Abstract
Percutaneous kyphoplasty (PKP) surgery is generally accepted as a minimally invasive treatment for osteoporotic vertebral compression fractures (OVCFs). However, hidden blood loss (HBL) caused by this procedure is usually disregarded. This study aimed to investigate the amount of HBL and its influencing factors after PKP surgery.
A total of 160 patients were retrospectively examined from January 2014 to January 2016, and their clinical and radiological data were recorded and analyzed. Preoperative and postoperative hematocrit (Hct) and hemoglobin (Hb) levels were also documented. HBL was calculated using Gross formula. Different factors, including gender, age, bone mineral density (BMD), number of fracture levels, hypertension, diabetes mellitus, operative time, percentage of vertebral height loss, percentage of vertebral height restoration, and cement leakage, were examined. Multivariate linear regression analysis was performed to elucidate the related clinical or radiological factors of HBL.
A total of 122 patients with 169 levels were eligible for inclusion in the study. The mean HBL was 279 ± 120 mL, and the postoperative Hb loss was 8.2 ± 3.9 g/L. Multivariate linear regression analysis revealed that HBL was positively associated with operative time (P = .000), percentage of vertebral height loss (P = .037), and percentage of vertebral height restoration (P = .000). By contrast, HBL was not associated with gender (P = .874), age (P = .148), BMD (P = .134), number of fracture levels (P = .079), hypertension (P = .259), diabetes mellitus (P = .495), and cement leakage (P = .975). The postoperative incidence of anemia significantly increased by 39.3% compared with that of the preoperative incidence (χ2 = 21.432, P = .000).
For patients with OVCFs, the amount of HBL after PKP is much larger than that observed perioperatively. Operative time, percentage of vertebral height loss, and percentage of vertebral height restoration are influencing factors of HBL.
Keywords: hidden blood loss, influencing factors, multivariate linear regression analysis, osteoporotic vertebral compression fractures, percutaneous kyphoplasty surgery
1. Introduction
In clinical practice, hidden blood loss (HBL) penetrating tissues, retained in a dead space, and lost due to hemolysis is often disregarded by orthopedic surgeons.[1] HBL may considerably affect postoperative outcomes, such as medical complications, increased blood transfusion risks, and prolonged hospitalization time.[2,3] HBL has been widely investigated in orthopedic surgeries since 2000,[2,4–7] and emerging data on knee and hip replacement surgeries have revealed that HBL varies from 26% to 56% of the total blood loss (TBL).[1,8,9] In a study on the treatment of intertrochanteric fractures, HBL was nearly 75% and 44% of the TBL in proximal femoral nail antirotation group and dynamic hip screw group, respectively.[10] In a work on anterior/posterior lumbar fusion surgery (ALIF/PLIF), HBL was approximately 40% of TBL.[2,4] However, few studies have considered HBL in percutaneous kyphoplasty (PKP) surgery.
PKP is a minimally invasive and effective treatment for osteoporotic vertebral compression fractures (OVCFs). It is performed by initially inserting an inflatable balloon through a pedicle and then injecting polymethylmethacrylate into a fractured vertebral body. In our clinical experience, PKP is associated with a relatively low perioperative blood loss because of small incision, reduced muscular dissection, and short operative time.
Correct information about the degree of blood loss can help prevent complications. However, HBL and its relevant influencing factors after PKP surgery have yet to be systematically investigated. In this study, we retrospectively reviewed patient data to determine the amount of HBL and identified the influencing factors of HBL after PKP surgery.
2. Materials and methods
2.1. Patients
The study design was reviewed and approved by the Ethics Committee of Chongqing General Hospital. Informed consent was obtained from all of the participants. A total of 160 patients, with 59 males and 101 females, with a complete disease history were analyzed between January 2014 and January 2016 in our hospital. Our inclusion criteria were age > 60 years, low-energy injury sustained within 2 weeks prior to admission, severe back pain (visual analogue score, VAS > 5), conservative treatment failures, acute OVCFs (confirmed by magnetic resonance imaging), decreased bone mineral density (BMD ≤ − 2.5 standard deviation, SD), and all of the patients underwent PKP surgery. Our exclusion criteria were old OVCFs, spine infection, suspected underlying malignant disease, spinal cord compression syndrome, severe cardiopulmonary comorbidity, major coagulopathy, and patients with liver cirrhosis or uremia. Basic clinical data, including gender, age, height, weight, body mass index (BMI), BMD, hypertension, diabetes mellitus, fracture location, number of fracture levels, preoperative and postoperative hematocrit (Hct) and hemoglobin (Hb), operative time, percentage of vertebral height loss, percentage of vertebral height restoration, and cement leakage were recorded.
