Abstract
Previously, we and others have shown that rodent neural progenitor cells (NPCs) can support functional recovery after cervical and thoracic transection injuries. To extend these observations to a more clinically relevant model of spinal cord injury, we performed unilateral midcervical contusion injuries in Fischer 344 rats. Two-weeks later, E14-derived syngeneic spinal cord-derived multi-potent NPCs were implanted into the lesion cavity. Control animals received either no grafts or fibroblast grafts. The NPCs differentiated into all three neural lineages (neurons, astrocytes, oligodendrocytes) and robustly extended axons into the host spinal cord caudal and rostral to the lesion. Graft-derived axons grew into host gray matter and expressed synaptic proteins in juxtaposition with host neurons. Animals that received NPC grafts exhibited significant recovery of forelimb motor function compared with the two control groups (analysis of variance p < 0.05). Thus, NPC grafts improve forelimb motor outcomes after clinically relevant cervical contusion injury. These benefits are observed when grafts are placed two weeks after injury, a time point that is more clinically practical than acute interventions, allowing time for patients to stabilize medically, simplifying enrollment in clinical trials, and enhancing predictability of spontaneous improvement in control groups.
Keywords: : functional recovery, neural progenitor cells, spinal cord cervical contusion
Introduction
Spinal cord injury (SCI) affects more than 280,000 persons in the United States today,1 and approximately half of injuries occur in cervical segments.1 Among persons with cervical injury, recovery of hand and upper limb function is the primary priority.2 Recent reports indicate that neural progenitor cell (NPC) transplantation into experimental rodent models of SCI can improve function of the hindlimbs after thoracic complete T3 spinal cord transection3 or thoracic contusion lesions,4 and can improve forelimb grasping ability after C4 lateral quadrant lesions.5
The approach of our group to NPC transplantation differs from other recent reports in that we attempt to fill lesion sites with cells in an effort to form new neural relays across the injury,3,5,6 whereas other investigators inject stem cells above and below lesion sites to provide primarily trophic support, minimize secondary injury, and promote remyelination.4,7,8 Given the benefits that we have observed with grafts of neural stem cells into lesion sites on functional outcomes after transection lesions, in the present study we aimed to determine whether transplants of NPCs would influence functional outcomes after more clinically relevant contusion lesions.9 Outcomes of these studies could influence the potential clinical translational path of efforts to reconstitute SCI sites with neural stem cells.
We studied a model of cervical contusion injury,10 examining whether Fischer 344 rat E14 spinal cord-derived NPCs survive when grafted into a contusion site two weeks post-injury (a clinically relevant time point for intervention). Lesion sites were not disturbed by the grafting procedure, other than passing a fine injection needle into the established lesion cavity. Anatomical and motor outcomes were examined over a three-month period.
Methods
Experimental design
Twenty-eight adult female Fisher 344 rats (10–14 weeks old, 200–250 g) were experimental subjects. This strain of rat is inbred, allowing for syngeneic cell transplants that are not rejected by the immune system. For behavioral analysis, three groups of animals were studied. All groups underwent a unilateral contusion at the C5 vertebra (C6 spinal cord level) (n = 7 animals per group) followed by a second surgery two weeks later. Group 1 received implants of syngeneic E14-derived spinal cord NPC grafts into the lesion site that were derived from green fluorescent protein (GFP+) rats (see below), allowing unequivocal tracking of cell fate and axon extension out from the graft; Group 2 received implants of GFP-expressing syngeneic fibroblasts into the lesion site, as a cellular graft control; and Group 3 received injections of cell diluent (fibrin +4 growth factor cocktail, see below) into the lesion cavity. Animals underwent biweekly forelimb functional testing on the Montoya staircase task and were sacrificed 12 weeks post-graft.
Three rats did not learn the task and thus were used for anatomical assessment of the graft at one month post-graft. Three weeks post-graft, these rats were injected with Fluoro-Gold into the ulnar nerve to retrogradely label motor neurons innervating forelimb musculature, to determine lesion/graft proximity to these motor neuron pools. In addition, four animals underwent unilateral C6 contusions and were sacrificed at two weeks post-lesion for histological assessment of the lesion cavity, to understand the nature of the lesion cavity at the time of cell grafting.
Embryonic day 14 NPC isolation and cell preparation
Spinal cord NPCs were isolated from syngeneic GFP-expressing Fischer 344 (F344) embryonic day 14 (E14) spinal cords, as described previously.3,11 Briefly, inbred transgenic F344 rats expressing enhanced green fluorescent protein (eGFP) under control of the ubiquitin promoter (F344-Tg [UBC-EGFP] F455Rrrc) were obtained from Rat Resource Research Center, University of Missouri. Timed pregnant embryos were collected from days 13.5–14.5 post-coitus and examined for eGFP expression with an ultraviolet flashlight (BlueStar). Spinal cords were dissected out from the fetus and the overlying meninges removed. Spinal cords were digested in 0.25% trypsin (ThermoFisher) at 37°C for 10 min. Fire-polished pipets were used to gently triturate the spinal cord tissue, and then centrifuged at 2500 rpm for 2 min. Cells were re-suspended in Neurobasal media (ThermoFisher), stained with 0.2% trypan blue, and live cells counted on a hemocytometer.
Surgical procedures
The National Institutes of Health (NIH) guidelines for laboratory animal care and safety were followed strictly. All procedures were performed under anesthesia with a combination (2 mL/kg) of ketamine (25 mg/mL) and Dormitor® (1 mg/mL).
