Abstract
Objectives
In mouse models of pancreatic cancer, IPI-926, an oral Hedgehog inhibitor, increases chemotherapy delivery by depleting tumor-associated stroma. This multicenter phase Ib study evaluated IPI-926 in combination with FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, oxaliplatin) in patients with advanced pancreatic cancer.
Methods
Patients were treated with once-daily IPI-926 plus FOLFIRINOX. A 3 + 3 dose escalation design was used, with cohort expansion at the maximum tolerated dose. A subset of patients underwent perfusion computed tomography to assess changes in tumor perfusion.
Results
The maximum tolerated dose was identified 1 dose level below standard FOLFIRINOX. Common treatment-related adverse events included liver function test abnormalities, neuropathy, nausea/vomiting, and diarrhea. Objective response rate was high (67%), and patients receiving IPI-926 maintenance showed further declines in CA19-9 levels even after FOLFIRINOX discontinuation. Treatment did not result in consistent increases in tumor perfusion. The study closed early when a separate phase II trial of IPI-926 plus gemcitabine indicated detrimental effects of this combination.
Conclusions
This is the first study to demonstrate the feasibility of using FOLFIRINOX as the chemotherapeutic backbone in a clinical trial design. Although robust antitumor activity and acceptable safety were observed with the addition of IPI-926 to this regimen, future development of Hedgehog inhibitors in pancreatic cancer seems unlikely.
Keywords: pancreatic cancer, Hedgehog, phase I, FOLFIRINOX, stroma
The Hedgehog (Hh) signaling pathway is important for normal mammalian embryonic development and adult tissue remodeling. 1 Aberrant activation of the Hh pathway, either through mutation of signaling components or constitutive expression of Hh pathway genes, has been shown to play a role in cancer initiation, growth, and metastasis2 and has been implicated in the pathogenesis of a number of solid tumors, including basal cell carcinoma,3 medulloblastoma,4 both non–small cell and small cell carcinoma of the lung,5,6 and pancreatic adenocarcinoma.7
IPI-926 (Infinity Pharmaceuticals, Cambridge, Mass) is a potent and specific inhibitor of Smoothened (Smo), a key signaling transmembrane protein in the Hh pathway. This agent has demonstrated clinical activity in Hh-dependent cancers, notably basal cell carcinomas, with inhibition of Hh activity confirmed by downregulation of Gli1 mRNA (an effector of Hh signaling) in skin biopsies.8 Moreover, evidence from a genetically engineered mouse model of pancreatic cancer demonstrated that IPI-926 can deplete tumor-associated stromal tissue and increase intratumoral mean vessel density.9 These changes resulted in enhanced delivery of concurrently administered systemic agents such as gemcitabine, leading to a decreased tumor burden and prolonged survival in this mouse model.
FOLFIRINOX, a biweekly regimen that includes infusional 5-fluorouracil (5-FU), leucovorin, irinotecan, and oxaliplatin, represents a frontline standard for patients with advanced pancreatic cancer and intact performance status. Results from a phase III clinical trial from France (PRODIGE 4/ACCORD 11) demonstrated a significant survival benefit of this regimen over gemcitabine, albeit with greater toxicity.10 Whether FOLFIRINOX can reliably serve as a chemotherapy platform for investigational combination with novel agents, however, has not been established. Based on the above preclinical findings showing a clear rationale for studying Hh pathway inhibitors in combination with chemotherapy, we conducted the current phase I clinical trial to evaluate the combination of IPI-926 and FOLFIRINOX in patients with advanced previously untreated pancreatic cancer.
MATERIALS AND METHODS
Study Design
This trial was conducted in 3 academic cancer centers (University of California San Francisco, University of Chicago, and University of Wisconsin), with institutional review board approval at each site. A standard 3 + 3 dose escalation study design with 4 dose levels was used to determine the maximum tolerated dose (MTD) of FOLFIRINOX in combination with IPI-926 (Table 1), with cohort expansion of up to 15 patients at MTD.
TABLE 1.
