Abstract
The temporal discrimination threshold (TDT) is the shortest time interval at which an observer can discriminate two sequential stimuli as being asynchronous (typically 30-50 ms). It has been shown to be abnormal (prolonged) in neurological disorders, including cervical dystonia, a phenotype of adult onset idiopathic isolated focal dystonia. The TDT is a quantitative measure of the ability to perceive rapid changes in the environment and is considered indicative of the behavior of the visual neurons in the superior colliculus, a key node in covert attentional orienting. This article sets out methods for measuring the TDT (including two hardware options and two modes of stimuli presentation). We also explore two approaches of data analysis and TDT calculation. The application of the assessment of temporal discrimination to the understanding of the pathogenesis of cervical dystonia and adult onset idiopathic isolated focal dystonia is also discussed.
Keywords: Neuroscience, Issue 131, Temporal Discrimination, Temporal Discrimination Threshold, Staircase, Random, Dystonia, Cervical Dystonia, Endophenotype
Introduction
Temporal discrimination describes a person's ability to discriminate, or perceive, rapid changes in their environment. The temporal discrimination threshold (TDT) is the shortest time interval at which an individual can perceive that two sequential sensory stimuli are asynchronous. Temporal discrimination has been shown to be abnormally prolonged in disorders affecting the basal ganglia, including dystonia1,2,3,4,5,6,7.
Dystonia is the third most common neurological movement disorder - after Parkinson's disease and Essential Tremor. It is characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive, movements or postures8. Dystonia can affect any part of the body. When it affects one body part it is known as focal dystonia8. Dystonia affecting the neck muscles is known as cervical dystonia, and is the most common phenotype of adult onset idiopathic isolated focal dystonia.9,10 The pathogenesis of cervical dystonia remains unknown; it is considered to be a genetic disorder with autosomal dominant inheritance and markedly reduced penetrance. Environmental factors are also considered important in relation to disease penetrance and expression.
The superior colliculus, a sensorimotor structure situated in the dorsal midbrain, is important for the rapid detection of environmental stimuli in the process of covert attentional orienting2,11,12. Visual stimuli access the superior colliculus rapidly through the retino-tectal magnocellular pathway. The TDT is a simple, objective measure believed to represent the processing of visual (and other sensory stimuli) in the superficial layers of the superior colliculus. The TDT has been studied in individuals with cervical dystonia, their unaffected relatives and healthy control participants. Compared to age- and sex-matched control participants, an abnormal TDT has high sensitivity (97%, 36 of 37 patients) and specificity (98-100%) in cervical dystonia1. An abnormal TDT has been found in 50% of unaffected first-degree female relatives of patients with cervical dystonia (14 of 25, aged 48 years or older), demonstrating age- and sex-related penetrance with autosomal dominant inheritance13,14. An abnormal TDT in unaffected relatives of cervical dystonia patients (compared to relatives with normal TDTs) is associated with increased putaminal volume (by voxel-based morphometry)15 and reduced putaminal activity (by fMRI)4. The superior colliculus is considered a significant node in the neuronal network, which is dysfunctional in cervical dystonia12. The assessment of temporal discrimination is regarded as providing important clues as to the pathomechanisms underlying cervical dystonia.
The goal of this article is to present two methods for measuring and analyzing temporal discrimination, as well as demonstrating the application of this method to studying the pathophysiology of cervical dystonia.
Protocol
The Medical Research Ethics Committee at St. Vincent's University Hospital, Dublin gave approval for the recruitment of patients with cervical dystonia, their siblings (unaffected by dystonia), and healthy controls, to participate in the protocol described below.
1. Hardware & Software Solutions
Note: Two hardware options have been developed to display visual stimuli with precise inter-stimulus intervals. Both were designed and built in-house at the Trinity Centre for Bioengineering, Trinity College Dublin, and have been previously described5,16. Those wishing to replicate the exact hardware solutions used herein may request same by contacting the Trinity Centre for Bioengineering directly. Alternatively, a full set of instructions including 3D printing files for the headset, instructions for the accompanying Arduino microcontroller, etc. can be downloaded from http://www.dystoniaresearch.ie/temporal-discrimination-threshold/. The stimuli presented in the table top approach may be generated using custom programs in Presentation (e.g., Neurobehavioural Systems), installed on a desktop computer and programmed to control the light-emitting diodes (LED) via the parallel port of the computer. Alternatively, as described below, the table top LEDs may be controlled via an Arduino microcontroller. Both the Presentation code and Arduino files are also available to download from the above link.
