Abstract
Biofouling refers to the unfavourable attachment and accumulation of marine sessile organisms (e.g. barnacles, mussels and tubeworms) on the solid surfaces immerged in ocean. The enormous economic loss caused by biofouling in combination with the severe environmental impacts induced by the current antifouling approaches entails the development of novel antifouling strategies with least environmental impact. Inspired by the superior antifouling performance of the leaves of mangrove tree Sonneratia apetala, here we propose to combat biofouling by using a surface with microscopic ridge-like morphology. Settlement tests with tubeworm larvae on polymeric replicas of S. apetala leaves confirm that the microscopic ridge-like surface morphology can effectively prevent biofouling. A contact mechanics-based model is then established to quantify the dependence of tubeworm settlement on the structural features of the microscopic ridge-like morphology, giving rise to theoretical guidelines to optimize the morphology for better antifouling performance. Under the direction of the obtained guidelines, a synthetic surface with microscopic ridge-like morphology is developed, exhibiting antifouling performance comparable to that of the S. apetala replica. Our results not only reveal the underlying mechanism accounting for the superior antifouling property of the S. apetala leaves, but also provide applicable guidance for the development of synthetic antifouling surfaces.
Keywords: surface morphology, antifouling, textured surface, surface topography, bio-adhesion
1. Introduction
Marine biofouling refers to the accumulation of biomolecules and organisms on surfaces of submerged structures in ocean [1–4]. It not only affects the appearance of the structures but also causes a range of substantial impairments to marine industry, including increasing frictional drag of ships [2], smothering oceanographic equipment [5] and accelerating structural deterioration [6]. Traditional approaches to tackling marine biofouling mainly applied paints incorporated with inorganic and organometallic biocides (e.g. copper compounds and tributyltin (TBT) compounds) [7]. However, many biocides have severe negative impacts on ecology and environment. For example, TBT compounds were found to cause defective growth of mollusc shells and debilitation of immunological defence in fishes [8,9]; therefore the use of TBT in antifouling paints has been banned since the early stage of the twenty-first century. As to the copper-based paints, even though their toxicity was claimed less than that of the TBT-based ones, there are still doubts concerning the effects of high copper concentration on certain marine organisms [10]. To lower the impact of antifouling paints on environment, organic biocides were adopted whose toxicity is still under scrutiny [11]. Developing antifouling strategies with least environmental impact is still required.
In nature, many animals and plants have evolved surfaces with excellent antifouling competence. Inspired by these natural antifouling surfaces [12,13], researchers have devoted themselves to the development of biomimetic chemical antifoulants such as enzymes [14] and metabolites isolated from marine microorganisms[15]. However, there are still challenges for the application of these biomimetic antifoulants including high cost, short-term efficacy and specificity. In addition to chemistry, structural traits of materials such as surface morphology were also found to play an important role in preventing biofouling [16–20]. For instance, surfaces with micropattern mimicking sharkskin were found effective in prohibiting the settlement of zoospores and cyprids [20]. A polymer coating with surface topography mimicking that of the skin of pilot whale Globicephala melas was found capable of reducing the settlement strength of Ulva zoospores [21]. Apart from animals, plants can also serve as paradigms for developing biomimetic antifouling surfaces. For instance, a replica of Trifolium leaf was proven to inhibit settlement of microalgae and facilitate cell release [22]. Recently, mangrove tree Sonneratia apetala (figure 1a) has attracted much attention for its unique leaves. As an intertidal plant, S. apetala is subject to marine fouling. Interestingly, the leaves of S. apetala, compared to its twigs and barks, are almost immune to biofouling. Proposed mechanisms accounting for such excellent antifouling property include low surface wettability, antifoulant of oleanolic acid and post-settlement detachment [23]. However, these may not be the most dominant mechanism because leaves of other mangrove species also share these mechanisms but exhibit much worse antifouling performance [23]. Here, we propose surface morphology as the dominant mechanism for the extraordinary antifouling competence of S. apetala leaves. To verify this hypothesis, we firstly prepare a polymeric replica that duplicates the surface morphology of a S. apetala leaf [24–26]. The antifouling performance of the replica is verified by attachment test with tubeworm larvae [27,28]. To gain deeper insights into the effects of surface morphology on antifouling performance, theoretical modelling is carried out, giving rise to guidelines for achieving better antifouling performance through morphology optimization. Finally, a biomimetic surface with microscopic ridge-like morphology is synthesized, showing comparable antifouling performance to that of the S. apetala leaves.
Figure 1.
The antifouling leaves of S. apetala and tubeworm H. elegans. (a) Sonneratia apetala living in intertidal zone. (b) A leaf of S. apetala. (c) Adult tubeworms H. elegans accumulated on a plastic bucket. (d) A larva of H. elegans with calcareous shell.
