Abstract
Paraquat is a nonselective contact herbicide of great toxicological importance, being associated with high mortality rates. Because of its high toxicity, the European Union withdrew it from its market in 2007. The aim of this study is to analyze all cases of paraquat poisoning hospitalized in French Guiana in order to assess their incidence and main characteristics.
Medical records of all paraquat intoxicated patients hospitalized from 2008 until 2015 were reviewed in this retrospective study.
Demographics, clinical presentation, and laboratory data were evaluated.
A total of 62 cases were reviewed. The incidence of paraquat poisoning was 3.8/100,000 inhabitants/year. There were 44 adults and 18 children younger than 16 years of age. The median ages were 31 years [18.08–75.25] in adults and 13.4 years [0.75–15.08] in children, respectively. The median duration of hospitalization was longer in children [15.5 days (1–24)] than in adults [2 days (1–30)], P < .01. The majority of cases was due to self-poisoning (84%).
Children had ingested a lower quantity of paraquat [48.8 mg/kg (10–571.1)] than adults [595.8 mg/kg (6–3636.4), P = .03]. There were more deaths among adults (65%) than in children (22%), P = .004. The severity and outcome was determined primarily by the amount of paraquat ingested.
In conclusion, French Guiana has the largest cohort of paraquat poisonings in the European Union. The major factor affecting the prognosis of patients was the ingested amount of paraquat. The administration of activated charcoal or Pemba, in situ, within the first hour after ingestion of paraquat is essential.
Keywords: activated charcoal, French Guiana, high death rate, high incidence, paraquat poisoning, Pemba
1. Introduction
Paraquat (1,1′-dimethyl-4,4′-bipyridylium dichloride) is one of the most widely used herbicides, discovered in 1955 and developed in the early 1960s by Imperial Chemical Industries, now Syngenta.[1,2] It held the largest share of the global herbicide market until recently overtaken by glyphosate.[3] Paraquat is sold in about 130 countries for use on large and small farms, plantations and estates, and in nonagricultural weed control. It is a quick acting, nonselective herbicide, which destroys green plant tissue on contact and by translocation within the plant.[4,5] However, this product is highly toxic for humans and most animals. Its ingestion (often for suicide) can cause multiple organ failure, including liver insufficiency and lung fibrosis that can be life-threatening because of respiratory failure. The paraquat inhibits the reduction of nicotinamide adenine dinucleotide phosphate (NADP) to nicotinamide adenine dinucleotide phosphate hydrogen (NADPH), resulting in the overproduction of reactive oxygen and nitrite species that destroy the lipids of cell membranes. As paraquat is taken up against a concentration gradient into the lung, there is inflammation, with leukocyte recruitment and late pulmonary fibrosis, leading to hypoxemia unresponsive to treatment. This is usually confirmed by radiological findings.[6,7]
Paraquat poisoning is very serious. Most victims, including those who have ingested a small amount, will die from Paraquat poisoning.[8–13] In the majority of cases, the cause of death is pulmonary fibrosis. Because of its high toxicity, the European Union withdrew paraquat from its market in 2007.[14] Paraquat is also banned in French Guiana.
French Guiana is an overseas department and region of France, situated on the north-eastern coast of South America (Fig. 1). French Guiana is bounded by the Amapa state of Brazil to the south and east, Suriname to the west, and the Atlantic Ocean to the north-east. The Maroni River forms the French Guiana–Suriname border in the west, and the Oyapock River forms the border with Brazil. The population of Guiana (about 250,000) consists of a majority of mixed African and French ancestry (Creoles), with minorities of metropolitan French, Brazilians, Surinamese, Haitians, and other Caribbeans, Chinese, and Laotians. As a part of France, French Guiana is part of the European Union and the Eurozone. While the sale of paraquat has been banned in French Guiana since 2007, this pesticide remains freely available in neighboring countries (Suriname, Brazil), making it accessible to residents of French Guiana, and therefore increasing the number of paraquat poisonings reported in French Guiana.[15–17]
Figure 1.
Map of French Guiana.
The aim of this study is to analyze all cases of paraquat poisoning hospitalized in the 3 main hospitals in French Guiana in order to assess their incidence and main characteristics.
2. Methods
2.1. Inclusion and exclusion criteria
From the 1st of January 2008 to the 31st of December 2015, we included all consecutive patients with paraquat poisoning, admitted in the 3 tertiary referral hospitals in French Guiana, localized in Cayenne, Kourou, and Saint-Laurent-du-Maroni (Fig. 1). The main exclusion criterion was a negative paraquaturia.
