Supplemental Digital Content is available in the text
Keywords: heart failure, Histamine H2 antagonists, meta-analysis
Abstract
Background:
Histamine H2 antagonists (H2RAs) have long been suggested to have beneficial effects on congestive heart failure (CHF). However, full agreement about the cardioprotective effects of H2RAs is still not reached yet. Therefore, this study aims to clarify the effects of H2RAs on myocardial function in CHF patients by meta-analysis.
Methods:
Electronic databases including PubMed, Embase, and Cochrane Library were retrieved. Randomized controlled trials comparing the cardiac effects of H2RAs and placebo or other medicines were collected. Pooled mean differences (MDs) with 95% confidence intervals (CIs) were calculated and meta-analysis was performed using RevMan 5.3 software.
Results:
A total of 10 studies (472 participants) were included in this meta-analysis. H2RAs exhibited significant negative inotropic and chronotropic effects to reduce heart rate (MD: –3.90; 95%CI: –7.07 to –0.73, P = .02). Furthermore, although H2RAs did not affect the blood pressure in health volunteers, they significantly decreased the blood pressure of CHF patients. Additionally, H2RAs were also associated with significant increase in pre-ejection period and the ratio of pre-ejection period to left ventricular ejection time.
Conclusion:
In summary, these findings showed that H2RAs exerted negative inotropic and chronotropic effects to reduce heart rate and blood pressure, which, similar to beta-adrenergic receptor blockers, might decrease myocardial oxygen demand and eventually result in improvement of CHF symptoms. These data provided further evidence for the effect of H2RAs on cardiac function and novel potential strategy for treatment of CHF.
1. Introduction
Congestive heart failure (CHF) is a clinical syndrome that is characterized by reduced cardiac output and other typical symptoms such as pulmonary edema and systemic venous congestion. It is the end stage of various cardiovascular diseases. Despite recent advances in the therapy, the mortality of CHF is still high worldwide[1] and new strategies for the prevention and treatment of CHF remain an unmet medical need.
Recently, it was found that histamine H2 receptor (H2R) was closely related to the development of various cardiovascular diseases such as myocardial ischemia,[2–4] hypertension,[5] myocardial infarction,[6] and CHF as well.[7] Similar to beta-adrenergic receptors, H2R is also a Gs-protein coupled receptor and is abundantly expressed in human cardiac myocytes,[8,9] whose activation induces positive chronotropic and inotropic responses[8–11] and contributes to the exacerbation of myocardial ischemia/reperfusion injury by inducing cardiomyocyte apoptosis.[12] As beta-adrenergic receptor blockers are well acknowledged as one of the first-line therapy drugs for CHF, blockade of H2R with H2R antagonists (H2RAs) is very probably a novel and promising therapeutic strategy for CHF patients.
H2RAs are commonly used to treat peptic ulcer, which have a relatively strong safety profile.[13] However, concerns about whether H2RAs are beneficial for CHF are matter of debate. Investigations regarding the cardioprotective effects of H2RAs yielded conflicting results and there is no conclusive definition for the efficacy of H2RAs in treatment of CHF. On one hand, both animal experiments and clinical trials indicated that H2RAs were beneficial for CHF. It was reported that blockade of H2Rs improved anaerobic myocardial metabolism, protected heart against ischemia reperfusion injury, and ameliorated development of heart failure in dogs.[14,15] Meanwhile, it was also reported that famotidine improved both symptoms and ventricular remodeling associated with CHF in a clinical trial.[16] Additionally, a recent study further revealed that H2RAs use was associated with a reduced CHF risk in a multiethnic cohort without cardiovascular diseases at baseline.[17] On the other hand, however, certain studies showed opposite conclusions that H2RAs failed to demonstrate any significant effects on cardiac parameters in both CHF patients[18,19] and healthy volunteers.[20] In addition, famotidine was even reported to cause a prolonged QT syndrome.[21] Therefore, whether H2RAs have a positive impact on cardiac parameters or whether H2RAs are cardioprotective in CHF patients still needs to be further clarified.
Based on these backgrounds, we prepared a meta-analysis that synthesizes the results from all available randomized controlled trials (RCTs) trying to provide necessary power to assess whether H2RAs are cardioprotective in CHF patients.
2. Materials and methods
2.1. Criteria for considering studies of this review
Eligibility criteria. The design of studies was RCTs. Participants eligible for inclusion were healthy volunteers and CHF patients who had taken H2RAs. Type of interventions: oral H2RAs (cimetidine, ranitidine, famotidine, roxatidine, nizatidine) were used in the experimental group and placebo or other conventional therapy medicines were used in the control group. Outcomes of the study include heart rate, stroke volume, cardiac output, pre-ejection period (PEP), ratio of pre-ejection period to left ventricular ejection time (PEP/LVET), fraction shorting (FS), systolic blood pressure (SBP), and diastolic blood pressure (DBP).
2.2. Search strategy
The following keywords, Histamine H2 antagonists, Histamine H2 receptor blockers, H2-receptor antagonist, ranitidine, famotidine, cimetidine, nizatidine, roxatidine, heart failure, cardiac failure, congestive heart failure, randomized controlled trials, randomized controlled trial and clinical trial, were used as search terms in the Cochrane library, MEDLINE, EMBASE, and PubMed until February 28, 2018. In addition, reference list of the included studies and reviews on this topic was manually scanned for additional possible studies. The search strategy for PubMed as an example is presented below.
#1 Histamine receptor antagonist OR H2RAs OR Histamine H2 receptor blockers OR Histamine H2 antagonists OR Famotidine OR Ranitidine OR Cimetidine OR Nizatidine OR Roxatidine
#2 Heart failure OR Congestive heart failure OR Cardiac failure
#3 Randomized controlled trials OR Controlled clinical trials OR Clinical trials
#4 #1 AND #2 AND #3.
