Abstract
Geographic atrophy (GA), the late stage of dry age-related macular degeneration is characterized by loss of the retinal pigment epithelial (RPE) layer, which leads to subsequent degeneration of vital retinal structures (e.g., photoreceptors) causing severe vision impairment. Similarly, RPE-loss and decrease in visual acuity is seen in long-term follow up of patients with advanced wet age-related macular degeneration (AMD) receiving intravitreal anti-vascular endothelial growth factor (VEGF) treatment. Therefore, on the one hand, it is fundamental to efficiently derive RPE cells from an unlimited source that could serve as replacement therapy. On the other hand, it is important to assess the behavior and integration of the derived cells in a model of the disease entailing surgical and imaging methods as close as possible to those applied in humans. Here, we provide a detailed protocol based on our previous publications that describes the generation of a preclinical model of GA using the albino rabbit eye, for evaluation of the human embryonic stem cell derived retinal pigment epithelial cells (hESC-RPE) in a clinically relevant setting. Differentiated hESC-RPE are transplanted into naive eyes or eyes with NaIO3-induced GA-like retinal degeneration using a 25 G transvitreal pars plana technique. Evaluation of degenerated and transplanted areas is performed by multimodal high-resolution non-invasive real-time imaging.
Keywords: Cellular Biology, Issue 131, Retinal Pigment Epithelial Cell, Human Embryonic Stem Cell, Large-eyed model, Geographic Atrophy, Suspension Subretinal Transplantation, Optical Coherence Tomography, Transvitreal Pars Plana
Introduction
This protocol describes the generation of a large-eyed preclinical model of geographic atrophy (GA) that allows the evaluation of integration of transplanted hESC-RPE in the subretinal space. The methods described in detail here have been used in 3 recent publications that demonstrate the production of an enriched, pure, and functional population of RPE cells from hESC1, as well as the creation of outer retinal damage and a GA-like phenotype induced by the subretinal injection of physiologic salt solutions (i.e., BSS and PBS) or NaIO3 in the rabbit eye2,3. We further demonstrated that sub-retinal suspension transplants of hESC-RPE form extensive functional monolayers with photoreceptor rescue capacity2.
Several advantages accompany the use of the rabbit eye for the generation of a GA model of disease. Firstly, the size of the rabbit eye, which is 70% the volume of an adult human eye, allows clinically meaningful transplantation using a cell density that is much lower than routinely used in small rodent eyes (1,000 cells/µL vs. 50,000 cells/µL)4,5. Secondly, surgery in rodents is usually transscleral through the choroid, which compromises the retinal barrier and potentially triggers an inflammatory response and a possible rejection6. Both factors together may lead to multilayering and clumping of transplanted cells, and an overall poor integration of the transplanted cells in a disrupted native retinal tissue. However, the large-eyed rabbit model allows performing a surgical technique with instrumentation identical to a clinical setting. Thirdly, a large-eyed model also permits high-resolution in vivo imaging and monitoring of the transplanted cells and the overlying retina through time1,2,3. Thus, we describe a clinically relevant and cost-efficient preclinical model that should be an attractive alternative to rodents for anyone with an interest in research of the normal and diseased retina and the sub-retinal space.
Protocol
The following protocol follows the animal care guidelines of Karolinska Instituet. All animal experiments using New Zealand albino rabbits (Table of Materials) have been approved by the regional animal ethics committee (Stockholms Norra Djurförsöksetiska Nämnd) (permit: dnr 56/15). The use of hESC (dnr 2011/745-31/3) and the transfer and manipulation of hESC-RPE (dnr 2013/813-31/2) is also in accordance with the Swedish legislation and Karolinska Institutet regulations, and has been approved by the regional human ethics committee (Regionala Etikprövningsnämnden i Stockholm).
1. Subretinal Injection of Sodium Iodate (NaIO3) into a Large-eyed Animal Model
Anesthetize animals by intramuscular administration in the thigh with a mixture of 35 mg/kg ketamine and 5 mg/kg xylazine in saline using a 30 G syringe, and dilate pupils with topical eyedrops using a mix of 0.75% cyclopentolate and 2.5% phenylephrine. Proper anesthetization is confirmed if the animal does not react to a hard pinch of its back leg.
