Abstract
Background:
Rhinoplasty is 1 of the most common aesthetic and reconstructive plastic surgical procedures performed within the United States. Yet, data on functional reconstructive open and closed rhinoplasty procedures with or without spreader graft placement are not definitive as only a few studies have examined both validated measurable objective and subjective outcomes of spreader grafting during rhinoplasty. The aim of this study was to utilize previously validated measures to assess objective, functional outcomes in patients who underwent open and closed rhinoplasty with spreader grafting.
Methods:
We performed a retrospective review of consecutive rhinoplasty patients. Patients with internal nasal valve insufficiency who underwent an open and closed approach rhinoplasty between 2007 and 2016 were studied. The Cottle test and Nasal Obstruction Symptom Evaluation survey was used to assess nasal obstruction. Patient-reported symptoms were recorded. Acoustic rhinometry was performed pre- and postoperatively. Average minimal cross-sectional area of the nose was measured.
Results:
One hundred seventy-eight patients were reviewed over a period of 8 years. Thirty-eight patients were included in this study. Of those, 30 patients underwent closed rhinoplasty and 8 open rhinoplasty. Mean age was 36.9 ± 18.4 years. The average cross-sectional area in closed and open rhinoplasty patients increased significantly (P = 0.019). There was a functional improvement in all presented cases using the Nasal Obstruction Symptom Evaluation scale evaluation.
Conclusions:
Closed rhinoplasty with spreader grafting may play a significant role in the treatment of nasal valve collapse. A closed approach rhinoplasty including spreader grafting is a viable option in select cases with objective and validated functional improvement.
INTRODUCTION
According to the 2015 American Society of Plastic Surgeons statistics report, cosmetic rhinoplasty was 1 of the 5 top cosmetic procedures (217,979) performed in the United States.1 When combined with internal nasal valve reconstruction, nose-reshaping procedures aim not only to improve patients’ quality of life but also to enhance their appearance.2,3 The use of spreader grafts increases the internal nasal valve angle and maintains the straightened position of the cartilaginous septum.4 Nasal valve dysfunction is 1 of the most common causes of chronic adult nasal obstruction, which can be quite symptomatic prompting a large number of patients to seek the procedure solely for functional purposes.5–7 Nasal valve treatment accounts for approximately 13% of cases undergoing functional nasal surgery.6,8 Internal nasal valve incompetence (INVI) is frequently overlooked and incorrectly attributed to other anatomical or physiologic causes.9 On physical examination, primary anatomic variations to consider are nasal valve narrowing, septal deviation, middle turbinate concha bullosa, inferior turbinate hypertrophy, choanal atresia, pyriform aperture stenosis, posttraumatic adhesions, or previous nasal surgery.3,10,11 Some physiologic causes to consider include sino-nasal inflammatory diseases, neoplasms, or medical/hormonal changes.3,12
Galen (AD 130–201) described nasal anatomy and function nearly 2,000 years ago. However, the term “internal nasal valve” was coined in 1903 by Mink.13 Since then, the abundance of techniques for the correction of nasal valve dysfunction, including spreaders, alar batten grafts, lateral crural strut grafts, butterfly grafts, splay grafts, and H-grafts or auto-spreader flaps, has evolved.13–16 The reconstruction of INVI can be performed by either external (open), or endonasal (closed) approaches.17–19 The open approach is perhaps more commonly used due to its advantages of improved visualization and, when utilizing grafts, potentially more accurate fixation of the cartilage grafts.20,21 The main disadvantages of this technique include the relative invasiveness of the procedure and the possibility of compromising the integrity of the middle nasal vault when the upper lateral cartilages (ULCs) are divided from the septum if the ULCs are disarticulated from underneath the nasal bones; postoperative swelling following an open approach to the nose is an additional significant consideration.19 The endonasal approach, which provides recovery without scar formation, is a similar procedure in select cases where there is less deformity; however, it is limiting because it does not clearly expose the anatomical structures.19 However, both approaches provide good aesthetic and functional outcomes.6,22 Nonetheless, surgeons strive to achieve the most achievable satisfactory results while minimizing the risks of compromising the integrity of the nose.11 Few studies have examined both validated measurable objective and subjective outcomes of spreader grafting during rhinoplasty.
The aim of our study was to assess a consecutive series of patients undergoing open or closed rhinoplasty with spreader grafting and to assess pre- and postoperative objective and functional outcomes using previously validated measurable tools. The study objective also more broadly aimed further knowledge in defining the indications and aimed to provide further knowledge objective outcome differences between open and closed approaches for rhinoplasty.
PATIENTS AND METHODS
Patient Recruitment
We conducted a retrospective review of 178 patients who underwent open or closed rhinoplasty over a 8-year period (2008–2016) at our academic medical center. Institutional review board approval was secured before the study. Inclusion criteria entailed patients older than 18 years of age of any race or gender who presented with cosmetic concerns for changing the appearance of the nose or functional nasal obstruction for more than 1 year. Rhinoplasty without spreader grafting and childhood nasal trauma served as exclusion criteria.
