Abstract
Rationale:
Direct-acting antivirals (DAAs) are the first-line treatment for patients with chronic hepatitis C virus (HCV) infection. However, its effects on patients with acute HCV infection are poorly understood, and the data for treatment of DAAs for genotype 2 acute monoinfection patients with HCV are lacking.
Patient concerns:
In this case report, a 26 year-old Chinese female acquired a tattoo and developed fatigue, nausea, and anorexia. Laboratory tests showed abnormal liver function.
Diagnoses:
Five months after the patient acquired a tattoo, laboratory tests showed anti-HCV antibody titers were 26.0 s/co, HCV RNA was 5.74×105 IU/mL, and HCV genotype was 2a. The patient was diagnosed with acute hepatitis C (AHC).
Interventions:
HCV RNA did not have spontaneous clearance 12 weeks after the infection of the patient. The patient received sofosbuvir (SOF) and daclatasvir (DCV) combination treatment for 12 weeks.
Outcomes:
Laboratory tests showed HCV RNA was undetectable at weeks 4, and anti-HCV antibody was in seroconversion at weeks 12 during treatment. The patient achieved a sustained virological response 36 weeks after the end of treatment.
Lessons:
Patients with acute HCV genotype 2 monoinfection would benefit from antiviral treatment with SOF and DCV.
Keywords: acute hepatitis C (AHC), case report, daclatasvir (DCV), direct-acting antivirals (DAAs), sofosbuvir (SOF)
1. Introduction
The number of patients with chronic hepatitis C virus (HCV) infection is estimated at 180 million,[1] and HCV infection is one of the main causes of chronic liver disease worldwide.[2] Approximately 75% to 85% acute HCV cases progress to chronic infection and up to 20% develop liver cirrhosis over 20 to 25 years.[3] The treatment of patients with chronic hepatitis C (CHC) has entered a new interferon (IFN)-free era.[4] Compared with pegylated (Peg)-IFN alfa plus ribavirin, the direct-acting antivirals (DAAs) therapy significantly increases the sustained virological response (SVR) rates and shortens the duration of treatment. However, the time and drug type of antiviral treatment for patients with acute hepatitis C (AHC) remain controversial.[5] Previous studies have shown that a satisfactory effect was achieved when acute HCV infection has been treated with Peg-IFN alfa-based therapies. However, therapy with Peg-IFN alfa can sometimes lead to severe side effects, and many patients cannot be treated with this drug because of contraindications.[6,7] Patients with AHC who are not eligible for Peg-IFN alfa should be considered for treatment with IFN-free regimens containing DAAs. Unfortunately, few data are available on the DAAs treatment for patients with AHC.
We report a young female with acute HCV genotype 2 monoinfection who was successfully treated with sofosbuvir (SOF) and daclatasvir (DCV) and had achieved SVR 36 weeks after the end of treatment.
2. Case report