2. Polydimethylsiloxane replica of Sonneratia apetala leaves
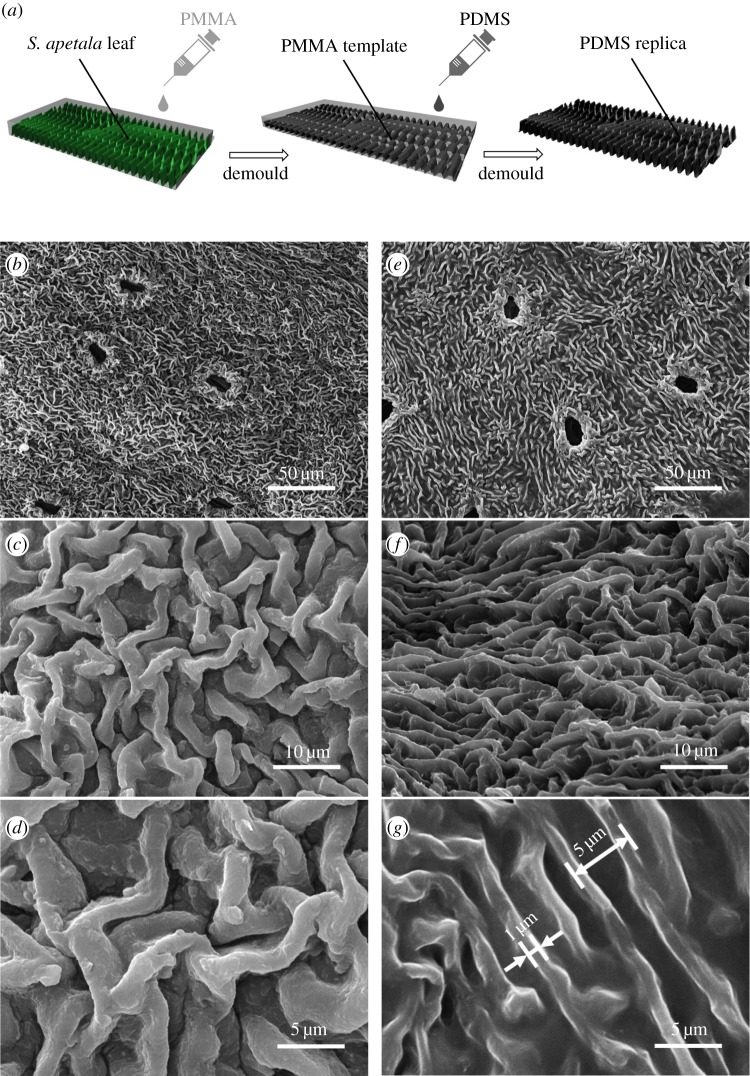
To investigate the effect of surface morphology on antifouling performance and meanwhile mask other possible factors such as bioactive compounds, polydimethylsiloxane (PDMS) replicas of S. apetala leaves are prepared by a moulding process as illustrated in figure 2a (see Material and methodologies for detailed description). Figure 2b–g shows the surface morphology of a PDMS replica in comparison with that of the S. apetala leaf used for duplication. Clearly, the PDMS replica faithfully duplicates the microscopic ridge-like morphology of the S. apetala leaf, of which the height and thickness of the ridges are around 5 µm and 1 µm while the inter-ridge spacing is around 5 µm. Moreover, PDMS blocks with similar size but flat surfaces are also prepared as the control specimens for antifouling performance test.
Figure 2.
(a) Schematic of the moulding process for preparing PDMS replica of S. apetala leaves. (b–d) Scanning electron microscopy images of a leaf surface of S. apetala compared to (e–g) those of its replica.
To examine the antifouling performance of the PDMS replicas of the S. apetala leaves, settlement tests of tubeworms are carried out with flat PDMS samples and glass slides used as the controls (see Material and methodologies for details). Figure 3 shows the number of settled tubeworms on different surfaces after 24 h and 48 h immersion. It can be seen that little change happens in the number of settled tubeworms during the period from 24 h to 48 h, implying that the settlement of tubeworms mainly takes place within the first 24 h after immersion. This is most probably because the tubeworms that are unable to attach on the solid surfaces within 24 h will not survive for long. The number of tubeworms settled on the PDMS replicas after 24 h immersion is less than 5% of those on the flat PDMS surfaces and glass slides. Moreover, no significant difference in the numbers of attached tubeworms is observed between the flat PDMS surfaces and glass slides, implying that surface morphology rather than material chemistry plays the dominant role in determining the settlement of tubeworms. This finding evokes an earlier similar study on the attachment of various fouling species on surfaces with microgrooves of different sizes [29]. It was concluded that the settlement of tubeworms on a textured surface is sensitive to the characteristic length scale of the texture. For a given type of texture, there exists an optimal characteristic length that can prohibit the settlement of tubeworms to the best extent. For the microscopic ridge-like surface morphology of S. apetala leaves, does the settlement of the tubeworms depend on its characteristic sizes? Are there any optimal characteristic lengths leading to better antifouling performance? To answer these questions, theoretical analysis is carried out to investigate the adhesion between a tubeworm and a surface with ridge-like morphology.
