Abstract
Chronic shortages of organs for transplantation have led to the use of marginal kidneys from donors after circulatory death with acute kidney injury (AKI), but the utilization of kidneys with severe AKI is not well established. We retrospectively analyzed eight kidney transplantation (KTx) cases from donation after circulatory death (DCD) with terminal creatinine (t-Cr) concentrations higher than 10.0 mg/dL and/or oliguria for more than 5 days (AKI network criteria: stage III). Although all patients showed delayed graft function, no cases of primary nonfunction (PNF) were found. Five patients maintained stable renal function for approximately 15.5, 10, 10, 5, and 0.5 years after KTx. Only 1 patient showed biopsy-proven acute rejection. Also, 2 patients developed graft failure: one attributable to chronic antibody mediated rejection at 11.3 years after KTx, and one attributable to recurrence of IgA nephropathy at 4.6 years after KTx. Kidneys with AKI stage III yielded great outcomes without the risk of primary nonfunction and rejection. Although the AKI kidneys were associated with delayed graft function, these results suggest that even the most severe kidneys with AKI stage III from DCD donors can be considered a valid alternative for recipients on a waiting list for KTx.
Recent efforts have been undertaken worldwide to expand donor pools, including the wider use of marginal kidneys from donation after circulatory death (DCD),1-4 of dual kidney transplants,5,6 and of revised graft perfusion methods for preventing reperfusion injury.7,8 Nevertheless, marginal kidneys from DCD have been regarded as inappropriate for donor pool expansion. These grafts have developed a higher frequency of delayed graft function (DGF), acute rejection, and graft loss because of several factors related to poor graft outcome, such as donor age, a history of cerebrovascular event (CVE) and hypertension, cold ischemic time (CIT), and acute kidney injury (AKI). By contrast, some earlier reports have described that 5-year graft and patient outcomes from DCD kidneys are compatible with those from donation after brain death kidneys.9,10 We also reported improved results for recipients of transplanted DCD kidneys.11
An analysis presented in the Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients Annual Report revealed that more than 20% of marginal kidneys have been declined for use in transplantation. Although AKI is frequently associated with multiple organ failure and death, most patients can fully recover renal function.12-16 Short-term follow-up showed that kidneys with AKI stage I and II are useful for strategies for discarded organs.17,18 Nevertheless, most kidneys included among these data were obtained from donation after brain death donors. Outcomes of DCD kidneys with AKI stage III remain controversial. This report describes cases of 8 patients who received kidneys from DCD donors with stage III AKI and presents analysis of the frequency of primary nonfunction (PNF) and DGF, pathological findings, and long-term graft outcomes.
CASE DESCRIPTION
The study was conducted in accordance with the principles of the Declaration of Helsinki. We retrospectively analyzed 8 kidney transplantations (KTx) of DCD kidneys with terminal creatinine (t-Cr) concentrations higher than 10.0 mg/dL and/or oliguria for more than 5 days (Table 1 and Figure 1). These were the worst 8 kidneys at our center in terms of t-Cr concentration.
TABLE 1.