- TDT hardware: Table-Top Method
- Mark an 'X', as a fixation point, on a black mat or sheet placed on the table in front of the participant.
- Ask the participant to position themselves so that they are sitting directly in front of the fixation point.
- Place the yellow light-emitting diode (LED) pairs (5 mm diameter, 90 cd/m2 luminance), encased in a box, on the table in front of the participant.
- Orient the box such that the LEDs are vertically aligned and positioned 7 ° from the subject's center point on the left and right side, as needed.
- Conduct this experiment in a darkened room. A small amount of background luminance may be required to enable the operator to see enough to run the experiment.
- Instruct the participant to focus on the fixation point at all times and not to look directly at the flashing LEDs.
- Connect the microcontroller to the LED box and follow the on-screen instructions displayed on the liquid crystal display of the microcontroller box, e.g., select presentation method: 'random' or 'staircase', and select mode: 'left top first', etc.
- Ask the participant to respond "same" or "different" following presentation of each stimulus pair, depending on whether they perceive the stimuli to be synchronous or asynchronous.
- Inform the participant when each trial is about to commence, by vocalizing the on-screen countdown from 5 - 0 s.
Figure 1: (a) Schematic of the design of the headset. A pair of yellow LEDs (5 mm diameter),and the red fixation LED (3 mm diameter), are placed on the left and right side of the participant via a head-mounted unit and made visible by way of reflection in the mirrors in front of the user. (b) Schematic 3D model of the headset. The headset was developed from laser-sintered nylon plastic, weighs 0.70 kg, has a low transparency index and is black in color to minimize light penetrance. (a and b) are reproduced, with slight modification, from Butler et al.16 with permission from IOP Publishing. (c) The LED stimulus box for table-top presentation.
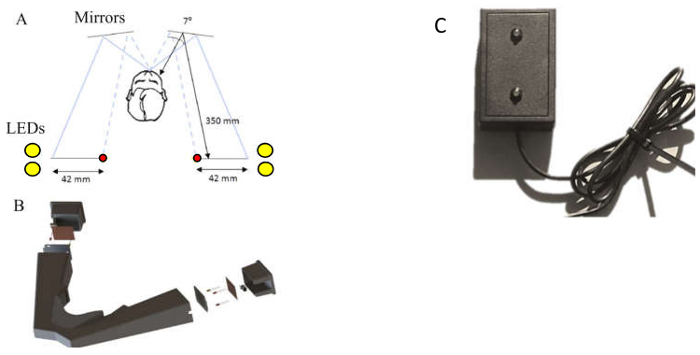
- TDT Hardware: Portable TDT Headset
- Conduct the experiment in any suitable location.
- Connect the microcontroller to the headset and follow the on-screen instructions displayed on the liquid crystal display of the microcontroller box, e.g., select presentation method: 'random' or 'staircase', and mode: 'left top first', etc.
- Direct the participant to position themselves with their elbows on a table in front of them. Then, holding the device in their hands, direct them to gently press their face into the rubber sealant surrounding the eyepiece, thereby sealing out ambient light.
- Instruct the participant to focus on the red fixation LED at all times and not to look directly at the flashing LEDs.
- Ask the participant to respond "same" or "different" following presentation of each stimulus pair, depending on whether they perceive the stimuli to be synchronous or asynchronous.
- Inform the participant when each trial is about to commence, by vocalizing the on-screen countdown from 5 - 0 s.
2. Stimulus Presentation
Note: Two approaches to stimulus presentation have been employed.
- Staircase method
- Select 'staircase' presentation; stimuli are presented every 5 s with the inter-stimulus interval starting at 0 and becoming progressively more asynchronous (increasing by 5 ms) each time.
- Select any of the four presentation modalities: (i) left top LED first (ii) left bottom LED first (iii) right top LED first, or (iv) right bottom LED first.
- Repeat step 2.1.2 so that each modality is run twice, resulting in a total of eight runs.
- Terminate the trial when a participant responds "different" for three consecutive pairs of stimuli.
- Random Presentation Method
- Select 'Random' presentation; stimuli pairs are presented every 5 s. The inter-stimulus interval varies, in a randomized fashion, from 0-100 ms.