Figure 3.
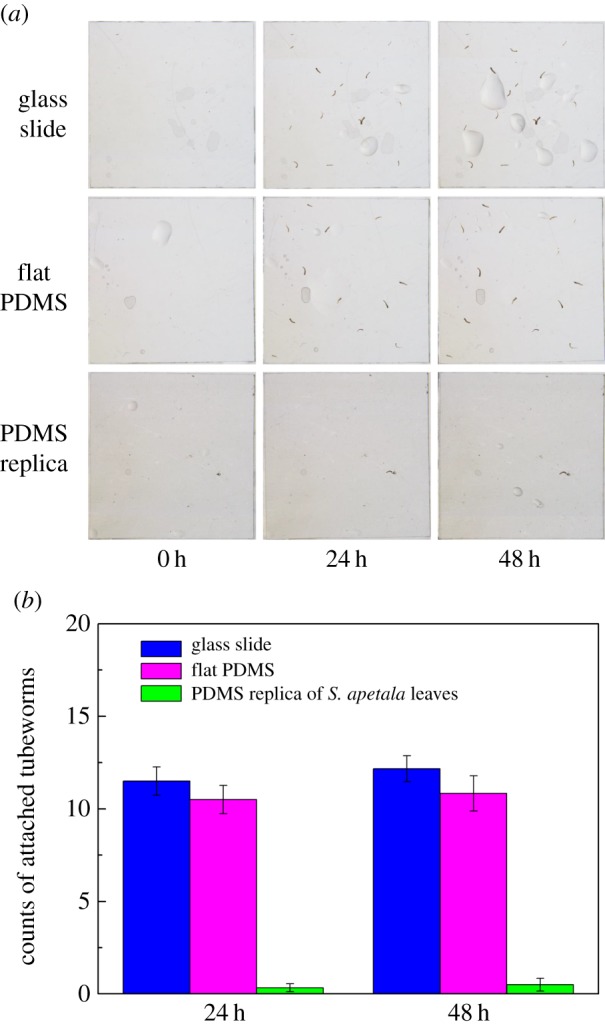
Antifouling performance of the PDMS replica of a S. apetala leaf. (a) Attachment of tubeworm larvae on glass slide, flat PDMS surface and PDMS replica of a S. apetala leaf after 0 h, 24 h and 48 h immersion. (b) Means ± s.e. (n = 6) of total counts of tubeworms attached on various surfaces after 24 h and 48 h.
3. Theoretical modelling
The effect of surface morphology on biofouling has long been recognized and studied [29–33]. One of the earliest theoretical attempts might be the attachment point theory, which indicated that textures with optimal characteristic sizes could prohibit the settlement of foulers. However, as an empirical description of the attachment of foulers on textured surfaces, the attachment point theory lacks physical basis, much less a quantitative estimation and prediction competence. To shed light on the size dependence of the antifouling performance of the ridge-like morphology, a quantitative model with physical basis, rigorous formulation and prediction competence is required.
For a tubeworm larva, we assume that the success rate of attachment on a textured surface depends on the maximum adhesion force that can be achieved between them. To quantify the adhesive force between a tubeworm and a textured surface shown in figure 2b, a mechanics model is established. Given the cylindrical shape of tubeworms and the microscopic ridge-like surface morphology of the S. apetala leaves, here we neglect the longitudinal dimension of the microscopic ridges and the disorder of their distribution and consider an adhesive contact problem between a two-dimensional (plane strain) cylinder and a substrate with wavy profile (figure 4a). Even though the fouling attachment involves complex chemical and biological processes, this simplified model is believed capable of capturing the mechanical essentials of fouling attachment.
Figure 4.
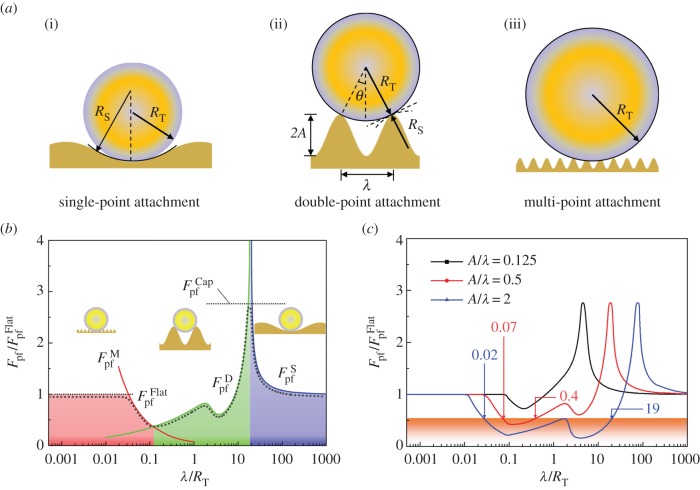
(a) Schematic of three possible configurations of an elastic cylinder in adhesive contact with a wavy substrate. (b) Variation of the normalized pull-off force with λ/RT for A/λ = 0.5. (c) Effect of A/λ on pull-off force.
