Abstract
Background
We aimed to evaluate changes in health-related quality of life (HRQoL) in patients 65 years or older from time of kidney transplantation (KTx) until 1 year postengraftment.
Methods
A single-center prospective study was conducted. HRQoL was measured pre-KTx and at 2, 6, and 12 months postengraftment using self-reported Kidney Disease and Quality of Life short-form version 1.3. Intraindividual scores before and after KTx were evaluated. Liu Comorbidity Index was registered at enlisting. short-form-36 scores were additionally compared with scores from an age-matched population.
Results
From January 1, 2013, until November 30, 2016, a total of 289 waitlisted patients were included. By September 1, 2017, 134 had reached 1 year postengraftment, and valid questionnaires were available in 120 (90%) patients. Mean age at KTx was 71.6 years (±4.3 years), 71% were male. Living donor was used in 21%, and preemptive KTx was performed in 30% of the recipients. Median waiting time for KTx from deceased donor was 16 months (range, 0.6-50.5 months). A total of 79 (66%) recipients had a Liu Comorbidity Index score of 3 or less.
All HRQoL scores except the domain social function improved at 2 months postengraftment and remained stable or continued to improve at 1 year.
HRQoL scores 12 months postengraftment were similar to those described in an age-matched general population except for the domain social function which remained at a significantly lower level. Time in dialysis was the most important variable associated with impaired HRQoL postengraftment.
Conclusions
HRQoL scores showed clinically significant improvement in older KTx recipients 1 year posttransplant.
Worldwide, an increasing number of patients 65 years or older with end-stage kidney disease (ESKD) are enlisted for kidney transplantation (KTx). Several papers have stated that patient and graft survival in this age group are acceptable1-5 and better than for patients remaining in dialysis.6,7 There is, however, very limited data describing health-related quality of life (HRQoL) in this population.8 Many elderly patients experience good HRQoL in modern dialysis treatment, and immunosuppressive drugs are known to have side effects with negative impact on HRQoL. The primary aspect of KTx is to extend the ESKD patients life span and maintain or increase their quality of life. HRQoL has been established as a key measurement of outcome after KTx.
A few retrospective publications indicate good HRQoL in older KTx recipients compared with patients in dialysis or age-matched controls,9-11 but the numbers of included recipients in these studies are small. Over a 10-year period, Humar et al12 were able to collect HRQoL data on 42 older KTx recipients (≥65 years) using short-form 36 (SF-36), and they described higher scores in mental health (MH) and social function compared with younger recipients and to an age-matched general population, whereas the scores in the physical domains were reduced.
Advanced age by itself is no longer an absolute contraindication for KTx. There is, however, a lack of knowledge regarding possible risk factors that may predict a poor outcome in older recipients after KTx, especially with respect to HRQoL.
The primary aim of the current study was to evaluate HRQoL longitudinally in patients 65 years or older, from KTx, and until 1 year post-KTx. Second, we wanted to investigate if degree of comorbidity registered before transplantation or any other clinical and demographic variables could influence on posttransplant HRQoL outcomes.
METHODS
Design and Study Population
A single-center, prospective observational study investigating changes in HRQoL before and after KTx in older kidney transplant recipients was conducted. All patients 65 years or older accepted for KTx and on the active transplantation waiting list at the Norwegian national transplantation center at Oslo University Hospital between January 1, 2013, and November 30, 2016, were prospectively invited to participate. Patients not understanding the Norwegian language and patients with cognitive dysfunction, making it impossible to complete the questionnaire, were excluded. Cognitive dysfunction was investigated as part of the preenlisting evaluation. Information regarding preenlisting evaluation of KTx candidates and distribution of HRQoL questionnaires before KTx has been described previously.13
The study was approved by the Regional Committee for Medical and Health Research Ethics, South East Norway (2012/527) and was carried out according to the Helsinki Declaration. All participants were given oral and written information about the study and written informed consent was obtained before inclusion.
HRQoL Assessment
HRQoL was assessed using the self-reporting Kidney Disease and Quality of Life Short Form version 1.3 (KDQOL)14 which has been validated in KTx recipients.15 KDQOL has previously been translated into Norwegian16 and validated in a Scandinavian population.17 The questionnaire consists of a generic and a disease specific part.
The generic part of the instrument (SF-36) consists of 8 multi-item scales that address domains of physical function (PF), role limitations due to physical problems, bodily pain (BP), general health (GH), vitality (VT), social function (SF), role limitations due to emotional problems, and MH.18 We calculated the SF-36 domain scores according to published guidelines converting question items to a 0 to 100 scale with higher scores reflecting better HRQoL. Loge and Kaasa19 have previously published normative SF-36 data based on questionnaires sampled from randomly drawn Norwegian citizens. Their publication present overall data as well as data grouped by age decades. We compared our results with the normative data from responders that are 70 years or older because the mean age of our population was 72 years.
