Abstract
Aim
To evaluate the dose distribution to the left anterior descending (LAD) coronary artery in patients treated with postoperative three-dimensional conformal radiotherapy (3DCRT).
Background
Postoperative radiotherapy may increase the risk of heart disease, particularly in patients with left-sided breast cancer. Clinical data on doses to the LAD are limited.
Materials and methods
Retrospective study of 14 patients who underwent postoperative 3DCRT for left breast cancer in 2014. All data were retrieved from medical records. Means, medians, ranges, and percentages were calculated.
Results
The mean dose to the LAD in patients with V25 < 1% was 0.12 cGy. Dmean, Dmax and V25 to the heart were, respectively, 3.7 Gy (range, 0.9–4.18), 40.3 Gy (9.28–62.9), and 1.59 cGy. The mean Dmean and Dmax values in the sample were 9.71 Gy and 33.2 Gy, respectively. The maximum dose to the LAD (D2%) ranged from 3.66 to 53.01 Gy. Due to the spacing of the CT slices (5 mm), it was not possible to completely contour the entire artery. The mean dose to the heart (3.3 Gy) was considered acceptable.
Conclusions
The maximum dose to the LAD was as high as 53 Gy, suggesting an increased risk of cardiac morbidity. This study underscores the value of contouring the LAD and the value of the breath hold technique to reduce maximum cardiac doses. Smaller CT cuts (2.5 mm) can improve contouring. Larger studies with long-term follow up are needed to determine the radiation tolerance dose for the LAD.
Keywords: Breast neoplasms, Breath holding, Coronary vessels, Radiation tolerance, Conformal radiotherapy
1. Background
The standard treatment for breast cancer is either breast-conserving surgery (BCS) or, in high-risk patients, mastectomy. In both cases, surgery is typically followed by adjuvant whole breast radiotherapy (WBRT). However, postoperative radiotherapy poses an increased risk of radiation-induced heart disease in patients with left-sided breast cancer caused by damage (micro- and macro-angiopathy) to the coronary arteries that may lead to myocardial fibrosis and coronary artery disease.1
Previously, the whole heart was considered a single organ at risk (OAR). However, numerous studies have shown that the impact of the radiation dose depends on the heart substructures and, thus, dose restrictions should be modified accordingly.2 Dose restriction to the whole heart to prevent pericarditis and cardiovascular mortality are well-established; however, the tolerance dose for the left anterior descending (LAD) coronary artery remains to be determined.3 This is highly relevant because irradiation of the internal mammary chain (IMC) by a direct beam and left tangential fields—which include the distal branches of the LAD—can increase the risk of late heart disease. Given that damage to even a small section of the LAD can lead to severe toxicity or even death, it is crucial to minimize the dose delivered to the artery. Nonetheless, in clinical practice, the LAD is not routinely contoured in left breast radiotherapy, in part because of the difficulties posed in delineating the narrow volume of this artery, but also because data from some studies have shown that it may not be necessary to specifically contour the coronary arteries provided that the Radiation Therapy Oncology Group (RTOG) guidelines are followed.4, 5
2. Aim
Although several studies have investigated the radiation dose to the LAD during left-sided irradiation,4, 6 more data are needed to better characterize the tolerance dose.3 In this context, the objective of the present study was to describe the dosimetric distribution of radiation doses to the LAD in a series of patients treated with three-dimensional conformal radiotherapy delivered with tangential fields.
3. Materials and methods
This retrospective study involved 14 patients treated for left breast cancer during the year 2014 at the Radiation Oncology Department of Médica Sur Hospital in Mexico. The whole heart and LAD were contoured separately.
3.1. Data collection
Relevant patient and treatment data—including tumour characteristics, treatment details, use of adjuvant endocrine treatment, chemotherapy, and recurrences—were obtained from patient medical records. The radiotherapy records were reviewed to classify the target areas: whole breast plus surgical bed after BCS, and regional lymph nodes (LN) located in the axillary, IMC, and supraclavicular (SCL) areas.
