Abstract
Rationale:
Brain metastasis (BM) is a rising challenge in forward-looking oncology, as its treatment choices are very limited, especially, after the failure of local treatment schemes.
Patient concerns:
We report on a 39-year-old Chinese woman who was diagnosed with stage IV triple-negative breast cancer(TNBC) with multiple brain, lung, and bone metastases. She had previously, undergone whole-brain radiation therapy. Paclitaxel, platinum, UTD1, capecitabine, gemcitabine, vinorelbine, and single-agent apatinib were then administered as first- to fifth-line therapies. She exhibited progression each time after a short period of disease stabilization.
Diagnoses:
Triple-negative breast cancer.
Interventions:
The patient chose treatment with apatinib+CPT-11+S-1 as the sixth-line therapy.
Outcomes:
A remarkable response of the brain, and lung metastases, and alleviation of the brain edema were achieved, and these effects persisted for 7 months.
Lessons:
We describe the significant anti-tumor effect of apatinib + CPT-11 + S-1 against BMs from breast cancer. This report is the first to suggest potential approaches to BM treatment using this scheme and describes the effects of an apatinib-containing regimen on BMs.
Keywords: apatinib, brain metastases, triple-negative breast cancer
1. Introduction
Breast cancer is the most common neoplasia worldwide. Approximately, between 40 to 45% of all breast cancer patients will develop metastasis, and the median survival time for these patients between 18 to 30 months.[1] Among all subtypes, triple-negative breast cancer (TNBC) has the highest frequency of brain metastasis (BM) (up to 46%), and the poorest prognosis, as the median survival time of these patients is less than 6 months.[2] As treatment schemes, whole-brain radiotherapy, and surgery are well known, and useful but limited. New cytotoxic, or cytostatic, agents and innovative drugs are being actively developed.
Apatinib is a small-molecular receptor tyrosine kinase inhibitor (TKI) with potential antiangiogenic, and antineoplastic function that selectively binds, and inhibits VEGFR-2. In preclinical data, apatinib exhibited highly, potent activity against solid tumors, effectively, inhibited the tube formation, proliferation, and migration, and of umbilical vein endothelial cells, blocked the budding of the rat aortic ring, reduced the growth of xenograft tumors,[3] and reversed ABCG2 (BCRP/MXR/ABCP)- and P-glycoprotein (ABCB1/MDR1)-mediated multidrug resistance.[4,5] In phase I, and phase II studies, apatinib exhibited exciting antineoplastic activity, and manageable toxicity.[6,7] Two phase II studies found that single-agent apatinib showed promising effects in heavily pretreated, metastatic non-TNBC with manageable toxicity.[8,9] The median progression-free survival (PFS) was between 4.0 to 3.3 months, and the overall survival (OS) was between 10.3 to 10.6 months. However, no studies have confirmed the value of apatinib, or its combination with chemotherapy in metastatic breast cancer (MBC), and neither of the 2 clinical trials involved BM patients. Recently, we used apatinib in combination with CPT-11 and S-1 to treat a refractory TNBC patient with BM, and achieved exciting results. This case is reported below.
2. Case report
In the year May 2016, BMs were found after fourth-line palliative chemotherapy in a 39-year-old woman with breast cancer, and multiple brain, lung, and bone metastases in our hospital. The patient complained of headache and epilepticus insultus. Magnetic resonance imaging (MRI) of the brain showed multiple metastatic lesions with edema at the cerebrum (Fig. 1A).
Figure 1.