Donor characteristics
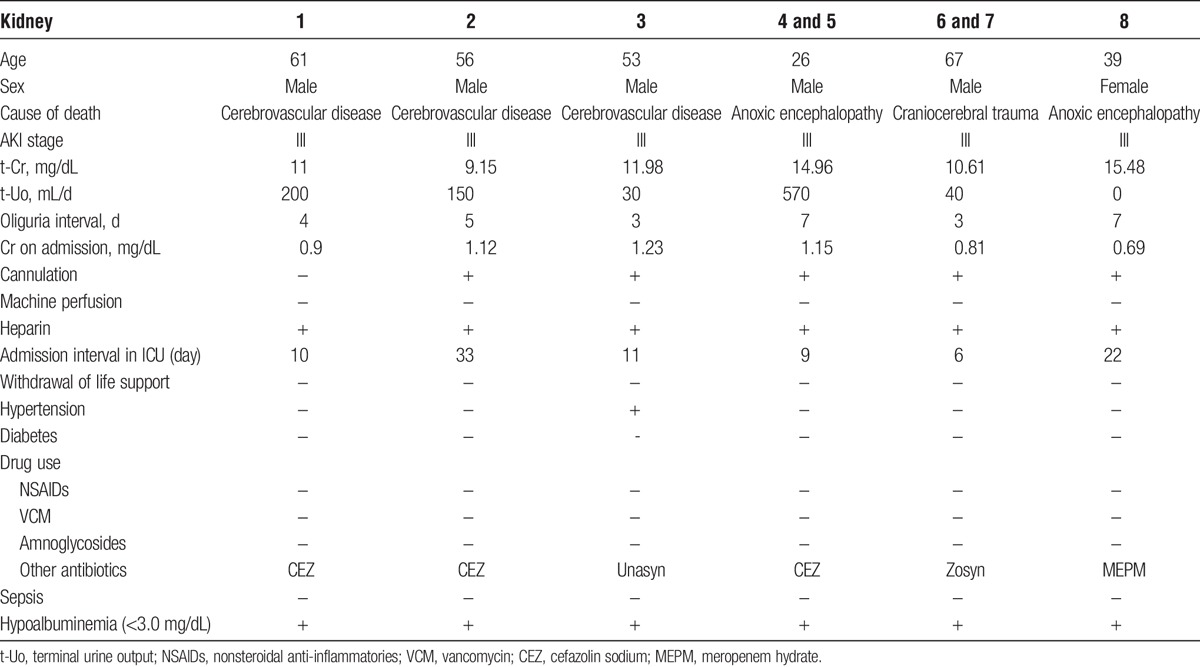
FIGURE 1.
Trends of serum Cr concentrations (mg/dL) and urine outputs (mL/d) in donors until donation. Patients 4/5 and patients 6/7 were transplanted grafts from the same donors.
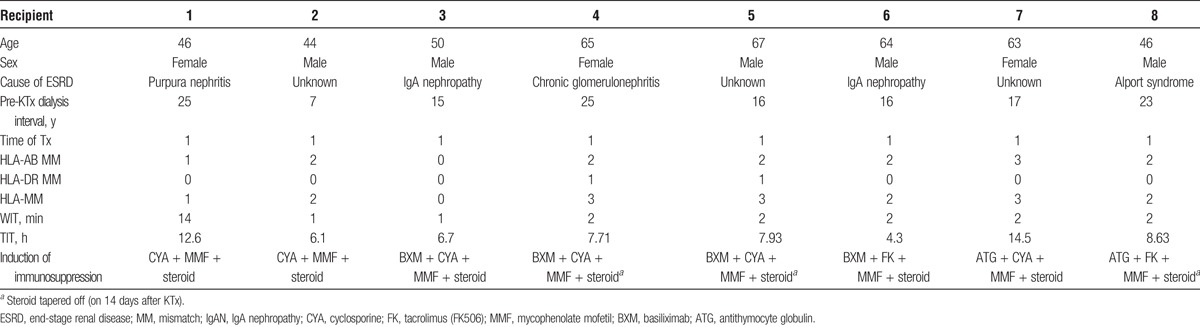
The mean donor age was 49.38 years (Table 1). All donors were classified as AKI stage III based on AKI network criteria.19,20 The averages of t-Cr and terminal urine output in donors were 12.34 mg/dL and 200 mL/d, respectively. Of the 6 donors, 2 had CVEs and 1 had CVEs and a history of HT. The average interval in the intensive care unit (ICU) after admission was 15.2 days. All donors had hypoalbuminemia. The mean recipient age was 55.63 years (Table 2). Recipients 4 and 5 were transplanted with a single graft from a donor. Recipients 6 and 7 were also transplanted with a single graft from another donor. The average interval of dialysis before KTx was 17.88 years. The mean warm ischemic time (WIT) was 3.25 minutes. The mean total ischemic time (TIT) was 8.56 hours. We used cyclosporine or tacrolimus, mycophenolate mofetil, and methylprednisolone as the basic immunosuppressive regimen. Basiliximab (20 mg/d, twice) or antithymocyte globulin (1.5 mg/kg per day, 4-5 times) was administered as an induction therapy. Where possible, methylprednisolone was tapered off on postoperative day 14 (14POD). The average of follow-up periods for recipients was 8.0 years.