3.2. Treatment
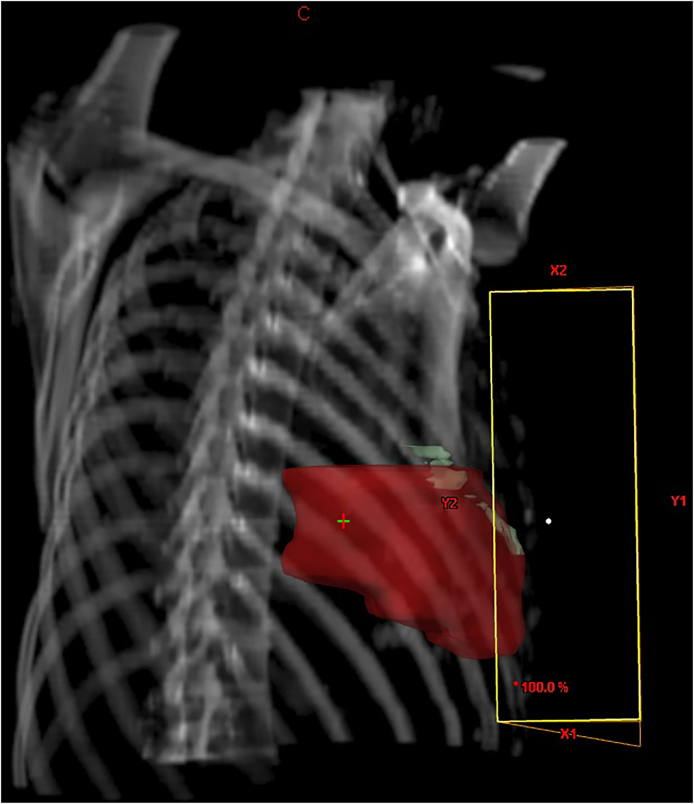
A simulation computed tomography (CT) scan was acquired with the patient in the supine position on an inclined treatment table, with the left arm extended upwards, in a positron-emission tomography (PET)-CT (Siemens Biograph 16). The gantry amplitude was 75 cm, field of view (FOV) of 6 mm, and scanner rotation time of 5.5 s. CT slices were obtained every 5 mm (Fig. 1). No contrast dye was administered.
Fig. 1.
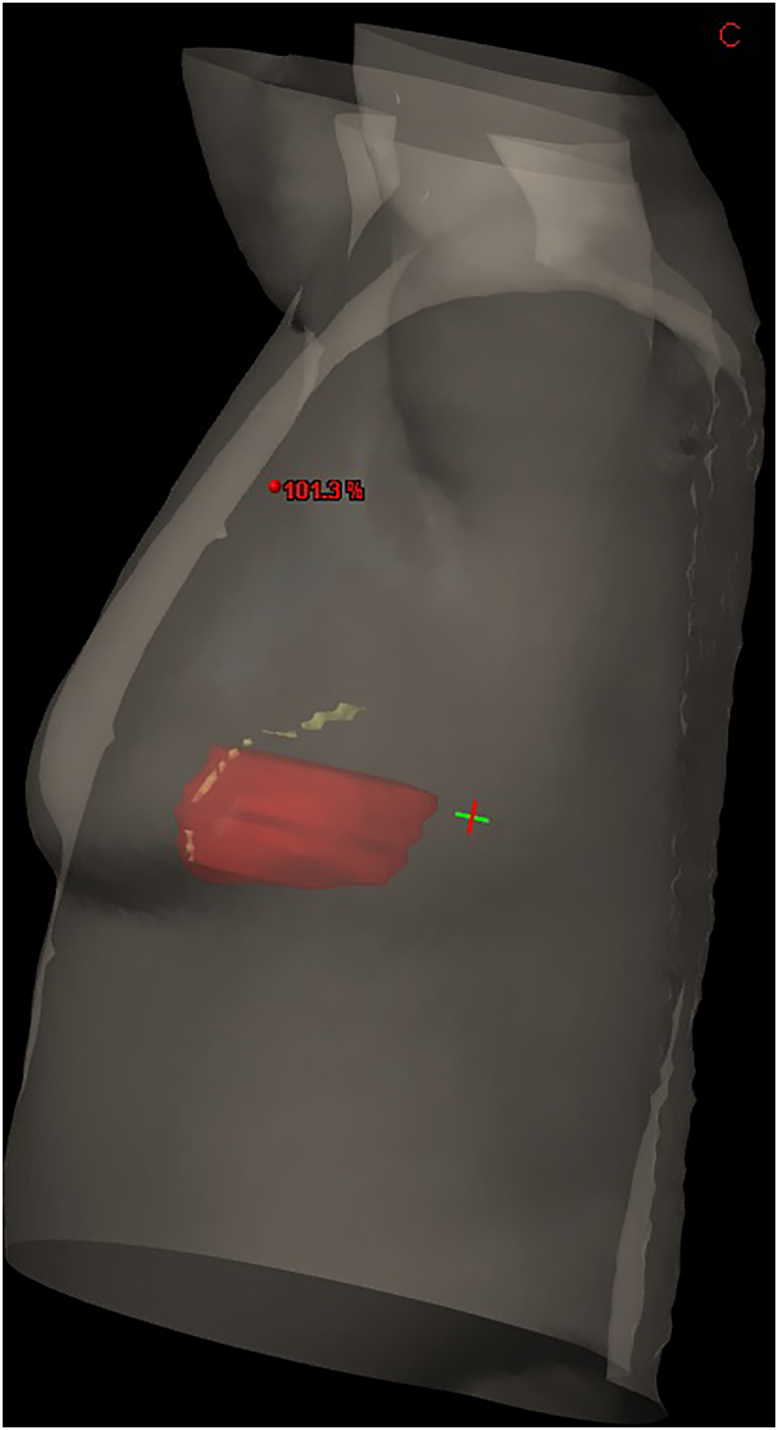
3D reconstruction of 5 mm thickness CT slices with a staggered LAD view.