- Select any of the four presentation modalities: (i) left top LED first (ii) left bottom LED first (iii) right top LED first, or (iv) right bottom LED first.
- Repeat step 2.2.2 so that each modality is run twice, resulting in a total of eight runs. Note: Each run is the same length and will complete automatically.
3. Data Analysis
- Single TDT value
- Using the data from the staircase method, highlight the first of the final three "different" responses for each of the eight runs. These are the threshold values for each run.
- Calculate the temporal discrimination threshold (TDT) for each participant by taking the median of the thresholds from each of their eight runs; resulting in a single TDT value (in milliseconds) per individual.
- Calculate the Zscore for each participant. Define the Zscore as the difference between the participant's TDT, and the mean TDT from an age-matched control population (
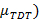 , divided by the standard deviation of the TDT values for that control population
, divided by the standard deviation of the TDT values for that control population 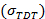 .
. 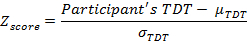
- Determine if the individual has a normal or abnormal TDT. A Zscore ≥ 2.5 is deemed to reflect an abnormal TDT.
- Distribution Analysis
- Using the data from the staircase method, encode the response data such that '0' corresponds to "same" and '1' corresponds to "different", Table 1.
- Download a free MATLAB.exe to perform the distribution analysis described below from http://www.dystoniaresearch.ie/temporal-discrimination-threshold/. See Butler et al.16 for a full description of this method. Alternatively, proceed as described below.
- Pad out the data to ensure all runs are the same length as the longest run. This is done by assuming all subsequent responses, following termination of a run, are "different", Table 1(b).
- Average responses across trials for each participant, Table 1(c). This can be plotted as a function of stimulus asynchrony.
- Fit this averaged or representative data with a cumulative Gaussian function. The mean of this distribution represents the point at which participants are equally likely to respond “same” or “different”. This point is referred to as the ‘point of subjective equality’ (PSE). The standard deviation of the Gaussian distribution, also referred to as the ‘just noticeable difference’ (JND), indicates how sensitive participants are to changes in temporal asynchrony around their mean.
- Extend the analysis by submitting the data to a non-parametric bootstrapping procedure in order to estimate the 95% confidence intervals for the TDT and the PSE and JND of the psychometric, cumulative Gaussian function. To do this, generate new representative data sets by random sampling with replacement from the original responses, Table 1(b), for each time step. Calculate the TDT and fit a new psychometric function for each representative data set16.
- Calculate the goodness of fit, or deviance (D), for each participant using the log-likelihood ratio,16,17
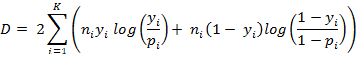 where K is the number of time points, ni is the number of repetitions at that time point, generally eight repetitions (four right and four left), yi is the observed proportion of asynchronous responses, pi is the proportion of asynchronous responses predicted by the fitted curve. A deviance value of 0 means a perfect fit.
where K is the number of time points, ni is the number of repetitions at that time point, generally eight repetitions (four right and four left), yi is the observed proportion of asynchronous responses, pi is the proportion of asynchronous responses predicted by the fitted curve. A deviance value of 0 means a perfect fit. - Plot the results. Note: Data from the random presentation approach can be analyzed to determine the single or distributed TDT as described in section 3 above for data arising from the staircase presentation method. However, due to the random presentation order of inter-stimuli intervals, these data must first be ordered (from smallest to largest inter-stimulus interval), prior to commencing the analysis described above, Table 2. In addition, it is not necessary to pad the data following random presentation as, by default, all runs are of equal length.
Representative Results
Examples of filled score sheets are provided in Tables 1 and 2, where these respectively represent results following staircase and random stimulus presentation methods. The thresholds for each run (the timing of the first of three stimulus pairs deemed to be 'different'), are highlighted. In the case of Table 1, the TDT is calculated as 25 ms (i.e., the median of 40, 25, 25, 25, 45, 25, 40, 10 ms). These data are taken from a 35 year old woman who participated in a previous study18. The mean and standard deviation for TDT values from women in this age bracket were 27.48 ms and 10.86 ms, respectively. Therefore the Z-score for this individual can be calculated as: ![]()
As this Zscore is below 2.5, this individual has a normal TDT.
Responses from the same individual following random stimulus presentation are shown in Table 2. Ordering these data is an important step prior to continuing with analysis.