2.2. Data extraction
We collected information on patient demographics, clinical presentation, hospital course, laboratory findings and outcomes, radiological studies, and clinical follow-up from the medical chart databases using Excel.
2.3. Ethical consideration and regulatory
Patients’ medical records were retrospectively reviewed, and all data collected were anonymized in standardized forms according to procedures of the Commission Nationale de l’Informatique et Libertés (the French information protection commission). Moreover, the patient anonymity was guaranteed.
2.4. Data analysis
We first describe the demographic characteristics, clinical features, laboratory findings, and outcomes. The variables compared between survivors and nonsurvivors are the amount of ingested paraquat, the serum creatinine level, the administered treatment, the timing of such treatment, and the Yamaguchi score and Acute Kidney Injury Network (AKIN) stage (predicting the prognosis for patients with acute paraquat intoxication), and the outcomes.
Data are presented in numbers and percentages for qualitative values, in means, standard deviations, and medians for quantitative values. Data are analyzed using R software (R Core Team 2015). Categorical variables were compared using the χ2 test or Fisher exact test (as appropriate) and continuous variables were compared with the Mann–Whitney ∗U∗ test. P values < .05 were considered statistically significant.
3. Results
3.1. Overall characteristics of patients
The annual incidence of paraquat poisoning was 3.8/100,000 inhabitants/year. There were more cases of paraquat poisoning in 2015 than in 2008 (Fig. 2). Sixty-two patients were included (30 males and 32 females). There were 44 adults and 18 children younger than 16 years. The median ages were 31 years [18.08–75.25] in adults and 13.4 years [0.75–15.08] in children. The median duration of hospitalization was higher in children [15.5 days (1–24)] than in adults [2 days (1–30)], P < .01. Regarding the place of residence of the patients, we noted that 67% came from Maroni (Saint Laurent, Papaichton, and Maripasoula), such as easier access to the chemical due to geographical location, near to the Suriname. No cases occurred in the east (mainly populated by Native Americans). Nearly 84% of children had no psychiatric history.
Figure 2.
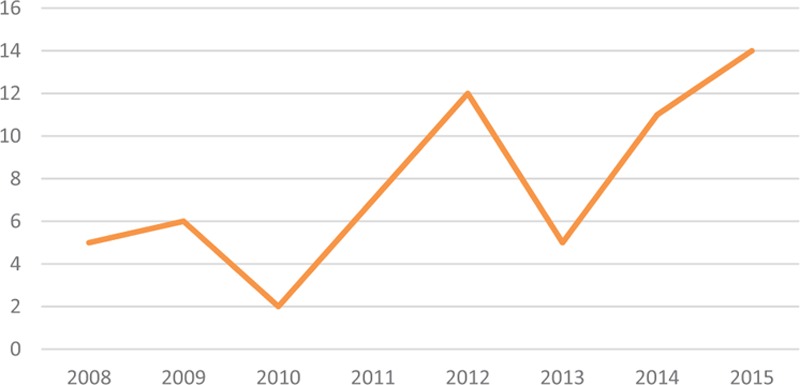
Annual number of paraquat poisonings.
3.2. Clinical and biological manifestations at presentation
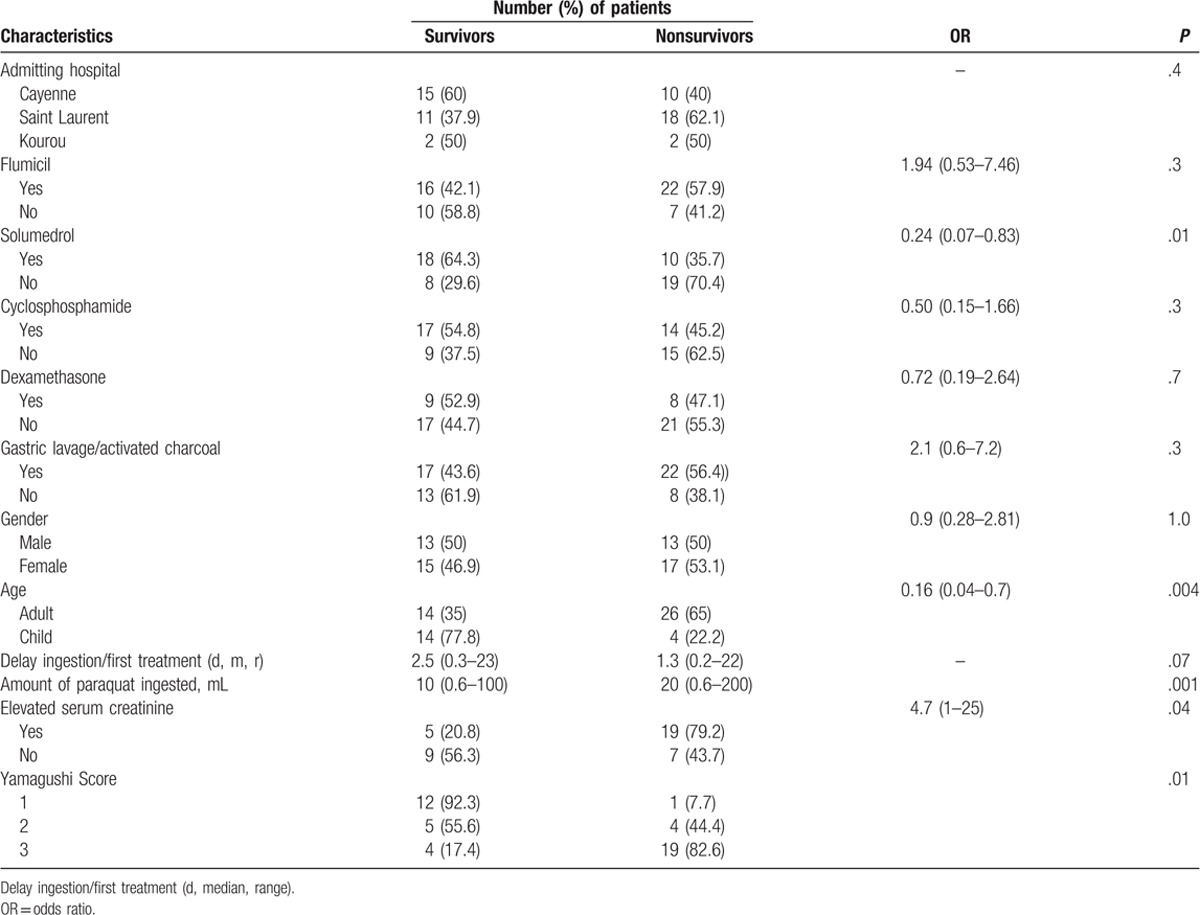
