Supplemental digital content is available in the text.
Abstract
Early allograft dysfunction (EAD) identifies allografts with marginal function soon after liver transplantation (LT) and is associated with poor LT outcomes. The impact of EAD on post-LT renal recovery, however, has not been studied. Data on 69 primary LT recipients (41 with and 28 without history of renal dysfunction) who received renal replacement therapy (RRT) for a median (range) of 9 (13-41) days before LT were retrospectively analyzed. Primary outcome was renal nonrecovery defined as RRT requirement 30 days from LT. Early allograft dysfunction developed in 21 (30%) patients, and 22 (32%) patients did not recover renal function. Early allograft dysfunction was more common in the renal nonrecovery group (50% vs 21%, P = 0.016). Multivariate logistic regression analysis demonstrated that EAD (odds ratio, 7.25; 95% confidence interval, 2.0-25.8; P = 0.002) and baseline serum creatinine (odds ratio, 3.37; 95% confidence interval, 1.4-8.1; P = 0.007) were independently associated with renal nonrecovery. History of renal dysfunction, duration of renal dysfunction, and duration of RRT were not related to renal recovery (P > 0.2 for all). Patients who had EAD and renal nonrecovery had the worst 1-, 3-, and 5-year patient survival, whereas those without EAD and recovered renal function had the best outcomes (P < 0.001). Post-LT EAD was independently associated with renal nonrecovery in LT recipients on RRT for a short duration before LT. Furthermore, EAD in the setting of renal nonrecovery resulted in the worst long-term survival. Measures to prevent EAD should be undertaken in LT recipients on RRT at time of LT.
Since the implementation of the Model for End-stage Liver Disease (MELD) score for allocation of liver grafts, the proportion of liver transplantation (LT) recipients with pre-LT renal dysfunction has increased.1,2 Previous reports identified predictors of post-LT renal recovery including pre-LT estimates of kidney function, duration of renal dysfunction, duration of pre-LT renal replacement therapy (RRT) and risk factors for chronic kidney disease (CKD), such as diabetes and hypertension.3-6 One important conclusion from these studies is that RRT duration before LT had a major impact on post-LT renal recovery with patients on RRT for less than 6 weeks duration having the highest likelihood of post-LT renal recovery.7,8 Nevertheless, only 65% to 75% of LT recipients who were on RRT for less than 6 weeks actually recovered kidney function post-LT with worse outcomes in patients who lacked renal recovery.8-10 Identifying predictors of post-LT renal recovery in those patients who were on RRT for short duration before LT is, therefore, essential to improve their renal recovery rate and the overall LT outcome. Multiple peri- and post-LT events affect post-LT renal recovery; we previously demonstrated that adverse intraoperative and postoperative LT events negatively impact post-LT renal recovery.11-13 However, few modifiable post-LT events have been correlated with post-LT renal recovery especially in recipients who were on RRT before LT.
Early allograft dysfunction (EAD) after LT affects both liver graft and patient survival and can be regarded as an intermediate and potentially modifiable factor that can affect post-LT renal recovery.14,15 The criteria of post-LT EAD were recently characterized and validated in different LT recipient cohorts which created an opportunity to study the effects of EAD on post-LT renal recovery in a standardized fashion.15 Our group previously demonstrated that EAD was associated with higher risks of new onset acute kidney injury (AKI) and end-stage renal disease post-LT.16 The aim of the current study was to examine the association between EAD and post-LT renal recovery in a cohort of LT recipients who were on RRT for a short period before LT. A secondary aim was to study the interaction between EAD and renal recovery on post-LT outcomes.
MATERIALS AND METHODS
Patient Population
The records of 117 adult LT recipients who were transplanted between January 2003 and December 2011 and were on RRT at time of LT were retrospectively reviewed. Simultaneous liver-kidney transplant recipients (n = 33), those who had previously received a LT (n = 6), and those who died (n = 7) or lost their graft and were retransplanted (n = 2) within the first week post-LT were also excluded from this study, leaving 69 primary LT alone recipients in the final analysis. Of these 69 patients, 41 (60%) had history of renal dysfunction before RRT initiation. Renal dysfunction was defined as any serum creatinine (Cr) ≥1.5 mg/dL in the 6 months preceding RRT initiation (detailed further below). EAD was defined by the presence of 1 or more of the following: (1) total bilirubin ≥10 mg/dL on postoperative day 7, (2) international normalized ratio of 1.6 or greater on postoperative day 7, and (3) alanine aminotransferase (ALT) or aspartate aminotransferase (AST) of 2000 IU/mL or greater within the first 7 postoperative days.15 The study was approved by the Mayo Clinic Institutional Review Board.
Primary Outcome
The primary outcome was renal nonrecovery, as defined by RRT requirement for longer than 30 days post-LT. Patients who died within 30 days from LT and were on RRT at time of death were considered to have not recovered renal function.