Dose Escalation Study Design
| Dose Level |
5-FU Bolus, mg/m2 |
5-FU Infusion, mg/m2×44–46 h |
Leucovorin, mg/m2 |
Oxaliplatin, mg/m2 |
Irinotecan, mg/m2 |
IPI-926, mg/d |
n | No. DLTs |
|---|---|---|---|---|---|---|---|---|
| −1 | — | 1600 | 400 | 50 | 120 | 130 | — | — |
| 1* | — | 1920 | 400 | 65 | 150 | 130 | 9 | 0 |
| 2 | — | 2400 | 400 | 85 | 180 | 130 | 6 | 2 |
| 3 | — | 2400 | 400 | 85 | 180 | 160 | — | — |
Starting dose level.
Dose-limiting toxicities (DLTs) were based on NCI-CTCAE version 4.0 criteria and defined as any of the following events occurring during the initial 5 weeks of study treatment: grade 3 or higher nonhematologic toxicity (not including nausea/vomiting or diarrhea occurring without optimal medical management and/ or resolving to grade ≤2 within 48 hours); grade 2 or higher seizures; grade 4 neutropenia lasting 5 or more days; grade 3 to 4 neutropenia with fever of 38.5°C or higher and/or infection requiring treatment; any grade 4 thrombocytopenia or grade 3 thrombocytopenia accompanied by grade 2 or higher hemorrhage; or predefined patterns of aspartate aminotransferase, alanine aminotransferase, and/or total bilirubin elevation without associated biliary obstruction. In addition, a delay in the start of cycle 2 of FOLFIRINOX/IPI-926 by more than 14 days, or the need to hold IPI-926 for more than 7 days during the DLT window, constituted a DLT if attributable to study treatment.
In addition to determination of MTD (the primary study end point), other end points included establishing the safety and toxicity of this combination; preliminary assessment of clinical efficacy, including progression-free survival (PFS), objective response rate by Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 criteria, and CA19-9 biomarker decline; and plasma concentrations and pharmacokinetic parameters of IPI-926 and its relevant metabolites.
In a subset of patients, perfusion computed tomography (CT) imaging using a 256-slice multidetector CT scan (Brilliance iCT; Philips Healthcare, Highland Heights, Ohio) was performed as part of an exploratory analysis to assess changes in tumor perfusion. The CT perfusion component used jog-mode to study an 8-cm longitudinal segment of the upper abdomen centered on the pancreatic tumor mass. After administration of approximately 50 mL of iohexol (Omnipaque; GE) 350 nonionic intravenous contrast, scans with 5-mm slice thickness were obtained at 2-second intervals for 60 seconds with a 5-second postinjection threshold. The x-ray parameters were 100 kV with 100 mAs. Perfusion data were subsequently analyzed by MIStar software (Apollo Medical Imaging Technology, Melbourne, Australia).
Main Eligibility Criteria
Patients were eligible if they had locally advanced or metastatic pancreatic adenocarcinoma for which they had received no prior systemic therapy (except in the adjuvant setting >6 months previously). Other key eligibility criteria included evidence of either, or both, RECIST-defined measurable disease or an elevated serum CA19-9 at baseline (≥2 times upper limits of normal [ULN]); ECOG (Eastern Cooperative Oncology Group) performance score 0 to 1; and adequate bone marrow, renal, and hepatic function. Patients with central nervous system metastases, coagulopathy, preexisting peripheral neuropathy more than grade 1, comorbid medical conditions conferring a high risk for treatment-related complications, concurrent active malignancy, or who required concurrent administration of strong CYP3A inhibitors, were excluded.
Study Treatment and Assessments
Enrolled patients received a 7-day run-in period with IPI-926 alone (days −7 to −1). FOLFIRINOX was then administered in 14-day cycles in identical fashion to the PRODIGE trial10 at doses prescribed for the given cohort, with the exception that bolus 5-FU was omitted for this study. Primary prophylaxis with either pegfilgrastim or filgrastim was required with each treatment cycle. A drug self-administration diary was used to track patients' compliance with IPI-926.
Tumor assessments consisted of CT scans performed at the end of every 4 treatment cycles (8 weeks), as well as serial measurements of serum CA19-9 in patients with elevated baseline levels. Sparse pharmacokinetic analyses were performed with measurement of plasma drug concentrations of IPI-926 and its main metabolite, IPI-541, at predefined time points. Given the known cumulative toxicities of FOLFIRINOX that may not meet the study's formal toxicity stopping rules, patients with stable disease or better after 8 to 12 cycles of chemotherapy were given the option of discontinuing FOLFIRINOX while remaining on IPI-926 maintenance therapy alone.