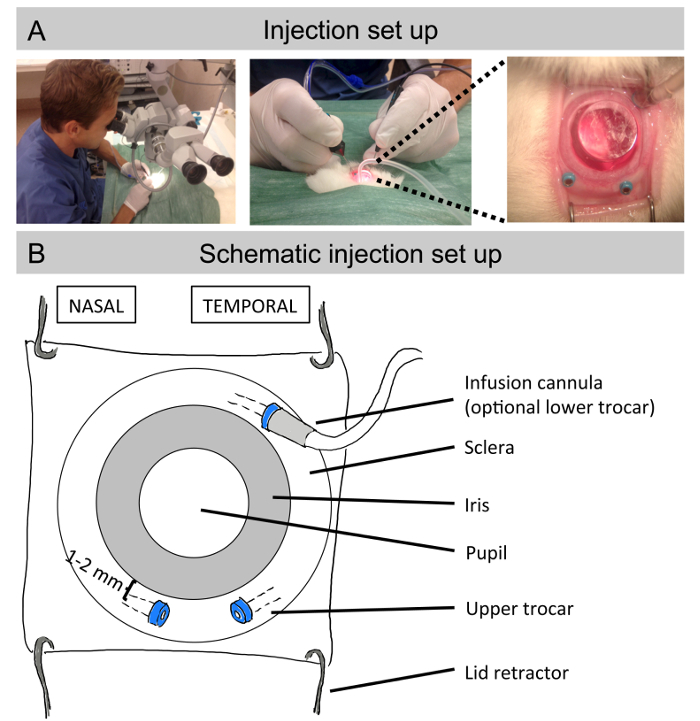
Place the rabbit under the surgical microscope with the head facing the surgeon (Figure 1A). Use a lid retractor to remove eyelids and nictitans membrane with a sterile cloth to minimize the risk of contamination. Use balanced salt solution (BSS) to prevent dryness while under anesthesia in both eyes.
- For microsurgery, use a 2-port (or optional 3-port) 25 G transvitreal pars plana technique with non-valved trocars for the insertion of microsurgical instruments (Figure 1B). The multifunction vitrectomy machine has ports to connect an infusion cannula, endoillumination, vitrector, and endolaser. For subretinal injections, only the endoillumination is mandatory, which makes a 2-port set-up sufficient. Insert the endoillumation through the upper left trocar and use the upper right trocar for the subretinal injection cannula.
- For a 3-port set-up, use the lower temporal trocar for the BSS infusion cannula. If optional instruments (such as a vitrector) are used, insert them through the upper right trocar.
- Insert the upper 2 trocars 1-2 mm from the limbus using clawed forceps to grab and displace the conjunctiva overlying the insertion site.
- Make sure the trocars are inserted transsclerally at a 30-45° limbus parallel angle, and proceed to the middle of the tip of the trocar. Then turn the trocar 90° and advance into the eye, aiming at the posterior pole of the eye. This procedure will avoid post-surgical leakage from the sclerotomies, and also decrease the risk of endophthalmitis (i.e., bacterial infection in the eye). See also Figure 2B for trocar positions.
- Put a single use flat contact lens on the cornea to visualize the retina, with synthetic tears as contact gel between the eye and the contact lens (Figure 1A).
- Draw 500 µL of NaIO3 into a 1 mL syringe connected to an extension tube (operated by the assistant) and a 38 G polytip cannula (operated by the surgeon).
- Insert the endoillumination probe through the upper left trocar and the injection cannula through the upper right trocar, and advance the cannula through the vitreous space towards the retina, aiming for the area just below the optic nerve head.
- Allow the tip of the cannula to slowly touch the retina until a focal whitening is visible. The injection itself will penetrate the retina, allowing for subretinal delivery. Do not let the cannula penetrate the retina, since this may cause hemorrhage.
