Abstract
Background:
Previous studies seem to show different effects of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) on cardiovascular (CV) events in hypertensive patients with type 2 diabetes mellitus (T2DM). Our objective was to analyze which are preferable on the incidence of all-cause mortality, CV death, and major CV events in hypertensive patients with T2DM.
Methods:
PubMed, MEDLINE, and EMBASE were searched for randomized controlled trials (RCTs) published up to June 2016 with ACEI or ARBs as the intervention for hypertensive patients with T2DM. The primary end points were all-cause mortality and CV death. The secondary end points were myocardial infarction (MI), stroke, heart failure (HF), and CV events. Two investigators extracted the information independently. Data were pooled using a fixed-effects model or a random-effects model if significant heterogeneity was present.
Results:
A total of 13 trials were included for analysis, 5 ACEI trials (24,976 patients) and 8 ARB trials (22,032 patients) followed for a mean of 3.8 years. Treatment with ACEI was associated with significantly reduction in all-cause mortality [odds ratio (OR) 0.87; 95% confidence interval (95% CI), 0.80–0.94], CV death (OR 0.81; 95% CI, 0.68–0.98), and other CV outcomes such as MI (0R 0.77; 95% CI, 0.66–0.90), stroke (OR 0.88; 95% CI, 0.78–0.99), HF (OR 0.65; 95% CI, 0.47–0.90), and CV events (OR 0.83; 95% CI, 0.73–0.95), whereas ARBs therapy had no significant reduction in the results of many primary and secondary outcomes.
Conclusion:
This meta-analysis suggests that treatment with ACEI showed a significant CV protection for all-cause mortality, CV death, and major CV events, whereas ARBs had no benefits on these outcomes except MI. In consideration of high mortality and morbidity, ACEI was preferable than ARBs on patients with hypertension and T2DM.
Keywords: ACEI, ARB, hypertension, meta-analysis, mortality, T2DM
1. Introduction
Hypertension and type 2 diabetes mellitus (T2DM) are the 2 primary risk of the cardiovascular (CV) events. People with both of them are at a higher risk of CV disease than those suffering from hypertension or T2DM alone.[1–3] As a leading cause of premature death, DM is associated with many macrovascular complications, and approximately 6.8% people who died from heart disease or stroke are because of DM.[4] Compared with those without DM, the rate of mortality from CV disease is 2 to 4-fold higher in people with DM.[5–7] The high blood pressure is another independent risk factor for CV disease, and treatment or control of hypertension can reduce death and hospitalization from CV disease.[8]
The renin-angiotensin-aldosterone system is a major regulatory system of CV function and the blockade of RAS activity is beneficial for CV disease.[9] It was recommended that patients with hypertension and DM should be treated with a pharmacologic therapy regimen that includes ACEI or ARB.[10] And, the use of the RAS blockade angiotensin-converting enzyme (ACE) inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) could reduce both CV mortality and morbidity.[11–13]
Although the therapy by ACEI or ARBs was recommended by the guidelines of 2013 European Society of Hypertension (ESH) and the European Society of Cardiology (ESC),[14] there still remains controversial in clinical for hypertensive patients with T2DM who treated with ACEI or ARBs. The recent meta-analysis by Cheng et al[15] showed that ACEI or ARBs had different effects on CV outcomes in patients with DM. And the meta-analysis by van Vark et al[16] showed that ACEI or ARBs had different effects on all-cause mortality in patients with hypertension. But there is no meta-analysis focused on the comparison of ACEI and ARBs on CV risk in hypertensive patients with T2DM.
Thus, we undertook the present meta-analysis aiming to compare the effects of ACEI or ARBs separately on the incidence of all-cause mortality, CV death, and major CV events, which included myocardial infarction (MI), stroke, and heart failure (HF) in the hypertensive patients with T2DM.