Distribution Analysis
Key stages in the distribution analysis are illustrated in Table 1 (data padding and response averaging) and Figure 2. The sample data used in this analysis is from the same subject as that discussed above and shown in Tables 1 and 2. The plots in Figure 2 are generated from the downloadable MATLAB.exe file. The left side shows the observed data, the cumulative Gaussian functions fitted to the bootstrapped data (following 2000 iterations), and the average cumulative Gaussian function. The goodness of fit measure is illustrated on the right-hand side. Also shown are the temporal discrimination thresholds, the fit parameters, the point of subjective equality (PSE), and just noticeable difference (JND) values. The right side shows goodness of fit measure the log likelihood ratio (deviance) for the observed data (red horizontal line) and the Monte-Carlo generated log likelihood ratio distribution and the 95% confidence intervals (dashed horizontal lines).
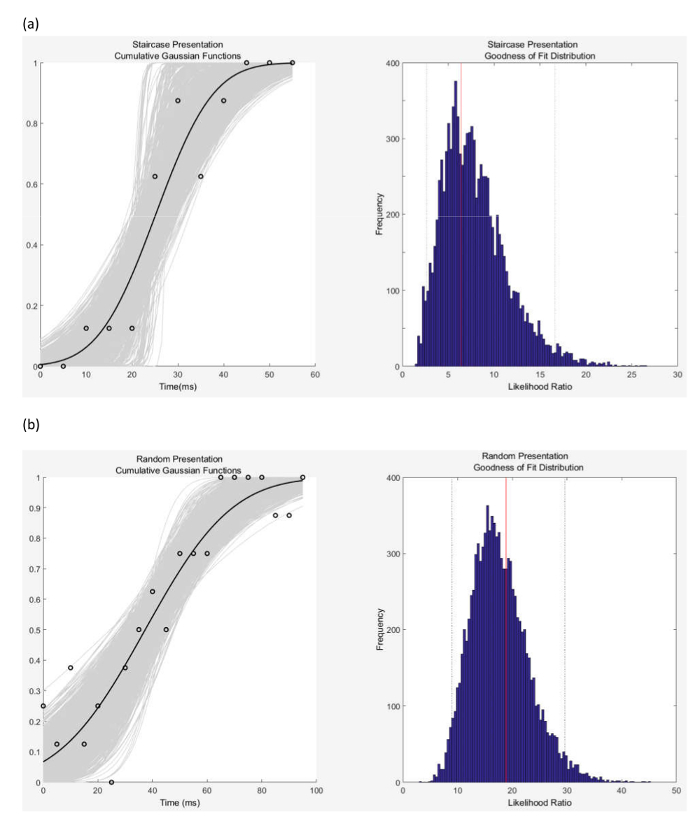
The same MATLAB executable exports the TDT, PSE and JND values and bootstrapped cut-offs of 2.5%, 25%, 50%, 75% and 97.5% confidence intervals as well as the goodness of fit or deviance and cutoffs to an excel file. Table 3 provides the outputs generated for the data in Tables 1 and 2. By way of comparison, the TDT values for staircase and random stimulus presentation methods, obtained by the standard method (median of the 8 thresholds), are 25 ms and 50 ms respectively; whereas Table 3 provides the TDT values obtained following bootstrapping of the data. These are 23.75 ms and 48.75 ms respectively.
Figure 2: The left-hand column shows the cumulative Gaussian Distributions for (a) results following the staircase method of stimulus presentation, and (b) the random method of stimulus presentation. The black dots show the original data (the proportion of perceived 'different' responses as a function of inter-stimulus interval, or temporal asynchrony). The light grey curves represent the 2000 Gaussian functions that were fitted to the bootstrapped data. The dark grey curve represents the average cumulative Gaussian function. Values for the Point of Subjective Equality (PSE) (mean) and Just Noticeable Difference (JND) (standard deviation) and the TDT value, calculated from the full distribution are detailed in Table 3. Please click here to view a larger version of this figure.
