Abstract
Aging is associated with decreased self-initiated use of effective elaborative encoding strategies. Little is currently known regarding what factors drive age differences in self-initiated encoding strategies. The present research investigated whether age differences in prefrontal gray matter integrity contribute to age differences in self-initiated elaborative encoding strategies. The relationships between age, prefrontal regional gray matter volumes, and overall use of self-initiated elaborative encoding strategies were examined in healthy younger and older adults. Gray matter volume was calculated from structural MRI scans using Freesurfer. Encoding strategy use was assessed by retrospective item-by-item strategy self-reports given after a verbal intentional encoding task. Left caudal middle frontal gray matter volume mediated the effect of age on overall self-initiated use of elaborative encoding strategies. This suggests that age-associated declines in prefrontal gray matter integrity significantly contribute to age-associated declines in effective encoding strategies.
Keywords: Aging, Encoding, Gray Matter Volume, Memory, Strategy Use
1. Introduction
Older adults’ episodic memory is impaired relative to younger adults’ (for a review see Balota et al., 2000), especially when no explicit instruction is given on how to encode studied information (i.e., unsupported intentional encoding; Hultsch et al., 1990; Sanders et al., 1980). Prior research suggests that age differences in self-initiated use of memory strategies during encoding contribute to age differences in episodic memory (Hertzog et al., 1998; Kirchhoff et al., 2012; Verhaeghen & Marcoen, 1994). When asked, older adults report using elaborative encoding strategies less frequently than younger adults during unsupported intentional encoding (Hertzog et al., 1998; Naveh-Benjamin et al., 2007; Verhaeghen & Marcoen, 1994). Elaborative encoding strategies rely on complex, highly effortful cognitive processes, such as thinking about the meaning of studied items, purposefully forming detailed visual images, and/or relating studied items to each other or one’s personal experiences (Craik & Lockhart, 1972; Kirchhoff et al., 2013; Richardson, 1998). Some common examples of elaborative encoding strategies include visual imagery, sentence generation, story generation, and personal relevance (Camp et al., 1983; Kirchhoff et al., 2012; Naveh-Benjamin et al., 2007). Non-elaborative strategies are simpler and require less controlled processing to initiate and maintain (Kirchhoff et al., 2013). Common examples include rote repetition (i.e., verbal rehearsal) and concentration (Kirchhoff et al., 2012; Naveh-Benjamin et al., 2007; Verhaeghen & Marcoen, 1994). Use of elaborative encoding strategies results in a greater ability to learn presented material than use of non-elaborative encoding strategies (Camp et al., 1983; Geiselman et al., 1982; Martin et al., 1965; Shaughnessy, 1981). Older adults are also more likely than younger adults to report not using any strategy to learn new information (Devolder & Pressley, 1992; Kirchhoff et al., 2012; Rowe & Schnore, 1971).
Importantly, age differences in encoding strategy use are not due to older adults’ inability to use elaborative encoding strategies. Instructing or training older adults to use elaborative encoding strategies can increase their use of those strategies and improve their memory performance (Kirchhoff et al., 2012; Miotto et al., 2014; Naveh-Benjamin et al., 2007). Thus, age differences in spontaneous encoding strategy use are most likely due to age differences in the ability to self-initiate complex effortful strategies rather than impairment in older adults’ ability to effectively use them.