2. Methods
2.1. Search strategy and study selection
We performed a systematic search of MEDLINE, EMBASE, and PubMed for randomized controlled trials (RCTs) published in English between January 2000 and June 2016 for relevant studies performed in hypertensive patients with T2DM. The following Medical subject heading terms and key words were used: hypertension and T2DM; CV mortality or events, all-cause mortality, MI, stroke, HF; ACEIs or ARBs; RCTs or clinical trials. We also searched reference lists from cited articles. The criteria for eligible studies were as follows: randomized clinical trials for hypertension with T2DM; treatment with ACEI or ARBs with control treatment (placebo or actives); the endpoints were all-cause mortality, CV mortality or events, MI, stroke, HF; the mean follow-up was more than 1 year; the age of patients should older than 55 years; and the total participant should more than 500. Finally, a total of 13 trials were included for our analysis, 5 ACEI trials (24,976 patients) and 8 ARB trials (22,032 patients). Studies were excluded if it was not RCT or it was not associated with the outcome we need clearly. We also excluded the study not published in English (Fig. 1).
Figure 1.
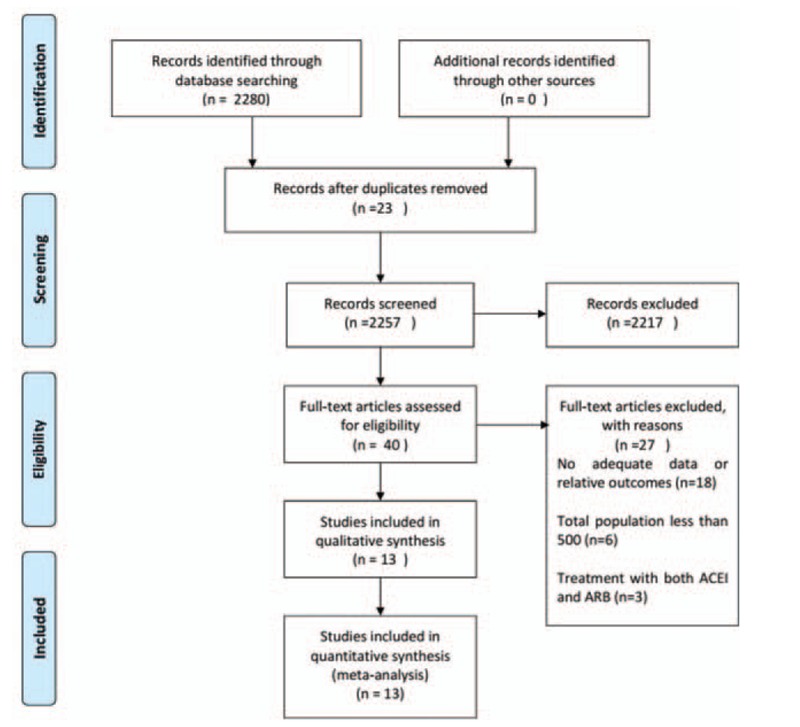
Flow diagram of trial search and selection process.
2.2. Data extraction
Two investigators independently extracted data and resolved differences by discussion from each study as the following information: first author name and study title, publication year, interventions, total participants, the percentage of male, the mean age at baseline, follow-up duration, the mean diastolic blood pressure and systolic blood pressure, and the results for each outcome.
2.3. Quality assessments
According to the guidelines in the Cochrane Handbook, the risk of bias table was used to evaluate the quality of the trials on the results by reviewing the randomization protocols and follow-up procedures adopted, which contains sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias (Fig. 2).
Figure 2.
