Cotransplantation of islets with mesenchymal stromal cells (MSCs) has been shown to improve islet transplantation outcome. Improved glycemia in graft recipients have been attributed to superior islet revascularization, maintenance of islet organization and morphology, and a multitude of immunomodulatory mechanisms. These effects are mediated by a vast array of soluble trophic MSC-derived factors and extracellular matrix production, and/or direct cell-cell contact mechanisms. Most of these studies have used experimental transplantation sites, such as the kidney, to facilitate the colocalization of large numbers of MSCs with the islet graft. However, there are notable disparities with the clinically preferred intraportal route which have implications for colocalization of the islets and MSCs. Thus, because of the differences in their size, islets lodge in small vessels of the hepatic microcirculation, whereas the smaller MSCs will most likely engraft in other sites, such as the microvasculature of the lungs.1
An alternative method to ensure colocalization of the MSCs with islets posttransplantation is to form composite MSC islets in vitro,2,3 before transplantation. This approach has demonstrated beneficial effects on islet revascularization and reductions in graft lymphocyte infiltration.3 We aimed to determine whether composite MSC islets transplanted to the clinically preferred intraportal site improve glycemia in diabetic mice. Composite MSC islets were formed by suspension coculture and visualized using a Nikon Biostation. Islet insulin secretory function in vitro was assessed by static secretion assays.4,5 Intraportal syngeneic islet transplantations (C57Bl/6 mice) were used4 to assess the in vivo function of MSC islet composites.
Mesenchymal stromal cells adhered to the surface of islets in a random manner and appeared to penetrate the islet structure within 8 hours (75 islets: 200 000 MSCs [Figures 1A and B]). There was limited interaction of MSCs with islets at lower MSC doses (20 000). We demonstrated an MSC dose-dependent potentiation of glucose-stimulated insulin secretion when islets were precultured with MSCs in suspension coculture to form composites (Figure 1C). The majority of MSCs at both doses remained in suspension and did not adhere to cocultured islets, so transplanting MSC islet composites greatly reduced the dose of MSCs delivered in the graft when compared with cotransplanting islets and MSCs at the renal subcapsular site. Average blood glucose concentrations were comparable between transplant groups for the first 3 weeks posttransplantation and were lower at 4 weeks posttransplantation in recipients of MSC islet composites (Figure 1D).
FIGURE 1.
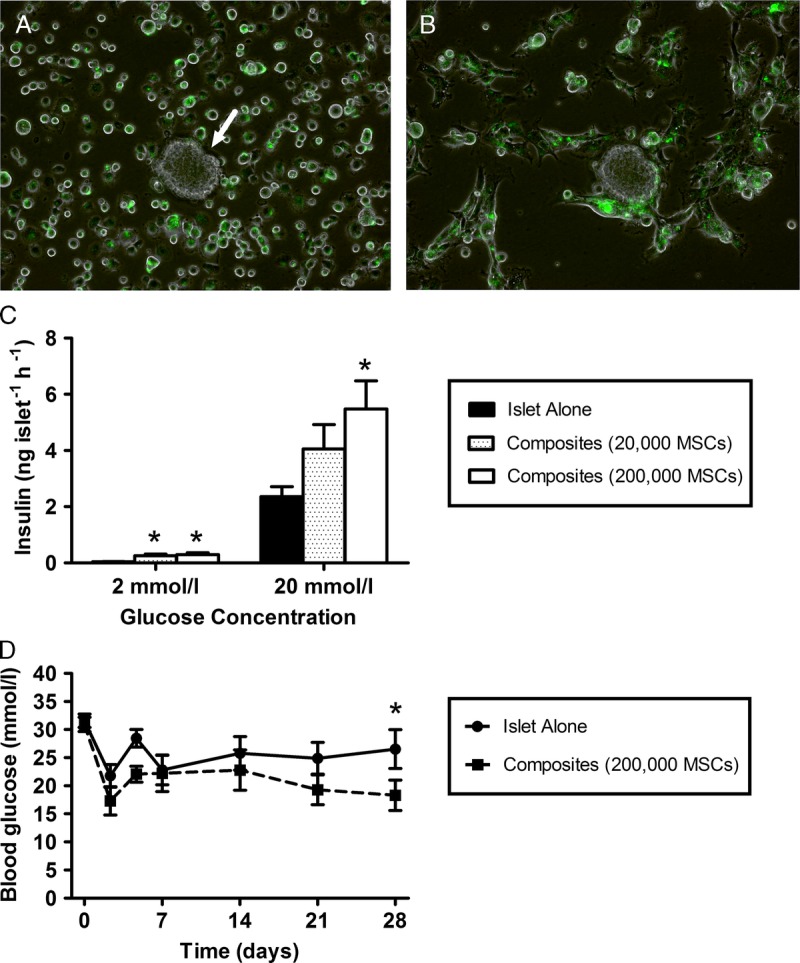
Seventy-five mouse islets were precultured with 200 000 fluorescently labeled (Qtracker 525 cell labeling kit; Invitrogen) mouse MSCs (green), per 35-mm dish, in suspension coculture for 24 hours to form composite MSC-islets. A single islet (white arrow) surrounded by MSCs (green) at the beginning of the coculture period (A). MSCs adhere to the edge of the islet after 2 hours of culture (B) and MSCs penetrate the islet core after 8 hours, leading to the formation of MSC-islet composites. Scale bar 100 μm. C, Insulin release at 2 and 20 mmol/L glucose of 10 replicates of 3 islets per Eppendorf tube, precultured alone (black bar) for 24 hours, or in suspension coculture with 20 000 (dotted bar), or 200 000 MSCs (white bar), to form MSC-islet composites, *P < 0.01 versus islets precultured alone at the same glucose concentration. D. Average blood glucose concentrations of mice receiving intraportal transplantations of 200 islets precultured with MSCs (at a ratio of 75 islets: 200 000 MSCs per 35-mm dish) in suspension coculture for 24 hours to form composites (dashed line) or islets precultured alone for 24 hours (solid line), *P < 0.05 vs. mice transplanted with MSC islet composites (repeated-measures ANOVA with Bonferroni post hoc test, n = 6 for both transplant groups). ANOVA, analysis of variance.