Statistical Analysis
Data were gathered and summarized with respect to demographic and baseline characteristics, safety observations and measurements, and efficacy observations and measurements. Each outcome measure was analyzed at each dose cohort and for the entire study population. Progression-free survival and overall survival were summarized according to the method of Kaplan and Meier. Descriptive statistics were used to summarize pharmacokinetic and perfusion CT results.
RESULTS
Patients
A total of 15 patients were enrolled on this study between October 2011 and December 2012. Baseline characteristics are shown in Table 2.
TABLE 2.
Patient Demographics (N = 15)
| Characteristic | No. Patients (%) |
|---|---|
| Mean age, y | 58.5 |
| <65 | 12 (80%) |
| >65 | 3 (20%) |
| Sex | |
| Male | 10 (67%) |
| Female | 5 (33%) |
| Stage | |
| Locally advanced | 3 (20%) |
| Metastatic | 12 (80%) |
| Tumor location | |
| Head | 6 (40%) |
| Other | 9 (60%) |
| Elevated CA19-9 | |
| (>2× ULN) | |
| Yes | 9 (60%) (median, 3994; range, 96–41,362 |
| No | 6 (40%) |
| Baseline ECOG | |
| performance status | |
| 0 | 8 (53%) |
| 1 | 7 (47%) |
| Prior surgical resection | |
| Yes | 1 (7%) |
| No | 14 (93%) |
Treatment Administration
Four patients were initially enrolled at dose level 1, none of whom experienced a protocol-defined DLT. At dose level 2, composed of FOLFIRINOX doses identical to those used in the original PRODIGE study (minus the 5-FU bolus), 2 of 6 patients experienced DLTs, both related to hepatotoxicity: one with transient elevation in alanine aminotransferase more than 10 times ULN (grade 3) and another with persistent grade 2 to 3 transaminitis lasting longer than 2 weeks and preventing timely administration of FOLFIRINOX cycle 2. Both of these were reversible after treatment was held. We therefore established dose level 1 as the MTD and enrolled an additional 5 patients at this dose level before study closure (refer to Table 1).
For the entire study cohort, patients received a median of 11 cycles of FOLFIRINOX (range, 1–12). Seven patients received the protocol-recommended maximum 12 cycles of chemotherapy. After discontinuing FOLFIRINOX, 9 patients continued on maintenance therapy with IPI-926 alone (range, 1.6–8.8 months; median, 2.8 months). Eleven patients did require dose reduction of some or all components of FOLFIRINOX, including 4 in whom oxaliplatin was discontinued altogether between cycles 8 and 10. Reasons for study discontinuation included disease progression (8 patients), toxicity (4 patients), patient preference (1 patient), and discontinuation of study drug supply (2 patients).
Adverse Events
The most common adverse events (any grade) included peripheral sensory neuropathy, nausea/vomiting, diarrhea, fatigue, anorexia, and liver function test abnormalities (both transaminitis and elevated alkaline phosphatase) (Table 3). These occurred with similar frequencies at both dose levels 1 and 2. Eight of 15 patients (53%) experienced at least 1 grade 3 or 4 adverse event. There were no deaths attributable to study treatment.
TABLE 3.
Toxicities Possibly or Likely Related to Study Treatment
| Common Toxicity Criteria Adverse Events (Version 4.0) (N = 15) |
||||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Laboratory | ||||
| Neutropenia | 1 | 1 | ||
| Anemia | 3 | 1 | ||
| Thrombocytopenia | 3 | 2 | ||
| Aspartate aminotransferase/alanine aminotransferase | 3 | 5 | 2 | |
| Alkaline phosphatase | 4 | 3 | 1 | |
| Nonlaboratory | ||||
| Peripheral neuropathy/cold sensitivity | 8 | 7 | ||
| Nausea/vomiting | 8 | 4 | 1 | |
| Diarrhea | 6 | 7 | ||
| Fatigue | 8 | 6 | ||
| Weight loss/anorexia | 4 | 5 | ||
| Mucositis | 4 | 1 | ||
| Infection | 3 | 2* | ||
| Thromboembolic event | 3 | 1 | ||
| Rash/dermatitis | 1 | 1 | ||
One Clostridium difficile enterocolitis, 1 epidural abscess.