Little is currently known about what factors contribute to age differences in self-initiated use of elaborative encoding strategies. One important factor may be age differences in prefrontal function. Prior research has shown that the prefrontal cortex plays an important role in supporting self-initiated use of elaborative encoding strategies. Individuals with prefrontal lesions report using elaborative encoding strategies less frequently than healthy controls during unsupported intentional encoding. They also report not using any encoding strategy at all more frequently than healthy controls (Gershberg & Shimamura, 1995). However, like healthy older adults, individuals with frontal lobe lesions can effectively use elaborative strategies during encoding when instructed to do so (Hirst & Volpe, 1988). Individual differences in brain activity in prefrontal cortex during unsupported intentional encoding have been shown to be positively correlated with individual differences in self-initiated encoding strategy use in younger adults (Kirchhoff & Buckner, 2006). Training older adults to use elaborative semantic encoding strategies leads to changes in prefrontal brain activity during unsupported intentional encoding which are positively correlated with behavioral improvements in self-initiated semantic encoding strategy use and memory performance (Kirchhoff et al., 2012). Structural neuroimaging research has shown that prefrontal cortex is one of the brain regions most affected by age (for reviews see Lockhart & DeCarli, 2014; Raz & Rodrigue, 2006), and negative associations between age and prefrontal gray matter volume have consistently been reported (Jernigan et al., 2001; Raz et al., 1997). Taken together, this prior research suggests that age-associated declines in prefrontal integrity may significantly contribute to age-associated declines in effective encoding strategies.
To begin to test the contribution of declines in prefrontal integrity to declines in effective encoding strategy use in older adults, our research group recently examined the relationships between age, prefrontal regional gray matter volumes, and semantic clustering during free recall on the California Verbal Learning Test (CVLT-II; Delis et al., 2000) in a large adult lifespan dataset (Kirchhoff et al., 2014). Semantic clustering scores (Bousfield, 1953) from the CVLT are the most common measure of self-initiated elaborative memory strategy use in the clinical psychology and cognitive neuroscience literatures. The scores measure the degree to which individuals consecutively report semantically related words during free recall after studying an unstructured word list. The degree of clustering is thought to reflect self-initiated semantic strategy use during encoding and/or retrieval (Schmitt et al., 1981; Weist, 1972). We found that gray matter volume in left caudal middle, right rostral middle, and left inferior frontal regions mediated the effect of age on semantic clustering. These results are consistent with the proposed contribution of age-associated declines in prefrontal integrity to age-associated declines in effective encoding strategies. However, a significant limitation of using semantic clustering scores to measure self-initiated strategic processing is that it is not possible to determine whether semantic clustering is driven by purposeful memory strategy use during encoding, recall, both encoding and recall, or neither memory stage. For example, semantic clustering during recall could reflect relatively automatic, non-purposeful grouping of semantically-related words rather than self-initiated use of elaborative memory strategies in some individuals. Thus, further research is needed to directly investigate whether there is a significant link between age differences in self-initiated elaborative encoding strategy use found in behavioral research and age differences in prefrontal integrity found in structural neuroimaging research.
The primary goal of the current study was to investigate whether age differences in prefrontal integrity make a significant contribution to age differences in effective encoding strategies by examining whether prefrontal regional gray matter volumes mediate the effect of age on self-initiated elaborative encoding strategies. Strategy use was assessed by retrospective item-by-item strategy self-reports following a verbal unsupported intentional encoding task. This allowed us to measure the frequency of self-initiated elaborative strategy use specifically during encoding. Based upon our prior work (Kirchhoff et al., 2014), we hypothesized that gray matter volume in left caudal middle, right rostral middle, and left inferior frontal cortex would mediate the effect of age on overall self-initiated use of elaborative encoding strategies in this study. The relationships between gray matter volume and encoding strategy use were also examined in prefrontal exploratory regions of interest (ROIs) because it is possible that prefrontal regions beyond those identified in our prior structural neuroimaging study mediate self-initiated use of elaborative encoding strategies.
2. Material and Methods
2.1 Participants
Thirty-eight younger (mean age = 25.0, range = 18 – 35, 20 female) and thirty-eight older (mean age = 70.4, range = 65 – 80, 23 female) adults participated in this study. All participants were right-handed native English-speakers with normal or corrected-to-normal vision. They reported no significant neurological history or active psychiatric conditions and were not taking any psychiatric medications. Participants were also screened for head injuries, untreated hypertension, heart disease, diabetes, kidney disease, thyroid conditions, chemotherapy treatments, and alcoholism. Older adults underwent screening for dementia using the Short-Blessed (Katzman et al., 1983). They all had weighted error scores less than seven (M = 1.3, SD = 1.5, Range = 0 – 4), indicating that they were non-demented. Study procedures were approved by the Human Studies Committees of Saint Louis University, the University of Missouri - St. Louis, and Washington University in St. Louis. Informed consent was obtained in accordance with their guidelines.
