Abstract
This study tests the hypothesis that amplitude modulation (AM) detection will be better under conditions where basilar membrane (BM) response growth is expected to be linear rather than compressive. This hypothesis was tested by (1) comparing AM detection for a tonal carrier as a function of carrier level for subjects with and without cochlear hearing impairment (HI), and by (2) comparing AM detection for carriers presented with and without an ipsilateral notched-noise precursor, under the assumption that the precursor linearizes BM responses. Average AM detection thresholds were approximately 5 dB better for subjects with HI than for subjects with normal hearing (NH) at moderate-level carriers. Average AM detection for low-to-moderate level carriers was approximately 2 dB better with the precursor than without the precursor for subjects with NH, whereas precursor effects were absent or smaller for subjects with HI. Although effect sizes were small and individual differences were noted, group differences are consistent with better AM detection for conditions where BM responses are less compressive due to cochlear hearing loss or due to a reduction in cochlear gain. These findings suggest the auditory system may quickly adjust to the local soundscape to increase effective AM depth and improve signal-to-noise ratios.
I. INTRODUCTION
Speech, music, and other real-world signals contain gross fluctuations in amplitude over time. Sensitivity to these fluctuations is important for sound source identification (Yost, 1991) and for conveying manner of articulation, syllable structure, voice onset time, and speech rate (Rosen, 1992). Adults with normal hearing (NH) are sensitive to sinusoidal amplitude modulation (AM) of noise carriers at AM depths corresponding to a 1 dB difference between AM peaks and valleys (Viemeister, 1979). For low modulation frequencies (<30 Hz) this sensitivity is constant across a wide range of noise carrier levels (Viemeister, 1979), or improves with increasing level for tonal carriers (Kohlrausch et al., 2000).
The finding that AM detection is constant or improves with increasing carrier level is inconsistent with basilar membrane (BM) response growth. For measurements in the base of the cochleae of laboratory animals, BM responses at the characteristic frequency (CF) grow linearly at low levels and compressively for moderate-to-high levels (Robles and Ruggero, 2001). This transition between linear and compressive response growth is the result of a progressively smaller contribution of cochlear amplification as a function of level, where this amplification is a result of outer hair cell (OHC) motility (Liberman et al., 2002). The transition from linear to compressive BM growth is expected to result in a progressively smaller modulation depth at the output of the cochlea (i.e., hereafter referred to as “effective” modulation depth) with increasing carrier level. This is due to compression reducing the effective modulation depth relative to the input modulation depth for moderate-to-high carrier levels. At lower input levels, where BM responses are linear, the effective modulation depth is expected to be similar to the input modulation depth. Under the assumption that a constant modulation depth will yield constant performance (Viemeister, 1979), AM detection for high-frequency carriers associated with the cochlear base is expected to decline with increasing carrier level due to the reduction in effective modulation depth, as has been shown through model simulations (Heinz et al., 2001).
The finding that AM detection as a function of carrier level is constant or improves rather than declines poses a challenge to understanding how AM detection is influenced by cochlear processes. Contributing to this challenge is the finding that excitation spreads basalward with increasing stimulus intensity, which may result in detection of AM through auditory filters remote from the carrier frequency (Strickland and Viemeister, 1997; Kohlrausch et al., 2000). Based on findings that BM responses grow linearly for off-CF stimuli (Ruggero et al., 1997), the envelope of high-level carriers is expected to be processed linearly through these off-CF filters, leading to improvements in AM detection with increasing carrier level, despite the expected level-dependent effects of cochlear compression (Millman and Bacon, 2008).
Using a short-duration (50 ms), narrowband-noise carrier centered on 5000 Hz and off-frequency noise to minimize the effects of the spread of excitation, Almishaal et al. (2017) measured AM detection as a function of carrier level to test the hypothesis that AM detection declines with increasing carrier level, as predicted from BM compression. Consistent with their hypothesis, AM detection declined as carrier level increased from 45 to 65 dB sound pressure level (SPL). To further assess the role of compression, Almishaal et al. (2017) compared AM detection for a carrier preceded by silence, or by a 200-ms, notched-noise precursor, under the assumption that the precursor linearizes BM responses (Strickland, 2008; Jennings et al., 2009; Yasin et al., 2013; Roverud and Strickland, 2015a). This assumption is based on the presumed effects of the medial olivocochlear (MOC) reflex, which decreases cochlear amplifier gain in response to acoustic stimulation (Guinan, 2006) with a time constant of approximately 70 ms (Backus and Guinan, 2006). AM detection with the precursor was better than detection without the precursor for mid-level carriers, resulting in monotonically decreasing AM detection thresholds with increasing carrier level. Almishaal et al. (2017) concluded that these results were consistent with a reduction in cochlear amplifier gain that could be attributed to the effects of the MOC reflex.
Cochlear hearing loss results in less-than-normal cochlear amplifier gain (Davis, 1983), which narrows the range of input levels subjected to cochlear compression (Yates et al., 1990). Thus, BM responses grow linearly over a wider range of input levels for subjects with hearing impairment (HI) than for subjects with NH (Oxenham and Bacon, 2003). The contribution of cochlear amplifier gain to BM excitation becomes progressively smaller with increasing SPL (Ruggero et al., 1997), thus MOC-mediated adjustments to OHC gain may be smaller than normal in subjects with HI, consistent with masking studies (Bacon and Takahashi, 1992; Strickland and Krishnan, 2005; Jennings et al., 2016) and model simulations (Strickland and Krishnan, 2005; Jennings et al., 2016).
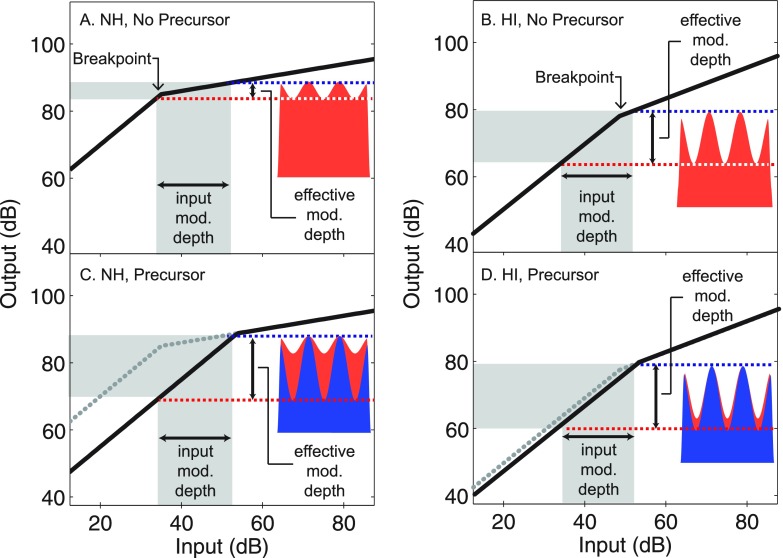
Expected improvements in AM detection due to reductions in cochlear amplifier gain are illustrated in Fig. 1, which plots schematized input/output (I/O) functions (black lines) at the output of an auditory filter centered on the carrier frequency for subjects with NH [Figs. 1(A) and 1(C)] and HI [Figs. 1(B) and 1(D)]. For subjects with NH, moderate-level, short-duration (e.g., 50 ms) carriers [Fig. 1(A)] are expected to undergo compression, which results in a smaller effective modulation depth (vertical double arrows) than the input modulation depth (horizontal double arrows). However, for subjects with HI [Fig. 1(B)], the effective modulation depth at comparable input levels is expected to be larger than normal due to less compressive response growth. Compression exponents in Fig. 1 were 0.2 dB/dB for subjects with NH based on experiments with laboratory animals (Ruggero et al., 1997; Rhode and Recio, 2000) and forward masking experiments with humans (Oxenham and Plack, 1997; Nelson et al., 2001; Plack and O'Hanlon, 2003). Compression exponents were 0.5 dB/dB for subjects with HI consistent with Rosengard et al. (2005); however, for some subjects with milder HI, compression exponents may be similar to normal (Plack et al., 2004; Dubno et al., 2007).
FIG. 1.
(Color online) Schematic illustration of the expected effects of a reduction in cochlear amplifier gain on effective amplitude modulation depth in NH (panels A, C) and HI (panels B, D) subjects. BM response growth, at the output of an auditory filter centered on the carrier frequency, grows linearly for low-level carriers, and compressively for mid-to-high level carriers (solid lines), where linear and compressive regions intersect at a breakpoint. The presentation of a precursor is assumed to reduce cochlear amplifier gain (dotted gray line vs solid black line), but less so in subjects with HI than in subjects with NH. This results in increases in the input levels corresponding to the compression breakpoint. For moderate-level carriers preceded by silence (panels A, B) the effective modulation (“mod.”) depth is smaller than the input modulation depth. Similarly, for moderate-level carriers preceded by a precursor (panels C, D) the effective modulation depth is roughly equal to the input modulation depth. The effective modulation depth for subjects with HI is assumed to be larger than that for subjects with NH in the no precursor condition (panels A,B) due to less compressive BM response growth in subjects with HI than in subjects with NH. The solid black lines in the top panels are replotted as gray dotted lines in the bottom panels. Horizontal double arrows show the input modulation depth. Horizontal dashed lines and vertical double arrows show the effective modulation depth. Waveforms on the right side of the panels are schematic representations of the amplitude modulated carrier at the output of the cochlea at the characteristic frequency. To facilitate comparison, waveforms from panels A and B are copied to panels C and D, respectively.