2.3. Data extraction and quality assessment
Data were extracted independently by 2 investigators and any disagreements were resolved by discussion or by asking a third investigator. We extracted the following items from each study: the first author's name, publication year, number of participants, participants’ age, participants’ sex, intervention, control type (placebo or other conventional therapy medicines), and outcomes (heart rate, stroke volume, cardiac output, PEP, PEP/LVET, FS, SBP, DBP).
2.4. Assessment of risk of bias in included studies
We assessed methodological quality using the Cochrane risk of bias tool and reported the results in a Risk of bias table. This tool assesses the following 7 domains of bias (decided as low risk, high risk, or unclear risk): sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Two reviewers assessed study quality independently and the assessments were verified by a third reviewer.
2.5. Ethical statement
All results and analyses were based on previous ethically-approved studies, thus no further ethical approval and patient consent are required.
2.6. Statistical analysis
Data analysis was performed by using Review Manger 5.3 software (Cochrane Collaboration, Oxford, UK). We expressed continuous variables as a mean difference (MD) or standardized mean difference (SMD) with 95% confidence interval (CI). For quantifying statistical heterogeneity, the calculation of the I2 statistic was used. A fixed-effect meta-analysis was performed when no heterogeneity was present. When substantial heterogeneity (I2 > 50%) was detected, a random-effect model was used to test the robustness of the findings or a descriptive analysis was presented instead of combining the results. The sensitivity analysis was conducted for each outcome measure to evaluate the contribution of each trial to the total estimate by removing single trial one at a time and performing the analysis based on the remaining trials. The statistical analysis was conducted in a 2-tailed manner. P < .05 was defined as statistically significant.
3. Results
3.1. Study selection and characteristics
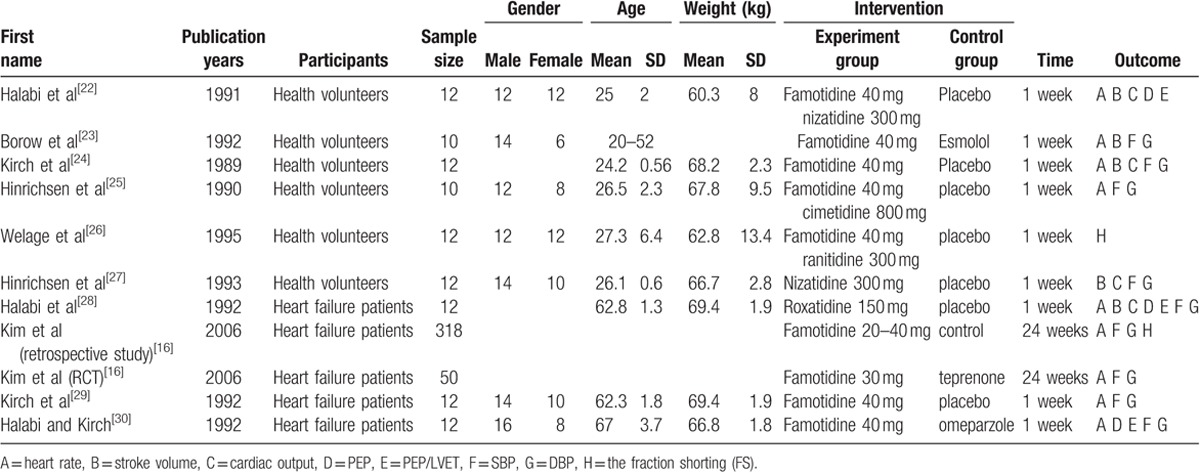
The detailed descriptions of search strategy and study selection process were shown in Fig. 1. There were 10 studies[16,22–30] with 472 participants in the present meta-analysis. The mean age of participants ranged from 20 to 64 years. The participants of 6 studies[22–27] were healthy volunteers and the rest studies enrolled CHF patients.[16,28–30] The characteristics of the included studies were summarized in Table 1.
Figure 1.
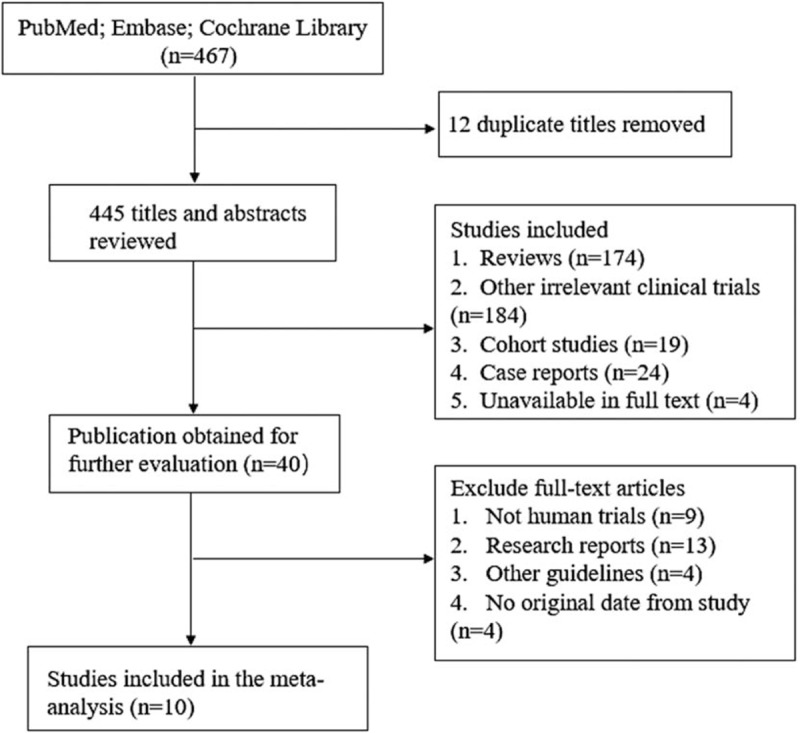
Flow diagram of eligible studies included in the meta-analysis.