Contouring of the LAD artery (Fig. 2) was performed by the attending radiation oncologist with the support of a radiologist. The heart was outlined according to the RTOG breast cancer contouring atlas.5 The Eclipse (v. 11.031) treatment planning system (TPS) was used (Varian Medical Systems; Palo Alto, CA, USA) with the AAA (anisotropic analytical algorithm) and PBC (pencil beam convolution) models.
Fig. 2.
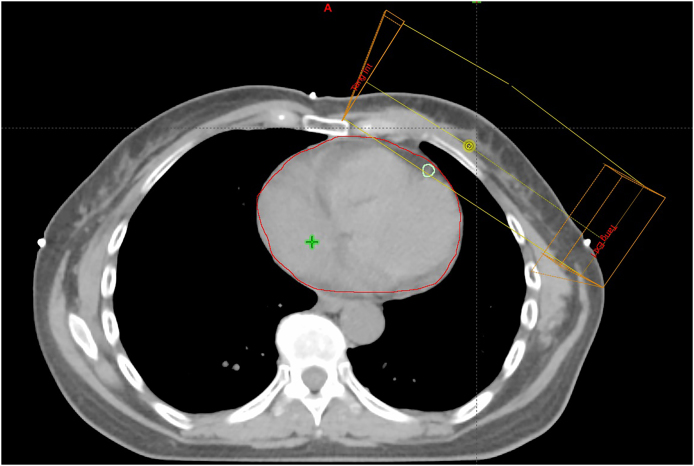
Transversal CT slice showing contoured LAD inside the tangential radiation fields.
The treatment technique used was opposite tangential fields with 6 MV photons (Fig. 3). In 6 cases (42%), due to patient anatomy, 18 MV photons were also used to achieve a homogeneous dose distribution.