2.2. Surgical technique and postoperative therapy
All of the operations were primary procedures and performed by an experienced surgeon (SZ) using local anesthesia. PKP surgery was carried out in accordance with a standard published technique, namely, bilateral pedicle approach, with a C-arm fluoroscope.[11] All of the patients were prescribed with osteoporotic medication, including calcium, vitamin D supplements, and antiresorptive agents after surgery.
2.3. Management of blood loss
No patient received blood transfusion throughout the assessment period. Low visible blood loss was detected intraoperatively but was disregarded. Therefore, HBL was approximated to TBL. No drainage was typically placed in any of the patients. All of the patients underwent a full blood count, including Hct, and Hb before the surgery and 2 or 3 days after the surgery. By this time, the patients were hemodynamically stable, and fluid shifts were largely completed.[9]
2.4. Calculation of patient's blood volume (PBV) and HBL
PBV was estimated in accordance with the formula of Nadler et al[12] as follows:
PBV (L) = k1 × height (m)3 + k2 × weight (kg) + k3, where k1 = 0.3669, k2 = 0.03219, and k3 = 0.6041 for males, and k1 = 0.3561, k2 = 0.03308, and k3 = 0.1833 for females. We assumed that the blood volume was the same on admission and on days 2 or 3 after surgery.
The TBL was calculated through the Gross formula[13] as follows:
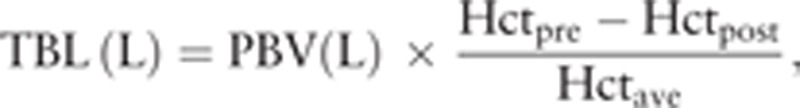 |
where Hctpre is the initial preoperative Hct, Hctpost is the Hct 2 or 3 days postoperation, and Hctave is the average of Hctpre and Hctpost.
The preoperative and postoperative Hb levels were used to calculate Hbloss in the perioperative period. Hbloss was calculated as follows:
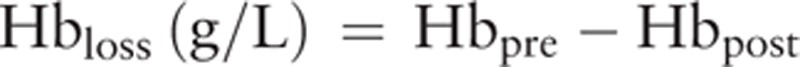 |
2.5. Calculation of the percentages of vertebral height loss and restoration
All of the included cases were examined using plain radiographs. Vertebral height (VBH) was measured at the point of the maximal collapse of the affected vertebral body. The percentages of vertebral height loss (HL, %) and vertebral height restoration (HR, %) were calculated with the following equations:
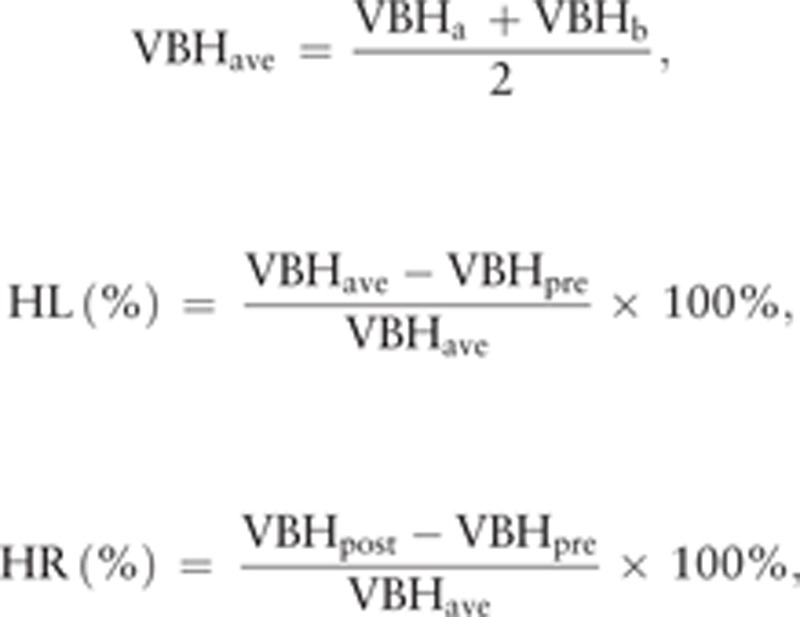 |
where VBHa and VBHb are 2 adjacent vertebral heights, VBHave is the average height of the 2 adjacent vertebrae, and VBHpre is the preoperative anterior or midline vertebral body height of the fractured vertebrae.