Data Collection and Post-LT Management
Baseline recipient demographics, donor, and transplant-related characteristics were recorded. Surgical techniques for organ procurement and the recipient operation have been described previously.17 All LT operations were performed via the piggyback technique without portocaval shunt, caval clamping, or veno-venous bypass. Post-LT immunosuppression management did not vary over the study period.13 All patients received induction with interleukin-2 receptor blocker to delay calcineurin inhibitor introduction. Intravenous methylprednisolone was initiated at time of LT followed by oral prednisone tapered to 0 mg/d by the third post-LT month. Calcineurin inhibitor with either tacrolimus (target trough level of 6-10 ng/mL in the first month and 5-8 ng/mL thereafter) was the primary immunosuppression medications. Trough tacrolimus levels obtained in the first month post-LT were used to calculate weekly average trough tacrolimus levels. Mycophenolate mofetil 1000 mg twice daily was started at LT with subsequent dose adjustment based on white blood cell and platelet counts as well as patient tolerance. Mycophenolate mofetil was discontinued by the fourth month after LT.
Renal function was measured using serial Cr before and after LT. The highest Cr in the 6 months before initiation of RRT was recorded and was considered to be the baseline serum Cr. The 4-point Modification of Diet in Renal Disease equation was used for the estimated glomerular filtration rate (eGFR) using the baseline Cr. Patients with baseline Cr of 1.5 mg/dL or greater were considered to have renal dysfunction before starting RRT. Duration of pre-LT renal dysfunction was measured from the time of pre-LT Cr of 1.5 mg/dL or greater until initiation of RRT, pre-LT RRT duration was measured from the time of RRT initiation to LT, and total pre-LT renal dysfunction duration was measured from the time of Cr of 1.5 mg/dL or greater until LT. The decision to initiate RRT was made by the treating nephrologist and was based on clinical criteria. All patients received intraoperative continuous RRT (CRRT) using the NxStage machine (NxStage Medical, Inc, Lawrence, MA) from 2003 to 2010 then the Prismaflex CRRT machine (Baxter Inc. Deerfield, I). A 0-mEq/L potassium bath was used as the default solution. Intraoperative CRRT was managed by the transplant anesthesiologist who adjusted the replacement fluid rate, volume removal rate, and the composition of the replacement solution. Post-LT, patients were managed in the intensive care unit by a transplant intensivist. The decision to stop RRT post-LT was made by the treating transplant nephrologist based on the presence of adequate urine output and absence of hyperkalemia.
Statistical Analysis
Baseline demographics, transplant, and donor-related factors were compared between the renal recovery and the nonrenal recovery groups. Categorical variables were presented as number (percentage) and were compared using the Pearson χ2 test. Normally distributed continuous variables were presented as mean ± standard deviation and were compared using the Student t test, whereas non-normally distributed continuous variables were presented as median (range) and were compared using the Mann-Whitney U test.
A univariate analysis was first performed to identify variables associated with 30-day renal nonrecovery. Variables that had a P value of 0.05 or less in the univariate analysis were included in a multivariate logistic regression model to identify independent predictors of renal nonrecovery at 30 days from LT. Odds ratio (OR) and 95% confidence intervals (CI) were estimated. The Kaplan Meier method and the log rank test were used to assess the effects of renal recovery and post-LT EAD development on patient and graft survivals. Statistical analysis was performed using IBM SPSS software (version 22) and STATA 12 (Stata Corp., College Station, TX).
RESULTS
The non–death-censored probability of post-LT renal recovery is demonstrated in Figure 1. As shown in Figure 1, the 30-day, 60-day and 1-year and 2-year probabilities of renal recovery were 69%, 78%, 84%, and 87%, respectively.
FIGURE 1.
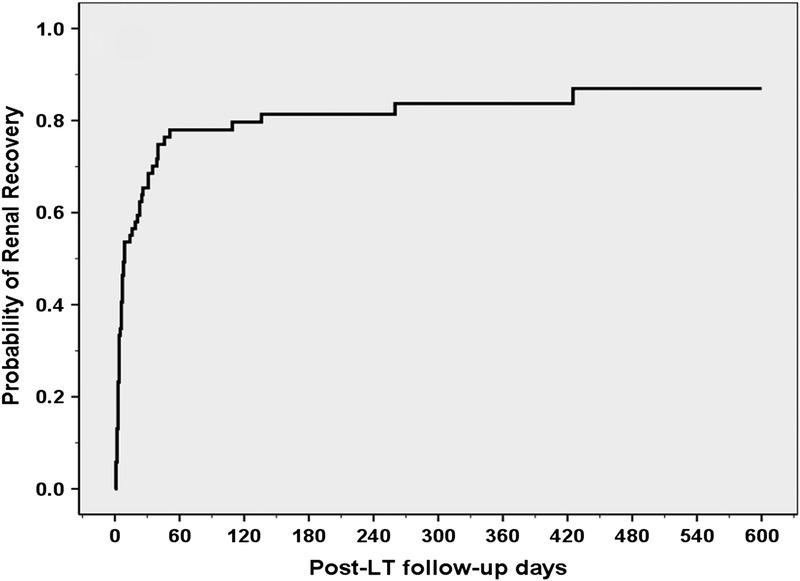
Show here is the probability of post-LT renal recovery. As shown, the 30-day, 60-day and 1-year and 2-year probabilities of renal recovery were 69%, 78%, 84%, and 87%, respectively.