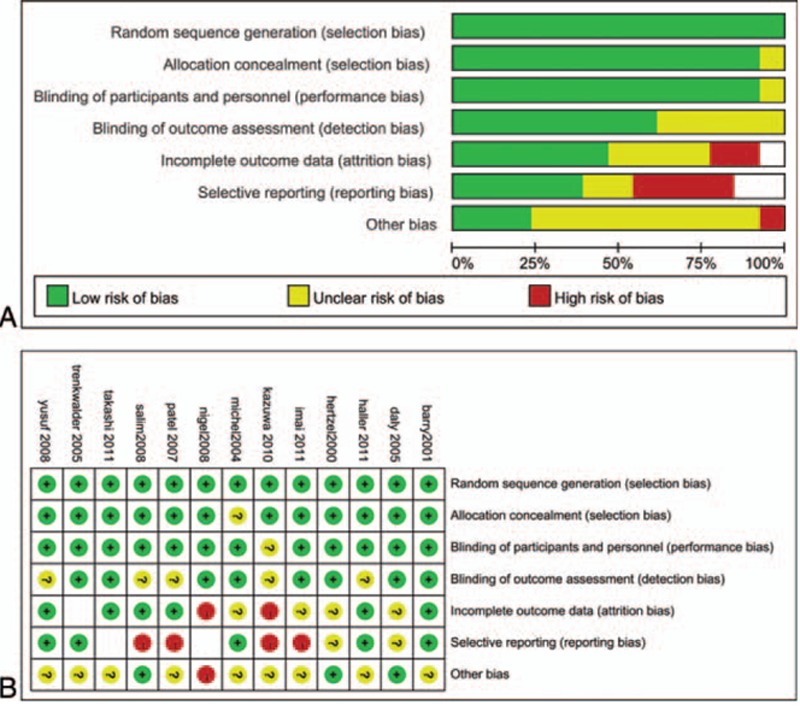
Risk of bias graph and risk of bias summary.
2.4. End points
The primary end points were all-cause mortality and CV mortality. The secondary end points were MI (including fatal and nonfatal MI), stroke (including fatal and non-fatal stroke), HF, CV events (including coronary artery bypass, percutaneous transluminal coronary angioplasty, death from CV causes, nonfatal MI, HF, etc). Every end point was not contained in all studies. Data on all-cause mortality were not available in SCOPE. Data on CV mortality and stroke were not available in RENNAL. Data on MI were not available in ADVANCE and SCOPE. Data on HF were not available in ADVANCE, SCOPE, ROADMAP, and CASE-J.
2.5. Statistical analysis
We calculated individual study odds ratio (OR) and 95% confidence interval (95% CI) and combined the estimates for OR using fixed-effects model or random-effect model, if there was any heterogeneity (P < .1, or I2 > 50%). The results were confirmed by the Mantel–Haenszel fixed-effect model to avoid small studies being overly weighted. The I2 statistic was used to evaluate the heterogeneity, judging values of 25%, 50%, and 75% was considered to be low, moderate, and high heterogeneity, respectively.[17] We further conducted a sensitivity analysis to explore the possible explanation for heterogeneity, which investigated the effect of a single study on overall risk factors by excluding 1 study once a time.[18] The potential publication bias was assessed using the funnel plot and Egger test. A 2-sided P value of less than .05 was considered statistically significant. All statistical analyses were done in Review Manager, version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark) except Egger test that was done in Stata 12.0 (Stata Corp, College Station, TX).