2.2 Neuropsychological assessment of cognitive function
A neuropsychological testing session was conducted prior to the MRI scanning session (range 0 – 32 days) to characterize participants’ crystallized intelligence (Vocabulary subtest of the Wechsler Adult Intelligence Scale - Third Edition, WAIS-III; Wechsler, 1997), verbal fluency (FAS; Spreen & Strauss, 1991), semantic fluency (Animal Naming Test; Spreen & Strauss, 1991), working memory capacity (Computation Span; McCabe et al., 2010), and processing speed (Digit Symbol-Coding subtest of the WAIS-III; Wechsler, 1997).
2.3 Memory tasks
2.3.1 Unsupported intentional encoding task
An unsupported intentional encoding task was performed during three fMRI scans that occurred within the same scanning sessions as the structural MRI scans. Stimuli were 108 4 –7 letter English words selected from the MRC Psycholinguistic Database (Wilson, 1988). Half of the words were concrete (e.g., paper) and half were abstract (e.g., power). Word lists were counterbalanced across participants and were matched for word frequency, length, and syllable count.
The unsupported intentional encoding scans had a block design and started with a 36 s fixation block, during which a plus sign was displayed in the center of a screen. The initial fixation block was followed by an intentional encoding task block that lasted 72 s, during which eighteen words were presented one at a time for 3,750 ms in the center of a screen. Each word was immediately followed by a fixation plus sign presented for 250 ms. A second fixation block then occurred for 28 s, and was followed by an additional 72 s intentional encoding task block and then a final 28 s fixation block. Participants were instructed to carefully study each word, and were told that their memory for the words would be tested later. However, they were not instructed to use any specific strategy or strategies to study the words, so they could study the presented words using any method that they chose. To ensure that participants were paying attention to the presented words, they were asked to press a button each time a word appeared on the screen.
2.3.2 Recognition memory test
Participants’ memory for the studied words was assessed with a Remember/Know/New recognition memory test (108 studied words and 108 new words; Tulving, 1985) performed immediately after they left the MRI scanner. Words were presented one at a time on a computer screen. Participants were instructed to make a Remember response if they could consciously remember specific details about the presentation of the word during encoding, such as specific thoughts they had while viewing the word or the context surrounding the word (e.g., a word that came directly before or after it). Participants were instructed to make a Know response if they thought that the word was presented during encoding, but they could not recall any specific details about its prior presentation. Remember responses are thought to be a measure of conscious recollection, while Know responses are thought to be a measure of familiarity in the absence of conscious recollection. Participants were instructed to make a New response if they thought that a word had not been presented during the intentional encoding task. Each word stayed on the screen until the participant made a response.
2.4 Strategy use assessment
Providing descriptions of common encoding strategies before encoding increases older adults’ use of elaborative strategies (Dunlosky & Hertzog, 2001). This strongly suggests that having younger and older adults report the strategies used to learn words during encoding would also alter their strategy use. Therefore, to measure truly self-initiated encoding strategy use, strategy reports must be made after encoding. Participants made retrospective item-by-item strategy reports during the recognition memory test in this study. They indicated by keypress what strategy or strategies they had used to study each word that they gave a Remember or Know response immediately after making the response. Strategy reports were not given for words that participants indicated were New. The strategy report options were rote repetition, visual imagery, sentence generation, personal relevance, other strategy, no strategy, and forgot strategy. Participants were instructed to choose forgot strategy if they knew they had used a strategy or strategies to study the word, but had forgotten the specific strategy or strategies they had employed. Participants were allowed to make more than one strategy response per word.