The disease specific part of the questionnaire includes 43 questions divided into 11 kidney-related items: Symptoms (Symp), effect of kidney disease (EKD), burden of kidney disease (BKD), work status, cognitive function (Cogn), quality of social interaction, sexual function, sleep, social support (Soc), dialysis staff encouragement, and patient satisfaction. In addition, the disease-specific part includes a final item asking the respondent to rate their total health on a 0 to 10 numerical rating scale, ranging from worst possible to best possible health.14 According to the manual, we linearly converted kidney-disease domain scores to a 0 to 100 scale in a similar manner as with SF-36 scores.20
Comorbidity
As part of our national standard pretransplant workup routine, comorbidity data according to the comorbidity index for dialysis patients described by Liu in 201021 have been prospectively registered at enlisting since 2012. The Liu Comorbidity Index score range from 0 (no comorbidity) to 21. The scores are categorized into 4 intervals in accordance with the original publication; 3 or less, 4 to 6, 7 to 9, and 10 or higher.21 In the regression analysis, we chose to use a comorbidity score higher than 75 percentile of the study population (Liu comorbidity score, >4) as an independent variable.
Graft Function
Graft function was evaluated as estimated glomeruli filtration rate (eGFR) calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.22
Statistical Analysis
Descriptive data are presented as numbers and percentage, mean with standard deviation (SD) or median with range. χ2 Test for contingency tables was used to detect differences in categorical variables. In the comparative analysis of HRQoL data, an independent sample t test was used for normal distributed data and the Mann-Whitney test for skewed distributed data. A P value less than 0.05 was considered statistically significant. A difference of half a standard deviation in mean score as reported by Norman et al23 was considered clinically significant. In the case of comparative analysis with the general population, we used a z score greater than 0.524 as a measure of clinical significance. The kidney-specific domains, dialysis staff encouragement and patient satisfaction, were not supposed to be completed by the preemptive patients and are not relevant after KTx. Comparing analyses for these domains were therefore consequently not performed. Similarly, because all patients were retired, work status was not included in the analyses.
Linear mixed-effect regression analysis models were used to detect changes in HRQoL scores over time. The 12 HRQoL domains were defined as outcome variables in the model. Fixed effect for time was used as categorical variable of interest. In addition, sex, age, marital status, comorbidity, time in dialysis, time on waiting list for KTx, eGFR at 1 year, donor age, and HLA mismatch were assessed for inclusion. Variables with P values less than 0.25 in univariable analysis were included as fixed factors in the multivariable analysis. Subsequently, multivariable linear mixed-effect models with manual backward elimination procedure were performed to identify risk factors associated with any change in HRQoL scores. All models included random intercept and an unstructured correlation matrix was used. In addition, GH, PF, EKD, Cogn, and total health were modeled with random slopes. Interaction effects between time and the fixed factors were checked by including product terms, one at a time, into the models. No significant interactions were observed. Due to multicollinearity between living donor and waiting time, and between “in dialysis” and “time in dialysis,” we chose to use time on waiting list and time in dialysis in our multivariable models because they were considered to be the most clinically relevant factors.
Missing items were handled by replacing the average value of the complete items (in each domain of SF-36) if a minimum of 50% of the items in the questionnaire were answered. If more than 50% of the items were missing, no scale was computed. No substitution for missing data was performed for kidney specific items. Questions not answered were calculated according to the scoring manual.20
RESULTS
Study Population
From January 1, 2013, to November 30, 2016, we included 289 patients from 437 eligible waitlisted patients. By September 1, 2017, a total of 193 were transplanted and 134 had reached 1 year postengraftment (Figure 1). The response rates were 87% at 2 months, 86% at 6 months, and 90% 1 year post-KTx, respectively.
FIGURE 1.
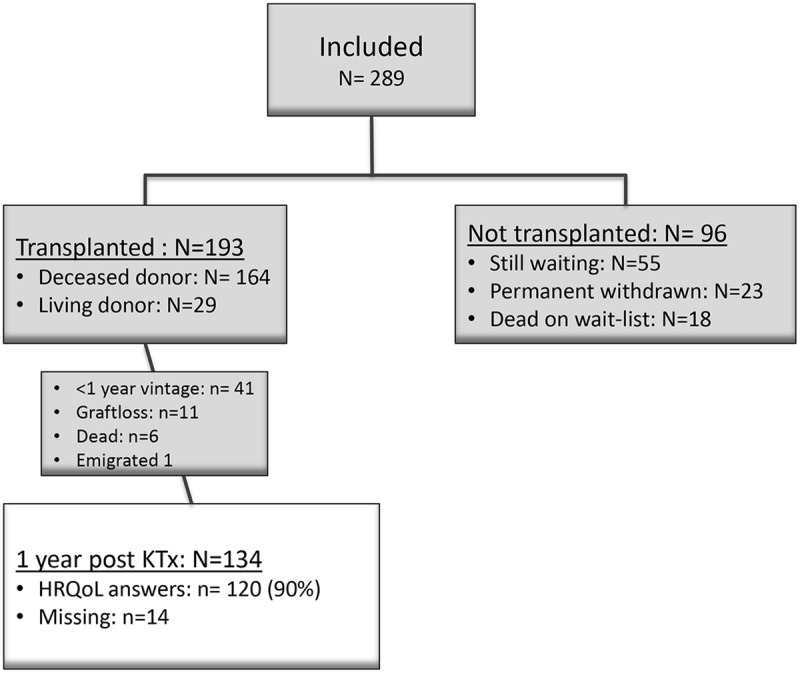
Study flowchart.