Because the majority of post-LT renal recovery occurred within the first 30 days from LT, we then focused on identifying predictors of post-LT renal recovery at this time point. Table 1 summarizes recipient, donor, transplant, and post–LT-related factors of the 47 (68%) patients who recovered renal function compared with the 22 (32%) patients who remained on RRT at 30 days from LT. Of the 22 patients who did not recover renal function, 2 patients died within 1 month while on RRT and the remaining 20 patients were on RRT 30 days from LT. The median (range) post-LT RRT duration for those who recovered renal function was 4 (1-30) days. As demonstrated in Table 1, both groups were well matched in terms of the recipients' age, sex, race, cause of end-stage liver disease, and biological MELD score. Baseline serum Cr was higher in the renal nonrecovery group (2.1 ± 1.4 mg/dL vs 1.4 ± 0.5 mg/dL, P = 0.04). eGFR was also lower in the renal nonrecovery group (44.1 ± 23.2 mL/min versus 54.7 ± 35.2 mL/min) but this did not reach statistical significance (P = 0.22). Of the 41 patients with renal dysfunction, 26 (64%) recovered renal function, whereas 15 (36%) were on RRT 1 month after LT (P = 0.31). As shown in Table 1, the duration of pre-RRT renal dysfunction was comparable between the 2 groups (P = 0.43). The median (range) duration of pre-LT RRT was also comparable between those who recovered (8 [1-41]) and those who did not recover (13 [1-36]) renal function (P = 0.27). When the duration of pre-RRT renal dysfunction and the duration of pre-LT RRT were combined, there was still no observed difference between the 2 groups (31 [7-180] days for the renal recovery and 45 [6-186] days for the renal nonrecovery [P = 0.46]).
TABLE 1.
Univariate analysis comparing baseline clinical characteristics, donor-, and transplant-related factors of 69 LT recipients who were on RRT at LT grouped according to post-LT renal recovery
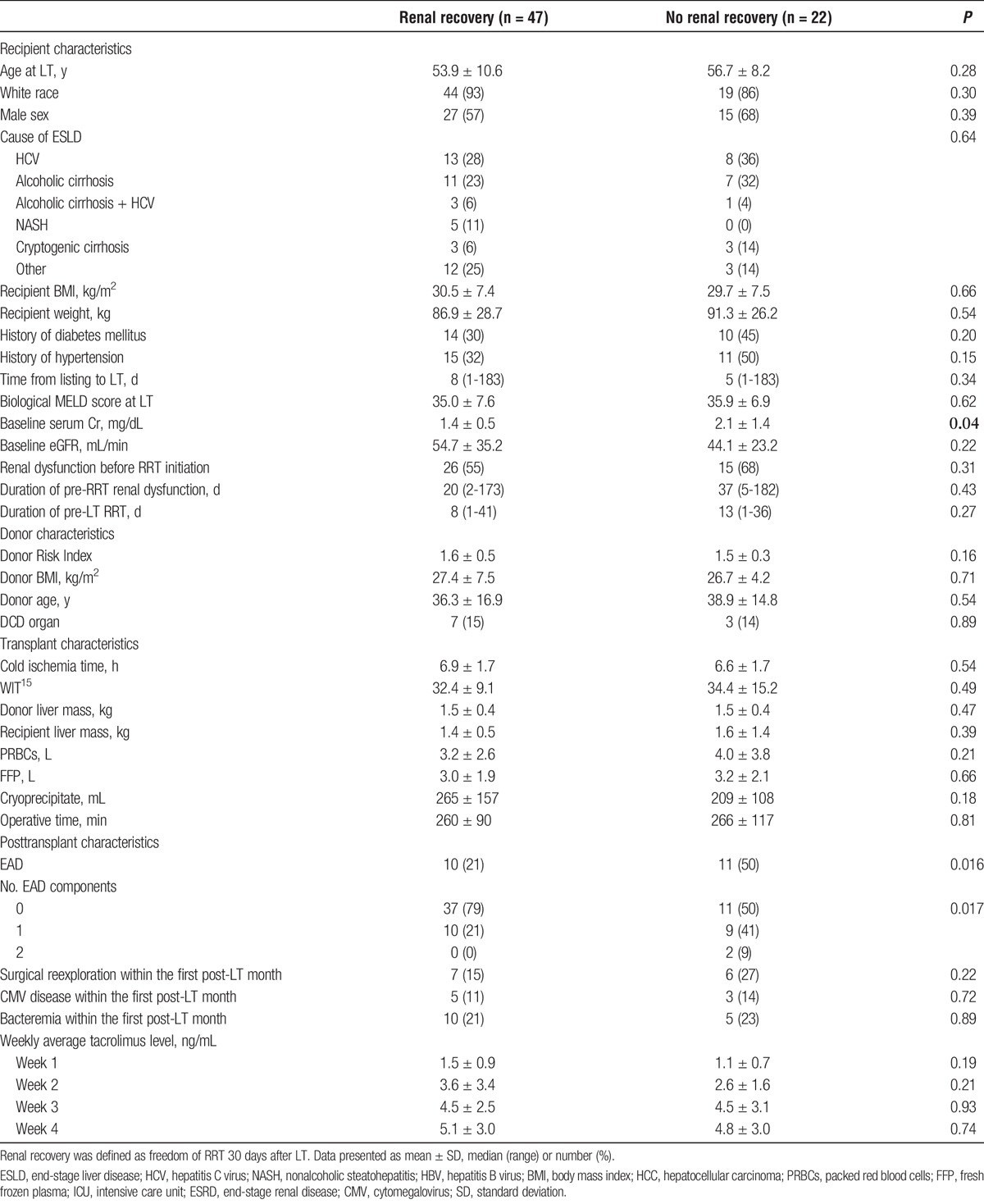
Transplant and post-LT factors including post-LT infection and reoperation rates as well as tacrolimus trough levels were comparable between the groups (Table 1) with the exception of EAD which was more common in the renal nonrecovery group (50% vs 21%, P = 0.016). The unadjusted OR (95% CI) of renal nonrecovery for patients who developed EAD was 