Abstract
Developing seeds constitute a strong sink for the plant and rely on the turnover and mobilization of carbon and nitrogen assimilates to supply the nutrients needed for their maturation. In large part these nutrients emanate from the vegetative organs including leaves and pod walls. Vegetative lipoxygenases (VLXs) accumulate in the paraveinal mesophyll cell layer of soybean (Glycine max L.) leaves where individual isoforms are proposed to play a role(s) as active enzymes or as transient storage proteins. VLXs also are prominent proteins in soybean pod walls, representing approximately 12% of the total soluble protein. Examining the temporal, tissue, and subcellular patterns of individual VLX isoform accumulation and of lipoxygenase activity through pod wall development indicates that VLXD is the principal VLX isoform playing a role in storage in this organ. The major accumulation of VLXD occurs just prior to seed fill within the endocarp middle zone, and protein extracted from this region shows relatively low levels of lipoxygenase activity, suggesting the middle zone may act as a storage tissue. Three other VLX isoforms, VLXA, VLXB, and VLXC colocalize to the cytoplasm of a single discrete cell layer in the mesocarp. Thus, the patterns of VLX cellular and subcellular localization in pod walls suggest independent functions for these different isoforms while also serving as specific markers for a novel cell layer in the pod wall.
Lipoxygenases (LOXs) and vegetative storage proteins (VSPα and VSPβ) play a role in the temporary storage of nitrogen in soybeans (Glycine max L.) (Staswick, 1989b; Staswick et al., 1991; Tranbarger et al., 1991; Stephenson et al., 1998; Fischer et al., 1999). Both VSPs and specific LOX isoforms accumulate in sink-regulated leaves and, upon onset of sink demand, decrease in abundance (Staswick, 1989b; Staswick et al., 1991; Bunker et al., 1995; Bunker and Grimes, 1996; Stephenson et al., 1998; Fischer et al., 1999). Individual LOX isoforms are found in discrete subcellular compartments within the paraveinal mesophyll (PVM) cell layer of soybean leaves (Stephenson et al., 1998; Fischer et al., 1999). The PVM is a reticulate layer of cells located between the spongy and palisade parenchyma and is postulated to function in the mobilization of assimilates into the minor veins (Fisher, 1967; Franceschi and Giaquinta, 1983a, 1983b; Bunker et al., 1995; Stephenson et al., 1998; Fischer et al., 1999).
In soybean, LOXs are organized in a large multigene family containing at least eight members. Three of these gene products are expressed primarily in seeds (LOX-1, -2, and -3; Shibata et al., 1987, 1988; Yenofsky et al., 1988). The other five gene products are present in vegetative tissues and germinating cotyledons, and we refer to these as vegetative lipoxygenase (VLXs) and designate the sequence as VLXA, VLXB, VLXC, VLXD, and VLXE (Bunker et al., 1995; Bunker and Grimes, 1996; Stephenson et al., 1998; Fischer et al., 1999). After sink limitation VLXD accumulates in vacuoles of the bundle sheath and PVM cell layer, whereas VLXA, B, and C localize to the cytosol of these cells (Fischer et al., 1999). In addition to the apparent role as VSPs, LOXs catalyze the addition of molecular oxygen to pentadiene moieties of polyunsaturated fatty acids. In plants, hydroperoxidation of linoleic (18:2) and linolenic acids (18:3) is the first step in the pathway leading to the biosynthesis of systemic signal molecules such as the jasmonates and epoxy and hydroxy fatty acids (Siedow, 1991; Rosahl, 1996).
Soybean pod walls function as a major nutrient reservoir for the developing seeds (Hanway and Weber, 1971; Thorne, 1979; Staswick, 1989a; Grimes et al., 1993). During seed development, starch, reducing sugars, and nitrogen mobilized from the pod wall account for over 12% of the dry weight of seeds (Thorne, 1979). Furthermore, total protein accumulates in the pod wall during development and then decreases by 4-fold during seed fill (Hanway and Weber, 1971). Grimes et al. (1993) demonstrated that soybean pod walls accumulate high amounts of LOX protein, which, together with VSPα, accumulate to approximately 45% of total pod wall proteins during early pod fill (Staswick, 1989a). These are the primary proteins to decline during major seed filling, decreasing prior to senescence and the turnover of Rubisco (Staswick, 1989a, 1989b). Pod wall LOX is also responsive to nitrogen status, increasing preferentially relative to other proteins when soil nitrogen is increased (Grimes et al., 1993).
Although the spatial and temporal pattern of accumulation of each VLX isoform has been thoroughly characterized in leaves (Fischer et al., 1999), little is known about VLX isoform accumulation patterns in the pericarp or pod wall. The objective of this study was to determine the cellular and subcellular localization patterns for the VLX isoforms and VSPα during pod development, and its transition from a sink tissue to a source tissue for the developing seeds.
RESULTS
LOX Protein Dynamics during Pod Wall Development
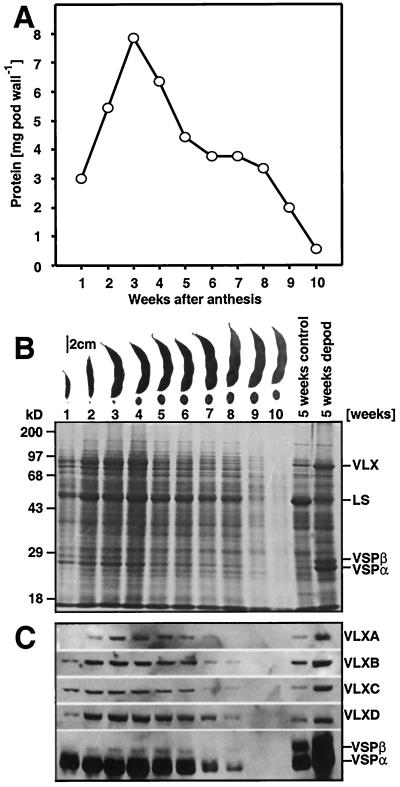
To examine the changes in protein composition in developing pod walls, proteins were extracted at weekly intervals and the total soluble protein concentration determined. Figure 1A shows weekly changes in total protein content on a per organ basis and verifies that the pericarp (pod wall) functions as a sink organ accumulating protein during the first 3 weeks of its maturation. After this, the protein content per organ decreases, reflecting the mobilization of stored pod wall protein into the developing seeds (Fig. 1A).