3. Results
3.1. Characteristics of the studies
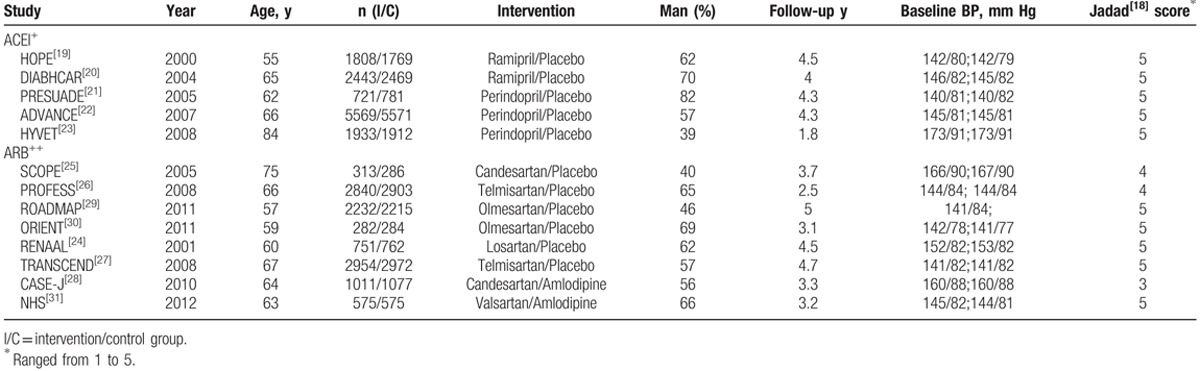
A total of 13 RCTs fulfilled our selection criteria for this meta-analysis, and their main characteristics are summarized in Table 1.[19–31] Among these trials, 5 trials (n = 24,976) compared ACEI with control therapy and 8 trials (n = 22,032) compared ARBs. The ACEI group was all compared with placebo, and the ARBs group was not. Of the 8 trials, CASE-J[28] and NHS[31] compared ARBs with active drugs, and the remaining studies compared ARBs with placebo. The mean follow-up duration was 3.8 years in the ACEI treatment and 4.2 years in the ARB treatment. The mean age was 66 years in ACEI and 64 years in ARB. The baseline level of blood pressure was more than 140/70 mm Hg. Patients in both groups were not significantly different in gender.
Table 1.
Baseline characteristics of study population in included trials.
3.2. Primary end points
3.2.1. Effects of ACEI on all-cause mortality and CV mortality
Treatment with ACEI was associated with significant reduction in all-cause mortality (OR: 0.87, 95% CI: 0.80–0.94, P = .0008), and there was a moderate heterogeneity (P = .09; I2 = 50%). The same result as ACEI therapy on the occurrence of CV death (OR: 0.81, 95% CI: 0.68–0.98, P = .03) compared with control group; the reduction was significant. But for the outcome of CV death, there was significant heterogeneity in this treatment (P = .04; I2 = 60%), although it was estimated by random-effects model instead (Figs. 3A and 4A) After excluding the DIABHCAR[20] studies by sensitivity analysis, the heterogeneity among the trials was not significant (P = .29, I2 = 21%). The funnel plot showed no suggestion of publication bias and the Egger test indicated no statistically significant reporting bias in both groups (P = .400; P = .643).
Figure 3.
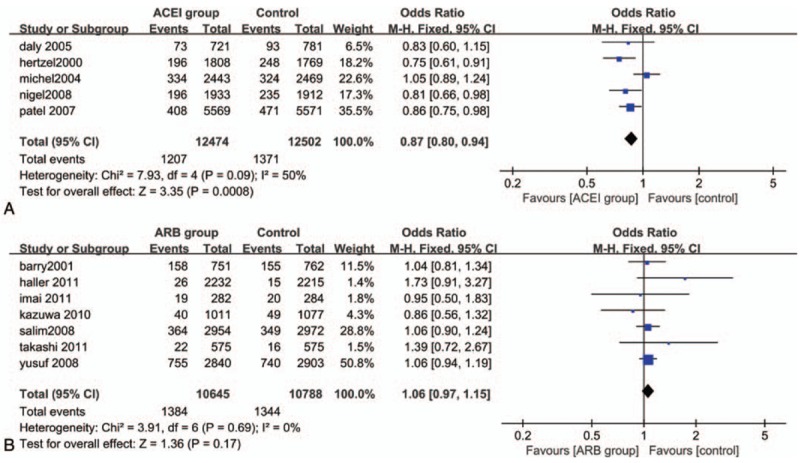
Forest plot for all-cause mortality. (A) Analyze comparing ACEI with control treatment; (B) Analyze comparing ARBs with control treatment. ACEI = angiotensin-converting enzyme inhibitors, ARB = angiotensin receptor blockers.
Figure 4.