Pharmacokinetics
Drug concentrations of IPI-926 and its main metabolite, IPI- 541,measured at predefined time points during the first 5 weeks of study treatment, are depicted graphically in Supplemental Figure 1 (Supplemental Digital Content, http://links.lww.com/MPA/A421). As expected, plasma levels of IPI-926 far exceeded those of IPI-541 at all time points. Patients reached relative steady state levels of IPI-926 by the end of the 1-week run-in period. Mean IPI-926 concentration 4 hours postdose on day 22, after patients had completed their first cycle of FOLFIRINOX, was 188 ng/mL. By comparison, in the first-in-human study of IPI-926 administered as monotherapy, mean Cmax (achieved at 3.13 hours) of this drug given at the same dose, by day 22, was 336 ng/dL.
Efficacy
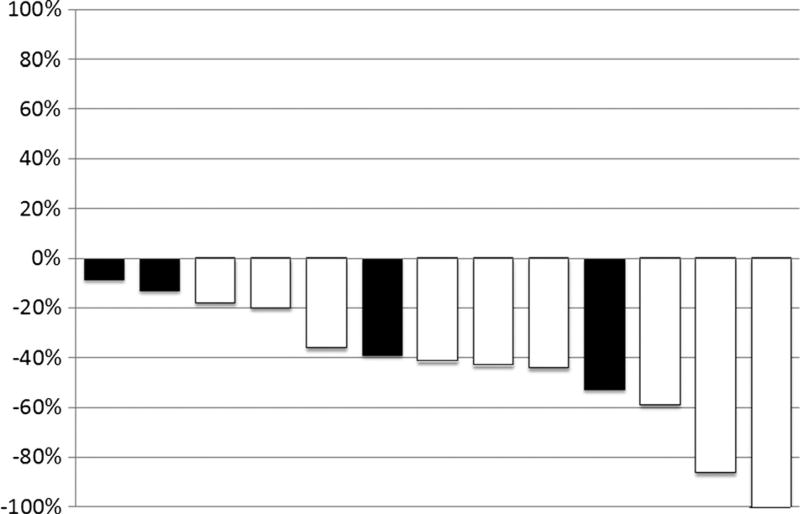
Nine of 15 patients (66.7%) demonstrated an unconfirmed objective response by RECIST criteria. A waterfall plot of best response to study treatment is shown in Figure 1. Six (66.7%) of 9 patients with an elevated CA19-9 level at baseline (>2 times ULN) experienced greater than 50% decline in this tumor marker, including 5 with a deep decline of 90% or more. Median PFS of the entire study cohort was 8.4 months.
Figure 1.
Waterfall plot demonstrating maximum change in target lesions from baseline in evaluable patients. Black bars indicate dose level 1; white bars, dose level 2.
Of the 9 patients who continued on maintenance therapy with IPI-926 alone after completing between 8 and 12 cycles of FOLFIRINOX, 4 showed continued stabilization of disease for an additional 4 months or longer. Interestingly, a continued decline in CA19-9 was observed in 4 of 5 patients (range of decline, 26.9%–97.7%) receiving IPI-926 monotherapy.
One patient with limited pulmonary metastases underwent resection of his primary pancreatic tail tumor, consisting of distal pancreatectomy with en bloc splenectomy, 11 months after study enrollment. Final pathology demonstrated residual node–positive cancer (1 of 19 positive lymph nodes); of note, the surgical specimen showed significant areas of higher-grade, more poorly differentiated tumor when compared with his pretreatment biopsy, which was classified as moderately differentiated (Supplemental Figure 2, Supplemental Digital Content, http://links.lww.com/MPA/A422).