For spinal cord C6 contusions, blunt dissection was used to expose the C5 and C6 vertebrae. A laminectomy was performed of the C5 vertebra exposing the C6 spinal cord. The lateral aspects of the C2 and C6 vertebra were clamped with Adelson forceps. A 2-mm round impactor tip was positioned over the side of the spinal cord corresponding to the preferred paw, as determined during behavioral pre-training. The Infinite Horizon impactor device (Precision Systems and Instrumentation) was used to delivery a force of 200 kDyns.10 The muscles were sutured and the skin stapled. Rats were given ampicillin (20 mg/kg, subcutaneously [SC]) and Banamine® (1 mg/kg, SC) in 3 mL lactated Ringer solution immediately after the surgical procedure, placed in a 37°C incubator, and 1 h later given Antisedan® (1 mg/kg, intramuscularly) to counteract the Dormitor. For three days post-operation, rats were given daily SC injections of a 3 mL solution containing lactated Ringer solution, ampicillin (20 mg/kg), Banamine (1 mg/kg).
Two weeks post-contusion, the animals were re-anesthetized with ketamine/Dormitor and placed in a spinal stereotax (Kopf). The lesion site was re-exposed by blunt dissection. Immediately before transplantation, 1.25 × 106 cells of dissociated E14 spinal cord-derived NPCs or fibroblasts were concentrated by centrifugation and resuspended in 5 μL of fibrinogen containing a 4-factor growth factor cocktail: 10 μg/mL Fibroblast Growth Factor-2 (FGF-2) 10 μg/mL Vascular Endothelial Growth Factor (VEGF) 50 μg/mL Brain Derived Neurotrophic Factor (BDNF) (all from Peprotech), and 50 μM of the calpain inhibitor, MDL28170 (Sigma).12
The laminectomy site was identified, and a total volume of 5 μL containing cells (1.5 × 106 cells) and grafting cocktail was injected directly into the lesion site using a Picospritzer II (General Valve). Cells were injected into three points (one point at the lesion epicenter and two points 0.5 mm rostral and caudal). The muscles were sutured, the skin stapled, and the rats were maintained in a 37°C incubator until awakened with Antisedan. Rats were given daily SC injections of a 3 mL solution containing lactated Ringer solution, ampicillin (20 mg/kg), and Banamine (1 mg/kg) for three days post-surgery.
To label motor neurons retrogradely, 1 μL of a 4% solution of Fluoro-Gold (Fluorochrome, Inc.) was loaded into a glass pulled pipet tip using a 5 μL Hamilton syringe, then attached to a Picospritzer. The pipet tip was carefully inserted freehand into the ulnar nerve at the level of the bicep and the fluid slowly pulsed with the Picospritzer. One week later, the animals were perfused with 4% paraformaldehyde (PFA).
Behavior testing
Before SCI, all rats were acclimated to the human tester and the testing apparatus. Rats were pre-trained for two to three weeks on the Montoya staircase (Lafayette Instrument Company) as described previously.13 Briefly, rats were allowed 15 min to consume sugar pellets ad libitum while in the apparatus. Animals were trained five days per week (2 × /day for 15 min) until they were able to retrieve seven pellets per side. For weekly or biweekly testing, rats were food deprived for 24 h, then placed in the Montoya staircase. Testers blinded to group identity recorded the number of pellets eaten, displaced, or dropped. Pellet retrieval accuracy was calculated as a percentage of sugar pellets displaced that were eaten.
Tissue preparation and immunohistochemistry
Rats were transcardially perfused with 0.9% NaCl2, followed by ice cold 4% PFA. Spinal cords were removed and post-fixed overnight in 4% PFA, then transferred to 30% sucrose in 0.1M phosphate buffer and cryoprotected for three days at 4°C. Horizontal tissue sections of a 1 cm block of spinal cord tissue containing the lesion/graft site were cut on a freezing microtome set to 25 μm. Serial tissue sections were collected in tissue cryoprotectant solution. A subset of slides were stained with 0.25% cresyl violet to label Nissl bodies or immunolabeled, as described below.
Fluorescent immunolabeling
For immunolabeling, one in 12 tissue sections was pooled and washed in tris-buffered saline (TBS). Nonspecific antibody binding was blocked for 1 h in a solution of 5% donkey serum in 0.25% triton-x in TBS (TBS++). Sections were transferred to primary antibody solution containing a mixture of primary antibodies (Table 1) overnight in TBS++ at 4°C. After TBS washes, sections were incubated in biotinylated donkey anti-chicken (5 μg/mL, Jackson Labs) in TBS++ for 1 h at room temperature (RT). After TBS washes, sections were incubated with Vectastain ABC reagent (1:200, Vector Labs) for 30 min at RT. After TBS washes, sections were incubated in biotinylated tyramide (1:2500 in 0.6% H2O2 in TBS) for 30 min at RT. Sections were then rinsed in TBS and incubated for 1 h at RT in TBS++ containing a mixture of Alexa-488 conjugated streptavidin and the appropriate donkey secondary antibodies conjugated to Alexa-568 or Alexa-647.
Table 1.
Fluorescent Immunolabeling
| Antibody | Labeling | Species | Vendor | Concentration |
|---|---|---|---|---|
| 5-HT | Serotonergic axons/terminal | Goat | Immunostar | 0.5 μg/mL |
| Chx10 | V2a spinal interneurons | Sheep | Abcam | 2 μg/mL |
| eGFP | Grafted cells | Chicken | Aves | 10 μg/mL |
| Gephyrin | Inhibitory post-synaptic density | Mouse | Synaptic Systems | 1 μg/mL |
| GFAP | Astrocyte processes | Rabbit | Agilent (Dako) | 1 μg/mL |
| Fluorogold | Tracer | Rabbit | Fluorochrome, Inc. | 1:50 dilution |
| Homer-1 | Excitatory post-synaptic density | Rabbit | Synaptic Systems | 1 μg/mL |
| MAP2 | Neuronal soma/dendrites | Mouse | Millipore Sigma | 1 μg/mL |
| NeuN | Neuronal nuclei | Mouse | Millipore Sigma | 1 μg/mL |
| RIP | Oligodendrocyte processes | Mouse | DSHB | 5 μg/mL |
| SOX9 | Astrocyte transcription factor | Goat | R&D Systems | 2 μg/mL |
| SYN | Pre-synaptic terminals | Rabbit | Proteintech | 2 μg/mL |
| SYN | Pre-synaptic terminals | Mouse | Genetex | 2 μg/mL |
5-HT, 5-hydroxytrytamine; eGFP, enhanced green fluorescent protein; GFAP, glial fibrillary acidic protein; MAP2, microtubule associated protein; RIP, RIP antigen; SOX9, SRY Homeobox-like Gene 9; SYN, synaptophysin.