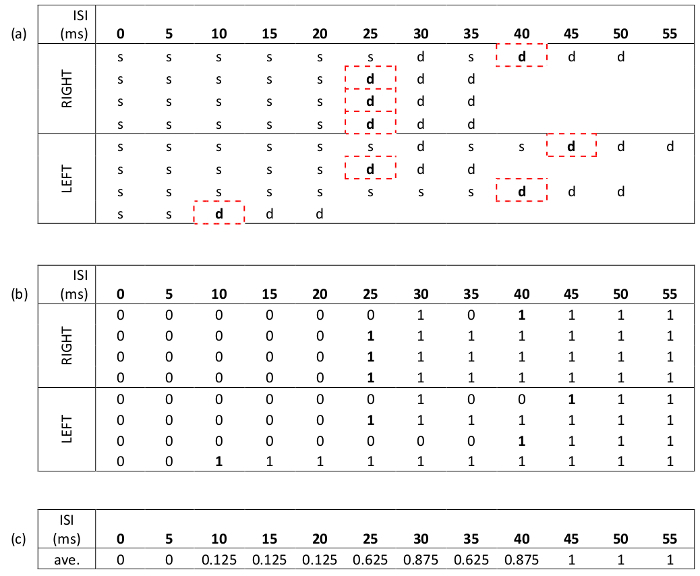 Table 1: Sample data following staircase presentation method, with inter-stimulus intervals (ISI) increasing by 5 ms each time. (a) Data shown for each of the two conditions (top LED first x2, and bottom LED first x2) for the right- and left-hand sides, giving a total of eight runs. 's' represents a response of 'same', and 'd', 'different'. The time intervals used to calculate the TDT are the ISI's corresponding to the first of three consecutive 'different' responses. Therefore, the TDT= 25 ms, the median of 40, 25, 25, 25, 45, 25, 40, and 10. (b) The same data as shown in (a), but encoded such that a '0' represents a response of 'same', and '1' represents 'different'. Data padding (to the longest run) is illustrated. This is a pre-processing step prior to applying the distribution analysis. (c) Averaged responses for each ISI. Note these values are used to generate the psychometric distribution and are plotted in Figure 2.
Table 1: Sample data following staircase presentation method, with inter-stimulus intervals (ISI) increasing by 5 ms each time. (a) Data shown for each of the two conditions (top LED first x2, and bottom LED first x2) for the right- and left-hand sides, giving a total of eight runs. 's' represents a response of 'same', and 'd', 'different'. The time intervals used to calculate the TDT are the ISI's corresponding to the first of three consecutive 'different' responses. Therefore, the TDT= 25 ms, the median of 40, 25, 25, 25, 45, 25, 40, and 10. (b) The same data as shown in (a), but encoded such that a '0' represents a response of 'same', and '1' represents 'different'. Data padding (to the longest run) is illustrated. This is a pre-processing step prior to applying the distribution analysis. (c) Averaged responses for each ISI. Note these values are used to generate the psychometric distribution and are plotted in Figure 2.
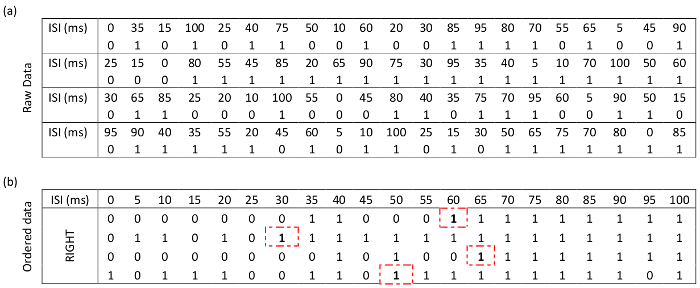 Table 2: Responses from the same participant as Table 1, this time stimuli are presented with randomized inter-stimulus intervals (ISI). (a) Data for the two conditions on the right-hand side (top LED first x2 and bottom LED first x2). For compactness, the data from the left-hand side are not shown here. However, all eight runs are used in all analysis. (b) The same data sorted by incrementing ISI. The threshold for each of the four right-hand side runs are indicated with dashed boxes.
Table 2: Responses from the same participant as Table 1, this time stimuli are presented with randomized inter-stimulus intervals (ISI). (a) Data for the two conditions on the right-hand side (top LED first x2 and bottom LED first x2). For compactness, the data from the left-hand side are not shown here. However, all eight runs are used in all analysis. (b) The same data sorted by incrementing ISI. The threshold for each of the four right-hand side runs are indicated with dashed boxes.
