studies compared them side by side in terms of vector safety and efficiency. One important quality attribute is the proportion of defective particles in rAAV stocks (Figure 2, output). For example, the quantity of empty particles has not been systematically investigated (or at least reported), and indeed, they can represent the majority of the viral particles in rAAV batches. This was the case for the clinical-grade scAAV2/8-FIX lot injected in patients with hemophilia B, which contained not more than 20% of full particles despite size-exclusion and anion-exchange chromatography purification steps.66
The most comprehensive study investigating the rAAV packaging rate was realized from clarified cell lysates in HEK293 cells.107 Vector genome and physical particles titration were determined by dot blot and ELISA, respectively, revealing that only 1%–18% of rAAV2 particles contain the transgene in cleared lysate. Using three plasmids, other groups consistently reported that 6% and 18% of rAAV2 total particles contained the FIX108 and SEAP56 transgene, respectively, at harvest as determined by qPCR and ELISA assay. Qu et al. highlighted the lot-to-lot disparity of the p/vg ratio, ranging from 3 to 30 when the triple-transfection production method was employed.108
Few studies have addressed this issue in the other platforms. The production of rAAV2-FIX vectors in A549-derived producer cell line was shown to generate 8%–13% of full particles at harvest.35 A recent study compared purified rAAV2 vectors produced in stable cell lines or in transfected HEK293 cells using analytical ultracentrifugation (AUC).109 The results indicated that rAAV produced in stable cell lines contained lower amounts of empty particles. The proportion of full (vector genome-containing) particles was up to 74% for a 3.4 kb-vector and 40%–45% for a 4.2 kb-vector, whereas the same vectors produced by triple-transfection contained only 8% and 10%–12% of full particles, respectively.
In parallel, using rHSV vectors, it seems that the packaging rate is in the same order of magnitude than other systems. Indeed, Kang et al.89 quantified 8% of the total assembled rAAV2 particles that incorporated the gfp transgene in crude lysate. In insect cells, Amine Kamen’s group110, 111 demonstrated that the baculovirus infection conditions commonly used for Sf9 cells infection, i.e., equal amount of the three baculoviruses, lead to a percentage of full virions ranging from 0.4% to 0.9%, as determined by real-time PCR and ELISA at harvest. In the more recent dual-baculovirus infection system, 7%–30% of total assembled particles harbor the rAAV genome in rAAV2 and rAAV8 (M.P.-B., unpublished data). However, many parameters are involved in the full-to-empty ratio fluctuation (rAAV genome length and sequence, serotype, helper virus, purification method, measurement technique, etc.), rendering it difficult to convey a clear message regarding the packaging efficiency in each production system.
Impurities Related to the Purification Process
Cell Lysis and Clarification
AAV particles are not only intracellular and can be released to the supernatant depending on the serotype and culture conditions,112 AAV particles are highly resistant to extreme conditions of pH, detergent, and temperature. Thus, several methods based on chemical or mechanical lysis are available to retrieve rAAV vectors from the cells. Rudimentary mechanical technique to liberate rAAV vectors from the cells is freeze/thaw cycles followed by a low-speed centrifugation clarification step. However, this technique is not appropriate for large-scale purification. On the contrary, microfluidization is an efficient and scalable process that consists in the passage of producer cells through a small-diameter fluid path under high pressure. It also shears cellular DNA and reduces crude lysate viscosity, facilitating clarification. The advantage of physical methods is the lack of additional chemical reagents. Efficient cell lysis can also be performed using detergent such as Triton X-100,113 whereas Benzonase treatment is usually applied after cell lysis to reduce viscosity and remove free nucleic acids. Following cell lysis, the insoluble cell components are removed by low-speed centrifugation for small-scale production, and polyethylene glycol (PEG) may be used to concentrate rAAV particles. For large-volume cultures, a filtration step is performed by depth filtration or tangential flow filtration (TFF) to prevent clogging. If chemical reagents are used, such as Triton and PEG, they should be quantified in the final product as a process-related impurity. These chemicals are usually quantified by high-performance liquid chromatography (HPLC) methods. The same is true for nucleases that can be measured by commercial ELISA kits.
Purification by Ultracentrifugation
For research-grade batches, rAAV vectors are traditionally and usually purified by density gradient ultracentrifugation, e.g., CsCl isopycnic gradients114 or iodixanol step gradients.115 Interestingly, the CsCl method allows the separation of the particles containing the genome from empty particles by exploiting its specific buoyant density. The conventional purification protocol included two rounds of CsCl gradient centrifugation followed by dialysis against the appropriate buffer in order to remove cytotoxic CsCl.114 In parallel, AAV vectors purified on discontinuous iodixanol gradients also show a high degree of purity but harbor more empty particles (∼20%) as compared with CsCl-purified vectors (<1%).80 Two advantages of iodixanol-based protocols over CsCl gradients are that the ultracentrifugation is shorter (2 versus 24 hr) and that iodixanol is not toxic for humans because it is often used as radiocontrast agent. The major drawback of the density gradients methods is that they are not suitable, in the first instance, for direct purification from a large volume of cleared lysate. Nevertheless, a CsCl continuous gradient centrifugation can be included in a large-scale purification scheme after a previous step of concentration, in particular for empty particles removal.
Purification of rAAV Vectors Using Affinity Columns
Heparin columns were initially used to purify rAAV2 vectors.52, 115, 116, 117 Then, heparin-affinity chromatography has been successfully demonstrated to purify rAAV3 and rAAV6 vectors.62 Based on the same principle, mucin, a sialic-acid-rich protein, was used for rAAV1,116 rAAV4, rAAV5, and rAAV6 vector purification because these serotypes can bind to the cell surface via O-linked and N-linked sialic acid moieties.
