Abstract
Novel nonspiro, fluorene‐based, small‐molecule hole transporting materials (HTMs) V1050 and V1061 are designed and synthesized using a facile three‐step synthetic route. The synthesized compounds exhibit amorphous nature with a high glass transition temperature, a good solubility, and decent thermal stability. The planar perovskite solar cells (PSCs) employing V1050 generated an excellent power conversion efficiency of 18.3%, which is comparable to 18.9% obtained with the state‐of‐the‐art Spiro‐OMeTAD. Importantly, the devices based on V1050 and V1061 show better stability compared to devices based on Spiro‐OMeTAD when aged without any encapsulation under uncontrolled humidity conditions (relative humidity around 60%) in the dark and under continuous full sun illumination.
Keywords: fluorene, materials science, organic conductor, perovskite, solar cells
1. Introduction
Due to the merits of intense absorption in an almost entire visible spectral region, perovskite materials first emerged as dye substitutes for dye‐sensitized solar cells with the liquid electrolyte showing a power conversion efficiency (PCE) around 4%.1 Since the first perovskite‐based solar cells (PSCs) suffered from fast degradation their architecture was shifted from liquid electrolyte‐based low PCE devices to solid‐state devices using small organic hole transporting molecule 2,2′,7,7′‐tetrakis(N,N‐di‐p‐methoxy‐phenylamine)‐9,9′‐spirobifluorene (Spiro‐OMeTAD).2 In consequence, the photovoltaic performances have skyrocketed to 21.1% for small‐area cells3 and 20.5% for cells4 with the active area larger than 1 cm2 in just a few years. Since then, Spiro‐OMeTAD became a standard material for the development of the new hole transporting materials (HTMs) for PSCs. In the majority of the state‐of‐the‐art devices, Spiro‐OMeTAD is used as a hole transporting material.5, 6, 7, 8 However, the tedious synthetic procedures and purification processes of this HTM make it cost ineffective and thus limit its application and commercialization.9 Moreover, the study by A. Binek et al. showed that Spiro‐OMeTAD can significantly contribute to the overall cost of materials required for the PSC manufacturing.10
To engineer HTMs which are considerably cheaper than Spiro‐OMeTAD, shorter reaction schemes with simple purification procedures are required. Successful examples include azomethine derivative EDOT‐OMeTPA,11 branched methoxydiphenylamine‐substituted fluorene derivatives V859 and V862,12 enamine derivative V950,13 and spiro[fluorene‐9,9′‐xanthene] (SFX)‐based materials SFXMeOTAD14 or X60,15 and X59.16 Some of them are reported to have equal or slightly better performance in comparison to that of Spiro‐OMeTAD.12, 15
Besides the low cost, HTMs should meet a number of other requirements, including excellent charge transporting properties, good energy matching with the perovskite, transparency to solar radiation, large Stokes shift, good solubility in organic solvents, morphologically stable film formation, and others. Meanwhile, numerous investigations are being carried out aiming to improve the efficiency of HTMs, however until now only few concrete recommendations have been made with regard to the molecular structure modification.17, 18, 19, 20, 21, 22, 23, 24
Recently, we have presented the synthesis of a new class of efficient HTMs.25, 26 Their structure consists of two 4,4′‐dimethoxydiphenylamine 3,6‐disubstituted carbazole fragments linked by a nonplanar unit. Synthesis of these HTMs was performed by a simple two‐step synthetic procedure providing a target product in high yield. Conveniently, the first step is a basic “click” type reaction and high reaction rates (≈10 min at room temperature), simple purification procedure (only filtration is required) and high yields are typical for this step. The performance of CH3NH3PbI3‐based PSC with these branched compounds was close to that of the Spiro‐OMeTAD in the same conditions.
In this work, we present the synthesis, characterization and photovoltaic performance of two new hole transporting materials, namely V1050 and V1061 possessing 4,4′‐dimethoxydiphenylamine 3,6‐disubstituted carbazole‐based hole transporting moieties and 9,9‐dialkyl‐9H‐fluorene as a central linking fragment (Figure 1 ).
Figure 1.
Structures of HTMs: V1050 and V1061.