Fig. 3.
Beam's eye view showing the LAD inside the tangential radiation fields.
The treatment plans were optimized individually using beam angles, wedges, and/or collimator angles. Dose-volume histograms (DVH) were created for the OARs. Dose restrictions to the heart were in accordance with RTOG recommendations, as follows: <5% of the heart volume should receive 40 Gy (Vheart 40 Gy = 5%) and less than 10% of the heart volume should receive 25 Gy (Vheart 25 Gy = 10%).
3.3. Statistical analysis
This was a descriptive study. Means, medians, ranges, and percentages were calculated as appropriate.
4. Results
4.1. Patient characteristics
Table 1 shows the demographic and tumour characteristics of the sample. Patients 39–83 years (mean, 56 years) and tumour size ranged from 2 cm to 2.7 cm (mean, 1.44 cm). Hormone receptors (HR) were positive in 9 patients and negative in 2 (data not available in 3 cases). Seven patients were diagnosed with invasive ductal carcinoma (IDC), two had ductal carcinoma in situ (DCIS), four presented both IDC and DCIS, and one had a mucinous carcinoma.
Table 1.
Patient and tumour characteristics.
| Patient number | Age, years | Tumour size (cm) | Hormone receptors | Histology | Other characteristics |
|---|---|---|---|---|---|
| 1 | 63 | 0.4 × 0.4 | Positive | IDC | |
| 2 | 56 | 0.9 | Positive | IDC + DCIS | |
| 3 | 83 | 1.5 × 1.2 | Negative | IDC | 1 positive lymph node |
| 4 | 39 | 1 | Positive | IDC | Mastectomy with reconstruction |
| 5 | 62 | 2.7 | Positive | IDC + DCIS | |
| 6 | 39 | 1 × 0.8 | Missing | IDC + DCIS | |
| 7 | 58 | 3 | Positive | IDC | Chemotherapy |
| 8 | 54 | 3 | Positive | Mucinous | |
| 9 | 66 | 1 | Positive | IDC | |
| 10 | 52 | 1 | Missing | DCIS | |
| 11 | 53 | 1.5 | Positive | IDC + DCIS | Chemotherapy |
| 12 | 41 | 1 | Positive | IDC | |
| 13 | 68 | 2 | Negative | IDC | |
| 14 | 55 | 0.2 | Missing | DCIS |
Abbreviations: IDC indicates invasive ductal carcinoma; DCIS, ductal carcinoma in situ.
Most patients (13/14; 92.9%) underwent BCS. However, one patient (patient number 4) was treated with mastectomy followed by immediate breast reconstruction (note that the radiation dose included the thoracic wall).
The prescribed radiation dose in all cases was 50 Gy in 25 sessions. Five patients received a boost to the surgical bed (15 Gy in 5 sessions); of these, three were treated with 9 MeV electrons, 1 with 12 MeV, and one with a reduced tangential field.
Table 2 shows the doses to 2% of the interpolated LAD volume, the heart volumes receiving >5 Gy and >25 Gy (V5 and V25) and Dmax and Dmean of both.
Table 2.
Dose distribution to the LAD and the whole heart.