For short-duration carriers preceded by precursors [Fig. 1(C)], cochlear amplifier gain is expected to decrease over the time course of the precursor for low-to-moderate input levels (Strickland, 2008), as illustrated by the 15-dB vertical distance between dashed gray and solid black lines. Gain reduction of this magnitude is within the range expected for MOC stimulation via electric shock in laboratory animals (Murugasu and Russell, 1996; Dolan et al., 1997; Russell and Murugasu, 1997; Cooper and Guinan, 2003), or as estimated via low-level (e.g., 40 dB SPL) ipsilateral acoustic stimulation in masking experiments with human subjects (Krull and Strickland, 2008; Jennings et al., 2009; Roverud and Strickland, 2010; Yasin et al., 2013). However, due to differences in species (e.g., guinea pig vs human) and stimulus type (i.e., electric shock vs acoustic stimulation), 15-dB gain reduction may be an overestimate and is used here for illustrative purposes only. A reduction in cochlear gain could result in linear rather than compressive BM responses over part of the I/O function [Fig. 1(C), shaded gray region] for subjects with NH. Linearization of BM responses increases the effective modulation depth over a range of moderate input levels, thus predicting better AM detection with a precursor than without a precursor. For low and high input levels, reductions in cochlear amplifier gain are not expected to increase effective modulation depth as BM responses will remain linear or compressive, respectively. For subjects with HI, precursors are expected to have little to no effect on effective modulation depth as BM responses remain linear across a wider range of levels and, therefore, are not affected by the presence of a precursor in the same way as for subjects with NH.
Improvements in effective AM depth depend on the amount of cochlear compression, and the input level delineating the linear and compressive regions of the I/O function (i.e., compression breakpoint, Fig. 1). Compression exponents and breakpoints, among other factors, are expected to vary among subjects with NH (Poling et al., 2012) and HI (Rosengard et al., 2005). Jennings et al. (2014) modeled individual differences in temporal masking curves (TMCs) in subjects with NH and HI and concluded that between-subject variability in on-frequency TMCs for subjects with NH was consistent with individual differences in cochlear compression exponents and breakpoints after controlling for other peripheral and central factors. The framework in Fig. 1 predicts that individual differences in cochlear compression exponents and breakpoints will result in individual differences in the effects of carrier level and the presence of the precursor on AM detection. Support for this comes from Roverud and Strickland (2015a,b) who observed appreciable individual differences in intensity discrimination thresholds in the presence (Roverud and Strickland, 2015b) and absence (Roverud and Strickland, 2015a) of precursors and were able to account for these differences using estimates of compression exponents and breakpoints obtained for individual subjects. Between-subject variability in MOC strength, as measured by otoacoustic emissions, has been shown to predict phoneme identification in noise for monosyllabic words (Giraud et al., 1997; Kumar and Vanaja, 2004) and psychometric function slope for words or sentences presented in noise (Mertes et al., 2017). This suggests that between-subject MOC strength may also influence individual differences in AM detection.
The framework illustrated in Fig. 1 leads to the following hypotheses, which were tested in this study by measuring AM detection as a function of the level of a 2000-Hz carrier with and without a precursor in subjects with NH and HI: (1) for subjects with HI, detection of AM within a short-duration carrier will be better than for subjects with NH (“better than normal”) for moderate-level carriers, consistent with less compressive BM response growth at those levels, (2) improvements in AM detection for moderate-level carriers presented with, compared to without, a precursor will be smaller than normal for subjects with HI, consistent with smaller MOC-mediated adjustments to cochlear amplifier gain, and (3) precursor and carrier level effects on AM detection will be marked by individual differences, consistent with individual differences in compression and MOC activity.
II. METHODS
A. Subjects
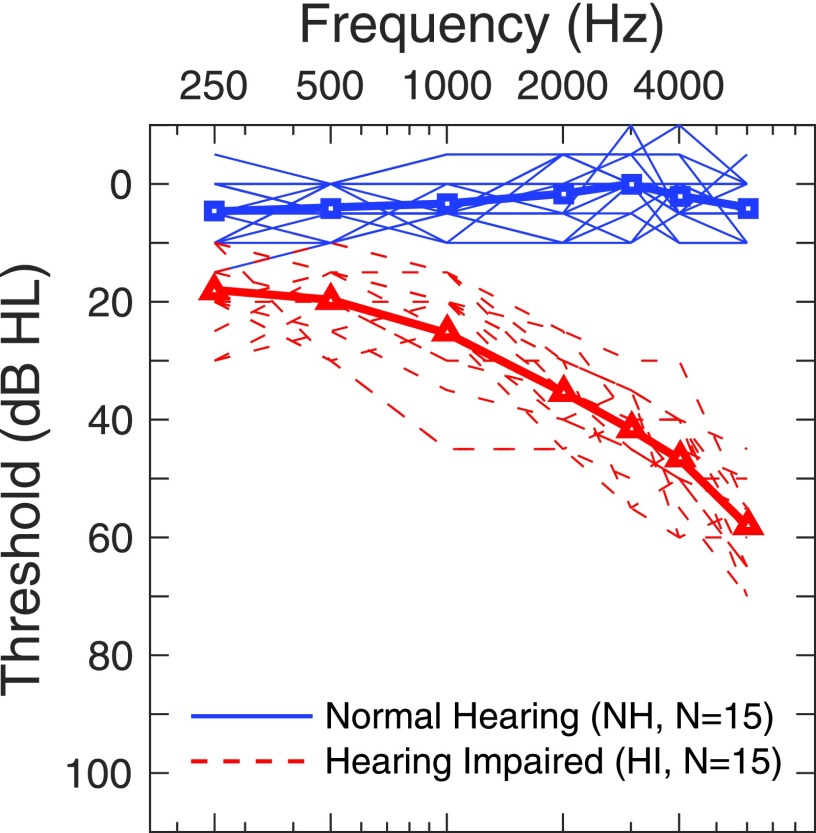
Thirty adults, ranging in age from 21 to 80 yr, participated in this experiment. Fifteen subjects with NH (mean age = 23.7 yr, 4 males) were <30 yr of age, and had thresholds ≤25 dB hearing level (HL) at audiometric frequencies between 250 and 8000 Hz. Fifteen subjects with HI (mean age = 73.2 yr, 8 males) were >60 yr of age and had mild-to-moderate sensorineural hearing loss. Eight subjects with NH and seven subjects with HI were tested at the University of Utah (U of U). The remaining subjects were tested at the Medical University of South Carolina (MUSC). All subjects had normal middle ear function as judged by a certified audiologist using results from clinical tympanometry (226 Hz probe). For subjects with NH, the right ear was selected as the test ear. For subjects with HI, the ear with the better audiometric thresholds at 2000 Hz (the frequency of the AM carrier) was selected as the test ear. The right ear served as the test ear for 8 of 15 subjects with HI. Audiometric thresholds for the test ear are shown in Fig. 2 for subjects with NH (squares) and HI (triangles), where thin and thick lines are individual and mean data, respectively.
FIG. 2.
(Color online) Audiometric thresholds (in dB HL) for subjects with NH (squares and solid lines) and HI (triangles and dashed lines). Thin lines are individual data, and thick lines and symbols are the mean data.
B. Apparatus and stimuli
A 2000 Hz (fc), 50-ms sinusoidal carrier was gated with 2-ms cos2 ramps and was sinusoidally amplitude modulated at a modulation frequency of 40 Hz. A short-duration carrier was used, as longer duration carriers may linearize BM response growth via a reduction in cochlear amplifier gain (Krull and Strickland, 2008; Jennings et al., 2009; Yasin et al., 2013; Roverud and Strickland, 2015a) and confound the comparison of AM detection between conditions with and without a precursor. In addition, 2000-Hz carriers were used to avoid frequencies with more than a mild-to-moderate hearing loss in subjects with HI, and a 40-Hz modulation frequency was chosen based on pilot data.1 Carrier levels were 45, 50, 55, 65, 75, and 85 dB SPL for subjects with NH and 60, 65, 75, 85, 95 dB SPL for subjects with HI. These levels correspond to sensation levels (SLs) ranging from 32 to 87 dB SL and 12 to 67 dB SL in subjects with NH and HI, respectively, relative to subjects' audiometric thresholds at 2000 Hz. To reduce the effects of off-frequency listening, a notched noise (Oxenham and Plack, 1997) was gated with the carrier and presented at 50 dB/Hz below the carrier level (Nelson et al., 2001). The inner frequency cutoffs of the notched noise were and (i.e., 1800 and 2400 Hz), and the outer frequency cutoffs were 1000 and 4000 Hz. The notched-noise precursor was 200 ms in duration, including 5-ms onset/offset ramps. A notched-noise precursor was used based on Almishaal et al. (2017), who reported that precursors with spectral energy at the carrier frequency resulted in forward masking in the modulation domain (Wojtczak and Viemeister, 2005). The precursor was presented immediately before the carrier (i.e., no delay between the offset of the precursor and the onset of the carrier). The precursor had a spectral notch extending from 1800 to 2200 Hz and outer frequency cutoffs of 600 and 3200 Hz, respectively. The spectra of the off-frequency noise and precursor were similar to Almishaal et al. (2017) after adjusting for the carrier frequency of this study (5000 Hz carriers were used in Almishaal et al., 2017). The precursor was presented at 40 dB SPL for subjects with NH and 55 dB SPL for subjects with HI. For the conditions without a precursor, silence preceded the carrier's onset and the timing of all other signals remained the same.
Stimuli were digitally generated using custom matlab (The MathWorks Inc., Natick, MA) software (Bidelman et al., 2015) and output through a LynxTWO-B (Lynx Studio Technology, Costa Mesa, CA) sound card (sampling rate, 44 100 Hz; 24-bit resolution) to the right earphone of EARTONE-5 A (3 M, Minneapolis, MN) insert earphones, which were driven by a headphone buffer [Tucker-Davis-Technologies (TDT), HB7, Alachua, FL). Identical equipment was used at both testing sites. Pilot testing (not shown) was obtained from three subjects with NH each at U of U and MUSC and confirmed that AM detection thresholds did not differ significantly between testing sites (t[5] = −0.54, p = 0.69). Data from this pilot experiment were not included in the final data set.