Study patients had either unilateral or bilateral internal nasal valve dysfunction resulting in chronic nasal obstruction, relieved using the Cottle maneuver. Each patient had their nose inspected and palpated for nasal bone anatomy, the strength of upper and lower lateral cartilages, and tip support. Reversible mucosal edema was examined in all patients before and after application of topical 1% phenylephrine. All patients received at least a minimum 4-week follow-up. The senior author performed all the presented operations.
Clinical Outcome Assessment (Nasal Obstruction Symptom Evaluation Scale and Acoustic Rhinomanometry)
Demographic data, information on comorbidities, nasal trauma, or prior surgical interventions was obtained from electronic medical records. Postoperative complications such as epistaxis, septal perforation, or unfavorable aesthetic outcome were noted. Completion and follow-up of the study occurred 4 weeks to 1 year following surgical intervention and was based on each patient’s follow-up assessment. The functional and aesthetic outcome was then determined. Functional outcome was determined by patients’ satisfaction level and measured by the Nasal Obstruction Symptom Evaluation (NOSE) instrument survey (0, not a problem; 1, very mild problem; 2, moderate problem; 3, fairly bad problem; 4, severe problem).23 Aesthetic outcome was determined by the authors’ aesthetic module added to the NOSE survey (0, looks worse; 1, no change; 2, looks better).
An acoustic rhinometer (Eccovision, HOOD Laboratories, Pembroke, Mass.) was used to assess nasal patency and nasal valve area. This measurement was based on the detection of acoustic reflection of a sound signal in the nose by structures within the nasal cavity providing measurements of the cross-sectional area of the nasal cavity as a function of the distance into the nasal cavity from the nasal sill.24,25 Two experienced technicians performed all acoustic rhinometry measurements. A minimum of 3 measurements were obtained for each side of the studied patients’ nasal passageways preoperatively and postoperatively. The cross-sectional area value (cm2) was measured each for the left and right sides, and the mean value was obtained (Fig. 1) at the internal nasal valve area. Pre- and interim 4-week interval postoperative measurements at the internal nasal valve region were obtained.
Fig. 1.
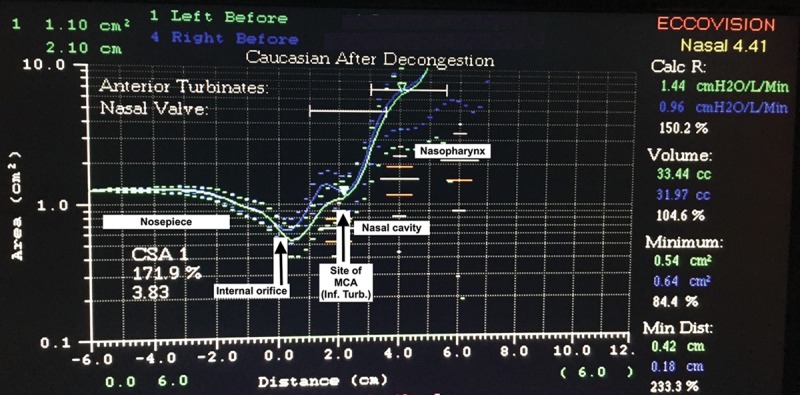
Example of acoustic rhinometry report. The x-axis represents distance from the nostril (at 0 cm), and the y-axis represents the nasal cross-sectional area (cm2). Inf. turb., inferior turbinate; MCA, minimal cross-sectional area.
Surgical Technique
Endonasal insertion of septal cartilage grafts between the ULCs and the nasal septum was performed similar to the original spreader grafting technique presented and popularized by Sheen26 in 1984 (Fig. 2). All the procedures were performed under general anesthesia. After infiltration with 1% lidocaine with 1:100,000 epinephrine, a modified Killian incision was designed on the left side, and a mucoperichondrial flap was then elevated in the standard fashion to perform the septoplasty and cartilage graft harvest. Unilateral or bilateral inferior turbinate reduction was performed and in-fracture and out-fracture were then performed using a Boies nasal elevator. Several millimeters caudal to the internal nasal valve on the right side, an intercartilaginous incision was then designed, and was localized with 1% lidocaine with 1:100,000 epinephrine. Spreader grafts were then fashioned on the back table into a rectangular shape with dimensions using harvested septal cartilage. An intercartilaginous incision was made, and dissecting scissors were used to dissect the ULC junction to the septum. The caudal aspect of the ULC was separated, and measured spreader grafts were inserted into the previously dissected unilateral or bilateral pockets within the internal nasal valve from the ULC to the septum. Open rhinoplasty approach consisted of an inverted “V” approach to the columella. Following vasoconstriction and marginal incisions, the nasal skin envelope was elevated. Dorsal reduction with a pull rasp and cartilaginous reduction was then performed. Following the elevation of the perichondrium of the ULC, the ULC was separated from the septum sequentially. Spreader graft cartilages were then placed in both pockets and secured with 5-0 Nylon sutures. The remainder of the rhinoplasty then proceeded. When necessary, additional procedure such as an osteotomy, dorsal hump resection, cartilage grafting, cartilage suture techniques, or dorsal augmentation, or alar batten graft was performed.
