Abstract
OBJECTIVES
Autoimmune pancreatitis (AIP) is an increasingly recognized disease entity, but data in children are limited. AIP presentation and outcome in children might differ from the adult experience. We aim to determine the characteristic features of AIP in children.
METHODS
Data about clinical symptoms, imaging, histology, and treatment were collected using two sources: (i) a systematic literature search and (ii) the INSPPIRE database, the largest international multicenter study of pancreatitis in children and the Cliniques Universitaires St-Luc (CUSL) registry.
RESULTS
We identified 48 AIP cases: 30 from literature review, 14 from INSPPIRE, and 4 from CUSL. The median age at diagnosis was 13 years (range 2–17 years). Abdominal pain (43/47, 91%) and/or obstructive jaundice (20/47, 42%) were the most common symptoms at diagnosis. Elevated serum IgG4 levels were only observed in 9/40 (22%) children. Cross-sectional imaging studies were abnormal in all children including hypointense global or focal gland enlargement (39/47, 83%), main pancreatic duct irregularity (30/47, 64%), and common bile duct stricture (26/47, 55%). A combination of lymphoplasmacytic inflammation, pancreatic fibrosis, and ductal granulocyte infiltration were the main histological findings (18/25, 72%). Children with AIP had a prompt clinical response to steroids. Complications of AIP included failure of exocrine (4/25, 16%) and endocrine (3/27, 11%) pancreas function.
CONCLUSIONS
Pediatric AIP has a distinct presentation with features similar to type 2 AIP in adults. This comprehensive report on the largest group of children with AIP to date is expected to help with the diagnosis and management of this disease and pave the way for future research studies.
INTRODUCTION
Autoimmune pancreatitis (AIP) is a distinctive type of pancreatitis first described in 1961 by Sarles et al. (1) as a particular form of chronic pancreatitis (CP) that was associated with hypergammaglobulinemia. In 1995, Yoshida et al. (2) labeled this newly recognized disease “autoimmune pancreatitis.” ICDC (International Consensus Diagnostic Criteria) for AIP (3) were established in 2011 and are mainly based on a combination of five cardinal features including pancreas histology and imaging findings, positive serology, presence of other autoimmune or inflammatory organ diseases, and prompt response to corticosteroids (3). AIP in adults is classified into two subgroups, characterized by differences in epidemiology, clinical profile, histopathology, natural history, and outcome (4). A high prevalence of AIP type 1 (lymphoplasmacytic sclerosing pancreatitis) is observed in Asia, whereas AIP type 2 (idiopathic duct-centric pancreatitis) mainly occurs in Western countries. A definitive diagnosis of idiopathic duct-centric pancreatitis requires histologic examination, whereas lymphoplasmacytic sclerosing pancreatitis can be diagnosed without (5).
In children, reports about AIP are scarce with a few published cases and case series (6–24). At the present time, pediatric gastroenterologists rely on the adult AIP guidelines to diagnose and manage AIP in children. However, initial observations and case reports suggest that the clinical presentation of AIP is different in children compared with adults, and that exclusive use of adult criteria may lead to underdiagnosis of AIP in children.
We aimed to summarize current available data to support identification of AIP in children, as AIP is the only form of CP for which targeted (anti-inflammatory) treatment is available. Though there are no data exploring the long-term outcome of pediatric AIP patients in a systematic manner, it is conceivable that treatment may affect the long-term outcome of these patients. Furthermore, summarizing the characteristic features of AIP in children is needed to facilitate stratification of these patients for future research studies.
In order to achieve these aims, we: (i) reviewed the literature to extract common characteristic features of children with a diagnosis of AIP and (ii) summarized characteristics reported in children with a diagnosis of AIP who are part of the largest international multicenter study of acute recurrent and chronic pancreatitis in children (INSPPIRE); this summary included several AIP cases collected at the Cliniques Universitaires St-Luc (CUSL) in Brussels, Belgium.
METHODS
Literature review
An electronic systematic literature search of PubMed (1946 on), Embase (1980 on), and CENTRAL (Cochrane Central Register of Controlled Trials) was performed up to September 2016. The key search terms included: “pediatric,” “children,” “lymphoplasmacytic sclerosing pancreatitis,” “idiopathic duct centric pancreatitis,” and “autoimmune pancreatitis.” The search was limited to human studies without any language restriction. Data concerning epidemiology (gender, age at presentation, ethnic origin), clinical symptoms, blood work, imaging and histologic findings, associated diseases, treatment, and outcome were extracted and summarized.