All the included patients had vomited before being admitted to the hospital. However, 22 patients (32%) had epigastric pain and inflammation of the oral mucosa. In 13 patients, paraquat was detected at the same time in gastric fluid and urine. These patients all had lesions on oesogastroduodenal fibroscopy. Fifty-five percent of the patients had a liver cytolysis (Table 1). The mean serum creatinine was significantly higher on admission in the deceased patients (81.7 μmol/L ± 34.4 vs 159.7 μmol/L ± 42.8). Fifty percent of adult patients with creatinine > 120 μmol/L on admission, died. In 2 patients, we did not have a second creatininemia, but the initial value was very high (737 and 446 μmol/L).
Table 1.
Comparative characteristics: survivors and nonsurvivors.
3.3. Emergency management of poisoned patients
The most common gastric decontamination method carried out for the patients was gastric lavage and charcoal in 44 cases (71%). Two patients received a mixture of water and Pemba immediately after ingesting paraquat, while waiting to be taken to hospital.
3.4. Medical treatments
In 31 cases (50%), treatment was carried out by corticosteroids, by cyclophosphamide in 36 cases (58%), and by N-acetyl cysteine (NAC) in 44 cases (71%). No patient has benefited from antioxidant. Only 1 patient was hemodialyzed.
3.5. Chest radiographic findings
Pulmonary radiography was performed in all patients. Only 15 patients had a chest computerized tomography scanner (CT scan). There were 4 cases of lung fibrosis, 1 pneumothorax, and 1 interstitial lung disease.
3.6. Outcomes
The duration of hospitalization was shorter in adults than in children (Table 1). The majority of cases were due to self-poisoning (84%). Children had ingested a lower quantity of paraquat [48.8 mg/kg (10–571.1)] than adults [595.8 mg/kg (6–3636.4), P = .03] (Fig. 3A, B). There was no difference in survival rate according to the given treatment. Among the 3 criminal poisonings, there were 2 children aged 5 and 8 years, whose mother made them drink paraquat before drinking herself. Both children survived and the mother died within 24 hours. The other patient (adult) described a criminal intoxication at work; he died 14 days after consuming the equivalent of 200 mL. We noted more deaths among patients with abnormal liver function during hospitalization. However, 7 patients with a normal liver function died within 24 hours of admission. They did not have a second liver function test performed. There were more deaths among adults (65%) than in children (22%), P = .004. The severity and outcome was determined primarily by the amount of paraquat ingested. Ninety-two percent of the patients with Yamaguchi score I > 1500–399 LogT survived (Fig. 4). Eighty-two percent of the patients with Yamaguchi score I < 930–399 LogT died. In multivariate analysis, the factor associated with death was the amount of paraquat ingested (P = .019).