In parallel, several antibodies that recognize different AAV capsid epitopes have been immobilized on a solid support matrix or surface. For example, A20 monoclonal antibody recognizes specifically assembled AAV particles of serotypes 2 and 3.2 It was immobilized on a HiTrap-Sepharose to efficiently purify rAAV2 particles.14 The most popular immunoaffinity column is the AVB Sepharose High Performance medium (GE Healthcare Life Sciences) that is adapted for purification of a broad spectrum of AAV serotypes (although at different efficiency depending on the serotype).20, 24, 118 The ligand of AVB column is a 14-kDa single-chain Llama antibody produced in yeast. The binding epitope of this antibody has been recently identified in the regions 326–331 and 656–672 of VP proteins, near the 5-fold symmetry axis.118 The AVB purification of AAV1, AAV2, AAV3B, AAV5, and AAVrh.10 is very efficient with a recovery ranging between 70% and 90%, whereas AAV8 and AAV9 poorly bind to this matrix. A brand-new immunoaffinity matrix has just been commercialized by Thermo Fisher Scientific, the POROS CaptureSelect AAVX Affinity Matrix. It has been designed for the purification of AAV particles of various serotypes (AAV1–8 and AAVrh.10) and will probably replace POROS CaptureSelect AAV8 and AAV9 affinity resins that are currently being used only for those serotypes. This new immunoaffinity matrix seems very promising and versatile as it is intended for the purification of different rAAV serotypes, according to the manufacturer. In contrast with density gradients, immunoaffinity chromatography is suitable for large-scale production,119 but unfortunately does not discriminate between empty and full particles. In terms of impurities, it is well recognized that affinity columns based on antibodies suffer from leaching, i.e., antibodies detaching from the support and found in the final product. Indeed, to quantify the residual ligands, both GE and Thermo Fisher commercialize its respective ELISA kits.
Vector Purification on IEX
The principle of IEX is the recovery of particles from the clarified cell lysate based on their net surface charge. The surface charge of AAV can be positive or negative depending on the environmental pH.
Several chromatography supports can be used as membrane absorbers (Mustang S), stationary monoliths (CIM), or particle-based adsorbents (Sepharose, POROS). For better purity, the purification protocol can be consolidated by a second column based on IEX, hydrophobic interaction chromatography (HIC),120 or size-exclusion chromatography (SEC)121 principle. Despite the small difference between empty and full particles isoelectric points (6.3 and 5.9, respectively),122 it has been shown that IEX can separate empty capsids from full genome-containing particles by optimizing elution conditions.108, 123, 124 Furthermore, the IEX-based processes are reproducible and can be automated and adapted to large-scale production in order to fulfill industrial manufacturing conditions. However, substantial development of IEX protocols is usually needed for the following reasons: (1) IEX is very sensitive to changes in the upstream biomass (cell density, media composition, pH, etc.), and (2) it is hard to establish an optimal method for all rAAV serotypes/variants because their capsid physicochemical properties differ. In terms of impurities, there is no need of specific chemical reagents other than salts and pH buffers, and the supports used for IEX do not suffer from leaching.
Formulation and rAAV Particle Stability
rAAV particles are usually formulated in saline buffers with pH between 6.5 and 8 (PBS, DPBS supplemented with MgCa, lactate Ringer’s solution, saline ocular solution, etc.) according to the optimal conditions for vector stability and to the application. AAV particles have been found to be remarkably stable at high temperature (up to 60°C for most of the serotypes)125, 126, 127 and in a pH ranging from 5.5 to 8.5.128 Despite the high resistance of AAV capsids to extreme conditions, other problems can occur such as particles aggregation. This can be a real issue for highly concentrated rAAV stocks, notably for rAAV2. It has been shown that particles aggregation can be minimized by increasing the ionic strength of the solution, preferably with divalent ions salts (∼200 mM magnesium sulfate).129, 130 Non-ionic surfactant can also be added at low concentration to avoid this phenomenon,131 such as Pluronic F68 poloxamer that was added at a volume concentration of 0.001% in the final product used for a hemophilia B clinical trial.65 Even if Pluronic F68 has been proven to increase in process recovery of rAAV2, rAAV4, rAAV5, rAAV8, and rAAV9,132 no effect was observed on rAAV2-purified product.129
Conclusions
The market authorization of Glybera (developed by uniQure using Sf9 cells) and the recent recommendation of the FDA to approve Luxturna, an AAV2 vector for the treatment of Leber congenital amaurosis (developed by Spark Therapeutics using HEK293 cells), demonstrate the maturity of AAV-based gene therapy drugs. Moreover, hundreds of pre-clinical and clinical studies conducted thus far have all provided supporting data for the use of AAV in the clinical setting, independently of the manufacturing method. Despite significant improvements in upstream and downstream processes, AAV stocks still contain significant amounts of impurities that should be minimized as much as possible during production, and quantified and identified accurately by improved analytical methods, such as HTS for nucleic acids and mass spectrometry for proteins. Nonetheless, with hundreds of patients enrolled in AAV trials up to date, no adverse events have been reported in relation to any of these impurities. In the near future, particular attention will be paid in those applications in which high doses of vectors will be administered systemically (>1 × 1014 vg/kg), for example, in Duchenne muscular dystrophy, because the total amount of impurities will be high and the route of administration is more prone to trigger immuno-toxicological responses. In the absence of comparative studies between manufacturing platforms, the field will have to rely on preclinical toxicology reports and phase I/II trial results to evaluate the safety and efficacy of the different production systems.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
Financial support to E.A.’s laboratory was provided by INSERM, University of Nantes, CHU of Nantes, Fondation d’Entreprises pour la Thérapie Génique en Pays de la Loire, and Région Pays de la Loire and Pre-industrial gene therapy consortium (Agence Nationale de la Recherche-investissements d’avenir, grant number 10-DPBS-0001).
References
- 1.Weitzman M.D., Kyöstiö S.R., Kotin R.M., Owens R.A. Adeno-associated virus (AAV) Rep proteins mediate complex formation between AAV DNA and its integration site in human DNA. Proc. Natl. Acad. Sci. USA. 1994;91:5808–5812. doi: 10.1073/pnas.91.13.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wistuba A., Kern A., Weger S., Grimm D., Kleinschmidt J.A. Subcellular compartmentalization of adeno-associated virus type 2 assembly. J. Virol. 1997;71:1341–1352. doi: 10.1128/jvi.71.2.1341-1352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermonat P.L., Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc. Natl. Acad. Sci. USA. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao G., Zhong L., Danos O. Exploiting natural diversity of AAV for the design of vectors with novel properties. Methods Mol. Biol. 2011;807:93–118. doi: 10.1007/978-1-61779-370-7_4. [DOI] [PubMed] [Google Scholar]
- 5.Rabinowitz J.E., Rolling F., Li C., Conrath H., Xiao W., Xiao X., Samulski R.J. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q., Wu Z., Zhang J., Firrman J., Wei H., Zhuang Z., Liu L., Miao L., Hu Y., Li D. A robust system for production of superabundant VP1 recombinant AAV vectors. Mol. Ther. Methods Clin. Dev. 2017;7:146–156. doi: 10.1016/j.omtm.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong B., Moore A.R., Dai J., Roberts S., Chu K., Kapranov P., Moss B., Xiao W. A concept of eliminating nonhomologous recombination for scalable and safe AAV vector generation for human gene therapy. Nucleic Acids Res. 2013;41:6609–6617. doi: 10.1093/nar/gkt404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galli A., Della Latta V., Bologna C., Pucciarelli D., Cipriani F., Backovic A., Cervelli T. Strategies to optimize capsid protein expression and single-stranded DNA formation of adeno-associated virus in Saccharomyces cerevisiae. J. Appl. Microbiol. 2017;123:414–428. doi: 10.1111/jam.13511. [DOI] [PubMed] [Google Scholar]
- 9.Backovic A., Cervelli T., Salvetti A., Zentilin L., Giacca M., Galli A. Capsid protein expression and adeno-associated virus like particles assembly in Saccharomyces cerevisiae. Microb. Cell Fact. 2012;11:124. doi: 10.1186/1475-2859-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thakur, S.S. (2012). Production of recombinant adeno-associated viral vectors in yeast. MS thesis (Gainesville, FL: University of Florida). http://ufdc.ufl.edu/UFE0044702/00001.
