Abstract
The objective of this study was to describe demographic and clinical features of ocular toxoplasmosis (OT) in Korean patients compared to those in other countries.
This retrospective study comprised 46 patients diagnosed with OT. All participants were recruited at the uveitis clinic in Seoul St. Mary's Hospital.
The mean age of patients was 54 years. Of 46 patients, 31 (67.4%) were females. Of all patients, 24 (52.2%) had definite eating history of wild boar meat or deer blood while 5 (10.9%) had history of close contact with cats. The most common forms of OT were vitritis (91.3%) combined with retinochoroiditis (65.2%). Active retinochoroidal lesion was located at the peripheral retina in 18 (39.1%) patients, central retina in 8 (17.4%) patients, and peripapillary retina in 4 (8.7%) patients. Seven (15.2%) cases were clinically diagnosed with typical OT without serologic evidence. Thirty-nine (84.8%) had serum IgG for toxoplasmosis. However, only 8 (17.4%) had serum IgM. In 65.2% of patients, there was no complication after treatment. The most common ocular complication was macular scar (8.7%).
The present study provides demographic and clinical characteristics of OT in Korea, a low endemic area of Toxoplasma gondii. Acquired infection is the major cause of OT in Korea. Even though Korea is a low endemic area of Toxoplasma gondii, OT is a preventable and common cause of acquired infectious uveitis.
Keywords: Korea, ocular toxoplasmosis, retinochoroiditis, uveitis
1. Introduction
Ocular toxoplasmosis (OT) has been reported as the most common cause of infectious posterior uveitis in immunocompetent patients.[1] The prevalence of OT ranges from 3.8% to 17.7% of infectious uveitis.[2] Ocular toxoplasmosis usually presents as posterior uveitis with a chorioretinal lesion associated with vitritis.[3,4]Toxoplasma gondii may induce a latent disease characterized by tissue cysts in various organs, thereby leading to delayed OT or recurrence from a retinochoroidal scar.[5] It has been estimated that approximately 2% of individuals experiencing toxoplasmosis will develop ocular manifestations, suggesting that 1 in 400 persons across the world might have posterior uveitis due to Toxoplasma gondii.[6]
Ocular toxoplasmosis sometimes causes visual impairment and blindness in the affected eye, even in young adults. In one previous study, 24% of OT patients developed legal blindness.[1] Because OT is primarily an infectious disease, it remains a preventable cause of blindness. In this aspect, epidemiologic studies on OT are important for further advancement in its knowledge and prevention.
However, there are limited data on the incidence, clinical characteristics, and disease course in Korea compared to other countries. This is because Korea is a low-endemic area of Toxoplasma gondii. Therefore, the objective of this study was to describe demographic data, clinical features, treatment strategies, and outcomes of OT in Korean patients in a tertiary referral center.
2. Materials and methods
We conducted a retrospective analysis of patients diagnosed as active OT at the uveitis clinic in Seoul St. Mary's Hospital in Korea. All participants with OT were recruited between March 2008 and April 2017. This study adhered to the tenets of the Declaration of Helsinki. All protocols were approved by the Institutional Review Board (IRB) of the Catholic University of Korea. The requirement for informed patient consent was waived by the IRB due to the retrospective nature of this study.
Inclusion criteria were proven diagnosis of OT and a minimum follow-up of six months. All patients who were suspected of OT were diagnosed by a uveitis specialist based on clinical features of OT. The diagnosis was supported by serologic tests. Typical OT was defined as an active creamy-white focal retinal lesion without associated pigmented retinochoroidal scar in either eye.[1] Active focal retinal lesions in the presence of old retinochoroidal scar were defined as recurrent OT. We routinely performed serologic tests, including serum IgG and IgM antibodies against Toxoplasma gondii using enzyme-linked immunosorbent assay (ELISA). Tests for syphilis and HIV infection were performed for each patient at baseline.