Data collection from INSPPIRE and CUSL
The INSPPIRE database collects data on demographics, clinical presentation, and longitudinal follow-up of children (onset of illness <19 years of age) with acute recurrent pancreatitis and CP from 18 participating international centers. We collected baseline and follow-up data from children that were assigned a diagnosis of AIP by the pediatric gastroenterologist of each participating center from 1 September 2012 to 31 December 2015. The collected information included age, gender, medical history, family history, clinical symptoms at presentation, biochemical results, imaging, histopathology, therapeutic interventions, treatment, and outcome. Similarly, all pediatric patients with a diagnosis of AIP who were followed at CUSL and who were part of the pediatric onset (<18 years) pancreatitis registry (1 January 2000 and 1 July 2015) were included. All participating centers have obtained approval from their institutional review boards. Consent was obtained from all participants or their parents for children <18 years.
Statistics
Summary data were presented as mean and s.d. for normal-distributed data or median and interquartile ranges for nonnormally distributed data. Fisher’s exact test was used for categorical variables and t-test analysis of variance was used for continuous variables. A P value of <0.05 was considered to be statistically significant.
RESULTS
Pediatric AIP literature review and cases from the INSPPIRE and CUSL database
A retrospective analysis of the literature identified 19 publications reporting 30 pediatric AIP cases (6–24). Furthermore, 14 of the 361 (3.9%) children enrolled in INSPPIRE were diagnosed with AIP by Pediatric Pancreatologists working in the participating centers. An additional 4 patients were recruited at CUSL.
Epidemiology and clinical presentation
AIP was found in patients with Caucasian, African, and Asian background, with slight male preponderance (23/43, 53% male). Median age at diagnosis was 13 years (range: 2–17 years).
Acute abdominal pain (43/47, 91%) and/or obstructive jaundice (20/47, 42%) were the most commonly reported clinical symptoms at diagnosis. (Table 1)
Table 1.
Summary of the clinical symptoms reported in children with AIP in the literature and in a multicentric study (INSPPIRE and CUSL)
| Baseline characteristics |
Literature (n=30 total) | INSPPIRE/CUSL (n=18 total) |
||
|---|---|---|---|---|
|
|
|
|
||
| Symptoms | No. of patients/ reported |
(%) | No. of patients/ reported |
(%) |
| Abdominal pain | 27/29 | (93%) | 16/18 | (89%) |
|
| ||||
| Obstructive jaundice | 10/29 | (34%) | 10/18 | (55%) |
|
| ||||
| Weight loss | 11/29 | (38%) | 3/9 | (33%) |
|
| ||||
| Nausea/vomiting | 6/29 | (21%) | 4/9 | (44%) |
|
| ||||
| Fatigue | 4/29 | (14%) | 4/9 | (44%) |
|
| ||||
| Abnormal stools | 1/29 | (3%) | 2/9 | (22%) |
AIP, autoimmune pancreatitis; CUSL, Cliniques Universitaires St-Luc.
Diagnostic evaluation
At the time point of diagnosis, an increase in amylase (median: 1.7× upper limit of normal (ULN), interquartile range (IQR): 1.3–4× ULN) and lipase (median: 4× ULN, IQR: 2.4–11×ULN) was observed in about half of the patients (10/23, 43% and 14/26, 54% respectively). A positive serology for IgG4 was found in only 22% (9/40) of the patients (median: 1.9× ULN, IQR: 1.2–2.5× ULN). Though not specific for AIP, increased levels of antinuclear antibody (ANA) were present in 29% (7/24) of the cases (Table 2).
Table 2.
Summary of serology, imaging, and histopathologic features in children with AIP
| Literature (n=30 total) | INSPPIRE/CUSL (n=18 total) | |||
|---|---|---|---|---|
| Serology and biochemistry | No. of positive/tested | (%) | No. of positive/tested | (%) |
| Increased lipase (>1× ULN) | 5/14 | (36%) | 9/12 | (75%) |
| Increased amylase (>1× ULN) | 6/15 | (40%) | 4/8 | (50%) |
| Increased serum IgG4 (>1× ULN) | 6/24 | (25%) | 3/16 | (19%) |
| ANA | 2/14 | (14%) | 5/10a | (50%) |
| ANCA | 4/7 | (57%) | 1/7 | (14%) |
| Anti-LKM, RF, SSA, SSB | 0/6 | (0%) | 0/4 | (0%) |
| Anti-DNA, ASCA | 0/5 | (0%) | 1/4 | (25%) |
| Imaging findings (diagnosis) | No. of patients/reported | (%) | No. of patients/reported | (%) |
| Gland enlargement mass/focal | 16/29 | (55%) | 9/18 | (50%) |
| Gland enlargement global | 9/29 | (31%) | 5/18 | (28%) |
| Capsule-like rim (MRCP) | 3/14 | (21%) | 2/18 | (11%) |
| Pancreas atrophy, pseudocysts, calcification | 0/29 | (0%) | 0/18 | (0%) |
| Main pancreatic duct irregularity | 22/29 | (76%) | 8/18 | (44%) |
| CBD stricture/tapering | 17/29 | (59%) | 9/18 | (50%) |
| CBD dilatation | 16/29 | (55%) | 9/18 | (50%) |
| Retroperitoneal fibrosis | 1/29 | (3%) | ND | — |
| Pancreas histopathology | No. of patients/reported | (%) | No. of patients/reported | (%) |
| Performed | 22 | 9 | ||
| Noninterpretable | 3 | (14%) | 2 | (22%) |
| Pancreas fibrosis | 12/19 | (63%) | 7/7 | (100%) |
| Venulitis/phlebitis | 2/19 | (11%) | 1/7 | (14%) |
| Lymphoplasmacytic infiltration | 19/19 | (100%) | 5/7 | (71%) |
| IgG4 (+) plasmacytes (>10/HPF) | 1/19 | (5%) | 0/7 | (0%) |
| Granulocytic epithelial lesion | 14/19 | (74%) | 4/7 | (57%) |
| Acinar atrophy | 3/19 | (16%) | 4/7 | (57%) |
AIP, autoimmune pancreatitis; ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; ASCA, anti-Saccharomyces cerevisiae antibody; CBD, common bile duct; CUSL, Cliniques Universitaires St-Luc; DNA, anti-DNA antibody; HPF, high-power field; LKM, liver kidney microsome antibody; MRCP, magnetic resonance cholangiopancreatography; ND, no data; RF, rheumatoid factor; SSA, SS-A/Ro antibody; SSB, SS-B/La antibody; ULN, upper limit of normal.