The coplanar central core was chosen in order to improve the efficiency of HTM, as was previously shown by Li and co‐workers.17 Furthermore, the properties and performance of newly synthesized HTMs were additionally compared to that of the state‐of‐the‐art Spiro‐OMeTAD.
2. Results and Discussions
2.1. Synthesis
The fluorene derivatives V1050 and V1061 were synthesized by a simple two‐step reaction from the key intermediates, the bromomethylfluorenes 1 and 3, as illustrated in Scheme 1 . To synthesize intermediate 3, a slightly modified literature procedure was employed to perform the tris(bromomethylation) of 9,9‐dimethylfluorene.27 In the next step, 3,6‐dibromo‐9H‐carbazole was reacted with 2,4,7‐tris(bromomethyl)‐9,9‐dimethyl‐9H‐fluorene (3) in the presence of KOH powder to provide the intermediate compound 4. Finally, the desired product V1050 was obtained by the palladium‐catalyzed Buchwald–Hartwig C–N cross‐coupling reaction of compound 4 with 4,4′‐dimethoxydiphenylamine. Surprisingly, di(bromomethylation) of 9,9‐dimethyl‐9H‐fluorene was unsuccessful and inseparable mixture of the reaction products was obtained by using two equivalents of paraformaldehyde. Presumably, the presence of the small methyl substituents at the 9th position markedly stipulates the formation of trisubstituted product as well. Therefore, as the reference compound, the hole transporting material V1061, containing 9,9‐diethyl‐9H‐fluorene as a central linking fragment and two equivalent 4,4′‐dimethoxydiphenylamine 3,6‐disubstituted carbazole‐based branches was also prepared according to the same approach. The final compounds V1050 and V1061 were isolated by column chromatography and precipitated from toluene or tetrahydrofuran into 15‐fold excess of n‐hexane. Obtained by such a procedure V1050 and V1061 were amorphous compounds. All our attempts to crystallize them were unsuccessful. The chemical structure of the synthesized V1050 and V1061 products were confirmed by 1H and 13C NMR as well as elemental analysis data. A more detailed procedure for the synthesis of hole transport materials is included in the Supporting Information.
Scheme 1.
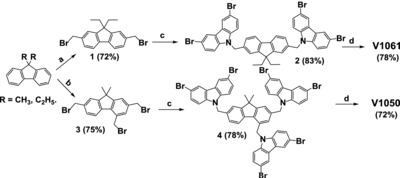
Synthesis route to HTMs V1050 and V1061: a) paraformaldehyde (2.2 equiv.), 33% HBr in HOAc, 65 –70 °C; b) paraformaldehyde (10.0 equiv), 33% HBr in HOAc, 85 –90 °C; c) 3,6‐dibromocarbazole, 85% KOH, THF, at r.t.; d) 4,4′‐dimethoxydiphenylamine, Pd(OAc)2, P(t‐Bu)3·BF3, NaOt‐Bu, at reflux of anhydrous toluene.
2.2. Thermal Properties
Thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC) were applied to measure the thermal stability of the above obtained HTMs. The weight loss as a function of temperature of V1050 and V1061 compounds is presented in Figure 2 a. A significant weight loss appears at around 400 °C and proceeds until complete decomposition of both materials at 600 °C.
Figure 2.
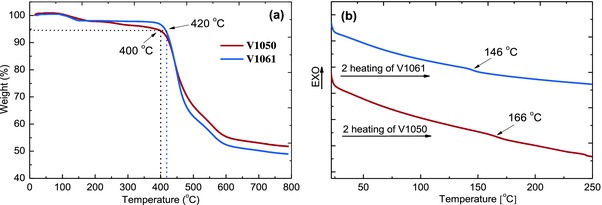
a) TGA curves of the V1050 and V1061 (heating rate 10 °C min−1), b) DSC second heating curves for the V1050 and V1061 (heating rate 10 °C min−1).
The decomposition temperature corresponding to a 5% weight loss (T d) is about 400 °C, indicating good thermal stability of V1050. HTM possessing two 4,4′‐dimethoxydiphenylamine 3,6‐disubstituted carbazole‐based branches shows slightly better thermal stability because T d is about 420 °C. From TGA measurements we conclude, that both fluorene‐based compounds are suitable for the application in the perovskite solar cells. Initial small (2–3%) weight loss at <150 °C can be attributed to the evaporation of the solvent traces remaining after the purification procedure.