Prior to solving the posed problem, it is worthwhile to introduce a useful result concerning the adhesion between an elastic cylinder and a flat substrate. Earlier studies indicated that the pull-off force between a cylinder (plane strain) and a flat substrate, which refers to the force required to separate them, is given by [34]
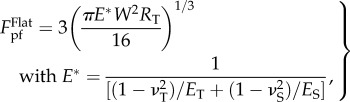 |
3.1 |
where ET, ES, νT, νS denote the elastic moduli and Poisson's ratios of the cylinder and the substrate, respectively, RT stands for the cross-sectional radius of the cylinder, and W represents the adhesion energy between the cylinder and substrate.
For a substrate with wavy profile, we assume that the profile is periodic and can be described by a trigonometric function 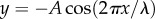 , where λ and A denote the wavelength and amplitude, two characteristic length scales of the profile, respectively. For simplicity, we assume A = 0.5λ for the moment. Cases with other amplitudes will be discussed later. Consider a tubeworm, which is modelled as a cylinder, in contact with such a wavy substrate. Three types of stable configurations may occur, depending on the relative sizes between the tubeworm and the wavy profile. If the wavelength of the profile λ is much larger than the tubeworm's radius RT, a stable configuration for the tubeworm is to rest on the trough of the groove, as shown in figure 4a(i). This configuration is called single-point attachment. Under this circumstance, the pull-off force is given by (see the electronic supplementary material for details)
, where λ and A denote the wavelength and amplitude, two characteristic length scales of the profile, respectively. For simplicity, we assume A = 0.5λ for the moment. Cases with other amplitudes will be discussed later. Consider a tubeworm, which is modelled as a cylinder, in contact with such a wavy substrate. Three types of stable configurations may occur, depending on the relative sizes between the tubeworm and the wavy profile. If the wavelength of the profile λ is much larger than the tubeworm's radius RT, a stable configuration for the tubeworm is to rest on the trough of the groove, as shown in figure 4a(i). This configuration is called single-point attachment. Under this circumstance, the pull-off force is given by (see the electronic supplementary material for details)
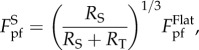 |
3.2 |
where RS represents the curvature radius at the trough of the substrate. Clearly, RS as a function of λ can be derived from the profile function given above. Considering the concave profile at the trough of the profile, one has 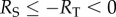 which implies that
which implies that 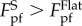 . It can be noticed that the pull-off force given in equation (3.2) goes to infinity when RS = −RT. Such unrealistic singularity is essentially attributed to the conventional parabolic approximation for circular profile in contact mechanics [34]. A reasonable cap of the pull-off force under the circumstance with RS = −RT is estimated to be (see the electronic supplementary material for details)
. It can be noticed that the pull-off force given in equation (3.2) goes to infinity when RS = −RT. Such unrealistic singularity is essentially attributed to the conventional parabolic approximation for circular profile in contact mechanics [34]. A reasonable cap of the pull-off force under the circumstance with RS = −RT is estimated to be (see the electronic supplementary material for details)
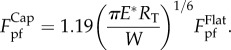 |
3.3 |
For the profile with intermediate wavelength λ, the spacing between two adjacent ridges is too narrow to accommodate a tubeworm at the trough. For stable attachment, a tubeworm has to straddle over a groove between two adjacent ridges, forming a configuration called double-point attachment (figure 4a(ii)). In this circumstance, the pull-off force is given by (see the electronic supplementary material for details)
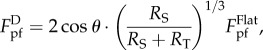 |
3.4 |
where RS represents the curvature radius of the substrate at the contact points, and θ is the contact angle designated in figure 4a(ii). Basic geometrical relations indicate that RS and θ are both functions of RT and 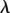 , so does
, so does 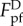 .
.
If the characteristic size of the profile λ is much smaller than the size of the tubeworm, more than two contact points will form between the tubeworm and substrate, giving rise to a multi-point attachment configuration, as shown in figure 4a(iii). In that case, an approximate solution to the pull-off force is given by (see electronic supplementary material for details)
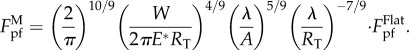 |
3.5 |
Nevertheless, equation (3.5) is applicable only to λ in a limited range because 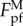 will go to infinity as λ approaches zero. Such unrealistic singularity is attributed to the neglect of the interaction between different contact points in our estimation (see the electronic supplementary material). Clearly, the wavy substrate will become flat and smooth as λ approaches zero. That is,
will go to infinity as λ approaches zero. Such unrealistic singularity is attributed to the neglect of the interaction between different contact points in our estimation (see the electronic supplementary material). Clearly, the wavy substrate will become flat and smooth as λ approaches zero. That is, 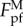 should asymptotically approach
should asymptotically approach 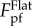 as λ approaches zero.
as λ approaches zero.