A 26 year-old Chinese female acquired a tattoo in July 2016 and developed fatigue, nausea, and anorexia in September 2016. In October 2016, laboratory tests showed albumin (ALB) of 46 g/L, total bilirubin (TBIL) of 18.6 μmol/L, direct bilirubin (DBIL) of 8.6 μmol/L, alanine aminotransferase (ALT) of 790 U/L, aspartate aminotransferase (AST) of 913 U/L, alkaline phosphatase (ALP) of 128 U/L, and glutamyl transpeptidase (GGT) of 335 U/L. Antihepatitis A virus (HAV)-IgM, anti-hepatitis E virus (HEV)-IgM, anti-HEV-IgG, HBsAg, anti-HCV antibody, and autoimmune antibodies were negative. The patient was given support treatment. In November 2016, laboratory tests showed ALB of 45 g/L, TBIL of 15.4 μmol/L, DBIL of 9.7 μmol/L, ALT of 165 U/L, AST of 71 U/L, ALP 94 of U/L, and GGT of 207 U/L. The treatment was then discontinued. Half a month later, she experienced fatigue and nausea again. On November 28, 2016, the patient was enrolled in our hospital for further treatment. The patient did not have any history of liver diseases. Physical examination did not show liver palms, spider nevus, hepatomegaly, splenomegaly, ascites, and lower extremity edema. Laboratory tests showed white blood cells at 8.16×109/L, red blood cells at 4.94×1012/L, platelets at 257×109/L, international standard ratio of 0.92, prothrombin activity of 121.4%, prothrombin time of 10.6 s, ALB of 46 g/L, ALT of 468 U/L, AST of 150 U/L, TBIL of 13.7 μmol/L, DBIL of 6.5 μmol/L, ALP of 124 U/L, GGT of 231 U/L, total bile acid of 29 μmol/L, and alpha fetoprotein of 10.28 ng/mL. Serum copper, cerulein, serum iron, ferritin, blood glucose, blood lipids, thyroid function, and immunoglobulin levels were normal. Autoimmune and tuberculosis antibodies were negative. Anti-HCV antibody titers were 26.0 s/co, HCV RNA was 5.74×105 IU/mL, HCV genotype was 2a, and IL28B rs12979860 was C/T. Anti-Epstein–Barr virus-IgM, anti-cytomegalovirus-IgM, anti-Herpes simplex virus-IgM, anti-parvovirus B19-IgM, anti-HAV-IgM, anti-HEV-IgM, anti-HEV-IgG, HBsAg, and anti-HIV were negative. Liver stiffness value was 7.3 kPa according to transient elastography (FibroScan). The patient underwent liver biopsy, and her liver pathology showed acute hepatitis and chronic trend (Fig. 1). The patient was diagnosed with AHC. She selected the treatment with DAAs from abroad because she was worried about the side effects of Peg-IFN alfa. We started the patient on an IFN-free combination therapy of SOF (400 mg/d) and DCV (60 mg/d) for 12 weeks. The patient was reviewed at treatment day 4, weeks 1, 2, 4, 8, 12, and at post-treatment weeks 12, 24, and 36. At treatment day 4, weeks 1 and 2, the HCV RNA viral load decreased to 1.25×103, 3.30×102, and 2.62×101 IU/mL, respectively. The results of liver function tests showed ALT of 27 U/L, AST of 26 U/L, TBIL of 7.5 μmol/L, and GGT of 162 U/L at week 1. At weeks 4, 12, and 24 (post-treatment weeks 12), 36 (post-treatment weeks 24), and 48 (post-treatment weeks 36), the HCV RNA viral load was undetectable (Fig. 2), and the liver function was normal (Fig. 3). At weeks 12, 24 (post-treatment weeks 12), 36 (post-treatment weeks 24), and 48 (post-treatment weeks 36), the anti-HCV antibody was negative (Fig. 2). At week 48 (post-treatment weeks 36), liver function tests showed ALT of 15 U/L, AST of 13 U/L, TBIL of 6.2 μmol/L, ALP of 66 U/L, GGT of 22 U/L, and ALB of 45 g/L. During treatment and post-treatment, no side effect was observed, and the blood cells, kidney function, and myocardial enzymes of the patient were normal.
Figure 1.
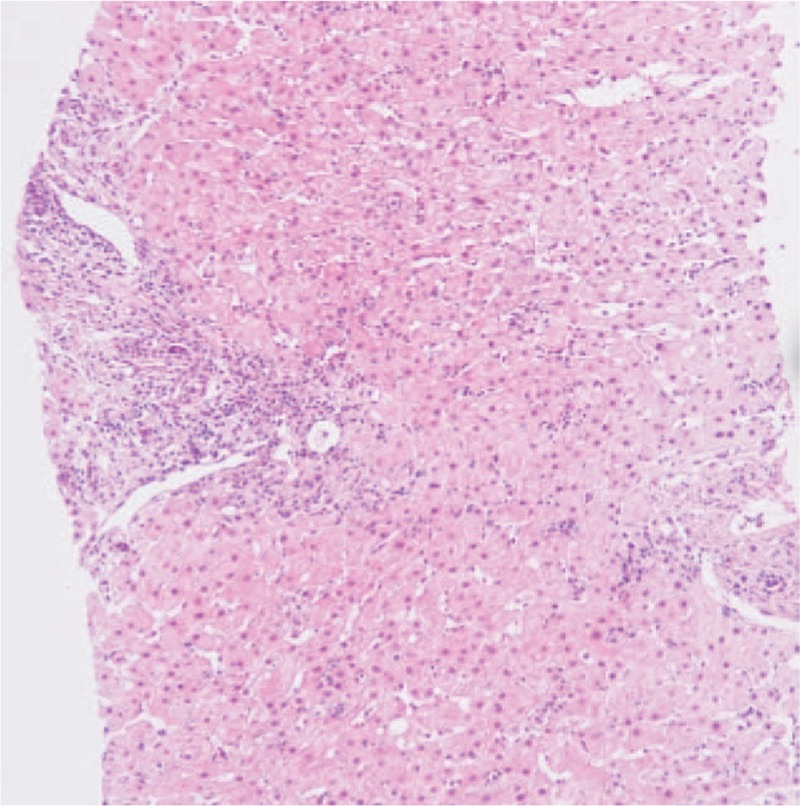
Microscopic examination of the liver (H&E, ×100): hepatocytes show diffuse hydropic degeneration, regional ballooning degeneration, few steatosis, pigmentation of pigment granules, and scattered spotty necrosis. Enlarged portal area and existence of lymphocyte predominant portal inflammation were observed.
Figure 2.