DSC analysis of the V1050 and V1061 has shown (Figure 2b) that after purification, the target compounds exist in an amorphous state with the glass transition temperatures of about 166 and 146 °C, respectively. The high glass transition temperature is in good agreement with one of Wirth postulates28 demonstrating that the glass transition temperature can be raised by increasing molecular size, incorporating additional bulky substituent into 4th position of the molecule, and enhancing molecular interaction, which can hinder molecular motions. To the best of our knowledge, V1050 exhibits one of the highest glass transition temperatures among amorphous HTMs applied in the PSCs, being 40 °C higher than that of Spiro‐OMeTAD (126 °C). A high glass transition temperature is an indicator of the more stable amorphous state and reduced tendency to crystallize. Recently, it has been observed that Spiro‐OMeTAD tends to crystallize in perovskite solar cells under device operating conditions,29 which in turn can lead to device degradation and failure over the longer term. Whereas, both V1050 and V1061 are entirely amorphous and do not show characteristic transitions of the crystalline state (Figures S1 and S2, Supporting Information). The superior properties of new HTMs can be attributed to the presence of the branched 4,4′‐dimethoxydiphenylamine 3,6‐disubstituted carbazole‐based moieties linked by bulky central fluorene core.
2.3. Optical and Photophysical Properties
The UV–vis absorption spectra of a new fluorene‐based organic HTMs measured in tetrahydrofuran (THF) solution is shown in Figure 3 a and compared with Spiro‐OMeTAD. The absorption spectra for both V1050 and V1061 HTMs are almost identical. This confirms that conjugation between 4,4′‐dimethoxydiphenylamine 3,6‐disubstituted carbazole‐based branches is absent. Both HTMs show intense π–π* absorption band with the maximum at 305 nm and weak low energy absorption at 375 nm corresponding to n–π* transitions. Furthermore, absorption of both compounds lies mainly in the UV region with a very weak undesirable absorption in the visible spectral range. Because a new hole transporting materials are more transparent to solar radiation, they become even more advantageous for the application in the PSC.30 The steady state absorption spectra of perovskite FA0.83Cs0.17Pb(I0.8Br0.2)3 films deposited on TiO2 with and without hole transporting materials are shown in Figure 3b. The absorption spectra show characteristic absorption onset at around 775 nm due to exciton absorption. All films reveal very similar signatures when measured above 550 nm, indicating that there is no apparent contribution of HTMs to the light absorption of perovskite solar cells.
Figure 3.
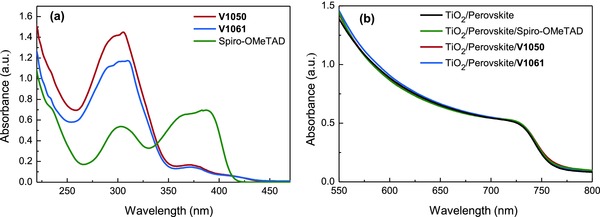
a) UV–vis absorption spectra of V1050, V1061, and Spiro‐OMeTAD, b) UV–vis absorption spectra of TiO2/perovskite (FA0.83Cs0.17Pb(I0.8Br0.2)3) film and perovskite (FA0.83Cs0.17Pb(I0.8Br0.2)3) deposited with Spiro‐OMeTAD and V1050, and V1061 hole transporting materials.
Here, we additionally performed time resolved photoluminescence (PL) decay measurements to study charge–carrier transport properties in perovskite films deposited on glass with and without HTMs (Figure 4 ). For pristine perovskite films, PL decays during 58 ns and reflects nongeminate electron–hole recombination30 being the dominating radiative channel in the neat perovskite films. When the perovskite films are covered with hole transporting materials, the PL decay rate for all three films becomes significantly reduced. The strong photoluminescence quenching indicates efficient extraction of holes at the perovskite/HTM interfaces.
Figure 4.