Among the 148 patients not included, 106 did not responded to the invitation, 21 did not meet the inclusion criteria, and 21 were reluctant to participate. The patients not included were younger (mean age, 69.8 ± 4.1 years vs 71.1 ± 4.1 years, P = 0.02). Sex and comorbidity score were comparable (data not shown).
During the first year after KTx, 11 patients lost their graft, and 6 patients died. The predominant reason for graft loss was primary nonfunction (n = 7). Causes of death were pulmonary infection (n = 3), cardiovascular disease (n = 2), and cachexia (n = 1). In these 6 recipients, mean age at KTx was somewhat higher than that in the entire study population (74.3 years vs 71.1 years), mean dialysis time before KTx was 29.4 months and 4 patients had a vascular disease as their ESKD diagnosis. Liu comorbidity score was comparable to the study population.
One patient was lost to follow-up because of emigration and two patients did not complete the HRQoL questionnaire postengraftment due to cognitive failure.
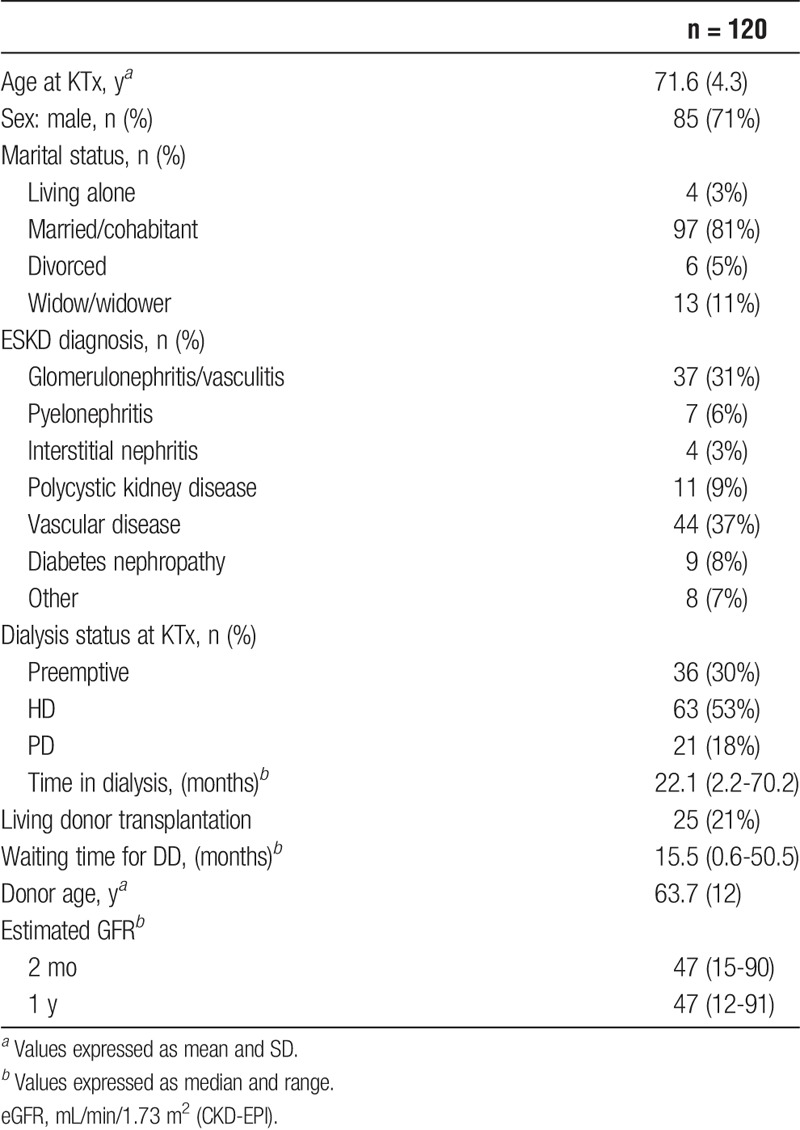
Valid 1-year questionnaires were available in 120 patients whom comprised the study population and only data from these patients were included in the final analyses. All patients were white. Demographic and clinical characteristics are shown in Table 1. The study population included 70% male KTx recipients which is in accordance with what we have described in previous observational studies from our center.25 Women had significantly older donors (67.4 years vs 62.1 years, P = 0.023), were more likely to be widow (23% vs 6%, P = 0.02), and less likely to be married (69% vs 86%, P = 0.04) than men. Otherwise, demographics did not differ between the sexes. Thirty-six recipients were transplanted preemptively, and 25 recipients received a kidney from a living donor. Median waiting time for a kidney from deceased was 16 months (range, 0.6-50.5).
TABLE 1.