- Inject 50 µL of NaIO3 subretinally over a 5 s period. Since there is a natural cleavage plane between the retina and the underlying choroid, a clearly visible semitransparent bleb should gradually form during the injection.
- During the injection, slowly retract the needle, but make sure the tip is maintained within the bleb to minimize reflux.
- After removal of the endoillumation and injection cannula, remove the trocars using clawed forceps, and apply light pressure for 30 s to the self-sealing suture-less sclerotomies using the tip or the blunt end of the forceps.
Post-surgically give 10 mL of saline subcutaneously to prevent dehydration. Do not give post-surgical topical steroids or antibiotics. For analgesics give 0.5 mL of buprenorphine 0.3 mg/ml subcutaneously after surgery, as well as the day after surgery.
After usage, wash all instruments by immersing them for a couple of seconds firstly in 70% ethanol, secondly in 45% ethanol, and lastly in distilled water. Dry them properly with a paper towel.
Attend animals until they regain sufficient consciousness and place them all single encaged. If required (e.g., for immunohistochemistry purposes), euthanize animals by intravenous injection of 100 mg/kg pentobarbital (see Table of Materials).
Wait 7 days to proceed with the transplantation of hESC-RPE cells.
2. Subretinal Transplantation of hESC-RPE Cells in Treated Animals
Administer 2 mg (100 µL) of intravitreal triamcinolone in anesthetized animals using a 30 g injection needle inserted 1-2 mm from the limbus in the lower temporal quadrant 1 week before transplantation of hESC-RPE, and re-administer it every 3 months. Make sure to point the tip towards the posterior pole of the eye to avoid a lens touch.
Culture a hESC-RPE monolayer as described previously1.
Remove cell differentiation media (see Table of Materials) of a 24-well confluent hESC-RPE monolayer and wash each well with 500 µL of PBS without Ca2+ and Mg2+. Repeat this action once more, for a total of 2 washes.
Discard supernatant, add 500 µL of trypsin per well, and incubate for 12 min at 37 °C.
Tilt the plate and carefully remove trypsin (cells should remain attached to the plate). Collect cells in 800 µL of fresh 37 °C prewarmed differentiation media by gentle pipetting, or even scraping if needed, to obtain a single cell suspension. NOTE: Use a 40 µm cell strainer if cell clumps are observed.
Count cells in a hemocytometer chamber using 0.2% Trypan Blue, according to the manufacturer's instructions.
Add 5 mL of differentiation media and centrifuge cells at room temperature, 300 x g for 5 min.
Discard supernatant and resuspend pellet in freshly filter-sterilized PBS (passed through a 25 mm syringe filter) to a final concentration of 1,000 cells/µL.
Aliquot the previous cell suspension into 600 µL aliquots and keep on ice until and during surgery.
Anesthetize animals by intramuscular administration in the thigh with a mixture of 35 mg/kg ketamine and 5 mg/kg xylazine in saline using a 30 G syringe. Dilate pupils with topical eyedrops using amix of 0.75% cyclopentolate and 2.5% phenylephrine. Proper anesthetization is confirmed if the animal does not react to a hard pinch in its back leg.
Place the rabbit with the head facing the surgeon. Use a lid retractor to remove eyelids and nictitans membrane with a sterile cloth to minimize the risk of contamination. Use BSS to prevent dryness while under anesthesia in both eyes.
- For microsurgery, use a 2-port (or optional 3-port) 25 G transvitreal pars plana technique with trocars placed in the same positions as described in step 1.3 and Figure 2. If the previous sclerotomies are visible, perform trocar insertion just adjacent, but not through these, to minimize the risk of postoperative leakage.
- Put a single use flat contact lens on the cornea to visualize the retina, with synthetic tears as the contact gel between the eye and the contact lens (Figure 1A).
- After proper tip positioning (see steps 1.3.5 and 1.3.6), inject 50 µL of a gently-mixed hESC-RPE suspension (50,000 cells) subretinally. Aim for the center of the NaIO3 pretreated area distinguished by a characteristic "metallic" endo-illumination reflex. The neurosensory retina should separate easily creating a clearly visible bleb. Flush the needle with sterile H2O in between rabbits/after use to avoid needle clogging due to cell clumps. Change the needle when clogging is noticed.