- 11.Snyder, R.O. April 2011. Production of recombinant AAV virions. U.S. patent 7927585 B2.
- 12.Barajas D., Aponte-Ubillus J.J., Akeefe H., Cinek T., Peltier J., Gold D. Generation of infectious recombinant Adeno-associated virus in Saccharomyces cerevisiae. PLoS ONE. 2017;12:e0173010. doi: 10.1371/journal.pone.0173010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urabe M., Ding C., Kotin R.M. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- 14.Grimm D., Kern A., Rittner K., Kleinschmidt J.A. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum. Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 15.Schnödt M., Schmeer M., Kracher B., Krüsemann C., Espinosa L.E., Grünert A., Fuchsluger T., Rischmüller A., Schleef M., Büning H. DNA minicircle technology improves purity of adeno-associated viral vector preparations. Mol. Ther. Nucleic Acids. 2016;5:e355. doi: 10.1038/mtna.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grieger J.C., Soltys S.M., Samulski R.J. Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Mol. Ther. 2016;24:287–297. doi: 10.1038/mt.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotin R.M., Snyder R.O. Manufacturing clinical grade recombinant adeno-associated virus using invertebrate cell lines. Hum. Gene Ther. 2017;28:350–360. doi: 10.1089/hum.2017.042. [DOI] [PubMed] [Google Scholar]
- 18.Virag T., Cecchini S., Kotin R.M. Producing recombinant adeno-associated virus in foster cells: overcoming production limitations using a baculovirus-insect cell expression strategy. Hum. Gene Ther. 2009;20:807–817. doi: 10.1089/hum.2009.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galibert L., Merten O.-W. Latest developments in the large-scale production of adeno-associated virus vectors in insect cells toward the treatment of neuromuscular diseases. J. Invertebr. Pathol. 2011;107(Suppl):S80–S93. doi: 10.1016/j.jip.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Smith R.H., Levy J.R., Kotin R.M. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 2009;17:1888–1896. doi: 10.1038/mt.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughn J.L., Goodwin R.H., Tompkins G.J., McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae) In Vitro. 1977;13:213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- 22.Aucoin M.G., Mena J.A., Kamen A.A. Bioprocessing of baculovirus vectors: a review. Curr. Gene Ther. 2010;10:174–186. doi: 10.2174/156652310791321288. [DOI] [PubMed] [Google Scholar]
- 23.Mietzsch M., Hering H., Hammer E.-M., Agbandje-McKenna M., Zolotukhin S., Heilbronn R. OneBac 2.0: Sf9 cell lines for production of AAV1, AAV2, and AAV8 vectors with minimal encapsidation of foreign DNA. Hum. Gene Ther. Methods. 2017;28:15–22. doi: 10.1089/hgtb.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mietzsch M., Grasse S., Zurawski C., Weger S., Bennett A., Agbandje-McKenna M., Muzyczka N., Zolotukhin S., Heilbronn R. OneBac: platform for scalable and high-titer production of adeno-associated virus serotype 1-12 vectors for gene therapy. Hum. Gene Ther. 2014;25:212–222. doi: 10.1089/hum.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Medicines Agency (EMA). (2012). Assessment report: Glybera. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002145/WC500135476.pdf.
- 26.Adamson-Small L., Potter M., Byrne B.J., Clément N. Sodium chloride enhances recombinant adeno-associated virus production in a serum-free suspension manufacturing platform using the herpes simplex virus system. Hum. Gene Ther. Methods. 2017;28:1–14. doi: 10.1089/hgtb.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas D.L., Wang L., Niamke J., Liu J., Kang W., Scotti M.M., Ye G.J., Veres G., Knop D.R. Scalable recombinant adeno-associated virus production using recombinant herpes simplex virus type 1 coinfection of suspension-adapted mammalian cells. Hum. Gene Ther. 2009;20:861–870. doi: 10.1089/hum.2009.004. [DOI] [PubMed] [Google Scholar]
- 28.Adamson-Small L., Potter M., Falk D.J., Cleaver B., Byrne B.J., Clément N. A scalable method for the production of high-titer and high-quality adeno-associated type 9 vectors using the HSV platform. Mol. Ther. Methods Clin. Dev. 2016;3:16031. doi: 10.1038/mtm.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chulay J.D., Ye G.-J., Thomas D.L., Knop D.R., Benson J.M., Hutt J.A., Wang G., Humphries M., Flotte T.R. Preclinical evaluation of a recombinant adeno-associated virus vector expressing human alpha-1 antitrypsin made using a recombinant herpes simplex virus production method. Hum. Gene Ther. 2011;22:155–165. doi: 10.1089/hum.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark K.R., Voulgaropoulou F., Fraley D.M., Johnson P.R. Cell lines for the production of recombinant adeno-associated virus. Hum. Gene Ther. 1995;6:1329–1341. doi: 10.1089/hum.1995.6.10-1329. [DOI] [PubMed] [Google Scholar]
- 31.Gao G.P., Qu G., Faust L.Z., Engdahl R.K., Xiao W., Hughes J.V., Zoltick P.W., Wilson J.M. High-titer adeno-associated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum. Gene Ther. 1998;9:2353–2362. doi: 10.1089/hum.1998.9.16-2353. [DOI] [PubMed] [Google Scholar]
- 32.Chadeuf G., Favre D., Tessier J., Provost N., Nony P., Kleinschmidt J., Moullier P., Salvetti A. Efficient recombinant adeno-associated virus production by a stable rep-cap HeLa cell line correlates with adenovirus-induced amplification of the integrated rep-cap genome. J. Gene Med. 2000;2:260–268. doi: 10.1002/1521-2254(200007/08)2:4<260::AID-JGM111>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Mathews L.C., Gray J.T., Gallagher M.R., Snyder R.O. Recombinant adeno-associated viral vector production using stable packaging and producer cell lines. Methods Enzymol. 2002;346:393–413. doi: 10.1016/s0076-6879(02)46068-5. [DOI] [PubMed] [Google Scholar]
- 34.Gao G., Qu G., Burnham M.S., Huang J., Chirmule N., Joshi B., Yu Q.C., Marsh J.A., Conceicao C.M., Wilson J.M. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum. Gene Ther. 2000;11:2079–2091. doi: 10.1089/104303400750001390. [DOI] [PubMed] [Google Scholar]
- 35.Farson D., Harding T.C., Tao L., Liu J., Powell S., Vimal V., Yendluri S., Koprivnikar K., Ho K., Twitty C. Development and characterization of a cell line for large-scale, serum-free production of recombinant adeno-associated viral vectors. J. Gene Med. 2004;6:1369–1381. doi: 10.1002/jgm.622. [DOI] [PubMed] [Google Scholar]