Exploratory Analysis: Perfusion CT Imaging
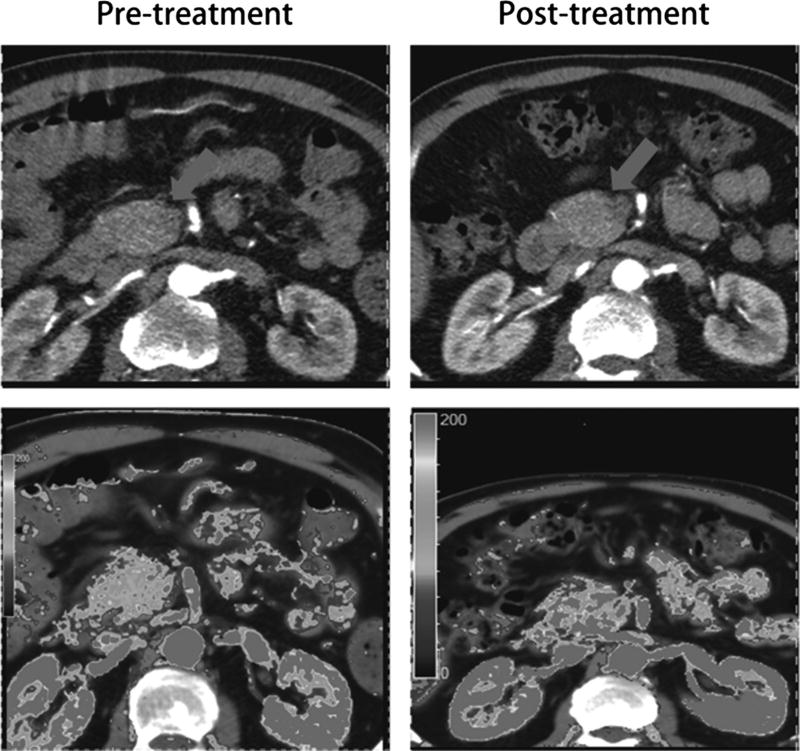
To test the hypothesis that inhibition of Hh signaling may produce stromal changes and result in improved vascularization of the primary pancreatic tumor, dynamic perfusion CT was performed in 6 patients at baseline and again after cycle 4 of FOLFIRINOX/IPI-926. For each patient, 3 regions of interest both pretreatment and posttreatment were examined, from which parametric mapping of multiple variables, including blood flow, blood volume, volume transfer constant, and permeability surface area product, were generated. Overall, no consistent trend was observed across this small patient sample to suggest increased tumor perfusion in response to study treatment (Supplemental Table 1, Supplemental Digital Content, http://links.lww.com/MPA/A423). Figure 2 shows an illustrative example in 1 patient in whom blood flow within the primary pancreatic tumor did increase posttreatment compared with baseline; of note, this patient demonstrated an objective radiographic response by RECIST.
Figure 2.
Computed tomographic images (upper panels, pancreatic tumor marked by arrow) with corresponding perfusion images (lower panels) in 1 patient, demonstrating increased blood flow after 2 months of study treatment.
DISCUSSION
This clinical trial was initiated at a time of growing interest in pharmacologic inhibitors of Hh signaling as potential therapy for pancreatic cancer. Multiple lines of evidence have supported a role for this pathway in pancreatic tumorigenesis. Aberrant expression of both Sonic Hh ligand and its associated signaling components, Patched (the cognate receptor for Hh ligands) and Smoothened (a coreceptor), is frequently seen in pancreatic cancer specimens, with increasing activity as tumors progress from early precursor (pancreatic intraepithelial neoplasia) lesions to invasive adenocarcinoma. 7,11–17 Moreover, studies involving global sequencing analysis have identified the Hh pathway as one of the central elements undergoing transformation in nearly all pancreatic cancers. 12 In pancreatic cancer cell lines7 and orthotopic xenograft models,18,19 pharmacologic inhibition of Hh signaling has demonstrated compelling evidence of antitumor activity. This pathway may also play an important role in maintenance of pancreatic cancer stem cells.20–22
The final piece of evidence supporting the therapeutic potential of Hh inhibitors was derived from studies conducted in a well-validated genetically engineered murine model of pancreatic cancer. In this KPC mouse (which expresses both oncogenic Kras and mutant p53 in pancreatic cells), Olive et al9 showed that IPI-926 could deplete the dense desmoplastic mesenchymal network that constitutes the stroma of pancreatic adenocarcinomas. This stromal microenvironment, which requires paracrine Hh signaling for its maintenance,23 presents a challenge to effective delivery of chemotherapy agents to the bulk of pancreatic tumor burden and represents a viable therapeutic target.