3.7 (1.2-10.9) (P = 0.019). Out of the 3 EAD criteria, only total bilirubin of 10 mg/dL or greater was significantly associated with renal nonrecovery (OR, 3.9; 95% CI, 1.2-3.7; P = 0.02), whereas day 7 international normalized ratio of 1.6 or greater and AST/ALT of 2000 IU/mL or greater with 7 days from LT were not (P < 0.30 for both). None of the patients who met 2 criteria for EAD recovered renal function within 30 days from LT. We then used the Kaplan-Meir method to calculate the 30-day probability of renal recover. The 30-day post-LT probability of renal recovery was 77% in the no EAD group and 48% in the EAD group (P = 0.02) (Figure 2). In contrast, the 30-day probability of renal recovery was 76% and 64% in patients with and without pre-LT renal dysfunction, respectively (P = 0.35) (Figure 1S, http://links.lww.com/TXD/A65). The probability of renal recovery at 30 days from LT was also not related to the duration of pre-LT RRT when the latter was divided into weekly intervals (P = 0.54) (Figure 2S, http://links.lww.com/TXD/A65).
FIGURE 2.
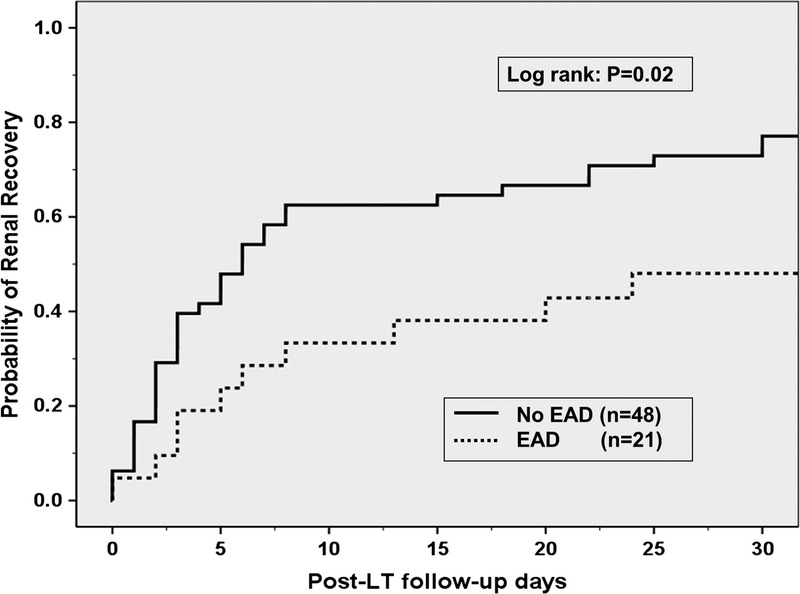
Thirty-day probability of post-LT renal recovery in 69 LT recipients on RRT at LT grouped by post-LT EAD. Recipients who developed post-LT EAD had lower probability of renal recovery (48%) compared with those who did not develop EAD (77%) (P = 0.02).
Table 2 summarizes the results of the multivariate logistic regression analysis for predictors of renal nonrecovery. Only EAD and baseline Cr were included in this analysis because these were the only 2 factors with P values of 0.05 or less on univariate analysis that correlated with renal nonrecovery. As demonstrated in Table 2, both post-LT EAD (OR, 7.25; 95% CI, 2.0-25.8, P = 0.002) and baseline Cr (OR, 3.37 for each 1 mg/dL increase in serum Cr; 95% CI, 1.4-8.1, P = 0.007) were independently associated with lack of renal recovery.
TABLE 2.
Results of multivariate analysis logistic regression analysis identifying factors independently associated with lack of post-LT renal recovery in 69 LT recipients on RRT at time transplantation

We repeated the analysis using renal recovery at 60 days from LT as the endpoint. Post-LT EAD remained independently associated with lack of renal recovery (OR, 3.8; 95% CI, 1.1-13.2, P = 0.03) after adjusting for baseline serum Cr.