- 36.Tessier J., Chadeuf G., Nony P., Avet-Loiseau H., Moullier P., Salvetti A. Characterization of adenovirus-induced inverted terminal repeat-independent amplification of integrated adeno-associated virus rep-cap sequences. J. Virol. 2001;75:375–383. doi: 10.1128/JVI.75.1.375-383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao G.P., Lu F., Sanmiguel J.C., Tran P.T., Abbas Z., Lynd K.S., Marsh J., Spinner N.B., Wilson J.M. Rep/Cap gene amplification and high-yield production of AAV in an A549 cell line expressing Rep/Cap. Mol. Ther. 2002;5:644–649. doi: 10.1006/mthe.2001.0591. [DOI] [PubMed] [Google Scholar]
- 38.Nony P., Tessier J., Chadeuf G., Ward P., Giraud A., Dugast M., Linden R.M., Moullier P., Salvetti A. Novel cis-acting replication element in the adeno-associated virus type 2 genome is involved in amplification of integrated rep-cap sequences. J. Virol. 2001;75:9991–9994. doi: 10.1128/JVI.75.20.9991-9994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.François A., Guilbaud M., Awedikian R., Chadeuf G., Moullier P., Salvetti A. The cellular TATA binding protein is required for rep-dependent replication of a minimal adeno-associated virus type 2 p5 element. J. Virol. 2005;79:11082–11094. doi: 10.1128/JVI.79.17.11082-11094.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark K.R., Voulgaropoulou F., Johnson P.R. A stable cell line carrying adenovirus-inducible rep and cap genes allows for infectivity titration of adeno-associated virus vectors. Gene Ther. 1996;3:1124–1132. [PubMed] [Google Scholar]
- 41.Liu X.L., Clark K.R., Johnson P.R. Production of recombinant adeno-associated virus vectors using a packaging cell line and a hybrid recombinant adenovirus. Gene Ther. 1999;6:293–299. doi: 10.1038/sj.gt.3300807. [DOI] [PubMed] [Google Scholar]
- 42.Toublanc E., Benraiss A., Bonnin D., Blouin V., Brument N., Cartier N., Epstein A.L., Moullier P., Salvetti A. Identification of a replication-defective herpes simplex virus for recombinant adeno-associated virus type 2 (rAAV2) particle assembly using stable producer cell lines. J. Gene Med. 2004;6:555–564. doi: 10.1002/jgm.542. [DOI] [PubMed] [Google Scholar]
- 43.Yang Q., Chen F., Trempe J.P. Characterization of cell lines that inducibly express the adeno-associated virus Rep proteins. J. Virol. 1994;68:4847–4856. doi: 10.1128/jvi.68.8.4847-4856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saudan P., Vlach J., Beard P. Inhibition of S-phase progression by adeno-associated virus Rep78 protein is mediated by hypophosphorylated pRb. EMBO J. 2000;19:4351–4361. doi: 10.1093/emboj/19.16.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z., Brister J.R., Im D.-S., Muzyczka N. Characterization of the adenoassociated virus Rep protein complex formed on the viral origin of DNA replication. Virology. 2003;313:364–376. doi: 10.1016/s0042-6822(03)00340-4. [DOI] [PubMed] [Google Scholar]
- 46.Berthet C., Raj K., Saudan P., Beard P. How adeno-associated virus Rep78 protein arrests cells completely in S phase. Proc. Natl. Acad. Sci. USA. 2005;102:13634–13639. doi: 10.1073/pnas.0504583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt M., Afione S., Kotin R.M. Adeno-associated virus type 2 Rep78 induces apoptosis through caspase activation independently of p53. J. Virol. 2000;74:9441–9450. doi: 10.1128/jvi.74.20.9441-9450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang L.S., Shi Y., Shenk T. Adeno-associated virus P5 promoter contains an adenovirus E1A-inducible element and a binding site for the major late transcription factor. J. Virol. 1989;63:3479–3488. doi: 10.1128/jvi.63.8.3479-3488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiao C., Wang B., Zhu X., Li J., Xiao X. A novel gene expression control system and its use in stable, high-titer 293 cell-based adeno-associated virus packaging cell lines. J. Virol. 2002;76:13015–13027. doi: 10.1128/JVI.76.24.13015-13027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizukami H., Okada T., Ogasawara Y., Matsushita T., Urabe M., Kume A., Ozawa K. Separate control of Rep and Cap expression using mutant and wild-type loxP sequences and improved packaging system for adeno-associated virus vector production. Mol. Biotechnol. 2004;27:7–14. doi: 10.1385/MB:27:1:07. [DOI] [PubMed] [Google Scholar]
- 51.Yuan Z., Qiao C., Hu P., Li J., Xiao X. A versatile adeno-associated virus vector producer cell line method for scalable vector production of different serotypes. Hum. Gene Ther. 2011;22:613–624. doi: 10.1089/hum.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark K.R., Liu X., McGrath J.P., Johnson P.R. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum. Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
- 53.Brument N., Morenweiser R., Blouin V., Toublanc E., Raimbaud I., Chérel Y., Folliot S., Gaden F., Boulanger P., Kroner-Lux G. A versatile and scalable two-step ion-exchange chromatography process for the purification of recombinant adeno-associated virus serotypes-2 and -5. Mol. Ther. 2002;6:678–686. doi: 10.1006/mthe.2002.0719. [DOI] [PubMed] [Google Scholar]
- 54.Flotte T., Carter B., Conrad C., Guggino W., Reynolds T., Rosenstein B., Taylor G., Walden S., Wetzel R. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum. Gene Ther. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- 55.Thorne B.A., Takeya R.K., Peluso R.W. Manufacturing recombinant adeno-associated viral vectors from producer cell clones. Hum. Gene Ther. 2009;20:707–714. doi: 10.1089/hum.2009.070. [DOI] [PubMed] [Google Scholar]
- 56.Martin J., Frederick A., Luo Y., Jackson R., Joubert M., Sol B., Poulin F., Pastor E., Armentano D., Wadsworth S., Vincent K. Generation and characterization of adeno-associated virus producer cell lines for research and preclinical vector production. Hum. Gene Ther. Methods. 2013;24:253–269. doi: 10.1089/hgtb.2013.046. [DOI] [PubMed] [Google Scholar]
- 57.Blouin V., Brument N., Toublanc E., Raimbaud I., Moullier P., Salvetti A. Improving rAAV production and purification: towards the definition of a scaleable process. J. Gene Med. 2004;6(Suppl 1):S223–S228. doi: 10.1002/jgm.505. [DOI] [PubMed] [Google Scholar]
- 58.Wright J.F. Product-related impurities in clinical-grade recombinant AAV vectors: characterization and risk assessment. Biomedicines. 2014;2:80–97. doi: 10.3390/biomedicines2010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.U.S. Food and Drug Administration (FDA). (2012). FDA briefing document: vaccines and related biological products advisory committee meeting: cell lines derived from human tumors for vaccine manufacture. http://www.nvic.org/cmstemplates/nvic/pdf/fda/fda-briefing-09192012.pdf.