2.6. Additional measurements
According to the World Health Organization, anemia is characterized by Hb levels of < 120 g/L for women and < 130 g/L for men.[14]
2.7. Statistical analysis
Data were expressed as means ± standard deviation or medians (25th–75th percentile). The normality of variables was assessed, and differences in mean and median values were evaluated using Student t test and Mann–Whitney U test, respectively. A χ2 test was adopted to compare the preoperative and postoperative incidence of anemia. Multivariate linear regression analysis was performed to evaluate the influencing factors associated with HBL. Among the qualitative variables, hypertension, diabetes mellitus, and cement leakage were set as “1,” whereas nonhypertension, nondiabetes mellitus, and nonleakage were set as “0.” A positive coefficient indicated a positive influence on the dependent variable (HBL), whereas a negative coefficient denoted a negative influence. All of the independent variables were incorporated into the model using the method of “Enter.” Data analyses were performed with SPSS version 19.0 (SPSS, Chicago, IL). A 2-sided P value of < .05 was considered statistically significant.
3. Results
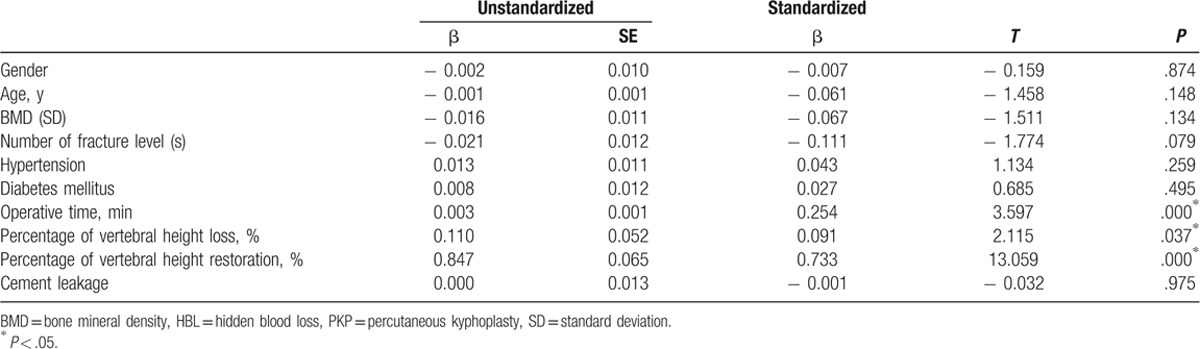
Thirty-eight patients were excluded, 20 patients were old OVCFs, 10 patients were age ≤ 60 years, 8 patients were BMD > − 2.5SD. As a result, a total of 122 patients with 169 levels were reviewed retrospectively. All of the patients underwent PKP for painful OVCFs. Their demographic data are summarized in Table 1. No differences in age (P = .361, Mann–Whitney U test) and BMI (P = .602, Student t test) were noted between male and female patients. Conversely, significant differences in height, weight, and BMD were found between male and female patients (P = .000, Mann–Whitney U test). The number of fracture levels, operation time, and HBL are shown in Table 2. The increase in operative time and amount of HBL was associated with the increasing number of fracture levels. The loss of Hb and Hct levels, operative time, percentage of vertebral height loss, percentage of vertebral height restoration, and HBL are listed in Table 3. The mean HBL was 279 ± 120 mL, and the mean Hb loss was 8.2 ± 3.9 g/L. Twenty-six patients suffered from preoperative anemia, and the number of patients with anemia increased to 74 after surgery (Fig. 1). This difference indicated that HBL could obviously increase the number of patients with anemia (χ2 = 21.432, P = .000). The operative time (P = .000), percentage of vertebral height loss (P = .037), and percentage of vertebral height restoration (P = .000) were positively correlated with HBL (Table 4). By contrast, gender (P = .874), age (P = .148), BMD (P = .134), number of fracture levels (P = .079), hypertension (P = .259), diabetes mellitus (P = .495), and cement leakage (P = .975) were not associated with HBL.
Table 1.
Patient's demographic information.