Measured ANA titers ranged between 1/80 and 1/640.
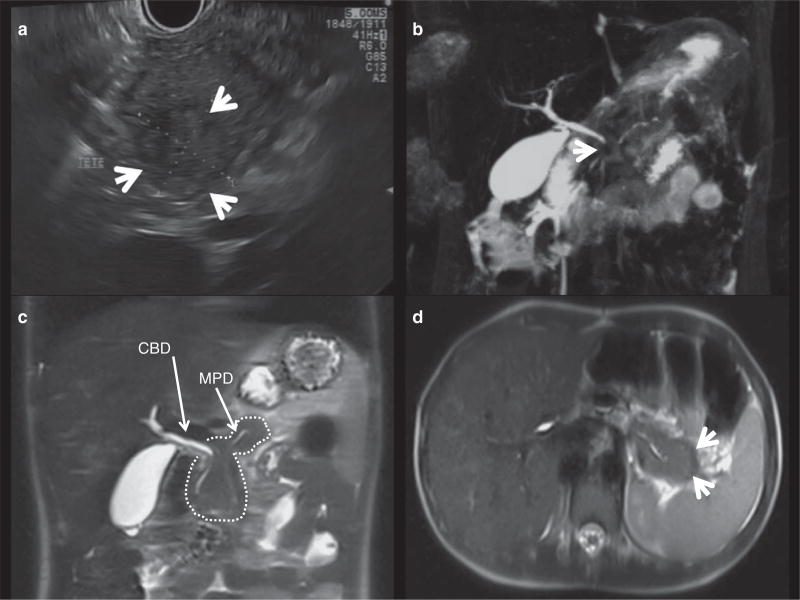
Imaging findings were abnormal in all reported patients (Table 2 and Figure 1). Transabdominal ultrasound was often the first available imaging modality. A hypoechoic and enlarged pancreas was described in 83% (39/47) children with an established diagnosis of AIP. In patients presenting with jaundice a proximal dilatation of the common bile duct (CBD) narrowing distally toward the pancreatic head was found in all cases. In all AIP patients magnetic resonance cholangiopancreatography (MRCP) imaging frequently showed global (17/47, 30%) or focal enlargements of the pancreas (25/47, 53%), demonstrated as hypointense areas on T1-weighted images. Irregularities of the main pancreatic duct were the most reported features (30/47, 64%), followed by a narrowed CBD (stricture or tapering, 26/47, 55%). A “capsule-like rim” (halo sign) was found in 5 of 32 children (16%). Five children of the INSPPIRE/CUSL cohort and 7 published cases underwent an endoscopic retrograde cholangiopancreatography (ERCP). ERCP findings correlated with those of MRCP in 11 patients; one patient showed narrowing of the CBD in ERCP that was not visualized on MRCP.
Figure 1.
Imaging features of autoimmune pancreatitis in children. (a) Mass lesion in the pancreatic head as shown by endoscopic ultrasound (arrows). (b) Magnetic resonance cholangiopancreatography (MRCP) T2-weighted three-dimensional (3D) cholangiogram reconstruction showing an irregular main pancreatic duct (MPD) in the pancreas tail, and no visible pancreas duct in the head. The common bile duct (CBD) is tapered in its proximal part by a pancreatic head mass (arrow). (c) MRCP T2-weighted HASTE (half-Fourier acquisition single-shot turbo spin-echo) coronal section showing a bulky pancreas (dashed contour). The MPD (arrow) and the distal CBD (arrow) are tapered by the enlarged pancreas head. (d) MRCP T2-weighted HASTE transversal section evidencing an abnormal capsule-like rim hypointense rehearsal in the pancreas periphery (arrow).