Fig. 2.
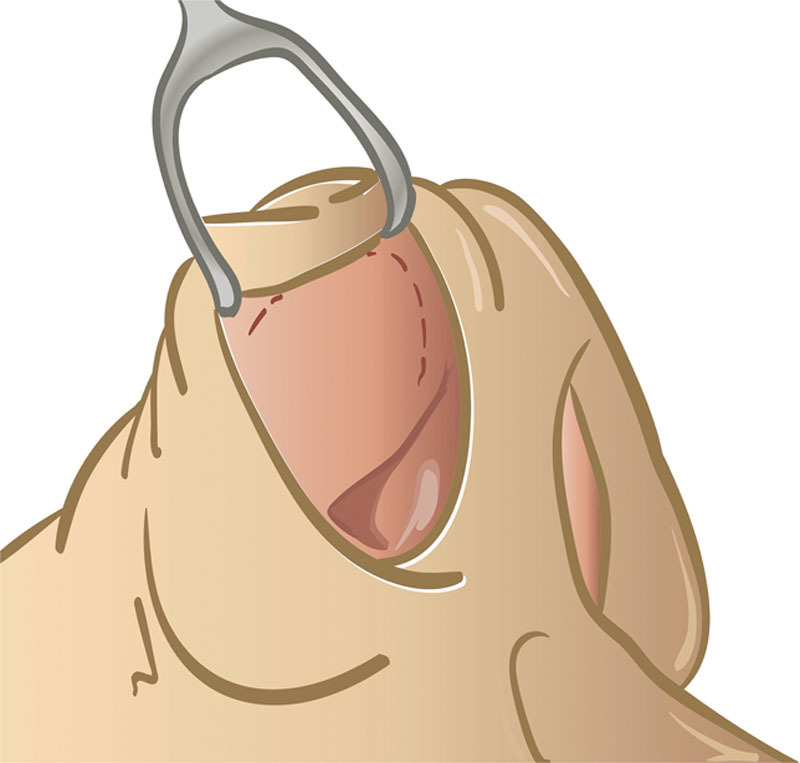
The intercartilaginous incision for spreader grafting technique through a closed approach.
Statistical Analysis
Descriptive statistics for patient characteristics are reported as a count or as a proportion of the overall patient cohort and subgroups of open or closed rhinoplasty with spreader grafting. Data analyses of the pre- and postoperative cross-sectional area measurements, as well as change in pre- and postoperative cross-sectional area measurements, were performed using Mann-Whitney U test with IBM SPSS version 22 (IBM Corp., Armonk, N.Y.). Significance was set at P < 0.05.
Ethical Approval
The patient information in this study is deidentified from Beth Israel Deaconess Medical Center patients’ medical records.
RESULTS
The overall characteristics are demonstrated in Table 1. A total of 38 consecutive patients met the inclusion criteria and were included in our study. Thirty patients underwent closed rhinoplasty with spreader grafting, and 8 patients underwent open rhinoplasty with spreader grafting to assess measurements in an open rhinoplasty patient population for comparison (Figs. 3–11). Spreader graft dimensions varied from 10–20 × 2–3 × 2–4 (mm) in the open rhinoplasty group and 10–15 × 2–3 × 2–3 (mm) in the closed rhinoplasty group. In the closed rhinoplasty cohort, 12 (40.0%) patients were female and 18 (60.0%) male. Mean age was 37.1 ± 12.5 (range, 22 – 65 years) years, and mean body mass index (BMI) was 24.8 ± 4.4 kg/m2. The majority of patients were Caucasian (n = 25, 83.4%), followed by African American (n = 2, 6.7%), Asian (n = 1, 3.3%), Hispanic (n = 1, 3.3%), and unknown ethnicity (n = 1, 3/3%). In this study cohort, a total of 24 patients (80.0%) underwent strictly functional nasal procedures, and 6 (20.0%) underwent functional reconstruction with a cosmetic component. And finally, 26 patients (86.7%) underwent primary septoplasty, and 4 patients (13.3%) underwent revision septoplasty. Patient comorbidity included asthma in 4 patients (13.3%), sleep apnea in 2 patients (6.6%), allergic rhinitis in 1 patient (3.3%), Ehlers-Danlos syndrome in 1 patient (3.3%), and diabetes in 1 patient (3.3%). Within the open rhinoplasty cohort, all patients were Caucasian females (100.0%), mean age was 36.9 ± 18.4 years, and mean BMI was 21.9 ± 1.2 kg/m2. A total of 5 patients (62.5%) underwent strictly functional nasal procedures, and 3 (37.5%) underwent functional reconstruction with a cosmetic component. And finally, 7 patients (87.5%) underwent primary septoplasty, and 1 patient (12.5%) underwent revision septoplasty. Patient comorbidity included chronic sinusitis in 3 patients (37.5%).