Participants’ strategy use frequency proportions were calculated by dividing the total number of correctly identified old words (i.e., words studied during the intentional encoding task given a Remember or Know response) for which a participant endorsed each strategy response option by the total number of correctly identified old words for which he/she gave a Remember or Know response during the recognition memory task. Consistent with prior characterizations of encoding strategies, the visual imagery, sentence generation, and personal relevance strategies were classified as elaborative encoding strategies and the rote repetition strategy was classified as a non-elaborative encoding strategy (Craik & Lockhart, 1972; Kirchhoff et al., 2013; Richardson, 1998). An elaborative strategy composite score was calculated for each participant by summing the number of correctly identified old words given a Remember or Know response for which he/she reported using one or more elaborative encoding strategy(ies) (visual imagery, sentence generation, and/or personal relevance) and then dividing by the total number of correctly identified old words that he/she gave a Remember or Know response during the recognition memory task. By calculating the strategy use frequency proportions using only studied words for which participants gave a Remember or Know response during the recognition memory test (i.e., hits), the strategy frequency proportions were not biased by differences in recognition performance between the two age groups.
The elaborative strategy composite score was used to identify brain regions that support self-initiated use of elaborative encoding strategies due to the substantial inter-individual variability in use of specific elaborative encoding strategies. Many individuals who frequently used elaborative encoding strategies in this study did not use all elaborative encoding strategies equally. Instead, they used one or two elaborative encoding strategies to learn most of the presented words, but did not use the other elaborative encoding strategy(ies) very frequently, if at all. Therefore, the most powerful way to identify regions that play an important role in supporting self-initiated elaborative encoding strategy use was to examine the relationships between prefrontal regional gray matter volumes and an elaborative strategy composite score instead of the relationships between prefrontal regional gray matter volumes and use of individual encoding strategies.
2.5 Structural neuroimaging data acquisition
One high resolution, T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) scan (TR = 2400 ms, TE = 3.08 ms, flip angle = 8°, T1 = 1000 ms, voxel size = 1.0 × 1.0 × 1.0 mm, slices = 176) was acquired for each participant using a Siemens 3.0 Tesla Tim TRIO MRI scanner (Erlangen, Germany). Cushions and a thermoplastic mask were used to minimize head movement.
2.6 Generation of regional gray matter volumes
Each participant’s MPRAGE image was visually inspected for excessive movement and image artifacts as a quality control. Regional gray matter volumes were generated using the FreeSurfer image analysis suite (version 5.3; http://surfer.nmr.mgh.harvard.edu/) (Desikan et al., 2006; Fischl et al., 2002; Fischl et al., 2004a). In FreeSurfer’s automated labeling procedure, each voxel in a T1-weighted image is assigned a neuroanatomical label based on probabilistic information derived from a manually labeled training set. Regional gray matter volumes generated using this procedure have been shown to have a high correspondence with manually traced volumes (Fischl et al., 2004b). Labeling of gray matter was manually checked and errors were fixed by removing incorrectly labeled areas and adding control points using FreeSurfer’s tkmedit tool by a research team member blind to participants’ strategy use. Gray matter volumes were adjusted for intracranial volume using a covariance approach (Buckner et al., 2004; Mathalon et al., 1993).