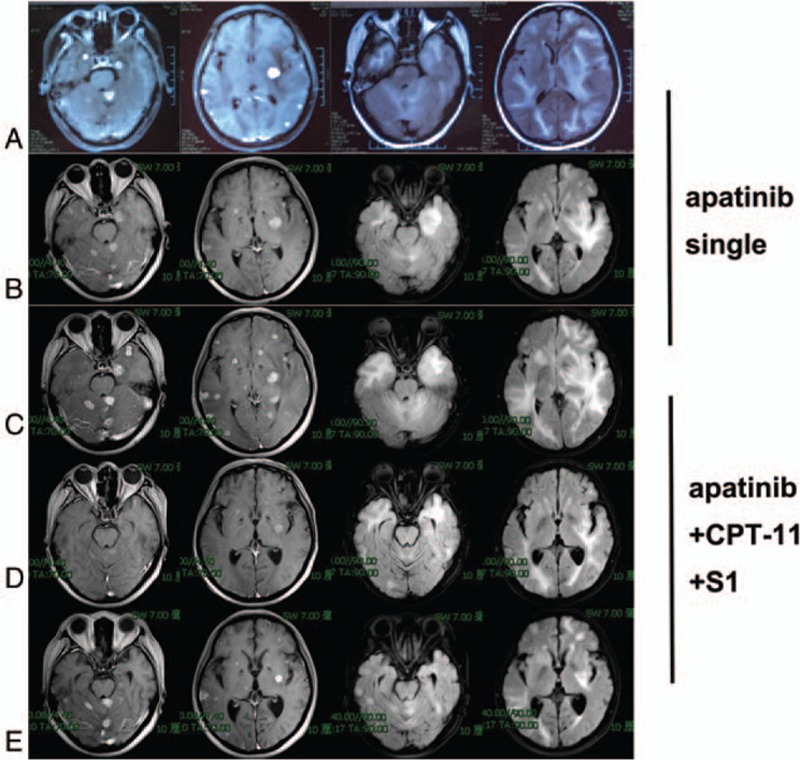
MRI of response to single-agent apatinib and apatinib+CPT-11+S1: (A) before single-agent apatinib, (B) after 3 months of single-agent apatinib, (C) after 7 months of single-agent apatinib, (D) after 2 cycles of apatinib + CPT-11+S1, (E) after 6 cycles of apatinib + CPT-11 + S1. MRI = Magnetic resonance imaging
Two and half years earlier, in the year December 2013, the patient underwent breast-conserving surgery with axillary lymph node dissection. Pathological evaluation confirmed invasive grade III ductal carcinoma within the breast tissue (ER- and PR-negative, HER2-, Ki67 35%). The pathological stage of the disease was cT2N0M0 stage IIB. The genetic subtype was triple-negative. As an adjuvant therapy, she received FEC (5-FU 500 mg/m2, epirubicin 100 mg/m2, cyclophosphamide 500 mg/m2) chemotherapy 3 times every 3 weeks followed by docetaxel (75 mg/m2) 3 times every 3 weeks. Seven months after surgery, she complained of headache. Multiple BMs were detected by MRI. A CT scan indicated multiple metastases in the bilateral lung, and bone. Whole-brain radiation therapy was performed. Paclitaxel, platinum, UTD1, capecitabine, gemcitabine, vinorelbine, and single-agent apatinib were then administered as first- to fifth-line therapy. The timeline of her past medical history is outlined in Table 1.
Table 1.
Summary of the timeline of the patient's past medical history.
In the year May 2016, the patient consulted our hospital for progressively, worsening headaches, and epilepticus insultus. A brain scan revealed multiple lesions with brain edema (Fig. 1A). After the patient signed the informed consent form, she received apatinib (500 mg po qd) as fifth-line therapy. Within a few days after the treatment of apatinib, an obvious improvement in the headache, and nervous system symptoms became evident, and corticosteroid treatment was terminated. The 3-month follow-up brain MRI (Fig. 1B), and chest CT (Fig. 2A) showed that the metastatic lesions remained stable. Apatinib monotherapy was continued. The brain (Fig. 1C), and lung (Fig. 2B), metastases progressed systemically, 7 months after the initiation of apatinib single therapy in the year November 2016. The patient experienced new episodes of severe dry cough, and worsening headache symptoms.
Figure 2.
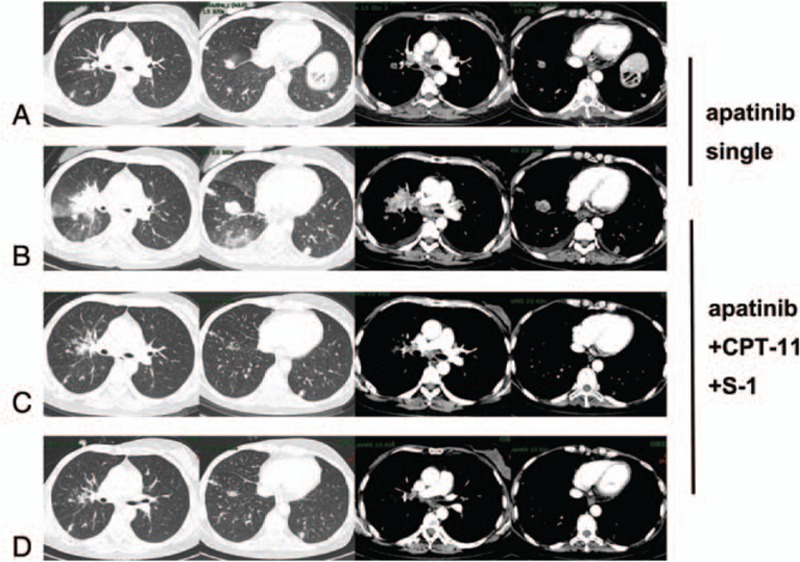
CT scan of response to single-agent apatinib and apatinib + CPT-11 + S1: (A) after 3 months of single-agent apatinib, (B) after 7 months of single-agent apatinib, (C) after 2 cycles of apatinib + CPT-11 + S1, (D) after 6 cycles of apatinib + CPT-11 + S1.