Figure 3.
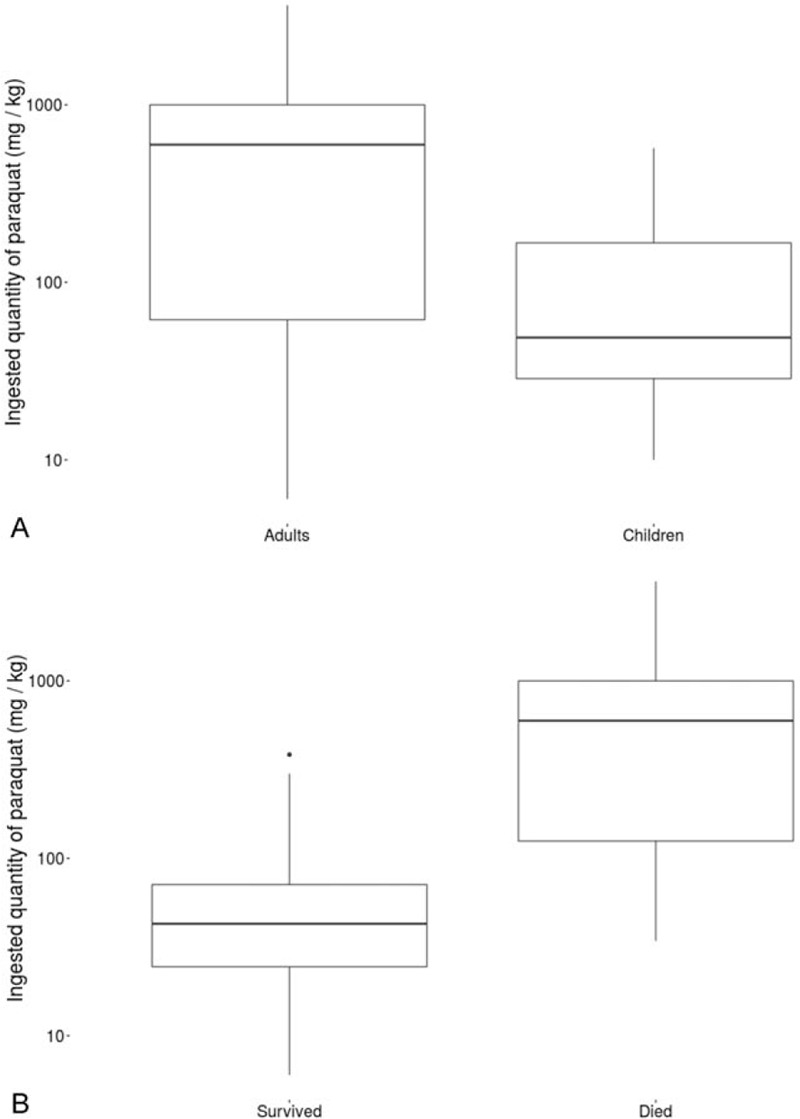
(A) Comparison of ingested quantity of paraquat between children and adults. (B) Outcome according to the quantity of paraquat ingested (P < .01).
Figure 4.
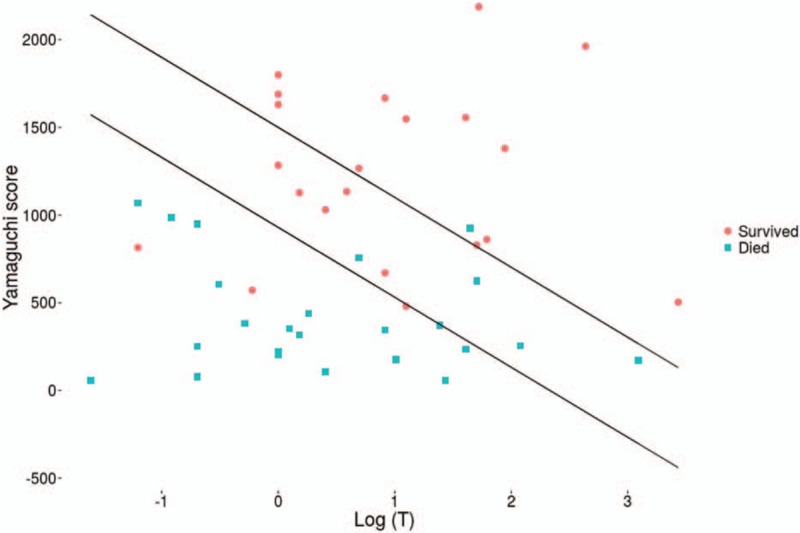
Outcome according to Yamaguchi score.