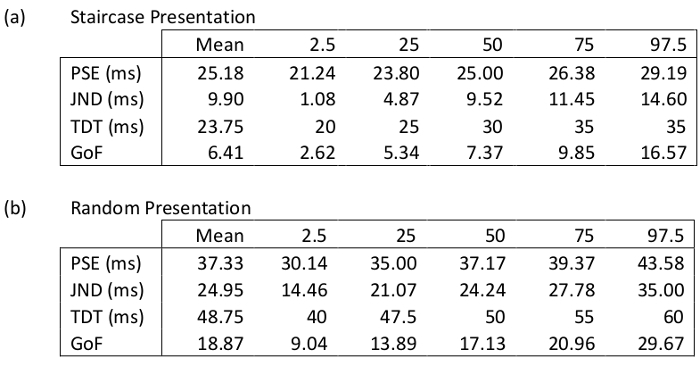 Table 3: Summary of Gaussian distribution and Goodness of Fit analysis for the results from the staircase presentation method shown in Table 1, and random presentation method shown in Table 2 (all data for this participant, e.g. total of eight runs (4 left and 4 right) have been used in above analysis). Point of subjective equality, PSE; just noticeable difference, JND; temporal discrimination, TDT; Goodness of Fit, GoF.
Table 3: Summary of Gaussian distribution and Goodness of Fit analysis for the results from the staircase presentation method shown in Table 1, and random presentation method shown in Table 2 (all data for this participant, e.g. total of eight runs (4 left and 4 right) have been used in above analysis). Point of subjective equality, PSE; just noticeable difference, JND; temporal discrimination, TDT; Goodness of Fit, GoF.
Discussion
TDT Measurement and Analysis
Two forms of apparatus (table-top and headset), two methods of stimulus presentation (staircase and random), and two approaches to data analysis (traditional and distribution) have been presented to illustrate how to measure and quantify a person's temporal discrimination ability. The portable headset provides a convenient hardware option that ensures consistency in distance and angles between the participant and the LED light sources while also allowing data to be collected in any convenient location. It, therefore, addresses some of the limitations associated with the table top approach, namely the need for controlled ambient lighting and the limited portability - typically requiring participants attend at a clinic or research center. The headset also guards against the possibility of variability in distance and angle between stimuli and participant during or between trials, potentially arising from positional adjustments by the participant. Molloy et al. compared the table-top and the headset approaches for stimulus delivery and found the headset to be reliable and accurate5. However, two potential weaknesses of the headset are that it presents the stimuli monocularly, i.e., only the left eye can see the stimuli presented on the left and vice versa; and the current design does not accommodate the wearing of glasses. Visual acuity may affect TDT performance, and as such one should always ascertain that participants have normal visual acuity. This is all the more important in the case of the headset approach, where glasses cannot be accommodated.
The 'staircase' approach is the most common method of stimulus presentation for visual and tactile temporal discrimination protocols6,7,14,15,19. A limitation of this technique, which presents non-randomized progressively asynchronous stimuli, is that it may possibly contribute to a potential learning effect. As an alternative, a randomized presentation modality was developed, allowing stimuli to be presented in a randomized manner. The possibility of the staircase method being subject to a learning effect was specifically tested by McGovern and colleagues16. The 'staircase' method was shown to be a robust approach with consistent results across repeated experiments18. Results from this earlier study, as shown above, revealed that randomized stimuli presentation method yields consistently longer TDT values compared with the existing staircase method (mean TDTRANDOM = 55.08 ms; mean TDTSTAIRCASE = 30.57 ms for 30 healthy controls)18. While both presentation methods are valid, the difference in resulting TDT values emphasizes the importance of maintaining uniformity in experimental technique selection within and across studies from a given laboratory. In addition, care should be taken when comparing absolute TDT values across studies (from patients and controls), and in the calculation of Zscores.
Two methods of data analysis have also been presented. The first, standard analysis method, results in a single threshold value for each of the eight runs, where that threshold is the inter-stimulus interval of the first of three stimulus pairs identified as being asynchronous. The median of the eight thresholds is taken as the TDT value for that participant. While this has proven to be reliable, it is nonetheless a single value. In order to overcome the potential limitation of assessing a person's temporal discrimination ability based on a single value, a more sophisticated approach has also been presented. In this instance, a participant's data is fitted with a cumulative Gaussian distribution and the mean and standard deviation extracted. In addition, the data are submitted to a non-parametric bootstrapped analysis to get 95% confidence intervals for each participant's data16. This method of data analysis offers the potential to gain deeper insight into differences in visual perception, particularly when examining differences within and between control and patient groups.