We changed the regimen to apatinib + CPT-11 + S1 due to the systemic progression. The cough, and headache, decreased rapidly. Cranial MRI after 2 cycles of therapy showed impressive reductions in the size of both the brain edema, and the contrast-enhancing lesions (Fig. 1D), and multiple lung metastases (Fig. 2C) were significantly, reduced in size, or disappeared. At the 5-month follow-up dynamic reexamination in the year April 2017, after 4 cycles of apatinib+CPT-11 + S-1 treatment, the MRI showed regression with systemically, stable disease. She was still receiving the same regimen, and no severe adverse events were detected during, or after the therapy. At the 7-month follow-up reexamination in the year July 2017, after the sixth cycle of treatment, MRI (Fig. 1E), and CT (Fig. 2D) confirmed new disease progression in the brain, and lung.
3. Discussion
The development of management strategies for BM in patients with TNBC is an important clinical challenge. We experienced a case in which the combination of apatinib with CPT-11 and S-1, effectively controlled refractory BM after the failure of whole-brain radiotherapy, and multi-line chemotherapy (paclitaxel, platinum, capecitabine, gemcitabine, and vinorelbine, as recommended by NCCN guidelines). The clinical regression of the brain, and lung metastases, and alleviation of the brain edema from breast cancer were sustained for 7 months. Why did this regimen exert such a strong inhibitory effect on brain, and lung metastases from breast cancer? We discuss the possible mechanism, and its implications for current, and potential future treatment strategies below.
Targeted therapies for patients with BM from TNBC are unfortunately, lacking, and chemotherapy is currently the only systemic option.[2] The blood-brain barrier (BBB) limits the crossing of drugs into the brain because of its very low permeability, and the expression of potent multispecific efflux transporters. These 2 mechanisms restrict the efficacy of some macromolecular drugs with potentially, therapeutic effects on brain diseases.[10] Some efflux proteins (P-glycoprotein /ABCB1, multidrug resistance proteins such as MrP, or ABCC, and breast cancer resistance proteins such as BCrP, or ABCg2) are responsible for the transfer of their substrates from the endothelial cells back into the blood, reducing the central bioavailability of many drugs.[11]
Vascular endothelial growth factor (VEGF), and its receptors (VEGFRs) play a significant role in the angiogenesis of breast carcinoma. Current research indicates that VEGF may provide a new mechanism for regulating the permeability of the BBB in vivo.[12] Moreover, previous studies have shown a fine balance between VEGF, and angiopoietin-2, a proapoptotic factor in angiogenesis in glioma models. The BBB is abnormal in cases of tumors >0.5 mm, which might compromise the integrity of the astrocytes, and endothelial cells.
The anti-VEGF monoclonal antibody bevacizumab, combined with first-line chemotherapy for patients with metastatic TNBC, significantly, improved the objective response rate (ORR), and PFS. This benefit has been confirmed by the meta-analysis of 3 randomized phase III trials (E2100, AVADO, RIBBON-1). Even in a second-line setting (RIBBON-2), the improvement in PFS with the administration of avastin to the TNBC subgroup was marked (median 6.0 vs. 2.7 months for chemotherapy plus avastin vs. chemotherapy alone; P = .0006), and the OS showed a tendency to improve.[13] Another therapeutic strategy to inhibit angiogenesis is the use of anti-VEGFR TKIs, such as sunitinib, and sorafenib; nevertheless, neither of these drugs exhibited a major impact on MBC.
