Abstract
Rationale:
Although metastases to the oral and maxillofacial region (OMR) are rare, the lung is the most common primary site metastasizing to the OMR.
Patient concerns:
An 83-year-old woman presented with reports of trismus, occlusal discomfort, swelling, and spontaneous pain in the right buccal region. Despite the absence of abnormal chest imaging findings, immunohistochemical analysis of biopsy specimens of the mandible and the thyroid indicated that the patient had multiple metastases from a lung poorly differentiated adenocarcinoma.
Diagnoses:
Metastases to the OMR and the thyroid from an undiscovered lung adenocarcinoma.
Interventions:
Gefitinib was started as first-line chemotherapy, and zoledronic acid was administered for bone metastases.
Outcomes:
Follow-up imaging examinations showed ossification and deformation of the right mandibular ramus and the condylar process. Although 2 years have passed since the first visit to our hospital, lung lesions have not been confirmed by imaging examinations.
Lessons:
Clinicians should consider the possibility that symptoms in the OMR may be the first clinical sign of an undiscovered distant primary tumor, and the primary tumors may not be detected by imaging examinations even when metastases to the OMR are revealed.
Keywords: imaging examinations, immunohistochemical staining, mandibular metastasis, occult lung adenocarcinoma
1. Introduction
Metastasizing to the oral and maxillofacial region (OMR) is rare, with 1% of all malignancies of that region.[1] In approximately 20%, oral metastases are discovered before the detection of the distant primary tumor.[1] The most common metastatic site in the craniofacial region is the mandible. The lung is the most common primary site metastasizing to the jaw, and the most common histologic type is squamous cell carcinoma, followed by adenocarcinoma.[2] According to previous literature, the primary lesions could be detected by imaging examination at the same time when the mandibular metastases were revealed, and patients sometimes had medical histories associated with the metastases.[1,2] The authors present a case of mandibular metastasis as the first clinical indication of occult lung adenocarcinoma with multiple metastases.
2. Consent
Written informed consent was obtained from the patient for publication of the case and any accompanying images.
3. Case report
An 83-year-old nonsmoking woman was referred to the Department of Dentistry and Oral Surgery in University of Fukui Hospital reporting trismus, occlusal discomfort, swelling, and spontaneous pain in the right buccal region for 4 months. Physical examination revealed facial asymmetry due to swelling in the right buccal region; however, she did not have a fever and respiratory symptoms. There were no abnormal findings in the oral mucosa, and the maximum interincisal opening was 36 mm with mandibular deviation toward the right side. Panoramic radiograph and lateral oblique transcranial projection revealed bone destruction of the right mandibular ramus and the condylar process (Fig. 1). Computed tomography (CT) scan and magnetic resonance imaging (MRI) revealed a tumorous lesion of the right mandibular ramus and the condylar process and metastases to cervical spine (C1, C2), brain, and cervical lymph node (Fig. 2). Whole-body 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) showed abnormally increased FDG uptake in the right mandibular ramus, cervical spine (C1, C2), thyroid, and lymph nodes (cervical, axillary, mediastinal) (Fig. 3). There were no abnormal findings on chest x-ray and chest CT scan. Although ultrasonography and mammography were performed, there were no findings suggestive of breast cancer. In laboratory examination, alkaline phosphatase test was 784 U/L (normal range: 107–340 U/L), carcinoembryonic antigen was 5 ng/mL (normal range: <5 ng/mL), and squamous cell carcinoma antigen was 0.6 ng/mL (normal range: <1.5 ng/mL). Thus, the patient was clinically diagnosed as having a malignant bone tumor of the mandible with multiple metastases at first.
Figure 1.
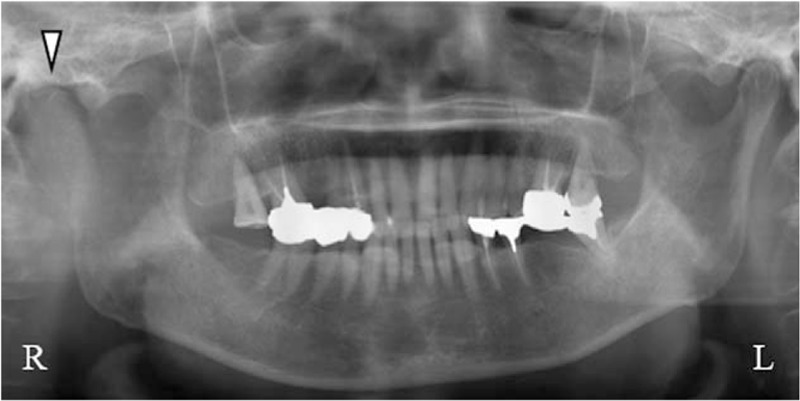
Panoramic radiograph at the first visit revealed bone destruction of the right mandibular ramus and the condylar process (Δ).