C. Procedures
Subjects were seated at a computer workstation inside a sound-attenuating booth. A three-interval, three-alternative forced-choice adaptive tracking procedure was used to measure AM depth (m), in dB, at threshold. During adaptive tracking, the modulation depth was adjusted using a two-down, one-up rule, which converged on the modulation depth necessary to achieve 70.7% correct AM detection (Levitt, 1971). The initial step size of the adaptive track, expressed as modulation depth in dB, was 8 dB for the first two reversals and 2 dB for the remaining ten reversals. AM detection threshold for a given track was defined as the average AM depth (in dB) of the final eight reversals. During a trial, observation intervals were marked by squares on a computer monitor and separated by 500 ms of silence. The off-frequency noise and precursor (when present) were generated independently before each interval. Sinusoidal amplitude modulation was applied to the carrier in a randomly assigned observation interval. The level of the carrier was constant across observation intervals to eliminate level cues (Viemeister, 1979). Subjects indicated the interval in which they perceived the modulation by pressing a button on a keyboard or by tapping a touchscreen monitor. Visual feedback was provided to indicate a correct or incorrect response. A training period preceded data collection. During training, two consecutive thresholds were measured on a representative sample of conditions, which included conditions with and without the precursor for three carrier levels. Thresholds measured during training were discarded.
During data collection, the order of carrier levels was randomized for each listener. For each carrier level, no precursor and precursor conditions were measured consecutively. Two threshold estimates were obtained for each condition and averaged to yield the final threshold for the condition. If the difference in thresholds of the two estimates was >5 dB, a third estimate was obtained, and the average of all three threshold estimates was taken as the final threshold. A third estimate was measured a total of four times in three different subjects (<1% of measured experimental conditions). Subjects HI10 and HI11 were unable to complete the AM detection task at the lowest carrier level (60 dB SPL) for the precursor and no precursor conditions. Similarly, subject HI13 was unable to complete the task at the lowest carrier level for the precursor condition. Finally, subject HI15 was unable to complete the task at the 65 dB SPL carrier level in the precursor and no precursor conditions.
Statistical analyses2 were conducted in the form of repeated-measures analyses of variance (ANOVA), independent t-tests, and paired t-tests. Significance was determined at α = 0.05 after correction for multiple comparisons via Bonferroni's method.
III. RESULTS
A. Precursor and carrier level effects: NH subjects
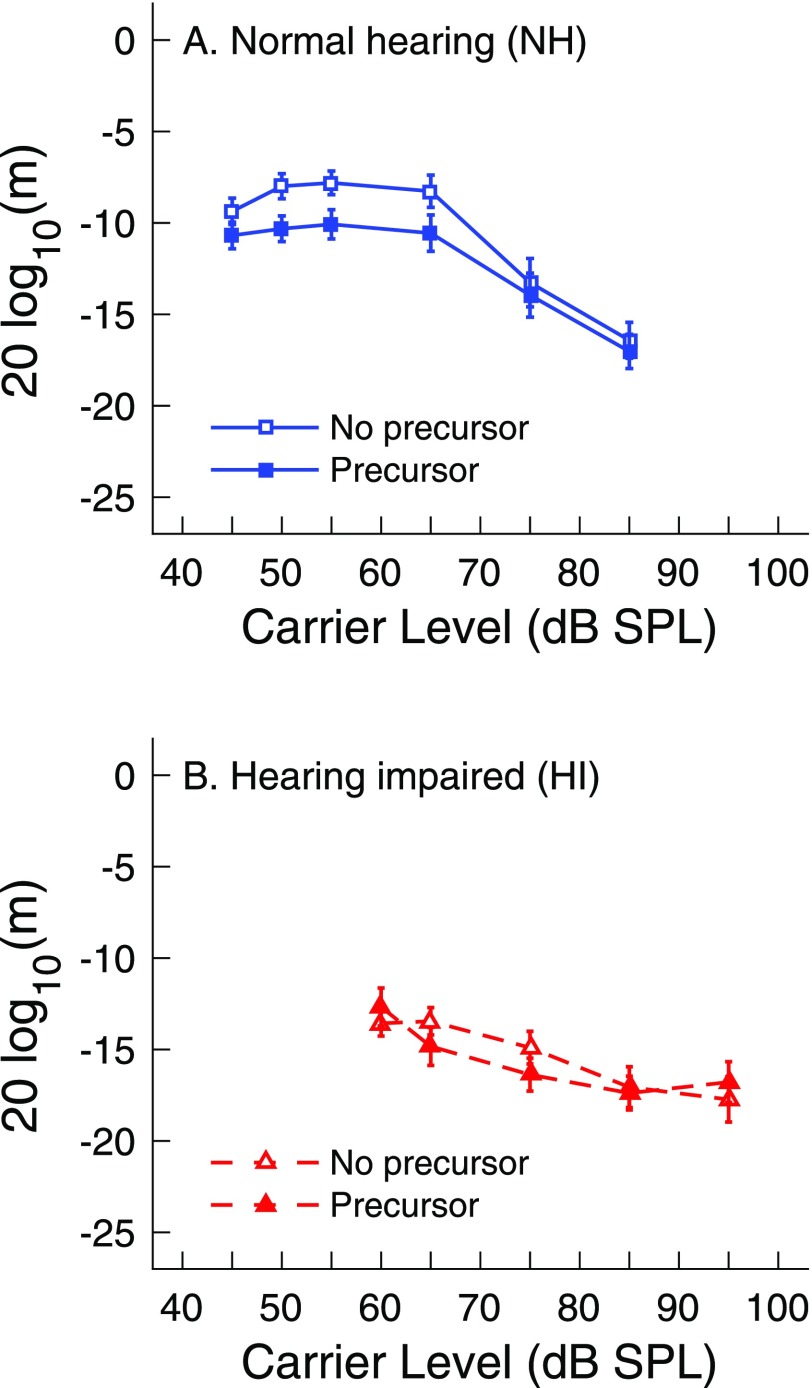
Average AM detection as a function of carrier level for subjects with NH is shown in Fig. 3(A) for conditions without (open squares) and with (closed squares) the precursor. Average AM detection for these subjects was poorer at moderate-level carriers compared to higher-level carriers, regardless of the absence or presence of the precursor. This was revealed by a two-way, repeated-measures ANOVA that showed significant main effects of precursor [F(1, 14) = 33.8, p < 0.001] and carrier level [F(5, 70) = 27.5, p < 0.001] and an insignificant precursor x carrier level interaction [F(5, 70) = 2.1, p = 0.1]. Post hoc testing revealed that, for the main effect of carrier level, AM detection was significantly better for the higher levels (65, 75, 85 dB SPL) compared to lower levels (45, 50, 55 dB SPL). This was true for the precursor [t(14) = 3.9, p < 0.005] and the no precursor [t(14) = 5.2, p < 0.001] conditions. For the main effect of precursor, post hoc testing revealed that AM detection was significantly better with, compared to without, a precursor [t(14) = 5.8, p < 0.001].
FIG. 3.
(Color online) Average amplitude modulation detection thresholds (m in dB) as a function of carrier level (in dB SPL). Carriers were preceded by silence (no precursor, open symbols) or by a notched-noise precursor (filled symbols) in subjects with NH (panel A) and HI (panel B). Error bars depict ±1 standard error of the mean.
B. Precursor and carrier level effects: HI subjects
Average AM detection as a function of carrier level for subjects with HI is shown in Fig. 3(B) for conditions without (open triangles) and with (closed triangles) the precursor. A two-way, repeated-measures ANOVA with carrier level (65, 75, 85, 95 dB SPL) and the presence of the precursor as within-subjects factors revealed an insignificant main effect of precursor [F(1, 13) = 3.0, p = 0.1],3 a significant main effect of carrier level [F(3, 39) = 4.1, p < 0.05], and a significant interaction between precursor x carrier level factors [F(3, 39) = 4.1, p < 0.05]. Results for the 60 dB SPL carrier were not included in the ANOVA, as AM detection was not measureable at this level in three subjects (HI10, HI11, HI13). Post hoc testing to determine the source of the significant effect of carrier level revealed statistically poorer AM detection at lower carrier levels (65 and 75 dB SPL) compared to higher carrier levels (85 and 95 dB SPL) in the no precursor condition [t(14) = 3.4, p < 0.005], but not in the precursor condition [t(14) = 1.6, p = 0.14]. Post hoc testing to determine the source of the precursor x carrier level interaction revealed that the average precursor effect (no precursor–precursor difference) for lower carrier levels (65 and 75 dB SPL) was significantly larger than that for higher carrier levels (85 and 95 dB SPL) [t(14) = 2.6, p < 0.05].