Table 1.
Characteristics of studies included in the meta-analysis.
3.2. Study quality
Nine studies[22–30] stated that participants had been randomized. But none of them clearly described the detailed random sequence generation and allocation concealment. Nine studies[22–30] reported the blinding of participants except 1 study.[16] The outcomes of all studies were reported completely and none of the participants were lost to follow-up. Reporting bias was unclear in all studies as the full and detailed protocol was not available (Figs. 2 and 3). We utilized the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria to assess the overall quality of evidence supporting the primary and secondary outcome.
Figure 2.
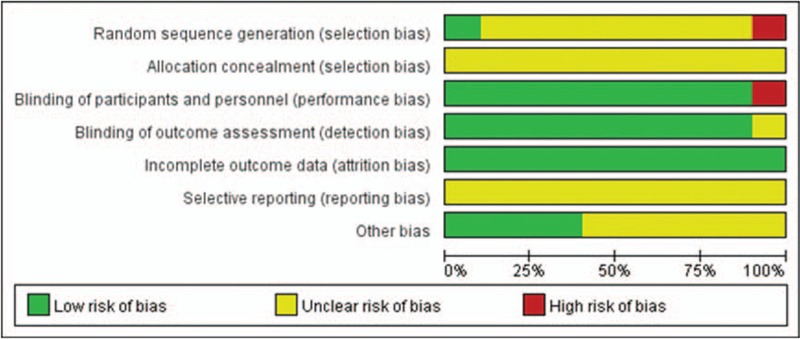
Risk of bias graph.
Figure 3.
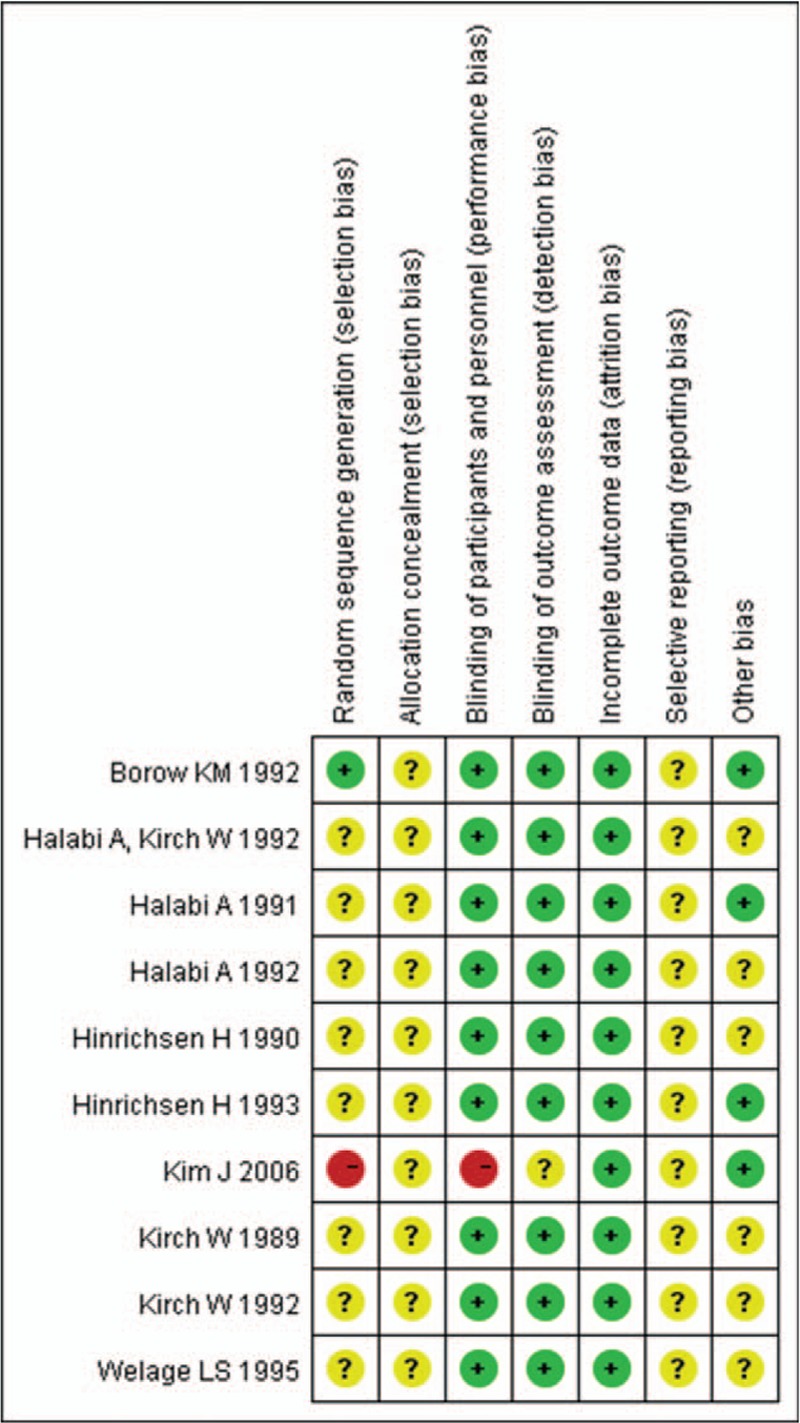
Risk of bias summary.