Figure 1.
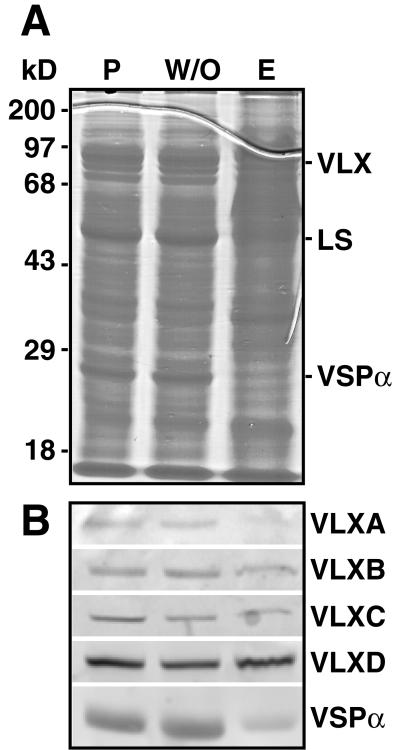
Changes in total pod wall protein and vegetative LOXs during soybean pod development. A, Total pod wall protein content per pod wall at weekly intervals during pod development. B, Pod walls were collected weekly, proteins extracted, resolved by SDS-PAGE, and analyzed by Coomassie Brilliant Blue staining. C, Immunoblotting using affinity-purified antipeptide antisera (for VLX isoforms) and a crude antisera for VSPα. B, Representative pods and seeds are shown at weekly intervals. B and C, “5 weeks control” and “5 weeks depod” refer to samples collected from soybean leaves 5 weeks postanthesis with pods present (“5 weeks control”) or from plants that had their pods removed daily for 5 weeks (“5 weeks depod”). LS refers to the large subunit of Rubisco. For pod walls, samples corresponding to 0.3% of a pod wall were loaded in each lane.
Since both VSPs and specific LOXs function as VSPs in soybean leaves, we next determined whether these proteins were present in the pod wall and quantified their change during pod development. Figure 1B demonstrates that both VLXs and VSPα accumulate in the pod wall during the first 3 to 4 weeks and then decrease. At week 3, VLXs and VSPα together represent approximately 24% of total soluble protein, each accounting for approximately 12% of pod wall protein. By week 7, VLXs and VSPα accounted for only approximately 8% of the total protein with VLX falling to approximately 3% and VSPα to approximately 5% by this time. The preferential change in VLXs and VSPα as compared with other soluble proteins levels suggests that these proteins are a significant source of nitrogen and carbon during this time of seed development. The level of the large subunit of Rubisco, for instance, remains relatively constant (approximately 17%) through pod wall development and decreases along with other proteins only after 8 weeks during pod senescence (Fig. 1B). However, all pod proteins are declining during these latter stages and represent potentially important nutrient sources for the developing seeds.
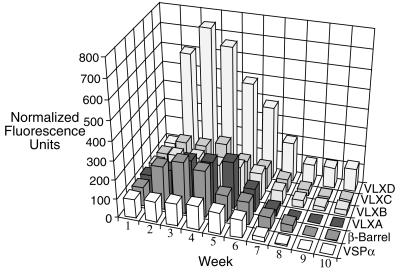
LOXs exist as a multigene family in soybeans, are differentially regulated, and accumulate in response to varying environmental conditions and signals (Bunker et al., 1995; Bunker and Grimes, 1996; Stephenson et al., 1998; Fischer et al., 1999). Hence, affinity-purified antipeptide VLX antibodies were used to determine the steady-state levels of individual isoforms during pod wall development. These antibodies are specific for individual VLX isoforms (Fischer et al., 1999). Immunoblots of pod wall proteins at weekly intervals show that VLXA, VLXB, VLXC, and VLXD are present in the approximately 94-kD band from the pod walls (Fig. 1C). Semiquantitative analysis of the individual isoform levels using fluorescent imaging, normalized to 100 fluorescent units at week 1, indicates that VLXD increases most dramatically during the first 3 weeks of pod development (Fig. 2). VLXD increases over 7-fold from its initial level at week 1, whereas the other VLX isoforms and VSPα increase by 0.5- to 2-fold (Fig. 2). This analysis suggests that VLXD functions as a major storage LOX isoform in developing pod walls since it is preferentially accumulated early during pod elongation prior to its presumed mobilization during seed fill.
Figure 2.
Semiquantitative changes in vegetative LOX isoforms and VSPα levels at weekly intervals during pod development. Data compiled and semiquantified from immunoblots shown in Figure 1C. The “β-barrel” refers to quantification with an antibody to the expressed N′-terminal β-barrel of VLXC, which cross-reacts with all soybean LOXs.
Cellular and Subcellular Localization of LOXs
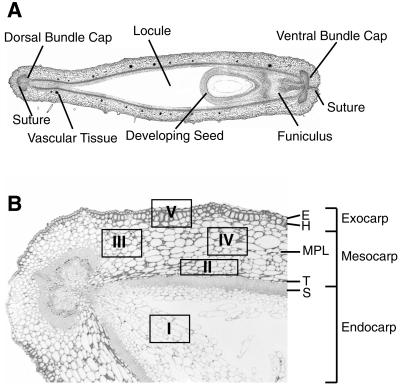
Figure 3A presents an overview of pod wall anatomy, whereas Figure 3B shows a light micrograph cross-section of a 3-week-old pod wall. The different shapes between these two reflect both different ages and pod-to-pod variability. The boxes and roman numerals on Figure 3B represent five regions of the pod wall that were repetitively sampled for staining with various antibodies used in this manuscript and will be referred to throughout this manuscript. Immunolocalization with all antibodies was performed with many sections replicated throughout the pod wall. The data presented in this manuscript show representative examples of regions and cells where VLX isoforms were present.
Figure 3.
Anatomy of the soybean pod wall. A, Cross-section of an entire 3-week-old pod. B, Bright field micrograph of a smaller region within a pod wall cross-section. Anatomical regions of the pod wall are indicated. The boxes with Roman numerals inside serve throughout this manuscript as reference points indicating where additional sections were obtained for further analysis. E, Epidermis; H, hypodermis; T, transition zone; S, sclerenchyma.