| Patient | LAD |
HEART |
||||||
|---|---|---|---|---|---|---|---|---|
| Volume, cm3 | Dmax (cGy) | Dmean (cGy) | D2% (cGy) | V 5 Gy (cGy) | V 25 Gy (cGy) | Dmax (cGy) | Dmean (cGy) | |
| 1 | 1.76 | 5316.40 | 2134.80 | 5301.90 | 11.40 | 5.75 | 5455.3 | 465.8 |
| 2 | 1.10 | 5285.70 | 3115.50 | 5225.20 | 5.26 | 1.90 | 5270.0 | 238.0 |
| 3 | 1.38 | 5231.90 | 2565.20 | 5187.20 | 9.46 | 3.60 | 5230.0 | 374.0 |
| 4 (mastectomy) | 1.82 | 5194.30 | 317.30 | 2263.80 | 7.10 | 2.62 | 5350.0 | 318.0 |
| 5 | 2.37 | 5165.60 | 1277.80 | 5029.90 | 5.21 | 1.83 | 4040.0 | 222.0 |
| 6 | 0.46 | 4035.50 | 260.70 | 2003.70 | 5.72 | 1.38 | 4280.0 | 212.0 |
| 7 | 1.48 | 3111.70 | 833.20 | 2427.30 | 3.43 | 0.03 | 3567.0 | 202.5 |
| 8 | 1.56 | 3019.80 | 1125.50 | 2197.80 | 7.83 | 1.01 | 6290.0 | 305.0 |
| 9 | 1.09 | 2719.50 | 324.50 | 759.30 | 0.34 | 0.00 | 928.3 | 181.6 |
| 10 | 0.92 | 2664.10 | 496.30 | 1123.60 | 11.40 | 3.99 | 5120.0 | 418.0 |
| 11 | 1.32 | 1893.10 | 300.60 | 977.40 | 2.03 | 0.12 | 3930.0 | 178.0 |
| 12 | 1.79 | 1529.60 | 353.10 | 806.90 | 0.69 | 0.01 | 3416.0 | 159.0 |
| 13 | 2.28 | 842.80 | 305.30 | 602.60 | 2.84 | 0.01 | 3395.0 | 184.7 |
| 14 | 1.76 | 481.70 | 188.50 | 366.10 | 0.79 | 0.01 | 3486.0 | 93.4 |
Abbreviations: LAD indicates left anterior descending artery; Dmax, maximum dose; Dmean, mean dose; D2%, maximum dose to the LAD; V, volume.
The mean dose to the LAD in patients with V25 < 1% was 0.12 cGy. Dmean, Dmax and V25 to the heart were, respectively, 3.7 Gy (range, 0.9–4.18), 40.3 Gy (9.28–62.9), and 1.59 cGy. The mean Dmean and Dmax values were 9.71 Gy and 33.2 Gy, respectively. The maximum dose to the LAD (D2%) varied widely, ranging from 3.66 to 53.01 Gy.
5. Discussion
This study evaluated the dose distribution to the heart and LAD in 14 patients treated with left-breast radiotherapy at our institution in the year 2014. The primary aim of this study was to measure the dose to the LAD. Our main finding is that the maximum dose (D2%) to the LAD varied widely, but could be as high as 53 Gy. Importantly, given the spacing of the CT slices (5 mm), it was not possible to completely contour the entire artery. The mean dose to the heart (3.3 Gy) was well within the acceptable range (3–13 Gy).
As numerous authors have noted,3, 6, 7, 8 the coronary arteries (particularly the LAD) are critical to the subsequent development of late radiation-induced cardiac morbidity.9 Although the heart is often considered a single OAR, in reality it is comprised of numerous different substructures, including the coronary arteries. As occurs in organs, such as the spinal cord, damage to any portion of the artery can have serious implications even if the rest of the artery remains undamaged. For this reason, it is crucial to limit the radiation dose to these arteries to the largest extent possible, especially considering that we do not know with any degree of accuracy what dose to these structures should be considered acceptable.7
5.1. LAD dose limitations
In the literature, the mean dose to the heart is highly variable and can range from 3 to 13 Gy. In our sample, the mean dose was 3.7 Gy, which is close to the lower end of reported ranges,3, 6, 7 although Becker-Schiebe8 reported an even lower mean dose (2.6 Gy). Similarly, the mean LAD doses in our sample (33.2 Gy) were consistent with those reported by other authors, including Taylor et al.10 and Nilsson et al.3
However, although it is important to assess mean doses to the heart and LAD, it is perhaps more crucial to evaluate the maximal doses to the LAD given the risk that high doses pose to the arteries. Although threshold doses to coronary arteries have not been determined, in the year 2009 the Danish Breast Cancer Cooperative Group (DBCG) recommended a maximum dose to the LAD of 20 Gy.7 In our sample, although the mean maximum and mean total doses to the heart were acceptable, several patients presented very high D2% (up to 53 Gy). These findings are consistent with data reported by Vennarini et al.,6 who found a D2% that ranged from 2.7 to 41.7 Gy. Therefore, based on the DBCG recommendations, the maximal doses in our sample and in that of Vennarini and colleagues exceed the recommended maximums. Clearly, to limit morbidity and mortality, this dose should be as low as feasible, especially to the LAD because, as noted above, damage to any segment of the coronary arteries could potentially have devastating consequences. Clearly, high doses to the narrow segments of the distal LAD are likely to increase the risk of cardiac morbidity. Consequently, it is essential to take steps to reduce the mean and maximal doses to the LAD whenever possible.