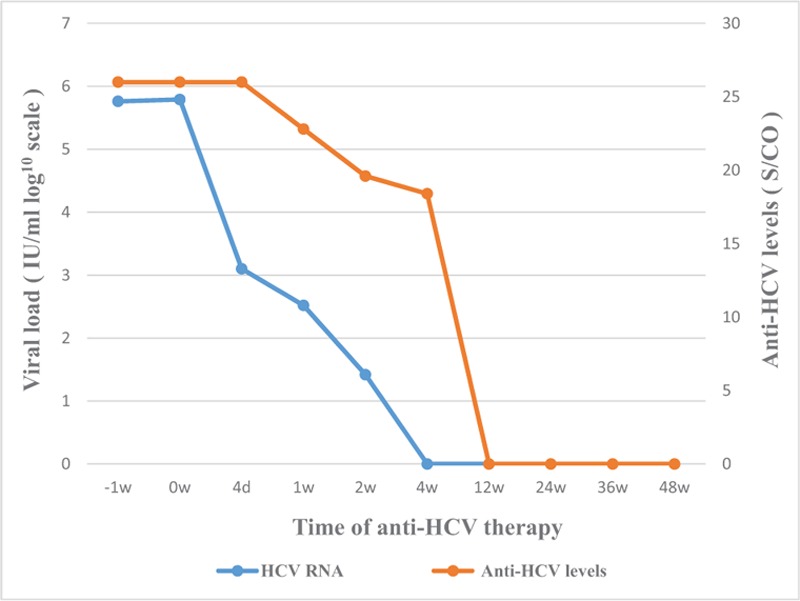
Dynamic trends of HCV RNA viral load and anti-HCV antibody after treatment with SOF and DCV of the patient: HCV RNA viral load was undetectable at weeks 4, and anti-HCV antibody underwent seroconversion at weeks 12. The patient achieved SVR 36 weeks after the end of treatment. DCV = daclatasvir, HCV = hepatitis C virus, SOF = sofosbuvir.
Figure 3.
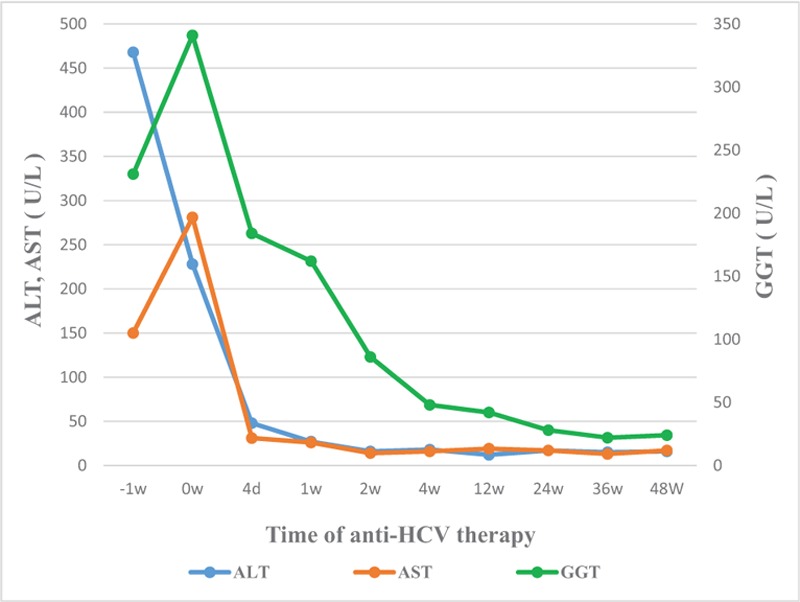
Dynamic trends of patient liver function after treatment with SOF and DCV: liver function gradually improved and returned to normal levels at weeks 4. DCV = daclatasvir, SOF = sofosbuvir.
3. Discussion
With the use of DAAs, the treatment of CHC patients has entered the IFN-free era. DAAs are recommended for patients with CHC by EASL and AASLD guidelines.[4,8] However, the data on DAAs therapy in patients with AHC are limited and mainly come from the studies on patients with HCV genotype 1 or 4. Approximately 77% of patients with acute genotype 1 or 4 HCV and HIV-1 coinfection achieved SVR12 after treatment with ledipasvir and sofosbuvir for 6 weeks,[9] whereas 100% of patients with acute genotype 1 monoinfection achieved SVR12 after treatment with ledipasvir and sofosbuvir fixed-dose combination for 6 weeks.[10] SOF and DCV were recommended to treat all genotypes of patients with CHC, which contained genotype 2 by EASL. SOF is a pan-genotypic nucleotide analogue inhibitor of the HCV NS5B RNA polymerase, whereas DCV is a pan-genotypic inhibitor of the HCV NS5A protein. DCV and SOF with or without RBV achieved high SVR12 and were well tolerated by patients with CHC.[11] No related research data are available on the treatment of AHC with DAAs for patients with genotype 2 monoinfection. Basing on the clinical study data of SOF and DCV for treatment of patients with genotype 2 CHC, we speculated that SOF and DCV may play a role in the treatment of patients with genotype 2 AHC. For this patient, both HCV RNA and anti-HCV antibody tested positive during 12 weeks after infection with no spontaneous clearance. The clinical features of the patient were as follows: IL28B rs12979860 was C/T, bilirubin level was low, and HCV RNA was persistently positive. In accordance with the diagnostic score for the prediction of spontaneous resolution of AHC patients,[12] these clinical features will easily lead to less chances of spontaneous clearance and increased chances of HCV chronic infection. The present study revealed that immediate treatment of acute HCV can improve the clinical outcomes and is highly cost-effective compared with deferring the treatment until the chronic phase of infection.[13] The patient was given antiviral treatment with SOF and DCV, and a satisfactory effect was achieved. HCV RNA viral load became undetectable, anti-HCV antibody underwent seroconversion, liver function returned to normal, and no adverse event occurred during treatment.