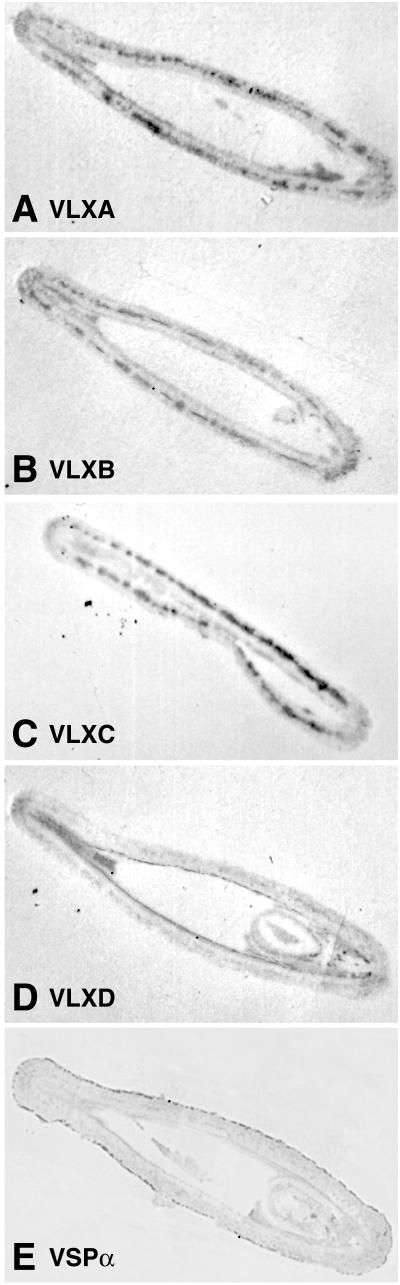
Tissue printing of pod walls was used to determine the distribution of the VLX isoforms and VSPα during pod development, and Figure 4 shows a sequence of sections taken at the middle seed locule from a 3-week-old pod with each section stained with a different antibody. VLXA, VLXB, and VLXC localize to a discrete layer within a narrow region in the mesocarp (Fig. 4). The cell layer marked by VLXA, VLXB, and VLXC did not fully circumnavigate the pod wall but terminated at each suture. VLXD is associated with the inner endocarp, whereas VSPα is seen primarily in the epidermal layer (Fig. 4). Examination of pod walls of other ages and areas indicated the individual isoforms to be distributed in the same pattern as observed in Figure 4.
Figure 4.
Distribution of VLX isoforms and VSPα in tissue prints derived from soybean pod wall cross-sections. A cross-section of the pod was made at the middle seed locule from a 3-week-old pod and the cut surface used to tissue print a nitrocellulose membrane. Membranes were developed using affinity-purified antipeptide antisera against the VLX isoforms. A, Stained with affinity-purified anti-VLXA peptide antiserum. B, Stained with affinity-purified anti-VLXB peptide antiserum. C, Stained with affinity-purified anti-VLXC peptide antiserum. D, Stained with affinity-purified anti-VLXD peptide antiserum. E, Stained with anti-VSPα antiserum. Arrows indicate regions of intense immunolabeling.
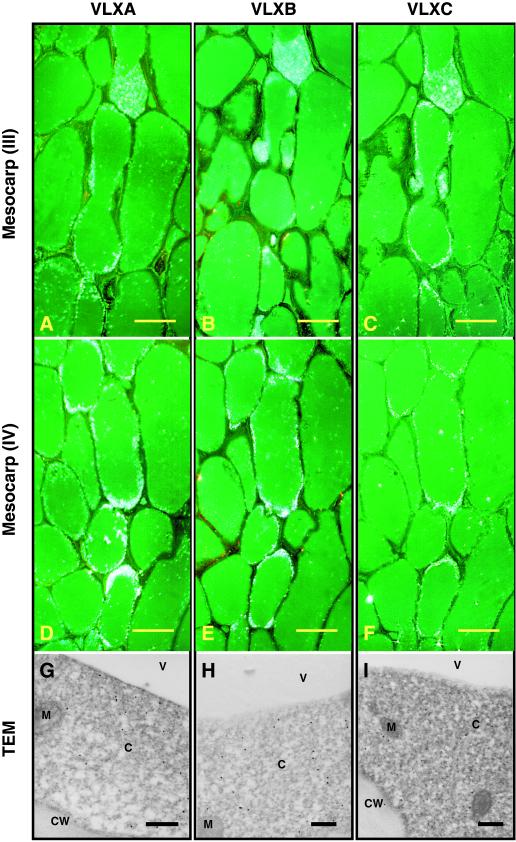
Figure 5 shows VLXA, VLXB, and VLXC localization patterns within two areas of the pod wall mesocarp indicated by regions III (Fig. 5, A–C) and IV (Fig. 5, D–F) from Figure 3B. Thus, epipolarized dark field microscopy verifies that VLXA, VLXB, and VLXC colocalize to a single discrete layer of cells within the mesocarp (Fig. 5). This cell layer, which we designate the mid-pericarp layer (MPL), runs the length of the pod wall outside the minor veins and ceases below the cleft where the thick sclerified walled cells form the dorsal and ventral bundle caps. Transmission electron microscopy (TEM) analysis shows that VLXA, VLXB, and VLXC are present in the cytosol of the MPL layer (Fig. 5, G–I). This pattern of cellular and subcellular localization for VLXA, VLXB, and VLXC is reminiscent of that seen in soybean leaves, where these isoforms specifically localize to the cytoplasm of the PVM (Fischer et al., 1999).
Figure 5.
Light and electron microscopy immunolocalization of VLXA, VLXB, and VLXC in 3-week-old soybean pod walls. Note the accumulation of these isoforms in a single cell layer, which confirms the observations seen in Figure 4. Sections were developed using affinity-purified antipeptide antisera for VLXA, VLXB, and VLXC. VLXA, VLXB, and VLXC colocalized to a distinct cell layer in the middle region of the mesocarp. Sections taken from regions III and IV (refer to Fig. 3B) illustrate this cellular localization. A through C, Sections taken from mesocarp region III stained with antisera against VLXA, VLXB, and VLXC, respectively. D through F, Sections taken from mesocarp region IV stained with antisera against VLXA, VLXB, and VLXC, respectively. Scale bar = 50 μm (A–F). G through I, TEM showing subcellular localization of VLXA, VLXB, and VLXC (respectively) in the cytosol of these cells. Scale bar = 500 nm (G–I). C, Cytoplasm; CW, cell walls; M, mitochondria; V, vacuole.