Patient and Graft Survival
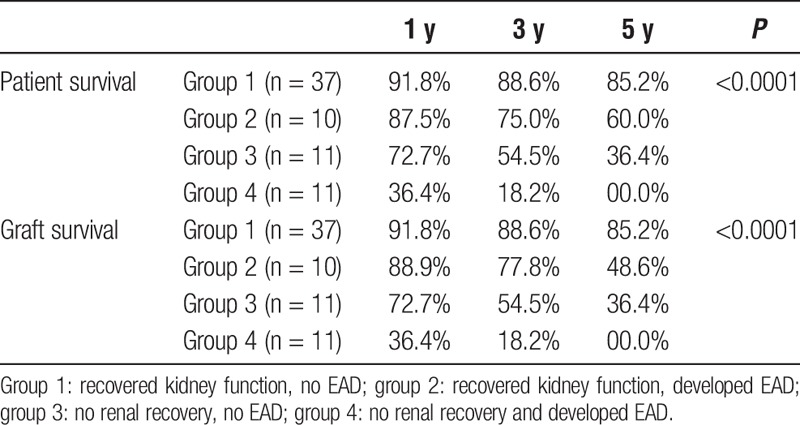
We then analysed patient and graft survival rates according to renal recovery and EAD development. Results of this analysis are presented in Figures 3A and B and Table 3. In this analysis, the 69 patients were divided into 4 groups. Group 1 (n = 37), recovered renal function within 30 days post-LT and did not develop EAD; group 2 (n = 10), recovered renal function within 30 days post-LT and developed EAD; group 3 (n = 11), did not recover renal function and did not develop EAD; group 4 (n = 11), patients with no renal recovery who also developed EAD. As demonstrated in Figures 2A, B and Table 3, patients who recovered renal function and did not develop EAD (group 1) had the best patient and graft survival rates, whereas those who did not recover renal function and developed EAD (group 4) had the worst outcomes. Patients who had EAD alone but recovered renal function (group 2) or failed to recover renal function but did not develop EAD (group 3) had intermediate outcomes.
FIGURE 3.
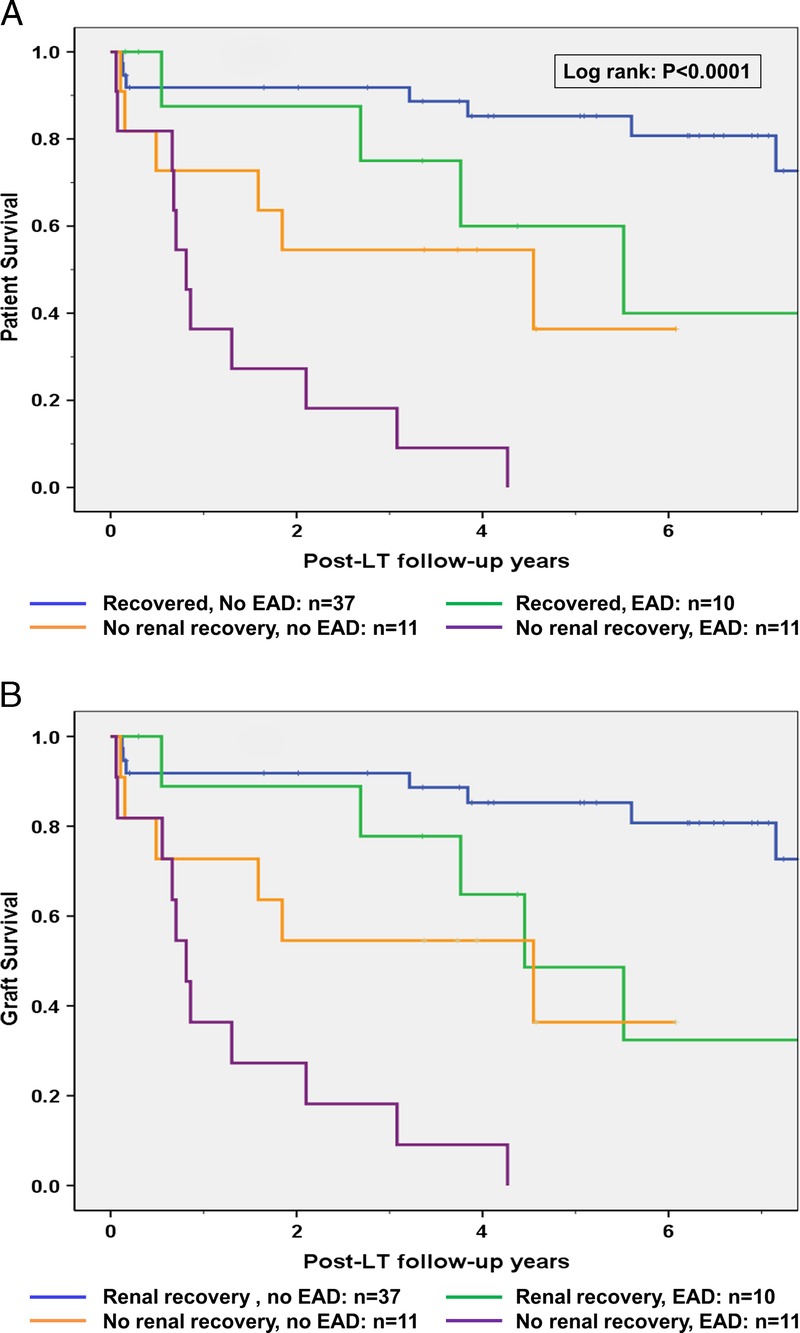
A and B, Patient (A) and liver graft (B) survival of 69 primary LT recipients grouped according to post-LT renal recovery and EAD development. Group 1: Recovered kidney function, no EAD (n = 37). Group 2: Recovered kidney function, developed EAD (n = 10). Group 3: No renal recovery, no EAD (n = 11). Group 4: No renal recovery and developed EAD (n = 11). As demonstrated in panels A and B, patients in group 1 had the best post-LT outcomes, whereas none in the patients in group 4 survived 5 years after LT.