Apatinib is a small-molecular receptor TKI with potential antiangiogenic, and antineoplastic function that selectively, binds, and inhibits VEGFR-2. Apatinib may inhibit VEGF-stimulated endothelial cell migration, and proliferation, and reduce tumor microvascular density. The exact mechanism by which apatinib affects brain lesions is still unclear; whether it can directly, affect the tumor microvascular structure, and/or the BBB, and/or can cross the BBB is unknown. Apatinib has been shown to inhibit the function of P-glycoprotein.[4,5] Additional studies are currently, being conducted to assess the role of apatinib in treating patients with malignant gliomas for whom almost all treatments (including temozolomide, bevacizumab, nimotuzumab, and reradiation) have failed.[14] Therefore, theoretically, apatinib might cross the BBB, penetrate brain tumors, and work with anticancer drugs at adequate concentrations. However, according to our experience, this report showed that single-agent VEGFR2 TKI might provide no benefit in the treatment of MBC caused by breast carcinoma. This patient showed progression of lung, and brain metastases after 7 months of apatinib monotherapy.
Irinotecan is an inhibitor of topoisomerase I that shows an effect on MBC with response rates between 20 to 30% as a single agent, and in combination with platinum-based therapy.[15] Irinotecan, whose ability to cross the BBB has been demonstrated in primary brain tumors,[16] exhibits a confirmed modest benefit when combined with iniparib in progressive TNBC BM. Preliminary research shows that tMZ, and CPt-11 are transported across the BBB by ABCB1 with brain/plasma ratios of 1.1, and 2.1, respectively.[17] Furthermore, the tolerance, and activity of irinotecan have been shown in the therapy of patients with extracranial MBC in a phase Ib setting.[18] S-1 is an oral fluorouracil that reverses the inhibition of the rate-limiting enzyme in 5-FU degradation, increasing the blood concentration, and enhancing the anti-tumor effect of 5-FU. Recurrent breast cancer treated with S-1 alone showed an RR of 30 to 41.7%, PFS of 5, and OS of 11.3 months with few adverse events.[19] A combination of irinotecan, and S-1 has been implemented in various doses for the treatment of advanced, or recurrent breast cancer with a response rate for BM of 50%. Torigoe et al found that irinotecan, and 5-FU combination therapy could induce an obvious anti-tumor effect in colon cancer cell lines in vitro.[20] They hypothesized that irinotecan reduced the activity of thymidylate synthase, a target enzyme of 5-FU, which regulates the cell cycle. S-1 has similar activities to those of 5-FU; the anti-tumor effect of irinotecan, and S-1 combination therapy might thus be improved through a similar mechanism.
Notably, the combination of apatinib with CPT-11, and S-1 produced a great effect. After 2 cycles of therapy, multiple lung metastases were significantly, reduced in size, or had disappeared, and the BMs had also decreased in size, or disappeared. An MRI confirmed that the cerebral edema was significantly, lower than before. The following mechanism might explain the curative effect of the combination regimens in this case. The antiangiogenic action of apatinib could have increased the transmission of CPT-11 and S-1. In addition, apatinib could have facilitated the passage of CPT-11, and S-1 across the BBB by reducing the function of P-glycoprotein.
In cancer patients, BMs are a common, and life-limiting complication. The symptoms of increased cranial pressure, and brain edema are most frequently, treated with steroids. However, steroids can give rise to various serious complications that further decrease the patient's quality of life. Apatinib is reported to be very effective in reducing brain damage, and peripheral edema in primary brain tumors. Here, we have noted our encouraging experience with apatinib treatment for the control of steroid-refractory brain edema in a patient with brain-metastasized breast cancer.
4. Conclusion
To the best of our knowledge, this report is the first to describe the successful use of apatinib in combination with CPT-11+S-1 to treat refractory BMs in a patient with TNBC. The efficacy in the patient was similar to a partial response (PR). These results suggest that a phase II clinical trial should be launched to further evaluate the efficacy of this treatment against brain-metastasized breast cancer.
Acknowledgments
The authors thank the patient for her participation and her agreement to the publication of the report.
Author contributions
Conceptualization: Yanxia Zhao.
Data curation: Yanxia Zhao, Qiuhui Li, yuxi Ma.
Formal analysis: Jie Xiong, yuxi Ma.
Funding acquisition: Yanxia Zhao, Gang Wu.
Investigation: Ting Hu, Yanxia Zhao, Gang Wu.