C. Effects of hearing loss
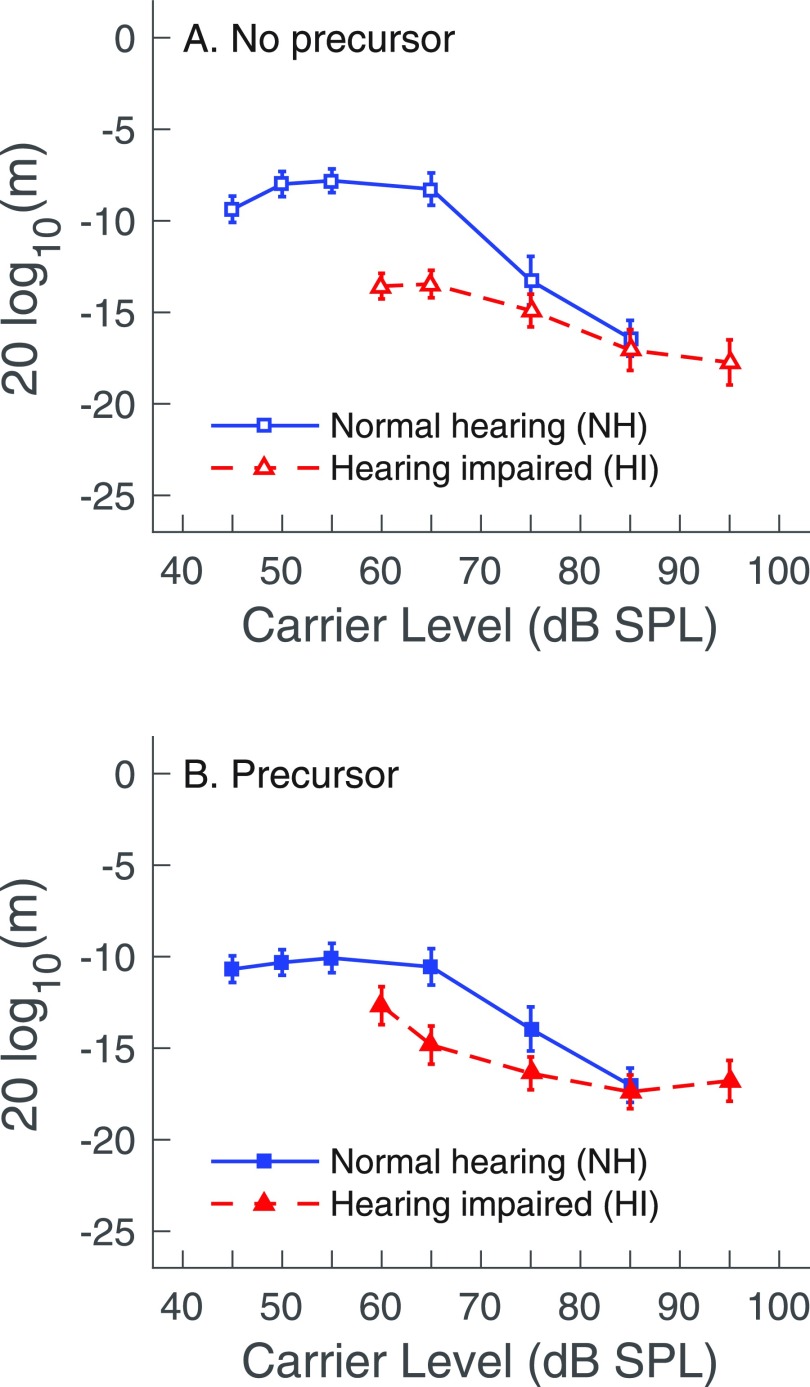
AM detection thresholds for three carrier levels (65, 75, and 85 dB SPL) were measured for all subjects with NH and HI. Average AM detection results from Fig. 3 are replotted in Fig. 4 to facilitate comparison of AM detection between subjects with NH and HI. A repeated-measures ANOVA with hearing status as the between-groups factor and carrier level and the presence of the precursor as within-subjects factors showed significant main effects of precursor [F(1, 27) = 17.4, p < 0.001] and of carrier level [F(2, 54) = 40.7, p < 0.001] and a significant carrier level x hearing status interaction [F(2, 54) = 7.3, p < 0.005]. The interactions for precursor x hearing status [F (1, 27) = 0.1, p = 0.8], precursor x carrier level [F(2, 54) = 3.2, p = 0.054], and precursor x carrier level x hearing status [F(2, 54) = 1.4, p = 0.3] did not reach significance. Hearing status as a between-subjects factor approached significance [F(1, 27) = 3.8, p = 0.06]. When evaluating the carrier level x hearing status interaction, post hoc testing revealed that AM detection thresholds were significantly poorer for subjects with NH than for subjects with HI, but only for the 65 dB SPL carrier. Specifically, group differences in AM detection (subjects with HI better than subjects with NH) for the 65 dB SPL carrier were 5.2 dB without the precursor [t(27) = 4.4, p < 0.001, Fig. 4(A)] and 4.3 dB with the precursor [t(27) = 2.9, p < 0.01, Fig. 4(B)].
FIG. 4.
(Color online) Average amplitude modulation detection thresholds (m in dB) replotted from Fig. 3. Thresholds for subjects with NH (squares) and HI (triangles) are compared for carriers preceded by silence (no precursor, A), or by a notched-noise precursor (B). Error bars depict ±1 standard error of the mean.
Due to the limited number of overlapping carrier levels among NH and HI groups, lower and higher carrier levels could not be included in the repeated-measures ANOVA with hearing status as the between-groups factor. When AM detection was grouped into lower and higher levels, average AM detection was significantly better with the precursor than without the precursor for carriers presented at the lower levels tested for each group [NH 45–55 dB SPL, t(14) = 5.3, p < 0.001; HI 65–75 dB SPL, t(14) = 2.5, p = 0.028] and for the higher levels tested for the NH group [NH 65–85 dB SPL, t(14) = 4.2, p < 0.005]. However, this was not the case for higher-level carriers for the HI group [HI 85–95 dB SPL, t(14) = −0.98, p = 0.34].
To compare precursor effects for subjects with NH and HI, the largest difference in AM thresholds with and without the precursor was computed for each subject, regardless of carrier level. Evaluating precursor effects based on the largest no precursor–precursor difference was preferred over other metrics because this metric is consistent with the expectation of individual differences in compression exponents and breakpoints (Poling et al., 2012; Jennings et al., 2014), and in MOC activity (Goodman et al., 2013). The use of this metric is based on the assumption that the greatest decrease in cochlear compression, as a result of the precursor, occurs at the carrier level with the largest no precursor–precursor difference. Consistent with this assumption, the largest no precursor–precursor difference was significantly greater for subjects with NH than for subjects with HI [t(28) = 3.0, p < 0.01].
D. Effects of precursor level and hearing loss
Smaller-than-normal precursor effects in subjects with HI may be due to differences in the SLs of the precursor, relative to subjects' detection thresholds for 2000 Hz pure tones (the mean was 35 dB SL for subjects with NH and 17 dB SL for subjects with HI). In other words, precursor effects may have been smaller than normal in subjects with HI due to lower audibility of the precursor. To test this hypothesis, four subjects with HI (HI1, HI4, HI5, HI6) returned and repeated the entire experiment with a 70 dB SPL precursor (equivalent to a mean SL of 32 dB SL). Results with the 70 dB SPL precursor (not shown) were similar to those for the 55 dB SPL precursor [solid triangles in Fig. 4(B)]. A two-way, repeated-measures ANOVA revealed that AM detection with the 70 dB SPL precursor was statistically equivalent to that of the 55 dB SPL precursor as indicated by an insignificant main effect of precursor level [F(1, 2) = 0.01, p = 0.9] and an insignificant interaction of carrier level x precursor level [F(4, 8) = 1.6, p = 0.3].
E. Individual differences
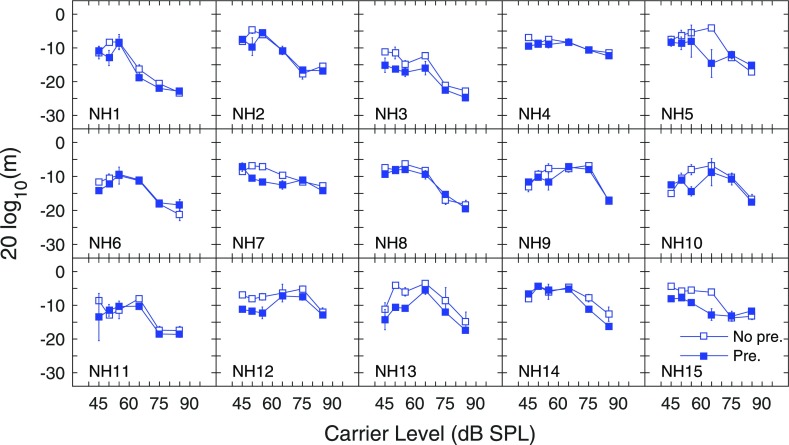
AM detection was marked by individual differences as illustrated in Figs. 5 and 6, which display thresholds for individual subjects with NH and HI, respectively. AM detection thresholds as a function of carrier level formed an inverted U-shaped pattern for most subjects with NH without a precursor (Fig. 5). The degree to which thresholds declined and then improved as a function of carrier level varied substantially among these subjects, as did the carrier level associated with poorest AM detection. When comparing AM detection with and without the precursor in subjects with NH, detection either improved with the precursor across a range of carrier levels (NH3, NH7, NH12, NH13, NH14, NH15), improved at one low-to-moderate carrier level (NH1, NH2, NH5, NH9, NH10, NH11), or remained constant (NH4, NH6, NH8).
FIG. 5.
(Color online) Amplitude modulation detection thresholds as a function of carrier level (in dB SPL) for carriers preceded by silence (No pre., open symbols), or by a notched-noise precursor (Pre., closed symbols) for individual subjects with NH. Error bars depict the standard error of the mean of the two (or three) threshold estimates obtained to compute the final threshold (see text).
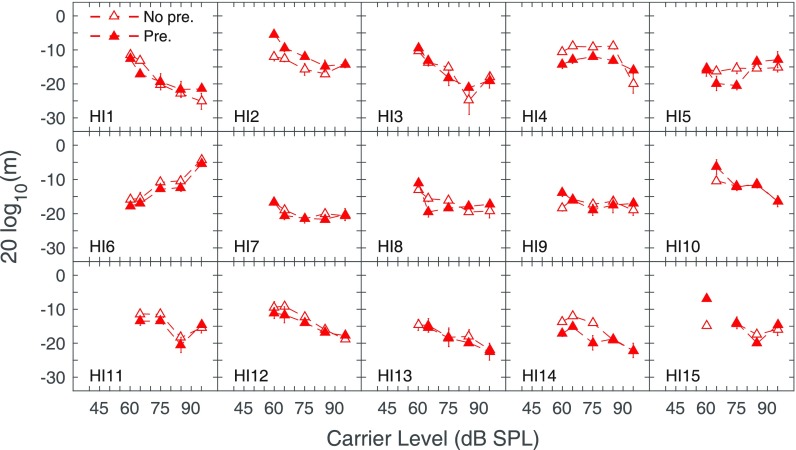
FIG. 6.
(Color online) Amplitude modulation detection thresholds as a function of carrier level (in dB SPL) for carriers preceded by silence (No pre., open symbols), or by a notched-noise precursor (Pre., closed symbols) for individual subjects with HI. Error bars depict the standard error of the mean of the two (or three) threshold estimates obtained to compute the final threshold (see text).