TABLE 3.
One-, 3-, and 5-year patient and graft survivals according to renal function recovery and post-LT EAD development in 69 LT recipients on RRT at time of transplantation
DISCUSSION
In this single-center retrospective study, we identified predictors of renal nonrecovery in 69 LT recipients who were on RRT for a median of 9 days before LT. Our results indicated that the majority of these patients (68%) recovered renal function and were free of RRT within 30 days from LT. However, we identified post-LT EAD development as an independent predictor of renal nonrecovery. The 30-day probability of renal recovery was 77% in the no EAD group and 48% in the EAD group (P = 0.02). Post-LT EAD had strong impact on post-LT renal recovery and was associated with an unadjusted 3.7-fold increased risk of RRT dependency at 1 month from LT. After adjusting for pre-LT renal function, patients who developed post-LT EAD had more than a sevenfold increased risk of renal nonrecovery. Of the 3 criteria that define EAD, total bilirubin greater than 10 mg/dL was the criterion that was significantly associated with renal nonrecovery. There was no significant correlation between presence of renal dysfunction before RRT initiation, its duration, or the pre-LT RRT duration and post-LT renal recovery. One important finding of our analysis was the cumulative effect of EAD and post-LT renal nonrecovery on post-LT outcomes: none of the patients who had EAD and did not recover renal function survived 5 years after transplantation (1-, 3-, and 5-year patient survival rates were 36.4%, 18.2%, and 0%, respectively), whereas those who did not develop EAD and recovered kidney function had excellent post-LT outcomes (1-, 3-, and 5-year patient survival rates were 91.8%, 88.6%, and 85.2%, respectively) which was almost comparable to LT outcomes in patients with normal pre-LT renal function. Recipients who developed either EAD or renal nonrecovery had intermediate outcomes.
Post-LT EAD has a strong impact on both short- and long-term post-LT outcomes and its effects extend beyond the liver graft.18,19 We, as well as others, have previously demonstrated that post-LT EAD as defined by the Olthoff criteria15 and hepatic ischemia reperfusion injury are associated with a higher risk of post-LT AKI even in patients with normal pre-LT renal function.16,20,21 In a prospective cohort study that included 80 adult LT recipients, AKI occurred in 26% of the patients and was associated with peak AST level and post-LT EAD development.20 The effect of EAD on post-LT renal recovery in LT recipients on RRT for short duration at time of LT has not been studied. The results of the current study indicated that LT recipients, who develop EAD, have a lower probability of recovering kidney function post-LT. It is important to mention that the association between EAD and renal nonrecovery persisted even after adjusting for baseline serum Cr and after considering alternative end points, such as RRT dependence 60 days from LT. We can infer from our results that prevention of EAD and early optimization of liver graft function may improve the probability of post-LT renal recovery especially in patients receiving RRT at time of LT. Factors associated with EAD development are well documented in the literature.14,16,22 Some of these factors, such as duration of cold and warm ischemia times (WITs), are modifiable and open to intervention. Previous reports have linked prolonged WIT with post-LT renal nonrecovery. For example, Laskey and colleagues12 demonstrated that each minute increase in WIT was associated with 8% to 9% increased risk of renal nonrecovery in a group of 40 LT recipients with pre-LT renal dysfunction. Another alternative to avoid EAD is to implement better graft preservation techniques at time of liver procurement especially in organs directed to recipients on RRT. One of these potential techniques is hypothermic machine perfusion of the liver graft which was associated with attenuated hepatic ischemia reperfusion injury, lower risk of EAD and lower probability of post-LT AKI in 31 LT-recipients who received extended criteria donor livers.23 Similarly, Mergental and colleagues24,25 used normothermic ex-vivo liver perfusion before transplantation of 5 livers at high risk of post-LT EAD. All recipients experienced immediate liver graft function and had excellent 1- and 3-month renal function. Others have shown similar results with subnormothermic machine perfusion.24 These data suggest that such strategies may reduce the risk of post-LT EAD and based on our results may subsequently improve the renal recovery rate in LT recipients who were on RRT at time of LT.
Poor pre-LT kidney function, especially RRT dependency before LT, has been associated with poor LT outcomes.1,26 One important finding of the current study is that the negative impact of poor pre-LT renal function on post-LT outcomes was almost abolished in patients who experienced immediate liver graft function (ie, no EAD) and recovered renal function in the first post-LT month. Our results demonstrated 1-, 3-, and 5-year patient and graft survival rates of 91.8%, 88.6%, and 85.2%, respectively, in this group of recipients (group 1, Figures 2A and B) which was comparable to outcomes of LT recipients who had normal renal function at time of LT in our center and in previous reports.26,27 Our results also demonstrated that recipients who developed EAD but recovered renal function had better 1-, 3-, and 5-year patient and graft survival rates compared with those recipients who did not recover renal function and did not develop EAD. These findings might suggest that post-LT renal recovery has a greater impact on post-LT outcomes than EAD development. Patients with both EAD and nonrenal recovery had the worst 1-, 3-, and 5-year survival rates with none of these patients surviving 5 years after LT. Taken together, our results indicated that there was a significant interaction between EAD and post-LT renal recovery on post-LT outcomes and that avoiding EAD in LT recipients with significant pre-LT renal dysfunction might not only alter the probability of post-LT renal recovery but might also improve post-LT survival of this group of patients who historically experienced poor post-LT outcomes.