Figure 2.
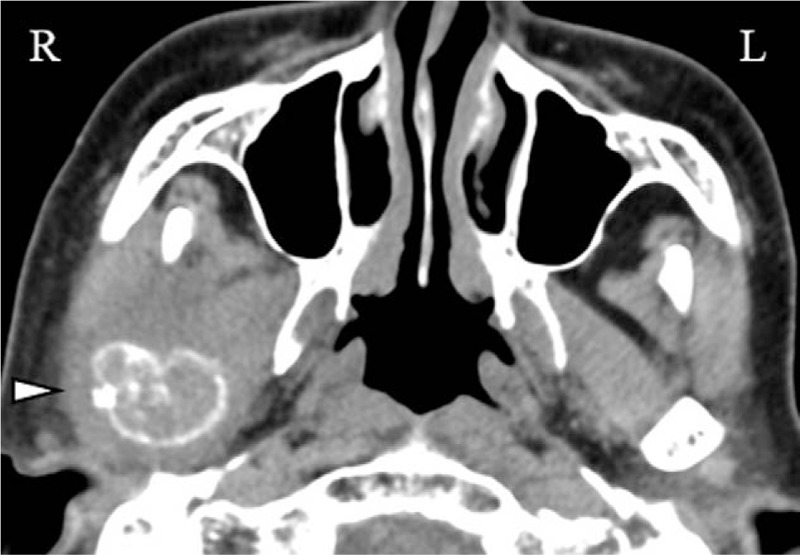
CT scan at the first visit revealed a tumorous lesion of the right mandibular ramus and the condylar process (Δ).
Figure 3.
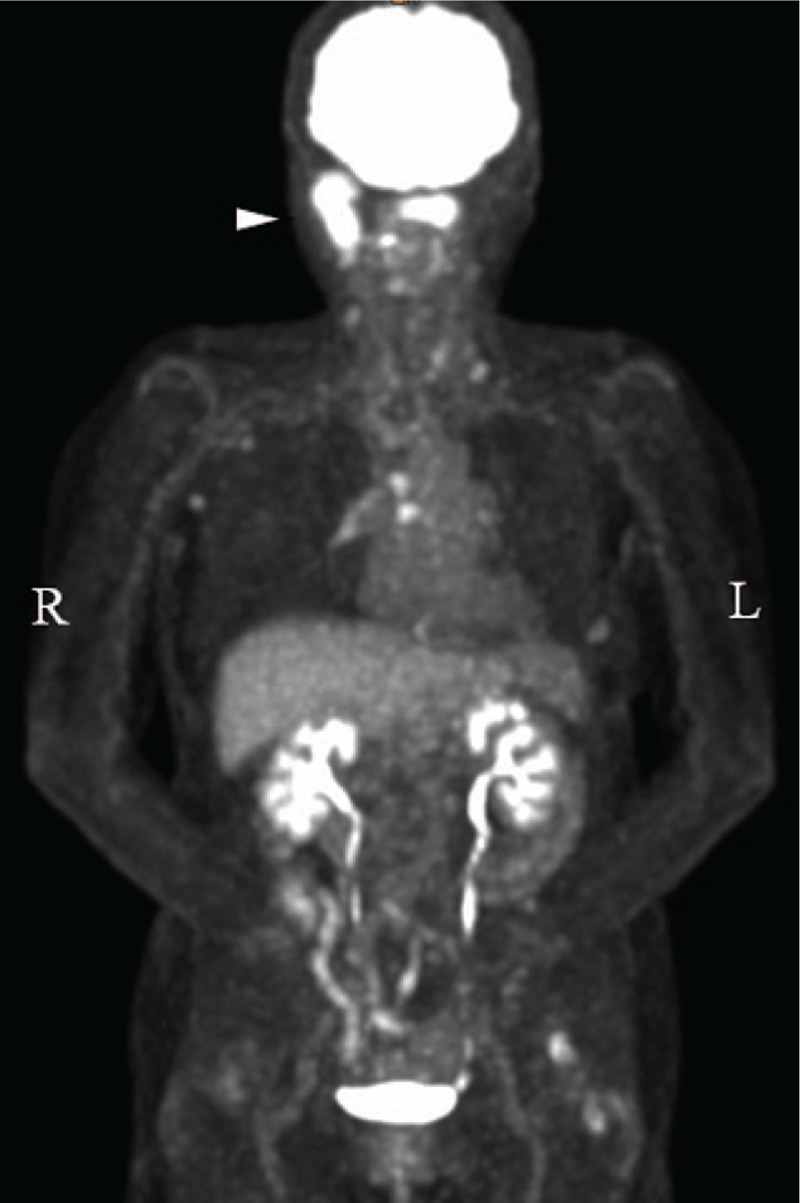
Whole-body FDG PET/CT showed abnormally increased FDG uptake in the right mandibular ramus (Δ), cervical spine, thyroid, and multiple lymph nodes.