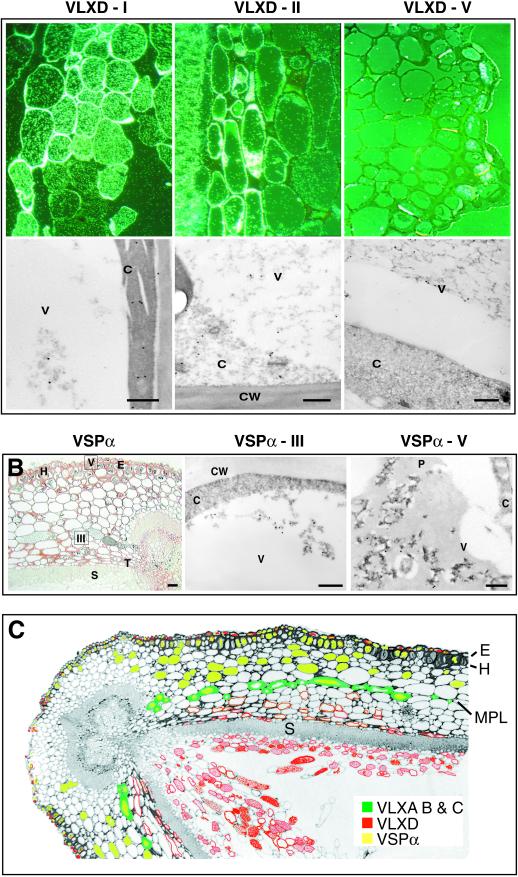
Figure 6A shows that VLXD labels three different regions of the pod wall: regions I, II, and V from Figure 3B. It is interesting that VLXD is not associated with the MPL (data not shown). The largest amount of VLXD appears in the loose parenchyma cells of the differentiated endocarp middle zone (region I; Fig. 6A, upper left panel). In this differentiated middle zone, TEM shows that most of the VLXD is within the cytoplasm with less pronounced labeling observed on the vacuolar proteinaceous material (Fig. 6A, bottom left panel). In the mesocarp (region II; Fig. 6A, upper center panel), VLXD labels both the cytoplasm and vacuolar material of cells interior to the MPL with increasing labeling toward and including the transition layer of the endocarp (Fig. 6A, bottom center panel). Consistent background labeling was noted in the thick cell walls of the sclerenchyma “S” layer in all antibody preparations, including nonimmune controls. In the exocarp, VLXD labels the epidermal cells (Fig. 6A, upper right panel), and TEM indicates that this isoform was present in the vacuoles of the outer epidermis (Fig. 6A, bottom right panel). The observation that VLXD is seen both in vacuoles (epidermal cells) and the cytosol (endocarp cells) may suggest that this antiserum is detecting another LOX isoform in pod walls or that VLXD exhibits dual localization. VSPα colocalizes with VLXD to the vacuoles of the outer epidermis but additionally labels the hypodermis and outer mesocarp cells (Fig. 6B; left panel) confirming the histochemical results for VSPα seen in Figure 4. The slight discrepancies in labeling between Figure 4 (histochemical) and Figure 6 (immunocytochemistry) probably reflect differences in resolution between these two techniques with immunocytochemistry being more sensitive. In addition, labeling was noted for VSPα in some vacuoles of the MPL, especially in those cells near the dorsal suture that contain visible proteinaceous material (Fig. 6B, left panel). This too is similar to the localization of VSPα to the PVM and to leaf epidermis (Fischer et al., 1999). TEM immunolocalization verifies the presence of VSPα in the vacuole of the MPL cells (Fig. 6B, center panel) and epidermal cells (Fig. 6B, right panel).
Figure 6.
Light and electron microscopy immunolocalization of VLXD and VSPα in 3-week-old soybean pod walls. A, Upper panels, epipolarized views of regions I (endocarp middle zone), II (mesocarp), and V (exocarp; refer to Fig. 3B) stained with antisera for VLXD. Bar = 50 μm. Lower panels, TEM immunolocalizaton of VLXD at the subcellular level from regions corresponding to those shown in the upper panels. Bar = 500 nm. B, Left, bright field micrograph showing cellular localization of VSPα in pod walls. Bar = 50 μm. Center, TEM immunolocalization of VSPα in region III (mesocarp). Bar = 500 nm. Right, TEM immunolocalization of VSPα in region V (exocarp). Bar = 500 nm. C, False-colored micrograph of a soybean pod wall indicating the immunolocalization patterns for VLXA, VLXB, VLXC, VLXD, and VSPα. P, Phenolics.
Figure 6C presents a summary of the immunolocalization results obtained from a 3-week-old pod wall. The majority of VLXD (red) is found in the endocarp, whereas VLXA, VLXB, and VLXC (green) mark the presence of a previously uncharacterized, but distinct, cell layer that we term the MPL. This figure also underscores the anatomical separation of the majority of VLXD to the endocarp middle zone away from VSPα and the other VLX isoforms in the MPL. The localization of VLXA, VLXB, and VLXC was consistent in all ages of tissue examined.
Immunolocalization indicates the middle zone of the endocarp to be the major site of VLXD accumulation, however, VSPα did not localize to this tissue. To test the validity of the VLXD immunolocalization, total proteins were extracted from isolated inner endocarp layers, pod walls without these endocarp cells, and from whole pod walls. Proteins were resolved on SDS-PAGE and visualized with Coomassie Brilliant Blue (Fig. 7A). The immunoblots shown in Figure 7B show that VLXD is enriched relative to VLXA, VLXB, and VLXC within the endocarp layers and confirms that VSPα is in low abundance within this tissue. Consistent high levels of VSPα and its segregation to the outer mesocarp and exocarp suggest it may be involved in temporary nutrient storage for early pod growth in addition to its role in nutrient redistribution during seed fill.
Figure 7.