Demographic information, clinical features, systemic or topical treatments and treatment outcomes were recorded. Ocular examinations including best corrected visual acuity (logarithm of the minimum angle of resolution, logMAR), noncontact pneumatic tonometry, and a thorough slit lamp examination were done for all subjects. Classification and grading of uveitis were done according to the Standardization of Uveitis Nomenclature (2005, SUN)[7] criteria. Treatment options for uveitis and selection of antitoxoplasmosis medications were at the physician's discretion.
Categorical data are expressed as absolute numbers while continuous data are presented as mean ± SD (95% confidence interval). Data were analyzed using the Statistical Package for Social Sciences (SPSS) for Windows ver. 23.0 (SPSS Inc., Chicago, IL).
3. Results
This retrospective study comprised 46 patients diagnosed with OT. Demographic and general characteristics of study participants are summarized in Table 1. Their mean age was 54 ± 12.2 years. Of these 46 patients, 31 (67.4%) were females. Twenty-four (52.2%) of 46 patients had definite eating history of wild boar meat or deer blood while 5 (10.9%) of 46 patients had a history of close contact with cats. Thirty (65.2%) patients had their first episode of OT at presentation. Fifteen (32.6%) patients had reported a previous episode while one (2.2%) patient reported congenital toxoplasmosis. For recurrent cases, their mean number of episodes of uveitis was 2.6 ± 0.8. Five (10.9%) patients showed bilateral involvement. For the remaining patients with unilateral involvement, 24 (52.2%) involved the right eye while 17 (36.9%) involved the left eye.
Table 1.
Demographic and general characteristics of study participants.

Details of clinical characteristics of patients are depicted in Table 2. The most common forms of OT were vitritis (91.3%) combined with retinochoroiditis (65.2%). The mean number of retinochoroidal lesions was 0.8 ± 0.8. Active retinochoroidal lesion was located at the peripheral retina in 18 (39.1%) patients, central retina in 8 (17.4%) patients, and peripapillary retina in 4 (8.7%) patients.
Table 2.
Clinical characteristics of study participants.
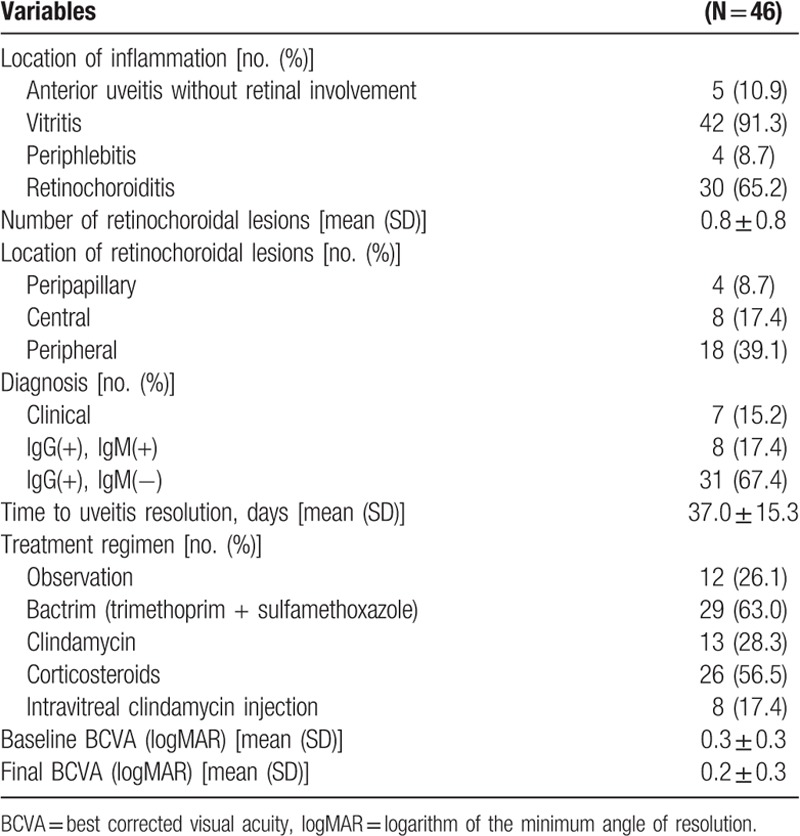
Seven (15.2%) of 47 patients were clinically diagnosed with typical OT without serologic evidence for antibodies against toxoplasmosis. A total of 39 (84.8%) patients had serum IgG antibodies for toxoplasmosis while 8 (17.4%) patients had serum IgM antibodies for toxoplasmosis. All patients showed negative serologic tests for syphilis or HIV infection.