Table 1.
Overall Patient Characteristics

Fig. 3.
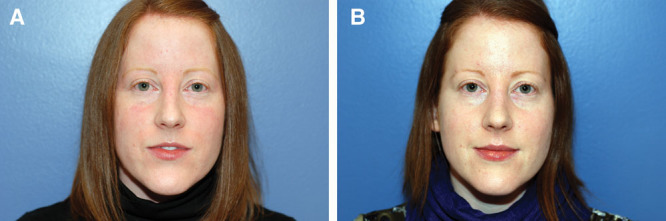
Preoperative (A) and postoperative (B) frontal images of a patient following closed approach for spreader grafting.
Fig. 11.

Pre- and postoperative nasal obstruction based on NOSE scale (0, not a problem; 1, very mild problem; 2, moderate problem; 3, fairly bad problem; 4, severe problem).
Fig. 4.

Preoperative (A) and postoperative (B) left 3/4 view images of a patient following closed approach for spreader grafting.
Fig. 5.

Preoperative (A) and postoperative (B) right 3/4 view images of a patient following closed approach for spreader grafting.
Fig. 6.

Preoperative (A) and postoperative (B) lateral images of a patient following closed approach for spreader grafting.
Fig. 7.

Preoperative (A) and postoperative (B) frontal images of a patient following open approach for spreader grafting.
Fig. 8.

Preoperative (A) and postoperative (B) left 3/4 view images of a patient following open approach for spreader grafting.
Fig. 9.

Preoperative (A) and postoperative (B) right 3/4 view images of a patient following open approach for spreader grafting.
Fig. 10.

Preoperative (A) and postoperative (B) lateral images of a patient following open approach for spreader grafting.
Primary cause of nasal obstruction was isolated trauma (n = 15, 39.4%), followed by congenital abnormality (n = 14, 36.6%), and previous surgery (n = 8, 21.0%) Autologous septal cartilage grafts were used in all cases. The closed cohort entailed 12 unilateral (8 left-sided and 4 right-sided) and 18 bilateral spreader graft insertions. Bilateral turbinate reduction was performed in 29 patients (96.7%) and unilateral in 1 patient (3.3%). Within the open cohort, there were 4 unilateral (2 left sided and 2 right sided) and 4 bilateral spreader graft insertions. Bilateral turbinate reduction was performed in all patients, indicated due to patient-reported congestion as part of a combination functional rhinoplasty.
Additional procedures were performed in 14 patients (10 closed and 4 open) (Table 2). In patients undergoing closed rhinoplasty, nasal valve reconstruction was combined with a dorsal hump reduction in 6 patients (20.0%), with supra-tip grafting in 1 patient (3.3%), polyp resection in 1 (3.3%) patient, lateral/medial osteotomies in 1 patient (3.3%), and septal perforation reconstruction in 1 patient (3.3%). Postoperative complication included epistaxis in 1 patient (3.3%) with a history of coagulopathy. This was resolved by surgical hematoma evacuation. In patients undergoing open rhinoplasty, nasal valve reconstruction was combined with a dorsal hump reduction in 1 patient (12.5%), with supra-tip grafting in 1 patient (12.5%), and alar batten grafts in 2 patients (25.0%). In distinction, there were no postoperative complications in this patient cohort.
Table 2.
Additional Procedures Performed Simultaneously with Spreader Grafting

Acoustic rhinometer measurements were completed consecutively to confirm an anatomic cause for decreased nasal resistance. The average nasal valve distance was set at 2.1 cm based on a normalized rhinometer plot for the internal nasal valve. The overall average cross-sectional area for the sides that underwent spreader grafting significantly increased from 0.63 ± 0.29 cm2 to 1.01 ± 0.78 cm2 (0.38 ± 0.78; P < 0.018). Separating patients into subgroups of open versus closed rhinoplasty with spreader grafting revealed a significant increase in cross-sectional area in the open group 0.58 ± 0.31 to 1.15 ± 0.95 (0.57 ± 0.81; P < 0.019). There was also an increase in cross-sectional area in the closed group but not statistically significant [0.68 ± 0.26 to 0.87 ± 0.56 (0.20 ± 0.65; P < 0.60)]. There was a statistically significant difference in the increase in cross-sectional area for open versus closed rhinoplasty with spreader grafting (0.57 ± 0.81 to 0.20 ± 0.65; P < 0.011; Table 3). No patients in this series required revisional surgery in this period of follow-up.