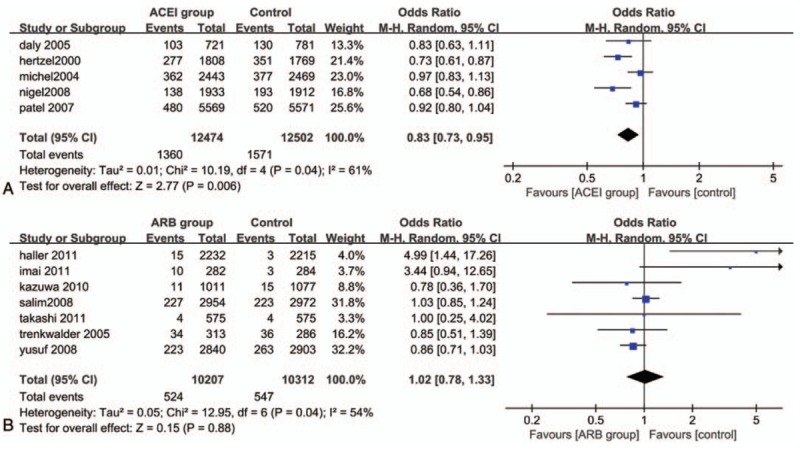
Forest plot for CV death. (A) Analyze comparing ACEI with control treatment; (B) Analyze comparing ARBs with control treatment. ACEI = angiotensin-converting enzyme inhibitors, ARB = angiotensin receptor blockers.
3.2.2. Effects of ARBs on all-cause mortality and CV mortality
There was no significant decrease in the occurrence of all-cause mortality (OR: 1.06, 95% CI: 0.97–1.15, I2 = 0%) and CV death (OR: 1.02, 95% CI: 0.78–1.33, I2 = 54%) when treatment was done with ARBs compared with control therapy. And both of them showed no statistical significant association (P = .17>.05, P = .88>.05) (Figs. 3B and 4B). There was no evidence of publication bias (P = .784; P = .389).
3.3. Secondary end points
3.3.1. Effects of ACEI on MI, STROKE, HF, and CV events
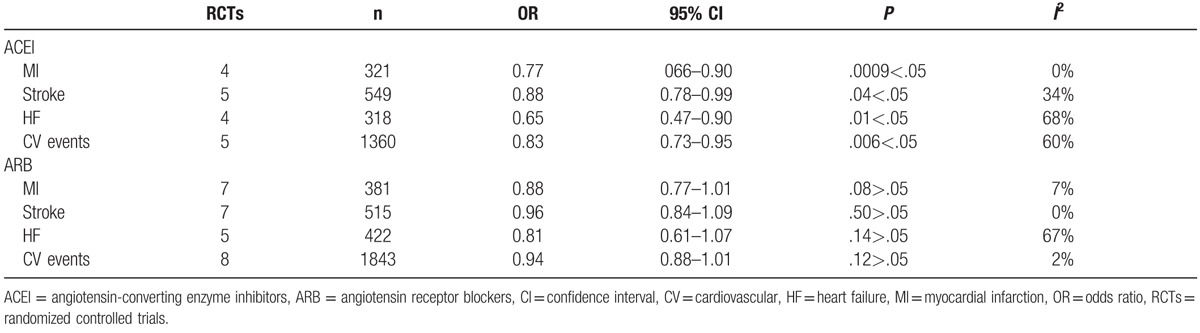
Four of five trials (PRESUADE,[21] HOPE,[19] DIANHCAR,[20] and HYVET[23]) assessed the effect of ACEI therapy on the occurrence of MI and HF. This treatment significantly reduced the occurrence of MI (OR: 0.77, 95% CI: 066–0.90, P = .0009 <.05, I2 = 0%) and HF (OR: 0.65, 95% CI: 0.47–0.90, P = .01 <.05, I2 = 68%). But there was significant heterogeneity for HF, after a sensitivity analysis of excluding the HYVET[23] studies, greater heterogeneity disappeared (P = .48, I2 = 0%). And there been no evidence of publication bias in both.