Our current findings demonstrate that the dose of MSCs that can be codelivered and colocalized at the site of individual islet engraftment by forming MSC islet composites is too low to promote early and robust improvements in graft function at the clinically preferred intraportal site. Lack of efficacy due to the low MSC dose will be further exacerbated by the loss of up to 90% of MSCs posttransplantation.3 These findings are of significant clinical importance because they highlight the need to interpret some of the promising experimental studies using extrahepatic sites with caution, in terms of translation to clinical practice. We propose that MSC cotransplantation strategies present superior translational benefit at alternative sites, such as the intramuscular site, where codelivery of therapeutically efficacious MSC doses can more likely be achieved. However, the issue of limited MSC survival and functional persistence in vivo remains a significant issue at these sites. Emerging evidence indicates tremendous potential in harnessing the MSC secretome as a “cell-free” regenerative strategy in numerous disease settings, including diabetes.5 The use of defined MSC biotherapeutics has obvious translational advantage, negating many safety and regulatory concerns of incorporating MSCs into clinical transplantation protocols. Because it is not possible to deliver high numbers of MSCs directly with the islets intraportally, alternative MSC-based strategies using defined dosing schedules of MSC-derived trophic factors5 may provide a preferable approach to improve the outcomes of intraportal islet transplantation.
Footnotes
Published online 19 March, 2018.
The authors are grateful to Diabetes UK for funding this study (06/0003387 and 11/0004290 to A.J.F.K. and P.M.J.).
The authors declare no conflicts of interest.
C.L.R. participated in research design, writing of the letter, performance of the research and data analysis. P.K.D. participated in research design, performance of the research and data analysis. S.J.S. participated in research design, performance of the research, and data analysis. M.G. participated in research design, performance of the research, and data analysis. A.J.F.K. participated in research design, writing of the letter and performance of the research. P.M.J. participated in research design and writing of the letter.
REFERENCES
- 1.Eggenhofer E, Benseler V, Kroemer A, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duprez IR, Johansson U, Nilsson B, et al. Preparatory studies of composite mesenchymal stem cell islets for application in intraportal islet transplantation. Ups J Med Sci. 2011;116:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fransson M, Brannstrom J, Duprez I, et al. Mesenchymal stromal cells support endothelial cell interactions in an intramuscular islet transplantation model. Regen Med Res. 2015;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rackham CL, Dhadda PK, Le Lay AM, et al. Preculturing islets with adipose-derived mesenchymal stromal cells is an effective strategy for improving transplantation efficiency at the clinically preferred intraportal site. Cell Med. 2014;7:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rackham CL, Vargas AE, Hawkes RG, et al. Annexin A1 is a key modulator of mesenchymal stromal cell-mediated improvements in islet function. Diabetes. 2016;65:129–139. [DOI] [PubMed] [Google Scholar]