Equations (3.2)–(3.5) comprise the whole picture of the variation of pull-off force with the characteristic length scale of surface profile, λ, as depicted in figure 4b. It can be seen that the pull-off force between a tubeworm and a rough surface could be either higher or lower than that on a flat surface, depending on the characteristic length scale of surface roughness. For a surface with sinusoidal profile as we assumed, the maximum pull-off force occurs when the wavelength λ is around 10 times of the tubeworm's diameter. The magnitude of the maximum pull-off force is proportional to 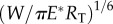 . The minimum pull-off force, which takes place when λ/RT = 0.1 is less than 45% of
. The minimum pull-off force, which takes place when λ/RT = 0.1 is less than 45% of 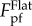 , implying the best antifouling performance of a profile with λ = 0.1 RT.
, implying the best antifouling performance of a profile with λ = 0.1 RT.
The analysis carried out so far is for a given ratio of A/λ = 0.5. To investigate the effects of profile amplitude A on the pull-off force, similar analysis is performed by taking A/λ = 0.125 and 2.0. Figure 4c compares the variations of the pull-off force as a function of λ/RT for these three cases. When A/λ = 0.125, there is only a small range of λ/RT giving pull-off force less than 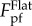 with minimum value around 0.7
with minimum value around 0.7 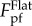 . With increase of A/λ, the range of λ/RT that gives rise to relatively lower pull-off force expands. When A/λ = 0.5, the range of λ/RT in which the pull-off force is less than 0.5
. With increase of A/λ, the range of λ/RT that gives rise to relatively lower pull-off force expands. When A/λ = 0.5, the range of λ/RT in which the pull-off force is less than 0.5 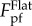 is 0.07–0.4. Such range extends to 0.02–19 when A/λ = 2. Considering the diversity of tubeworms in size, above results imply that high ridges can tackle the fouling of tubeworms of different sizes better. For S. apetala leaves and their PDMS replicas, the inter-ridge spacing is around 5 µm which is around 10% of the radius of a tubeworm larva (approx. 50 µm). In the light of figure 4b,c, such ratio of λ/RT should give rise to a lower pull-off force and therefore better antifouling performance as confirmed by the attachment tests above. Additionally, figure 4b,c also implies practical guidelines for the design of antifouling surface with ridge-like morphology. That is, high ridges, and proper inter-ridge spacing should be adopted.
is 0.07–0.4. Such range extends to 0.02–19 when A/λ = 2. Considering the diversity of tubeworms in size, above results imply that high ridges can tackle the fouling of tubeworms of different sizes better. For S. apetala leaves and their PDMS replicas, the inter-ridge spacing is around 5 µm which is around 10% of the radius of a tubeworm larva (approx. 50 µm). In the light of figure 4b,c, such ratio of λ/RT should give rise to a lower pull-off force and therefore better antifouling performance as confirmed by the attachment tests above. Additionally, figure 4b,c also implies practical guidelines for the design of antifouling surface with ridge-like morphology. That is, high ridges, and proper inter-ridge spacing should be adopted.
4. Synthetic antifouling surface with microscopic ridge-like morphology
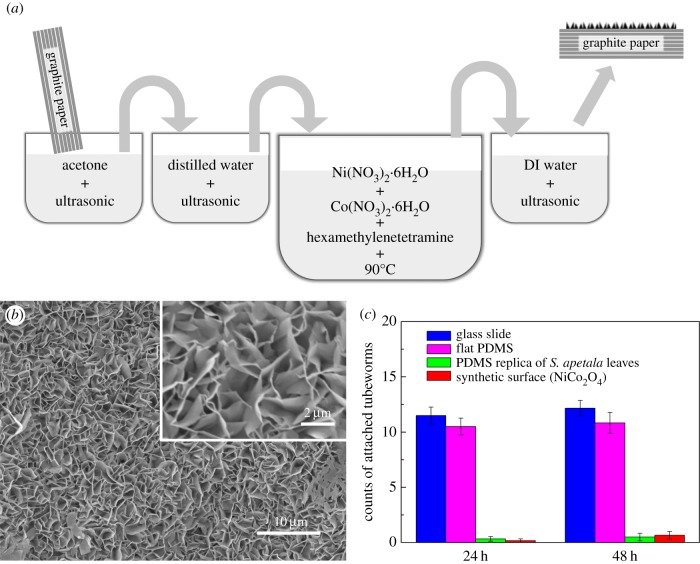
Above theoretical modelling indicates that high ridges with appropriate inter-ridge spacing are crucial for better antifouling performance. To further verify this finding and overcome the size limitation of replica of the natural antifouling surfaces, a synthetic surface with microscopic ridge-like morphology is prepared by water bath method with graphite paper (GP) in aqueous solution containing nickel and cobalt nitrates (figure 5a and Material and methodologies). Figure 5 shows the scanning electron microscopy images of the obtained surface which is covered with nanoflakes vertically situated on the underlying graphite substrate, giving rise to microscopic ridge-like morphology similar to that of the S. apetala leaves. The nanoflakes, which are identified as NiCo2O4, are around tens of nanometres in thickness and a few micrometres in lateral dimensions. The inter-ridge spacing ranges from several hundred nanometres to several micrometres. The ratios of λ/RT and A/λ are estimated to be in the ranges of 0.01–0.1 and 2–20, respectively, which, according to figure 4b,c, implies great antifouling potential. Settlement test with tubeworm larvae is also carried out for such synthetic ridge-like surfaces. The numbers of the settled tubeworms after 24 h and 48 h immersion are less than 10% of those on the glass slides and flat PDMS surfaces, as shown in figure 5c. Such outstanding antifouling performance is comparable with that of the PDMS replica of the S. apetala leaves. Certainly, for a practical antifouling application, such NiCo2O4 nanoflake coating is still at the embryo stage. Its mechanical robustness and drag force in flow fields would be the topics of interest for further investigation.