A biopsy of the right mandibular ramus was performed under general anesthesia, and histopathologic examination revealed a proliferation of atypical cells with large irregular nuclei (Fig. 4). Immunohistochemical staining was shown as follows: keratin (K) AE1/AE3+, K7+, K20–, thyroid transcription factor 1 (TTF-1)+, Napsin A+, thyroglobulin–, and vimentin+. Immunohistochemical staining of the thyroid fine-needle aspiration biopsy specimen was shown as follows: TTF-1+, Napsin A+, and paired box gene 8 (PAX8)–. Also, mutation analysis identified an L858R point mutation in the epidermal growth factor receptor (EGFR) gene. Despite the absence of abnormal chest imaging findings, these results indicated that the patient had multiple metastases from a lung poorly differentiated adenocarcinoma, cT0N2M1b, stage IV. Gefitinib was then started as first-line chemotherapy based on testing results for mutations in EGFR, and zoledronic acid was administered for bone metastases at the Department of Respiratory Medicine in University of Fukui Hospital.
Figure 4.
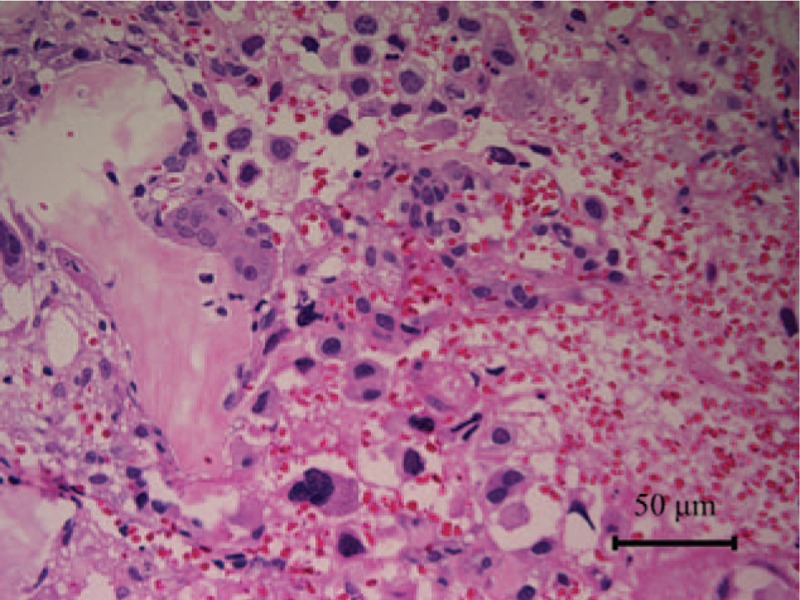
Histopathologic examination of the mandibular biopsy specimens revealed a proliferation of atypical cells with large irregular nuclei.
A follow-up panoramic radiograph and CT scan showed ossification and deformation of the right mandibular ramus and the condylar process (Figs. 5 and 6). Although 2 years have passed since the first visit to our hospital, lung lesions have not been confirmed by imaging examinations.
Figure 5.
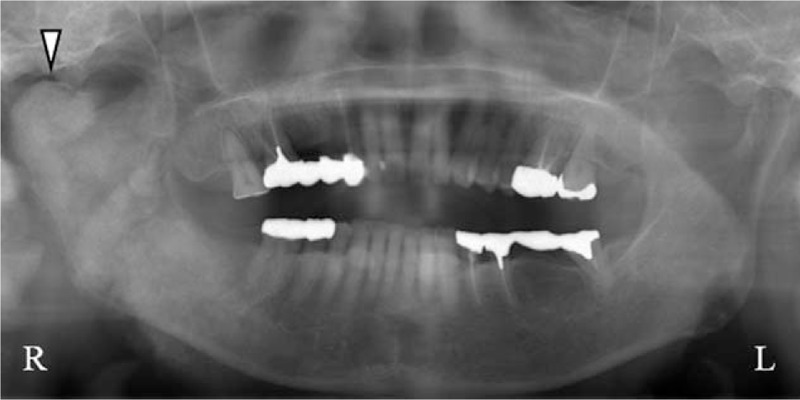
Panoramic radiograph examination performed 2 years after the first visit. The image shows ossification and deformation of the right mandibular ramus and the condylar process (Δ).