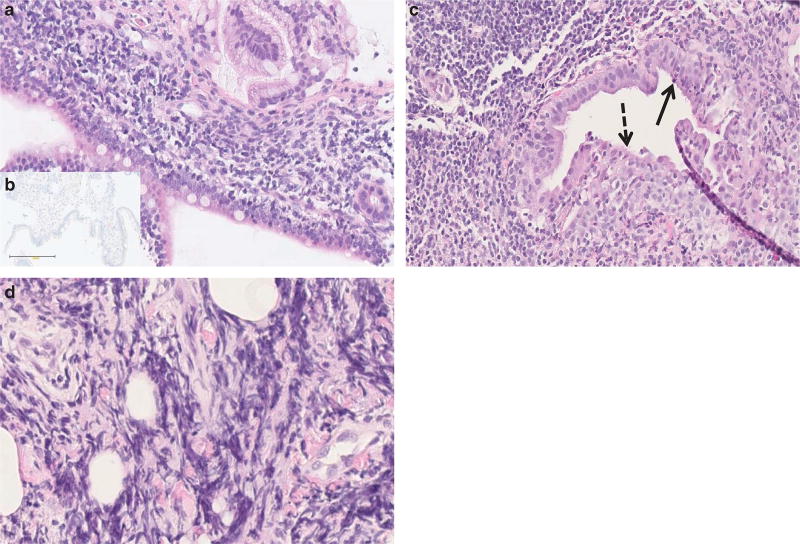
Interpretable histologic results were available for 26 children (Table 2 and Figure 2); of these, 16 were obtained using endoscopic ultrasound-guided pancreas biopsies and 10 by open-surgical biopsies. Taken together, the majority (18/26, 69%) of the pediatric AIP patients demonstrated a combination of lymphoplasmacytic infiltration, pancreatic fibrosis, and granulocyte infiltration of the pancreatic duct epithelium and lumen (also called granulocytic epithelial lesion). Positive IgG4 staining was found in one child (1/26, 4%).
Figure 2.
Pancreas histopathology in pediatric autoimmune pancreatitis (AIP). Representative histopathologic features on pancreas core biopsies in children with autoimmune pancreatitis (hematoxylin and eosin (H&E) staining). (a) Dense lymphoplasmacytic infiltration of the major papilla (original magnification ×20). (b) Negative IgG4 staining of the plasma cells (original magnification ×20). (c) Granulocytic epithelial lesion (arrow) and focal epithelial duct destruction (dashed arrow). The chorion is infiltrated by lymphoplasmatic cells (original magnification ×20). (d) Storiform fibrosis (original magnification ×40).
Other organ involvement
AIP as part of an IgG4-related multisystemic disease was uncommon (2/45, 4%). Twelve AIP children (12/45, 27%) developed other autoimmune/inflammatory diseases including Crohn’s disease, ulcerative colitis, glomerulonephritis, and hemolytic anemia (Table 3).
Table 3.
Other organ involvement in pediatric AIP
| Literature (n=30 total) | INSPPIRE/CUSL (n=18 total) |
|||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Other organ involvement |
No. of patients/ reported |
(%) | No. of patients/ reported |
(%) | ||
| IgG4-related diseasea | 1/29 | (3%) | 1/16 | (6%) | ||
|
| ||||||
| Crohn’s disease | 1/29 |
|
1/16 |
|
||
|
|
|
|
||||
| Ulcerative colitis | 4/29 | → (27%) | 3/16 | →(25%) | ||
|
|
|
|
||||
| Other autoimmune disease | 3/29 | 0/16 | ||||
AIP, autoimmune pancreatitis; CUSL, Cliniques Universitaires St-Luc.
IgG4-related systemic disease is defined as a multisystemic disease combining a variety of extrapancreatic lesions (such as lacrimal and salivary gland lesions, hilar lymphadenopathy, interstitial lung disease, sclerosing cholangitis, retroperitoneal fibrosis, and tubulointerstitial nephritis) and AIP (32).
Therapy
The main treatment approach to AIP was the use of prednisone. The doses and duration of corticosteroid regimen differed significantly among centers. Prednisone doses ranged from 1 to 1.5 mg/kg/day with a maximum of 30 to 120 mg/day orally for 15 to 50 days in adolescents, followed by a progressive taper over 2 to 24 months. Eight children (8/46, 17%) were clinically monitored, but did not receive any corticosteroid therapy (Table 4).
Table 4.