Figure 5.
(a) Schematic of the synthesis process of antifouling surface. (b) Scanning electron microscopy images of a synthetic surface with microscopic ridge-like morphology. (c) Means ± s.e. (n = 6) of the total counts of tubeworms attached on the synthetic surfaces with microscopic ridge-like morphology.
5. Conclusion
Inspired by the superior antifouling performance of S. apetala leaves, in this work we investigated the effect of surface morphology on antifouling performance. It was demonstrated that the excellent antifouling performance of the S. apetala leaves can be attributed to their microscopic ridge-like surface morphology. Theoretical modelling further indicated that high ridge and proper inter-ridge spacing would reduce the attachment probability of foulers and therefore facilitate antifouling. According to this guidance, a biomimetic surface with microscopic ridge-like morphology was synthesized. The follow-up attachment tests reconfirmed the feasibility of using ridge-like surface morphology to control biofouling. As a preliminary study, our present work mainly focused on the size effect of surface morphology, while the disorder effect of the microscopic ridge-like pattern was neglected for the moment. Such idealization allowed us to investigate the size effect of the morphology analytically. It is evident that the pull-off force between the fouler and substrate also depends on the disorder degree of the pattern, mechanical properties and adhesion energy of the substrate. The quantitative effects of these factors on the antifouling performance are still unclear and expected for in-depth investigations. Moreover, in our current study we applied tubeworms to test the antifouling performance. The applicability of the current microscopic ridge-like morphology to other fouling species remains unclear and deserves further study.
6. Material and methodologies
6.1. Preparation of polydimethylsiloxane replicas of Sonneratia apetala leaves
Firstly, the S. apetala leaves were cleaned using acetone and dried in air. Then, a poly(methyl methacrylate) (PMMA) solution (acetone as solvent) was poured directly onto a treated leaf's surface. After curing of the PMMA, the leaf was peeled off from the PMMA, giving rise to a negative PMMA mould duplicating the morphology of the leaf. Then, PDMS base and cross-linker were mixed in a volume ratio of 10 : 1. The obtained mixture was poured onto the negative PMMA mould in a glass dish. The whole set was placed in a desiccator for low-pressure degassing for 1 h. After curing at room temperature for 48 h, the PDMS replica was obtained after demoulding from the PMMA mould.
6.2. Attachment test with tubeworm larvae
Prior to test, all surface specimens to be tested were immersed into sterile deionized water for sufficient wetting. Then the wetted specimens were placed into a petri dish, which contained 20 ml seawater and around 100 tubeworm larvae (approx. 100 µm in width, approx. 300 µm in length; see electronic supplementary material for the culturing details). To ensure the repeatability of the results to be obtained, six replicas were prepared in parallel. All the test groups were kept in an ambient environment for 48 h in a 12 L : 12 D cycle. During the test process, the settled tubeworms were counted carefully with the aid of an optical microscope at 24 h and 48 h. All the specimens were dip-rinsed three times in filtered seawater to remove any unsettled tubeworms if available.
6.3. Synthesis of biomimetic antifouling surface
A piece of GP with size of 20 × 40 × 0.5 mm (width × length × thickness) was rinsed with acetone and distilled (DI) water, and then dried in a vacuum oven overnight at 80°C. The treated GP was then immersed into a solution made by dissolving 0.5 mmol Ni(NO3)2·6H2O, 1 mmol Co(NO3)2·6H2O and 2.2 mmol hexamethylenetetramine into 20 ml DI water and 10 ml ethanol. The solution together with GP was transferred to a Teflon-lined stainless-steel autoclave and kept at 90°C for 6 h. After cooling down to room temperature, the GP covered with NiCo2O4 nanoflakes was obtained, which was ready for antifouling test after thorough rinsing with DI water and ethanol followed by drying at 60°C for 12 h.
Supplementary Material
Acknowledgements
We acknowledge Ms Yuan Meng and Ms Camilla Campanati from the University of Hong Kong for helping in culturing tubeworms.
Data accessibility
The datasets supporting this article are included in the main paper and have been uploaded as the electronic supplementary material. Additional data related to this paper may be requested from the authors.