In general, AM detection without a precursor improved monotonically as a function of carrier level for most subjects with HI (Fig. 6), although thresholds in some subjects declined slightly from the lowest carrier level before improving (HI4, HI9, HI14), or declined slightly at the highest carrier levels (HI2, HI3, HI11). For other subjects with HI, AM detection in the no precursor condition had flat (HI5), rising (HI6), or complex (HI15) patterns as a function of carrier level. Compared to thresholds without a precursor, AM detection remained constant (HI7, HI13), or declined (HI2, HI10, HI15) with the presence of the precursor. For other subjects with HI, AM detection with the precursor improved at some lower levels and declined at higher levels (HI1, HI5, HI8). Finally, for the remainder of subjects with HI, AM detection improved appreciably (HI4, HI14), or minimally (HI6, HI11, HI12), with the presence of the precursor.
IV. DISCUSSION
A. Hypotheses
The results of this study show that, for moderate-level carriers, average AM detection is better than normal for subjects with HI (Fig. 4, 65 dB SPL carrier), and that improvements in average AM detection from the addition of a notched-noise precursor are smaller than normal for subjects with HI (Fig. 3).
1. Better-than-normal AM detection for subjects with HI
Better-than-normal AM detection for subjects with HI has been reported in previous studies. For subjects with unilateral and bilateral HI, Glasberg and Moore (1989) evaluated the relationship between speech perception and several psychoacoustic measures, including AM detection. For subjects with unilateral HI, average AM detection for 500, 1000, and 2000 Hz tonal carriers modulated at 4 Hz was better in the impaired ear compared to the normal ear for some subjects when carriers were presented at equal SPLs and for all subjects when carriers were presented at equal SLs. Glasberg and Moore (1989) interpreted this finding as evidence for loudness recruitment. In a later study, Moore et al. (1992) measured temporal modulation transfer functions (TMTFs) in subjects with unilateral and bilateral HI. They observed better-than-normal AM detection for modulation frequencies between 16 and 256 Hz in the impaired ears of subjects with unilateral HI. A similar result was found by Bacon and Gleitman (1992) who measured TMTFs for broadband noise carriers and reported better AM detection in subjects with flat HI compared to subjects with NH at some carrier SLs.
Better-than-normal AM detection at moderate-level carriers for subjects with HI in the current study extends interpretations from previous studies (e.g., Glasberg and Moore, 1989; Moore et al., 1992) to include the effects of cochlear compression. Specifically, findings from this study are consistent with the hypothesis that reduced compression of BM responses, attributed to cochlear hearing loss, results in larger effective AM depths in subjects with HI than in subjects with NH [Figs. 1(A) and 1(B)]. A similar conclusion was recently offered by Wallaert et al. (2017) who measured and modeled AM detection as a function of modulation cycles and modulation frequency in subjects with NH and HI. Linear response growth has also been invoked to explain why subjects with HI have better-than-normal detection thresholds for short sinusoidal probes presented at the onset of moderate-level, long-duration maskers in studies concerned with psychophysical overshoot (Schmidt and Zwicker, 1991; von Klitzing and Kohlrausch, 1994; Strickland and Krishnan, 2005).
2. Smaller-than-normal precursor effects for subjects with HI
The larger precursor effect in subjects with NH than subjects with HI is consistent with the hypothesis that a reduction in cochlear amplifier gain results in greater improvements in the effective AM depth in subjects with NH than in subjects with HI (Fig. 1). This is consistent with subjects with NH having greater cochlear compression than subjects with HI, resulting in their poorer AM detection thresholds without a precursor. Thus, the smaller-than-normal precursor effect for subjects with HI is primarily due to better-than-normal AM detection without a precursor, consistent with less compression in subjects with HI, compared to subjects with NH.
Differences in the effect of a precursor between subjects with NH and HI are unlikely due to group differences in carrier SL. For SLs common to both groups (37–62 dB SL), average AM detection was 6.2 and 8.0 dB poorer for subjects with NH than for subjects with HI for precursor and no precursor conditions, respectively. Similarly, smaller-than-normal precursor effects in subjects with HI are unlikely due to the differences in the audibility of the precursor. Although the wide range of hearing thresholds among subjects with HI results in between-subject differences in the audibility of the constant 55 dB SPL precursor, the follow-up study with a 70 dB SPL precursor revealed that a 15 dB increase in precursor level had an insignificant effect on AM detection.
3. Findings consistent with residual compression
Better AM detection at moderate carrier levels (60, 65, 75 dB SPL) with, than without, the precursor in some subjects with HI (e.g. HI04, HI14) is inconsistent with cochlear hearing loss resulting in linear BM response growth. These findings may be better explained by assuming these subjects with HI have some residual compression, which is associated with residual cochlear gain. Residual compression in subjects with HI is consistent with behavioral studies that inferred BM response functions for basal and mid-turn cochlear locations using masking techniques (Plack et al., 2004; Rosengard et al., 2005).
On average, AM detection with 65 dB SPL carriers in the precursor condition was better for subjects with HI than for subjects with NH [squares and triangles in Fig. 4(B)]. This is inconsistent with the prediction that BM responses grow linearly in the precursor condition at moderate-level carriers for subjects with NH and HI, which predicts similar AM detection, regardless of hearing status. Instead, these results for the precursor condition are consistent with (1) greater compression for subjects with NH than HI, and/or (2) the precursor producing greater forward masking of the AM signal in subjects with NH than in subjects with HI, as discussed in Sec. IV A 4.
4. Effects of precursor level
Cochlear amplifier gain decreases as the level of an acoustic MOC elicitor increases (Warren and Liberman, 1989). In the context of Fig. 1, this corresponds to increases in the input level of the compression breakpoint with increasing precursor level. Thus, based on prior physiological findings and the framework illustrated in Fig. 1, the range of levels with linear BM response growth is expected to increase with increasing precursor level for subjects with NH. One explanation of the smaller-than-normal precursor effect in subjects with HI is that precursor levels were not high enough to sufficiently reduce cochlear amplifier gain in subjects with HI. Data collected with the higher level precursor (70 dB SPL vs 55 dB SPL) in four subjects with HI are inconsistent with this explanation. For low carrier levels, the higher level, 70 dB SPL precursor resulted in higher (poorer) AM detection than in the no precursor condition, consistent with the 70 dB SPL precursor producing forward masking of the AM carrier. This suggests that the larger precursor effect in subjects with NH compared to subjects with HI was not due to differences in precursor audibility. Alternatively, less cochlear amplifier gain in subjects with HI than subjects with NH may limit improvements in AM detection with increasing precursor level in subjects with HI, as they have less gain available to adjust over the time course of the precursor. Finally, better AM detection in the presence of the 55 dB SPL precursor for subjects with HI than for subjects with NH may be due to higher precursor SLs in subjects with NH. Specifically, the higher SL precursor may have produced greater forward masking of low-level AM carriers in subjects with NH, compared to subjects with HI.
5. Effects of carrier level
Improvements in AM detection from moderate to high level carriers in subjects with NH and HI are consistent with the upward spread of excitation and detection of AM through the tails of the excitation pattern as suggested by Long and Cullen (1985), based on the intensity discrimination model of Florentine and Buus (1981). Because BM responses grow linearly at the tails of the excitation pattern for cochleae with normal and abnormal OHC function (Ruggero et al., 1997), AM detection thresholds are expected to be similar for subjects with NH and HI at high carrier levels. Improvements in AM detection with increasing carrier level also suggest that the notched noise used to restrict off-frequency listening was not effective at the highest carrier levels, as was also found by Almishaal et al. (2017). Alternatively, better AM detection at high levels, compared to moderate levels, is consistent with BM responses becoming less compressive with increasing level (Ruggero and Rich, 1991). However, a reduction in BM compression at high levels begins at signal levels >85 dB SPL (Ruggero et al., 1997), which is higher than the range of levels where AM detection began to improve in the current study (i.e., 55–75 dB SPL). Finally, phase locking improves with increasing level (Joris and Yin, 1992). Greater phase locking at high carrier levels may lead to improved neural encoding of the temporal envelope of the AM carrier, thus leading to better AM detection at higher compared to lower carrier levels.
6. Individual differences
Although the influence of carrier level on the precursor effect was marked by individual differences, the finding that the largest precursor effect was usually at low- or moderate-level carriers is consistent with the framework illustrated in Fig. 1. For example, precursor effects at isolated moderate levels (NH1, NH2, NH5, NH9, NH10, NH11, HI1, HI8) are consistent with linearization of BM responses for a narrow range of input levels, where levels below and above this range are similarly linear or similarly compressed, regardless of the presence of the precursor. In some of these cases (NH1, NH2, NH5, NH10), AM detection with a precursor is better at moderate than at lower levels. This may be due to AM detection at the lowest levels being elevated by the internal noise of subjects, creating a near-threshold effect (e.g., Plack and Skeels, 2007). Precursor effects for a range of several low-to-moderate carrier levels (NH3, NH7, NH12, NH13, NH15, HI4, HI14) are consistent with linearization of BM responses over a wide range of carrier levels. Precursor effects at the lowest carrier level (NH3, NH4, NH6, NH11, NH12, NH13, NH15, HI4, HI14) are consistent with a compression breakpoint below the lowest level tested. Thus, individual differences may be partly due to even slight differences in peripheral processing among subjects. Specifically, subjects may differ in terms of (1) compression exponents and breakpoints, even among subjects with NH who have similar audiometric thresholds (Poling et al., 2012; Jennings et al., 2014), and (2) the amount of cochlear gain reduction elicited by the constant-level precursor, which is consistent with the finding that MOC strength varies among animals of the same species (Maison and Liberman, 2000). Although individual differences can be partially explained by the framework illustrated in Fig. 1, sources of individual differences cannot be determined from the current data without objective measures of cochlear compression, MOC strength, and other potential mechanisms. Among these other mechanisms is the inherent variability of AM detection measurements; however, as indicated by the standard error values reported in Figs. 5 and 6 and in Almishaal et al. (2017), the contribution of measurement error to individual differences in this AM detection task is likely small.