4. Discussion
Our study shows an increase in the annual incidence of paraquat intoxications in French Guiana. There is no recent study on the incidence of paraquat poisoning in neighboring countries. In Korea, the annual number of paraquat poisoning cases has decreased, as well as the incidence of all suicides with pesticides, between 2011 and 2014.[12] This was due to the paraquat ban, and suggests that paraquat will not commonly be used for suicide in the future. In countries that continue to authorize the unrestricted sale of paraquat, this incidence may be higher.[18] In our review, the median age of patients with paraquat poisoning was 27.4 years and 51.6% were men. This is the same distribution as in the Giuanese population pyramid (https://populationpyramid.net/french-guiana/2016/). Although the number of deceased patients with abnormal liver function is probably underestimated, these data were consistent with the literature.[19] The occurrence of liver cytolysis was comparable in 3 studies (55% in French Guiana, 68% in China, and 66% in Taiwan). Few patients underwent gastroscopy. This investigation can give an indication of the quantity of ingested paraquat. The presence of gastric lesions is associated with ingestion of large amounts of paraquat. The 13 patients who had a positive paraquat gastric fluid also had a positive paraquaturia. We discuss the value of this test, because there is no previous publication looking at paraquat levels in gastric fluid. Overall, the only interest in paraquatemia lies in its prognostic value. In a large prospective cohort study in Sri Lanka, about 451 patients were assessed using 5 methods and predicting outcome in function of the paraquatemia (https://populationpyramid.net/french-guiana/2016/). In that study, the surviving patients were followed for 3 months to detect delayed death. All methods underestimated the number of deaths.[20–22] The most recent study shows that the paraquatemia is a good marker for survival except when ingested paraquat concentrations were low.[23] Furthermore, given the delay in getting the result of this test in French Guiana (technical complex test, done in Mainland France and therefore at a high cost), we can discuss the need to get this test result, unless the test becomes technically feasible in French Guiana. Laboratories performing quantitative analysis of paraquat are few and the results are often known only several days to several weeks after the poisoning (often after death). In 2009, a study was performed to develop an alternative to plasma paraquat dosage.[24] It showed that it was sufficient to use the same method for the detection of urinary paraquat, substituting 2 mL of urine for 2-mL plasma. A color change to dark blue was associated with mortality in 100% of cases. There was only a mortality of 50% if the color change was not so pronounced. This method is easy to use and could be implemented in French Guiana.
In our study, 50% of adult patients with creatinine > 120 μmol/L on admission, died. This result was not significant, but there was probably an underestimation of kidney dysfunction on admission. The presence of renal failure on admission carries a poor prognosis.[25] A Korean study in 2009 on 278 patients showed that an elevated creatinine level over 120 μmol/L on admission was a significant predictor of mortality (P < .01).[26] About 51.4% of our patients were in this category. The majority of poisonings was intentional and this is consistent with literature.[27–29] Indeed, self-poisoning has become a response to an increase in emotional distress among young adults.[28,30]
There are limited studies of paraquat poisoning in children. A prospective study on 146 Chinese children hospitalized for paraquat poisoning reported similar results to ours.[8] In children over 10 years of age, the majority of exposures were intentional. Death rates in children were similar (22% in Guyana, against 22.7% in China, and 33.3% in Taiwan). There were more deaths among patients with renal failure. Similarly, the mean serum creatinine was significantly higher on admission in the deceased patients (81.7 μmol/L ± 34.4 vs 159.7 μmol/L ± 42.8). Nearly 84% of children had no psychiatric history. Thus, one can suspect a predominantly impulsive mode of action in our patients, which corroborates a study in Suriname in 2009, showing that 82% of patients hospitalized for suicide attempts committed these on impulse.[27] Regarding the Yamaguchi predictive score, 92% of patients having a score I > 1500–399 log have survived, while by definition, the score speaks of 90%. In contrast, 82% of patients with a score <950–399 log T have died, when the score speaks of a probability of death greater than 95%. So, the SIPP score and paraquatemia are better scores but not achievable in French Guiana.[31]
Regarding the place of residence of the patients, we noted that 67% came from Maroni (Saint Laurent, Papaichton, and Maripasoula) and no cases occurred found in the east (mainly populated by Native Americans who were using another mode of suicide by hanging). It is likely that this poisoning mainly concerned Bushinenge. In Suriname, 70% of patients who committed suicide used a pesticide. In addition, 17% of these patients were Bushinenge (representing 36% of Surinamese mainly from the east of the country against 30% in French Guiana).[32]
There is no specific treatment for paraquat poisoning. In our study, we noted the absence of an exact protocol or consistent approach for management of paraquat-intoxicated patients. According to the literature, the use of activated carbon significantly reduces mortality compared with Earth Foulon or Bentonite. In fact, activated carbon absorbs paraquat best. It is recommended to use it as soon as possible after paraquat ingestion [33,34] (within the first hour after ingestion). The number of case in this study are too small to demonstrate the potential efficacy of digestive decontamination, but the statistical analysis shows a significant trend (P = .09). Removing this dangerous product to prevent attempts of suicide and therefore suicidal deaths might result in a reduction in the incidence of paraquat poisoning. Paraquat poisoning and its mortality have disappeared from mainland France and other countries where the sale of the product has been banned.