Demographic and clinical characteristics
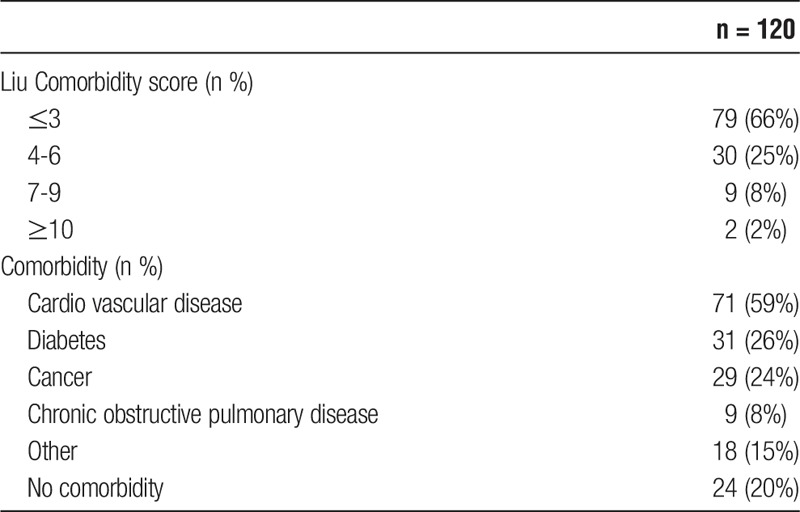
The majority of the recipients (n = 79, 66%) evaluated as low-risk recipients with a pre-KTx Liu comorbidity score of 3 or less (Table 2). There were no major differences regarding comorbidity between the sexes, except that a significant higher proportion of males had cardiovascular disease (68% vs 37%, P = 0.002).
TABLE 2.
Comorbidity
HRQoL After KTx
The pre-KTx HRQoL evaluation was obtained within the last 6 months (mean, 3.2 months) before KTx. Overall HRQoL scores improved already 2 months after KTx and remained stable during the first year (Figure 2). The improvement in mean scores from pre-KTx until 1 year post-KTx in role limitations due to physical problems (24-50), GH (50-68), VT (41-60), Symp (76-83), EKD (70-85), and BKD (37-79) were considered clinically significant with a difference of more than ½ SD. The domain SF remained unchanged at a level of 65. Also, the mean scores in PF (65_74), MH (77-82), Cogn (89-92), and Soc (87-91) increased, but were not judged as clinically significant (Figure 3).
FIGURE 2.
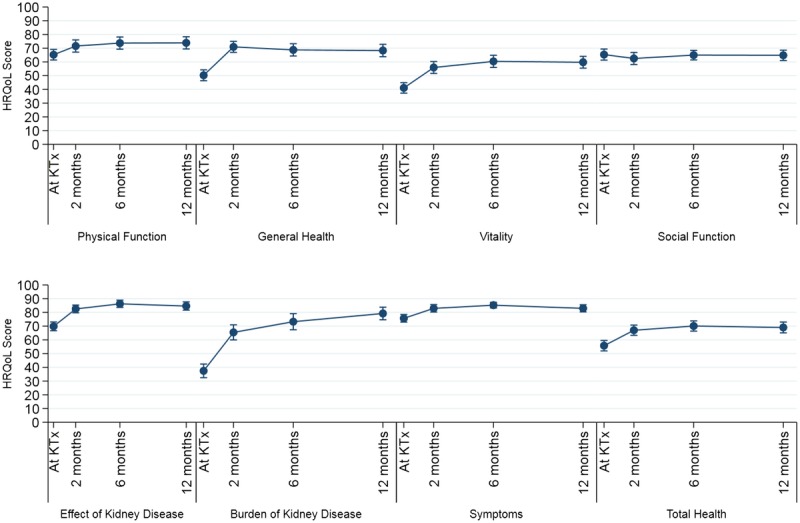
Changes in HRQoL from KTx to 1 year postengraftment.
FIGURE 3.
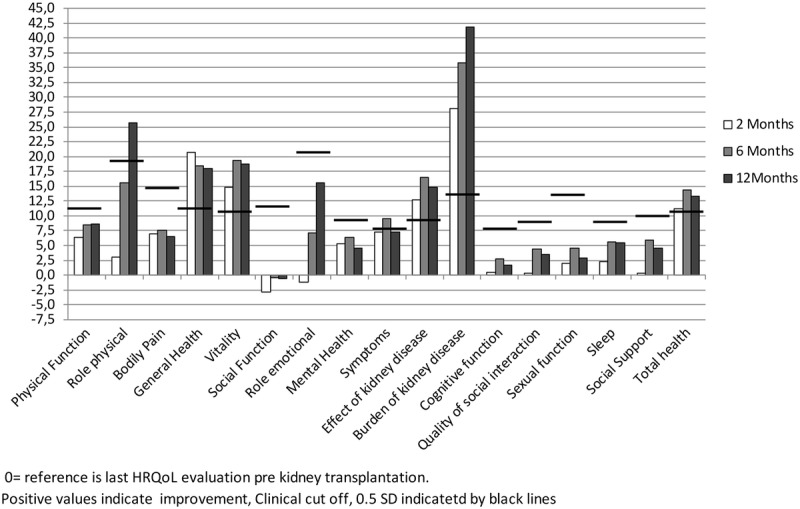
Changes in HRQoL scores at 2, 6, and 12 months after KTx compared to pretransplantation.
The evaluation of the disease-specific item scoring “total health” revealed a clinically and statistically significant increase in mean score from 56 (±20.6) at KTx to 67 (±18.9) 2 months after KTx, with further increase to 70 (±18.9) after 6 months, and then remained stable with a score of 69 (±21.4) at 1 year.