Distribution of vegetative LOXs in pod wall tissues. Three-week-old pods were harvested and proteins extracted from pod walls (P), the inner endocarp (E), and the pod wall without the inner endocarp (W/O). A, Equal quantities of protein (10 μg) were resolved by SDS-PAGE and stained with Coomassie Brilliant Blue. B, After SDS-PAGE, proteins were immunoblotted using antisera to affinity- purified anti-peptide VLXA, VLXB, VLXC, or VLXD antisera and VSPα antisera.
In Vitro Activity of Pod Wall LOXs
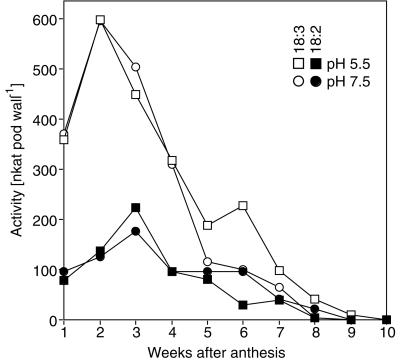
To allow comparison of total LOX protein levels and their patterns of cellular and subcellular localization to their potential enzymatic activity in the pod wall, we examined LOX activity in pod wall extracts. Figure 8 expresses LOX activity, using both 18:3 and 18:2 as substrates at pH 5.5 and 7.5, on a per-organ basis from the pod wall. These data indicate that total LOX activity peaks at 2 weeks with 18:3 as substrate and at 3 weeks with 18:2 as substrate at both pH 5.5 and 7.5. It is somewhat surprising that LOX activity decreases so sharply after this initial peak since immunoblotting (Fig. 1C) shows that substantial amounts of VLXA, VLXB, VLXC, and VLXD are present through 5 weeks postanthesis.
Figure 8.
LOX activity at weekly intervals during pod wall development. Pod walls were harvested at weekly intervals, extracted, and assayed for LOX activity at pH 5.5 and 7.5 using 18:3 or 18:2 fatty acids as substrates.
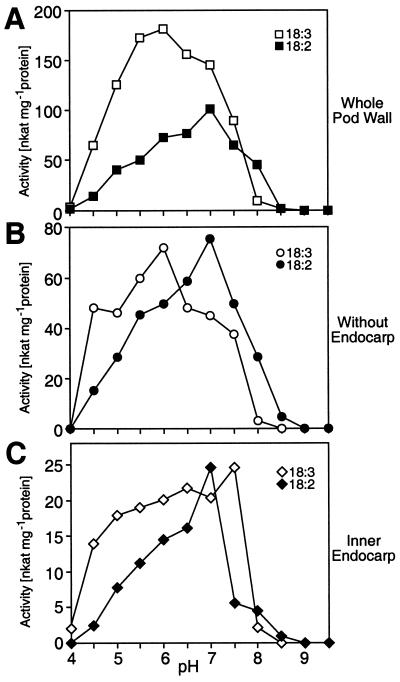
Fischer et al. (1999) demonstrated that individual VLX isoforms have distinct pH optima when expressed as fusion proteins. Our immunolocalization data indicate that VLXD is primarily associated with the inner endocarp and that VLXA, VLXB, and VLXC mark the MPL cell layer. Thus, to determine whether specific activities, substrate preference, or pH optima varied between these different sites of localization, proteins were extracted from whole pod walls, the inner endocarp, and pod walls without endocarp. These extracts were subjected to LOX activity assays over a pH range of 4.0 to 9.5. With the data expressed on an equal protein basis, whole pod wall extracts demonstrated a strong preference for 18:3 at pH 6 and a lower preference for 18:2 at pH 7 (Fig. 9A). When the endocarp was removed, there was equal preference for both 18:2 and 18:3 substrates with pH optima of 6 and 7, respectively (Fig. 9B). In isolation the inner endocarp exhibited the lowest level of LOX activity but retained a nearly equivalent preference for 18:2 and 18:3 substrates with pH optima of 7.0 to 7.5. These data, compared with Fischer et al. (1999), suggest that the different VLX isoforms are potentially active in vivo.
Figure 9.
Characterization of LOX activity in different pod wall tissues. A, pH profile of LOX activity extracted from whole pod walls using 18:3 or 18:2 as substrate. B, pH profile of LOX activity extracted from pod walls without inner endocarp layers using 18:3 or 18:2 fatty acids as substrate. C, pH profile of LOX activity extracted from inner endocarp layers using 18:3 or 18:2 as substrate.
DISCUSSION
Soybean pod walls act as temporary storage sites where the VSPs (VSPα and LOX) act as pericarp storage proteins that preferentially accumulate during pod fill and then decrease during seed development (Staswick, 1989a, 1989b; Grimes et al., 1993). By examining changes in the accumulation and distribution of VSPα and each VLX isoform during pod wall development, we conclude that both VSPα and VLXD act as pericarp storage proteins and identify discrete regions of the pod wall that appear to function as storage tissues. VLXA, VLXB, and VLXC localize to and identify a unique cell layer within the mesocarp, which we term the MPL. Although the function of the MPL is currently unknown, a more detailed investigation of this novel cell layer is presented in Dubbs and Grimes (2000).
In previous reports, examining individual VLX isoform accumulation and subcellular pattern of accumulation in soybean leaves, we showed that VLXD is the principal VLX isoform responsible for nitrogen storage in PVM vacuoles (Stephenson et al., 1998; Fischer et al., 1999). The observed pattern of VLXD accumulation and depletion in pod walls also implicate VLXD as a principal storage protein in this organ. Between weeks 3 and 4 the amount of protein per pod wall peaks and then decreases, signifying the conversion of the pod wall from a sink to a source tissue, and VLXD most strongly correlates with this conversion. In soybean leaves, VLXD accumulates in the vacuolar compartment after a conversion of these vacuoles to a storage function, which is marked by the accumulation of δ-tonoplast intrinsic protein (Jauh et al., 1998; Stephenson et al., 1998; Fischer et al., 1999). In pod walls, however, VLXD localizes within both the vacuole and the cytoplasm of cells in the endocarp middle zone. This may indicate either a cross-reaction of the anti-VLXD antibody with another LOX that we have not yet identified or the possibility that VLXD localizes to discrete subcellular compartments. Late embryogenesis abundant proteins, for instance, have been reported to associate with multiple subcellular compartments (Marttila et al., 1996). In this regard, it is interesting to note that VLXD lacks a typical N′-terminal leader peptide (Nakamura and Matsuoka, 1993; Bunker et al., 1995; Stephenson et al., 1998; Fischer et al., 1999) and is postulated to be transported by an autophagic process directly at the tonoplast (Fischer et al., 1999).