In some cases, it is difficult to accurately determine the amount ingested. Moreover, the severity and outcome of paraquat poisoning are determined primarily by the amount ingested.[22,35,36] Given all these data, how can we reduce suicide attempts by paraquat ingestion in French Guiana? Several studies have attempted to address this increasingly common problem.[29] The pesticide industry has set up educational campaigns for safer use of pesticides.[37] Thus, the ban on paraquat in Suriname would be an essential measure to decrease cases of paraquat poisoning in French Guiana and Suriname. What role is there for the use of Pemba? Two patients received a mixture of water and pemba immediately after ingesting paraquat, while waiting to be taken to hospital. Obviously, we lack information on this decision and it is difficult to establish a causal relationship between intake of pemba and survival, as there are many confounding factors (lower ingested amount, for example). Pemba is local clay, otherwise known as kaolin, often used by Bushinenge in western French Guiana. It is prepared in a traditional way and is sold as balls of different sizes. Recently, a more addictive and dangerous use of Pamba in pregnant women was studied, and its anti-reflux properties were assessed.[38] In fact, regular use causes anemia due to iron deficiency. In 1968, a study showed that 80% of paraquat was absorbed by Kaolin in the environment.[39] It would be interesting to assess the paraquat absorbing capacity of Pemba in a laboratory setting. If these tests were conclusive, we could consider providing the rural health centers with Pemba, or simply inform people that in case of paraquat poisoning and pending medical evacuation, Pemba could be used in secondary prevention, to reduce intestinal paraquat absorption.
This study has some limitations. First, it cannot be considered as an exhaustive evaluation of paraquat poisoning in French Guiana, because it is based only on reports from hospitalized patients. As the records from the rural health centers were not computerized, it was very difficult to gather all the necessary information. While most patients seen at these centers were then transferred to 1 of the 3 hospitals and therefore included in this study, we cannot exclude that some patients have remained on site, because transferring them was impossible or they refused. Finally, the large area of French Guiana makes it plausible that some patients have died of poisoning at home, without timely transfer to a health center. A significant number of patients were excluded from the study because of a lack of laboratory diagnosis. Although the number of paraquat poisoning cases is underestimated, this data analysis allowed drawing a composite sketch of this problem in French Guiana.
5. Conclusion
With an incidence rate of at least 3.8 cases/100,000 inhabitants/year, French Guiana has the largest rate of paraquat poisoning in the European Union, with a high mortality rate of 52%. The major factor affecting the prognosis of patients was the ingested amount of paraquat. No treatment has proven its effectiveness on mortality. The preventive administration of activated charcoal or Pemba, in situ, within the first hour after ingestion of paraquat is essential. Extensive pharmacological research on pemba may be needed. An outright ban on the sale of Paraquat in Suriname seems to be the best way to eradicate this scourge from French Guiana.
Acknowledgments
The authors would like to thank Pr Mathieu Nacher from the Centre d’Investigation Clinique Antilles-Guyane, Cayenne Hospital, Rue des flamboyants, BP 6006, 97306 Cayenne Cedex, French Guiana for English correction.
Author contributions
Conceptualization: C. Merlin, N. ELENGA.
Data curation: B. Ntab, C. Merlin, K-A. Dinh-Van, M. Sobesky, N. ELENGA, R. Kom-Tchameni, R. Le Guern, Y-M. Ducrot.
Formal analysis: R. Le Guern.
Investigation: C. Merlin, M. Pradier, N. ELENGA, R. Kom-Tchameni, Y-M. Ducrot.
Methodology: C. Merlin, N. ELENGA, R. Le Guern.
Project administration: B. Ntab.
Resources: B. Ntab, K-A. Dinh-Van, M. Sobesky.