TABLE 2.
Recipient characteristics
All recipients developed DGF (Table 3). However, all transplanted kidneys functioned sufficiently without maintenance dialysis within 1 month after KTx (the average interval and times of dialysis after KTx were 13.25 days and 6.25 times). The serum Cr at 1 week after KTx remained high (the average was 9.21 mg/dL). However, that of 1 and 6 months after KTx decreased over time (respective averages were 3.03 and 1.62 mg/dL). Moreover, the lowest serum Cr and the highest estimated glomerular filtration rate (respective averages were 1.14 mg/dL and 52.85 mL/min per 1.73 m2) showed excellent outcomes.
TABLE 3.
Outcomes of post-KTx
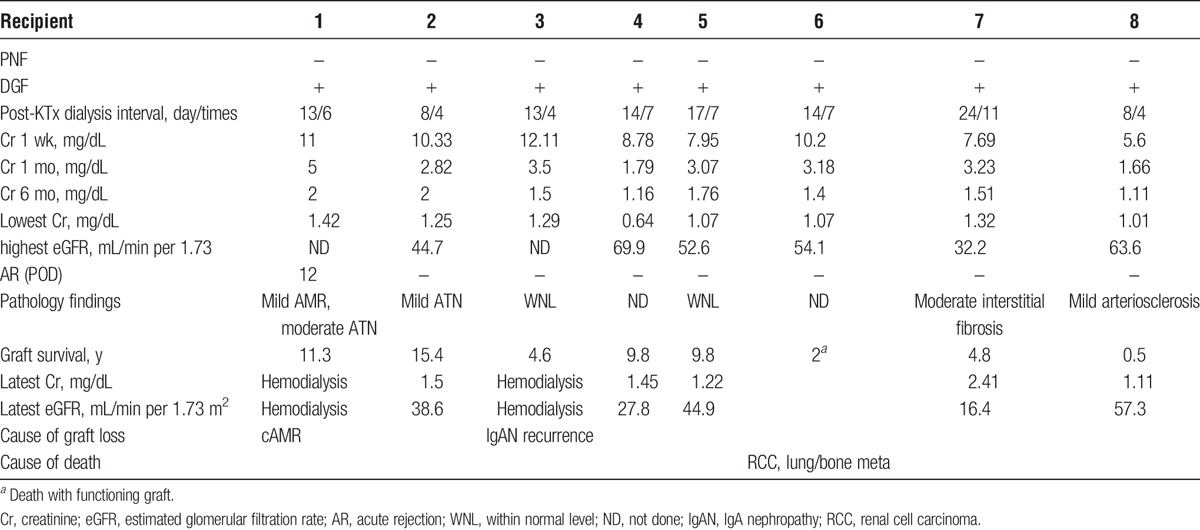
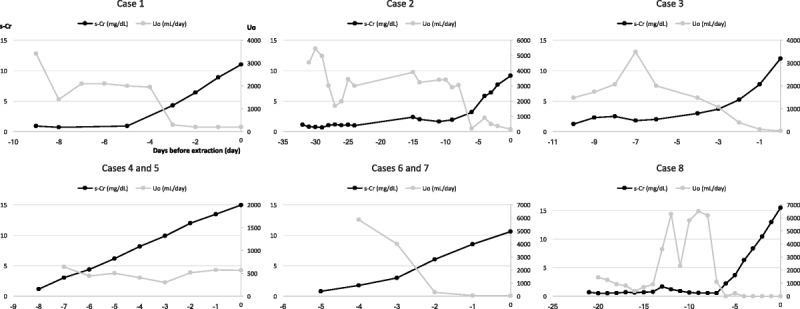
Except for patient 1, who suffered biopsy-proven acute antibody-mediated rejection (AMR) on 12POD, all severe AKI kidneys recovered spontaneously from acute tubular necrosis (ATN) within 1 month and exhibited no evidence of rejection with decreasing serum Cr concentration (less than 3.5 mg/dL) (Table 3). During 6 months to 2 years of follow-up, all grafts were functional of stable serum Cr concentrations (less than 2.0 mg/dL) with the longest graft survival of more than 15 years (patient 2) (Table 3 and Figure 2). Patient 1 was treated with 500 mg supplementary methylprednisolone for 2 days and tapered by 4 mg/d. After treatment for acute AMR, the kidney graft function was well maintained for 10 years. However, she developed chronic AMR at 11 years after KTx and returned to hemodialysis. Patient 3 exhibited stable renal function until 2 years after KTx. At that point, he was found to have microhematuria. Graft biopsy confirmed the lack of rejection. Nevertheless, the subsequent recurrence of his original disease was noted: IgA nephropathy. Despite maintaining basic immunosuppression with low-dose methylprednisolone (4 mg/d), he lost his kidney graft at 2.6 years after diagnosis by recurrence of IgA nephropathy. Patient 6 maintained excellent renal function for 2 years with a standard immunosuppression protocol. After suffering from unexpected diaphyseal fracture on his left femur, he was diagnosed as having cancer on his right kidney with multiple lung and bone metastases. This fracture was attributable to bone metastasis. He died of respiratory insufficiency with a functioning kidney graft at 1 month after diagnosis. Patients 2, 4, 5, 7, and 8 have exhibited normal renal function, respectively, for approximately 15.5, 10, 10, 5, and 0.5 years after KTx.