Figure 6.
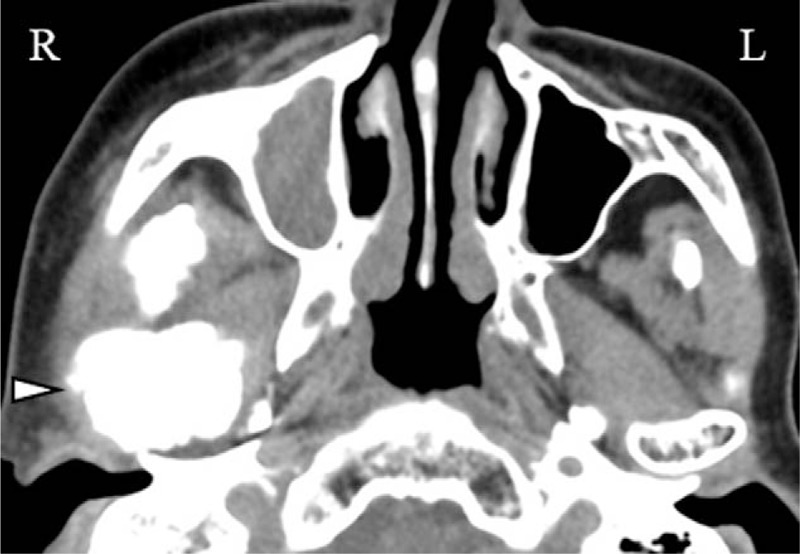
CT examination performed 2 years after the first visit showing ossification and deformation of the right condylar and coronoid processes (Δ).
4. Discussion
Cancer of unknown primary (CUP) is defined as the presence of metastases proved by cytologic or histologic examinations and the absence of a primary tumor despite performing standardized diagnostic procedures.[3] The primary site was identified at autopsy in 50% to 75% of the patients with CUP, and the most common primary site was the lung.[3] Most patients with CUP have metastasis of moderately or poorly differentiated adenocarcinoma or carcinoma to the viscera, bone, or brain.[3] Patients with CUP usually exhibit an atypical natural history, and they usually have a poor prognosis, with survival typically ranging from 7 to 11 months.[3] Previous literature has shown that the role of FDG-PET is limited, with a primary lesion detection rate of approximately 40% in patients with CUP.[4] Examinations of biopsy specimens are mandatory, and immunohistochemistry is the gold standard for diagnosing CUP.[5] Keratin expression patterns of the biopsy specimen of this patient were consistent with the diagnosis of epithelial tumor, such as lung adenocarcinoma, breast ductal carcinoma, ovarian serous papillary carcinoma, and endometrial adenocarcinoma.[5] TTF1 is a highly specific marker for primary lung adenocarcinomas, and Napsin A is a very sensitive marker for detecting lung adenocarcinomas.[5] Although the coexpression of Napsin A and TTF-1 strongly suggests a possibility of lung adenocarcinoma, it should be noted that they also can be detected in extrapulmonary adenocarcinomas and that a small fraction of primary lung adenocarcinomas does not show their coexpression.[5,6] PAX8 is useful for distinguishing primary lung adenocarcinoma from carcinomas metastatic to the lung, and the negative PAX8 expression in this patient was consistent with the diagnosis of primary lung adenocarcinoma.[6] Thyroglobulin is an excellent marker of papillary and follicular thyroid carcinomas, and it showed negative in this patient.[5] Previous literature has reported that the positive vimentin expression was detected with approximately 30% of patients with nonsmall cell lung cancer, including adenocarcinoma, and it was associated with the occurrence of metastasis and poor prognosis.[7] The patient was initially considered to have malignant bone tumor of the mandible on the basis of imaging examinations because no abnormal findings suggested lung cancer on chest x-ray examination, chest CT, and FDG-PET. Lung adenocarcinoma was diagnosed based on results of histopathologic examination and immunohistochemical staining for biopsy specimens of the mandible and the thyroid, as described above. This case indicated the limitations of imaging examinations and the importance of biopsy of suspected metastatic lesions in case of CUP.