Application of TDT to understanding the pathophysiology of Cervical Dystonia
While it is likely that cortical processing is relevant in temporal discrimination20, the evidence suggests that in cervical dystonia abnormal temporal discrimination reflects primarily a disorder in a network involving the superior colliculus and basal ganglia4,21. An abnormal TDT can be interpreted as an impaired ability to detect or discriminate environmental change. The superior colliculus, in the dorsal midbrain, plays a critical role in detecting and reacting to salient stimuli22. Although a complex structure, it can be functionally separated into two layers22. The visuosensory neurons in the superficial layer receive direct input from the visual system, whereas the premotor and cephalomotor neurons in the deep layer have multiple projections, including control of the muscles of the eyes, neck and head. Superior collicular activity is modulated by gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter23. Inhibitory GABAergic activity limits the duration of the transient burst response in both the visuosensory neurons in the superficial layer and the premotor neurons in the deep layer of the superior colliculus24. In response to a visual stimulus, the visual neurons in the superficial layer exhibit a transient 'ON' response. GABAergic inhibition then silences this response, enabling the neurons to be ready to respond again when they detect a change in the environment as the visual stimulus is turned off. If there is insufficient GABA, these neurons may become dysfunctionally active24. It is hypothesized that insufficient GABAergic inhibition results in prolonged duration firing of the visual neurons, giving rise to abnormal temporal discrimination, and prolonged TDT values. In addition, the abnormal movements characteristic of cervical dystonia are hypothesized to also result from insufficient GABAergic inhibition, this time by the cephalomotor neurons in the deep layers of the superior colliculus.
An endophenotype is a subclinical marker of genetic carriage that can help us understand disease pathomechanisms. The TDT is proposed as a potential endophenotype for adult onset focal dystonia2,4 and has been found to be abnormal in up to 97% of patients and approximately 50% of their clinically unaffected relatives1,3,4. In addition, abnormal TDT has been shown to follow an age- and sex-related pattern similar to that of cervical dystonia14,25. These findings suggest autosomal dominant inheritance and support the use of the TDT as an endophenotype for adult onset focal dystonia, and in particular, cervical dystonia.
This article has provided a guide on how to measure and analyze a participant's visual temporal discrimination. In addition, with the aid of animated graphics in the video, the application of TDT to the study of cervical dystonia has been outlined both in the context of it being a reliable endophenotype, and as a potential tool to shed light on the pathomechanisms of this disorder.
Disclosures
Rebecca B Beck, Eavan M Mc Govern, John Butler, Dorina Birsanu, Brendan Quinlivan, Ines Beiser, Shruti Narasimham have no funding sources, financial disclosures or conflict of interests to declare. Michael Hutchinson receives research grants from Dystonia Ireland, the Health Research Board of Ireland (CSA-2012-5), Foundation for Dystonia Research (Belgium) and the Irish Institute of Clinical Neuroscience. Sean O'Riordan reports receiving a speaker's honorarium from Abbvie. Richard Reilly receives funding from Science Foundation Ireland, Enterprise Ireland andthe Health Research Board of Ireland.
Acknowledgments
This research was supported by grants from the Health Research Board, Dystonia Ireland, Science Foundation Ireland and the Irish Institute for Clinical Neuroscience.