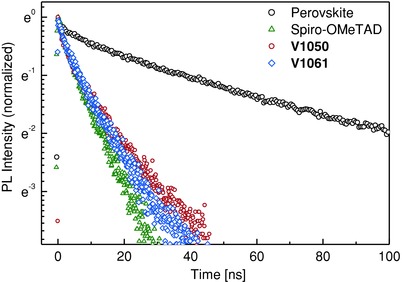
Photoluminescence decay kinetics of lead halide perovskite (FA0.83Cs0.17Pb(I0.8Br0.2)3) films deposited on glass substrates comprising V1050, V1061, and Spiro‐OMeTAD hole transporting layers. Photoluminescence lifetimes were monitored at the emission maximum at 770 nm upon excitation at 470 nm.
To evaluate the efficiency of the charge transfer properties from perovskite to HTMs from time‐resolved PL measurements, we used previously proposed protocol.31 The calculated charge‐transfer time and efficiency values are presented in Table 1 . The charge transfer efficiency values of perovskite solar cell devices comprising new HTMs are comparable to that obtained with commercially available Spiro‐OMeTAD. The superior hole collection was also consistent with photovoltaic measurements of devices, which showed similar power conversion efficiencies between all devices.
Table 1.
Charge–transfer time (τCT) and efficiency (CTE) calculated for perovskite films employing various HTMs
| HTM | τ [ns] | CTE [%] |
|---|---|---|
| V1050 | 12.8 | 81.9 |
| V1061 | 15.9 | 78.4 |
| Spiro‐OMeTAD | 9.6 | 85.8 |
2.4. Photoelectrical Properties
Xerographic time‐of‐flight technique was used to characterize charge transporting properties of the synthesized HTMs. The measured charge mobility values of V1050 and V1061 are found to be comparable to the values measured for Spiro‐OMeTAD. Values of charge mobility defining parameters: zero field mobility (µ0) and the mobility at the electric field of 6.4 × 105 V cm−1 are given in Table 2 . The measured hole–drift mobility for V1061 was 3.0 × 10−6 cm2 V−1 s−1, while for V1050 – 1.5 × 10−6 cm2 V−1 s−1 at weak electric fields. Interestingly, the charge mobility values of the new HTMs at strong field strength coming closer to the values measured for Spiro‐OMeTAD (Figure 5 , Table 2). Lower hole drift mobility values could be attributed to the presence of the bulky central fluorene fragment, which is linked to the 4,4′‐dimethoxydiphenylamine 3,6‐disubstituted carbazole‐based hole transporting moieties by nonconjugated bonds. Conformational freedom increases disorder of molecules in the films, thus lowering mobility values.
Table 2.
Thermal and photoelectrical properties of V1050, V1061, and Spiro‐OMeTAD
| HTM | T g a) [°C] | T dec b) [°C] | I p c) [eV] | µ 0 d) [cm2 V−1 s−1] | µ e) [cm2 V−1 s−1] |
|---|---|---|---|---|---|
| V1050 | 166 | 400 | 5.11 | 1.5 × 10−6 | 1.7 × 10−4 |
| V1061 | 146 | 420 | 5.10 | 3.0 × 10−6 | 2.0 × 10−4 |
| Spiro‐OMeTAD26 | 126 | 449 | 5.00 | 4.1 × 10−5 | 5.0 × 10−4 |
Determined by DSC: scan rate = 10 °C min−1, N2 atmosphere; second run
Thermal decomposition temperature was registered at 5% weight loss
Ionization potential was measured by the photoemission in air method from films
Mobility value at zero field strength
Mobility value at 6.4 × 105 V cm−1 field strength.
Figure 5.
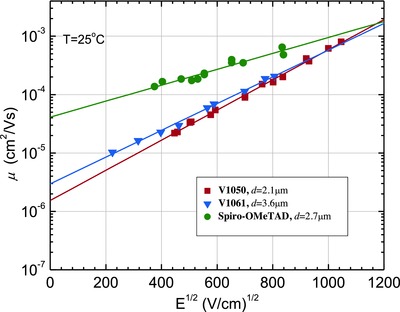
Electric field dependencies of the hole‐drift mobilities (µ) in charge transport layers of V1050, V1061, and Spiro‐OMeTAD, with corresponding linear fits represented by solid lines.
To determine the highest occupied molecular orbital (HOMO) energy level of V1050 and V1061, solid state ionization potential (I p) was measured by photoelectron spectroscopy in air method32 and results are presented in Table 2; the measurement error is evaluated as 0.03 eV. Compounds V1050 and V1061 have almost the same I p values, 5.11 and 5.10 eV, respectively (Figures S3 and S4, Supporting Information). The estimated I p values are very closed to the value of Spiro‐OMeTAD (5.00 eV) and compatible for application in perovskite solar cell devices to ensure efficient hole transfer at the interface.