The predominance of VLXD in the endocarp middle zone and its subsequent depletion indicate this tissue may function in storage and assimilate mobilization, conducting nutrients along the endocarp to the funiculus and into the developing seed. This is a previously undefined function for this tissue, which in broad bean has been interpreted to function in maintaining optimal moisture conditions around the developing seed (Kaniewski, 1968; Esau, 1977). LOX has also been reported within the endocarp and mesocarp in very early stages of pea pericarp development (Rodríguez-Concepcíon et al., 1996). However, at ages over 3 DPA, this LOX was absent, and Rodriguez-Concepcion et al. (1996) conclude that LOX is associated with early cell growth and expansion.
Another region implicated to act as a storage tissue is the outer mesocarp and exocarp. The vegetative storage protein VSPα is a major pod wall protein and localizes within vacuoles of these cells and colocalizes with VLXD within the outer epidermis. The pattern of accumulation for VSPα is not only spatially separated from the endocarp middle zone, but it is also temporally separated from VLXD as the highest relative concentrations of VSPα occur early in pod wall growth and thus may represent an additional role for VSPα in nutrient storage for the initial rapid growth and development of the pod wall.
LOXs, in addition to any role in nitrogen storage, probably function in lipid hydroperoxidation as well. Pod wall LOX activity assays indicate that one or more VLX isoform is an active enzyme. The pattern of VLXA, VLXB, and VLXC accumulation does not indicate a major role for these isoforms in nitrogen storage but does correspond to the observed changes in LOX activity over the development of the pod wall. The occurrence of these isoforms within the cytoplasm of the MPL may indicate a novel physiological function for these isoforms and this layer in lipid metabolism (Fischer et al., 1999) or another function.
In summary we have shown VLXD and VSPα act as pericarp storage proteins that are found in separate, defined regions of the pod wall. The differentiation and development of the endocarp middle zone and the accumulation of VLXD to these cells implies this region is acting as a major storage tissue that developing seeds can use during seed fill. VLXA, VLXB, and VLXC localize to a single cell layer in the mid-pericarp region and thus serve as specific markers for this MPL region. A similarly positioned cell layer in Phaseolus was shown to label with a general LOX antibody (Eiben and Slusarenko, 1994) and might be homologous to the MPL described here. The occurrence of this layer and the specificity of VLX isoform labeling suggest a biochemical function separate from that of protein storage.
MATERIALS AND METHODS
Plant Material
Soybean (Glycine max L. Merrill cv Wye) plants were grown under greenhouse conditions with supplemental light of 16 h at variable intensities, depending on ambient light, not falling below a photosynthetic photon flux density of 150 μmol m−2 s−1. Plants were thinned to one plant per 1-gallon pot and fertilized weekly with excess 500 μg μL−1 nitrogen. Developmental stages of soybean pods grown under greenhouse conditions were determined using the protocol of Peterson et al. (1992). Pods were collected weekly between 6 and 7 h into the photoperiod, and an age series including the process of pod development, seed development through pod dehiscence (approximately 10 weeks from day of anthesis) was obtained. Three-seeded pods from the first four nodes of racemes containing seven to 10 flowers were used as they are more likely to set pods than those that are more distal (Brun and Betts, 1984).
Protein Extraction, Gel Electrophoresis, Immunoblotting, and LOX Assays
Total soluble protein was extracted as per Grimes et al. (1993). Seeds were separated from pod walls when possible (due to their small size, seed removal from 1-week-old pods was not feasible). After seed removal, the fresh weight of pod walls was determined, and the tissue was frozen in liquid nitrogen and dried overnight in a Virtis Lyophilizer (no. 6201 3130, Virtis, Gardiner, NY). After determination of the dry weight, the tissue was stored with desiccant at −80°C. Dry tissue was ground to a powder in an electric coffee mill for 1 min, transferred to a room temperature mortar and pestle, and ground for an additional 40 s using 1 mL of extraction buffer per 0.5 g fresh weight. The extraction medium consisted of 25 mm Tricine (N-[tris(hydroxymethyl)methyl]Gly), pH 7.5, 1% (w/v) insoluble polyvinylpolypyrrolidone, 1 mm EDTA, 10 mm β-mercaptoethanol, 10 μm leupeptin, 1 μm pepstatin, and 0.57 mm phenylmethylsulfonyl fluoride. These extracts were centrifuged for 10 min at 15,000g at 4°C, and the supernatants were assayed for protein concentration with protein assay reagent (Bio-Rad Laboratories, Hercules, CA). Aliquots of these extracts were stored at −80°C prior to analysis of LOX activities.
For some experiments, total soluble protein was also separately extracted from whole pod walls, the endocarp, and pod walls minus the endocarp. To accomplish this, 4- to 6-week-old postanthesis pods were split along their dorsal and ventral sutures. The inner layer of the endocarp (middle zone and inner epidermis) was pulled away from the sclerenchyma cells (Gijzen et al., 1999), frozen in liquid nitrogen, lyophilized, and protein extracted as above. Total soluble protein was also extracted from the remaining pod walls without these endocarp layers.
For SDS-PAGE, extracts were mixed (1:1, v/v) with 2× Laemmli sample buffer (Laemmli, 1970) and boiled for 5 min. Protein extracts from weekly harvested pod walls were loaded on 12% (w/v) SDS-PAGE gels on a per organ basis. Each lane represents total soluble protein extracted from 0.3% of a pod wall. The SDS-PAGE gels for pod wall endocarp were loaded on an equal protein basis (usually 15 μg). To allow semiquantification of LOX, VSP, and protein levels, gels were stained with Sypro Red (Molecular Probes, Eugene, OR), analyzed using a Storm 860 FluorImager (Molecular Dynamics, Sunnyvale, CA), and quantified using ImageQuant software (Molecular Dynamics). These gels were then stained with Coomassie Brilliant Blue R-250 (Bio-Rad).