Treatment modalities reported in children with AIP
| Literature (n=30 total) |
INSPPIRE/CUSL (n=18 total) |
|||
|---|---|---|---|---|
|
|
|
|||
| Treatment | No. of patients/ reported |
(%) | No. of patients/ reported |
(%) |
| Whipple procedure | 2/28 | (7%) | 0/18 | (0%) |
|
| ||||
| Partial pancreatectomy | 3/28 | (10%) | 0/18 | (0%) |
|
| ||||
| Biliary/pancreatic duct stenting | 4/28 | (14%) | 4/18 | (22%) |
|
| ||||
| Choledochoduodenostomy | 0/28 | (0%) | 1/18 | (6%) |
|
| ||||
| Prednisone | 19/28 | (68%) | 10/18 | (55%) |
|
| ||||
| No treatment | 3/28 | (11%) | 5/18 | (28%) |
AIP, autoimmune pancreatitis; CUSL, Cliniques Universitaires St-Luc.
Response to therapy and early disease recurrence
Patients with AIP showed good short-term clinical response to corticosteroids (27/29, 93%) defined as clinical symptom resolution. The 8 patients who did not receive corticosteroids improved without further treatment. Insufficient data were available to analyze the time delay between the start of corticosteroid treatment and symptom resolution or to compare the time of clinical response between corticosteroid-treated and untreated patients. Imaging reevaluation, when done (n=12/29 cases in literature and n=13/18 INSPPIRE/CUSL), was performed 1 to 11 months after treatment initiation and showed resolution of the initial radiologic findings in all but 4 children.
AIP recurrence was documented for 8/39 patients (17%), 6 of those had been previously treated with corticosteroids. Two of the six children with AIP relapse had only a partial response to the induction course but were successfully brought in remission after adding mycophenolate mofetil or 6-mercaptopurine, respectively.
Pancreatic function and long-term outcome
The reported median follow-up time was 21 months (IQR: 12.5–48) following prednisone induction as compared with 33 months (IQR: 14–48.5) for those not treated with corticosteroids (P=0.42). Impaired exocrine function requiring pancreatic enzyme replacement therapy and insulin-dependent diabetes mellitus occurred with a frequency of 16% (4/25) and 11% (3/27), respectively. On follow-up cross-sectional imaging (mean: 4.5 months, range: 1–11 months) 8 patients (8/13, 61%) of the INSPPIRE/CUSL cohort demonstrated pancreatic parenchymal atrophy (Table 5).
Table 5.
Summary of outcome in children with AIP
| Literature (n=30 total) |
INSPPIRE/CUSL (n=18 total) |
|||
|---|---|---|---|---|
|
|
|
|||
| Outcome | No. of patients/ reported |
(%) | No. of positive/ reported |
(%) |
| AIP relapse | 4/23 | (17%) | 4/16 | (25%) |
|
| ||||
| Exocrine pancreatic insufficiency requiring PERT | 1/7 | (14%) | 3/18 | (17%) |
|
| ||||
| Diabetes mellitus | 1/9 | (11%) | 2/18 | (11%) |
AIP, autoimmune pancreatitis; CUSL, Cliniques Universitaires St-Luc; PERT, pancreatic enzyme replacement therapy.
DISCUSSION
AIP is a distinct form of pancreatitis that also occurs in children. By utilizing data from a large pediatric multicenter cohort study and information from previously reported cases, we have now derived the characteristic features of AIP in children. This comprehensive report will enable us to recognize pediatric patients with AIP that will stimulate further studies.
According to our findings, a diagnosis of AIP in children can be established based on the combination of specific clinical symptoms at presentation and distinct findings on cross-sectional imaging. This is in contrast to adult diagnostic AIP criteria that are much more comprehensive and based on HISORt (histology, imaging, serology, the presence of other organ involvement, and response to therapy) criteria. The majority of children had symptoms of abdominal pain and/or obstructive jaundice in combination with focal pancreas enlargement, main pancreatic duct irregularities, and distal CBD narrowing on cross-sectional imaging. In fact abdominal pain, weight loss, and fatigue were more consistently reported in children (89–93%, 33–38%, and 14–44% respectively) than in adults, with 60–75% (4) of adult AIP patients presenting with painless jaundice, 15% with weight loss, and 9% with fatigue (25).
Though most of the imaging findings of the pancreas are not specific for AIP, in combination with the clinical symptoms, however, certain findings as listed in Table 2 are highly suggestive of AIP. A capsule-like rim enhancement of the pancreas was infrequent in children but its occurrence appears to be pathognomonic for the disease. A pancreatic head enlargement or nonencapsulated mass lesions have been reported more frequently. Although rare, a focal pancreas enhancement needs to be differentiated from pancreatic malignant tumors such as pancreatoblastoma or solid pseudopapillar epithelial neoplasms that are often encapsulated and express cystic and solid components. Lymphoma is another very rare, differential cause for focal pancreas enhancements. Compared with adults (4), focal rather than diffuse pancreas enlargements were more frequent in children. Main pancreatic duct irregularities and distal CBD narrowing are nonspecific findings and might also be seen in patients with CP or biliopancreatic diseases of any other etiology or even in primary sclerosing cholangitis type 1 (26), though adults now include this specific type of primary sclerosing cholangitis to the diagnostic criteria for AIP (27). More detailed description of the pancreas and ductal pathology using cross-sectional imaging is challenged by the limitations of the MRCP resolution in young children (14) and the less frequent use of ERCP compared with adults (28).