Table 3.
Cross-sectional Area Open Versus Closed

Seventeen NOSE surveys were returned after a 12-month period representing a 57% response rate (Table 4). All surveys were filled in by patients undergoing closed rhinoplasty. A comparison of pre- and postoperative nasal obstruction based on each patient’s subjective survey assessment showed significant improvement in airway passage in all cases (Fig. 3). Regarding the aesthetic aspect of the procedure, 1 patient (5.9%) reported a worsening of nasal shape, 15 (88.0%) patients reported no change, and 1 (5.9%) patient reported an improvement in the appearance of the nose. Average follow-up time was 3 years. P value was < 0.0001 between all groups.
Table 4.
Preoperative and Postoperative Comparison (After 12 Months) of NOSE Scores between Patients*

DISCUSSION
The nose functions as a physiologic airway resistor, accounting for approximately 50% of total airway resistance.27–29 Disruption of nasal aerodynamics is determined by alterations in the shape and function of the nasal cavities. INVI can be of static or dynamic origin.28,30 Numerous studies have reported that the incidence of airway impairment following aesthetic rhinoplasty ranges from 10% to 54%.8,31–33 Internal nasal valve insufficiency is often overlooked as a primary cause of obstruction. It can present as a congenital abnormality or occur following iatrogenic collapse of the nasal valve.28,34,35 In our study, 36.8% of the patient population suffered from a congenital abnormality of the internal nasal valve. One factor that should be carefully assessed is childhood nasal trauma, which could mistakenly be grouped into the congenital abnormality population. Prior nasal trauma accounted for 42.1% in our total patient population. Another important factor is the presence of bony or cartilaginous septum influencing airway obstruction, such as in the case of septal deviation or bone spurs. Correction of these problems can help alleviate nasal obstructive symptoms and play an important role in functional rhinoplasty.
In 1984, Sheen26 presented a series of 3 patients, describing a new technique for endonasal spreader grafting. He stated that a significant group of primary or secondary rhinoplasty cases required middle nasal vault reconstruction. Accordingly, to increase the angle at the internal valve and recreate the dorsal roof, spreader grafting provides an ideal approach. Sheen implemented and developed Cottle’s and Skoog’s idea of combining a functional and aesthetic rhinoplasty approach.36,37
Despite the development of new surgical techniques over the years, spreader grafting remains the cornerstone for internal nasal valve reconstruction.4 The external (open) rhinoplasty approach has gained in popularity over the last several decades, especially as a form of secondary rhinoplasty.17,38 Lee et al.20 reported that 72% of surgeons use the open approach for primary rhinoplasty, whereas the remaining 28% implement a closed approach. For secondary rhinoplasty, 76% of surveyed practitioners reported using the open approach. Nevertheless, the open approach is still more commonly used in aesthetic surgery due to improved exposure and ease and precision in graft placement.4,17,38 Moreover, other advantages such as preservation of mucosal vascular bridges can be achieved with the open approach. The final operative technique depends mostly on the individual surgeon’s preference, patient preference, and surgical plan.3,10 Despite this, the closed approach with spreader grafting has reported advantages: no visible scars, precise, tailor-made spreader graft pockets, preservation of mucosal vascular bridges, less swelling, and shorter operation time.39,40 Some of its limitations include poor visualization, complex dissection, inability to be used in patients with smaller nasal anatomy, in patients with inverted V deformity or after prior-performed open rhinoplasty, and when an external scar exists.40 However, for the experienced rhinoplasty surgeon, a closed approach may still be feasible in these challenging circumstances as well.
Although spreader grafting may be useful in alleviating nasal obstructive symptoms by improving mid vault collapse, authors have noted negative impacts on the aesthetic outcome, such as a wider dorsum with less defined dorsal aesthetic lines. It may be interesting to evaluate whether there is a certain balance point for aesthetic outcome and functional outcomes. Four unilateral and 4 bilateral procedures were performed requiring turbinate reduction with in/out fracturing. Yoo and Jen41 presented a similar approach, although the authors performed the turbinate surgery only in 23 (56%) of 41 consecutive patients. In the current study, spreader grafting in conjunction with turbinate surgery was performed in all patients. Indeed, it is notable that for this functional study multiple maneuvers were performed for functional nasal improvement, and, in this study population, it would be a challenge to separate out individual procedures during the treatment plan for study purposes; however, acoustic rhinometry was focused in studying primarily the internal nasal valve area specifically. In 7 patients (18.4%), dorsal hump reduction was performed to achieve aesthetic goals.