5.2. Strategies to minimize irradiation dose of the LAD
One strategy to minimize damage to the LAD is to specifically contour this area. However, as we found in the present study, when CT slices are acquired every 5 mm, portions of the LAD are not visible on the CT scan. The coronary arteries are narrow structures—the lumen diameter of the proximal LAD is 3.7 (±0.4) mm while the distal one is only 1.9 (±0.4) mm11; consequently, the 5 mm gap between slices can easily miss this artery, making proper contouring difficult or impossible. For this reason, smaller cuts are needed for complete visualization of this structure. To overcome this problem, at our institution we recently (September 2015) starting using 2.5 mm cuts for image acquisition, which allows a better visualization of the artery in the simulation CT. However, it is worth noting that while 2.5 mm slices improve visualization, given the narrowness of the LAD (particularly the distal LAD), this is still less than ideal.
Numerous other strategies to minimize dose to the heart have been proposed, including two-tangential or multi-beam intensity-modulated radiotherapy (IMRT), respiratory gated radiotherapy, breath holding techniques, and prone patient positioning. Studies have shown that deep inspiration breath hold (DIBH) reduces the dose to the heart and LAD during left-sided radiotherapy.12 Yeung et al. found that DIBH in patients with left-sided breast cancer was associated with 43.5% reduction in Dmean of the LAD, as compared to free breathing WBRT. While there is not yet any clinical evidence showing reduced cardiac toxicity or morbidity when using DIBH, numerous planning studies13, 14, 15 have demonstrated a decreased dose to cardiac structures when compared to free breathing.
At our institution, we recently implemented the breath hold technique, based on our institutional experience and on the other published reports, such as that by Becker-Schiebe et al.8 Those authors conducted a study of 130 patients to determine whether the mean heart dose—the most commonly used parameter to determine the cardiac risk of radiotherapy—is correlated with the dose to the LAD during breathing-adapted radiotherapy. In that study, 71 free-breathing patients were compared to 59 gated patients. The use of breathing-adapted RT significantly reduced the Dmean heart from 2.7 to 2.4 Gy, while the Dmean LAD decreased from 11.1 to 9.3 Gy (p = 0.04).
5.3. Study strengths and limitations
The main limitations of this study are the small sample size and the retrospective design. However, given the scarcity of concrete clinical data on radiation dose to the LAD, this study provides valuable data to help determine whether the LAD should be specifically contoured.
6. Conclusion
In this sample of 14 patients, the range of maximum doses to the left anterior descending coronary artery varied widely, with doses as high as 54 Gy in some cases. Although the precise dose constraints for the LAD have not yet been established, the available evidence indicates that this should be kept as low as possible in patients with left-sided breast cancer. The findings presented in this study led us to implement changes in routine clinical practice—primarily the use of the breath hold technique and a decrease in CT slice size from 5 mm to 2.5 mm—at our institution in order to reduce the radiation dose to the heart. The radiation tolerance dose for the coronary arteries remains uncertain and larger studies with long-term follow-up are needed to establish a dose–response relationship between the dose and coronary artery stenosis.
Financial disclosure
None declared.