- During the injection, slowly retract the needle but make sure the tip is maintained within the bleb to minimize reflux.
- After removal of the endoillumation and injection cannula, remove the trocars using clawed forceps, and apply light pressure for 30 s to the self-sealing suture-less sclerotomies using the tip or the blunt end of the forceps.
Post-surgically give 10 mL of saline subcutaneously to prevent dehydration. Do not give post-surgical topical steroids or antibiotics. For analgesics give 0.5 mL of buprenorphine 0.3 mg/ml subcutaneously after surgery, as well as the day after surgery.
After usage, wash all instruments by immersing them for a couple of seconds, firstly in 70% ethanol, secondly in 45% ethanol, and lastly in distilled water. Dry them properly with a paper towel.
Attend animals until they regain sufficient consciousness and place them all single encaged. If required (e.g. for immunohistochemistry purposes), euthanize animals by intravenous injection of 100 mg/kg pentobarbital.
3. In Vivo Retinal and Subretinal Imaging
- Use a spectral domain optical coherence tomography (SD-OCT) device with the accompanying software (see Table of Materials) to obtain cross-sectional b-scans of the treated animals, according to manufacturer's instructions. To avoid image blurring, make sure to keep the cornea moist by flushing with topical saline every 30-60 s.
- Place the anesthetized and pupil-dilated animals (see steps 1.1 and 2.10) in an adjustable mount to obtain an unobstructed path from the instrument light source to the rabbit retina.
- Obtain at least 3 OCT scans with simultaneous infrared-confocal scanning laser ophthalmoscopy (IR-cSLO) reflectance reference images representing the upper, central, and lower portion of the injected area.
- Obtain en-face fundus images with cSLO blue, green, infrared, and multicolor laser reflectance (i.e., multiple simultaneous laser colors), respectively.
- Capture blue light autofluorescence (BAF) images using the blue-light laser capability of the SD-OCT device.
Representative Results
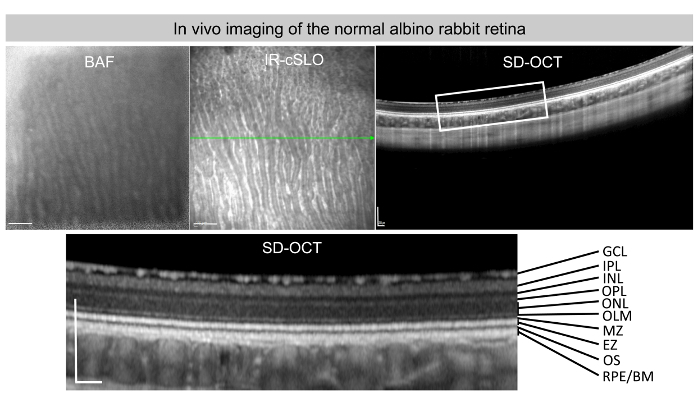
Representative in vivo images of BAF, IR-cSLO, and SD-OCT of a normal albino rabbit retina are shown in Figure 2. Note the different retinal layers with their distinctive levels of light reflection captured by the SD-OCT instrument.
In Figures 1A and Figure 1B, the setup to create sub-retinal blebs is illustrated: a lid retractor is positioned, subjecting the eye lids to allow the insertion of 3 trocars (Figure 1B) 1-2 mm far from the limbus, in order to avoid a lens touch, and at a 30-45 ˚ limbus parallel angle. Trocars will enable the introduction of an optional infusion cannula, in addition to a light, and the injection needle through the sclera. A dilated pupil together with a flat contact lens will facilitate a view of the eye fundus and the sub-retinal space while the surgical procedure is performed.