In all 5 trials together, we can find significant reduction in stroke treatment with ACEI (OR: 0.88, 95% CI: 0.78–0.99, P = .04<.05, I2 = 34%) and in CV events (OR: 0.83, 95% CI: 0.73–0.95, P = .006<.05, I2 = 60%). By excluding DIABHCAR[20] and ADVANCE studies,[22] the significant heterogeneity disappeared (P = .56, I2 = 0%) for CV events end point, and there was no publication bias (Table 2).
Table 2.
The secondary end points effects on MI, STROKE, HF, and CV event.
3.3.2. Effects of ARBs in MI, STROKE, HF, and CV events
In our meta-analysis, there was no significant reduction in the occurrence of MI (OR: 0.88, 95% CI: 0.77–1.01, P = .08>.05, I2 = 7%), stroke (OR: 0.96, 95% CI: 0.84–1.09, P = .50>.05, I2 = 0%), and CV events (OR: 0.94, 95% CI: 0.88–1.01, P = .12>.05, I2 = 2%). The heterogeneity between these trials is low. There was a clearly apparent decrease in HF end point (OR: 0.81, 95% CI: 0.61–1.07). However, it has not been statistically significant and the heterogeneity is apparent (P = .14>.05, I2 = 67%) (Table 2).
4. Discussion
RAS blockade is one of the first-line treatments for the hypertensive patients with T2DM, and there was no doubt regarding the CV protection of ACEI in the hypertensive patients with T2DM. But the CV protective effect of ARBs in the hypertensive patients with T2DM was equivocal. With regard to the previous studies and meta-analyses, our study showed that, for the primary end points, treatment with ACEI significantly reduced the occurrence of all-cause mortality by approximately 13% and CV death by approximately 19%. On the contrary, ARBs therapy had no significant effect on all-cause mortality and CV death. On the secondary end points, ACEI can also reduce the occurrence of major CV events by 17% significantly, which contained MI by 23% and HF by 35%. No more like previous study, there was no clearly apparent beneficial effect on stroke[15]; our study showed a 12% significant reduction. Compared with previous meta-analysis by Cheng et al,[15] there were also some differences among the ARBs therapy. Previous studies showed that there was no significant decrease in the occurrence of major CV events and MI in the patients with DM when treatment was done with ARBs; however, it was with a 30% reduction in HF.[15] However, our meta-analysis showed that ARBs therapy did not significantly affect the occurrence of MI, STROKE, and CV events, but the only reduction of HF by 19% had no statistical significance. These differences may contribute to the criteria we made. In our meta-analysis, we limited the criteria for eligible studies at the baseline of previous study: the age of patients should be older than 55 years; the total participants should more than 500; and the patients must be diagnosed with both hypertension and T2DM. Thus, our study included 13 RCTs finally and the purpose was more obvious.
According to our analysis, it is confirmed that ACEI therapy trend to a less fatal CV events than treatment with ARBs compared with placebo. But there was significant heterogeneity between the include studies in the result of CV death (I2 = 60%) treatment with ACEI in our research, when changed into random-effect model the heterogeneity still existed. So, we further conducted a sensitivity analysis to explore the possible explanation for heterogeneity. We found that the heterogeneity was mainly caused to DIABHCAR[20] studies; when this trial was excluded, the significant heterogeneity disappeared (I2 = 21%). The DIABHCAR[20] studies showed that Ramipril group resulted in more mortality than placebo group in hypertensive patients with T2DM, while other trials showed less mortality compared with control group. The data of DIABHCAR were associated with a trend to more mortality compared with placebo (OR: 1.08). Heterogeneity may be closely related to its design, the post hoc observations, dosage, or administration, which were attributed to the DIABHCAR study. It is revealed that among the different ACEIs, the heterogeneity may exist.