Increased serum IgG4 is very suggestive of AIP in adults, but this marker is of limited value as only 22% of the children had IgG4 levels above the ULN. In comparison, about 65% of adult AIP type 1 and 25% of adult AIP type 2 patients have elevated IgG4 levels. This suggests that children may more commonly follow the disease presentation of AIP type 2 or may have a distinct AIP pattern that may not necessarily fall into either category.
A diagnosis of AIP can be confirmed by the histopathological identification of pathognomonic and well-described features of an autoinflammatory process in the pancreas tissue specimen. Frequent finding of granulocytic epithelial lesion in pediatric pancreas tissue specimen would be consistent with type 2 AIP as per adult criteria. However, because of the increased clinical recognition of AIP as well as a growing familiarity with AIP-type imaging findings, adult gastroenterologists often times waive a histopathological diagnosis and start a trial with corticosteroids that itself is used as a diagnostic criterion to confirm the diagnosis of AIP (3,4).
The distinction of two AIP subgroups such as those described in adults is difficult to establish in children. Only one child had autoimmune pancreatitis in the context of an IgG4-related systemic disease and could thus clearly be classified as having an AIP type 1. IgG4 serology and immunostaining is rarely positive in children with AIP. Although granulocytic epithelial lesion is more frequently found in the pancreas of children with AIP compared with adults, histopathological features of pediatric AIP often include a combination of granulocytic epithelial lesion, lymphoplasmacytic infiltration, and fibrosis. If adult AIP criteria were applied to the pediatric AIP population, children would most likely be classified as having type 2 AIP. However, as histology of pancreas biopsies in children with AIP have not yet been validated in relation to the disease phenotype (3,8), we cannot at this point exclude the possibility that AIP in children may yet follow a distinct disease pattern.
A total of 27% of children and 10–20% of adults (5) diagnosed with AIP have concurrent immune/inflammatory diseases, especially ulcerative colitis. It remains to be elucidated whether the pancreas inflammation drives the intestinal inflammation in these cases or whether the intestinal inflammation spreads to the pancreas.
The current standard treatment for AIP is corticosteroid therapy. The therapeutic approach to AIP has significantly evolved over time. In the early days, surgery was undertaken to confirm the nature of a pancreatic mass and/or drain an obstructed bile duct. ERCP was used for CBD stenting in children with obstructive jaundice. This approach has now mainly been replaced by a trial of corticosteroid therapy. The corticosteroid induction dose used in children (1–1.5 mg/kg/day) as captured in our study was higher than the recommended dose (0.6–1 mg/kg/day) in adults with AIP. Of the children, 92% clinically improved with corticosteroids. Interestingly, 17% of the AIP patients had disease resolution without any treatment. Although corticosteroid use seems beneficial in the short term in adults with AIP, it remains unclear whether it has an impact on the long-term outcome of patients (29). In the absence of any comparative or outcome studies in children, we believe that a time-limited corticosteroid treatment course to treat the acute symptoms of pancreatitis, which may also prevent long-term complications of pancreatic insufficiency, is justified. However, further studies are needed to determine whether the advantages of steroid therapy outbalance the potential side effects of this therapy, particularly in children. In this regard, it will be helpful to obtain control imaging about 3 months after starting corticosteroids to evaluate for normalization of the pancreatic imaging findings, confirming the diagnosis of AIP.
This study comprises the most comprehensive collection of new AIP cases in children based on a multicenter collaboration. INSPPIRE started enrolling children with acute recurrent pancreatitis and CP in 2012, gathering diagnostic and longitudinal follow-up data to elucidate the natural history of pancreatitis of different etiologies. In order to ensure a complete collection of AIP cases and to prevent center-specific biases in establishing a diagnosis of AIP, we not only searched the INSPPIRE database with respect to specific MRCP findings such as pancreatic masses and biliary strictures, but also additionally asked individual investigators for reevaluation of all patients with a diagnosis of AIP to confirm the diagnosis.
The main limitations of this study are the limited data on mid- and long-term outcome especially with respect to the exocrine and endocrine pancreas function, and also regarding the development of other immune/inflammatory conditions. For some patients, other causes of pancreatitis including genetic forms were not completely excluded. However, recent adult data have shown that the presence of mutations in pancreatitis-related genes and AIP are not mutually exclusive (30,31).