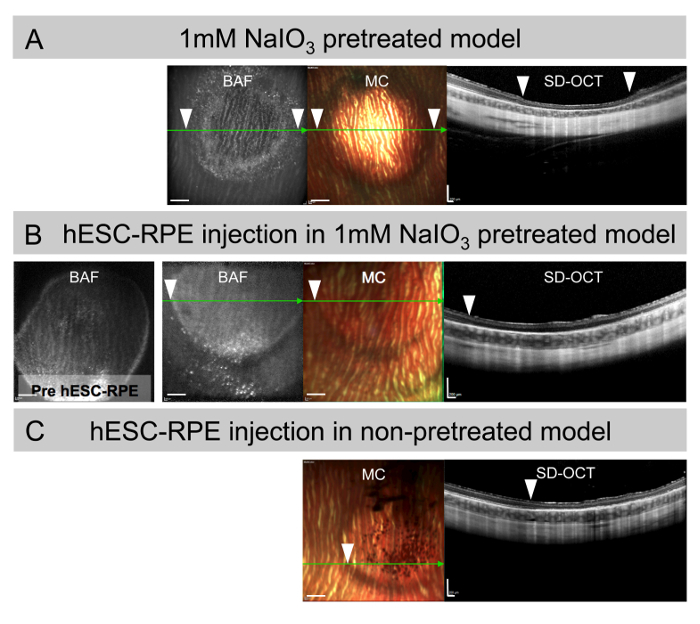
After the injection of 50 µL of 1 mM NaIO3 solution, a bleb is created in the subretinal space that will resolve and progressively degenerate the outer retina, as shown in the SD-OCT image in Figure 3A three months after injection. Appreciate the thinning of the outermost neuroretinal layers in the SD-OCT and the hypo-BAF areas, corresponding to RPE loss thus re-creating a GA-like phenotype. Upon identification of the area of damage, 50,000 hESC-RPE in a 50 µL volume is re-injected for transplantation, creating a second bleb that resolves as shown in the BAF and multi-color (MC) images in Figure 3B. Recognize the mild to moderate hyper-BAF areas with a focal increased hyper-BAF inferiorly on the borderline of the area of damage, indicative of chronic stress of the native RPE shown in the SD-OCT scans together with the absence of pigmented areas in the MC image. The bleb corresponding to the injection of 50 µL of hESC-RPE (50,000 cells) in non-pretreated eyes showing patches of pigmented cells in the (MC) image and well-preserved retinal structures in the SD-OCT scan is shown for comparison (Figure 3C).
Collectively, this procedure and methodology allow the study of the integration of subretinal suspension transplants of hESC-RPE in a relevant model for GA.
Figure 1: Injection set up. (A) Pictures depicting the surgical set-up for sub-retinal injections. The animal is placed under the surgical microscope (left) and the surgeon holds the endoillumination probe inserted through the left trocar in the left hand, and the intravitreal surgical instrument (e.g. subretinal injection cannula) inserted through the left trocar in the right hand (middle). A flat contact lens placed on the cornea allows a magnified view of the fundus during the surgical procedure (right). (B) Close schematic view of the albino rabbit eye with the lid retractor in place and the nipple from the trocars inserted 1-2 mm deep from the limbus and at a 30-45° parallel angle, together with the trocars that facilitate the introduction of an optional infusion cannula, a light, and the needle for injection. A flat contact lens situated on top of the pupil will help to get a better view of the eye fundus during the surgical procedure. Please click here to view a larger version of this figure.
Figure 2: Normal albino rabbit retina in vivo imaging.In vivo BAF, IR-cSLO, and SD-OCT images representing a normal albino rabbit retina. A blow up of the SD-OCT with the corresponding labeling of the different retinal layers is seen in the SD-OCT image: GCL (ganglion cell layer), IPL (inner plexiform layer), INL (inner nuclear layer), OPL (outer plexiform layer), ONL (outer nuclear layer), OLM (outer limiting membrane), EZ (ellipsoid zone), OS (outer segments), RPE (retinal pigment epithelium), and BM (Bruch's membrane). Scale bars = 1 mm (BAF and IR-cSLO images), 200 µm (SD-OCT images). Please click here to view a larger version of this figure.