Our research included 13 RCTs that contained more than 24,926 patients and 22,032 patients treatment with ACEI or ARBs separately. The strength of our meta-analysis is important for the clinical questions, rigorously select criteria, comprehensive quality assessment, a large number of participants, and consistency of results. Moreover, protective effects of ACEIs and ARBs regarding major CV events among T2DM and hypertensive patients showed that our manuscript is valuable. However, some limitations have to be mentioned in our study. First, search of the literature may not find all eligible studies, although the funnel plot and Egger test did not show a significant publication bias. And some studies were excluded because they did not report the number of CV events clearly. Furthermore, we searched studies published only in English. Second, there was a great deal of variation between the populations we studied, such as, different ACEI or ARBs, different dosages of treatment and control group, different follow-up times and different combination treatments, and participants with other concomitant conditions or background therapy we used in our study. Therefore, more studies should be performed to evaluate the difference between ACEI and ARBs on the CV protective effect in hypertensive patients with T2DM.
5. Conclusion
Treatment with ACEI significantly reduced the occurrence of all-cause mortality, CV death, and major CV events, including MI, stroke, and HF compared with control groups. Whereas ARBs did not benefit the advantages in these outcomes. Therefore, because of the high mortality and morbidly rates for hypertensive patients with T2DM, ACEI is more preferable than ARBs.
Author contributions
Conceptualization: Xiaodan lv, Yingshi Zhang, Qi Song, Qingchun Zhao.
Data curation: Xiaodan lv, Yingshi Zhang, Qi Song.
Formal analysis: Xiaodan lv, Yingshi Zhang, Yixuan Niu, Qingchun Zhao.
Funding acquisition: Xiaodan lv, Yingshi Zhang.
Investigation: Xiaodan lv, Yingshi Zhang.
Methodology: Xiaodan lv, Yingshi Zhang, Qi Song.
Project administration: Xiaodan lv, Yingshi Zhang, Qi Song.
Resources: Xiaodan lv, Yingshi Zhang.
Software: Xiaodan lv, Yingshi Zhang, Qingchun Zhao.
Supervision: Xiaodan lv, Yingshi Zhang.
Validation: Xiaodan lv, Yingshi Zhang.
Visualization: Xiaodan lv, Yingshi Zhang.
Writing – original draft: Xiaodan lv, Yingshi Zhang.
Footnotes
Abbreviations: ACEI = angiotensin-converting enzyme inhibitors, ARB = angiotensin-receptor blockers, CI = confidence interval, CV = cardiovascular, HF = heart failure, MI = myocardial infarction, OR = odds ratio, RCTs = randomized controlled trials, T2DM = type 2 diabetes mellitus.
Equal contribution: X. lv and Y. Zhang.
Ethical approval was not necessary, because our manuscript is a systematic review and meta-analysis. The data are derived from the original study rather than directly from the patient.
The authors report no conflicts of interest.