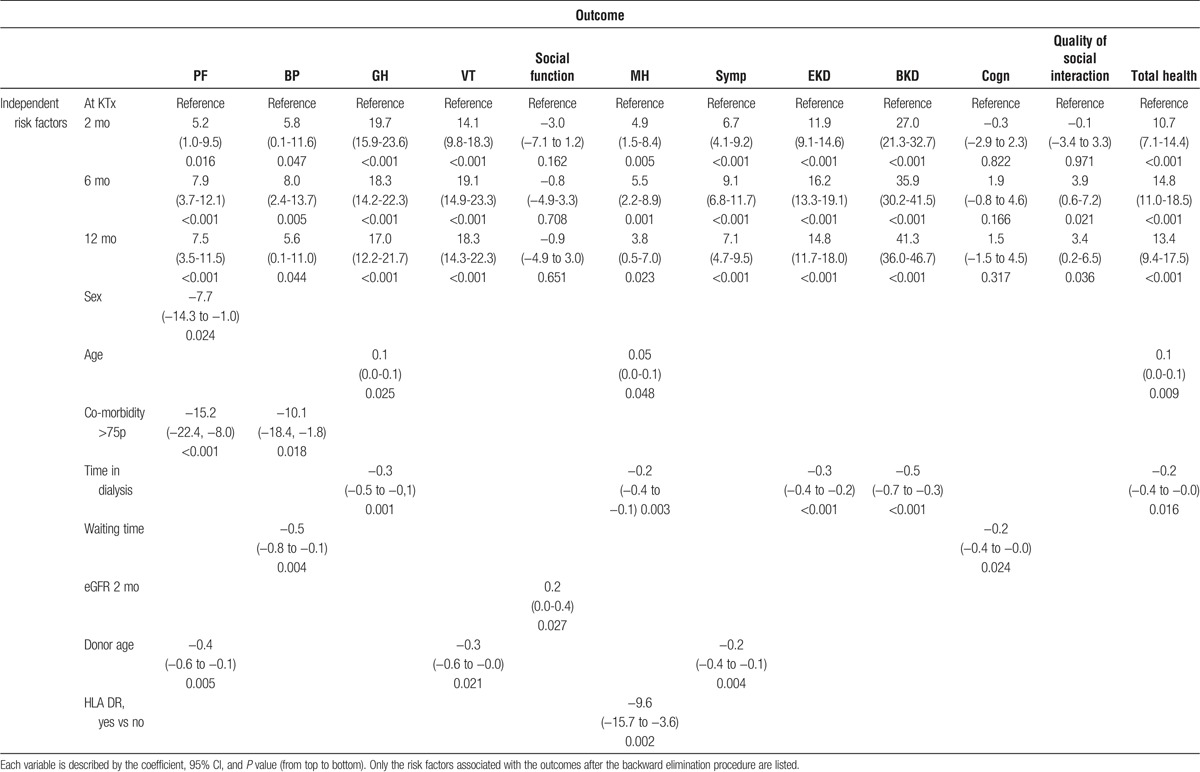
Total time in dialysis was the most important factor associated with changes in HRQoL scores, waiting time had a limited implication (Table 3). Additionally, recipient-related factors with impact on HRQoL were sex, comorbidity, and age. The improvement in PF was higher in women than in men. High comorbidity was associated with poorer PF and BP. Increasing age was associated with improvement in GH, MH, and total health expression scores. Furthermore, the following donor and transplant factors were analyzed; graft function expressed as eGFR (CKD-EPI), donor age, and number of HLA-DR mismatches. Graft function was only associated with SF after KTx and donor age was associated with changes in PF, VT, and Symp. Marital status did not have any impact on changes in HRQoL.
TABLE 3.
Linear mixed-effect regression analysis (multivariable) at 2, 6, and 12 months after KTx
Compared with the general Norwegian population older than 70 years, both male and female KTx had comparable HRQoL scores 1 year after engraftment except for SF for which the score was lower in the study population (Figure 4). For male patients, the decrease in SF score also reached clinical significance.
FIGURE 4.
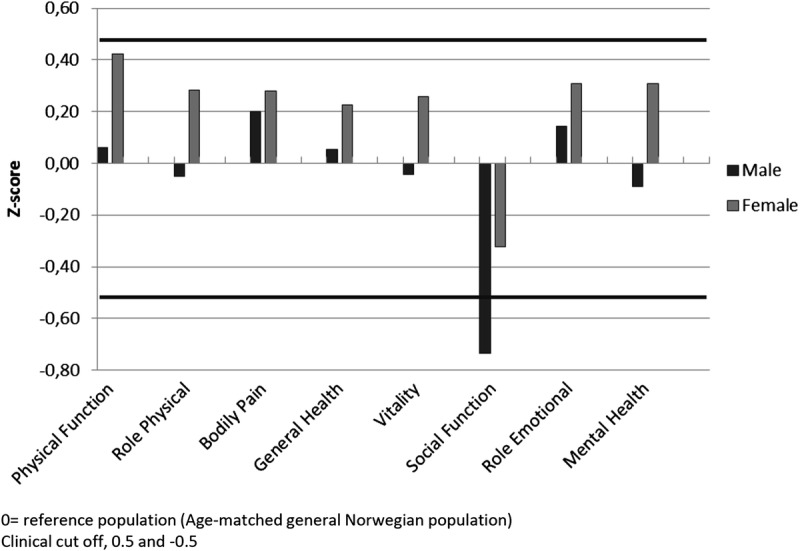
HRQoL scores at 12 months after KTx compared with the age-matched general Norwegian population, presented as z scores.