Conflict of interest
None declared.
Acknowledgments
We would like to thank Bradley Londres for his invaluable assistance in improving the writing in this manuscript.
References
- 1.Group EBCTC Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst – Monogr. 2010;41(41):162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng M., Moran J.M., Koelling T. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol. 2011;79(1):10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilsson G., Witt Nyström P., Isacsson U. Radiation dose distribution in coronary arteries in breast cancer radiotherapy. Acta Oncol (Madr) 2016;55(8):959–963. doi: 10.1080/0284186X.2016.1182209. [DOI] [PubMed] [Google Scholar]
- 4.Shekel E., Levin D., Epstein D., Tova Y., Zalmanov-Faermann S., Pfeffer R. Is contouring the left anterior descending (LAD) artery necessary for left-breast patients? A retrospective comparison between treated and revised plans. Int J Radiat Oncol. 2014;90(1):S265. [Google Scholar]
- 5.Duane F., Aznar M.C., Bartlett F. A cardiac contouring atlas for radiotherapy. Radiother Oncol. 2017;122(3):416–422. doi: 10.1016/j.radonc.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vennarini S., Fournier-Bidoz N., Aristei C. Visualisation of the left anterior descending coronary artery on CT images used for breast radiotherapy planning. Br J Radiol. 2013;86(1025):20120643. doi: 10.1259/bjr.20120643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Offersen B., Højris I., Overgaard M. Radiation-induced heart morbidity after adjuvant radiotherapy of early breast cancer – is it still an issue? Radiother Oncol. 2011;100(2):157–159. doi: 10.1016/j.radonc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Becker-Schiebe M., Stockhammer M., Hoffmann W., Wetzel F., Franz H. Does mean heart dose sufficiently reflect coronary artery exposure in left-sided breast cancer radiotherapy? Strahlentherapie Und Onkol. 2016;192(9):624–631. doi: 10.1007/s00066-016-1011-y. [DOI] [PubMed] [Google Scholar]
- 9.Di Franco R., Ravo V., Nieddu V. Detection of a numeric value predictive of increased dose to left anterior descending coronary artery (LAD) in radiotherapy of breast cancer. Springerplus. 2016;5(1):841. doi: 10.1186/s40064-016-2399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor C.W., Povall J.M., McGale P. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol. 2008;72(2):501–507. doi: 10.1016/j.ijrobp.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 11.Dodge J.T., Brown B.G., Bolson E.L., Dodge H.T. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation. 1992;86(1) doi: 10.1161/01.cir.86.1.232. [DOI] [PubMed] [Google Scholar]
- 12.Yeung R., Conroy L., Long K. Cardiac dose reduction with deep inspiration breath hold for left-sided breast cancer radiotherapy patients with and without regional nodal irradiation. Radiat Oncol. 2015;10(1):200. doi: 10.1186/s13014-015-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hjelstuen M.H.B., Mjaaland I., Vikström J., Dybvik K.I. Radiation during deep inspiration allows loco-regional treatment of left breast and axillary-, supraclavicular- and internal mammary lymph nodes without compromising target coverage or dose restrictions to organs at risk. Acta Oncol (Madr) 2012;51(3):333–344. doi: 10.3109/0284186X.2011.618510. [DOI] [PubMed] [Google Scholar]
- 14.Hayden A.J., Rains M., Tiver K. Deep inspiration breath hold technique reduces heart dose from radiotherapy for left-sided breast cancer. J Med Imaging Radiat Oncol. 2012;56(4):464–472. doi: 10.1111/j.1754-9485.2012.02405.x. [DOI] [PubMed] [Google Scholar]
- 15.Nissen H.D., Appelt A.L. Improved heart, lung and target dose with deep inspiration breath hold in a large clinical series of breast cancer patients. Radiother Oncol. 2013;106(1):28–32. doi: 10.1016/j.radonc.2012.10.016. [DOI] [PubMed] [Google Scholar]