FIGURE 2.
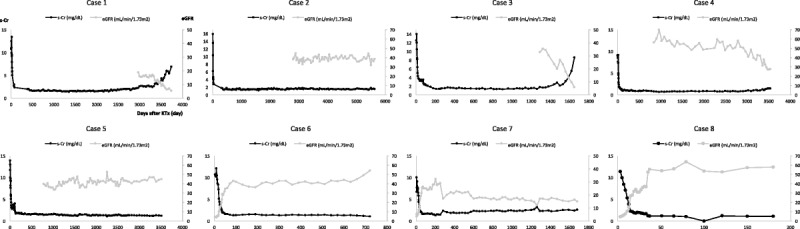
Trends of serum Cr concentrations (mg/dL) and eGFR (mL/min per 1.73 m2) in recipients after KTx. Patients 4/5 and patients 6/7 were transplanted grafts from the same donors.
DISCUSSION
The average waiting time for dialysis patients to receive kidney grafts in Japan is 13 years, as opposed to the 4 to 6 years prevailing in most countries.21,22 The number of recipients on a waiting list for KTx has been increasing. Therefore, DCD donors have become an accepted organ resource to alleviate the chronic shortage of kidney grafts. Based on the criteria used at our center,11 we have frequently used marginal kidneys from DCD donors.
Several investigators have reported excellent graft outcomes using DCD kidneys with AKI.1,3,23,24 By contrast, grafts with AKI stage III before extraction have been regarded as high risk and as inappropriate for KTx: approximately 35% kidneys were discarded because of increased t-Cr and/or oliguria.17 In a healthy person, AKI can usually be attributed to exacerbation of renal function because of hypoxia, ischemia, or nephrotoxicity. However, AKI is regarded as a reversible condition, which can recover spontaneously with tubule regeneration.12-15 By graft biopsy, ATN before KTx revealed no significant relation with 1-year kidney graft survival.25 Although AKI kidneys are well associated with a higher frequency of DGF, some recent large cohort studies have demonstrated that renal function with AKI stage III at 6 months after KTx is comparable to those of kidneys with no AKI or with AKI stage I or II.17,18 As expected, grafts of AKI stage III from DCD donor involved a higher frequency of DGF. Nevertheless, no cases of PNF were reported: these severe AKI kidneys functioned sufficiently within 1 month after KTx. Thereafter, they maintained excellent graft function not only during short-term but also at long-term follow-up for over 15 years. Ugarte et al26 reported that the PNF of AKI kidneys was not a higher percentage than that of no-AKI kidneys, which is consistent with our observations.
Prolonged CIT exhibited a negative effect on the frequency of DGF and long-term graft survival in DCD kidneys.27 According to the DCD protocol in single institutions, limited WIT and TIT are within 45 to 60 minutes and 24 hours. The American Society of Transplant Surgeons recommends minimization of WIT and TIT.28 Family consent was provided for prearrest cannulation. Therefore, 5 donors were subjected quickly to regional in situ cold perfusion after the declaration of death. Our analysis showed that the mean WIT (3.25 minutes) and TIT (8.56 hours) were much shorter, suggesting that CIT minimization can facilitate quick recovery from DGF because of AKI.