4. Conclusions
The presented case reveals that patients with AHC are less prone to obtain spontaneous clearance. Early DAAs treatment can be provided to help patients avoid chronic liver disease caused by HCV infection. This case supports the hypothesis that patients with acute HCV genotype 2 monoinfection would benefit from antiviral treatment with sofosbuvir and daclatasvir. We look forward to large-scale clinical studies that will confirm the effectiveness of DAAs in treating AHC. Now might be the time to revisit the treatment guidelines for patients with AHC.
Acknowledgments
Thanks to Prof. Guang De Zhou from the Department of Pathology of 302 Military Hospital, Beijing, China to for providing pathological pictures to our study.
Author contributions
Funding acquisition: Chen Li.
Investigation: Chen Li.
Methodology: Chen Li.
Resources: Chen Li.
Writing – original draft: Chen Li.
Conceptualization: JinHua Hu.
Project administration: JinHua Hu.
Footnotes
Abbreviations: AHC = acute hepatitis C, ALB = albumin, ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CHC = chronic hepatitis C, DAAs = direct-acting antivirals, DBIL = direct bilirubin, DCV = daclatasvir, GGT = glutamyl transpeptidase, HAV = hepatitis A virus, HCV = hepatitis C virus, HEV = hepatitis E virus, SOF = sofosbuvir, SVR = sustained virological response, TBIL = total bilirubin.
Ethics approval statement: Not applicable as this is a single case report, not a study.
Consent for publication: Written permission to publish this case report was obtained from the patient.
Funding: This study was supported by innovation projects of Beijing 302 Military Hospital of China (no. YNKT2014007).
The authors have no conflicts of interest to disclose.
References
- [1].Messina JP, Humphreys I, Flaxman A, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015;61:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect 2011;17:107–15. [DOI] [PubMed] [Google Scholar]
- [3].Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol 2007;42:513–21. [DOI] [PubMed] [Google Scholar]
- [4].AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015;62:932–54. [DOI] [PubMed] [Google Scholar]
- [5].Kalafateli M, Buzzetti E, Thorburn D, et al. Pharmacological interventions for acute hepatitis C infection: an attempted network meta-analysis. Cochrane Database Syst Rev 2017;3:CD011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wiegand J, Buggisch P, Boecher W, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology 2006;43:250–6. [DOI] [PubMed] [Google Scholar]
- [7].Santantonio T, Fasano M, Sagnelli E, et al. Acute hepatitis C: a 24-week course of pegylated interferon α-2b versus a 12-week course of pegylated interferon α-2b alone or with ribavirin. Hepatology 2014;59:2101–9. [DOI] [PubMed] [Google Scholar]
- [8].European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017;66:153–94. [DOI] [PubMed] [Google Scholar]
- [9].Rockstroh JK, Bhagani S, Hyland RH, et al. Ledipasvir-sofosbuvir for 6 weeks to treat acute hepatitis C virus genotype 1 or 4 infection in patients with HIV coinfection: an open-label, single-arm trial. Lancet Gastroenterol Hepatol 2017;2:347–53. [DOI] [PubMed] [Google Scholar]
- [10].Deterding K, Spinner CD, Schott E, et al. Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet Acute HCV IV): an open-label, single-arm, phase 2 study. Lancet Infect Dis 2017;17:215–22. [DOI] [PubMed] [Google Scholar]
- [11].Welzel TM, Petersen J, Herzer K, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut 2016;65:1861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Beinhardt S, Payer BA, Datz C, et al. A diagnostic score for the prediction of spontaneous resolution of acute hepatitis C virus infection. J Hepatol 2013;59:972–7. [DOI] [PubMed] [Google Scholar]
- [13].Bethea E, Chen Q, Hur C, et al. Should we treat acute hepatitis C? A decision and cost-effectiveness analysis. Hepatology 2017;67:837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