Methodology: Ting Hu, Cuiwei Liu, Jie Xiong.
Project administration: Cuiwei Liu.
Resources: Qiuhui Li.
Software: Ting Hu, Qiuhui Li.
Writing – original draft: Ting Hu.
Writing – review & editing: Ting Hu.
Footnotes
Abbreviations: BBB = blood-brain barrier, BM = brain metastasis, MBC = metastatic breast cancer, MRI = Magnetic resonance imaging, OS = overall survival, PFS = progression-free survival, TKI = tyrosine kinase inhibitor, TNBC = triple-negative breast cancer.
This case report was approved by the Union Hospital of Huazhong University of Science and Technology. Written informed consent was obtained from the patient for the publication of this case report and accompanying images. Methods: The Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (IORG No: IORG0003571) gave a final approval for the study. This Ethics Committee is constituted and functioned in accordance with ICH-GCP, GCP in China and Declaration of Helsinki (2013). The patient has signed informed consent.
The authors report that there are no conflicts of interest in this work.
References
- [1].Witzel I, Oliveira-Ferrer L, Pantel K, et al. Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res 2016;18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Costa R, Carneiro BA, Wainwright DA, et al. Developmental therapeutics for patients with breast cancer and central nervous system metastasis: current landscape and future perspectives. Ann Oncol 2016;28:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Desi Devel Ther 2015;9:6075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tong XZ, Wang F, Liang S, et al. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. Biochem Pharmacol 2012;83:586–97. [DOI] [PubMed] [Google Scholar]
- [5].Mi Y, Liang Y, Huang H, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res 2010;70:7981-7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fontanella C, Ongaro E, Bolzonello S, et al. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med 2014;2:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhou N, Liu C, Hou H, et al. Response to apatinib in chemotherapy-failed advanced spindle cell breast carcinoma. Oncotarget 2016;7:72373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014;14:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hu XC, Zhang J, Xu BH, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2014;135:1961–9. [DOI] [PubMed] [Google Scholar]
- [10].He C, Cai P, Li J, et al. Blood-brain barrier-penetrating amphiphilic polymer nanoparticles deliver docetaxel for the treatment of brain metastases of triple negative breast cancer. J Control Release 2016;246:98-L109. [DOI] [PubMed] [Google Scholar]
- [11].Cordon-Cardo C, O’Brien JP, Casals D, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci 1989;86:695–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ay I, Francis JW, Jr BR. VEGF increases blood-brain barrier permeability to Evans blue dye and tetanus toxin fragment C but not adeno-associated virus in ALS mice. Brain Res 2008;1234:198–205. [DOI] [PubMed] [Google Scholar]
- [13].Hamilton EP, Blackwell KL. Safety of bevacizumab in patients with metastatic breast cancer. Oncology 2011;80:314–25. [DOI] [PubMed] [Google Scholar]
- [14].Zhang H, Chen F, Wang Z, et al. Successful treatment with apatinib for refractory recurrent malignant gliomas: a case series. Onco Targets Ther 2017;10:837-L845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].O'Shaughnessy J, Weckstein DJ, Vukelja SJ. Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer. Breast Cancer Research and Treatment 2007;106(supplement 1):S32–3. [Google Scholar]
- [16].Vredenburgh JJ, Desjardins A, Herndon JE, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007;25:4722–9. [DOI] [PubMed] [Google Scholar]
- [17].Goldwirt L, Beccaria K, Carpentier A, et al. Irinotecan and temozolomide brain distribution: a focus on ABCB1. Cancer Chemother Pharmacol 2014;74:185–93. [DOI] [PubMed] [Google Scholar]
- [18].Sengupta S, Rojas R, Mahadevan A, et al. CPT-11/bevacizumab for the treatment of refractory brain metastases in patients with HER2–neu-positive breast cancer. Oxf Med Case Reports 2015;2015:254–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tsuji W, Ishiguro H, Tanaka S, et al. Orally administered S-1 suppresses circulating endothelial cell counts in metastatic breast cancer patients. Int J Clin Oncol 2014;19:452–9. [DOI] [PubMed] [Google Scholar]
- [20].Otsuka H, Fujii T, Toh U, et al. Phase II clinical trial of metronomic chemotherapy with combined irinotecan and tegafur-gimeracil-oteracil potassium in metastatic and recurrent breast cancer. Breast Cancer 2015;22:335–42. [DOI] [PubMed] [Google Scholar]