One of our main concerns was pre- and postoperative evaluation of nasal congestion. To date, there is no agreement on which technique is the most reliable.42–44 Objective evaluations of spreader graft placement are challenging because current measurement methods fail to correlate with patient symptom scores.45 As described by Pawar et al.43 patient satisfaction continues to remain 1 of the most important outcome measurements. To make a reliable assessment, in our study, we implemented both a previously validated patient self-evaluation module (NOSE score) and objective acoustic rhinometry. According to de Pochat et al.46 there was a association in acoustic rhinometry improvement with subjective self-reported assessment of nasal patency.
Consistent with previous published data, a majority of patients expressed satisfaction with the acquired functional improvement; only 1 patient was dissatisfied with the aesthetic aspect after surgery.4,47–50
In this study, besides author’s aesthetic module in NOSE survey, we utilized the acoustic rhinometer to provide objective evidence of the utility of endonasal spreader graft when performed in conjunction with nasal septoplasty and inferior turbinoplasty. In agreement with previous published data, we found an increased nasal valve area (0.38 ± 0.78; P < 0.018) when comparing preoperative and postoperative acoustic rhinometry measurements.51 In our study, we observed the objective results of acoustic rhinometry and obtained patient satisfaction through oral patient feedback. According to de Pochat et al.,46 there was an association in acoustic rhinometry improvement with subjective self-reported assessment of nasal patency. Consistent with previous published data, a majority of patients expressed satisfaction with the acquired functional improvement; no patients were dissatisfied with the aesthetic aspect after surgery.4,47–50
Though a prospective study with objective data and functional outcomes measures, this single-center study is limited by its small sample size. Furthermore, it is difficult to isolate the effects of spreader grafting with respect to concomitantly performed turbinectomy and septoplasty procedures; however, with acoustic rhinometry, the authors have attempted 1 method of data collection specific to the internal nasal valve. Acoustic rhinometry was studied at the internal valve area specifically, as the most narrow portion of the nasal airway. However, this study may add an additional perspective to the growing body of knowledge of open and closed approach spreader grafting rhinoplasty and potentially help to define indications for open versus closed approaches for rhinoplasty. Additionally, like any surgical procedure, closed rhinoplasty success is based on proper patient selection and surgeon experience. Although the primary advantages of the closed approach are no visible scars and shorter recovery time, it should be noted that closed approach rhinoplasty remains as 1 of the more challenging aesthetic surgery procedures. By the same token, open rhinoplasty can offer more exposure of the surgical field and can have better functional results objectively, as seen in the significant difference in cross-sectional area change postoperatively; although cross-sectional area increased in both open and closed cases and all patients reported relief of nasal obstruction, the increase in cross-sectional area was significantly greater in the open cohort.
CONCLUSIONS
Rhinoplasty is regarded as a 1 of the most difficult aesthetic and functional surgeries with both open and closed approaches utilized by expert rhinoplasty surgeons. Despite this, spreader grafting plays an invaluable role in the treatment and even prevention of nasal valve collapse as it widens and supports the nasal valve area. In this study, the authors have described objective and subjective data to help distinguish differences between open and closed rhinoplasty utilizing spreader grafts. Open rhinoplasty approaches offer open access to the midvault area; however, current closed rhinoplasty with spreader grafting may play a significant role in the treatment of nasal valve collapse with similar results without having a cutaneous incision. Closed approach rhinoplasty including spreader grafting is a viable option in select cases with objective and validated functional improvement.
Footnotes
Disclaimer: The Beth Israel Deaconess Medical Center patients’ medical records are the source of information used in this study. Data extrapolated, statistical analysis performed, and conclusions reached are the result of the work done by authors of this study.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.American Society of Plastic Surgeons (ASPS) statistics report 2015. Available at http://www.plasticsurgery.org/news/2016/new-statistics-reflect-the-changing-face-of-plastic-surgery.html. Accessed March 1, 2016.