Most commonly used antitoxoplasmosis medication was Bactrim (trimethoprim/sulfamethoxazole, 63.0%) in addition to oral corticosteroids (56.5%). Interestingly, 12 (26.1%) of 46 patients did not receive any antitoxoplasma treatment. Eight of 46 patients (17.4%) were treated with intravitreal clindamycin injection.
Initial best-corrected visual acuity was 0.3 ± 0.3. The best-corrected visual acuity after uveitis resolution was 0.2 ± 0.3. Mean time to uveitis resolution was 37 ± 15.3 days.
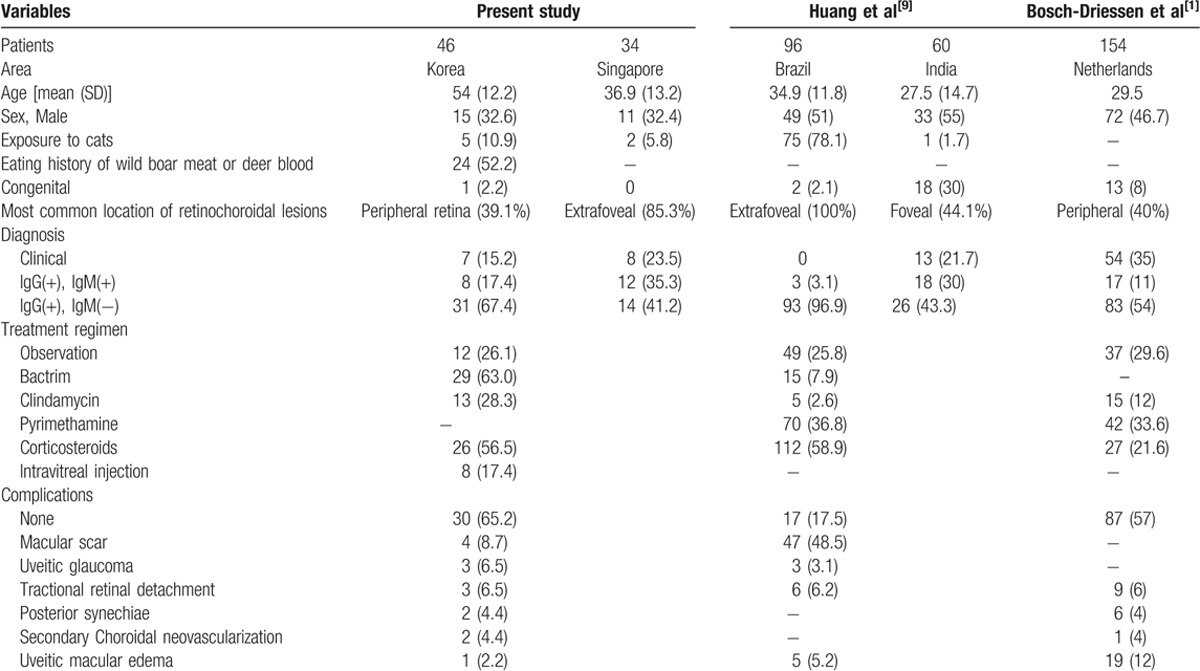
Ocular complications observed in study participants are listed in Table 3. In 65.2% of patients, there was no ocular complication after treatment. The most common complication was macular scar shown in 4 (8.7%) of 46 patients. Other complications included uveitic glaucoma (6.5%), secondary tractional retinal detachment (6.5%), posterior synechiae (4.4%), secondary choroidal neovascularization (4.4%), and uveitic macular edema (2.2%). Results of comparison for clinical characteristics of OT based on previous publications from different countries are shown in Table 4.
Table 3.
Incidence of complications of ocular toxoplasmosis.

Table 4.