- 60.Wright J.F., Zelenaia O. Vector characterization methods for quality control testing of recombinant adeno-associated viruses. Methods Mol. Biol. 2011;737:247–278. doi: 10.1007/978-1-61779-095-9_11. [DOI] [PubMed] [Google Scholar]
- 61.Flotte T.R., Barraza-Ortiz X., Solow R., Afione S.A., Carter B.J., Guggino W.B. An improved system for packaging recombinant adeno-associated virus vectors capable of in vivo transduction. Gene Ther. 1995;2:29–37. [PubMed] [Google Scholar]
- 62.Halbert C.L., Allen J.M., Miller A.D. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J. Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halbert C.L., Metzger M.J., Lam S.L., Miller A.D. Capsid-expressing DNA in AAV vectors and its elimination by use of an oversize capsid gene for vector production. Gene Ther. 2011;18:411–417. doi: 10.1038/gt.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lecomte E., Tournaire B., Cogné B., Dupont J.-B., Lindenbaum P., Martin-Fontaine M., Broucque F., Robin C., Hebben M., Merten O.W. Advanced characterization of DNA molecules in rAAV vector preparations by single-stranded virus next-generation sequencing. Mol. Ther. Nucleic Acids. 2015;4:e260. doi: 10.1038/mtna.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hauck B., Murphy S.L., Smith P.H., Qu G., Liu X., Zelenaia O., Mingozzi F., Sommer J.M., High K.A., Wright J.F. Undetectable transcription of cap in a clinical AAV vector: implications for preformed capsid in immune responses. Mol. Ther. 2009;17:144–152. doi: 10.1038/mt.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allay J.A., Sleep S., Long S., Tillman D.M., Clark R., Carney G., Fagone P., McIntosh J.H., Nienhuis A.W., Davidoff A.M. Good manufacturing practice production of self-complementary serotype 8 adeno-associated viral vector for a hemophilia B clinical trial. Hum. Gene Ther. 2011;22:595–604. doi: 10.1089/hum.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye G.J., Scotti M.M., Liu J., Wang L., Knop D.R., Veres G. Clearance and characterization of residual HSV DNA in recombinant adeno-associated virus produced by an HSV complementation system. Gene Ther. 2011;18:135–144. doi: 10.1038/gt.2010.102. [DOI] [PubMed] [Google Scholar]
- 68.Penaud-Budloo M., Lecomte E., Guy-Duché A., Saleun S., Roulet A., Lopez-Roques C., Tournaire B., Cogné B., Léger A., Blouin V. Accurate identification and quantification of DNA species by next-generation sequencing in adeno-associated viral vectors produced in insect cells. Hum. Gene Ther. Methods. 2017;28:148–162. doi: 10.1089/hgtb.2016.185. [DOI] [PubMed] [Google Scholar]
- 69.Chadeuf G., Ciron C., Moullier P., Salvetti A. Evidence for encapsidation of prokaryotic sequences during recombinant adeno-associated virus production and their in vivo persistence after vector delivery. Mol. Ther. 2005;12:744–753. doi: 10.1016/j.ymthe.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Nambiar B., Cornell Sookdeo C., Berthelette P., Jackson R., Piraino S., Burnham B., Nass S., Souza D., O’Riordan C.R., Vincent K.A. Characteristics of minimally oversized adeno-associated virus vectors encoding human factor VIII generated using producer cell lines and triple transfection. Hum. Gene Ther. Methods. 2017;28:23–38. doi: 10.1089/hgtb.2016.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye G.-J., Budzynski E., Sonnentag P., Miller P.E., Sharma A.K., Ver Hoeve J.N., Howard K., Knop D.R., Neuringer M., McGill T. Safety and biodistribution evaluation in Cynomolgus macaques of rAAV2tYF-CB-hRS1, a recombinant adeno-associated virus vector expressing retinoschisin. Hum. Gene Ther. Clin. Dev. 2015;26:165–176. doi: 10.1089/humc.2015.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye G.J., Budzynski E., Sonnentag P., Nork T.M., Miller P.E., McPherson L., Ver Hoeve J.N., Smith L.M., Arndt T., Mandapati S. Safety and biodistribution evaluation in CNGB3-deficient mice of rAAV2tYF-PR1.7-hCNGB3, a recombinant AAV vector for treatment of achromatopsia. Hum. Gene Ther. Clin. Dev. 2016;27:27–36. doi: 10.1089/humc.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye G.J., Budzynski E., Sonnentag P., Nork T.M., Miller P.E., Sharma A.K., Ver Hoeve J.N., Smith L.M., Arndt T., Calcedo R. Safety and biodistribution evaluation in Cynomolgus macaques of rAAV2tYF-PR1.7-hCNGB3, a recombinant AAV vector for treatment of achromatopsia. Hum. Gene Ther. Clin. Dev. 2016;27:37–48. doi: 10.1089/humc.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye G.-J., Conlon T., Erger K., Sonnentag P., Sharma A.K., Howard K., Knop D.R., Chulay J.D. Safety and biodistribution evaluation of rAAV2tYF-CB-hRS1, a recombinant adeno-associated virus vector expressing retinoschisin, in RS1-deficient mice. Hum. Gene Ther. Clin. Dev. 2015;26:177–184. doi: 10.1089/humc.2015.077. [DOI] [PubMed] [Google Scholar]
- 75.Flotte T.R., Trapnell B.C., Humphries M., Carey B., Calcedo R., Rouhani F., Campbell-Thompson M., Yachnis A.T., Sandhaus R.A., McElvaney N.G. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum. Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith P.H., Wright F.J., Qu G., Patarroyo-White S., Parker A., Sommer J.M. Packaging of host cell and plasmid DNA into recombinant adeno-associated virus particles produced by triple transfection. Mol. Ther. 2003;7:S348. [Google Scholar]
- 77.Brimble M., Zhou J., Morton C., Meagher M., Nathwani A.C., Gray J.T., Davidoff A.M. AAV preparations contain contamination from DNA sequences in production plasmids directly outside of the ITRs. Mol. Ther. 2016;24(Suppl 1):S218–S219. [Google Scholar]