DISCUSSION
In this nationwide longitudinal study of kidney transplant recipients 65 years or older, we found that HRQoL scores were improved already 2 months after KTx compared with the latest pre-KTx evaluation. HRQoL scores remained stable or even increased further at 6 and 12 months after KTx. The improved HRQoL was associated with short time in dialysis and low comorbidity. The KTx recipients in this population had HRQoL scores comparable to the general age-matched Norwegian population.
As shown, improved PF was associated with low comorbidity. Cardiovascular disease is known to affect HRQoL. The female cohort had a significantly lower prevalence of cardiovascular disease compared with men ahead of KTx which may explain the difference in improvement of PF. There were also a higher percent of the women without any comorbidity at all, although this was only of borderline significance.
As far as we know, this is the first study after HRQoL prospectively from time of KTx until 1 year after engraftment in older kidney transplant recipients. In 2016, Kostro et al26 published data describing changes between pretransplant and 1 year posttransplant HRQoL scores in a population of 69 KTx recipients with a mean age of 47 years (range, 18-76 years). They only included recipients with well-functioning grafts. Like our study, Kostro et al26 also demonstrated clinical and statistical improvements of HRQoL scores after transplantation. The main difference from their study is that we have included more and older patients disregarding graft function.
In a previous Norwegian study involving younger recipients, an improvement of HRQoL after KTx has been described.27 We found a corresponding improvement in older recipients. In contrast to previous published studies, we compared HRQoL scores after KTx with a HRQoL evaluation performed at a maximum of 6 months before KTx. The improvement was evident already 2 months after engraftment, which is somewhat surprising because one could expect that older recipients would have a longer and more complicated postoperative rehabilitation period than younger recipients. Experiencing normalization of the kidney function and happiness over actually getting a KTx may influence the early HRQoL scores in this life experienced population.
The majority of the study population received a kidney from a deceased donor. We have previously reported a decrease in HRQoL while the patients were on the waiting list.13 Time in dialysis was associated with reduced HRQoL scores and has been shown to be a risk factor for impaired HRQoL after KTx in nonelderly recipients28 and also a risk factor for mortality in older recipients.29 This may indicate that suitable patients should be transplanted as early as possible while preemptive or at least in the start of dialysis treatment. However, for most of the transplant centers, it will be close to impossible to achieve this with average waiting time ranging from 3 to 8 years.30,31 At our center, the average waiting time for deceased donor KTx has been relatively short (15 months for 1st KTx), and this may have contributed to the good overall HRQoL we report in this population. Dialysis treatment is also known to have a negative impact on social life32 and one of our expectations was that the patients' social life, which decreased during waiting time,13 would benefit from transplantation. To our surprise, this did not happen even though other domains as VT, effect, and BKD improved. In the regression analysis, eGFR after 2 months was a significant variable associated with reduced social function. Having a suboptimal graft function leads to extended treatment and complications might occur. In the first months after KTx, patients with suboptimal graft function spend almost all of their time at the hospital and the rehabilitation time is delayed. Furthermore, life with a new kidney leads to new routines, possible side-effects of immunosuppressive drugs and time for social life is possibly still limited. In addition, with aging, the social life with friends of similar age might generally decrease and time is mainly spent together with close relatives, despite a successful KTx.
It has previously been demonstrated that survival in older KTx recipients in the modern transplantation era is good.4,5,33 In our cohort, overall comorbidity according to the Liu score was low. The majority had a comorbidity score of 3 or lower at time of acceptance for KTx and for the 75 percentile was 4. During the first year after KTx, 6 patients (5%) died, suggesting that the current preenlisting medical evaluation of elderly ESKD patients for KTx is acceptable.
Few studies have identified patients who do not experience an increase in HRQoL after KTx. McAdams-DeMarco et al34-36 showed that frail recipients are at risk of adverse outcome after KTx with delayed graft function, longer hospitalization after engraftment, immunosuppressive intolerance and increased mortality. For more than a decade we have at our center had an increased focus on immunosuppression in this elderly population.37,38 To avoid rejection but simultaneously have a low calcineurin inhibitor trough concentration seems important.39 Even though the KDQOL questionnaire does not ask about side effects from immunosuppressive medication, our overall impression was that this was of minor concern for the patients. In a recent publication, McAdams-DeMarco et al40 have also shown that frail recipients experience improvement in post-KTx HRQoL. There is however no consensus on how frailty should be measured. Regardless of this, we did, unfortunately, not measure frailty in our study. Because our population consist of patients selected as suitable for KTx based on strict medical criteria and have low comorbidity scores (Table 2), they are not likely to have high frailty scores.
Donor age was comparable to recipient age and did not influence on post-KTx HRQoL. In our transplant program as well as in the Eurotransplant Senior Program, use of extended criteria donor kidneys for age matched recipients is well established with good results.29,41 It has ensured kidneys for the oldest ESKD population without competing with the younger recipients and is an important factor when discussing overall waiting time.