In conclusion, AIP is a unique form of pancreatitis. Pediatric AIP has a distinct presentation with features similar to type 2 AIP in adults. The diagnosis of pediatric AIP relies on the combination of clinical symptoms and distinct pancreatic parenchymal, pancreatic duct, and/or bile duct imaging abnormalities, possibly complemented with histopathological findings. The primary choice of therapy is corticosteroids with potential long-term sequelae that include exocrine pancreatic insufficiency and diabetes. We hope that our summary will lead to improved recognition of AIP in children, thus paving the way for future multicentric prospective studies.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Autoimmune pancreatitis (AIP) is a distinctive type of pancreatitis that can also occur in children.
-
✓
Disease-specific diagnostic criteria have been developed for AIP in adults, but not for children.
WHAT IS NEW HERE
-
✓
Pediatric AIP has a distinct presentation with features similar to type 2 AIP in adults.
-
✓
Abdominal pain is the most common symptom and serum IgG4 is rarely elevated in children with AIP.
-
✓
The diagnosis of pediatric AIP can be made based on typical clinical presentation and imaging findings.
Acknowledgments
Financial support: This work was supported by NIH DK096327 (to A.U.), DK108334 (to A.U.), UL1 TR000442 (CTSA), National Pancreas Foundation (to A.U.), and REDCap. I.S. is supported by the Restracomp Grant and a Fondation St-Luc Grant.
M.L. is a consultant for AbbVie and Nordmark Arzneimittel, is in the Board of Directors of the National Pancreas Association, and receives royalties from Millipore. T.G. received a research grant from Vertex Pharmaceuticals.
Footnotes
CONFLICT OF INTEREST
Potential competing interests: The other authors declare no conflict of interest.
Guarantor of the article: Tanja Gonska, MD.
Specific author contributions: I.S.: study design, acquisition of data, analysis and interpretation of data, statistical analysis, drafting of the manuscript. J.J.P., S.F., M.W., and U.S.: member of the AIP working group within INSPPIRE, acquisition of data, and critical revision of the manuscript for important intellectual content. M.A.-E.-H., B.B., D.S.F., C.G., M.J.G., M.B.H., R.W.H., S.Z.H., T.K.L., Q.L., M.L., M.M., V.M., C.Y.O., E.R.P., D.A.P., J.F.P., S.J.S., D.T., and S.W.: critical revision of the manuscript. A.U.: member of the AIP working group within INSPPIRE, acquisition of data, critical revision of the manuscript for important intellectual content, obtained funding, and study supervision. T.G.: member of the AIP working group within INSPPIRE, study concept and design, acquisition of data, drafting and critical revision of the manuscript for important intellectual content, and study supervision.
References
- 1.Sarles H, Sarles JC, Muratore R, et al. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688–98. doi: 10.1007/BF02232341. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida K, Toki F, Takeuchi T, et al. Chronic pancreatitis caused by an auto- immune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–8. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 3.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–8. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 4.Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology. 2015;149:39–51. doi: 10.1053/j.gastro.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 2013;62:1771–6. doi: 10.1136/gutjnl-2012-303617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ectors N, Maillet B, Aerts R, et al. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997;41:263–8. doi: 10.1136/gut.41.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Notohara K, Burgart LJ, Yadav D, et al. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27:1119–27. doi: 10.1097/00000478-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Zamboni G, Luttges J, Capelli P, et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552–63. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- 9.Fukumori K, Shakado S, Miyahara T, et al. Atypical manifestations of pancreatitis with autoimmune phenomenon in an adolescent female. Intern Med. 2005;44:886–91. doi: 10.2169/internalmedicine.44.886. [DOI] [PubMed] [Google Scholar]
- 10.Bartholomew SV, Zigman A, Sheppard B. Lymphoplasmacytic sclerosing pancreatitis presenting as a pancreatic head mass in a child: case report and management recommendations. J Pediatr Surg. 2006;41:e23–5. doi: 10.1016/j.jpedsurg.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 11.Blejter J, Weller S, Pace R, et al. Autoimmune pancreatitis: an adolescent case and review of literature. J Pediatr Surg. 2008;43:1368–72. doi: 10.1016/j.jpedsurg.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 12.Detlefsen S, Mohr Drewes A, Vyberg M, et al. Diagnosis of autoimmune pancreatitis by core needle biopsy: application of six microscopic criteria. Virchows Arch. 2009;454:531–9. doi: 10.1007/s00428-009-0747-5. [DOI] [PubMed] [Google Scholar]