Authors' contributions
H.Y. conceived the idea. J.F., H.Z., Z.G., D.-Q.F. and V.T. carried out the experiments. H.Y. and J.F. performed the theoretical modelling and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The research is supported by the General Research Fund from Hong Kong RGC (PolyU 152193/14E; HKU 17304914 and 17303517) and the National Marine Economic Development Demonstration Project in Xiamen (16CZB023SF12).
References
- 1.Callow JA, Callow ME. 2011. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2, 244 ( 10.1038/ncomms1251) [DOI] [PubMed] [Google Scholar]
- 2.Schultz MP. 2007. Effects of coating roughness and biofouling on ship resistance and powering. Biofouling 23, 331–341. ( 10.1080/08927010701461974) [DOI] [PubMed] [Google Scholar]
- 3.Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2, 95–108. ( 10.1038/nrmicro821) [DOI] [PubMed] [Google Scholar]
- 4.Clare AS. 1996. Marine natural product antifoulants: status and potential. Biofouling 9, 211–229. ( 10.1080/08927019609378304) [DOI] [Google Scholar]
- 5.Qian PY, Rittschof D, Sreedhar B. 2000. Macrofouling in unidirectional flow: miniature pipes as experimental models for studying the interaction of flow and surface characteristics on the attachment of barnacle, bryozoan and polychaete larvae. Mar. Ecol. Prog. Ser. 207, 109–121. ( 10.3354/meps207109) [DOI] [Google Scholar]
- 6.Tesler AB, Kim P, Kolle S, Howell C, Ahanotu O, Aizenberg J. 2015. Extremely durable biofouling-resistant metallic surfaces based on electrodeposited nanoporous tungstite films on steel. Nat. Commun. 6, 8649 ( 10.1038/ncomms9649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenhahn A, Ederth T, Pettitt ME. 2008. Advanced nanostructures for the control of biofouling: the FP6 EU Integrated Project AMBIO. Biointerphases 3, 1–5. ( 10.1116/1.2844718) [DOI] [PubMed] [Google Scholar]
- 8.Champ MA. 2000. A review of organotin regulatory strategies, pending actions, related costs and benefits. Sci. Total Environ. 258, 21–71. ( 10.1016/s0048-9697(00)00506-4) [DOI] [PubMed] [Google Scholar]
- 9.Yebra DM, Kiil S, Dam-Johansen K. 2004. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 50, 75–104. ( 10.1016/j.porgcoat.2003.06.001) [DOI] [Google Scholar]
- 10.Almeida E, Diamantino TC, de Sousa O. 2007. Marine paints: the particular case of antifouling paints. Prog. Org. Coat. 59, 2–20. ( 10.1016/j.porgcoat.2007.01.017) [DOI] [Google Scholar]
- 11.Voulvoulis N, Scrimshaw MD, Lester JN. 2002. Comparative environmental assessment of biocides used in antifouling paints. Chemosphere 47, 789–795. ( 10.1016/s0045-6535(01)00336-8) [DOI] [PubMed] [Google Scholar]
- 12.Ralston E, Swain G. 2009. Bioinspiration—the solution for biofouling control? Bioinspir. Biomim. 4, 015007 ( 10.1088/1748-3182/4/1/015007) [DOI] [PubMed] [Google Scholar]
- 13.Bixler GD, Bhushan B. 2012. Biofouling: lessons from nature. Phil. Trans. R. Soc. A 370, 2381–2417. ( 10.1098/rsta.2011.0502) [DOI] [PubMed] [Google Scholar]
- 14.Cordeiro AL, Werner C. 2011. Enzymes for antifouling strategies. J. Adhes. Sci. Technol. 25, 2317–2344. ( 10.1163/016942411X574961) [DOI] [Google Scholar]
- 15.Dobretsov S, Dahms H-U, Qian P-Y. 2006. Inhibition of biofouling by marine microorganisms and their metabolites. Biofouling 22, 43–54. ( 10.1080/08927010500504784) [DOI] [PubMed] [Google Scholar]
- 16.Falconnet D, Csucs G, Grandin HM, Textor M. 2006. Surface engineering approaches to micropattern surfaces for cell-based assays. Biomaterials 27, 3044–3063. ( 10.1016/j.biomaterials.2005.12.024) [DOI] [PubMed] [Google Scholar]
- 17.Du T, Ma SH, Pei XW, Wang ST, Zhou F. 2017. Bio-inspired design and fabrication of micro/nano-brush dual structural surfaces for switchable oil adhesion and antifouling. Small 13, 1602020 ( 10.1002/smll.201602020) [DOI] [PubMed] [Google Scholar]
- 18.Shivapooja P, Wang Q, Orihuela B, Rittschof D, Lopez GP, Zhao X. 2013. Bioinspired surfaces with dynamic topography for active control of biofouling. Adv. Mater. 25, 1430–1434. ( 10.1002/adma.201203374) [DOI] [PubMed] [Google Scholar]