3.3. Primary outcome
3.3.1. Heart rate
Three included studies[16,23,24] involving 390 participants reported heart rate change after administration of H2RAs. Compared with control group, H2RAs slightly decreased the heart rate after administration (MD: –3.90; 95%CI: –7.07 to –0.73, P = .02) (Fig. 4). Considering relatively high heterogeneity (I2 = 91%), we explored whether heterogeneity was explained within the 2 subgroups (i.e., health volunteers subgroup and CHF patients subgroup). H2RAs also reduced heart rate in health volunteers (MD: –3.00; 95%CI: –5.06 to –0.94, P = .004) with significantly decreased heterogeneity (I2 = 0%). In CHF patients, the decreasing trend of H2RAs still existed with the P value at the edge of significance (MD: –4.52; 95%CI: –9.42 to 0.38, P = .07). However, the heterogeneity still remained (I2 = 97%) due to the limited number of related studies (Fig. 4).
Figure 4.

Forest plot for comparison of heart rate between H2RAs and control group. H2RAs = Histamine H2 antagonists.
3.3.2. Stroke volume
Stroke volume was reported in 3 studies[22,27,28] involving 36 participants at 1.5, 3, and 6 hours after 1 week administration of placebo or H2RAs. As shown in Fig. 5, there was no significant difference between the 2 groups on stroke volume change at 1.5, 3, and 6 hours after 1 week administration (P > .05), indicating that H2RAs had no influence on stroke volume. The sensitivity analysis also showed no significant difference on stroke volume between placebo and H2RAs at 1.5, 3, and 6 hours after removing 1 study,[28] in which the participants were CHF patients (Supplementary Fig. 1). Then, we explored whether heterogeneity was explained within health volunteers and CHF patients subgroup. There was still no significant difference between the 2 groups on stroke volume change at 1.5, 3, and 6 hours in health volunteers (P > .05, Supplementary Fig. 2). However, H2RAs significantly declined stroke volume at 3 hours (MD: –4.00; 95%CI: –6.58 to –1.42, P = .002) and 6 hours (MD: –3.90; 95%CI: –5.90 to –1.90, P = .001) in CHF patients (Supplementary Fig. 3).
Figure 5.

Forest plot for comparison of stroke volume between H2RAs and placebo group at 1.5, 3, and 6 hours after 1 week administration. H2RAs = Histamine H2 antagonists.
3.3.3. Cardiac output
Three studies[22,27,28] involving 36 participants reported the cardiac output at 1.5, 3, and 6 hours after successive administration of H2RAs or placebo for 1 week. Cardiac output was significantly reduced at 1.5 hours (MD: –0.28; 95%CI: –0.54 to –0.03, P = .03), 3 hours (MD: –0.37; 95%CI: –0.48 to –0.25, P < .001), and 6 hours (MD: –0.13; 95%CI: –0.24 to –0.02, P = .02) after administration of H2RAs (Fig. 6). In sensitivity analysis, although there was no significant difference on cardiac output change between 2 groups at 6 hours after drug administration (P = .43), the statistical difference still remained at 1.5 hours (MD: –0.37; 95%CI: –0.53 to –0.21, P < .001) and 3 hours (MD: –0.43; 95%CI: –0.59 to –0.27, P < .001) after removing 1 study,[28] in which the participants were CHF patients (Supplementary Fig. 4). We next explored whether heterogeneity was explained within subgroups. Cardiac output was significantly reduced in H2RAs group at 1.5 hours (MD: –0.37; 95%CI: –0.53 to –0.21, P < .001) and 3 hours (MD: –0.43; 95%CI: –0.59 to –0.27, P < .001), but showed no significant difference at 6 hours (P = .43) after administration in health volunteers (Supplementary Fig. 5). In CHF patients, H2RAs significantly reduced cardiac output at 3 hours (MD: –0.30; 95%CI: –0.46 to –0.14, P = .002) and 6 hours (MD: –0.20; 95%CI: –0.36 to –0.04, P = .01) (Supplementary Fig. 6).
Figure 6.

Forest plot for comparison of cardiac output between H2RAs and placebo group at 1.5, 3, and 6 hours after 1 week administration. H2RAs = Histamine H2 antagonists.
3.3.4. PEP
Three studies[22,28,30] involving 36 participants reported PEP at 1.5, 3, and 6 hours after 1 week administration of H2RAs or placebo. Compared with placebo, H2RAs were associated with significant increase in PEP at 1.5 hours (MD: 9.26; 95%CI: 7.12–11.40, P < .001) and 3 hours (MD: 9.55; 95%CI: 2.38–16.73, P = .009) but not at 6 hours (P = .38) after 1 week administration (Fig. 7). In sensitivity analysis, significant difference still remained between 2 groups at 1.5 hours (MD: 9.25; 95%CI: 5.92–12.58, P < .001) but not at 3 hours (P = .08) and 6 hours (P = .70) after removing 1 study,[22] in which the participants were healthy volunteers (Supplementary Fig. 7). Furthermore, we also explored whether heterogeneity was explained within subgroups. PEP was significantly increased in H2RAs group at 1.5 hours (MD: 9.40; 95%CI: 6.24–12.56, P < .001) and 3 hours (MD: 11.00; 95%CI: 7.02–14.98, P < .001) in health volunteers (Supplementary Fig. 8), and also at 1.5 hours (MD: 9.25; 95%CI: 5.92–12.58, P < .001) in CHF patients (Supplementary Fig. 9).
Figure 7.