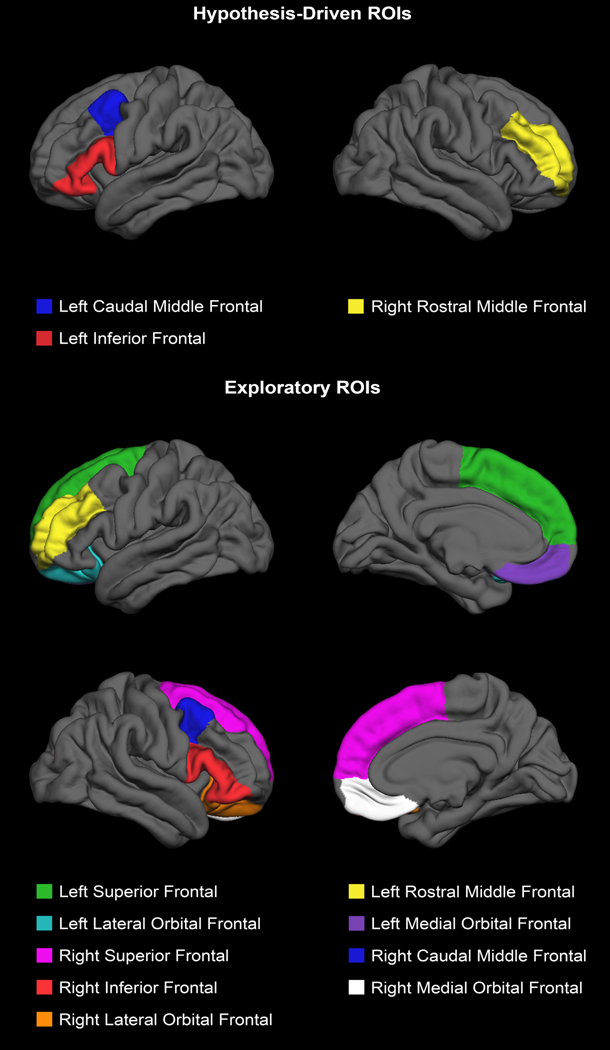
There were three hypothesis-driven prefrontal gray matter ROIs for this study: left caudal middle, right rostral middle, and left inferior frontal cortex. Nine exploratory prefrontal gray matter ROIs were also examined: left superior, left rostral middle, left medial orbital, left lateral orbital, right superior, right caudal middle, right inferior, right medial orbital, and right lateral orbital frontal cortex (see Figure 1 for ROI locations). In total, the exploratory ROIs included all prefrontal gray matter outside of the hypothesis-driven ROIs except for a small portion of the frontal pole. ROIs were identified using the Desikan-Killiany atlas (Desikan et al., 2006) within FreeSurfer. Gray matter volumes in the inferior frontal ROIs were calculated by summing gray matter volumes in the pars opercularis, pars triangularis, and pars orbitalis.
Fig. 1.
Hypothesis-driven and exploratory regions of interest (ROIs) displayed on the FreeSurfer template brain.
2.7 Data analysis
2.7.1 Outlier analysis and covariates
Years of education, recognition memory (hits - false alarms), strategy use, and regional gray matter volume data were examined for both univariate and multivariate outliers by calculating z statistics and a Mahalanobis D2 score for each participant. Univariate z scores greater than four standard deviations from the mean, and multivariate Mahalanobis D2 scores with p values less than 0.001, were considered to be outliers. One younger adult participant’s right hemisphere caudal middle frontal gray matter volume was determined to be a univariate outlier. A new value was imputed via the expectation-maximization algorithm in SPSS (version 19), which estimated the score based on all other data. All Mahalanobis D2 scores were greater than 0.001, indicating that there were no multivariate outliers.
Years of education, recognition memory performance, elaborative strategy use frequency, and regional gray matter volumes were transformed into z scores for data analyses. Age was treated as a dichotomous categorical variable (young versus old). Zero-order correlations between sex, years of education, and all predictor and outcome variables were examined (α = 0.05, one-tailed). Both sex and years of education were significantly correlated with at least one predictor or outcome variable, and therefore were treated as covariates in statistical analyses.
2.7.2 Age, strategy use, and recognition memory performance
Planned independent samples t-tests and partial correlations were conducted to examine the effects of age on memory performance and self-initiated use of encoding strategies. In addition, a partial correlation was conducted to examine the relationship between elaborative encoding strategy use and memory performance. A one-tailed α of 0.05 was used for these analyses because we had a priori hypotheses regarding the directions of the relationships among the examined variables based on previous research. Specifically, we hypothesized that recognition memory performance and use of elaborative encoding strategies would be negatively associated with age (Hertzog et al., 1998; Naveh-Benjamin et al., 2007; Parkin & Walter, 1992; Verhaeghen & Marcoen, 1994) and that use of elaborative encoding strategies would be positively associated with recognition memory performance (Camp et al., 1983; Geiselman et al., 1982; Martin et al., 1965; Shaughnessy, 1981). We also hypothesized that use of a non-elaborative rote repetition strategy and reports of no strategy use would be positively associated with age (Devolder & Pressley, 1992; Kirchhoff et al., 2012; Naveh-Benjamin et al., 2007; Rowe & Schnore, 1971).