To counterstain all nuclei, 4',6-diamidino-2-phenylindole (DAPI, 0.5 μg/mL, Sigma-Aldrich) was added for the final 5 min. Sections were washed in TBS, mounted on gelatin-subbed slides, air-dried, and coverslipped with Mowiol mounting medium. For labeling of post-synaptic proteins (gephyrin and Homer1), sections were first incubated in pepsin (1 mg/mL in 0.2N HCl diluted in H2O) for 6 min at 37°C. Sections were then washed in TBS and incubated in primary antibodies and visualized with fluorescent conjugated secondary antibodies, as described above.
Imaging
Fluorescent images were acquired with a Keyence all-in-one microscope or an Olympus FV1000 confocal microscope. Images were imported into Photoshop CS6 and minimally altered for brightness and contrast.
Statistical analysis
In all quantification procedures, observers were blinded to the nature of the experimental manipulations. For behavioral outcomes, multiple group comparisons were made by repeated measures two-way analysis of variance (ANOVA) (group vs. time) with a significance level of 95% followed by Fisher's post hoc testing. Data are presented as mean ± standard error of the mean (SEM). The final numbers of animals per group that were assessed were n = 7 in the NPC group, n = 7 in the fibroblast group, n = 7 in the lesion only group.
Results
Contusion histology and impact parameters
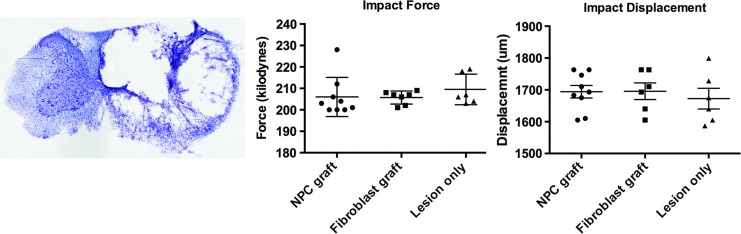
Unilateral cervical contusion lesions generated substantial loss of right-sided spinal cord parenchyma, with extensive cavitation and a small rim of spared tissue (Fig. 1A). The Infinite Horizons Impactor was set to deliver a force 200 kDyn, resulting in actual forces (mean ± SEM) of 206 ± 3 kDyns (NPC graft), 206 ± 1 kDyns (fibroblast graft), and 210 ± 3 kDyns (lesion only); these values did not differ significantly among groups (Fig. 1B). Displacement of the spinal cord (mean ± SEM) in the dorsoventral axis was 1694 ± 20 μm (NPC graft), 1696 ± 26 μm (fibroblast graft), 1672 ± 33 μm (fibrin +4-growth factors); these values did not differ significantly between groups (Fig. 1C).
FIG. 1.
Unilateral cervical contusion lesions. (A) Thionin stained transverse section of lesion site, two weeks post-contusion, demonstrating morphology of lesion cavity at time of cell grafting. (B) Impact force, and (C) impact displacement among groups. There are no significant differences between groups. Scale bar: 1 mm. NPC, neural progenitor cell.
NPCs and fibroblasts survive and fill the contusion lesion cavity 3 months post-grafting
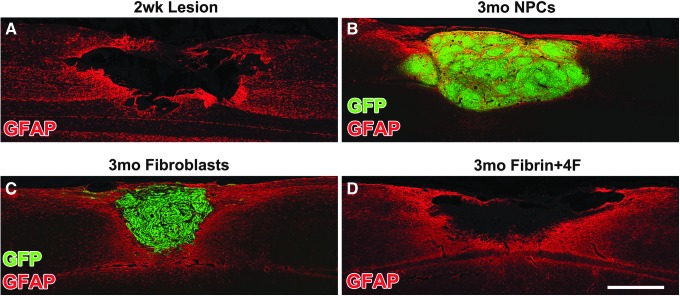
To assess the morphology of the lesion site at the time of grafting (two weeks post-contusion), a subgroup of animals underwent a unilateral C6 contusion lesion and were sacrificed two weeks later. Glial fibrillary acidic protein (GFAP)-immunolabeling showed cavitation at the impact site, indicating that a lesion cavity had formed into which cells could be injected (Fig. 1A, 2A). Thus, remaining animals that were part of functional studies underwent transplantation of NPCs, fibroblasts, or fibrin +4 growth factors into the lesion cavity, two weeks post-injury. Twelve weeks later, GFP-expressing NPCs or fibroblasts consistently filled the lesion cavity (Fig. 2 and Supplemental Fig. 1; see online supplementary material at www.liebertpub.com/neu).
FIG. 2.
Grafts in lesion site. (A) Glial fibrillary acidic protein (GFAP) labeled horizontal section, two weeks post-contusion, at the time point that cells were grafted. There is a cystic lesion cavity into which cells can be injected in an attempt to fill the lesion cavity. (B) Three months post-grafting, green fluorescent protein (GFP)-labeled neural progenitor cells (NPCs) fill the lesion cavity. (C) Similarly, three months post-grafting, GFP-labeled fibroblast grafts also fill the lesion cavity. (D) Fibrin +4 growth factor injected contusion cavity, 3.5 months post-lesion. Scale bar: 500 μm.