Figure 3: Subretinal suspension injections in the large-eyed rabbit model. (A) BAF, MC, and SD-OCT images of subretinal injection of 1 mM NaIO3 3 months after damage induction. Note the hypo-BAF surrounded by hyper-BAF areas corresponding to the bleb and the degenerated neuroretina in the SD-OCT. (B) BAF, MC, and SD-OCT images of eyes pretreated with 1 mM NaIO3 for 1 week followed by subretinal transplantation of 50,000 hESC-RPE in suspension and analyzed 3 months after transplantation. Note the hyper-BAF areas in the BAF image, the absence of integrated pigmented cells in the MC and the atrophic neuroretina in the SD-OCT. (C) MC and SD-OCT images corresponding to non-pretreated naive eyes 3 months after subretinal transplantation of hESC-RPE in suspension. Note the pigmented areas on the MC image and the preserved neuroretinal structure on the SD-OCT. SD-OCT scan planes are marked (green arrow). White arrowheads mark the border of blebs in the en-face and SD-OCT images. Scale bars = 1 mm (BAF and MC images), 200 µm (SD-OCT images). Please click here to view a larger version of this figure.
Discussion
In this protocol, the generation of a large-eyed model of GA and its preclinical use for evaluating hESC-RPE integration in vivo is described.
For translation of regenerative therapies for GA and related diseases into the clinic7, it is important to develop and optimize methods that faithfully capture the clinical methods for transplantation and imaging. The rabbit is in this aspect attractive: it has a relatively large eye that permits intraocular surgery and use of standard imaging, and is cheap and easily housed compared to other large-eyed animals.
We describe the use of standard transvitreal 25-gauge pars plana vitrectomy technique for the creation of sub-retinal blebs either to induce sub-retinal damage or to transplant hESC-RPE. Initially, we used a 3-port set-up, as illustrated in Figure 1B, with the intention of using the infusion port (trocar), in case a vitrectomy was performed during the procedure. However, since we have not found a vitrectomy to be necessary for applying sub-retinal injections, the infusion port has been omitted. Nevertheless, the third port option should remain if the procedure is further adapted and a vitrectomy is performed.
The large-eyed rabbit is a well-established ocular model with accumulated data on anatomy and physiology over the past centuries8. Moreover, rabbits are easy to handle and breed, economically attractive (e.g., purchase, housing, and keeping), and readily available compared to other large-eyed mammal models. A major advantage of a large eyed-model is that it enables the use of high-resolution clinical imaging techniques, which in turn allow for tracking of transplanted hESC-RPE cells in the subretinal space over time. However, despite lagomorphs being phylogenetically closer to humans than rodents, it must be stated that they possess a merangiotic retina and a visual streak, compared to a holangiotic retina and a fovea present in primates9. Merangiotic retina means that most of the blood supply of the inner retina is derived from the choriocapillaris, a difference that one needs to consider since it can increase the risk of lesion and hemorrhage. One experimental advantage is that it allows for modeling earlier stages of GA following subretinal blebs of physiologic solutions alone, as it has been previously demonstrated1,3. These studies suggested that a subretinal bleb causing temporal retinal hypoxia in the merangiotic milieu may be enough to cause photoreceptor death; however, in this context, RPE loss has been shown to most likely be generated by the sub-retinal flow induced by using a 1 mL syringe and a 50 µL bleb volume, a random phenomenon that in turn potentiates neuro-retinal atrophy. Therefore, the bleb volume can have a direct effect on the retinal stretch damage caused, meaning that the larger the volume used (e.g., 100 µL, as has been used in other studies10), the more detrimental it can become.
A critical step to ensure integration of the injected cells is to avoid reflux of cells into the vitreous while injecting, and the positioning of the needle in the subretinal space. If positioned too deep, the outer retinal barrier/choroid will be penetrated and immune cells may invade the sub-retinal space, causing immune rejection despite the use of immunosuppression. Another important aspect for transplantation success is the state of the vitreous. In the present model, vitrectomy is not performed in order to minimize reflux and surgical trauma. However, it has been noticed that in some eyes it is difficult to get a proper tip position on the retina as the tip gets stuck in the pre-retinal vitreous interphase leading to the formation of small or even no blebs. Although it is a relatively rare event, it does indicate that variability of the rabbit vitreous needs to be taken into account during surgery and post-surgical analysis.