The present study originally was intended to inform a subsequent national cooperative group trial evaluating IPI-926 for the treatment of locally advanced pancreatic cancer, a clinical context in which Hh signaling inhibition was felt to be of potential greatest utility. However, as our trial was still accruing, early results became available from a separate phase II trial sponsored by Infinity Pharmaceuticals (IPI-926-03), in which patients with metastatic pancreatic cancer were randomized to receive gemcitabine plus either IPI-926 or placebo.24 In that trial, patients receiving IPI-926 had a shorter median survival time and more rapid rate of disease progression compared with the placebo-containing arm, resulting in the study being voluntarily halted. Moreover, a subsequent phase Ib/randomized phase II study of gemcitabine plus another Hh inhibitor (vismodegib; Genentech, South San Francisco, Calif ) also failed to improve progression-free or overall survival compared with gemcitabine plus placebo.25 After careful consideration and with explicit disclosure to all study patients, we opted to continue our phase I trial for several months longer before stopping enrollment short of the intended full cohort expansion.
The reasons why the promising preclinical results of Hh inhibitors in pancreatic cancer have not translated into similar success in the clinical arena are uncertain, although recent “postclinical” data from 2 separate groups have indicated the potential detrimental effects of depleting the stromal compartment in pancreatic cancer via altering the biology of the tumor.26,27 In one such study, conditional deletion of Shh in a transgenic mouse model of pancreatic cancer resulted in more aggressive, undifferentiated, and highly proliferative tumors, suggesting that tumor stroma may play an important role in restraining, as opposed to supporting, tumor growth.26 This same study found that long-term treatment with IPI-926, alone or in combination with gemcitabine, yielded the same outcome. Intriguingly, the 1 patient in our trial for whom we had a posttreatment histopathologic specimen available for analysis did in fact show areas of higher-grade, more poorly differentiated tumor compared with baseline, although this finding could also be caused by inherent tumor heterogeneity.
However, at the same time, we note that our study, although limited by small numbers, did show high rates of response and prolonged PFS, suggesting that the chemotherapeutic backbone to which a novel targeted agent is added represents a critical consideration in trial design. Indeed, our study is important as it represents, to our knowledge, the first example of a clinical trial in pancreatic cancer to demonstrate the feasibility of using FOLFIRINOX as a cytotoxic backbone on which to build in the evaluation of a novel drug. Given the broadening array of therapeutic options that are now available for advanced pancreatic cancer, including not only FOLFIRINOX but also gemcitabine in combination with nab-paclitaxel,28,29 clinical trial design for this disease indication is evolving.30 Designing a clinical trial that incorporates a more intensive multidrug chemotherapy regimen becomes correspondingly more complex because it requires development of very specific rules to guide dose reduction of each separate component of FOLFIRINOX while, at the same time for phase I dose escalation studies, limiting the number of dose cohorts for practical purposes. We were especially vigilant about the potential overlapping and additive toxicities of IPI-926 and FOLFIRINOX, notably hepatotoxicity and gastrointestinal side effects; although these adverse events were observed in a majority of patients, they were primarily grades 1 and 2 in nature.
Two other interesting observations from our study are worth noting. First, several patients demonstrated a marked decline in serum CA19-9 levels, along with durable disease control radiographically, while maintained on IPI-926 alone, indicating the efficacy of this agent independent of chemotherapy coadministration. Second, the correlative radiographic studies did not show a consistent increase in tumor perfusion for patients receiving study treatment. Both these findings suggest that the mechanisms of action of Hh inhibitors likely extend beyond their effects solely on peritumoral stroma.
In conclusion, we found evidence of robust antitumor activity and acceptable safety with the addition of IPI-926 to FOLFIRINOX, establishing the feasibility of using FOLFIRINOX as a chemotherapeutic platform on which to build in pancreatic cancer trial design. The relative contribution of IPI-926 to these promising results, however, cannot be determined from this small study. Despite our encouraging findings, the future of Hh pathway inhibitors as a therapy for pancreatic cancer seems uncertain, even while other stroma-depleting therapies continue to be actively investigated. We propose that the specific chemotherapy regimen with which this class of agents is partnered may make a significant difference in its therapeutic efficacy and warrants further exploration.