B. Other considerations
1. Size of precursor effects
The effect of the precursor on AM detection in subjects with NH was smaller in this study compared to Almishaal et al. (2017). In their study, detection of 20-Hz AM applied to a 50-ms narrowband carrier centered on 5000 Hz was 3–7 dB better with than without the precursor for carrier levels > 65 dB SPL for all seven subjects with NH. This contrasts with the current study where precursor effects were typically largest for carrier levels <65 dB SPL and were marked by individual differences. One explanation relates to stimulus differences. For example, the carrier frequency (2000 Hz) and modulation frequency (40 Hz) in this study were lower and higher than Almishaal et al. (2017), respectively. Higher modulation frequencies are expected to result in a smaller precursor effect based on previous studies comparing TMTFs with and without a precursor (Almishaal et al., 2017) and for studies comparing continuous and gated carrier presentations (Sheft and Yost, 1990; Yost and Sheft, 1997). Yost and Sheft (1997) showed that the gated/continuous difference, which is analogous to the precursor effect, decreased with increasing modulation frequency up to 64 Hz for 125-ms tonal carriers, consistent with smaller precursor effects for 40-Hz than for 20-Hz modulation frequencies. Moreover, the gated/continuous difference is larger for 4000 Hz tonal carriers than for 500 and 1000 Hz carriers (Yost and Sheft, 1997), consistent with smaller precursor effects for 2000 Hz than for 5000 Hz carriers.
2. Potential applications for speech recognition
Better-than-normal AM detection and masking thresholds in overshoot conditions (e.g., Bacon and Takahashi, 1992) is in contrast to poorer-than-normal speech understanding in background noise in subjects with HI, as quantified by signal-to-noise ratio (SNR) loss (e.g., Killion et al., 2004). The effects of cochlear compression on SNR are illustrated by defining the effective SNR as the difference in cochlear output to a speech and noise mixture compared to noise alone. Cochlear compression is expected to decrease the effective SNR compared to the input SNR for auditory filters where the input SNR is positive (Noordhoek and Drullman, 1997; Edwards, 2004). In everyday listening environments, SNRs are generally greater than 0 dB (Pearsons et al., 1977; Smeds et al., 2015). A potential role of the MOC reflex is to adjust cochlear amplifier gain in a frequency specific manner to facilitate perception of target sounds and improve effective SNRs (Guinan, 2006; Chintanpalli et al., 2012). For subjects with HI, parts of the speech spectrum are expected to be processed linearly and result in favorable effective SNRs; however, other parts of the spectrum are near threshold or inaudible. Dysfunction of the cochlear amplifier in individuals with cochlear hearing loss necessarily limits adjustment of OHC gain. Thus, one consequence of cochlear hearing loss may be a decreased ability to adjust cochlear amplifier gain to improve effective SNRs.
The 2-dB precursor effect in AM detection for low-to-moderate level carriers corresponds to an intensity difference of 4 dB based on the formula from Long and Cullen (1985), which converts the modulation index to intensity difference limens. This 4-dB improvement is consistent with the improvements in intensity discrimination for short (30 ms), moderate-level tonal pedestals presented near the end of long (150 ms) compared to short (50 ms) ipsilateral broadband noise (Roverud and Strickland, 2015a). Small improvements in effective AM depth may contribute to better speech recognition in background noise in real-world listening. Improved effective AM depth from a reduction in cochlear amplifier gain is expected to increase the contrast between peaks and valleys of the speech envelope, the coding of which is important for speech understanding (Shannon et al., 1995). Similarly, reductions in cochlear amplifier gain occur at low-to-moderate levels; thus, cochlear responses are expected to decrease more for lower level background noise than for higher level speech, resulting in increased effective SNRs. Small increases in SNRs for listening to sentences in noise can result in large increases in percent correct recognition scores. For example, McArdle et al. (2005) reported a 15% improvement in recognition scores for each decibel improvement in SNR for sentences presented in multi-talker babble.
3. Effects of age
Subjects from NH and HI groups were not matched in age. Thus, age differences are potentially responsible for better AM detection thresholds and smaller-than-normal precursor effects in subjects with HI. However, this explanation is not supported by previous studies reporting poorer AM detection thresholds (He et al., 2008; Kumar and Sangamanatha, 2011; Wallaert et al., 2016) and temporal resolution (Fitzgibbons and Gordon-Salant, 2010) for older adults with NH compared to younger adults with NH. Better-than-normal AM detection for subjects with HI does not indicate that age has no effect on AM thresholds in older subjects with HI. Instead, these findings suggest that the effects of less compressive BM growth on AM detection outweigh the detrimental effects of age in older subjects with HI, resulting in better-than-normal AM detection. Younger subjects with HI are needed to fully examine the relative contribution of age and hearing loss to AM detection.
4. Challenges and future research
AM detection was marked by large individual differences in the current study. Thus, the group data were sometimes a poor representation of AM detection for an individual NH or HI subject. Sources of individual differences in psychoacoustic tasks originate from the peripheral (Bharadwaj et al., 2015) and central (Moore, 2015) auditory nervous systems and the interaction of these systems (Jennings et al., 2014). Evidence is lacking on the peripheral and central mechanisms responsible for individual differences and the unique contributions of these mechanisms. This poses a challenge when attempting to control for individual differences in behavioral measures of auditory processing. In the current study, individual differences were large for subjects with NH and HI, despite the expectation that older subjects with HI have less cochlear compression (Oxenham and Bacon, 2003). Thus, the less compressive BM responses expected from subjects with HI do not appear to greatly decrease individual differences for detection of AM with a short-duration carrier. Future research is needed to identify mechanisms of individual differences and understand how hearing impairment influences these mechanisms. The success of identifying mechanisms of individual differences will likely depend on the development and refinement of accurate and reliable behavioral and physiological measures of peripheral and central auditory processing.
Although results from this study are consistent with better AM detection under conditions where cochlear responses are expected to be linear, the extent to which these effects apply to speech recognition in noise is unknown. As a first approximation, such effects are expected to increase the contrast between peak and troughs of the speech envelope and improve SNR. Future research is needed to compare speech recognition in noise for conditions where cochlear responses are expected to be linear and compressed. Such research may reveal the extent to which more compressive rather than less compressive cochlear responses lead to poorer speech recognition in noise. Indeed, speech recognition as a function of off-frequency masker level declines more than predicted from the articulation index for subjects with lower compression breakpoints than for subjects with higher breakpoints (Dubno et al., 2007). This finding is consistent with relatively poorer effective SNRs for compressive than for linear cochlear responses (see also Horwitz et al., 2007). Finally, further research is needed to test the extent to which individuals with cochlear hearing loss have a reduced ability to adapt cochlear amplifier gain to changes in the acoustic environment.
V. SUMMARY AND CONCLUSIONS
Results of this study show that AM detection with and without a precursor in subjects with NH and HI is consistent with expected BM growth based on the gain of the cochlear amplifier (Fig. 1). Specifically, less cochlear amplification in subjects with HI results in less compressive BM response growth, favorable effective AM depths, and better-than-normal AM detection at moderate-level carriers. Similarly, reduced cochlear amplifier gain over the time course of the precursor results in linearization of BM responses and better AM detection for low-to-moderate level carriers in most subjects with NH and some subjects with HI.
Differences in age between subjects with NH and HI do not explain better-than-normal AM detection in older subjects with HI because poorer-than-normal AM detection and temporal resolution are expected in older adults.
AM detection was marked by individual differences. These differences may be related to individualized compression exponents and breakpoints, MOC strength, or to more central factors. The contribution of these mechanisms to individual differences is unknown without independent measures of peripheral and central auditory function.
Improvements in AM detection with compared to without a precursor are consistent with the auditory system adjusting to the local soundscape such that effective AM depth improves quickly after acoustic stimulation. This adjustment may facilitate speech recognition in noisy backgrounds by increasing the contrast between peaks and troughs of the speech envelope and improving SNRs.
ACKNOWLEDGMENTS
This work was supported in part by Grant No. K23 DC014752 from NIH/NIDCD (PI: Jennings) and Grant Nos. R01 DC000184 and P50 DC000422 (PI: Dubno) from NIH/NIDCD; the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the MUSC and NIH/NCRR Grant No. UL1 RR029882. Part of this investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant No. C06 RR14516 from the National Center for Research Resources, National Institutes of Health. The authors thank Sarah Hargus Ferguson for facilitating access to hearing-impaired subjects through the University of Utah's Senior Ears participant pool. Michael Simpson (U of U) assisted with data collection. Olivia Piper (U of U) assisted with proofreading this manuscript.
Portions of this research were presented at the Fortieth Annual Mid-Winter Meeting of the Association for Research in Otolaryngology (2017).
Footnotes
Initially, pilot data were collected on younger and older subjects with NH. These data revealed that some older subjects with NH could not perform the task when the modulation frequency was 20 Hz; however, performance was stable when the modulation frequency was 40 Hz. The 40 Hz modulation frequency was used for the remaining pilot data and experimental data to avoid the possibility that older subjects with HI may have similar difficulty performing the task.
The following were coded as missing data in the statistical analyses: (1) AM detection for HI15 for the 65 dB SPL carrier in the precursor and no precursor conditions, and (2) AM detection for HI5 in the follow-up study involving a 70 dB SPL precursor (see text).
Due to missing data for HI15 with 65 dB SPL carriers, all ANOVA tests that included HI subjects were evaluated on 14 rather than 15 subjects with HI.