- 2.Most SP. Analysis of outcomes after functional rhinoplasty using a disease-specific quality-of-life instrument. Arch Facial Plast Surg. 2006;8:306–309.. [DOI] [PubMed] [Google Scholar]
- 3.Rohrich RJ, Ahmad J. A practical approach to rhinoplasty. Plast Reconstr Surg. 2016;137:725e–746e.. [DOI] [PubMed] [Google Scholar]
- 4.Rohrich RJ, Hollier LH. Use of spreader grafts in the external approach to rhinoplasty. Clin Plast Surg. 1996;23:255–262.. [PubMed] [Google Scholar]
- 5.Ballert JA, Park SS. Functional considerations in revision rhinoplasty. Facial Plast Surg. 2008;24:348–357.. [DOI] [PubMed] [Google Scholar]
- 6.Schlosser RJ, Park SS. Surgery for the dysfunctional nasal valve. Cadaveric analysis and clinical outcomes. Arch Facial Plast Surg. 1999;1:105–110.. [DOI] [PubMed] [Google Scholar]
- 7.Angelos PC, Been MJ, Toriumi DM. Contemporary review of rhinoplasty. Arch Facial Plast Surg. 2012;14:238–247.. [DOI] [PubMed] [Google Scholar]
- 8.Elwany S, Thabet H. Obstruction of the nasal valve. J Laryngol Otol. 1996;110:221–224.. [DOI] [PubMed] [Google Scholar]
- 9.Bewick JC, Buchanan MA, Frosh AC. Internal nasal valve incompetence is effectively treated using batten graft functional rhinoplasty. Int J Otolaryngol. 2013;2013:734795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohrich RJ, Ahmad J. Rhinoplasty. Plast Reconstr Surg. 2011;128:49e–73e.. [DOI] [PubMed] [Google Scholar]
- 11.Sajjadian A, Guyuron B. Primary rhinoplasty. Aesthet Surg J. 2010;30:527–539.; quiz 540. [DOI] [PubMed] [Google Scholar]
- 12.Becker DG, Becker SS. Treatment of nasal obstruction from nasal valve collapse with alar batten grafts. J Long Term Eff Med Implants. 2003;13:259–269.. [DOI] [PubMed] [Google Scholar]
- 13.Mink PJ. Le nez comme voie respiratorie. Presse Otolaryngol, Belgium 1903;21:481–496.. [Google Scholar]
- 14.Kim DW, Rodriguez-Bruno K. Functional rhinoplasty. Facial Plast Surg Clin North Am. 2009;17:115–131, vii.. [DOI] [PubMed] [Google Scholar]
- 15.Seyhan A. Method for middle vault reconstruction in primary rhinoplasty: upper lateral cartilage bending. Plast Reconstr Surg. 1997;100:1941–1943.. [DOI] [PubMed] [Google Scholar]
- 16.Tastan E, Demirci M, Aydin E, et al. A novel method for internal nasal valve reconstruction: H-graft technique. Laryngoscope. 2011;121:480–486.. [DOI] [PubMed] [Google Scholar]
- 17.Gunter JP, Rohrich RJ. External approach for secondary rhinoplasty. Plast Reconstr Surg. 1987;80:161–174.. [DOI] [PubMed] [Google Scholar]
- 18.Gunter JP. The merits of the open approach in rhinoplasty. Plast Reconstr Surg. 1997;99:863–867.. [DOI] [PubMed] [Google Scholar]
- 19.Constantian MB. Differing characteristics in 100 consecutive secondary rhinoplasty patients following closed versus open surgical approaches. Plast Reconstr Surg. 2002;109:2097–2111.. [DOI] [PubMed] [Google Scholar]
- 20.Lee M, Unger JG, Gryskiewicz J, et al. Current clinical practices of the Rhinoplasty Society members. Ann Plast Surg. 2013;71:453–455.. [DOI] [PubMed] [Google Scholar]
- 21.Ponsky D, Eshraghi Y, Guyuron B. The frequency of surgical maneuvers during open rhinoplasty. Plast Reconstr Surg. 2010;126:240–244.. [DOI] [PubMed] [Google Scholar]
- 22.Bloching MB. Disorders of the nasal valve area. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2007;6:Doc07. [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart MG, Witsell DL, Smith TL, et al. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130:157–163.. [DOI] [PubMed] [Google Scholar]
- 24.Erickson B, Hurowitz R, Jeffery C, et al. Acoustic rhinometry and video endoscopic scoring to evaluate postoperative outcomes in endonasal spreader graft surgery with septoplasty and turbinoplasty for nasal valve collapse. J Otolaryngol Head Neck Surg. 2016;45:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acoustic rhinometry. Available at http://rhinometer.com/. Accessed February 1, 2016.