References
- Bradley D, et al. Temporal discrimination thresholds in adult-onset primary torsion dystonia: an analysis by task type and by dystonia phenotype. J Neurol. 2012;259(1):77–82. doi: 10.1007/s00415-011-6125-7. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, et al. The endophenotype and the phenotype: temporal discrimination and adult-onset dystonia. Mov Disord. 2013;28(13):1766–1774. doi: 10.1002/mds.25676. [DOI] [PubMed] [Google Scholar]
- Kimmich O, et al. Sporadic adult onset primary torsion dystonia is a genetic disorder by the temporal discrimination test. Brain. 2011;134(Pt 9):2656–2663. doi: 10.1093/brain/awr194. [DOI] [PubMed] [Google Scholar]
- Kimmich O, et al. Temporal discrimination, a cervical dystonia endophenotype: penetrance and functional correlates. Mov Disord. 2014;29(6):804–811. doi: 10.1002/mds.25822. [DOI] [PubMed] [Google Scholar]
- Molloy A, et al. A headset method for measuring the visual temporal discrimination threshold in cervical dystonia. Tremor Other Hyperkinet Mov (N Y) 2014;4:249. doi: 10.7916/D8TD9VF6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termsarasab P, et al. Neural correlates of abnormal sensory discrimination in laryngeal dystonia. Neuroimage Clin. 2016;10:18–26. doi: 10.1016/j.nicl.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorio M, et al. Defective temporal processing of sensory stimuli in DYT1 mutation carriers: a new endophenotype of dystonia? Brain. 2007;130(1):134–142. doi: 10.1093/brain/awl283. [DOI] [PubMed] [Google Scholar]
- Albanese A, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28(7):863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG, Muenter MD, Aronson A, Kurland LT, Melton LJ., 3rd Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov Disord. 1988;3(3):188–194. doi: 10.1002/mds.870030302. [DOI] [PubMed] [Google Scholar]
- Williams L, et al. Epidemiological, clinical and genetic aspects of adult onset isolated focal dystonia in Ireland. Eur J Neurol. 2016. [DOI] [PubMed]
- Bell AH, Munoz DP. Activity in the superior colliculus reflects dynamic interactions between voluntary and involuntary influences on orienting behaviour. Eur J Neurosci. 2008;28(8):1654–1660. doi: 10.1111/j.1460-9568.2008.06393.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, et al. Cervical dystonia: a disorder of the midbrain network for covert attentional orienting. Front Neurol. 2014;5:54. doi: 10.3389/fneur.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LJ, et al. Young Women do it Better: Sexual Dimorphism in Temporal Discrimination. Front Neurol. 2015;6:258. doi: 10.3389/fneur.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JS, et al. Age-Related Sexual Dimorphism in Temporal Discrimination and in Adult-Onset Dystonia Suggests GABAergic Mechanisms. Front Neurol. 2015;6:258. doi: 10.3389/fneur.2015.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D, et al. Temporal discrimination threshold: VBM evidence for an endophenotype in adult onset primary torsion dystonia. Brain. 2009;132(Pt 9):2327–2335. doi: 10.1093/brain/awp156. [DOI] [PubMed] [Google Scholar]
- Butler JS, et al. Non-parametric bootstrapping method for measuring the temporal discrimination threshold for movement disorders. J Neural Eng. 2015;12(4):046026. doi: 10.1088/1741-2560/12/4/046026. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001;63(8):1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- McGovern EM, et al. A comparison of stimulus presentation methods in temporal discrimination testing. Physiol Meas. 2017;38(2):N57–N64. doi: 10.1088/1361-6579/38/2/N57. [DOI] [PubMed] [Google Scholar]
- Scontrini A, et al. Somatosensory temporal discrimination in patients with primary focal dystonia. J Neurol Neurosurg Psychiatry. 2009;80(12):1315–1319. doi: 10.1136/jnnp.2009.178236. [DOI] [PubMed] [Google Scholar]
- Nardella A, et al. Inferior parietal lobule encodes visual temporal resolution processes contributing to the critical flicker frequency threshold in humans. PLoS One. 2014;9(6):e98948. doi: 10.1371/journal.pone.0098948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor MA, Macaluso E, Day BL, Frackowiak RS. Putaminal activity is related to perceptual certainty. Neuroimage. 2008;41(1):123–129. doi: 10.1016/j.neuroimage.2008.01.034. [DOI] [PubMed] [Google Scholar]
- Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol. 2009;102(5):2581–2593. doi: 10.1152/jn.00498.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Endo T, Saito Y. The visuo-motor pathway in the local circuit of the rat superior colliculus. J Neurosci. 1998;18(20):8496–8504. doi: 10.1523/JNEUROSCI.18-20-08496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda K, Isa T. GABAergic mechanisms for shaping transient visual responses in the mouse superior colliculus. Neuroscience. 2013;235:129–140. doi: 10.1016/j.neuroscience.2012.12.061. [DOI] [PubMed] [Google Scholar]
- Ramos VF, Esquenazi A, Villegas MA, Wu T, Hallett M. Temporal discrimination threshold with healthy aging. Neurobiol Aging. 2016;43:174–179. doi: 10.1016/j.neurobiolaging.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