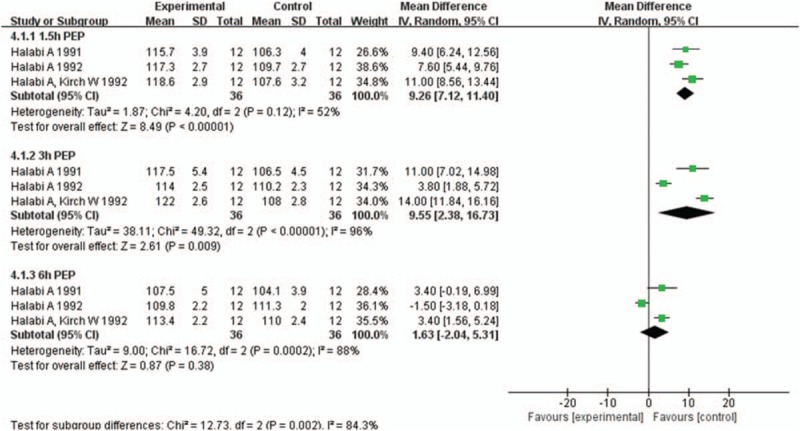
Forest plot for comparison of pre-ejection period (PEP) between H2RAs and placebo group at 1.5, 3, and 6 hours after 1 week administration. H2RAs = Histamine H2 antagonists.
3.3.5. PEP/LVET
Three studies[22,28,30] involving 36 participants reported PEP/LVET at 1.5, 3, and 6 hours after 1 week administration of H2RAs or placebo. Compared with placebo, H2RAs significantly increased the ratio of PEP/LVET at 1.5 hours (MD: 0.03; 95%CI: 0.02–0.04, P < .001), 3 hours (MD: 0.03; 95%CI: 0.01–0.05, P = .008) after 1 week administration. At 6 hours after administration, there was no significant difference between placebo and H2RAs group (P = .23) (Fig. 8). In sensitivity analysis, we removed 1 study,[22] in which the participants were health volunteers. H2RAs significantly increased the ratio of PEP/LVET at 1.5 hours (MD: 0.03; 95%CI: 0.02–0.04, P < .001) but not at 3 hours (P = .14) or 6 hours (P = .73) after administration (Supplementary Fig. 10). In the following subgroup analysis, PEP/LVET was significantly increased in H2RAs group at 1.5 hours (MD: 0.03; 95%CI: 0.01–0.04, P < .001) and 3 hours (MD: 0.04; 95%CI: 0.02–0.05, P < .001), but showed no significant difference at 6 hours (P = .09) after administration in health volunteers (Supplementary Fig. 11). In CHF patients, PEP/LVET was significantly increased in H2RAs group at 1.5 hours (MD: 0.03; 95%CI: 0.02–0.04, P < .001) but not at 3 hours (P = .14) or 6 hours (P = .73) after administration (Supplementary Fig. 12).
Figure 8.
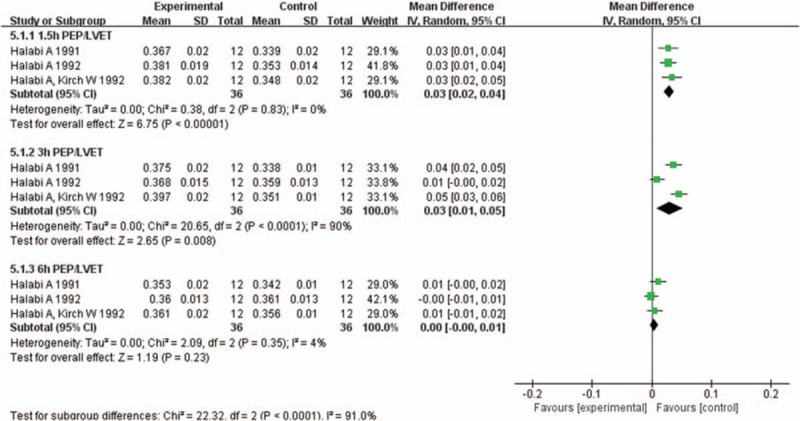
Forest plot for comparison of the ratio of pre-ejection period to left ventricular ejection time (PEP/LVET) between H2RAs and placebo group at 1.5, 3, and 6 hours after 1 week administration. H2RAs = Histamine H2 antagonists.
3.3.6. FS, SBP, and DBP
Two included studies[16,26] involving 330 participants compared the effect of H2RAs on FS change with controlled medicines. H2RAs were more effective for reducing FS (MD: –1.00; 95%CI: –1.22 to –0.78, P < .001) (Fig. 9A). Three included studies[16,23,24] involving 390 participants compared the effect of H2RAs with control group on SBP change. There was significant difference between 2 groups on SBP change (MD: –4.39; 95%CI: –7.67 to –1.11, P = .009) (Fig. 9B). Subgroup analysis showed that there was no significant difference between 2 groups in SBP in healthy volunteers (P = .31), but a significant reduce in SBP was observed in CHF patients (MD: –6.44; 95%CI: –7.68 to –5.20, P < .001) (Supplementary Fig. 13). Three included studies[16,23,24] involving 390 participants compared the effect of H2RAs with control group on DBP change. There was also significant difference between 2 groups on DBP change (MD: –3.56; 95%CI: –6.88 to –0.23, P = .04) (Fig. 9C). In subgroup analysis, H2RAs also did not have significant effect on DBP in healthy volunteers (P = .89), but significantly reduced DBP in CHF patients (MD: –6.42; 95%CI: –9.36 to –3.49, P < .001) (Supplementary Fig. 14).
Figure 9.