References
- 1. Almishaal, A. , Bidelman, G. M. , and Jennings, S. G. (2017). “ Notched-noise precursors improve detection of low-frequency amplitude modulation,” J. Acoust. Soc. Am. 141, 324–333. 10.1121/1.4973912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Backus, B. C. , and Guinan, J. J. (2006). “ Time-course of the human medial olivocochlear reflex,” J. Acoust. Soc. Am. 119, 2889–2904. 10.1121/1.2169918 [DOI] [PubMed] [Google Scholar]
- 3. Bacon, S. P. , and Gleitman, R. M. (1992). “ Modulation detection in subjects with relatively flat hearing losses,” J. Speech. Hear. Res. 35, 642–653. 10.1044/jshr.3503.642 [DOI] [PubMed] [Google Scholar]
- 4. Bacon, S. P. , and Takahashi, G. A. (1992). “ Overshoot in normal-hearing and hearing-impaired subjects,” J. Acoust. Soc. Am. 91, 2865–2871. 10.1121/1.402967 [DOI] [PubMed] [Google Scholar]
- 5. Bharadwaj, H. M. , Masud, S. , Mehraei, G. , Verhulst, S. , and Shinn-Cunningham, B. G. (2015). “ Individual differences reveal correlates of hidden hearing deficits,” J. Neurosci. 35, 2161–2172. 10.1523/JNEUROSCI.3915-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bidelman, G. M. , Jennings, S. G. , and Strickland, E. A. (2015). “ PsyAcoustX: A flexible matlab® package for psychoacoustics research,” Front. Psychol. 6, 1498. 10.3389/fpsyg.2015.01498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chintanpalli, A. , Jennings, S. G. , Heinz, M. G. , and Strickland, E. A. (2012). “ Modeling the anti-masking effects of the olivocochlear reflex in auditory nerve responses to tones in sustained noise,” J. Assoc. Res. Otolaryngol. 13, 219–235. 10.1007/s10162-011-0310-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper, N. P. , and Guinan, J. J. (2003). “ Separate mechanical processes underlie fast and slow effects of medial olivocochlear efferent activity,” J. Physiol. 548, 307–312. 10.1113/jphysiol.2003.039081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis, H. (1983). “ An active process in cochlear mechanics,” Hear. Res. 9, 79–90. 10.1016/0378-5955(83)90136-3 [DOI] [PubMed] [Google Scholar]
- 10. Dolan, D. F. , Guo, M. H. , and Nuttall, A. L. (1997). “ Frequency-dependent enhancement of basilar membrane velocity during olivocochlear bundle stimulation,” J. Acoust. Soc. Am. 102, 3587–3596. 10.1121/1.421008 [DOI] [PubMed] [Google Scholar]
- 11. Dubno, J. R. , Horwitz, A. R. , and Ahlstrom, J. B. (2007). “ Estimates of basilar-membrane nonlinearity effects on masking of tones and speech,” Ear. Hear. 28, 2–17. 10.1097/AUD.0b013e3180310212 [DOI] [PubMed] [Google Scholar]
- 12. Edwards, B. (2004). “ Hearing aids and hearing impairment,” in Speech Processing in the Auditory System ( Springer, New York: ), pp. 339–421. [Google Scholar]
- 13. Fitzgibbons, P. J. , and Gordon-Salant, S. (2010). “ Behavioral studies with aging humans: Hearing sensitivity and psychoacoustics,” in The Aging Auditory System, edited by Gordon-Salant S., Frisina R. D., Popper A. N., and Fay R. R. ( Springer, New York: ), pp. 111–134. [Google Scholar]
- 14. Florentine, M. , and Buus, S. (1981). “ An excitation-pattern model for intensity discrimination,” J. Acoust. Soc. Am. 70, 1646–1654. 10.1121/1.387219 [DOI] [Google Scholar]
- 15. Giraud, A. L. , Garnier, S. , Micheyl, C. , Lina, G. , Chays, A. , and Chery-Croze, S. (1997). “ Auditory efferents involved in speech-in-noise intelligibility,” NeuroRep. 8, 1779–1783. 10.1097/00001756-199705060-00042 [DOI] [PubMed] [Google Scholar]
- 16. Glasberg, B. , and Moore, B. C. (1989). “ Psychoacoustic abilities of subjects with unilateral and bilateral cochlear hearing impairments and their relationship to the ability to understand speech,” Scand. Audiol. Suppl. 32, 1–25. [PubMed] [Google Scholar]
- 17. Goodman, S. S. , Mertes, I. B. , Lewis, J. D. , and Weissbeck, D. K. (2013). “ Medial olivocochlear-induced transient-evoked otoacoustic emission amplitude shifts in individual subjects,” J. Assoc. Res. Otolaryngol. 14, 829–842. 10.1007/s10162-013-0409-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guinan, J. J. (2006). “ Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans,” Ear. Hear. 27, 589–607. 10.1097/01.aud.0000240507.83072.e7 [DOI] [PubMed] [Google Scholar]
- 19. He, N. J. , Mills, J. H. , Ahlstrom, J. B. , and Dubno, J. R. (2008). “ Age-related differences in the temporal modulation transfer function with pure-tone carriers,” J. Acoust. Soc. Am. 124, 3841–3849. 10.1121/1.2998779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heinz, M. G. , Colburn, H. S. , and Carney, L. H. (2001). “ Rate and timing cues associated with the cochlear amplifier: Level discrimination based on monaural cross-frequency coincidence detection,” J. Acoust. Soc. Am. 110, 2065–2084. 10.1121/1.1404977 [DOI] [PubMed] [Google Scholar]
- 21. Horwitz, A. R. , Ahlstrom, J. B. , and Dubno, J. R. (2007). “ Speech recognition in noise: Estimating effects of compressive nonlinearities in the basilar-membrane response,” Ear. Hear. 28, 682–693. 10.1097/AUD.0b013e31812f7156 [DOI] [PubMed] [Google Scholar]
- 22. Jennings, S. G. , Ahlstrom, J. B. , and Dubno, J. R. (2014). “ Computational modeling of individual differences in behavioral estimates of cochlear nonlinearities,” J. Assoc. Res. Otolaryngol. 15, 945–960. 10.1007/s10162-014-0486-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jennings, S. G. , Ahlstrom, J. B. , and Dubno, J. R. (2016). “ Effects of age and hearing loss on overshoot,” J. Acoust. Soc. Am. 140, 2481–2493. 10.1121/1.4964267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jennings, S. G. , Strickland, E. A. , and Heinz, M. G. (2009). “ Precursor effects on behavioral estimates of frequency selectivity and gain in forward masking,” J. Acoust. Soc. Am. 125, 2172–2181. 10.1121/1.3081383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Joris, P. X. , and Yin, T. C. (1992). “ Responses to amplitude-modulated tones in the auditory nerve of the cat,” J. Acoust. Soc. Am. 91, 215–232. 10.1121/1.402757 [DOI] [PubMed] [Google Scholar]
- 26. Killion, M. C. , Niquette, P. A. , Gudmundsen, G. I. , Revit, L. J. , and Banerjee, S. (2004). “ Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners,” J. Acoust. Soc. Am. 116, 2395–2405. 10.1121/1.1784440 [DOI] [PubMed] [Google Scholar]
- 27. Kohlrausch, A. , Fassel, R. , and Dau, T. (2000). “ The influence of carrier level and frequency on modulation and beat-detection thresholds for sinusoidal carriers,” J. Acoust. Soc. Am. 108, 723–734. 10.1121/1.429605 [DOI] [PubMed] [Google Scholar]
- 28. Krull, V. , and Strickland, E. A. (2008). “ The effect of a precursor on growth of forward masking,” J. Acoust. Soc. Am. 123, 4352–4357. 10.1121/1.2912440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar, A. U. , and Sangamanatha, A. V. (2011). “ Temporal processing abilities across different age groups,” J. Am. Acad. Audiol. 22, 5–12. 10.3766/jaaa.22.1.2 [DOI] [PubMed] [Google Scholar]
- 30. Kumar, U. A. , and Vanaja, C. S. (2004). “ Functioning of olivocochlear bundle and speech perception in noise,” Ear. Hear. 25, 142–146. 10.1097/01.AUD.0000120363.56591.E6 [DOI] [PubMed] [Google Scholar]
- 31. Levitt, H. (1971). “ Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- 32. Liberman, M. C. , Gao, J. , He, D. Z. , Wu, X. , Jia, S. , and Zuo, J. (2002). “ Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier,” Nature 419, 300–304. 10.1038/nature01059 [DOI] [PubMed] [Google Scholar]
- 33. Long, G. R. , and Cullen, J. K., Jr. (1985). “ Intensity difference limens at high frequencies,” J. Acoust. Soc. Am. 78, 507–513. 10.1121/1.392472 [DOI] [PubMed] [Google Scholar]
- 34. Maison, S. F. , and Liberman, M. C. (2000). “ Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength,” J. Neurosci. 20, 4701–4707. 10.1523/JNEUROSCI.20-12-04701.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McArdle, R. A. , Wilson, R. H. , and Burks, C. A. (2005). “ Speech recognition in multitalker babble using digits, words, and sentences,” J. Am. Acad. Audiol. 16, 726–739; quiz 763–724. 10.3766/jaaa.16.9.9 [DOI] [PubMed] [Google Scholar]
- 36. Mertes, I. B. , Wilbanks, E. C. , and Leek, M. R. (2017). “ Olivocochlear efferent activity is associated with the slope of the psychometric function of speech recognition in noise,” Ear. Hear. (published online). 10.1097/AUD.0000000000000514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Millman, R. E. , and Bacon, S. P. (2008). “ The influence of spread of excitation on the detection of amplitude modulation imposed on sinusoidal carriers at high levels,” J. Acoust. Soc. Am. 123, 1008–1016. 10.1121/1.2816575 [DOI] [PubMed] [Google Scholar]
- 38. Moore, B. C. , Shailer, M. J. , and Schooneveldt, G. P. (1992). “ Temporal modulation transfer functions for band-limited noise in subjects with cochlear hearing loss,” Br. J. Audiol. 26, 229–237. 10.3109/03005369209076641 [DOI] [PubMed] [Google Scholar]