NPCs differentiate into subtypes of neurons and astrocytes
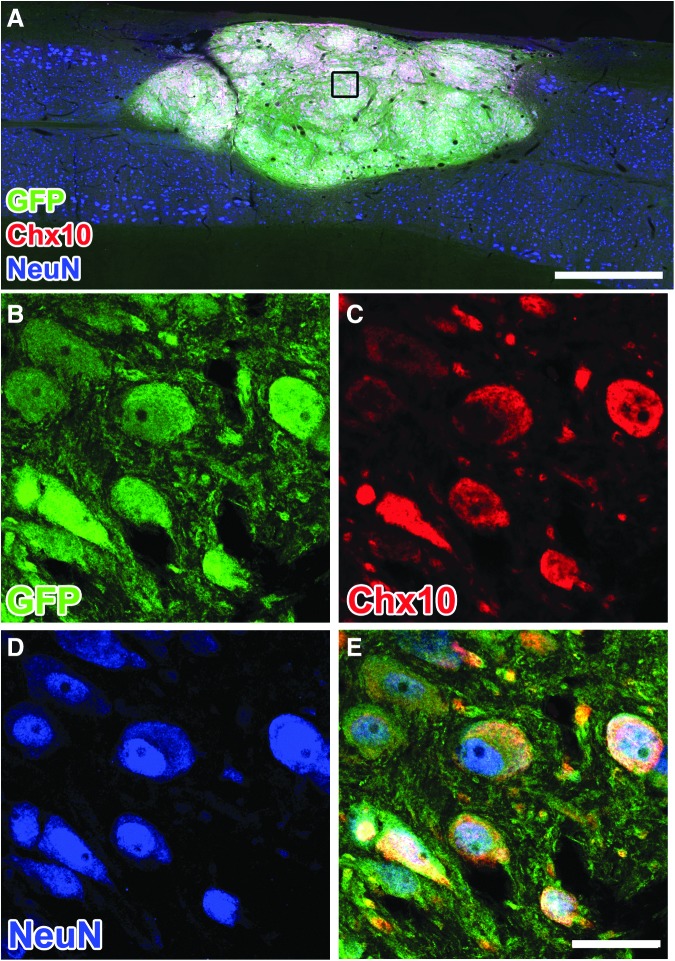
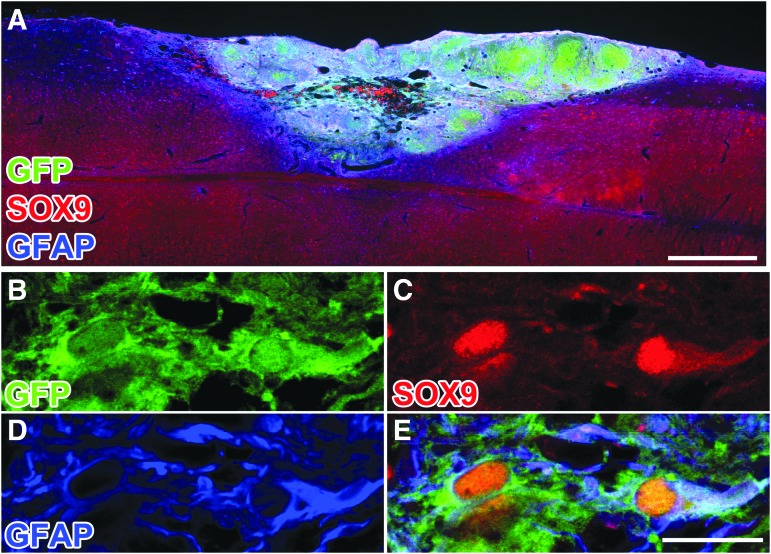
Twelve weeks post-grafting, GFP-expressing cells within NPC grafts expressed markers of mature neurons (NeuN; Fig. 3), with some neurons expressing a marker of V2a interneurons (Chx10; Fig. 3). The NPCs also differentiated into SOX9-positive, GFAP-positive astrocytes within the graft (Fig. 4). The percentage of GFP-labeled grafted cells that co-labeled for NeuN (neurons14) or SOX9 (astrocytes15) was 44.6 ± 8.3% and 23.6 ± 6.1%, respectively. These proportions are similar to our previous observations.3,5
FIG. 3.
Neural progenitor cell (NPC) grafts express mature neuronal and spinal interneuronal markers. (A–D) Three months post-grafting, (B) green fluorescent protein (GFP)-labeled cells express markers of (C) V2a interneurons, Chx10, and (D) mature neurons, NeuN. Scale bars: 500 μm in A; 30 μm in B–D.
FIG. 4.
Neural progenitor cell (NPC) grafts express mature astrocyte markers. (A) Low power image three months post-grafting, NPC graft immunolabeled for green fluorescent protein (GFP), SOX9, and glial fibrillary acidic protein (GFAP). (B–E) Single optical confocal section within the graft, immunolabeled for GFP-positive NPCs and SOX9/GFAP-positive astrocytes. Scale bars: 500 μm in A; 30 μm in B–D.