Generally, for marginal kidneys, a donor older than 60 years adversely affects graft survival from DCD kidney.3,29 Some earlier reports have described that AKI in older patients develops to end-stage renal disease much more easily than in younger patients.30-32 Kane-Gill et al33 reported that a history of HT, drugs, and mechanical ventilation play a crucially important role in the development to AKI for elderly patients. In addition, the length of stay in the ICU and hypoalbuminemia (<3.0-3.5 mg/dL) were found to have a significant effect on the progress to AKI.34,35 Results suggest that the comorbidity of severe AKI in aged donors, such as patients 1, 6, and 7 in this study, represent an important risk factor affecting long-term graft survival.
In conclusion, kidneys with AKI stage III, which were the worst 8 cases in terms of t-Cr concentrations before extraction, yielded excellent outcomes with no increase in the risk of PNF and rejection. This report describes that a single DCD kidney with t-Cr concentrations greater than 10.0 mg/dL and oliguria can be considered a valid alternative for recipients on a waiting list for KTx.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Tamotsu Tojimbara for helpful advice. Additionally, the authors thank Dr. Takeshi Hachisuka, Dr. Sayaka Morita, and Dr. Akira Kondo for their daily suggestions and contributions to this study.
Footnotes
Published online 19 March, 2018.
The authors declare no funding or conflicts of interest.
Y.T., K.I., I.N., and S.F. participated in research design. Y.T., K.I., and S.F. participated in the writing of the article. Y.T., K.I., Y.O., K.M., K.K., A.S., T.M., I.K., K.K., I.N., and S.F. participated in the performance of the research. Y.T. and K.I. contributed new reagents or analytic tools. Y.T. and K.I. participated in data analysis.
REFERENCES
- 1.Tomita Y, Tojimbara T, Iwadoh K, et al. Outcomes of kidney transplantation from circulatory death donors with increased terminal creatinine levels in serum. Transplantation. 2016;100:1532–1540. [DOI] [PubMed] [Google Scholar]
- 2.Tomita Y, Tojimbara T, Iwadoh K, et al. Long-term outcomes in kidney transplantation from expanded-criteria donors after circulatory death. Transplant Proc. 2017;49:45–48. [DOI] [PubMed] [Google Scholar]
- 3.Nagaraja P, Roberts GW, Stephens M, et al. Impact of expanded criteria variables on outcomes of kidney transplantation from donors after cardiac death. Transplantation. 2015;99:226–231. [DOI] [PubMed] [Google Scholar]
- 4.Heilman RL, Mathur A, Smith ML, et al. Increasing the use of kidneys from unconventional and high-risk deceased donors. Am J Transplant. 2016;16:3086–3092. [DOI] [PubMed] [Google Scholar]
- 5.Mallon DH, Riddiough GE, Summers DM, et al. Successful transplantation of kidneys from elderly circulatory death donors by using microscopic and macroscopic characteristics to guide single or dual implantation. Am J Transplant. 2015;15:2931–2939. [DOI] [PubMed] [Google Scholar]
- 6.Stratta RJ, Farney AC, Orlando G, et al. Dual kidney transplants from adult marginal donors successfully expand the limited deceased donor organ pool. Clin Transplant. 2016;30:380–392. [DOI] [PubMed] [Google Scholar]
- 7.Demiselle J, Augusto JF, Videcoq M, et al. Transplantation of kidneys from uncontrolled donation after circulatory determination of death: comparison with brain death donors with or without extended criteria and impact of normothermic regional perfusion. Transpl Int. 2016;29:432–442. [DOI] [PubMed] [Google Scholar]
- 8.O'Callaghan JM, Knight SR, Morgan RD, et al. A National Registry Analysis of kidney allografts preserved with Marshall's solution in the United Kingdom. Transplantation. 2016;100:2447–2452. [DOI] [PubMed] [Google Scholar]
- 9.Summers DM, Johnson RJ, Allen J, et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet. 2010;376:1303–1311. [DOI] [PubMed] [Google Scholar]
- 10.Doshi MD, Hunsicker LG. Short- and long-term outcomes with the use of kidneys and livers donated after cardiac death. Am J Transplant. 2007;7:122–129. [DOI] [PubMed] [Google Scholar]