- 13.Gargouri L, Ponsot P, Viala J, et al. Recurrent autoimmune pancreatitis in a 10-year-old boy. J Pediatr Gastroenterol Nutr. 2009;48:374–7. doi: 10.1097/mpg.0b013e3181826dca. [DOI] [PubMed] [Google Scholar]
- 14.Refaat R, Harth M, Proschek P, et al. Autoimmune pancreatitis in an 11-year-old boy. Pediatr Radiol. 2009;39:389–92. doi: 10.1007/s00247-008-1132-2. [DOI] [PubMed] [Google Scholar]
- 15.Takase M, Imai T, Nozaki F. Relapsing autoimmune pancreatitis in a 14-year-old girl. J Nippon Med Sch. 2010;77:29–34. doi: 10.1272/jnms.77.29. [DOI] [PubMed] [Google Scholar]
- 16.Mannion M, Cron RQ. Successful treatment of pediatric IgG4 related systemic disease with mycophenolate mofetil: case report and a review of the pediatric autoimmune pancreatitis literature. Pediatr Rheumatol Online J. 2011;9:1. doi: 10.1186/1546-0096-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedlander J, Quiros JA, Morgan T, et al. Diagnosis of autoimmune pancreatitis vs neoplasms in children with pancreatic mass and biliary obstruction. Clin Gastroenterol Hepatol. 2012;10:1051–5.e1. doi: 10.1016/j.cgh.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Fujii LL, Chari ST, El-Youssef M, et al. Pediatric pancreatic EUS-guided trucut biopsy for evaluation of autoimmune pancreatitis. Gastrointest Endosc. 2013;77:824–8. doi: 10.1016/j.gie.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Murata S, Yoden A, Aomatsu T, et al. Three cases of childhood-onset autoimmune pancreatitis. Nihon Shokakibyo Gakkai Zasshi. 2014;111:1632–9. [PubMed] [Google Scholar]
- 20.Zen Y, Grammatikopoulos T, Hadzic N. Autoimmune pancreatitis in children: insights into the diagnostic challenge. J Pediatr Gastroenterol Nutr. 2014;59:e42–5. doi: 10.1097/MPG.0b013e3182994559. [DOI] [PubMed] [Google Scholar]
- 21.Scheers I, Ergun M, Aouattah T, et al. Diagnostic and therapeutic roles of endoscopic ultrasound in pediatric pancreaticobiliary disorders. J Pediatr Gastroenterol Nutr. 2015;61:238–47. doi: 10.1097/MPG.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 22.Lurz E, Gonska T. Pancreatic head mass leading to transient obstructive jaundice and diabetes mellitus in an adolescent. Gastroenterology. 2015;149:e9–10. doi: 10.1053/j.gastro.2015.04.051. [DOI] [PubMed] [Google Scholar]
- 23.Bolia R, Chong SY, Coleman L, et al. Autoimmune pancreatitis and IgG4 related disease in three children. ACG Case Rep J. 2016;3:e115. doi: 10.14309/crj.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel Z, Patel S, Grendell J, et al. Type 2 autoimmune pancreatitis: case report of a 9-year-old female and a review of the literature. Clin J Gastroenterol. 2015;8:421–5. doi: 10.1007/s12328-015-0615-6. [DOI] [PubMed] [Google Scholar]
- 25.Okazaki K, Kawa S, Kamisawa T, et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis 2013 I. Concept and diagnosis of autoimmune pancreatitis. J Gastroenterol. 2014;49:567–88. doi: 10.1007/s00535-014-0942-2. [DOI] [PubMed] [Google Scholar]
- 26.Nakazawa T, Ohara H, Sano H, et al. Schematic classification of sclerosing cholangitis with autoimmune pancreatitis by cholangiography. Pancreas. 2006;32:229. doi: 10.1097/01.mpa.0000202941.85955.07. [DOI] [PubMed] [Google Scholar]
- 27.Bowlus CL, Olson KA, Gershwin ME. Evaluation of indeterminate biliary strictures. Nat Rev Gastroenterol Hepatol. 2016;13:28–37. doi: 10.1038/nrgastro.2015.182. [DOI] [PubMed] [Google Scholar]
- 28.Chavhan GB, Babyn PS, Manson D, et al. Pediatric MR cholangiopancreatography: principles, technique, and clinical applications. Radiographics. 2008;28:1951–62. doi: 10.1148/rg.287085031. [DOI] [PubMed] [Google Scholar]
- 29.Hirano K, Tada M, Isayama H, et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut. 2007;56:1719–24. doi: 10.1136/gut.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao F, Li YM, Hong GL, et al. PRSS1_p.Leu81Met mutation results in autoimmune pancreatitis. World J Gastroenterol. 2013;19:3332–8. doi: 10.3748/wjg.v19.i21.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang MC, Jan IS, Liang PC, et al. Human cationic trypsinogen but not serine peptidase inhibitor, Kazal type 1 variants increase the risk of type 1 autoimmune pancreatitis. J Gastroenterol Hepatol. 2014;29:2038–42. doi: 10.1111/jgh.12649. [DOI] [PubMed] [Google Scholar]
- 32.Kawa S, Okazaki K, Kamisawa T, et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 II. Extrapancreatic lesions, differential diagnosis. J Gastroenterol. 2014;49:765–84. doi: 10.1007/s00535-014-0944-0. [DOI] [PubMed] [Google Scholar]