Additionally, the proper injection of triamcinolon is crucial to avoid obscuring the fundus by white steroid crystals, which will then hamper surgery, and post-surgical fundus visualization by SD-OCT. To minimize this problem, the injection of triamcinolon should be done in the lower temporal quadrant. Similarly, trocars should be placed 1-2 mm from the limbus to avoid vitreous hemorrhage from the ciliary body and touching the unproportionally large rabbit lens with the needle. A lens touch will cause a cataract that will in turn impede proper visualization of the sub-retinal space during postoperative imaging.
Steroids, including triamcinolone, may themselves cause cataracts over time. We have not observed this following triamcinolone administration for up to 8 months. However, it is likely that steroid-induced cataracts will develop if administering triamcinolone over extensive periods of time.
The described methods can be used both for surgical training and for analysis of cell-integration and function as described here and in our previous publications. The method also has high relevance for administration of sub-retinal viral vectors now in clinical trial for several hereditary retinal dystrophies. Future modifications may include transplantation of more complex materials, such as sheet carriers and biogels. As new clinical imaging methods emerge, these will be easily adapted to the rabbit eye, possibly without modifications.
In conclusion, the large-eyed rabbit model is demonstrated to be a relevant pre-clinical model that has several advantages compared to rodent models for i) recapitulating different stages of GA, ii) applying patient-relevant surgical and imaging methods, and iii) evaluating integration of donor cells to be used as cell replacement therapy for GA and related disorders.
Disclosures
None of the authors have competing interests or conflicting interests.
Acknowledgments
This study was supported by grants from the Karolinska Institute, the Crown Princess Margareta's Foundation for the Visually Impaired, the Edwin Jordan Foundation for Ophthalmological Research, the Swedish Eye Foundation, the King Gustav V Foundation, the ARMEC Lindeberg Foundation, and the Cronqvist Foundation.
References
- Plaza Reyes A, et al. Xeno-Free and Defined Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells Functionally Integrate in a Large-Eyed Preclinical Model. Stem Cell Rep. 2016;6(1):9–17. doi: 10.1016/j.stemcr.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartuma H, et al. In Vivo Imaging of Subretinal Bleb-Induced Outer Retinal Degeneration in the Rabbit. Invest Ophthalmol Vis Sci. 2015;56(4):2423–2430. doi: 10.1167/iovs.14-16208. [DOI] [PubMed] [Google Scholar]
- Petrus-Reurer S, et al. Integration of Subretinal Suspension Transplants of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in a Large-Eyed Model of Geographic Atrophy. Invest Ophthalmol Vis Sci. 2017;58(2):1314–1322. doi: 10.1167/iovs.16-20738. [DOI] [PubMed] [Google Scholar]
- Carido M, et al. Characterization of a mouse model with complete RPE loss and its use for RPE cell transplantation. Invest Ophthalmol Vis Sci. 2014;55(8):5431–5444. doi: 10.1167/iovs.14-14325. [DOI] [PubMed] [Google Scholar]
- Lund RD, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8(3):189–199. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- Vugler A, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol. 2008;214(2):347–361. doi: 10.1016/j.expneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- Hughes A. A schematic eye for the rabbit. Vision Res. 1972;12(1):123–138. doi: 10.1016/0042-6989(72)90143-5. [DOI] [PubMed] [Google Scholar]
- Blanch RJ, Ahmed Z, Berry M, Scott RA, Logan A. Animal models of retinal injury. Invest Ophthalmol Vis Sci. 2012;53(6):2913–2920. doi: 10.1167/iovs.11-8564. [DOI] [PubMed] [Google Scholar]
- Nork TM, et al. Functional and anatomic consequences of subretinal dosing in the cynomolgus macaque. Arch Ophthalmol. 2012;130(1):65–75. doi: 10.1001/archophthalmol.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]