Supervision: D. Mathieu, G. Egmann, H. Kallel, J-M. Dueymes, M. Pradier, M. Mathieu-Nolf, N. ELENGA, R. Kom-Tchameni, Y-M. Ducrot.
Validation: N. ELENGA.
Writing – original draft: C. Merlin, N. ELENGA.
Writing – review & editing: D. Mathieu, G. Egmann, H. Kallel, J-M. Dueymes, M. Mathieu-Nolf, N. ELENGA.
Footnotes
Abbreviations: AKIN = Acute Kidney Injury Network, CT scan = computerized tomography scanner, NAC = N-acetyl cysteine, NADP = nicotinamide adenine dinucleotide phosphate, NADPH = nicotinamide adenine dinucleotide phosphate hydrogen.
There is no fund related to this study.
The authors have no conflict of interest to declare.
References
- [1].Roberts TR, Dyson JS, Lane MCG. Deactivation of the biological activity of paraquat in the soil environment: a review of long-term environmental fate. J Agric Food Chem 2002;50:3623–31. [DOI] [PubMed] [Google Scholar]
- [2].Dyson JS. Ecological safety of paraquat with particular reference to soil. Planter 1997;73:467–78. [Google Scholar]
- [3].El Mhammedi MA, Bakasse M, Chtaini A. Electrochemical studies and square wave voltammetry of paraquat at natural phosphate modified carbon paste electrode. J Hazard Mater 2007;145:1–7. [DOI] [PubMed] [Google Scholar]
- [4].Moustakas M, Malea P, Zafeirakoglou A, et al. Photochemical changes and oxidative damage in the aquatic macrophyte Cymodocea nodosa exposed to paraquat-induced oxidative stress. Pestic Biochem Physiol 2016;126:28–34. [DOI] [PubMed] [Google Scholar]
- [5].Oracz K, El-Maarouf-Bouteau H, Kranner I, et al. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol 2009;150:494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee SH, Lee KS, Ahn JM, et al. Paraquat poisoning of the lung: thin-section CT findings. Radiology 1995;195:271–4. [DOI] [PubMed] [Google Scholar]
- [7].Liu ZN, Zhao M, Zheng Q, et al. Inhibitory effects of rosiglitazone on paraquat-induced acute lung injury in rats. Acta Pharmacol Sin 2013;34:1317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Duan Y, Wang Z. To explore the characteristics of fatality in children poisoned by paraquat: with analysis of 146 cases. Int J Artif Organs 2016;39:51–5. [DOI] [PubMed] [Google Scholar]
- [9].Kim HJ, Kim HK, Lee H, et al. Toxicokinetics of paraquat in Korean patients with acute poisoning. Korean J Physiol Pharmacol 2016;20:35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cha ES, Chang SS, Gunnell D, et al. Impact of paraquat regulation on suicide in South Korea. Int J Epidemiol 2016;45:470–9. [DOI] [PubMed] [Google Scholar]
- [11].Kanchan T, Bakkannavar SM, Acharya PR. Paraquat poisoning: analysis of an uncommon cause of fatal poisoning from Manipal, South India. Toxicol Int 2015;22:30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee JW, Hwang IW, Kim JW, et al. Common pesticides used in suicide attempts following the 2012 Paraquat Ban in Korea. J Korean Med Sci 2015;30:1517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Delirrad M, Majidi M, Boushehri B. Clinical features and prognosis of paraquat poisoning: a review of 41 cases. Int J Clin Exp Med 2015;8:8122–8. [PMC free article] [PubMed] [Google Scholar]
- [14].EUR-Lex. Judgment of the Court of First Instance: European Directive 91/414/EEC case T-229/04. Available at: http://eur-lex. europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:62004TJ0229. Accessed September 28, 2012. [Google Scholar]
- [15].Kervégant M, Merigot L, Glaizal M, et al. Paraquat poisonings in France during the European ban: experience of the Poison Control Center in Marseille. J Med Toxicol 2013;9:144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Conso F. Paraquat poisoning: experience of poison control centers in France. Vet Hum Toxicol 1979;21 Suppl:112–3. [PubMed] [Google Scholar]
- [17].Kervégant M, Schmitt C, Martin E, et al. Self poisonings with paraquat in French Guiana : persistent use during suicidal behavior in French overseas territories. Ann Toxicol Anal 2013;25:71–3. [Google Scholar]
- [18].Perriëns J, Van der Stuyft P, Chee H, et al. The epidemiology of paraquat intoxications in Surinam. Trop Geogr Med 1989;41:266–9. [PubMed] [Google Scholar]