- 26.Sheen JH. Spreader graft: a method of reconstructing the roof of the middle nasal vault following rhinoplasty. Plast Reconstr Surg. 1984;73:230–239.. [PubMed] [Google Scholar]
- 27.Sulsenti G, Palma P. Tailored nasal surgery for normalization of nasal resistance. Facial Plast Surg. 1996;12:333–345.. [DOI] [PubMed] [Google Scholar]
- 28.Howard BK, Rohrich RJ. Understanding the nasal airway: principles and practice. Plast Reconstr Surg. 2002;109:1128–1146.; quiz 1145. [DOI] [PubMed] [Google Scholar]
- 29.Guyuron B. Soft tissue functional anatomy of the nose. Aesthet Surg J. 2006;26:733–735.. [DOI] [PubMed] [Google Scholar]
- 30.Sciuto S, Bernardeschi D. Upper lateral cartilage suspension over dorsal grafts: a treatment for internal nasal valve dynamic incompetence. Facial Plast Surg. 1999;15:309–316.. [DOI] [PubMed] [Google Scholar]
- 31.Beekhuis GJ. Nasal obstruction after rhinoplasty: etiology, and techniques for correction. Laryngoscope. 1976;86:540–548.. [DOI] [PubMed] [Google Scholar]
- 32.Pontell J, Slavit DH, Kern EB. The role of outfracture in correcting post-rhinoplasty nasal obstruction. Ear Nose Throat J. 1998;77:106–108, 111.. [PubMed] [Google Scholar]
- 33.Grymer LF. Reduction rhinoplasty and nasal patency: change in the cross-sectional area of the nose evaluated by acoustic rhinometry. Laryngoscope. 1995;105:429–431.. [DOI] [PubMed] [Google Scholar]
- 34.Araco A, Gravante G, Gentile P, et al. Iatrogenic collapse of the nasal valve after aesthetic rhinoplasty. Scand J Plast Reconstr Surg Hand Surg. 2007;41:293–296.. [DOI] [PubMed] [Google Scholar]
- 35.Khosh MM, Jen A, Honrado C, et al. Nasal valve reconstruction: experience in 53 consecutive patients. Arch Facial Plast Surg. 2004;6:167–171.. [DOI] [PubMed] [Google Scholar]
- 36.Cottle MH. Nasal roof repair and hump removal. AMA Arch Otolaryngol. 1954;60:408–414.. [DOI] [PubMed] [Google Scholar]
- 37.Skoog T. A method of hump reduction in rhinoplasty. A technique for preservation of the nasal roof. Arch Otolaryngol. 1966;83:283–287.. [DOI] [PubMed] [Google Scholar]
- 38.Rohrich RJ, Lee MR. External approach for secondary rhinoplasty: advances over the past 25 years. Plast Reconstr Surg. 2013;131:404–416.. [DOI] [PubMed] [Google Scholar]
- 39.Schiffman MA, Giuseppe AD. Advanced Aesthetic Rhinoplasty: Art, Science, and New Clinical Techniques. 2013Springer Science & Business Media. [Google Scholar]
- 40.Hettiaratchy S, Griffiths M, Ali F, et al. Plastic Surgery: A Problem Based Approach. 2011Springer Science & Business Media. [Google Scholar]
- 41.Yoo DB, Jen A. Endonasal placement of spreader grafts: experience in 41 consecutive patients. Arch Facial Plast Surg. 2012;14:318–322.. [DOI] [PubMed] [Google Scholar]
- 42.Teymoortash A, Fasunla JA, Sazgar AA. The value of spreader grafts in rhinoplasty: a critical review. Eur Arch Otorhinolaryngol. 2012;269:1411–1416.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pawar SS, Garcia GJ, Kimbell JS, et al. Objective measures in aesthetic and functional nasal surgery: perspectives on nasal form and function. Facial Plast Surg. 2010;26:320–327.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilberg O, Jackson AC, Swift DL, et al. Acoustic rhinometry: evaluation of nasal cavity geometry by acoustic reflection. J Appl Physiol (1985). 1989;66:295–303.. [DOI] [PubMed] [Google Scholar]
- 45.Beck DO, Kenkel JM. Evidence-based medicine: rhinoplasty. Plast Reconstr Surg. 2014;134:1356–1371.. [DOI] [PubMed] [Google Scholar]
- 46.de Pochat VD, Alonso N, Mendes RR, et al. Nasal patency after open rhinoplasty with spreader grafts. J Plast Reconstr Aesthet Surg. 2012;65:732–738.. [DOI] [PubMed] [Google Scholar]
- 47.Ingels KJ, Orhan KS, van Heerbeek N. The effect of spreader grafts on nasal dorsal width in patients with nasal valve insufficiency. Arch Facial Plast Surg. 2008;10:354–356.. [DOI] [PubMed] [Google Scholar]
- 48.Yeung A, Hassouneh B, Kim DW. Outcome of nasal valve obstruction after functional and aesthetic-functional rhinoplasty. JAMA Facial Plast Surg. 2016;18:128–134.. [DOI] [PubMed] [Google Scholar]
- 49.André RF, Paun SH, Vuyk HD. Endonasal spreader graft placement as treatment for internal nasal valve insufficiency: no need to divide the upper lateral cartilages from the septum. Arch Facial Plast Surg. 2004;6:36–40.. [DOI] [PubMed] [Google Scholar]
- 50.Guyuron B, Bokhari F. Patient satisfaction following rhinoplasty. Aesthetic Plast Surg. 1996;20:153–157.. [DOI] [PubMed] [Google Scholar]
- 51.Huang C, Manarey CR, Anand VK. Endoscopic placement of spreader grafts in the nasal valve. Otolaryngol Head Neck Surg. 2006;134:1001–1005.. [DOI] [PubMed] [Google Scholar]