NPCs extend axons into host spinal cord and express synaptic markers
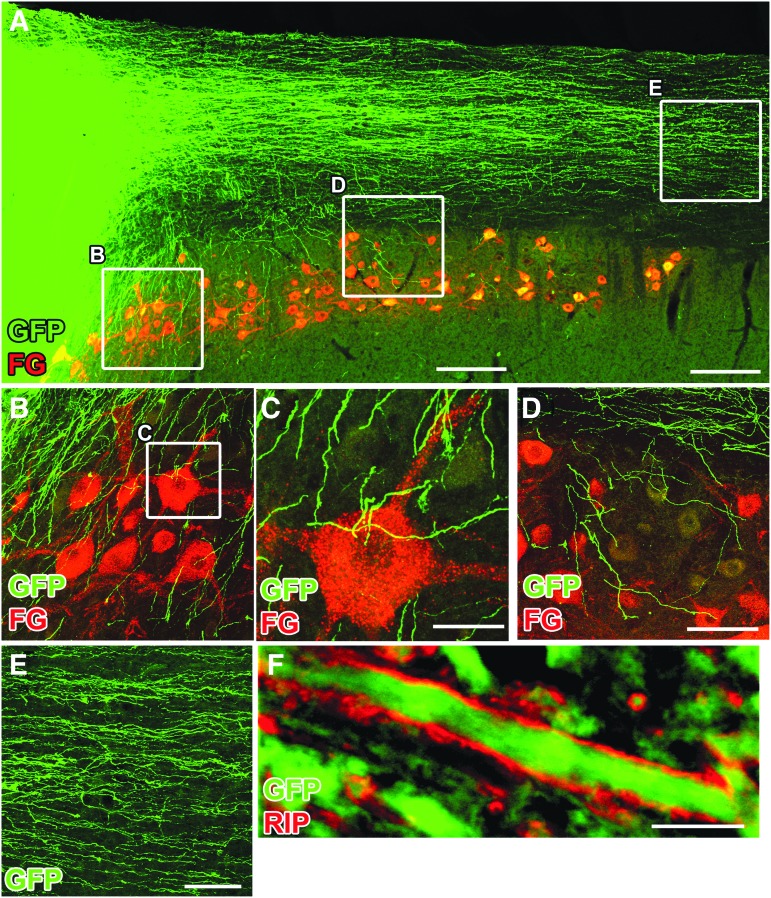
Previously, we observed robust outgrowth of GFP-expressing axons from NPCs grafted into either thoracic or cervical transection lesions.3,5,6 In the present experiment, we also observed extensive growth of GFP-labeled axons into the host spinal cord both caudal and rostral to the lesion site, consistent with previous observations (Fig. 5). Importantly, graft-derived GFP+ axons were surrounded by host RIP+ oligodendrocytes (Fig. 5F). This finding is important because it indicates that axons readily emerge from grafts into contusion lesion sites without removal of peri-lesion “scar” at the time of grafting.
FIG. 5.
Neural progenitor cell graft-derived axons extend into the distal host spinal cord. Horizontal section immunolabeled for green fluorescent protein GFP and Fluoro-Gold (FG); injected into ulnar nerve to label host motor neurons. (A) Graft-derived, GFP-expressing axons extend caudally in large numbers. Boxes indicate regions shown at higher magnification in B–D. (B) Graft-derived axons extend into regions of host motor neurons. (C) GFP-labeled axons adjacent to host motor neuron (3-μm thick optical section). (D) More caudally, GFP-labeled axons continue to branch off from the white matter to penetrate into host motor neuron pools and other regions of spinal cord gray matter. (E) Graft-derived axons extending caudally in white matter. (F) Graft-derived GFP+ axon surrounded by host-derived RIP-labeled oligodendrocyte process. Scale bars: 100 μm in B–E; 2 μm in F.
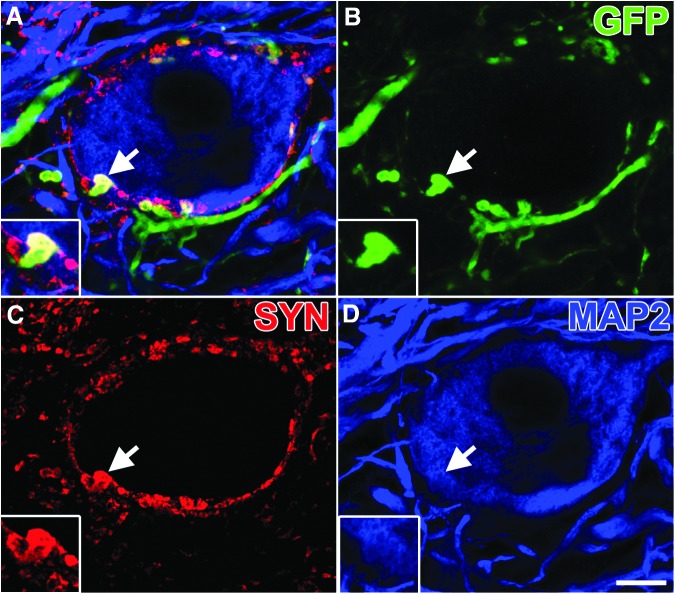
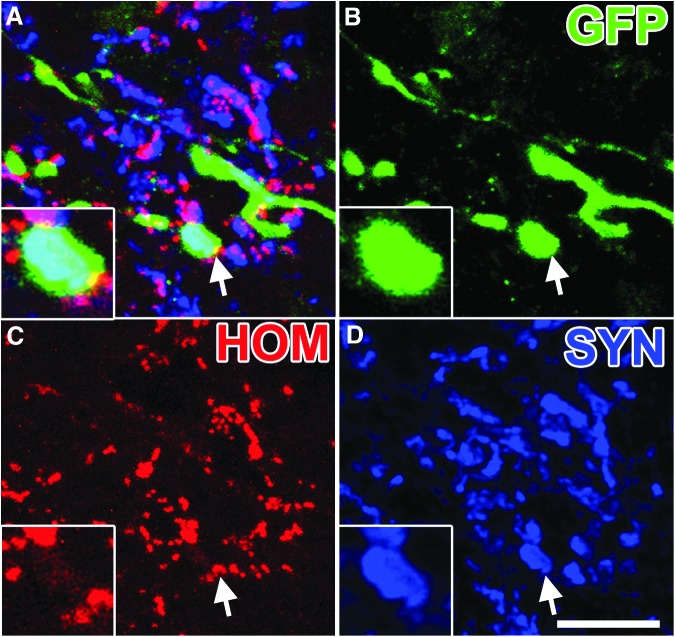
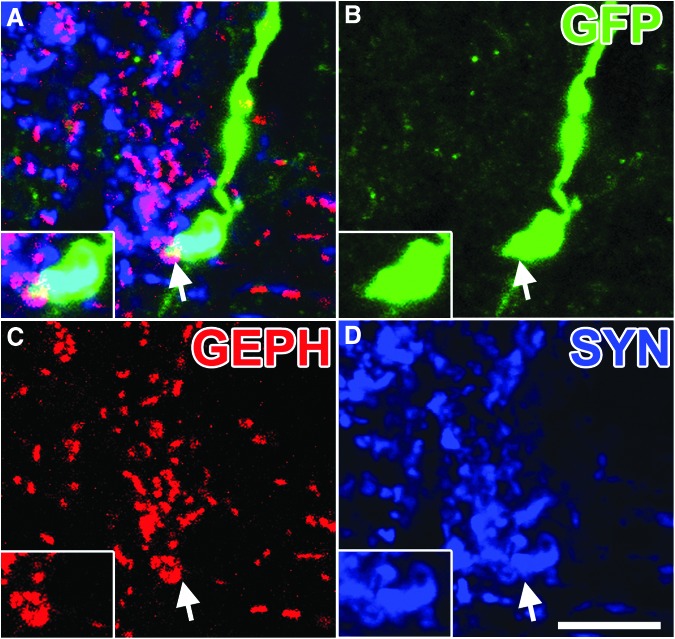
In the host spinal cord distal to the lesion site, numerous GFP-labeled graft-derived axons converged onto host neurons to form bouton-like structures that co-localized with the pre-synaptic protein, synaptophysin (Fig. 6). To further characterize the synaptic identity of graft-derived synapses, we immunolabeled for Homer1, a marker of excitatory post-synaptic densities16 and gephyrin, a marker of inhibitory post-synaptic densities.17 The GFP-labeled synaptophysin-positive terminals co-localized with both Homer1 (Fig. 7) and gephyrin (Fig. 8).