References
- [1].Yamagishi S. Cardiovascular disease in recent onset diabetes mellitus. J Cardiol 2011;57:257–62. [DOI] [PubMed] [Google Scholar]
- [2].Assmann G, Schulte H. Diabetes mellitus and hypertension in the elderly: concomitant hyperlipidemia and coronary heart disease risk. Am J Cardiol 1989;63:33H–7H. [DOI] [PubMed] [Google Scholar]
- [3].Mancia G. The association of hypertension and diabetes: prevalence, cardiovascular risk and protection by blood pressure reduction. Acta Diabetol 2005;42(Suppl 1):S17–25. [DOI] [PubMed] [Google Scholar]
- [4].International Diabetes Federation. The Diabetes Atlas. 4th ed. Brussels: International Diabetes Federation; 2009. [Google Scholar]
- [5].Lloyd-Jones D, Adams RJ, Brown TM, et al. Writing Group Members; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010;121:e46–215. [DOI] [PubMed] [Google Scholar]
- [6].Schroll M, Larsen S. A ten-year prospective study, 1964-1974, of cardiovascular risk factors in men and women from the Glostrup population born in 1914: multivariate analyses. Dan Med Bull 1981;28:236–51. [PubMed] [Google Scholar]
- [7].Sarnak MJ, Levey AS, Schoolwerth AC, et al. American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003;108:2154–69. [DOI] [PubMed] [Google Scholar]
- [8].Campbell NR, Brant R, Johansen H, et al. Increases in antihypertensive prescriptions and reductions in cardiovascular events in Canada. Hypertension 2009;53:128–34. [DOI] [PubMed] [Google Scholar]
- [9].Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol 2006;98:121–8. [DOI] [PubMed] [Google Scholar]
- [10].American Diabetes Association. Executive summary: standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl 1):S4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Turnbull F, Neal B, Algert C, et al. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med 2005;165:1410–9. [DOI] [PubMed] [Google Scholar]
- [12].Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med 2000;342:145–53. [DOI] [PubMed] [Google Scholar]
- [13].Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362:782–8. [DOI] [PubMed] [Google Scholar]
- [14].Taylor J. 2013 ESH/ESC guidelines for the management of arterial hypertension. Eur Heart J 2013;34:2108–9. [PubMed] [Google Scholar]
- [15].Cheng J, Zhang W, Zhang X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med 2014;174:773–85. [DOI] [PubMed] [Google Scholar]
- [16].van Vark LC, Bertrand M, Akkerhuis KM, et al. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin-angiotensinaldosterone system inhibitors involving 158,998 patients. Eur Heart J 2012;33:2088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [19].Lonn E, Yusuf S, Hoogwerf B, et al. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–9. [PubMed] [Google Scholar]
- [20].Marre M, Lievre M, Chatellier G, et al. DIABHYCAR Study Investigators. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomised, double blind, placebo controlled trial (the DIABHYCAR study). BMJ 2004;328:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Daly CA, Fox KM, Remme WJ, et al. EUROPA Investigators. The effect of perindopril on cardiovascular morbidity and mortality in patients with diabetes in the EUROPA study: results from the PERSUADE substudy. Eur Heart J 2005;26:1369–78. [DOI] [PubMed] [Google Scholar]
- [22].Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370:829–40. [DOI] [PubMed] [Google Scholar]
- [23].Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–98. [DOI] [PubMed] [Google Scholar]
- [24].Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–9. [DOI] [PubMed] [Google Scholar]
- [25].Trenkwalder P, Elmfeldt D, Hofman A, et al. Study on Cognition and Prognosis in the Elderly (SCOPE). The Study on Cognition and Prognosis in the Elderly (SCOPE)—major CV events and stroke in subgroups of patients. Blood Press 2005;14:31–7. [DOI] [PubMed] [Google Scholar]
- [26].Yusuf S, Diener HC, Sacco RL, et al. PRoFESS Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008;359:1225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yusuf S, Teo K, Anderson C, et al. Telmisartan Randomised AssessmeNt Study in ACEiswcDI. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008;372:1174–83. [DOI] [PubMed] [Google Scholar]
- [28].Ogihara T, Fujimoto A, Nakao K, et al. Group C-JT. ARB candesartan and CCB amlodipine in hypertensive patients: the CASE-J trial. Expert Rev Cardiovasc Ther 2008;6:1195–201. [DOI] [PubMed] [Google Scholar]
- [29].Haller H, Ito S, Izzo JL, Jr, et al. ROADMAP Trial Investigators. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 2011;364:907–17. [DOI] [PubMed] [Google Scholar]
- [30].Imai E, Chan JC, Ito S, et al. ORIENT Study Investigators. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia 2011;54:2978–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Muramatsu T, Matsushita K, Yamashita K, et al. Comparison between valsartan and amlodipine regarding cardiovascular morbidity and mortality in hypertensive patients with glucose intolerance: NAGOYA HEART Study. Hypertension 2012;59:580–6. [DOI] [PubMed] [Google Scholar]