- 11.Tojimbara T, Fuchinoue S, Iwadoh K, et al. Improved outcomes of renal transplantation from cardiac death donors: a 30-year single center experience. Am J Transplant. 2007;7:609–617. [DOI] [PubMed] [Google Scholar]
- 12.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. [DOI] [PubMed] [Google Scholar]
- 13.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abosaif NY, Tolba YA, Heap M, et al. The outcome of acute renal failure in the intensive care unit according to RIFLE: model application, sensitivity, and predictability. Am J Kidney Dis. 2005;46:1038–1048. [DOI] [PubMed] [Google Scholar]
- 15.Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basile DP, Donohoe D, Roethe K, et al. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–F899. [DOI] [PubMed] [Google Scholar]
- 17.Hall IE, Schröppel B, Doshi MD, et al. Associations of deceased donor kidney injury with kidney discard and function after transplantation. Am J Transplant. 2015;15:1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boffa C, van de Leemkolk F, Curnow E, et al. Transplantation of kidneys from donors with acute kidney injury: friend or foe? Am J Transplant. 2017;17:411–419. [DOI] [PubMed] [Google Scholar]
- 19.Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692–1702. [DOI] [PubMed] [Google Scholar]
- 20.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 annual data report: kidney. Am J Transplant. 2017;17 Suppl 1:21–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziemann M, Heßler N, König IR, et al. Unacceptable human leucocyte antigens for organ offers in the era of organ shortage: influence on waiting time before kidney transplantation. Nephrol Dial Transplant. 2017;32:880–889. [DOI] [PubMed] [Google Scholar]
- 23.Farney AC, Rogers J, Orlando G, et al. Evolving experience using kidneys from deceased donors with terminal acute kidney injury. J Am Coll Surg. 2013;216:645–655; discussion 655–646. [DOI] [PubMed] [Google Scholar]
- 24.Heilman RL, Smith ML, Kurian SM, et al. Transplanting kidneys from deceased donors with severe acute kidney injury. Am J Transplant. 2015;15:2143–2151. [DOI] [PubMed] [Google Scholar]
- 25.Hall IE, Reese PP, Weng FL, et al. Preimplant histologic acute tubular necrosis and allograft outcomes. Clin J Am Soc Nephrol. 2014;9:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ugarte R, Kraus E, Montgomery RA, et al. Excellent outcomes after transplantation of deceased donor kidneys with high terminal creatinine and mild pathologic lesions. Transplantation. 2005;80:794–800. [DOI] [PubMed] [Google Scholar]
- 27.Salahudeen AK, Haider N, May W. Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int. 2004;65:713–718. [DOI] [PubMed] [Google Scholar]
- 28.Reich DJ, Mulligan DC, Abt PL, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004–2011. [DOI] [PubMed] [Google Scholar]
- 29.Callaghan CJ, Mumford L, Pankhurst L, et al. Early outcomes of the new UK deceased donor kidney fast-track offering scheme. Transplantation. 2017;101:2888–2897. [DOI] [PubMed] [Google Scholar]
- 30.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. [DOI] [PubMed] [Google Scholar]
- 31.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt R, Coca S, Kanbay M, et al. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis. 2008;52:262–271. [DOI] [PubMed] [Google Scholar]
- 33.Kane-Gill SL, Sileanu FE, Murugan R, et al. Risk factors for acute kidney injury in older adults with critical illness: a retrospective cohort study. Am J Kidney Dis. 2015;65:860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujinaga J, Kuriyama A, Shimada N. Incidence and risk factors of acute kidney injury in the Japanese trauma population: a prospective cohort study. Injury. 2017;48:2145–2149. [DOI] [PubMed] [Google Scholar]
- 35.Shao M, Wang S, Parameswaran PK. Hypoalbuminemia: a risk factor for acute kidney injury development and progression to chronic kidney disease in critically ill patients. Int Urol Nephrol. 2017;49:295–302. [DOI] [PubMed] [Google Scholar]