- 39. Moore, D. R. (2015). “ Sources of pathology underlying listening disorders in children,” Int. J. Psychophysiol. 95, 125–134. 10.1016/j.ijpsycho.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 40. Murugasu, E. , and Russell, I. J. (1996). “ The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea,” J. Neurosci. 16, 325–332. 10.1523/JNEUROSCI.16-01-00325.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nelson, D. A. , Schroder, A. C. , and Wojtczak, M. (2001). “ A new procedure for measuring peripheral compression in normal-hearing and hearing-impaired listeners,” J. Acoust. Soc. Am. 110, 2045–2064. 10.1121/1.1404439 [DOI] [PubMed] [Google Scholar]
- 42. Noordhoek, I. M. , and Drullman, R. (1997). “ Effect of reducing temporal intensity modulations on sentence intelligibility,” J. Acoust. Soc. Am. 101, 498–502. 10.1121/1.417993 [DOI] [PubMed] [Google Scholar]
- 43. Oxenham, A. J. , and Bacon, S. P. (2003). “ Cochlear compression: Perceptual measures and implications for normal and impaired hearing,” Ear. Hear. 24, 352–366. 10.1097/01.AUD.0000090470.73934.78 [DOI] [PubMed] [Google Scholar]
- 44. Oxenham, A. J. , and Plack, C. J. (1997). “ A behavioral measure of basilar-membrane nonlinearity in listeners with normal and impaired hearing,” J. Acoust. Soc. Am. 101, 3666–3675. 10.1121/1.418327 [DOI] [PubMed] [Google Scholar]
- 45. Pearsons, K. S. , Bennett, R. L. , and Fidell, S. (1977). Speech Levels in Various Noise Environments ( Office of Health and Ecological Effects, Office of Research and Development, US EPA, Washington, DC: ). [Google Scholar]
- 46. Plack, C. J. , Drga, V. , and Lopez-Poveda, E. A. (2004). “ Inferred basilar-membrane response functions for listeners with mild to moderate sensorineural hearing loss,” J. Acoust. Soc. Am. 115, 1684–1695. 10.1121/1.1675812 [DOI] [PubMed] [Google Scholar]
- 47. Plack, C. J. , and O'Hanlon, C. G. (2003). “ Forward masking additivity and auditory compression at low and high frequencies,” J. Assoc. Res. Otolaryngol. 4, 405–415. 10.1007/s10162-002-3056-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Plack, C. J. , and Skeels, V. (2007). “ Temporal integration and compression near absolute threshold in normal and impaired ears,” J. Acoust. Soc. Am. 122, 2236–2244. 10.1121/1.2769829 [DOI] [PubMed] [Google Scholar]
- 49. Poling, G. L. , Horwitz, A. R. , Ahlstrom, J. B. , and Dubno, J. R. (2012). “ Individual differences in behavioral estimates of cochlear nonlinearities,” J. Assoc. Res. Otolaryngol. 13, 91–108. 10.1007/s10162-011-0291-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rhode, W. S. , and Recio, A. (2000). “ Study of mechanical motions in the basal region of the chinchilla cochlea,” J. Acoust. Soc. Am. 107, 3317–3332. 10.1121/1.429404 [DOI] [PubMed] [Google Scholar]
- 51. Robles, L. , and Ruggero, M. A. (2001). “ Mechanics of the mammalian cochlea,” Physiol. Rev. 81, 1305–1352. 10.1152/physrev.2001.81.3.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rosen, S. (1992). “ Temporal information in speech: Acoustic, auditory and linguistic aspects,” Philos. Trans. R. Soc. London B. Biol. Sci. 336, 367–373. 10.1098/rstb.1992.0070 [DOI] [PubMed] [Google Scholar]
- 53. Rosengard, P. S. , Oxenham, A. J. , and Braida, L. D. (2005). “ Comparing different estimates of cochlear compression in listeners with normal and impaired hearing,” J. Acoust. Soc. Am. 117, 3028–3041. 10.1121/1.1883367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roverud, E. , and Strickland, E. A. (2010). “ The time course of cochlear gain reduction measured using a more efficient psychophysical technique,” J. Acoust. Soc. Am. 128, 1203–1214. 10.1121/1.3473695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roverud, E. , and Strickland, E. A. (2015a). “ The effects of ipsilateral, contralateral, and bilateral broadband noise on the mid-level hump in intensity discrimination,” J. Acoust. Soc. Am. 138, 3245–3261. 10.1121/1.4935515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roverud, E. , and Strickland, E. A. (2015b). “ Exploring the source of the mid-level hump for intensity discrimination in quiet and the effects of noise,” J. Acoust. Soc. Am. 137, 1318–1335. 10.1121/1.4908243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ruggero, M. A. , and Rich, N. C. (1991). “ Furosemide alters organ of corti mechanics: Evidence for feedback of outer hair cells upon the basilar membrane,” J. Neurosci. 11, 1057–1067. 10.1523/JNEUROSCI.11-04-01057.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ruggero, M. A. , Rich, N. C. , Recio, A. , Narayan, S. S. , and Robles, L. (1997). “ Basilar-membrane responses to tones at the base of the chinchilla cochlea,” J. Acoust. Soc. Am. 101, 2151–2163. 10.1121/1.418265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Russell, I. J. , and Murugasu, E. (1997). “ Medial efferent inhibition suppresses basilar membrane responses to near characteristic frequency tones of moderate to high intensities,” J. Acoust. Soc. Am. 102, 1734–1738. 10.1121/1.420083 [DOI] [PubMed] [Google Scholar]
- 60. Schmidt, S. , and Zwicker, E. (1991). “ The effect of masker spectral asymmetry on overshoot in simultaneous masking,” J. Acoust. Soc. Am. 89, 1324–1330. 10.1121/1.400656 [DOI] [PubMed] [Google Scholar]
- 61. Shannon, R. V. , Zeng, F. G. , Kamath, V. , Wygonski, J. , and Ekelid, M. (1995). “ Speech recognition with primarily temporal cues,” Science 270, 303–304. 10.1126/science.270.5234.303 [DOI] [PubMed] [Google Scholar]
- 62. Sheft, S. , and Yost, W. A. (1990). “ Temporal integration in amplitude modulation detection,” J. Acoust. Soc. Am. 88, 796–805. 10.1121/1.399729 [DOI] [PubMed] [Google Scholar]
- 63. Smeds, K. , Wolters, F. , and Rung, M. (2015). “ Estimation of signal-to-noise ratios in realistic sound scenarios,” J. Am. Acad. Audiol. 26, 183–196. 10.3766/jaaa.26.2.7 [DOI] [PubMed] [Google Scholar]
- 64. Strickland, E. A. (2008). “ The relationship between precursor level and the temporal effect,” J. Acoust. Soc. Am. 123, 946–954. 10.1121/1.2821977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Strickland, E. A. , and Krishnan, L. A. (2005). “ The temporal effect in listeners with mild to moderate cochlear hearing impairment,” J. Acoust. Soc. Am. 118, 3211–3217. 10.1121/1.2074787 [DOI] [PubMed] [Google Scholar]
- 66. Strickland, E. A. , and Viemeister, N. F. (1997). “ The effects of frequency region and bandwidth on the temporal modulation transfer function,” J. Acoust. Soc. Am. 102, 1799–1810. 10.1121/1.419617 [DOI] [PubMed] [Google Scholar]
- 67. Viemeister, N. F. (1979). “ Temporal modulation transfer functions based upon modulation thresholds,” J. Acoust. Soc. Am. 66, 1364–1380. 10.1121/1.383531 [DOI] [PubMed] [Google Scholar]
- 68. von Klitzing, R. , and Kohlrausch, A. (1994). “ Effect of masker level on overshoot in running- and frozen-noise maskers,” J. Acoust. Soc. Am. 95, 2192–2201. 10.1121/1.408679 [DOI] [PubMed] [Google Scholar]
- 69. Wallaert, N. , Moore, B. C. , Ewert, S. D. , and Lorenzi, C. (2017). “ Sensorineural hearing loss enhances auditory sensitivity and temporal integration for amplitude modulation,” J. Acoust. Soc. Am. 141, 971–980. 10.1121/1.4976080 [DOI] [PubMed] [Google Scholar]
- 70. Wallaert, N. , Moore, B. C. , and Lorenzi, C. (2016). “ Comparing the effects of age on amplitude modulation and frequency modulation detection,” J. Acoust. Soc. Am. 139, 3088–3096. 10.1121/1.4953019 [DOI] [PubMed] [Google Scholar]
- 71. Warren, E. H., III , and Liberman, M. C. (1989). “ Effects of contralateral sound on auditory-nerve responses. II. Dependence on stimulus variables,” Hear. Res. 37, 105–121. 10.1016/0378-5955(89)90033-6 [DOI] [PubMed] [Google Scholar]
- 72. Wojtczak, M. , and Viemeister, N. F. (2005). “ Forward masking of amplitude modulation: Basic characteristics,” J. Acoust. Soc. Am. 118, 3198–3210. 10.1121/1.2042970 [DOI] [PubMed] [Google Scholar]
- 73. Yasin, I. , Drga, V. , and Plack, C. J. (2013). “ Estimating peripheral gain and compression using fixed-duration masking curves,” J. Acoust. Soc. Am. 133, 4145–4155. 10.1121/1.4802827 [DOI] [PubMed] [Google Scholar]
- 74. Yates, G. K. , Winter, I. M. , and Robertson, D. (1990). “ Basilar-membrane nonlinearity determines auditory-nerve rate-intensity functions and cochlear dynamic-range,” Hear. Res. 45, 203–219. 10.1016/0378-5955(90)90121-5 [DOI] [PubMed] [Google Scholar]
- 75. Yost, W. A. (1991). “ Auditory image perception and analysis: The basis for hearing,” Hear. Res. 56, 8–18. 10.1016/0378-5955(91)90148-3 [DOI] [PubMed] [Google Scholar]
- 76. Yost, W. , and Sheft, S. (1997). “ Temporal modulation transfer functions for tonal stimuli: Gated versus continuous conditions,” Aud. Neurosci. 3, 401–414. [Google Scholar]