FIG. 6.
Neural progenitor cell axons form putative synaptic boutons within host gray matter. (A) Single optical section within the gray matter 3 mm caudal of the lesion/graft, immunolabeled for (B) green fluorescent protein (GFP), (C) synaptophysin (SYN), and (D) MAP2. Multiple GFP+/SYN+ are seen surrounding a microtubule associated protein (MAP2)+ neuron. Arrow indicates region of inset showing a GFP+/SYN+ puncta. Scale bar: 10 μm.
FIG. 7.
Neural progenitor cell axons form putative excitatory synaptic contacts within host gray matter. (A) Single optical section within the gray matter 3 mm caudal of the lesion/graft, immunolabeled for (B) green fluorescent protein (GFP), (C) Homer1 (HOM), and (D) synaptophysin (SYN). Arrow indicates region of inset showing a GFP+/HOM+/SYN+ puncta. Scale bar: 10 μm.
FIG. 8.
Neural progenitor cell axons form putative inhibitory synaptic contacts within host gray matter. (A) Single optical section within the gray matter 3 mm caudal of the lesion/graft, immunolabeled for (B) green fluorescent protein (GFP), (C) gephyrin (GEPH), and (D) synaptophysin (SYN). Arrow indicates region of inset showing a GFP+/GEPH+/SYN+ puncta. Scale bar: 10 μm.
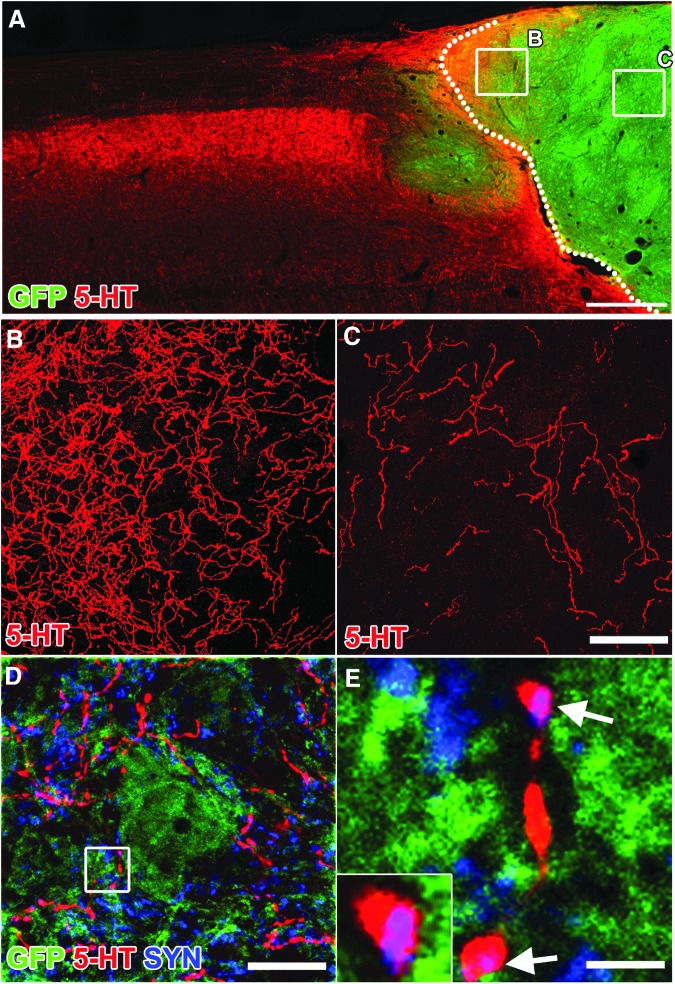
Host serotonergic axons penetrate NPC grafts
Host serotonergic axons that modulate motor activity also penetrated NPC grafts (Fig. 9A–C). Serotonergic axons labeled for 5HT exhibited co-localization with the pre-synaptic marker synaptophysin adjacent to GFP-expressing cells, suggesting putative synapse formation (Fig. 9D).
FIG. 9.