Proteins resolved by SDS-PAGE were electroblotted to nitrocellulose membranes according to Towbin et al. (1979). Blots were blocked for 1 h in 5% (w/v) non-fat dry milk in TTBS (0.2% [v/v] Tween 20, 25 mm Tris [tris(hydroxymethyl)aminomethane], 140 mm NaCl, and 5 mm KCl, pH 7.4). After blocking, the blots were incubated with primary antibody for 1 h in TTBS containing 1% (w/v) milk and 0.1% (v/v) Tween 20. Affinity-purified peptide specific antibodies to VLXA, VLXB, VLXC, and VLXD were used (Stephenson et al., 1998; Fischer et al., 1999) as well as anti-VSPα and an antibody to the β-barrel domain of VLXC (used as a nonisoform-specific LOX antibody). The secondary antibody was goat-anti-rabbit IgG alkaline phosphatase conjugate diluted 1:10,000 in the same TTBS solution. Antigens were visualized using enzymatic chemofluorescence detection (Amersham, Buckinghamshire, UK) with a Storm 860 FluorImager (Molecular Dynamics). Results were analyzed with ImageQuant software (Molecular Dynamics) using the “volume method” for quantifying individual blots (this semiquantification method is valid only for comparing the results from separate individual immunoblot preparations). All electrophoretic analyses were done at least three times with different groups of plants, and representative results are shown. LOX activities were measured with an oxygen electrode (Hansatech DW1, Hansatech, Norfolk, UK) as outlined in Fischer et al. (1999).
Tissue Prints
Pods were examined at 2, 3, and 5 weeks postanthesis. Three transverse section sample sites were selected from each pod: at the constricted area near the pistil end, near the pedicel-constricted area, and at the middle locule. Cut sections produced an exudate so were blotted on filter paper several times before pressing onto nitrocellulose. Resulting tissue prints were processed following the protocol of Ye and Varner (1991). Incubation with primary antibody was done overnight for anti-VLX isoforms or for 4 h with anti-VSPα. Positive results were noted by development of a region-specific blue coloration. Developed tissue prints were rinsed with distilled water, air dried, and photographed using an Olympus C-35AD (Olympus Optical, Tokyo) attached to a dissecting microscope (Wild M5A, Wild Leitz, Heerbrug, Switzerland).
Immunolocalization
Approximately 1-mm-thick cross-sectional pieces of tissue were made of the pod wall locule at an area opposite the middle seed. Tissue was fixed for 2 d on a rotator in 1.25% (v/v) glutaraldehyde plus 2% (v/v) paraformaldehyde in 50 mm PIPES (1,4-piperazinediethanesulfonic acid) buffer, pH 7.2. Tissue was washed three times over 1 h in buffer alone then dehydrated and infiltrated into LR White resin in a microwave processor (Pelco 3450 Lab, Ted Pella, Redding, CA) following the procedure of Giberson and Demaree (1995) and Giberson et al. (1997).
Thick sections (600 nm) were made and dried onto Silane-Prep slides (Sigma, St. Louis). Thin sections (100 nm) were made and placed on nickel grids. Immunolocalization was done similarly for both thin and thick sections. Sections were blocked for 1 h in TTBS using 1.5% (w/v) bovine serum albumin in 10 mm Tris (pH 7.2) using 500 mm NaCl with 0.3% (v/v) Tween 20 (2% [w/v] ovalbumin was used in place of the bovine serum albumin for the anti-VLXD antibody). To each blocker 0.5% (w/v) soluble polyvinylpyrrolidone was added to decrease antibody adhering to phenolics. The concentration of primary antibody and the length of incubation were dependent upon the affinity of the antibody used. All primary antibodies were applied to multiple sections within each anatomical region of the pod wall. Thus, each figure presented is representative of results replicated at several times. Furthermore, the data presented only show where positive results were obtained as inclusion of all immunolocalization results would be unwieldy. Sections were washed five times in blocking solution and then incubated with secondary reagent for 1 h (thick sections were incubated with 5-nm gold-linked goat-anti-rabbit serum 1:50 [Amersham]; thin sections were exposed to 1:50 protein A/G 20-nm gold [Amersham]). Sections were then washed for 1.5 h in blocking solution, then blocking solution without protein, and finally in filtered distilled, deionized water. Thick sections were allowed to dry slightly, then silver enhanced for 10 min (Amersham), stained with 0.5% (w/v) Safranin-O for 1 min, rinsed, and imaged using a microscope (Leitz Aristoplan, Midland, Ontario) with an Orthomat attachment for micrographs. Micrographs of both bright field and dark field (using epipolarized light) were obtained. Thin sections were stained for 3 min in 2% (w/v) uranyl acetate:1% (w/v) potassium permanganate (2:1), rinsed, and examined in an electron microscope (JEM-1200ex, JOEL, Tokyo). For all antibodies, negative controls were performed using either nonimmune or preimmune serum. To conserve space, these negative controls are not included, but none showed significant labeling.
ACKNOWLEDGMENTS
The authors are indebted to Dr. Andreas Fischer for critical evaluation of the manuscript and to Aaron Elmer for assistance with the figures. The authors also thank Dr. Paul Staswick (University of Nebraska, Lincoln) for the VSPα antibody.
Footnotes
This work was supported by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (grant nos. 9703353 and 9903498 to H.D.G.).