2.5. Perovskite Solar Cells
Currently one of the best and most reproducible results are obtained with “triple‐cation” composition of perovskite, containing MA+, FA+, and inorganic Cs+ cations.3 However, MA+ is a volatile cation and can cause degradation of the perovskite absorber film. Thus, during the last years, MA+‐free architectures have been extensively studied, where FA+ cation is combined with Cs+, which provides higher stability.33, 34, 35, 36 SnO2 was used as an electron transporting layer, as it can be deposited at low temperatures (annealing temperature–180 °C), and thus is suitable material for the application in tandem solar cells.37
The nonspiro, fluorene‐based compounds V1050 and V1061 were tested in perovskite solar cells employing planar FTO/SnO2/FA0.83Cs0.17Pb(I0.8Br0.2)3/HTM/Au architecture. Figure 6 shows current–voltage characteristics of the best devices employing V1050, V1061, and Spiro‐OMeTAD in planar PSC. The optimized champion device efficiency for V1050, V1061, Spiro‐OMeTAD, and corresponding photovoltaic performance parameters are shown in Figure 6. The V1050 HTM‐based device shows the device efficiency of 18.3% (J sc = 22.0 mA cm−2, V OC = 1.05 V, and FF = 79.5%). This is comparable to the state‐of‐the‐art material Spiro‐OMeTAD on a like‐to‐like comparison (PCE = 18.9%, J sc = 22.4 mA cm−2, V OC = 1.08 V, and FF = 77.9%). A bit lower PCE of 16.7% (Figure 6) was recorded with J sc = 21.6 mA cm−2, V OC = 0.96 V, and FF = 79.7% in case of V1061. Efficiencies at stabilized power output (SPO) of tested compounds are shown in Figure S5 in the Supporting Information and are 16.1%, 15.0%, and 17.3%, respectively. External quantum efficiency (EQE) spectra were recorded for typical device for each HTMs in Figure S6 (Supporting Information). The same trend was observed from the measured J–V curves. The statistical distribution of the PSC parameters employing investigated HTMs is shown in Figure S7 in the Supporting Information and is indicating high reproducibility of PSC devices. Furthermore, Figure S8 (Supporting Information) shows J–V curves from both forward‐bias to short‐circuit and short‐circuit to forward‐bias current–voltage sweeps of typical device from both sweeps. While hysteresis is quite pronounced, it is comparable between the mentioning HTMs, thus reverse scans can be used as representatives for the HTMs performance analysis.
Figure 6.
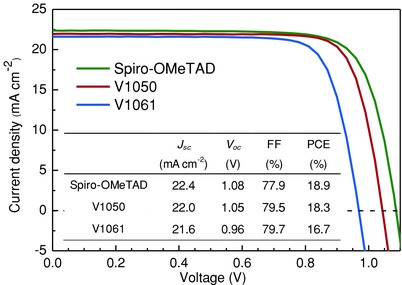
Best performing J–V characteristics (reverse scan) of the best devices employing V1050, V1061, and Spiro‐OMeTAD.
From the SEM cross‐section images, it can be seen, that new fluorene‐based compounds form a uniform morphology on top of perovskite layer as similar as Spiro‐OMeTAD (Figure 7 ).
Figure 7.
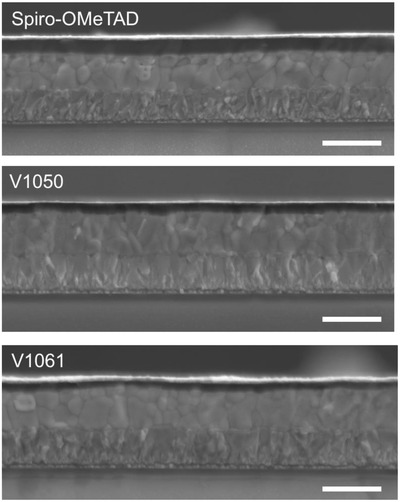
Cross‐sectional SEM microscopy image of the devices with Spiro‐OMeTAD, V1050, and V1061 as a HTM. Scale bar equals to 1000 nm.