Host serotonergic axons penetrate neural progenitor cell (NPC) grafts and form putative synaptic boutons. Horizontal section immunolabeled for 5-HT in NPC grafted animal. (A) Low magnification image of 5-HT axons extending into green fluorescent protein (GFP)+ graft. (B,C) High magnification confocal z-stacks of 5-HT axons extending into graft (from regions boxed in A). (D) Single optical confocal section within the graft immunolabeled for GFP, 5-HT, and synaptophysin (SYN). (E) High magnification of box in D indicating co-labeling of 5-HT and SYN (arrows, purple color) adjacent to GFP+ cell body. Scale bars: 500 μm in A; 100 μm in B,C; 30 μm in D; 5 μm in E.
NPCs support Recovery of Forelimb Function
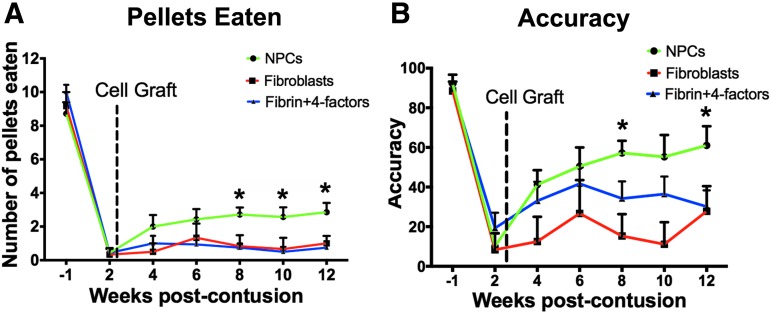
Grafts of NPCs significantly improved skilled forelimb grasping on the Montoya staircase task (Fig. 10). Control lesioned rats exhibited severe and persistent deficits in skilled reaching, recovering to a mean pellet retrieval score of only 0.75 ± 1.04 (mean ± SEM, n = 7) by week 12 after injury (Fig. 10). Similarly, recipients of control, fibroblast cell grafts exhibited severe deficits that were stable over time (1.00 ± 1.10 retrieved pellets), an amount that did not differ from animals that received fibrin +4 growth factors (Fig. 10). In contrast, the NPC-grafted group recovered to a mean of 2.86 ± 1.46 retrieved pellets by week 12, an amount that significantly exceeded the performance of both control groups (p < 0.01, repeated measures ANOVA; post hoc Fisher test p < 0.05, comparing NPC graft group to both control groups, Fig. 10).
FIG. 10.
Forelimb recovery after neural progenitor cell (NPC) grafting. (A) Unilateral C6 contusions caused severe deficits in forelimb grasping on the injured side. Subjects were tested in the Montoya staircase. Number of pellets retrieved after NPC graft (n = 7) was significantly improved compared with fibrin +4-growth factor graft (n = 7) or fibroblast graft (n = 7; p = 0.01, repeated measures analysis of variance (ANOVA); *post hoc p < 0.01). (B) NPC-grafted rats recovered significantly better than either lesion only or fibroblast groups on staircase accuracy (repeated measures ANOVA, p < 0.05).
Discussion
Findings of the present study indicate that rat NPCs can survive and integrate when transplanted directly into spinal cord contusion lesions. Rodent NPCs survive and fill the lesion site, differentiate into neurons and glia by 3 months, and extend numerous axonal projections into the host spinal cord. These axons express pre-synaptic proteins in close apposition to host neurons and post-synaptic proteins, suggesting synapse formation. Moreover, NPC grafts support significant improvement in skilled forelimb function relative to control lesioned animals. These beneficial functional effects are not a simple function of mechanical fill of the lesion site, because a control cell graft consisting of fibroblasts fails to improve functional outcomes.
A previous study grafting spinal cord NPCs also reported improvement in forelimb function, using a transection model (dorsal over-hemisection)18 rather than a contusion model. In previous studies of NPC grafting to spinal cord lesion sites, graft fill of lesions cavities was difficult to achieve consistently;19,20 for this reason, we derived a grafting “cocktail,”12 which results in consistent graft fill and survival in the contusion lesion.
Our transplantation procedure was performed by inserting the grafting needle through the dura overlying the contusion site. This is a relatively noninvasive procedure; we specifically did not open the lesion cavity or disrupt the spinal cord above and below the lesion, hypothetically reducing the risk of causing spinal cord damage. The fact that grafts survived, integrated into the lesion site, and extended axons in large numbers into the host indicates that there is no need to “freshen” the lesion site to enable graft integration with host or the resulting graft-derived axon penetration into the host, or host axon penetration into the graft. This offers a potentially meaningful safety advantage in considering human translation.
This study did not attempt to address mechanisms associated with graft-mediated improvement in forelimb function. Previous studies in a model of spinal cord transection demonstrate that E14-derived multi-potent NPCs form electrophysiological relays across the lesion.3,21 It is unlikely that grafts in the present study influenced functional outcomes through a neuroprotective mechanism, because grafts were placed two weeks after injury, a time delay that is very unlikely to preserve injured tissue. Methods to study how grafts affect functional outcomes in models of incomplete SCI could utilize delayed graft silencing (e.g., using designer receptor exclusively activated by designer drug 22) or elimination4 in future studies.
The NPCs were grafted into the lesion cavity two weeks post-injury, a delay after injury that is clinically relevant: by this time, most patients with SCI have stabilized medically and are more likely to be able to tolerate an invasive procedure. Moreover, enrollment in clinical trials is simpler and more successful when attempted within two weeks of injury compared with acute interventional trials that must enroll patients within hours or days after injury. In addition, the ability to predict spontaneous clinical improvement in injured patients when assessed two weeks post-injury is superior to the accuracy of predictions made within hours or days of injury23; consequently, adequate statistical powering of clinical trials is achieved with up to 50% fewer patients,23 reducing the cost and complexity of clinical trials. Thus, two-week delays provide time for careful clinical assessment, informed consent, and more reliable prediction of long-term outcome.
Supplementary Material
Acknowledgments
We thank Nicole Armstrong for technical assistance. Supported by the by Merit Review (I01 RX001045) and the Gordon Mansfield Collaborative Consortium for Spinal Cord Injury from the U.S. Department of Veterans Affairs, the NIH (NS42291), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation and the Bernard and Anne Spitzer Charitable Trust.