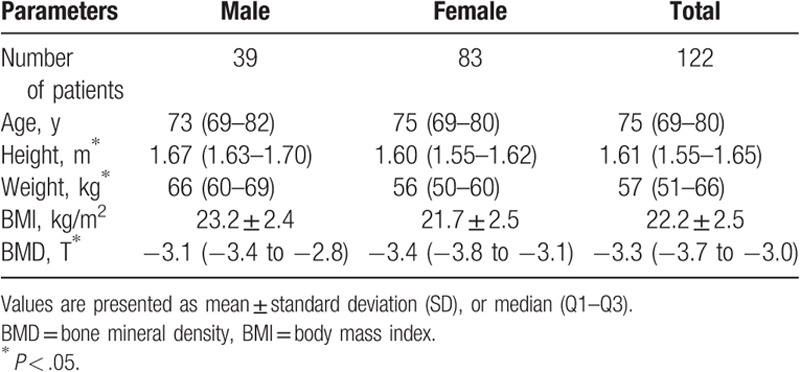
Table 2.
Number and involved levels.
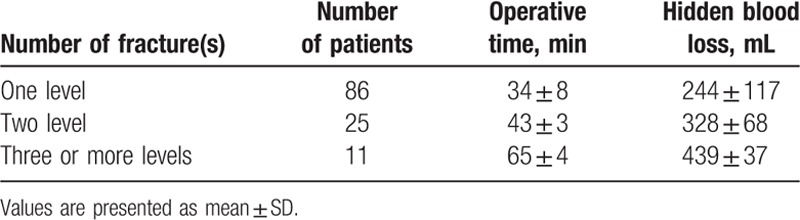
Table 3.
Clinical results in the patients after PKP surgery.

Figure 1.
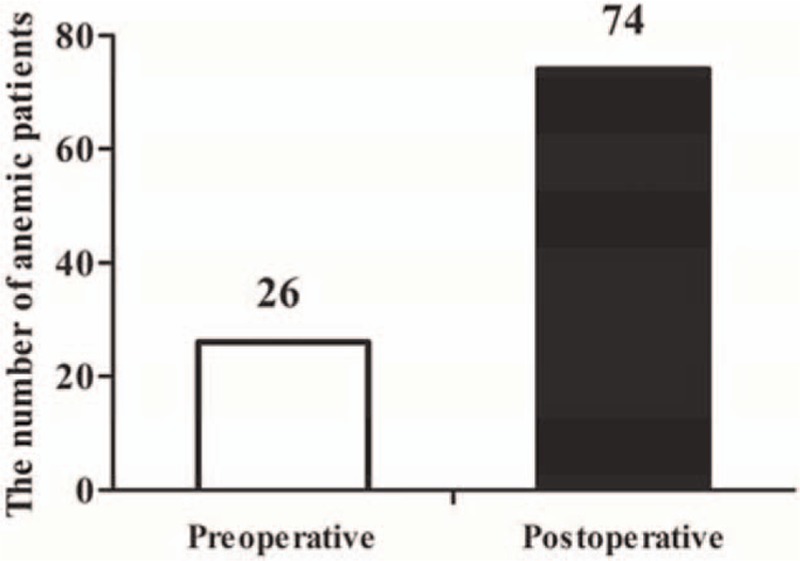
The number of anemic patients.
Table 4.
Multiple linear regression analysis on influential factors of HBL after PKP surgery.
4. Discussion
Studies on HBL after orthopedic surgery have mostly focused on total hip arthroplasty (THA), total knee arthroplasty (TKA), and ALIF/PLIF surgery but have rarely explored PKP. PKP involves a smaller incision, shorter operative time, less visible intraoperative blood loss, and less hemodynamic interference than THA, TKA, and ALIF/PLIF do. HBL is often disregarded in clinical practice. In this study, 279 ± 120 mL HBL and 8.2 ± 3.9 g/L Hb were obtained in the perioperative period. In addition, 48 patients with normal preoperative Hb levels developed anemia. This result was similar to that of Wu et al,[6] and the obtained amount was much greater than that of visible intraoperative blood loss. Excessive blood loss in the perioperative period can be associated with direct and indirect complications, especially for elderly patients with OVCFs. The correct information about HBL can improve patients’ safety and decrease the potential adverse effects of anemia.
The mechanisms of HBL possibly involve extravasation into tissue compartments, blood hemolysis, and ongoing blood loss.[1,15–17] However, influencing factors associated with the amount of HBL have yet to be elucidated. In this study, we performed multiple linear regression analysis to examine the associated factors. The results suggested that operative time, percentage of vertebral height loss, and percentage of vertebral height restoration were correlated with HBL.