In the current study, an elevated total bilirubin greater than 10 mg/dL at day 7 from LT was the main factor associated with lack of renal recovery (OR, 3.9; 95% CI, 1.2-3.7; P = 0.02). This finding is similar to a previous observation that correlated elevated total bilirubin level at day 7 from LT with renal nonrecovery in a group of 28 patients with hepatorenal syndrome before LT.9 Our group previously reported that an elevated bilirubin level was also associated with a higher risk of post-LT new-onset AKI in patients who were not on RRT at time of LT.16 Collectively, these findings are consistent with the known nephrotoxic effect of bilirubin and suggest that extra-corporeal removal of bilirubin might be beneficial in enhancing renal recovery in patients who develop hyperbilirubinemia post-LT.28-30
Previous reports linked donation after cardiac death (DCD) LT to higher rates of post-LT renal dysfunction.31 Donation after cardiac death LT is a risk factor for developing EAD.14,16 In the current study, 10 patients received DCD LT and 59 patients received donation after brain death LT. Although the rate of EAD was higher in DCD recipients (50%) compared with their donation after brain death counterparts (28%), this did not reach statistical significance (P = 0.15) probably due to the small number of patients. Yet, 7 (70%) of the DCD LT recipients were free of RRT and recovered kidney function 1 month after LT. We found that DCD LT per se was not an absolute risk for renal nonrecovery or EAD development in our cohort. As a result, we believe that the use of DCD organs can be justified in this high-risk group of LT recipients. Additional studies with larger number of patients are needed to clearly define the effect of DCD LT on post-LT renal recovery.
Recipients in the renal nonrecovery group were older, were on RRT for longer duration, and had higher proportion of patients with diabetes, hypertension, and history of renal dysfunction. However, none of these factors statistically correlated with post-LT renal recovery probably due to the small number of patients in the current study. Therefore, the correlation between these aforementioned factors and renal nonrecovery in this study should be interpreted with caution. However, despite the small number of patients, the current study identified post-LT EAD to be independently associated with renal nonrecovery even after adjusting for pre-LT baseline serum Cr.
This study is limited by its retrospective nature and the small number of patients involved that could have affected the results. However, we were able to identify a homogenous group of LT recipients who had AKI and were on RRT for a median of 9 days before LT and therefore had the highest probability of post-LT renal recovery. We also choose RRT requirement for more than 30 days from LT as a hard and easily reproducible outcome to study the effects of EAD on post-LT renal recovery. Even when we used an alternative definition for renal nonrecovery as RRT requirement for more than 60 days from LT, EAD was still associated with lack of renal recovery at this time point. The 1-month renal recovery rate of 68% was comparable to previous studies that determined that the rate of renal recovery in patients with pre-LT renal dysfunction, including those on short course of pre-LT RRT that varied from 65% to 75%.8,9 This indicates that despite the small number of patients, our definition for renal recovery was reproducible and reflective of real-life renal recovery rates in other transplant centres. The current study also lacks information regarding long-term kidney function as our main focus was to identify early events that impacted renal recovery at 30 days from LT.
In conclusion, we have identified post-LT EAD as a clinically important factor that affected renal nonrecovery in LT recipients with short duration of RRT before LT. We have demonstrated that there was significant interplay between EAD, post-LT renal recovery, and post-LT outcomes. Measures to prevent EAD development or mitigate its effects may be beneficial in improving post-LT renal recovery and survival rates in this group of patients.
Supplementary Material
Footnotes
Published online 14 March, 2018.
Funding provided internally by Mayo Clinic Collaborative in Transplant Research and Outcomes.
The authors declare no conflicts of interest.