Supplementary Material
Acknowledgments
Research support for this study was provided by Infinity Pharmaceuticals.
Dr Ko received financial support (paid directly to his institution) from Infinity Pharmaceuticals to support conduct of this investigator-initiated study.
Footnotes
The other authors declare no conflict of interest.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
References
- 1.Ingham PW. Signalling by hedgehog family proteins in Drosophila and vertebrate development. Curr Opin Genet Dev. 1995;5:492–498. doi: 10.1016/0959-437x(95)90054-k. [DOI] [PubMed] [Google Scholar]
- 2.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 3.Oro AE, Higgins KM, Hu Z, et al. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 4.Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Blanco J, Schilling NS, Tokhunts R, et al. The hedgehog processing pathway is required for NSCLC growth and survival. Oncogene. 2013;32:2335–2345. doi: 10.1038/onc.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park KS, Martelotto LG, Peifer M, et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat Med. 2011;17:1504–1508. doi: 10.1038/nm.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jimeno A, Weiss GJ, Miller WH, Jr, et al. Phase I study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clin Cancer Res. 2013;19:2766–2774. doi: 10.1158/1078-0432.CCR-12-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 11.Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 12.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu MS, Yang PY, Yeh TS. Sonic hedgehog signaling pathway in pancreatic cystic neoplasms and ductal adenocarcinoma. Pancreas. 2007;34:340–346. doi: 10.1097/mpa.0b013e3180333ab5. [DOI] [PubMed] [Google Scholar]
- 14.Morton JP, Lewis BC. Shh signaling and pancreatic cancer: implications for therapy? Cell Cycle. 2007;6:1553–1557. doi: 10.4161/cc.6.13.4467. [DOI] [PubMed] [Google Scholar]
- 15.Morton JP, Mongeau ME, Klimstra DS, et al. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci U S A. 2007;104:5103–5108. doi: 10.1073/pnas.0701158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quint K, Stintzing S, Alinger B, et al. The expression pattern of PDX-1, SHH, Patched and Gli-1 is associated with pathological and clinical features in human pancreatic cancer. Pancreatology. 2009;9:116–126. doi: 10.1159/000178882. [DOI] [PubMed] [Google Scholar]
- 17.Steg A, Vickers SM, Eloubeidi M, et al. Hedgehog pathway expression in heterogeneous pancreatic adenocarcinoma: implications for the molecular analysis of clinically available biopsies. Diagn Mol Pathol. 2007;16:229–237. doi: 10.1097/PDM.0b013e31811edc7e. [DOI] [PubMed] [Google Scholar]
- 18.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldmann G, Fendrich V, McGovern K, et al. An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol Cancer Ther. 2008;7:2725–2735. doi: 10.1158/1535-7163.MCT-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Lee HK, Cho SY, et al. Identification of osteogenic purmorphamine derivatives. Mol Cells. 2008;26:380–386. [PubMed] [Google Scholar]
- 22.Mueller MT, Hermann PC, Witthauer J, et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–1113. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 23.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 24. [Accessed January 27, 2012];Infinity Pharmaceuticals company Web site. Available at: http://phx.corporate-ir.net/phoenix.zhtml?c=121941&p=irol-newsArticle_print&ID=1653550&highlight=
- 25.Catenacci D, Bahary N, Nattam S, et al. Final analysis of a phase IB/randomized phase II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer: A University of Chicago phase II consortium study [Abstract 4012] J Clin Oncol. 2013;31(suppl) doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Jesus-Acosta A, O'Dwyer PJ, Ramanathan RK, et al. A phase II study of vismodegib, a hedgehog pathway inhibitor, combined with gemcitabine and nab-paclitaxel in patients with untreated metastatic pancreatic ductal adenocarcinoma [Abstract 257] J Clin Oncol. 2014;32(suppl 3) doi: 10.1038/s41416-019-0683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Loon K, Espinoza AM, Fogelman DR, et al. Should combination chemotherapy serve as the backbone in clinical trials of advanced pancreatic cancer? A pooled analysis of phase ii trials of gemcitabine-containing doublets plus bevacizumab. Pancreas. 2014;43:343–349. doi: 10.1097/MPA.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.