Our study population had SF-36 scores comparable to the age-matched general population. Even though our study population comprises a selected group, the recipients will, as individuals in the age-matched general population, struggle with health disabilities. Scoring of HRQoL will refer to the life of the person, not to the kidney and the results will probably be influenced by the total health situation. When comparing our HRQoL results with those of the general population, we assume that the burden of comorbidities is the same so that observed differences in score possibly can be attributed to the KTx.
When selecting ESKD patients 65 years or older for KTx, the challenge is to know who will benefit from a transplantation and who will not. Our current data and previous publication42 show that utilization of comorbidity indexes may not improve the selection of elderly patients for enlisting. HRQoL measurement is well established as one evaluation to select candidates, but questionnaires are often time consuming both for the patients and the clinicians. The numerical rating scale is used in many setting, that is, to measure changes in chronic pain over time. Question 22 in the KDQOL questionnaire asks the patients to evaluate their total health on a scale from 0 to 10. Our results indicate that the total health score reflects the scores of the GH domain and the regression analysis shows that the same independent factors influence both scores. In a clinical setting, the numerical rating scale may be quick to perform and interpret as a complementation to more comprehensive questionnaires to measure changes in HRQoL over time.
Study Limitations and Strengths
This study has some limitations. We have only 1 single validated instrument to measure HRQoL. The KDQOL questionnaire is, however, the most commonly used measure of HRQoL in patients with kidney disease. It was developed specifically for the ESKD population, and it has been evaluated in a population of KTx recipients. Unfortunately, the instrument does not capture post-KTx side effects of immunosuppression or concerns regarding graft function. We have not evaluated frailty, which is also a limitation of the study. The longitudinal nature of the study is a clear strength. The focus on HRQoL, both in the immediate postengraftment phase which is a critical time of recovery, and at 6 + 12 months is important. A long-term HRQoL evaluation is however still missing and would be of scientific value and clinical importance. Finally, we believe that we have a good comparator because HRQoL evaluations were performed regularly before engraftment in a safe and familiar environment (at home). The fact that no patient was lost to follow-up is also a definitive strength of the study.
CONCLUSIONS
In this cohort, with low comorbidity score, HRQoL after KTx was improved when compared with pre-KTx. One year after KTx the HRQoL was comparable to the age-matched general population. Short waiting time for KTx was associated with good HRQoL outcome. Consequently, we recommend preemptive KTx, possibly with a living donor, as the optimal choice for the old recipient. Further studies are needed to measure changes in HRQoL for this population in a longer perspective.
Footnotes
Published online 1 March, 2018.
This project has received financially support from the Norwegian Extra Foundation for Health and Rehabilitation through EXTRA funds.
The authors declare no conflict of interests.
K.L., K.M., and K.H. designed and wrote the article. K.L., C.B., T.B., K.H., and K.M. participated in data analysis. M.A., N.v.d.L., T.B., C.B., A.V.R., P.D.L., and A.H. participated in writing and evaluating the article.
REFERENCES
- 1.Tekin S, Yavuz HA, Yuksel Y, et al. Renal transplantation in recipients older than 65 years: retrospective analysis of the results of a 4-year (2008–2012) Experience. Transplant Proc. 2015;47:1356–1359. [DOI] [PubMed] [Google Scholar]
- 2.Cho H, Yu H, Shin E, et al. Risk factors for graft failure and death following geriatric renal transplantation. PLoS One. 2016;11:e0153410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dempster NJ, Ceresa CD, Aitken E, et al. Outcomes following renal transplantation in older people: a retrospective cohort study. BMC Geriatr. 2013;13:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heldal K, Hartmann A, Grootendorst DC, et al. Benefit of kidney transplantation beyond 70 years of age. Nephrol Dial Transplant. 2010;25:1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lonning K, Midtvedt K, Leivestad T, et al. Are octogenarians with end-stage renal disease candidates for renal transplantation? Transplantation. 2016;100:2705–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao PS, Merion RM, Ashby VB, et al. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83:1069–1074. [DOI] [PubMed] [Google Scholar]
- 7.Pippias M, Kramer A, Noordzij M, et al. The European Renal Association-European Dialysis and Transplant Association Registry Annual Report 2014: a summary. Clin Kidney J. 2017;10:154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balogun SA, Balogun R, Philbrick J, et al. Quality of life, perceptions, and health satisfaction of older adults with end-stage renal disease: a systematic review. J Am Geriatr Soc. 2017;65:777–785. [DOI] [PubMed] [Google Scholar]
- 9.Rebollo P, Ortega F, Baltar JM, et al. Health-related quality of life (HRQOL) in end stage renal disease (ESRD) patients over 65 years. Geriatr Nephrol Urol. 1998;8:85–94. [DOI] [PubMed] [Google Scholar]