- 19.Brzozowska AM, Parra-Velandia FJ, Quintana R, Zhu XY, Lee SSC, Chin-Sing L, Janczewski D, Teo SLM, Vancso JG. 2014. Biomimicking micropatterned surfaces and their effect on marine biofouling. Langmuir 30, 9165–9175. ( 10.1021/la502006s) [DOI] [PubMed] [Google Scholar]
- 20.Schumacher JF, Aldred N, Callow ME, Finlay JA, Callow JA, Clare AS, Brennan AB. 2007. Species-specific engineered antifouling topographies: correlations between the settlement of algal zoospores and barnacle cyprids. Biofouling 23, 307–317. ( 10.1080/08927010701393276) [DOI] [PubMed] [Google Scholar]
- 21.Cao XY, et al. 2010. Interaction of zoospores of the green alga Ulva with bioinspired micro- and nanostructured surfaces prepared by polyelectrolyte layer-by-layer self-assembly. Adv. Funct. Mater. 20, 1984–1993. ( 10.1002/adfm.201000242) [DOI] [Google Scholar]
- 22.Wan F, Pei XW, Yu B, Ye Q, Zhou F, Xue QJ. 2012. Grafting polymer brushes on biomimetic structural surfaces for anti-algae fouling and foul release. ACS Appl. Mater. Interfaces 4, 4557–4565. ( 10.1021/am300912w) [DOI] [PubMed] [Google Scholar]
- 23.Feng DQ, Wang W, Wang X, Qiu Y, Ke CH. 2016. Low barnacle fouling on leaves of the mangrove plant Sonneratia apetala and possible anti-barnacle defense strategies. Mar. Ecol. Prog. Ser. 544, 169–182. ( 10.3354/meps11585) [DOI] [Google Scholar]
- 24.Ismail AE, Grest GS, Heine DR, Stevens MJ, Tsige M. 2009. Interfacial structure and dynamics of siloxane systems: PDMS–vapor and PDMS–water. Macromolecules 42, 3186–3194. ( 10.1021/ma802805y) [DOI] [Google Scholar]
- 25.Efimenko K, Finlay J, Callow ME, Callow JA, Genzer J. 2009. Development and testing of hierarchically wrinkled coatings for marine antifouling. ACS Appl. Mater. Interfaces 1, 1031–1040. ( 10.1021/am9000562) [DOI] [PubMed] [Google Scholar]
- 26.Banerjee I, Pangule RC, Kane RS. 2011. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 23, 690–718. ( 10.1002/adma.201001215) [DOI] [PubMed] [Google Scholar]
- 27.Shikuma NJ, Pilhofer M, Weiss GL, Hadfield MG, Jensen GJ, Newman DK. 2014. Marine tubeworm metamorphosis induced by arrays of bacterial phage tail-like structures. Science 343, 529–533. ( 10.1126/science.1246794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadfield MG, Nedved BT, Wilbur S, Koehl MAR. 2014. Biofilm cue for larval settlement in Hydroides elegans (Polychaeta): is contact necessary? Mar. Biol. 161, 2577-2587. ( 10.1007/s00227-014-2529-0) [DOI] [Google Scholar]
- 29.Scardino AJ, Guenther J, de Nys R. 2008. Attachment point theory revisited: the fouling response to a microtextured matrix. Biofouling 24, 45–53. ( 10.1080/08927010701784391) [DOI] [PubMed] [Google Scholar]
- 30.Decker JT, Sheats JT, Brennan AB. 2014. Engineered antifouling microtopographies: surface pattern effects on cell distribution. Langmuir 30, 15 212–15 218. ( 10.1021/la504215b) [DOI] [PubMed] [Google Scholar]
- 31.Decker JT, Kirschner CM, Long CJ, Finlay JA, Callow ME, Callow JA, Brennan AB. 2013. Engineered antifouling microtopographies: an energetic model that predicts cell attachment. Langmuir 29, 13 023–13 030. ( 10.1021/la402952u) [DOI] [PubMed] [Google Scholar]
- 32.Scardino AJ, Harvey E, De Nys R. 2006. Testing attachment point theory: diatom attachment on microtextured polyimide biomimics. Biofouling 22, 55–60. ( 10.1080/08927010500506094) [DOI] [PubMed] [Google Scholar]
- 33.Brzozowska AM, Maassen S, Rong RGZ, Benke PI, Lim CS, Marzinelli EM, Janczewski D, Teo SLM, Vancso GJ. 2017. Effect of variations in micropatterns and surface modulus on marine fouling of engineering polymers. ACS Appl. Mater. Interfaces 9, 17 509–17 517. ( 10.1021/acsami.6b14262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhury MK, Weaver T, Hui CY, Kramer EJ. 1996. Adhesive contact of cylindrical lens and a flat sheet. J. Appl. Phys. 80, 30–37. ( 10.1063/1.362819) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are included in the main paper and have been uploaded as the electronic supplementary material. Additional data related to this paper may be requested from the authors.