Our statistical analysis showed that the patients who had long operative time suffered from more HBL than those who have short operative time. Although the duration of surgery was directly related to the number of fracture levels of the patients who underwent PKP, no correlation was noted between HBL and the number of fracture levels. This result contradicts those of Wu et al[6] and Ju et al.[4] Possibly because 14 mid-thoracic vertebral fractures were included in our study, the pedicle of the mid-thoracic vertebrae was smaller and the operation difficulty was higher than those of the thoracolumbar or lumbar vertebrae, the time of surgery for 1 level of the mid-thoracic vertebral fracture was often longer than that for 2 or 3 levels of lumbar fracture, and the operative time was shorter than that in the ALIF/PLIF surgery, although it had 3 levels or more of PKP surgery. In lumbar fusion surgery, the percentage of HBL was similar at 1 and 2 levels of operation and was not significantly different at 3 or more levels of operation. It increased the visible blood loss (surgery bleeding plus drainage).[2,18] In our study, the intraoperative and postoperative visible blood loss were disregarded, thereby possible underestimating the amount of HBL.
In the present study, HBL was directly related to vertebral fracture severity and enhanced height restoration. Vertebrae involve the cancellous bone, which is rich in blood supply. When a guide cannula is fixed, the expansion of the vertebral cavity can cause internal bleeding. An enhanced vertebral height restoration may result in an enlarged cavity, and gaps around the vertebrae may widen.[19] Vertebral cavities and intramuscular gaps also provide storage cavities for HBL.
No association was found between cement leakage and HBL. The HBL of the patients with leakage were not significantly higher than that of the patients with nonleakage. Gao et al[20] found that cortical disruption, large cement volume, and low BMD were strong predictors of cement leakage. Pre-existing cortical defects can cause persistent vertebral bleeding, which can significantly increase the volume of HBL. Polymethylmethacrylate, the most commonly used bone cement, may be an important factor inducing hemolysis because of its toxicity to cells and heat loss during solidification may result in thermal necrosis.[6] Our results differed from those of other studies possibly because of the decreased cement leakage (17/122) and amount of injected cement in our work. An abundant blood supply in the intravertebral body can bring large heat during cement solidification. As such, the correlation between cement and hemolysis should be further explored.
HBL seemed unaffected by age, gender, BMD, hypertension, and diabetes mellitus in this study. This result differed from those in THA, TKA, and intertrochanteric fracture surgery and treatment[10,21–23] likely because of variations in diagnosis, incision, surgical sites, and surgical techniques.
This study had several limitations. First, our study involved a retrospective analysis and a small sample size. As such, our findings should be performed in future prospective studies with a large number of patients. Second, the effects of hemodilution related to intravenous fluid infusion in the perioperative period were underestimated. Finally, the multilevel vertebral body fractures mainly included the thoracolumbar and lumbar areas but not the mid-thoracic vertebrae. Further research should involve more patients with mid-thoracic fractures.
5. Conclusion
PKP surgery is associated with substantial HBL. Operative time, percentage of vertebral height loss, and percentage of vertebral height restoration are the influencing factors of HBL. However, HBL seems unaffected by age, gender, BMD, number of fracture levels, hypertension, diabetes mellitus, and cement leakage. Further clinical research should focus on these factors to reduce HBL. Accurate perioperative HBL assessment can help prevent complications and improve rehabilitation.
Acknowledgment
The authors thank Yu Lang (Department of Statistics, Chongqing General Hospital) who provided statistical support.
Author contributions
Formal analysis: Daigui Cao, Shengli Zhang, Zujian Tan.
Funding acquisition: Daigui Cao.
Investigation: Daigui Cao, Shengli Zhang, Kai Shen.
Methodology: Daigui Cao.
Writing — original draft: Daigui Cao, Shengli Zhang.
Supervision: Fubin Yang, Zujian Tan.
Writing — review and editing: Zujian Tan.
Footnotes
Abbreviations: ALIF = anterior lumbar fusion surgery, BMD = bone mineral density, BMI = body mass index, Hb = hemoglobin, HBL = hidden blood loss, Hct = hematocrit, HL = height loss, HR = height restoration, OVCFs = osteoporotic vertebral compression fractures, PBV = patient's blood volume, PKP = percutaneous kyphoplasty, PLIF = posterior lumbar fusion surgery, SD = standard deviation, TBL = total blood loss, THA = total hip arthroplasty, TKA = total knee arthroplasty, VAS = visual analogue score, VBH = vertebral height.
DC and SZ contributed equally to this work and should be considered co-first authors.
This study was funded by Science and Technology Foundation of Yuzhong District in Chongqing (20160126), and the Medical and Technological Innovation Fund of Chongqing General Hospital (2016MSXM04).