Lung carcinomas are most often detected in metastatic stage IV, and the common sites of metastases are brain, bones, and adrenal glands.[8] Lung carcinomas metastasize via the lymphatic route and blood vessels, and the distant metastases via blood vessels usually occur earlier than those via the lymphatic route.[8] The pathogenesis of the mandibular metastasis is not fully understood; however, it is believed that the main mechanism of metastasis to the oral region is hematogenous associated with Batson venous plexus.[9] The most common metastatic site in the craniofacial region is the mandibular molar and premolar region because abrupt angulation of the vessel in this region causes slowing of the bloodstream, and it helps malignant cells deposit there.[9]
The combined appearance of atypical symptoms in the OMR, such as toothache, lip anesthesia, and buccal swelling, suggests the presence of malignant disease.[6] Furthermore, mandibular metastasis sometimes mimics temporomandibular disorders, osteomyelitis, and trigeminal neuralgia.[9] Clinicians may misdiagnose the presence of a malignant lesion as a dental disease, such as periodontal disease, because most odontogenic infections cause pain and swelling in the orofacial region.[2] Establishing an accurate diagnosis as soon as possible is important because the interval between the occurrence of metastasis to the oral region and patient death is usually a few months.[1] Accurate medical history of the patient with atypical symptoms in the OMR must be obtained because metastatic lesion to the OMR may be the first clinical indication of an undiscovered distant primary tumor or CUP.
5. Conclusions
A potential risk of misdiagnosis exists in patients with CUP. Clinicians should consider the possibility that symptoms in the orofacial region may be the first clinical sign of an undiscovered distant primary tumor, and the primary tumors may not be detected by imaging examinations even when the mandibular metastases were revealed.
Author contributions
Conceptualization: Shinpei Matsuda.
Data curation: Shinpei Matsuda.
Writing – original draft: Shinpei Matsuda.
Writing – review & editing: Hisato Yoshida, Hitoshi Yoshimura, Kazuo Sano, Shinpei Matsuda, Yoshiaki Imamura, Yukihiro Umeda.
Footnotes
Abbreviations: CT = computed tomography, CUP = cancer of unknown primary, EGFR = epidermal growth factor receptor, FDG PET = 18F-fluorodeoxyglucose positron emission tomography, K = keratin, MRI = magnetic resonance imaging, OMR = oral and maxillofacial region, PAX8 = paired box gene 8, TTF-1 = thyroid transcription factor 1.
The authors declare no conflicts of interest.
References
- [1].Pereira-Filho VA, Chaves MD, Haddad J, et al. Mandible metastasis as the first sign from primary adenocarcinoma of the lung. Gen Dent 2007;55:224–7. [PubMed] [Google Scholar]
- [2].Gultekin SE, Senguven B, Isik Gonul I, et al. Unusual presentation of an adenocarcinoma of the lung metastasizing to the mandible, including molecular analysis and a review of the literature. J Oral Maxillofac Surg 2016;74:2007.e1-8. [DOI] [PubMed] [Google Scholar]
- [3].Pentheroudakis G, Greco FA, Pavlidis N. Molecular assignment of tissue of origin in cancer of unknown primary may not predict response to therapy or outcome: a systematic literature review. Cancer Treat Rev 2009;35:221–7. [DOI] [PubMed] [Google Scholar]
- [4].Burglin SA, Hess S, Høilund-Carlsen PF, et al. 18F-FDG PET/CT for detection of the primary tumor in adults with extracervical metastases from cancer of unknown primary: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kandalaft PL, Gown AM. Practical applications in immunohistochemistry: carcinomas of unknown primary site. Arch Pathol Lab Med 2016;140:508–23. [DOI] [PubMed] [Google Scholar]
- [6].Ye J, Hameed O, Findeis-Hosey JJ, et al. Diagnostic utility of PAX8, TTF-1 and napsin A for discriminating metastatic carcinoma from primary adenocarcinoma of the lung. Biotech Histochem 2012;87:30–4. [DOI] [PubMed] [Google Scholar]
- [7].Tadokoro A, Kanaji N, Liu D, et al. Vimentin regulates invasiveness and is a poor prognostic marker in non-small cell lung cancer. Anticancer Res 2016;36:1545–51. [PubMed] [Google Scholar]
- [8].Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev 2016;35:75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yasar F, Oz G, Dolanmaz D, et al. Mandibular metastasis in a patient with pulmonary adenocarcinoma. Dentomaxillofac Radiol 2006;35:383–5. [DOI] [PubMed] [Google Scholar]