Comparison between previous publications reporting epidemiology and clinical characteristics of ocular toxoplasmosis and the present study.
4. Discussion
In the present study, we demonstrated the clinical characteristics of OT in Korean patients. OT has been reported as the most common cause of infectious posterior uveitis in immunocompetent patients.[1] Globally, there are wide variations in the distribution of OT among uveitis. OT is more common in South America, Central America, the Caribbean, and parts of tropical Africa than that in Europe and Northern America It is relatively rare in Korea and China.[8]
The mean age of our study group was 54 ± 12.2 years. This is older than that in OT patients in European studies, ranging from 29.5 to 50.2 years.[9,10] In Korean patients, the percentage of bilateral cases (10.9%) was lower compared to that (32%) of a European study.[1]
Lifestyles and dietary habits might support a presumed source of infection. In Western countries, it is common to keep cats indoors, thus exposing the population to a risk of toxoplasmosis infection.[11] Moreover, ingestion of tissue cysts in raw/undercooked meat from several intermediate animals might be the main cause of infection in some countries.[11–13] In our study population, only 10.9% had a history of exposure to cats while 52.2% of our subjects had a definite history of eating wild boar meat or deer blood. This might be due to a misbelief in Korea that raw viscera of wild animals have beneficial effects on man's stamina.[14] In Brazil, the prevalence of congenital toxoplasmosis has been reported to be up to 80%.[15] However, only 1 (2.2%) patient had a pre-existing retinochoroidal scar in our study, suggesting that acquired OT is more common in Korea.
Serum IgG and IgM antibodies to Toxoplasma gondii will develop within 1 to 2 weeks after infection.[16] For patients who are suspected of having acute toxoplasmosis infection, IgG antibody could be initially analyzed. Negative result of IgG can rule out toxoplasmosis diagnosis in immunocompetent patient.[17] However, unfortunately, serologic diagnosis of active OT is insensitive. This might be due to local production of antibodies.[18,19] In our patient population, 84.8% had serum IgG antibodies for toxoplasmosis. However, 15.2% of them were clinically diagnosed without serologic evidence of antibodies against toxoplasmosis. Therefore, an early clinical suspicion in the real world may allow preservation of vision and limiting the rate of ocular complications.
In immunocompetent patients, a chorioretinal lesion associated with OT is usually self-limiting. It will resolve spontaneously in 4 to 8 weeks.[20] Complications of OT are not very devastating in most cases. In our study population, 65.2% showed no ocular complication after acute OT. Macular scar (8.7%) was the most common complication.
Our study has some limitations. First, our study was performed at a single center. Therefore, referral bias might have influenced our data. Second, due to the low prevalence of OT in Korea, the sample size was small. Despite these limitations, this is the largest case series in Korean patients. We believe that this retrospective analysis provides useful epidemiological data of OT in a Korean population.
Even though Korea is a low endemic area of Toxoplasma gondii, OT is a preventable and common cause of infectious uveitis. Given its vision threatening potential, clinicians should consider toxoplasmosis as a possible cause of posterior uveitis. Acquired infection is the major cause of OT in Korea. Preventing OT should be directed toward prevention of food-borne infection. Pregnant women especially should be informed about consuming well-cooked meat and observing hygiene when keeping cats. We recommend serologic tests including IgM and IgG antibodies against Toxoplasma gondii for suspicious cases.
Author contributions
Conceptualization: Mirinae Kim.
Data curation: Mirinae Kim, Seung Yong Choi, Jae Yon Won.
Formal analysis: Mirinae Kim.
Funding acquisition: Young-Hoon Park.
Investigation: Mirinae Kim, Young-Hoon Park.
Methodology: Mirinae Kim, Young-Hoon Park.
Supervision: Young-Hoon Park.
Writing – original draft: Mirinae Kim.
Writing – review & editing: Young-Hoon Park.
Footnotes
Abbreviations: BCVA = best corrected visual acuity, ELISA = enzyme-linked immunosorbent assay, IRB = Institutional Review Board, logMAR = logarithm of the minimum angle of resolution, OT = ocular toxoplasmosis, SUN = Standardization of Uveitis Nomenclature.