The authors have no conflicts of interest to disclose.
References
- [1].Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty?. Correct blood loss management should take hidden loss into account. Knee 2000;7:151–5. [DOI] [PubMed] [Google Scholar]
- [2].Smorgick Y, Baker KC, Bachison CC, et al. Hidden blood loss during posterior spine fusion surgery. Spine J 2013;13:877–81. [DOI] [PubMed] [Google Scholar]
- [3].Mirza SB, Campion J, Dixon JH, et al. Efficacy and economics of postoperative blood salvage in patients undergoing elective total hip replacement. Ann R Coll Surg Engl 2007;89:777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ju H, Hart RA. Hidden blood loss in anterior lumbar interbody fusion (ALIF) surgery. Orthop Traumatol Surg Res 2016;102:67–70. [DOI] [PubMed] [Google Scholar]
- [5].Xie J, Ma J, Yao H, et al. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss after primary total knee arthroplasty without tourniquet: a randomized clinical trial. J Arthroplasty 2016;31:2458–64. [DOI] [PubMed] [Google Scholar]
- [6].Wu YS, Zhang H, Zheng WH, et al. Hidden blood loss and the influential factors after percutaneous kyphoplasty surgery. Eur Spine J 2017;26:1878–83. [DOI] [PubMed] [Google Scholar]
- [7].Lei J, Zhang B, Cong Y, et al. Tranexamic acid reduces hidden blood loss in the treatment of intertrochanteric fractures with PFNA: a single-center randomized controlled trial. J Orthop Surg Res 2017;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li B, Wen Y, Wu H, et al. The effect of tourniquet use on hidden blood loss in total knee arthroplasty. Int Orthop 2009;33:1263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br 2004;86:561–5. [PubMed] [Google Scholar]
- [10].Yu W, Zhang X, Wu R, et al. The visible and hidden blood loss of Asia proximal femoral nail anti-rotation and dynamic hip screw in the treatment of intertrochanteric fractures of elderly high-risk patients: a retrospective comparative study with a minimum 3 years of follow-up. BMC Musculoskelet Disord 2016;17:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee JH, Lee DO, Lee JH, et al. Comparison of radiological and clinical results of balloon kyphoplasty according to anterior height loss in the osteoporotic vertebral fracture. Spine J 2014;14:2281–9. [DOI] [PubMed] [Google Scholar]
- [12].Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery 1962;51:224–32. [PubMed] [Google Scholar]
- [13].Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology 1983;58:277–80. [DOI] [PubMed] [Google Scholar]
- [14].Beghé C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med 2004;116(suppl 7A):3S–10S. [DOI] [PubMed] [Google Scholar]
- [15].Pattison E, Protheroe K, Pringle RM, et al. Reduction in haemoglobin after knee joint surgery. Ann Rheum Dis 1973;32:582–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Erskine JG, Fraser C, Simpson R, et al. Blood loss with knee joint replacement. J R Coll Surg Edinb 1981;26:295–7. [PubMed] [Google Scholar]
- [17].Faris PM, Ritter MA, Keating EM, et al. Unwashed filtered shed blood collected after knee and hip arthroplasties. A source of autologous red blood cells. J Bone Joint Surg Am 1991;73:1169–78. [PubMed] [Google Scholar]
- [18].Xu D, Ren Z, Chen X, et al. The further exploration of hidden blood loss in posterior lumbar fusion surgery. Orthop Traumatol Surg Res 2017;103:527–30. [DOI] [PubMed] [Google Scholar]
- [19].Guglielmino A, Sorbello M, Barbagallo G, et al. Osteoporotic vertebral compression fracture pain (back pain): our experience with balloon kyphoplasty. Minerva Anestesiol 2007;73:77–100. [PubMed] [Google Scholar]
- [20].Gao C, Zong M, Wang WT, et al. Analysis of risk factors causing short-term cement leakages and long-term complications after percutaneous kyphoplasty for osteoporotic vertebral compression fractures. Acta Radiol 2018;59:577–85. [DOI] [PubMed] [Google Scholar]
- [21].Zhao J, Li J, Zheng W, et al. Low body mass index and blood loss in primary total hip arthroplasty: results from 236 consecutive ankylosing spondylitis patients. Biomed Res Int 2014;2014:742393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Miao K, Ni S, Zhou X, et al. Hidden blood loss and its influential factors after total hip arthroplasty. J Orthop Surg Res 2015;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br 2006;88:1053–9. [DOI] [PubMed] [Google Scholar]