- 78.Gray J.T., Zolotukhin S. Design and construction of functional AAV vectors. Methods Mol. Biol. 2011;807:25–46. doi: 10.1007/978-1-61779-370-7_2. [DOI] [PubMed] [Google Scholar]
- 79.Dong B., Duan X., Chow H.Y., Chen L., Lu H., Wu W., Hauck B., Wright F., Kapranov P., Xiao W. Proteomics analysis of co-purifying cellular proteins associated with rAAV vectors. PLoS ONE. 2014;9:e86453. doi: 10.1371/journal.pone.0086453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strobel B., Miller F.D., Rist W., Lamla T. Comparative analysis of cesium chloride- and iodixanol-based purification of recombinant adeno-associated viral vectors for preclinical applications. Hum. Gene Ther. Methods. 2015;26:147–157. doi: 10.1089/hgtb.2015.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Satkunanathan S., Wheeler J., Thorpe R., Zhao Y. Establishment of a novel cell line for the enhanced production of recombinant adeno-associated virus vectors for gene therapy. Hum. Gene Ther. 2014;25:929–941. doi: 10.1089/hum.2014.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qiu J., Brown K.E. A 110-kDa nuclear shuttle protein, nucleolin, specifically binds to adeno-associated virus type 2 (AAV-2) capsid. Virology. 1999;257:373–382. doi: 10.1006/viro.1999.9664. [DOI] [PubMed] [Google Scholar]
- 83.Johnson J.S., Samulski R.J. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J. Virol. 2009;83:2632–2644. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bevington J.M., Needham P.G., Verrill K.C., Collaco R.F., Basrur V., Trempe J.P. Adeno-associated virus interactions with B23/Nucleophosmin: identification of sub-nucleolar virion regions. Virology. 2007;357:102–113. doi: 10.1016/j.virol.2006.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Denard J., Jenny C., Blouin V., Moullier P., Svinartchouk F. Different protein composition and functional properties of adeno-associated virus-6 vector manufactured from the culture medium and cell lysates. Mol. Ther. Methods Clin. Dev. 2014;1:14031. doi: 10.1038/mtm.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Denard J., Beley C., Kotin R., Lai-Kuen R., Blot S., Leh H., Asokan A., Samulski R.J., Moullier P., Voit T. Human galectin 3 binding protein interacts with recombinant adeno-associated virus type 6. J. Virol. 2012;86:6620–6631. doi: 10.1128/JVI.00297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muruve D.A. The innate immune response to adenovirus vectors. Hum. Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 88.Thorne B.A., Quigley P., Nichols G., Moore C., Pastor E., Price D., Ament J.W., Takeya R.K., Peluso R.W. Characterizing clearance of helper adenovirus by a clinical rAAV1 manufacturing process. Biologicals. 2008;36:7–18. doi: 10.1016/j.biologicals.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 89.Kang W., Wang L., Harrell H., Liu J., Thomas D.L., Mayfield T.L., Scotti M.M., Ye G.J., Veres G., Knop D.R. An efficient rHSV-based complementation system for the production of multiple rAAV vector serotypes. Gene Ther. 2009;16:229–239. doi: 10.1038/gt.2008.158. [DOI] [PubMed] [Google Scholar]
- 90.van Loo N.D., Fortunati E., Ehlert E., Rabelink M., Grosveld F., Scholte B.J. Baculovirus infection of nondividing mammalian cells: mechanisms of entry and nuclear transport of capsids. J. Virol. 2001;75:961–970. doi: 10.1128/JVI.75.2.961-970.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kost T.A., Condreay J.P. Recombinant baculoviruses as mammalian cell gene-delivery vectors. Trends Biotechnol. 2002;20:173–180. doi: 10.1016/s0167-7799(01)01911-4. [DOI] [PubMed] [Google Scholar]
- 92.Laakkonen J.P., Kaikkonen M.U., Ronkainen P.H.A., Ihalainen T.O., Niskanen E.A., Häkkinen M., Salminen M., Kulomaa M.S., Ylä-Herttuala S., Airenne K.J., Vihinen-Ranta M. Baculovirus-mediated immediate-early gene expression and nuclear reorganization in human cells. Cell. Microbiol. 2008;10:667–681. doi: 10.1111/j.1462-5822.2007.01074.x. [DOI] [PubMed] [Google Scholar]
- 93.Fujita R., Matsuyama T., Yamagishi J., Sahara K., Asano S., Bando H. Expression of Autographa californica multiple nucleopolyhedrovirus genes in mammalian cells and upregulation of the host beta-actin gene. J. Virol. 2006;80:2390–2395. doi: 10.1128/JVI.80.5.2390-2395.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abe T., Matsuura Y. Host innate immune responses induced by baculovirus in mammals. Curr. Gene Ther. 2010;10:226–231. doi: 10.2174/156652310791321279. [DOI] [PubMed] [Google Scholar]
- 95.Margine I., Martinez-Gil L., Chou Y.Y., Krammer F. Residual baculovirus in insect cell-derived influenza virus-like particle preparations enhances immunogenicity. PLoS ONE. 2012;7:e51559. doi: 10.1371/journal.pone.0051559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rueda P., Fominaya J., Langeveld J.P., Bruschke C., Vela C., Casal J.I. Effect of different baculovirus inactivation procedures on the integrity and immunogenicity of porcine parvovirus-like particles. Vaccine. 2000;19:726–734. doi: 10.1016/s0264-410x(00)00259-0. [DOI] [PubMed] [Google Scholar]
- 97.Grein T.A., Michalsky R., Czermak P. Virus separation using membranes. Methods Mol. Biol. 2014;1104:459–491. doi: 10.1007/978-1-62703-733-4_26. [DOI] [PubMed] [Google Scholar]
- 98.Hermens, W.T.J.M.C., & Smith, J.P. March 2013. Removal of contaminating viruses from AAV preparations. World Intellectual Property Organization Patent 2013/036118A1.