This research was supported by a grant (2016R1A6A1A03010528) of the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Republic of Korea.
The authors have no conflicts of interest to disclose.
References
- [1].Bosch-Driessen LE, Berendschot TT, Ongkosuwito JV, et al. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology 2002;109:869–78. [DOI] [PubMed] [Google Scholar]
- [2].Rathinam SR, Namperumalsamy P. Global variation and pattern changes in epidemiology of uveitis. Indian J Ophthalmol 2007;55:173–83. [DOI] [PubMed] [Google Scholar]
- [3].McCannel CA, Holland GN, Helm CJ, et al. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol 1996;121:35–46. [DOI] [PubMed] [Google Scholar]
- [4].Balasundaram MB, Andavar R, Palaniswamy M, et al. Outbreak of acquired ocular toxoplasmosis involving 248 patients. Arch Ophthalmol 2010;128:28–32. [DOI] [PubMed] [Google Scholar]
- [5].Labalette P, Delhaes L, Margaron F, et al. Ocular toxoplasmosis after the fifth decade. Am J Ophthalmol 2002;133:506–15. [DOI] [PubMed] [Google Scholar]
- [6].Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol 2003;136:973–88. [DOI] [PubMed] [Google Scholar]
- [7].Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Petersen E, Kijlstra A, Stanford M. Epidemiology of ocular toxoplasmosis. Ocul Immunol Inflamm 2012;20:68–75. [DOI] [PubMed] [Google Scholar]
- [9].Huang PK, Jianping C, Vasconcelos-Santos DV, et al. Ocular toxoplasmosis in tropical areas: analysis and outcome of 190 patients from a multicenter collaborative study. Ocul Immunol Inflamm 2017;1–8. doi: 10.1080/09273948.2017.1367407. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [10].Stanford MR, See SE, Jones LV, et al. Antibiotics for toxoplasmic retinochoroiditis: an evidence-based systematic review. Ophthalmology 2003;110:926–31. quiz 31-2. [DOI] [PubMed] [Google Scholar]
- [11].Tsirouki T, Dastiridou A, Symeonidis C, et al. A focus on the epidemiology of uveitis. Ocul Immunol Inflamm 2018;26:2–16. [DOI] [PubMed] [Google Scholar]
- [12].Dodds EM. Toxoplasmosis. Curr Opin Ophthalmol 2006;17:557–61. [DOI] [PubMed] [Google Scholar]
- [13].Wakefield D, Chang JH. Epidemiology of uveitis. Int Ophthalmol Clin 2005;45:1–3. [DOI] [PubMed] [Google Scholar]
- [14].Park YH, Han JH, Nam HW. Clinical features of ocular toxoplasmosis in Korean patients. Korean J Parasitol 2011;49:167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vasconcelos-Santos DV, Machado Azevedo DO, Campos WR, et al. Congenital toxoplasmosis in southeastern Brazil: results of early ophthalmologic examination of a large cohort of neonates. Ophthalmology 2009;116:2199–205. e1. [DOI] [PubMed] [Google Scholar]
- [16].Marcolino PT, Silva DA, Leser PG, et al. Molecular markers in acute and chronic phases of human toxoplasmosis: determination of immunoglobulin G avidity by Western blotting. Clin Diagn Lab Immunol 2000;7:384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ozgonul C, Besirli CG. Recent developments in the diagnosis and treatment of ocular toxoplasmosis. Ophthalmic Res 2017;57:1–2. [DOI] [PubMed] [Google Scholar]
- [18].Rothova A, van Knapen F, Baarsma GS, et al. Serology in ocular toxoplasmosis. Br J Ophthalmol 1986;70:615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Holliman RE, Stevens PJ, Duffy KT, et al. Serological investigation of ocular toxoplasmosis. Br J Ophthalmol 1991;75:353–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Butler NJ, Furtado JM, Winthrop KL, et al. Ocular toxoplasmosis II: clinical features, pathology and management. Clin Exp Ophthalmol 2013;41:95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]