H.M.W., D.D.L., and C.B.T. participated in research design. H.M.W., D.D.L., A.P.K., and C.B.T. participated in the writing of the article. H.M.W., D.D.L., K.P.C., M.L.M., L.M., D.L., A.P.K., and C.B.T. participated in the performance of the research. H.M.W., D.D.L., K.P.C., and D.L. participated in data analysis. H.M.W. and C.B.T. provided the final approval.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Gonwa TA, McBride MA, Anderson K, et al. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6:2651–2659. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Schaubel DE, Guidinger MK, et al. Impact of MELD-Based allocation on end‐stage renal disease after liver transplantation. Am J Transplant. 2011;11:2372–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Goodrich NP, Schaubel DE, et al. Patient-specific prediction of ESRD after liver transplantation. J Am Soc Nephrol. 2013;24:2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P, Goodrich NP, Zhang M, et al. Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin J Am Soc Nephrol. 2013;8:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burra P, Senzolo M, Masier A, et al. Factors influencing renal function after liver transplantation. Results from the MOST, an international observational study. Dig Liver Dis. 2009;41:350–356. [DOI] [PubMed] [Google Scholar]
- 6.Ruebner R, Goldberg D, Abt PL, et al. Risk of end‐stage renal disease among liver transplant recipients with pretransplant renal dysfunction. Am J Transplant. 2012;12:2958–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong F, Leung W, Al Beshir M, et al. Outcomes of patients with cirrhosis and hepatorenal syndrome type 1 treated with liver transplantation. Liver Transpl. 2015;21:300–307. [DOI] [PubMed] [Google Scholar]
- 8.Northup PG, Argo CK, Bakhru MR, et al. Pretransplant predictors of recovery of renal function after liver transplantation. Liver Transpl. 2010;16:440–446. [DOI] [PubMed] [Google Scholar]
- 9.Marik PE, Wood K, Starzl TE. The course of type 1 hepato-renal syndrome post liver transplantation. Nephrol Dial Transplant. 2006;21:478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonwa TA, Mai ML, Melton LB, et al. Renal replacement therapy and orthotopic liver transplantation: the role of continuous veno-venous hemodialysis. Transplantation. 2001;71:1424–1428. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Singhapricha T, Hu KQ, et al. Postliver transplant acute renal injury and failure by the RIFLE criteria in patients with normal pretransplant serum creatinine concentrations: a matched study. Transplantation. 2011;91:348–353. [DOI] [PubMed] [Google Scholar]
- 12.Laskey HL, Schomaker N, Hung KW, et al. Predicting renal recovery after liver transplant with severe pretransplant subacute kidney injury: the impact of warm ischemia time. Liver Transpl. 2016;22:1085–1091. [DOI] [PubMed] [Google Scholar]
- 13.Wadei HM, Heckman MG, Rawal B, et al. Renal outcomes of liver transplant recipients who had pretransplant kidney biopsy. Transplantation. 2014;98:1323–1330. [DOI] [PubMed] [Google Scholar]
- 14.Lee DD, Singh A, Burns JM, et al. Early allograft dysfunction in liver transplantation with donation after cardiac death donors results in inferior survival. Liver Transpl. 2014;20:1447–1453. [DOI] [PubMed] [Google Scholar]
- 15.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943–949. [DOI] [PubMed] [Google Scholar]
- 16.Wadei HM, Lee DD, Croome KP, et al. Early allograft dysfunction after liver transplantation is associated with short‐ and long‐term kidney function impairment. Am J Transplant. 2016;16:850–859. [DOI] [PubMed] [Google Scholar]
- 17.Taner CB, Bulatao IG, Willingham DL, et al. Events in procurement as risk factors for ischemic cholangiopathy in liver transplantation using donation after cardiac death donors. Liver Transpl. 2012;18:100–111. [DOI] [PubMed] [Google Scholar]
- 18.Friedman BH, Wolf JH, Wang L, et al. Serum cytokine profiles associated with early allograft dysfunction in patients undergoing liver transplantation. Liver Transpl. 2012;18:166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurian SM, Fouraschen SM, Langfelder P, et al. Genomic profiles and predictors of early allograft dysfunction after human liver transplantation. Am J Transplant. 2015;15:1605–1614. [DOI] [PubMed] [Google Scholar]
- 20.Jochmans I, Meurisse N, Neyrinck A, et al. Hepatic ischemia/reperfusion injury associates with acute kidney injury in liver transplantation: prospective cohort study. Liver Transpl. 2017;23:634–644. [DOI] [PubMed] [Google Scholar]
- 21.Leithead JA, Armstrong MJ, Corbett C, et al. Hepatic ischemia reperfusion injury is associated with acute kidney injury following donation after brain death liver transplantation. Transpl Int. 2013;26:1116–1125. [DOI] [PubMed] [Google Scholar]
- 22.Deschenes M. Early allograft dysfunction: causes, recognition, and management. Liver Transpl. 2013;19(Suppl 2):S6–S8. [DOI] [PubMed] [Google Scholar]
- 23.Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant. 2015;15:161–169. [DOI] [PubMed] [Google Scholar]
- 24.Bruinsma BG, Yeh H, Özer S, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant. 2014;14:1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mergental H, Perera M, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex‐situ evaluation. Am J Transplant. 2016;16:3235–3245. [DOI] [PubMed] [Google Scholar]
- 26.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179–1185. [DOI] [PubMed] [Google Scholar]
- 27.Lee DD, Croome KP, Perry DK, et al. Liver transplantation at Mayo Clinic Florida. Clin Transpl. 2014:83–90. [PubMed] [Google Scholar]
- 28.Esposito P, Rampino T, Sileno G, et al. Selective bilirubin removal: a treatment of jaundice-related kidney injury? Kidney Int. 2013;84:624–625. [DOI] [PubMed] [Google Scholar]
- 29.van Slambrouck CM, Salem F, Meehan SM, et al. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013;84:192–197. [DOI] [PubMed] [Google Scholar]
- 30.Ott R, Rupprecht H, Born G, et al. Plasma separation and bilirubin adsorption after complicated liver transplantation: a therapeutic approach to excessive hyperbilirubinemia. Transplantation. 1998;65:434–437. [DOI] [PubMed] [Google Scholar]
- 31.Leithead JA, Tariciotti L, Gunson B, et al. Donation after cardiac death liver transplant recipients have an increased frequency of acute kidney injury. Am J Transplant. 2012;12:965–975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