- 99.Tjia S.T., zu Altenschildesche G.M., Doerfler W. Autographa californica nuclear polyhedrosis virus (AcNPV) DNA does not persist in mass cultures of mammalian cells. Virology. 1983;125:107–117. doi: 10.1016/0042-6822(83)90067-3. [DOI] [PubMed] [Google Scholar]
- 100.Rice S.A., Knipe D.M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J. Virol. 1990;64:1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Knop D.R., Harrell H. Bioreactor production of recombinant herpes simplex virus vectors. Biotechnol. Prog. 2007;23:715–721. doi: 10.1021/bp060373p. [DOI] [PubMed] [Google Scholar]
- 102.Ye G.J., Scotti M.M., Thomas D.L., Wang L., Knop D.R., Chulay J.D. Herpes simplex virus clearance during purification of a recombinant adeno-associated virus serotype 1 vector. Hum. Gene Ther. Clin. Dev. 2014;25:212–217. doi: 10.1089/humc.2014.060. [DOI] [PubMed] [Google Scholar]
- 103.Mueller C., Gernoux G., Gruntman A.M., Borel F., Reeves E.P., Calcedo R., Rouhani F.N., Yachnis A., Humphries M., Campbell-Thompson M. 5 year expression and neutrophil defect repair after gene therapy in alpha-1 antitrypsin deficiency. Mol. Ther. 2017;25:1387–1394. doi: 10.1016/j.ymthe.2017.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leonard J.N., Ferstl P., Delgado A., Schaffer D.V. Enhanced preparation of adeno-associated viral vectors by using high hydrostatic pressure to selectively inactivate helper adenovirus. Biotechnol. Bioeng. 2007;97:1170–1179. doi: 10.1002/bit.21355. [DOI] [PubMed] [Google Scholar]
- 105.Maxwell I.H., Maxwell F., Schaack J. An adenovirus type 5 mutant with the preterminal protein gene deleted efficiently provides helper functions for the production of recombinant adeno-associated virus. J. Virol. 1998;72:8371–8373. doi: 10.1128/jvi.72.10.8371-8373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jenny C., Toublanc E., Danos O., Merten O.W. Evaluation of a serum-free medium for the production of rAAV-2 using HeLa derived producer cells. Cytotechnology. 2005;49:11–23. doi: 10.1007/s10616-005-5361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grimm D., Kern A., Pawlita M., Ferrari F., Samulski R., Kleinschmidt J. Titration of AAV-2 particles via a novel capsid ELISA: packaging of genomes can limit production of recombinant AAV-2. Gene Ther. 1999;6:1322–1330. doi: 10.1038/sj.gt.3300946. [DOI] [PubMed] [Google Scholar]
- 108.Qu G., Bahr-Davidson J., Prado J., Tai A., Cataniag F., McDonnell J., Zhou J., Hauck B., Luna J., Sommer J.M. Separation of adeno-associated virus type 2 empty particles from genome containing vectors by anion-exchange column chromatography. J. Virol. Methods. 2007;140:183–192. doi: 10.1016/j.jviromet.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 109.Burnham B., Nass S., Kong E., Mattingly M., Woodcock D., Song A., Wadsworth S., Cheng S.H., Scaria A., O’Riordan C.R. Analytical ultracentrifugation as an approach to characterize recombinant adeno-associated viral vectors. Hum. Gene Ther. Methods. 2015;26:228–242. doi: 10.1089/hgtb.2015.048. [DOI] [PubMed] [Google Scholar]
- 110.Aucoin M.G., Perrier M., Kamen A.A. Production of adeno-associated viral vectors in insect cells using triple infection: optimization of baculovirus concentration ratios. Biotechnol. Bioeng. 2006;95:1081–1092. doi: 10.1002/bit.21069. [DOI] [PubMed] [Google Scholar]
- 111.Aucoin M.G., Perrier M., Kamen A.A. Improving AAV vector yield in insect cells by modulating the temperature after infection. Biotechnol. Bioeng. 2007;97:1501–1509. doi: 10.1002/bit.21364. [DOI] [PubMed] [Google Scholar]
- 112.Vandenberghe L.H., Xiao R., Lock M., Lin J., Korn M., Wilson J.M. Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum. Gene Ther. 2010;21:1251–1257. doi: 10.1089/hum.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dias Florencio G., Precigout G., Beley C., Buclez P.-O., Garcia L., Benchaouir R. Simple downstream process based on detergent treatment improves yield and in vivo transduction efficacy of adeno-associated virus vectors. Mol. Ther. Methods Clin. Dev. 2015;2:15024. doi: 10.1038/mtm.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ayuso E., Mingozzi F., Montane J., Leon X., Anguela X.M., Haurigot V., Edmonson S.A., Africa L., Zhou S., High K.A. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 2010;17:503–510. doi: 10.1038/gt.2009.157. [DOI] [PubMed] [Google Scholar]
- 115.Zolotukhin S., Byrne B.J., Mason E., Zolotukhin I., Potter M., Chesnut K., Summerford C., Samulski R.J., Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 116.Auricchio A., Hildinger M., O’Connor E., Gao G.P., Wilson J.M. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum. Gene Ther. 2001;12:71–76. doi: 10.1089/104303401450988. [DOI] [PubMed] [Google Scholar]
- 117.Anderson R., Macdonald I., Corbett T., Whiteway A., Prentice H.G. A method for the preparation of highly purified adeno-associated virus using affinity column chromatography, protease digestion and solvent extraction. J. Virol. Methods. 2000;85:23–34. doi: 10.1016/s0166-0934(99)00150-0. [DOI] [PubMed] [Google Scholar]