To give good performance, HTM should effectively block the electrons and transport holes from perovskite to the Au electrode. Thus, it is important for the HTMs to have excellent film forming ability. As from the SEM cross‐section images no significant difference could be observed, contact angle measurement of the HTM droplets on the perovskite film was performed (Figure S9, Supporting Information). From the results it can be seen, that V1050 solution has the lowest contact angle of 9°, which could potentially lead to a better film forming ability of V1050.
Additionally, we also studied the stability of fabricated champion devices without any encapsulation at uncontrollable humidity conditions (relative humidity ≈ 60%, temperature 22 °C, in dark condition) for our newly developed V1050, V1061 and standard Spiro‐OMeTAD HTMs. It can be seen (Figure 8 ) that the PCE values diminished down ≈20% after 330 h for Spiro‐OMeTAD based devices. However, the devices based on V1050 and V1061 are seen to be more stable with observed efficiency reduced only ≈6% at the same conditions. The device stability test results revealed that the improved stability of V1050 and V1061‐based cells compared to Spiro‐OMeTAD‐based device could be attributed to the uniform HTM capping layer on the top of the perovskite layer, preventing the moisture penetration into the perovskite layer, which is in agreement with the result from HTM contact angle measurement on top of perovskite film. The overall good performance of PCE and stability of V1050 over Spiro‐OMeTAD provide a promising alternative replacement for high performance PSCs.
Figure 8.
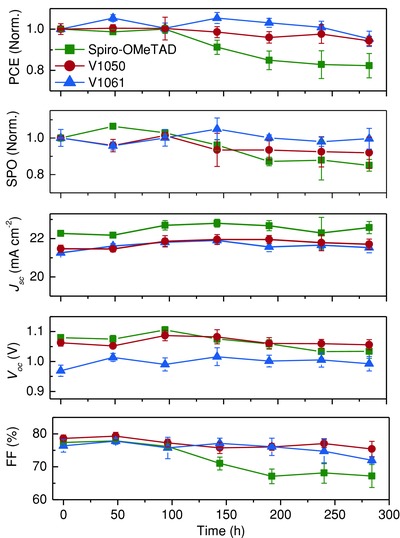
Transition of the PV performance parameters (PCE, SPO, J sc, V oc, and FF) of the PSCs with V1050, V1061, and Spiro‐OMeTAD.
3. Conclusions
In conclusion, a new promising nonspiro fluorene‐based hole transport materials V1050 and V1061 were synthesized and characterized. The synthesis of these HTMs consists of three steps starting from the commercially available materials. Solar cells using V1050 exhibit PCEs of 18.3% which is comparable to the performance of PSC comprising Spiro‐OMeTAD (18.9%) as HTM. Compared with Spiro‐OMeTAD, new HTM additionally shows several significant advantages: it has much facile synthesis, has high glass transition temperature (166 °C) and does not form the crystalline state. Moreover, this new HTM also exhibits better environmental stability compared to Spiro‐OMeTAD. We believe that the V1050 can be a useful alternative HTM to Spiro‐OMeTAD for perovskite solar cells, thus bringing PSCs closer to commercial production.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Supplementary
Acknowledgements
Š.D. and N.S. contributed equally to this work. The authors acknowledge funding from the Research Council of Lithuania via grant (Grant No. LAT‐07/2016), funding from the European Union Seventh Framework Programme [FP7/2007–2013] under grant agreement no. 604032 of the MESO project, and E. Kamarauskas for measurements of ionization potential.
Daškevičiūtė Š., Sakai N., Franckevičius M., Daškevičienė M., Magomedov A., Jankauskas V., Snaith H. J., Getautis V., Adv. Sci. 2018, 5, 1700811 https://doi.org/10.1002/advs.201700811
Contributor Information
Henry J. Snaith, Email: henry.snaith@physics.ox.ac.uk
Vytautas Getautis, Email: vytautas.getautis@ktu.lt.