- 10.Rebollo P, Ortega F, Baltar JM, et al. Is the loss of health-related quality of life during renal replacement therapy lower in elderly patients than in younger patients? Nephrol Dial Transplant. 2001;16:1675–1680. [DOI] [PubMed] [Google Scholar]
- 11.Weber M, Faravardeh A, Jackson S, et al. Quality of life in elderly kidney transplant recipients. J Am Geriatr Soc. 2014;62:1877–1882. [DOI] [PubMed] [Google Scholar]
- 12.Humar A, Denny R, Matas AJ, et al. Graft and quality of life outcomes in older recipients of a kidney transplant. Exp Clin Transplant. 2003;1:69–72. [PubMed] [Google Scholar]
- 13.Lonning K, Midtvedt K, Bernklev T, et al. Changes in health related quality of life in older candidates waiting for kidney transplantation. Nephrology (Carlton). 2017. [DOI] [PubMed] [Google Scholar]
- 14.Hays RD, Kallich JD, Mapes DL, et al. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3:329–338. [DOI] [PubMed] [Google Scholar]
- 15.Barotfi S, Molnar MZ, Almasi C, et al. Validation of the Kidney Disease Quality of Life-Short Form questionnaire in kidney transplant patients. J Psychosom Res. 2006;60:495–504. [DOI] [PubMed] [Google Scholar]
- 16.Østhus TB, Dammen T, Sandvik L, et al. Health-related quality of life and depression in dialysis patients: associations with current smoking. Scand J Urol Nephrol. 2010;44:46–55. [DOI] [PubMed] [Google Scholar]
- 17.Molsted S, Heaf J, Prescott L, et al. Reliability testing of the Danish version of the Kidney Disease Quality of Life Short Form. Scand J Urol Nephrol. 2005;39:498–502. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 19.Loge JH, Kaasa S. Short form 36 (SF-36) health survey: normative data from the general Norwegian population. Scand J Soc Med. 1998;26:250–258. [PubMed] [Google Scholar]
- 20.Hays RD, Kallich JD, Mapes DL, et al. Kidney Disease Quality of Life Short Form (KDQOL-SF ™), Version 1.3: A Manual for Use and Scoring. Santa Monica, CA: RAND Corporation; 1997. Available from: http://www.rand.org/pubs/papers/P7994.html. [Google Scholar]
- 21.Liu J, Huang Z, Gilbertson DT, et al. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77:141–151. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. [DOI] [PubMed] [Google Scholar]
- 24. Kirkwood BR, Sterne JAC. Essential medical statistics. 2nd ed. ed. Malden: Blackwell; 2003. [Google Scholar]
- 25.Heldal K, Leivestad T, Hartmann A, et al. Kidney transplantation in the elderly—the Norwegian experience. Nephrol Dial Transplant. 2008;23:1026–1031. [DOI] [PubMed] [Google Scholar]
- 26.Kostro JZ, Hellmann A, Kobiela J, et al. Quality of life after kidney transplantation: a prospective study. Transplant Proc. 2016;48:50–54. [DOI] [PubMed] [Google Scholar]
- 27.von der Lippe N, Waldum B, Brekke FB, et al. From dialysis to transplantation: a 5-year longitudinal study on self-reported quality of life. BMC Nephrol. 2014;15:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griva K, Davenport A, Newman SP. Health-related quality of life and long-term survival and graft failure in kidney transplantation: a 12-year follow-up study. Transplantation. 2013;95:740–749. [DOI] [PubMed] [Google Scholar]
- 29.Foss A, Heldal K, Scott H, et al. Kidneys from deceased donors more than 75 years perform acceptably after transplantation. Transplantation. 2009;87:1437–1441. [DOI] [PubMed] [Google Scholar]
- 30.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 annual data report: kidney. Am J Transplant. 2017;17 Suppl 1:21–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Branger P, Samuel U. Annual Report 2016, Eurotransplant International Foundation. Annual report. Eurotransplant International Foundation, 2017. [Google Scholar]
- 32.Pagels AA, Söderkvist BK, Medin C, et al. Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes. 2012;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAdams-DeMarco MA, James N, Salter ML, et al. Trends in kidney transplant outcomes in older adults. J Am Geriatr Soc. 2014;62:2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, length of stay, and mortality in kidney transplant recipients: a national registry and prospective cohort study. Ann Surg. 2017;266:1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13:2091–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heldal K, Hartmann A, Leivestad T, et al. Risk variables associated with the outcome of kidney recipients >70 years of age in the new millennium. Nephrol Dial Transplant. 2011;26:2706–2711. [DOI] [PubMed] [Google Scholar]
- 38.Falck P, Asberg A, Byberg KT, et al. Reduced elimination of cyclosporine A in elderly (>65 years) kidney transplant recipients. Transplantation. 2008;86:1379–1383. [DOI] [PubMed] [Google Scholar]
- 39.Storset E, Asberg A, Hartmann A, et al. Low-target tacrolimus in de novo standard risk renal transplant recipients: a single-centre experience. Nephrology (Carlton). 2016;21:821–827. [DOI] [PubMed] [Google Scholar]
- 40.McAdams-DeMarco MA, Olorundare IO, Ying H, et al. Frailty and postkidney transplant health-related quality of life. Transplantation. 2018;102:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frei U, Noeldeke J, Machold-Fabrizii V, et al. Prospective age-matching in elderly kidney transplant recipients—a 5-year analysis of the Eurotransplant Senior Program. Am J Transplant. 2008;8:50–57. [DOI] [PubMed] [Google Scholar]
- 42.Heldal K, Hartmann A, Leivestad T, et al. Clinical outcomes in elderly kidney transplant recipients are related to acute rejection episodes rather than pretransplant comorbidity. Transplantation. 2009;87:1045–1051. [DOI] [PubMed] [Google Scholar]