2.7.3 Hypothesis-driven and exploratory ROI analyses
Partial correlations between age group and gray matter volume in the hypothesis-driven and exploratory ROIs were conducted to examine the effects of age on prefrontal integrity. In addition, partial correlations between gray matter volume in the hypothesis-driven and exploratory ROIs and elaborative strategy composite scores were conducted to investigate which prefrontal regions support self-initiated use of elaborative encoding strategies. Exploratory ROI partial correlations were corrected for multiple comparisons using a False Discovery Rate (FDR) correction (Benjamini & Hochberg, 1995). A one-tailed α of 0.05 was used for these analyses because we had a priori hypotheses regarding the directions of the relationships among the examined variables based on previous research. We hypothesized that gray matter volume would be negatively associated with age (Jernigan et al., 2001; Kirchhoff et al., 2014; Raz et al., 1997). We also hypothesized that prefrontal gray matter volume would be positively associated with self-initiated elaborative encoding strategies because prior studies of the relationships between prefrontal gray matter volume and semantic clustering have only reported significant positive correlations (Kirchhoff et al., 2014; Matsui et al., 2008).
2.7.4 Hierarchical linear regression analyses
A hierarchical linear regression analysis was conducted to examine whether the relationship between self-initiated use of elaborative encoding strategies and recognition memory performance varied as a function of age group to test whether there were age differences in the efficacy of these strategies. This analysis was two-tailed (α = 0.05) because we did not have a specific a priori hypothesis regarding how the relationship between elaborative encoding strategy use frequency and recognition memory performance may vary with age.
A hierarchical linear regression analysis was also conducted to examine whether the relationship between gray matter volume and self-initiated use of elaborative encoding strategies varies as a function of age group in prefrontal ROIs with significant partial correlations between gray matter volume and self-initiated elaborative encoding strategies. This analysis was two-tailed (α = 0.05) because we did not have specific a priori hypotheses regarding how the relationship between prefrontal regional gray matter volumes and self-initiated use of elaborative encoding strategies may vary with age.
2.7.5 Mediation analysis
The PROCESS mediation analysis software program (http://www.processmacro.org/) (Hayes, 2013) was used to examine whether prefrontal regional gray matter volume mediated the age group effect on self-initiated elaborative encoding strategies. This program uses a logistic regression-based path analytic framework to estimate indirect effects and tests whether the indirect effects are significantly different from zero by implementing bias-corrected bootstrapped (20,000 samples) confidence intervals. A ninety-five percent bias-corrected confidence interval was used to determine the statistical significance of the indirect effects. Standardized residuals controlling for sex and years of education were used in the mediation analysis.
3. Results
3.1 Relationships between age, strategy use, and recognition memory performance
Participants’ demographic characteristics and performance on the neuropsychological tests are summarized in Table 1. Older adults had significantly worse recognition memory performance (hits - false alarms M = 0.23, SD = 0.17, d’ = 0.71) than younger adults (M = 0.34, SD = 0.17, d’ = 1.06; t = −2.84, p = 0.003, Cohen’s d = −0.65). Older adults had fewer hits (M = 0.52, SD = 0.21) than younger adults (M = 0.61, SD = 0.19; t = −2.00, p = 0.025, d = −0.45), but older (M = 0.29, SD = 0.19) and younger adults’ (M = 0.27, SD = 0.18) false alarm rates did not significantly differ (t = 0.35, p = 0.364, d = 0.11).
Table 1.
Demographic characteristics and neuropsychological test performance.
| Younger (n = 38) |
Older (n = 38) |
|
|---|---|---|
| Female/Male | 20/18 | 23/15 |
| Age* | 25.0 (4.8) | 70.4 (3.7) |
| Education (years) | 15.5 (2.2) | 16.3 (2.6) |
| Short-Blessed | — | 1.3 (1.5) |
| WAIS-III Vocabulary | 52.9 (7.7) | 56.3 (8.8) |
| FAS | 47.1 (9.1) | 43.0 (13.6) |
| Animal Naming | 23.6 (3.4) | 21.9 (4.4) |
| Computation Spana* | .76 (.11)b | .65 (.13) |
| WAIS-III Digit Symbol* | 85.7 (11.2)b | 66.9 (13.7) |
Means and standard deviations (in parentheses) for younger and older adults’ demographic characteristics and raw scores on the neuropsychological tests.