References
- 1. Kojima A., Teshima K., Shirai Y., Miyasaka T., J. Am. Chem. Soc. 2009, 131, 6050. [DOI] [PubMed] [Google Scholar]
- 2. Stranks S. D., Snaith H. J., Nat. Nanotechnol. 2015, 10, 391. [DOI] [PubMed] [Google Scholar]
- 3. Saliba M., Matsui T., Seo J.‐Y., Domanski K., Correa‐Baena J.‐P., Nazeeruddin M. K., Zakeeruddin S. M., Tress W., Abate A., Hagfeldt A., Grätzel M., Energy Environ. Sci. 2016, 9, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li X., Bi D., Yi C., Décoppet J.‐D., Luo J., Zakeeruddin S. M., Hagfeldt A., Grätzel M., Science 2016, 353, 58. [DOI] [PubMed] [Google Scholar]
- 5. Saliba M., Matsui T., Domanski K., Seo J.‐Y., Ummadisingu A., Zakeeruddin S. M., Correa‐Baena J.‐P., Tress W. R., Abate A., Hagfeldt A., Grätzel M., Science 2016, 354, 206. [DOI] [PubMed] [Google Scholar]
- 6. Son D.‐Y., Lee J.‐W., Choi Y. J., Jang I.‐H., Lee S., Yoo P. J., Shin H., Ahn N., Choi M., Kim D., Park N.‐G., Nat. Energy 2016, 1, 16081. [Google Scholar]
- 7. Zhang Y., Grancini G., Feng Y., Asiri A. M., Nazeeruddin M. K., ACS Energy Lett. 2017, 2, 802. [Google Scholar]
- 8. Grancini G., Rolda‐Carmona C., Zimmermann I., Mosconi E., Lee X., Martineau D., Narbey S., Oswald F., De Angelis F., Graetzel M., Nazeeruddin M. K., Nat. Commun. 2017, 8, 15684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu P., Xu B., Hua Y., Cheng M., Aitola K., Sveinbjörnsson K., Zhang J., Boschloo G., Sun L., Kloo L., J. Power Sources 2017, 344, 11. [Google Scholar]
- 10. Binek A., Petrus M. L., Huber N., Bristow H., Hu Y., Bein T., Docampo P., ACS Appl. Mater. Interfaces 2016, 8, 12881. [DOI] [PubMed] [Google Scholar]
- 11. Petrus M. L., Bein T., Dingemans T. J., Docampo P., J. Mater. Chem. A 2015, 3, 12159. [Google Scholar]
- 12. Malinauskas T., Saliba M., Matsui T., Daskeviciene M., Urnikaite S., Gratia P., Send R., Wonneberger H., Bruder I., Graetzel M., Getautis V., Nazeeruddin M. K., Energy Environ. Sci. 2016, 9, 1681. [Google Scholar]
- 13. Daskeviciene M., Paek S., Wang Z., Malinauskas T., Jokubauskaite G., Rakstys K., Cho K. T., Magomedov A., Jankauskas V., Ahmad S., Snaith H. J., Getautis V., Nazeeruddin M. K., Nano Energy 2017, 32, 551. [Google Scholar]
- 14. Maciejczyk M., Ivaturi A., Robertson N., J. Mater. Chem. A 2016, 4, 4855. [Google Scholar]
- 15. Xu B., Bi D., Hua Y., Liu P., Cheng M., Grätzel M., Kloo L., Hagfeldt A., Sun L., Energy Environ. Sci. 2016, 9, 873. [Google Scholar]
- 16. Bi D., Xu B., Gao P., Sun L., Grätzel M., Hagfeldt A., Nano Energy 2016, 23, 138. [Google Scholar]
- 17. Chi W.‐J., Sun P.‐P., Li Z.‐S., Nanoscale 2016, 8, 17752. [DOI] [PubMed] [Google Scholar]
- 18. Zhu L., Shan Y., Wang R., Liu D., Zhong C., Song Q., Wu F., Chem. Eur. J. 2017, 23, 4373. [DOI] [PubMed] [Google Scholar]
- 19. García‐Benito I., Zimmermann I., Urieta‐Mora J., Aragó J., Molina‐Ontoria A., Ortí E., Martín N., Nazeeruddin M. K., J. Mater. Chem. A 2017, 5, 8317. [Google Scholar]