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.National Spinal Cord Injury Statistical Center (2016). Spinal Cord Injury (SCI) Facts and Figures at a Glance. Available at: www.nscisc.uab.edu Accessed January31, 2018
- 2.Anderson K.D., Friden J., and Lieber R.L. (2009). Acceptable benefits and risks associated with surgically improving arm function in individuals living with cervical spinal cord injury. Spinal Cord 47, 334–338 [DOI] [PubMed] [Google Scholar]
- 3.Lu P., Wang Y., Graham L., McHale K., Gao M., Wu D., Brock J., Blesch A., Rosenzweig E.S., Havton L.A., Zheng B., Conner J.M., Marsala M., and Tuszynski M.H. (2012). Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150, 1264–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings B.J., Uchida N., Tamaki S.J., Salazar D.L., Hooshmand M., Summers R., Gage F.H., and Anderson A.J. (2005). Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. U. S. A. 102, 14069–14074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadoya K., Lu P., Nguyen K., Lee-Kubli C., Kumamaru H., Yao L., Knackert J., Poplawski G., Dulin J.N., Strobl H., Takashima Y., Biane J., Conner J., Zhang S.C., and Tuszynski M.H. (2016). Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med. 22, 479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu P., Woodruff G., Wang Y., Graham L., Hunt M., Wu D., Boehle E., Ahmad R., Poplawski G., Brock J., Goldstein L.S., and Tuszynski M.H. (2014). Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 83, 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keirstead H.S., Nistor G., Bernal G., Totoiu M., Cloutier F., Sharp K., and Steward O. (2005). Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 25, 4694–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cizkova D., Kakinohana O., Kucharova K., Marsala S., Johe K., Hazel T., Hefferan M.P., and Marsala M. (2007). Functional recovery in rats with ischemic paraplegia after spinal grafting of human spinal stem cells. Neuroscience 147, 546–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon B.K., Soril L.J., Bacon M., Beattie M.S., Blesch A., Bresnahan J.C., Bunge M.B., Dunlop S.A., Fehlings M.G., Ferguson A.R., Hill C.E., Karimi-Abdolrezaee S., Lu P., McDonald J.W., Muller H.W., Oudega M., Rosenzweig E.S., Reier P.J., Silver J., Sykova E., Xu X.M., Guest J.D., and Tetzlaff W. (2013). Demonstrating efficacy in preclinical studies of cellular therapies for spinal cord injury—how much is enough? Exp. Neurol. 248, 30–44 [DOI] [PubMed] [Google Scholar]
- 10.Scheff S.W., Rabchevsky A.G., Fugaccia I., Main J.A., and Lumpp J.E., Jr (2003). Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma 20, 179–193 [DOI] [PubMed] [Google Scholar]
- 11.Lu P., Graham L., Wang Y., Wu D., and Tuszynski M. (2014). Promotion of survival and differentiation of neural stem cells with fibrin and growth factor cocktails after severe spinal cord injury. J. Vis. Exp. e50641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson J. and Lu P. (2017). Optimization of trophic support for neural stem cell grafts in sites of spinal cord injury. Exp. Neurol. 291, 87–97 [DOI] [PubMed] [Google Scholar]
- 13.Montoya C.P., Campbell-Hope L.J., Pemberton K.D., and Dunnett S.B. (1991). The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J. Neurosci. Methods 36, 219–228 [DOI] [PubMed] [Google Scholar]
- 14.Mullen R.J., Buck C.R., and Smith A.M. (1992). NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201–211 [DOI] [PubMed] [Google Scholar]
- 15.Sun W., Cornwell A., Li J., Peng S., Osorio M.J., Aalling N., Wang S., Benraiss A., Lou N., Goldman S.A., and Nedergaard M. (2017). SOX9 Is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J. Neurosci. 37, 4493–4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soloviev M.M., Ciruela F., Chan W.Y., and McIlhinney R.A. (2000). Molecular characterisation of two structurally distinct groups of human homers, generated by extensive alternative splicing. J. Mol. Biol. 295, 1185–1200 [DOI] [PubMed] [Google Scholar]
- 17.Kneussel M. and Betz H. (2000). Receptors, gephyrin and gephyrin-associated proteins: novel insights into the assembly of inhibitory postsynaptic membrane specializations. J. Physiol. 525 Pt 1, 1-– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynskey J.V., Sandhu F.A., Dai H.N., McAtee M., Slotkin J.R., MacArthur L., and Bregman B.S. (2006). Delayed intervention with transplants and neurotrophic factors supports recovery of forelimb function after cervical spinal cord injury in adult rats. J. Neurotrauma 23, 617–634 [DOI] [PubMed] [Google Scholar]
- 19.Jakeman L.B. and Reier P.J. (1991). Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J. Comp. Neurol. 307, 311–334 [DOI] [PubMed] [Google Scholar]
- 20.Bregman B.S., McAtee M., Dai H.N., and Kuhn P.L. (1997). Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol 148, 475–494 [DOI] [PubMed] [Google Scholar]
- 21.Bonner J.F., Connors T.M., Silverman W.F., Kowalski D.P., Lemay M.A., and Fischer I. (2011). Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J. Neurosci. 31, 4675–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armbruster B.N., Li X., Pausch M.H., Herlitze S., and Roth B.L. (2007). Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. U. S. A. 104, 5163–5168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fawcett J.W., Curt A., Steeves J.D., Coleman W.P., Tuszynski M.H., Lammertse D., Bartlett P.F., Blight A.R., Dietz V., Ditunno J., Dobkin B.H., Havton L.A., Ellaway P.H., Fehlings M.G., Privat A., Grossman R., Guest J.D., Kleitman N., Nakamura M., Gaviria M., and Short D. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45, 190–205 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.