LITERATURE CITED
- Brun W, Betts K. Source/sink relations of abscising and nonabscising soybean flowers: partitioning of fixed carbon within a raceme. Plant Physiol. 1984;75:187–191. doi: 10.1104/pp.75.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker TW, Grimes HD. Nucleotide sequence of vlxB, a vegetative lipoxygenase from soybean (accession no. U50075) (PGR 96-024) Plant Physiol. 1996;111:348. [Google Scholar]
- Bunker TW, Koetje DS, Stephenson LC, Creelman RA, Mullet JE, Grimes HD. Sink limitation induces the expression of multiple soybean vegetative lipoxygenase mRNAs while the endogenous jasmonic acid level remains low. Plant Cell. 1995;7:1319–1331. doi: 10.1105/tpc.7.8.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbs WE, Grimes HD. The mid-pericarp cell layer in soybean pod walls is a multicellular compartment enriched in specific lipoxygenase isoforms. Plant Physiol. 2000;123:1281–1288. doi: 10.1104/pp.123.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiben HG, Slusarenko AJ. Complex spatial and temporal expression of lipoxygenase genes during Phaseolus vulgaris(L.) development. Plant J. 1994;5:123–135. doi: 10.1046/j.1365-313x.1994.5010123.x. [DOI] [PubMed] [Google Scholar]
- Esau K. The fruit. In: Esau K, editor. Anatomy of Seed Plants. Ed 2. New York: John Wiley & Sons; 1977. pp. 429–454. [Google Scholar]
- Fischer AM, Dubbs WE, Baker R, Fuller MA, Stephenson LC, Grimes HD. Protein dynamics, activity, and cellular localization of soybean lipoxygenases indicate distinct functional roles for individual isoforms. Plant J. 1999;19:543–554. doi: 10.1046/j.1365-313x.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- Fisher DB. An unusual layer of cells in the mesophyll of the soybean leaf. Bot Gaz. 1967;128:215–221. [Google Scholar]
- Franceschi VR, Giaquinta RT. The paraveinal mesophyll of soybean leaves in relation to assimilate transfer and compartmentation: I. Ultrastructure and histochemistry during vegetative development. Planta. 1983a;157:411–421. doi: 10.1007/BF00397198. [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Giaquinta RT. The paraveinal mesophyll of soybean leaves in relation to assimilate transfer and compartmentation: II. Structural, metabolic and compartmental changes during reproductive growth. Planta. 1983b;157:422–431. doi: 10.1007/BF00397199. [DOI] [PubMed] [Google Scholar]
- Giberson R, Demaree R. Microwave fixation: understanding the variables to achieve rapid reproducible results. Microsc Res Tech. 1995;32:246–254. doi: 10.1002/jemt.1070320307. [DOI] [PubMed] [Google Scholar]
- Giberson R, Demaree R, Dordhausen R. Four-hour processing of clinical/diagnostic specimens for electron microscopy using microwave technique. J Vet Diagn Investig. 1997;9:61–67. doi: 10.1177/104063879700900111. [DOI] [PubMed] [Google Scholar]
- Gijzen M, Miller SS, Kuflu K, Buzzell RI, Miki BLA. Hydrophobic protein synthesized in the pod endocarp adheres to the seed surface. Plant Physiol. 1999;120:951–959. doi: 10.1104/pp.120.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes HD, Tranbarger TJ, Franceschi VR. Expression and accumulation patterns of nitrogen-responsive lipoxygenase in soybeans. Plant Physiol. 1993;103:457–466. doi: 10.1104/pp.103.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanway J, Weber C. Accumulation of N, P, and K by soybean (Glycine maxL. Merrill) plants. Agron J. 1971;63:406–408. [Google Scholar]
- Jauh GY, Fischer AM, Grimes HD, Ryan CA, Rogers JC. δ-Tonoplast intrinsic protein defines unique plant vacuole functions. Proc Natl Acad Sci USA. 1998;95:12995–12999. doi: 10.1073/pnas.95.22.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniewski K. Hairs in the loculus of the broad bean (Vicia fabaL.) fruit. Bull Acad Pol Sci Cl V. 1968;41:585–594. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marttila S, Tenhola T, Mikkonen A. A barley (Hordeum vulgareL.) LEA3 protein, HVA1, is abundant in protein storage vacuoles. Planta. 1996;199:602–611. [Google Scholar]
- Nakamura K, Matsuoka K. Protein targeting to the vacuole in plant cells. Plant Physiol. 1993;101:1–5. doi: 10.1104/pp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CM, Mosjidis C, Dute RR, Westgate ME. A flower and pod staging system for soybean. Ann Bot. 1992;69:59–67. [Google Scholar]
- Rodríguez-Concepcíon M, Gómez MD, Beltrán J-P. Immunolocalization of lipoxygenase in pea (Pisum sativumL.) carpels. Plant Cell Rep. 1996;15:620–626. doi: 10.1007/BF00232465. [DOI] [PubMed] [Google Scholar]
- Rosahl S. Lipoxygenases in plants: their role in development and stress response. Z Naturforsch. 1996;51c:123–138. doi: 10.1515/znc-1996-3-401. [DOI] [PubMed] [Google Scholar]
- Shibata D, Steczko J, Dixon JE, Andrews PC, Hermodson M, Axelrod B. Primary structure of soybean lipoxygenase L-2. J Biol Chem. 1988;263:6816–6821. [PubMed] [Google Scholar]
- Shibata D, Steczko J, Dixon JE, Hermodson M, Yazdanparast R, Axelrod B. Primary structure of soybean lipoxygenase-1. J Biol Chem. 1987;262:10080–10085. [PubMed] [Google Scholar]
- Siedow JN. Plant lipoxygenase: structure and function. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:145–188. [Google Scholar]
- Staswick PE. Preferential loss of an abundant storage protein from soybean pods during seed development. Plant Physiol. 1989a;90:1252–1255. doi: 10.1104/pp.90.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE. Developmental regulation and the influence of plant sinks on vegetative storage protein gene expression in soybean leaves. Plant Physiol. 1989b;89:309–315. doi: 10.1104/pp.89.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Huang JF, Rhee Y. Nitrogen and methyl jasmonate induction of soybean vegetative storage protein genes. Plant Physiol. 1991;96:130–136. doi: 10.1104/pp.96.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson LC, Bunker TW, Dubbs WE, Grimes HD. Specific soybean lipoxygenases localize to discrete subcellular compartments and their mRNAs are differentially regulated by source-sink status. Plant Physiol. 1998;116:923–933. doi: 10.1104/pp.116.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne JH. Assimilate redistribution from soybean pod wall during seed development. Agron J. 1979;71:812–816. [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranbarger TJ, Franceschi VR, Hildebrand DF, Grimes HD. The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell. 1991;3:973–987. doi: 10.1105/tpc.3.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z-H, Varner JE. Tissue-specific expression of cell wall proteins in developing soybean tissues. Plant Cell. 1991;3:23–37. doi: 10.1105/tpc.3.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenofsky RL, Fine M, Liu C. Isolation and characterization of a soybean (Glycine max) lipoxygenase-3 gene. Mol Gen Genet. 1988;211:215–222. [Google Scholar]