- 20. Nishimura H., Ishida N., Shimazaki A., Wakamiya A., Saeki A., Scott L. T., Murata Y., J. Am. Chem. Soc. 2015, 137, 15656. [DOI] [PubMed] [Google Scholar]
- 21. Huang C., Fu W., Li C.‐Z., Zhang Z., Qiu W., Shi M., Heremans P., Jen A. K.‐Y., Chen H., J. Am. Chem. Soc. 2016, 138, 2528. [DOI] [PubMed] [Google Scholar]
- 22. Sung S.‐D., Kang M.‐S., Choi I.‐T., Kim H.‐M., Kim H., Hong M.‐P., Kim H.‐K., Lee W.‐I., Chem. Commun. 2014, 50, 14161. [DOI] [PubMed] [Google Scholar]
- 23. Kang M.‐S., Sung S.‐D., Kim I.‐T. C.‐H., Hong M.‐P., Kim J., Lee W.‐I., Kim H.‐K., ACS Appl. Mater. Interfaces 2015, 7, 22213. [DOI] [PubMed] [Google Scholar]
- 24. Lu C., Choi I.‐T., Kim J., Kim H.‐K., J. Mater. Chem. A 2017, 5, 20263. [Google Scholar]
- 25. Gratia P., Magomedov A., Malinauskas T., Daskeviciene M., Abate A., Ahmad S., Grätzel M., Getautis V., Nazeeruddin M. K., Angew. Chem., Int. Ed. 2015, 54, 11409. [DOI] [PubMed] [Google Scholar]
- 26. Magomedov A., Sakai N., Kamarauskas E., Jokubauskaitė G., Franckevičius M., Jankauskas V., Snaith H. J., Getautis V., Chem. ‐ Asian J. 2017, 12, 958. [DOI] [PubMed] [Google Scholar]
- 27. Yao S., Belfield K. D., J. Org. Chem. 2005, 70, 5126. [DOI] [PubMed] [Google Scholar]
- 28. Wirth H. O., Angew. Makromol. Chem. 1991, 185, 329. [Google Scholar]
- 29. Malinauskas T., Tomkute‐Luksiene D., Sens R., Daskeviciene M., Send R., Wonneberger H., Jankauskas V., Bruder I., Getautis V., ACS Appl. Mater. Interfaces 2015, 7, 11107. [DOI] [PubMed] [Google Scholar]
- 30. Augulis R., Franckevičius M., Abramavičius V., Abramavičius D., Zakeeruddin S. M., Grätzel M., Gulbinas V., Adv. Energy. Mater. 2017, 6, 1700405. [Google Scholar]
- 31. Kang M. S., Sung S. D., Choi I. T., Kim H., Hong M. P., Kim J., Lee W. I., Kim H. K., ACS Appl. Mater. Interfaces 2015, 7, 22213. [DOI] [PubMed] [Google Scholar]
- 32. Kirkus M., Tsai M.‐H., Grazulevicius J. V., Wu C.‐C., Chi L.‐C., Wong K.‐T., Synth. Met. 2009, 159, 729. [Google Scholar]
- 33. McMeekin D. P., Sadoughi G., Rehman W., Eperon G. E., Saliba M., Hörantner M. T., Haghighirad A., Sakai N., Korte L., Rech B., Johnston M. B., Herz L. M., Snaith H. J., Science 2016, 351, 151. [DOI] [PubMed] [Google Scholar]
- 34. Li Z., Yang M., Park J.‐S., Wei S.‐H., Berry J. J., Zhu K., Chem. Mater. 2016, 28, 284. [Google Scholar]
- 35. Yi C., Luo J., Meloni S., Boziki A., Ashari‐Astani N., Grätzel C., Zakeeruddin S. M., Röthlisberger U., Grätzel M., Energy Environ. Sci. 2016, 9, 656. [Google Scholar]
- 36. Qiu W., Ray A., Jaysankar M., Merckx T., Bastos J. P., Cheyns D., Gehlhaar R., Poortmans J., Heremans P., Adv. Funct. Mater. 2017, 27, 1700920. [Google Scholar]
- 37. Albrecht S., Saliba M., Correa Baena J. P., Lang F., Kegelmann L., Mews M., Steier L., Abate A., Rappich J., Korte L., Schlatmann R., Nazeeruddin M. K., Hagfeldt A., Gratzel M., Rech B., Energy Environ. Sci. 2016, 9, 81. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary