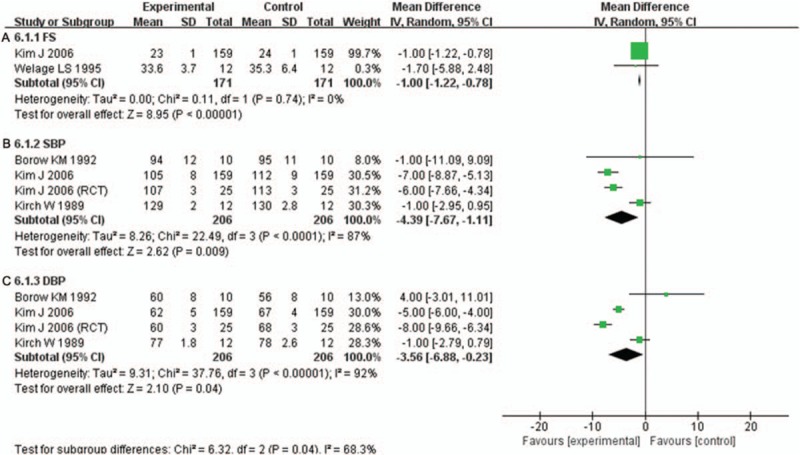
A. Forest plot for comparison of fraction shorting (FS) between H2RAs and control group. B. Forest plot for comparison of systolic blood pressure (SBP) between H2RAs and control group. C. Forest plot for comparison of diastolic blood pressure (DBP) between H2RAs and control group. H2RAs = Histamine H2 antagonists.
4. Discussion
To the best of our knowledge, this is the first meta-analysis to evaluate the cardioprotective effect of H2RAs in both health and CHF participants. CHF is a multifactorial process and factors involved in its initiation and progression are rather complicated and yet to be defined. It was recently reported that chronic activation of the sympathetic nervous system increased myocardial oxygen demand, ischemia and oxidative stress, mediated clinical progression of cardiac remodeling, and worsened CHF.[31] We previously found that histamine was a novel sympathetic neurotransmitter, which activated myocardial H2Rs and increased the intracellular cAMP level of cardiomyocytes.[32–34] In this regard, H2RAs might have similar anti-CHF effects as beta-adrenergic receptor blockers, which were highlighted as valid therapeutic tools in CHF according to numerous trials.[16,17,35] However, the potential cardioprotective value of H2RAs in CHF patients was largely neglected so far due to the lack of systematic clinical evidence. Our present work may provide further comprehensive insight into the value of H2RAs in future treatment of CHF.
4.1. Summary of main results
According to the present data, we found that H2RAs had both positive and negative influence on CHF patients. On one hand, administration of H2RAs significantly reduced the heart rate. These findings demonstrated a typical positive cardioprotective effect of H2RAs in CHF patients, in whom reduced heart rate would decrease myocardial oxygen demand, inhibit the myocardial apoptosis, and eventually result in inhibition of cardiac remolding.[36] Furthermore, H2RAs were found to decrease SBP and DBP in CHF patients but not in health volunteers, which indicated that H2RAs could also be beneficial to people with hypertension. On the other hand, H2RAs use was demonstrated to be associated with significant reduce of the cardiac output, which showed its negative inotropic effects on myocardial contractility. This is reasonable since H2R, much like beta-adrenergic receptor as we mentioned above, is also a Gs-protein coupled receptor sharing a common downstream pathway with beta-adrenergic receptor.[10,11,36] Considering that CHF is simply a state of inadequate systolic function, H2RAs might also be contraindicated for certain CHF patients on account of its negative effects such as reducing cardiac output.[22,27,28] However, it should be noted that, despite the significant negative inotropic and chronotropic effects, beta-adrenergic receptor blockers are still currently used as first-line anti-CHF drugs in clinic as they exert their effects by inhibiting the sympathetic nervous system, decreasing myocardial oxygen demand, and reducing cardiac pre- and after-load, which finally result in improvement of CHF symptoms.[31,37,38] Therefore, H2RAs may have the same cardioprotective effect as beta-adrenergic receptor blockers. Furthermore, the target of H2RAs might not be merely limited in H2Rs expressed in cardiac myocytes. In fact, in addition to cardiac myocytes, other cardiac cells such as cardiac endothelial and fibroblasts also express H2Rs,[36] which might mediate additional roles during the development of cardiac remodeling and CHF and are very likely to be novel targets of H2RAs although the possible underlying molecular mechanisms are unclear and greatly warranted to be further explored. Therefore, H2RAs probably have broad prospects in the treatment of cardiovascular diseases.
4.2. Overall completeness and applicability of evidence
Ten trials involving 472 participants were included in our meta-analysis. The trials included healthy volunteers and CHF patients and all participants finished the corresponding trials. The outcomes of all studies were reported completely and none of the participants were lost to follow-up, indicating relatively fine completeness of evidence in the present study. Furthermore, our present data provided reasonable evidence to suggest that H2RAs had certain cardioprotective effects in CHF patients, which, similar to beta-adrenergic receptor blockers, reflected its potential roles for clinical treatment of CHF. However, it should be mentioned that all the present available cardiovascular outcomes merely reflected short-term effects of H2RAs and long-term outcomes, such as cardiac remodeling index and 5-year survival rate, were unfortunately missing. Therefore, these results should be interpreted with caution and need to be considered carefully in future clinical practice. In this regard, original investigations based on high-quality and well-designed RCTs are especially needed in the future.
4.3. Quality of evidence
We used the GRADE approach to assess the quality of the evidence for the 2 primary outcomes (cardiac output and stroke volume). We graded the quality of the estimate of effect for the outcomes as low since some included studies did not clearly describe the randomization method or allocation concealment.[22,27,28] Meanwhile, there was imprecision of estimate relating to the small number of participants, which led us to downgrade the quality of the evidence (Fig. 10).
Figure 10.
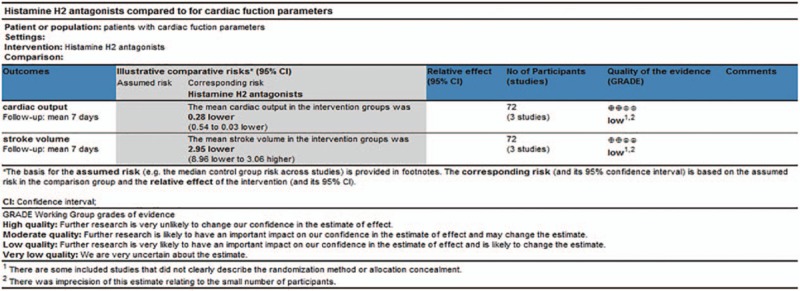
Quality of evidence in GRADE. GRADE = Grading of Recommendations Assessment, Development and Evaluation.
4.4. Potential bias and limitations in the review
We confessed several limitations in our review. Firstly, a low number of participants were considered in each trail. In addition, certain number of papers included in the present meta-analysis came from the same research group,[22,24,25,27–30] which might weaken the evidence quality. Secondly, some included studies did not clearly describe the randomization method or allocation concealment. In this regard, possibility of publication bias and other bias exists in our meta-analysis. An additional limitation is that there was no standardization of the H2RAs dose. So the dose ranges were broad and further studies based on narrow dose ranges are needed with a large number of participants. Moreover, none of the included studies reported the effects of different kinds of H2RAs on CHF patients. Finally, we did not generate a funnel plot to reveal any publication bias because there were too few included trials. Therefore, further studies, especially large-scale double-blind randomization trials, are required to provide more credible data for H2RAs treatment in CHF.
5. Conclusion
In summary, the findings of our meta-analyses showed that H2RAs reduced heart rate, cardiac output and FS. Meanwhile, H2RAs were associated with a distinguished increase in PEP and PEP/LVET. Therefore, H2RAs may decrease myocardial oxygen demand and improve CHF symptoms, which would eventually be beneficial for CHF patients. The conclusion of this meta-analysis provided evidence for the effect of H2RAs on cardiac function and novel potential strategy for treatment of CHF.
Author contributions
Conceptualization: Gong-Hao He.
Data curation: Juan Zhang, Ping Wang.
Formal analysis: Juan Zhang, Ping Wang, Xiao-Qian Lin.
Funding acquisition: Gong-Hao He.
Investigation: Wen-Ke Cai, Suo-Chao Fu, Gong-Hao He.
Methodology: Juan Zhang, Wen-Ke Cai, Xiao-Qian Lin.
Project administration: Gong-Hao He.
Resources: Juan Zhang, Wen-Ke Cai.
Software: Juan Zhang, Zheng Zhang, Ju Feng.
Validation: Juan Zhang.
Visualization: Gong-Hao He.
Writing – original draft: Juan Zhang.
Writing – review & editing: Zheng Zhang, Suo-Chao Fu, Gong-Hao He.
Supplementary Material
Footnotes
Abbreviations: CHF = congestive heart failure, CI = confidence interval, DBP = diastolic blood pressure, FS = fraction shorting, H2RA = Histamine H2 antagonist, MD = mean difference, PEP = pre-ejection period, PEP/LVET = ratio of pre-ejection period to left ventricular ejection time, RCT = randomized controlled trial, SBP = systolic blood pressure.
JZ, WKC, and ZZ have contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (no. 81460560), the National Science Foundation for Post-doctoral Scientists of China (no. 2013M532122), and the Applied Basic Research Program of Yunnan Province (no. 2017FB134).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Norton C, Georgiopoulou VV, Kalogeropoulos AP, et al. Epidemiology and cost of advanced heart failure. Prog Cardiovasc Dis 2011;54:78–85. [DOI] [PubMed] [Google Scholar]
- [2].Maintz L, Schwarzer V, Bieber T, et al. Effects of histamine and diamine oxidase activities on pregnancy: a critical review. Hum Reprod Update 2008;14:485–95. [DOI] [PubMed] [Google Scholar]
- [3].Patella V, Marinò I, Arbustini E, et al. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation 1998;97:971–8. [DOI] [PubMed] [Google Scholar]
- [4].Mackins CJ, Kano S, Seyedi N, et al. Cardiac mast cell-derived rennin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest 2006;116:1063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shiota N, Rysä J, Kovanen PT, et al. A role for cardiac mast cells in the pathogenesis of hypertensive heart disease. J Hypertens 2003;21:1935–44. [DOI] [PubMed] [Google Scholar]
- [6].Kupreishvili K, Fuijkschot WW, Vonk AB, et al. Mast cells are increased in the media of coronary lesions in patients with myocardial infarction and may favor atherosclerotic plaque instability. J Cardiol 2017;69:548–54. [DOI] [PubMed] [Google Scholar]
- [7].Hara M, Ono K, Hwang MW, et al. Evidence for a role of mast cells in the evolution to congestive heart failure. J Exp Med 2002;195:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Matsuda N, Jesmin S, Takahashi Y, et al. Histamine H1 and H2 receptor gene and protein levels are differentially expressed in the hearts of rodents and humans. J Pharmacol Exp Ther 2004;309:786–95. [DOI] [PubMed] [Google Scholar]
- [9].Parsons ME, Ganellin CR. Histamine and its receptors. Br J Pharmacol 2006;147(suppl):S127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Skovgaard N, Moller K, Gesser H, et al. Histamine induces postprandial tachycardia through a direct effect on cardiac H2-receptors in pythons. Am J Physiol Regul Integr Comp Physiol 2009;296:R774–85. [DOI] [PubMed] [Google Scholar]
- [11].Bristow MR, Ginsburg R, Harrison DC. Histamine and the heart: the other receptor system. Am J Cardiol 1982;49:249–51. [DOI] [PubMed] [Google Scholar]
- [12].Luo T, Chen B, Zhao Z, et al. Histamine H2 receptor activation exacerbates myocardial ischemia/reperfusion injury by disturbing mitochondrial and endothelial function. Basic Res Cardiol 2013;108:342. [DOI] [PubMed] [Google Scholar]
- [13].Jacobson BC, Ferris TG, Shea TL, et al. Who is using chronic acid suppression therapy and why? Am J Gastroenterol 2003;98:51–8. [DOI] [PubMed] [Google Scholar]
- [14].Takahama H, Asanuma H, Sanada S, et al. A histamine H2 receptor blocker ameliorates development of heart failure in dogs independently of beta-adrenergic receptor blockade. Basic Res Cardiol 2010;105:787–94. [DOI] [PubMed] [Google Scholar]
- [15].Asanuma H, Minamino T, Ogai A, et al. Blockade of histamine H2 receptors protects the heart against ischemia and reperfusion injury in dogs. J Mol Cell Cardiol 2006;40:666–74. [DOI] [PubMed] [Google Scholar]
- [16].Kim J, Ogai A, Nakatani S, et al. Impact of blockade of histamine H2 receptors on chronic heart failure revealed by retrospective and prospective randomized studies. J Am Coll Cardiol 2006;48:1378–84. [DOI] [PubMed] [Google Scholar]
- [17].Leary PJ, Tedford RJ, Bluemke DA, et al. Histamine H2 receptor antagonists, left ventricular morphology, and heart failure risk: The MESA study. J Am Coll Cardiol 2016;67:1544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Solomon SD, Wolff S, Jarboe LA, et al. Effects of histamine type 2-receptor antagonists cimetidine and famotidine on left ventricular systolic function in chronic congestive heart failure. Am J Cardiol 1993;72:1163–6. [DOI] [PubMed] [Google Scholar]
- [19].Lucas BD, Jr, Williams MA, Mohiuddin SM, et al. Effect of oral H2-receptor antagonists on left ventricular systolic function and exercise capacity in patients with chronic stable heart failure. Pharmacotherapy 1998;18:824–30. [PubMed] [Google Scholar]
- [20].Salmon P, Fitzgerald D, Kenny M. No effect of famotidine on cardiac performance by noninvasive hemodynamic measurements. Clin Pharmacol Ther 1991;49:589–95. [DOI] [PubMed] [Google Scholar]
- [21].Lee KW, Kayser SR, Hongo RH, et al. Famotidine and long QT syndrome. Am J Cardiol 2004;93:1325–7. [DOI] [PubMed] [Google Scholar]
- [22].Halabi A, Kirch W. Negative chronotropic effects of nizatidine. Gut 1991;32:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Borow KM, Ehler D, Berlin R, et al. Influence of histamine receptors on basal left ventricular contractile tone in humans: assessment using the H2 receptor antagonist famotidine and the beta-adrenoceptor antagonist esmolol as pharmacologic probes. J Am Coll Cardiol 1992;19:1229–36. [DOI] [PubMed] [Google Scholar]
- [24].Kirch W, Halabi A, Linde M, et al. Negative effects of famotidine on cardiac performance assessed by noninvasive hemodynamic measurements. Gastroenterology 1989;96:1388–92. [DOI] [PubMed] [Google Scholar]
- [25].Hinrichsen H, Halab A, Kirch W. Hemodynarnic effects of different H2-receptor antagonists. Clin Pharmacol Ther 1990;48:302–8. [DOI] [PubMed] [Google Scholar]
- [26].Welage LS, Dunn-Kucharski VA, Berardi RR, et al. Comparative evaluation of the hemodynamic effects of oral cimetidine, ranitidine, and famotidine as determined by echocardiography. Pharmacotherapy 1995;15:158–63. [PubMed] [Google Scholar]
- [27].Hinrichsen H, Halabi A, Fuhrmann G, et al. Dose-dependent heart rate reducing effect of nizatidine, a histamine H2-receptor antagonist. Br J Clin Pharmacol 1993;35:461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Halabi A, Nokhodian A, Kirch W. Haemodynamic effects of roxatidine, an H2-receptor antagonist. Clin Investig 1992;70:118–21. [DOI] [PubMed] [Google Scholar]
- [29].Kirch W, Halabi A, Hinrichsen H. Hemodvnarnic effects ot quinidine and famotidhe in patients with congestive heart failure. Clin Pharmacol Ther 1992;51:325–33. [DOI] [PubMed] [Google Scholar]
- [30].Halabi A, Kirch W. Cardiovascular effects of omeprazole and famotidine. Scand J Gastroenterol 1992;27:753–6. [DOI] [PubMed] [Google Scholar]
- [31].Triposkiadis F, Karayannis G, Giamouzis G, et al. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 2009;54:1747–62. [DOI] [PubMed] [Google Scholar]
- [32].Li M, Hu J, Chen Z, et al. Evidence for histamine as a neurotransmitter in the cardiac sympathetic nervous system. Am J Physiol Heart Circ Physiol 2006;291:H45–51. [DOI] [PubMed] [Google Scholar]
- [33].He G, Hu J, Ma X, et al. Sympathetic histamine exerts different pre- and post-synaptic functions according to the frequencies of nerve stimulation in guinea pig vas deferens. J Neurochem 2008;106:1710–9. [DOI] [PubMed] [Google Scholar]
- [34].He GH, Hu J, Li T, et al. Arrhythmogenic effect of sympathetic histamine in mouse hearts subjected to acute ischemia. Mol Med 2012;18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Barrese V, Taglialatela M. New advances in beta blocker therapy in heart failure. Front Physiol 2013;4:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zeng Z, Seng L, Li X, et al. Disruption of histamine H2 receptor slows heart failure progression through reducing myocardial apoptosis and fibrosis. Clin Sci (Lond) 2014;127:435–48. [DOI] [PubMed] [Google Scholar]
- [37].Talan MI, Ahmet I, Xiao RP, et al. β2 AR agonists in treatment of chronic heart failure: long path to translation. J Mol Cell Cardiol 2011;54:529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bernstein D, Fajardo G, Zhao MM. The role of β-adrenergic receptors in heart failure: differential regulation of cardiotoxicity and cardioprotection. Prog Pediatr Cardiol 2011;31:35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