- [19].Hong SY, Yang DH, Hwang KY. Associations between laboratory parameters and outcome of paraquat poisoning. Toxicol Lett 2000;118:53–9. [DOI] [PubMed] [Google Scholar]
- [20].Senarathna L, Eddleston M, Wilks MF, et al. Prediction of outcome after paraquat poisoning by measurement of the plasma paraquat concentration. QJM Int J Med 2009;102:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Scherrmann JM, Houze P, Bismuth C, et al. Prognostic value of plasma and urine paraquat concentration. Hum Exp Toxicol 1987;6:91–3. [DOI] [PubMed] [Google Scholar]
- [22].Sawada Y, Yamamoto I, Hirokane T, et al. Severity index of paraquat poisoning. Lancet Lond Engl 1988;1:1333. [DOI] [PubMed] [Google Scholar]
- [23].Gil H-W, Kang M-S, Yang J-O, et al. Association between plasma paraquat level and outcome of paraquat poisoning in 375 paraquat poisoning patients. Clin Toxicol 2008;46:515–8. [DOI] [PubMed] [Google Scholar]
- [24].Koo J-R, Yoon J-W, Han S-J, et al. Rapid analysis of plasma paraquat using sodium dithionite as a predictor of outcome in acute paraquat poisoning. Am J Med Sci 2009;338:373–7. [DOI] [PubMed] [Google Scholar]
- [25].Kim S, Gil H-W, Yang J-O, et al. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol Dial Transplant 2009;24:1226–32. [DOI] [PubMed] [Google Scholar]
- [26].Roberts DM, Wilks MF, Roberts MS, et al. Changes in the concentrations of creatinine, cystatin C and NGAL in patients with acute paraquat self-poisoning. Toxicol Lett 2011;202:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].van Spijker BAJ, Graafsma T, Dullaart HIA, et al. Impulsive but fatal self-poisoning with pesticides among South Asians in Nickerie, Suriname. Crisis 2009;30:102–5. [DOI] [PubMed] [Google Scholar]
- [28].Gunnell D, Eddleston M. Suicide by intentional ingestion of pesticides: a continuing tragedy in developing countries. Int J Epidemiol 2003;32:902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Onyon LJ, Volans GN. The epidemiology and prevention of paraquat poisoning. Hum Exp Toxicol 1987;6:19–29. [DOI] [PubMed] [Google Scholar]
- [30].Phillips MR, Yang G, Zhang Y, et al. Risk factors for suicide in China: a national case-control psychological autopsy study. Lancet 2002;360:1728–36. [DOI] [PubMed] [Google Scholar]
- [31].Dinis-Oliveira RJ, Duarte JA, Remião F, et al. Single high dose dexamethasone treatment decreases the pathological score and increases the survival rate of paraquat-intoxicated rats. Toxicology 2006;227:73–85. [DOI] [PubMed] [Google Scholar]
- [32].The Epidemiology of Paraquat Intoxications in Surinam. - Abstract - Europe PMC [Internet]. Available at: http://europepmc.org/abstract/med/2595808 3912. Accessed September 23, 2016.
- [33].University of California, Riverside, U.S.A. Academic Press, Krieger R. Handbook of Pesticide Toxicology, Two-Volume Set: Principles and Agents. 2001;1025 p. [Google Scholar]
- [34].Meredith TJ, Vale JA. Treatment of paraquat poisoning in man: methods to prevent absorption. Hum Toxicol 1987;6:49–55. [DOI] [PubMed] [Google Scholar]
- [35].Fengjun J, Wen Z, Taoning W, et al. Analysis of risk factors for prognosis of patients with acute paraquat intoxication. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015;27:906–10. [PubMed] [Google Scholar]
- [36].Kavousi-Gharbi S, Jalli R, Rasekhi-Kazerouni A, et al. Discernment scheme for paraquat poisoning: a five-year experience in Shiraz, Iran. World J Exp Med 2017;7:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Murray DL, Taylor PL. Claim no easy victories: evaluating the pesticide industry's global safe use campaign. World Dev 2000;28:1735–49. [Google Scholar]
- [38].Périnat R. Rapport sur l’enquête Pemba 2012 dans l’Ouest Guyanais (Rapport du RPG, 2013) [Internet]. Available at: www.mdr-973.fr/reseau-perinat/...reseau-perinat.../149_3334d3474a24297464294a4. Accessed September 22, 2016.
- [39].Weber JB, Weed SB. Adsorption and desorption of diquat, paraquat, and prometone by montmorillonitic and kaolinitic clay minerals1. Soil Sci Soc Am J 1968;32:485. [Google Scholar]