- 118.Wang Q., Lock M., Prongay A.J., Alvira M.R., Petkov B., Wilson J.M. Identification of an adeno-associated virus binding epitope for AVB sepharose affinity resin. Mol. Ther. Methods Clin. Dev. 2015;2:15040. doi: 10.1038/mtm.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cecchini S., Virag T., Kotin R.M. Reproducible high yields of recombinant adeno-associated virus produced using invertebrate cells in 0.02- to 200-liter cultures. Hum. Gene Ther. 2011;22:1021–1030. doi: 10.1089/hum.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chahal P.S., Aucoin M.G., Kamen A. Primary recovery and chromatographic purification of adeno-associated virus type 2 produced by baculovirus/insect cell system. J. Virol. Methods. 2007;139:61–70. doi: 10.1016/j.jviromet.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 121.Smith R.H., Yang L., Kotin R.M. Chromatography-based purification of adeno-associated virus. Methods Mol. Biol. 2008;434:37–54. doi: 10.1007/978-1-60327-248-3_4. [DOI] [PubMed] [Google Scholar]
- 122.Venkatakrishnan B., Yarbrough J., Domsic J., Bennett A., Bothner B., Kozyreva O.G., Samulski R.J., Muzyczka N., McKenna R., Agbandje-McKenna M. Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking. J. Virol. 2013;87:4974–4984. doi: 10.1128/JVI.02524-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Urabe M., Nakakura T., Xin K.-Q., Obara Y., Mizukami H., Kume A., Kotin R.M., Ozawa K. Scalable generation of high-titer recombinant adeno-associated virus type 5 in insect cells. J. Virol. 2006;80:1874–1885. doi: 10.1128/JVI.80.4.1874-1885.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Okada T., Nonaka-Sarukawa M., Uchibori R., Kinoshita K., Hayashita-Kinoh H., Nitahara-Kasahara Y., Takeda S., Ozawa K. Scalable purification of adeno-associated virus serotype 1 (AAV1) and AAV8 vectors, using dual ion-exchange adsorptive membranes. Hum. Gene Ther. 2009;20:1013–1021. doi: 10.1089/hum.2009.006. [DOI] [PubMed] [Google Scholar]
- 125.Rayaprolu V., Kruse S., Kant R., Venkatakrishnan B., Movahed N., Brooke D., Lins B., Bennett A., Potter T., McKenna R. Comparative analysis of adeno-associated virus capsid stability and dynamics. J. Virol. 2013;87:13150–13160. doi: 10.1128/JVI.01415-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pacouret S., Bouzelha M., Shelke R., Andres-Mateos E., Xiao R., Maurer A., Mevel M., Turunen H., Barungi T., Penaud-Budloo M. AAV-ID: a rapid and robust assay for batch-to-batch consistency evaluation of AAV preparations. Mol. Ther. 2017;25:1375–1386. doi: 10.1016/j.ymthe.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bennett A., Patel S., Mietzsch M., Jose A., Lins-Austin B., Yu J.C., Bothner B., McKenna R., Agbandje-McKenna M. Thermal stability as a determinant of AAV serotype identity. Mol. Ther. Methods Clin. Dev. 2017;6:171–182. doi: 10.1016/j.omtm.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gruntman A.M., Su L., Su Q., Gao G., Mueller C., Flotte T.R. Stability and compatibility of recombinant adeno-associated virus under conditions commonly encountered in human gene therapy trials. Hum. Gene Ther. Methods. 2015;26:71–76. doi: 10.1089/hgtb.2015.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wright J.F., Le T., Prado J., Bahr-Davidson J., Smith P.H., Zhen Z., Sommer J.M., Pierce G.F., Qu G. Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation. Mol. Ther. 2005;12:171–178. doi: 10.1016/j.ymthe.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 130.Turnbull A.E., Skulimowski A., Smythe J.A., Alexander I.E. Adeno-associated virus vectors show variable dependence on divalent cations for thermostability: implications for purification and handling. Hum. Gene Ther. 2000;11:629–635. doi: 10.1089/10430340050015815. [DOI] [PubMed] [Google Scholar]
- 131.Guo P., El-Gohary Y., Prasadan K., Shiota C., Xiao X., Wiersch J., Paredes J., Tulachan S., Gittes G.K. Rapid and simplified purification of recombinant adeno-associated virus. J. Virol. Methods. 2012;183:139–146. doi: 10.1016/j.jviromet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiao R., Andres-Mateos E., Barungi T., Qiu H., Vandenberghe L.H. Downstream AAV recovery dramatically increased by non-ionic surfactant PF68. Mol. Ther. 2017;25(Suppl 1):S329. [Google Scholar]
- 133.Louis N., Evelegh C., Graham F.L. Cloning and sequencing of the cellular-viral junctions from the human adenovirus type 5 transformed 293 cell line. Virology. 1997;233:423–429. doi: 10.1006/viro.1997.8597. [DOI] [PubMed] [Google Scholar]
- 134.DuBridge R.B., Tang P., Hsia H.C., Leong P.M., Miller J.H., Calos M.P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Corden S.A., Sant-Cassia L.J., Easton A.J., Morris A.G. The integration of HPV-18 DNA in cervical carcinoma. Mol. Pathol. 1999;52:275–282. doi: 10.1136/mp.52.5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Giard D.J., Aaronson S.A., Todaro G.J., Arnstein P., Kersey J.H., Dosik H., Parks W.P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 137.Stoker M., MacPherson I. Syrian hamster fibroblast cell line Bhk21 and its derivatives. Nature. 1964;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- 138.Wright J.F. Manufacturing and characterizing AAV-based vectors for use in clinical studies. Gene Ther. 2008;15:840–848. doi: 10.1038/gt.2008.65. [DOI] [PubMed] [Google Scholar]
- 139.Snyder R.O., Audit M., Francis J.D. rAAV vector product characterization and stability studies. Methods Mol. Biol. 2011;807:405–428. doi: 10.1007/978-1-61779-370-7_17. [DOI] [PubMed] [Google Scholar]
- 140.Robert M.A., Chahal P.S., Audy A., Kamen A., Gilbert R., Gaillet B. Manufacturing of recombinant adeno-associated viruses using mammalian expression platforms. Biotechnol. J. 2017;12:1600193. doi: 10.1002/biot.201600193. [DOI] [PubMed] [Google Scholar]
- 141.Samulski R.J., Chang L.S., Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J. Virol. 1987;61:3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]