WAIS-III: Wechsler Adult Intelligence Scale-III.
Partial-credit unit scoring (Conway et al., 2005).
n = 37.
p < 0.001 (two-tailed).
The frequency with which younger and older adults made each strategy response during the retrospective item-by-item strategy assessment is presented in Table 2. Older adults had significantly lower elaborative strategy composite scores than younger adults (t = −1.97, p = 0.027, d = −0.47). For the individual elaborative strategies, older adults reported using the sentence generation (t = −1.91, p = 0.031, d = −0.42) and personal relevance (t = −2.79, p = 0.004, d = −0.69) strategies significantly less frequently than younger adults. The frequency of their visual imagery strategy reports did not significantly differ from younger adults’ (t = −1.05, p = 0.149, d = −0.24). There were no significant age differences in the frequency of non-elaborative rote repetition strategy reports (t = −0.04, p = 0.486, d = −0.00). Overall, older adults reported using more than one encoding strategy significantly less frequently (M = 0.12, SD = 0.21) than younger adults (M = 0.23, SD = 0.21; t = −2.33, p = 0.012, d = −0.52). Consistent with prior research, older adults reported using no strategy to encode words significantly more frequently than younger adults (t = 1.68, p = 0.049, d = 0.38). Importantly, the frequency of forgot strategy responses did not significantly differ for older versus younger adults (t = −0.22, p = 0.413, d = −0.08), suggesting that older adults were not more likely to forget what strategies they used during encoding than younger adults. Age group was significantly negatively correlated with both recognition memory performance (pr = −0.33, p = 0.002) and elaborative encoding strategy use (pr = −0.23, p = 0.025) controlling for sex and years of education.
Table 2.
Strategy use frequency proportions for younger and older adults.
| Elaborative Strategy* |
Sentence Generation* |
Personal Relevance* |
Visual Imagery |
Rote Repetition |
Other Strategy |
No Strategy* |
Forgot Strategy |
|
|---|---|---|---|---|---|---|---|---|
| Younger Adults | .57 (.24) | .17 (.22) | .26 (.16) | .33 (.22) | .23 (.27) | .12 (.19) | .11 (.16) | .11 (.10) |
| Older Adults | .45 (.27) | .09 (.15) | .15 (.16) | .28 (.20) | .23 (.31) | .10 (.21) | .20 (.29) | .10 (.15) |
Means and standard deviations (in parentheses) for the proportion of hits (i.e., old words given Remember or Know responses) for which younger and older adults made each strategy response on the retrospective item-by-item strategy assessment.
Significant age difference (p < 0.05, one-tailed).
Finally, elaborative encoding strategy use was significantly positively correlated with recognition memory performance (pr = 0.27, p = 0.011) controlling for sex and years of education. A hierarchical linear regression analysis explored whether age moderated this relationship. Recognition memory performance was the dependent variable. The first step of the model which contained sex and years of education was not significant (ΔR2 = 0.005, F(2,73) = 0.19, p = 0.832). The second step of the model which contained age group and the elaborative strategy composite score was significant (ΔR2 = 0.146, F(2,71) = 6.09, p = 0.004). Importantly, the last step of the model which contained an age group x elaborative strategy composite score interaction term was not significant (ΔR2 = 0.000, F(1,70) = 0.00, p = 0.954). This indicates that age did not significantly moderate the positive relationship between elaborative encoding strategy use and recognition memory performance, suggesting that elaborative encoding strategies were similarly effective for younger and older adults in this study. (See Supplementary Results for analyses of the relationships between age, elaborative encoding strategy use, and Remember responses.)
3.2 Volumetric correlates of self-initiated elaborative encoding strategies
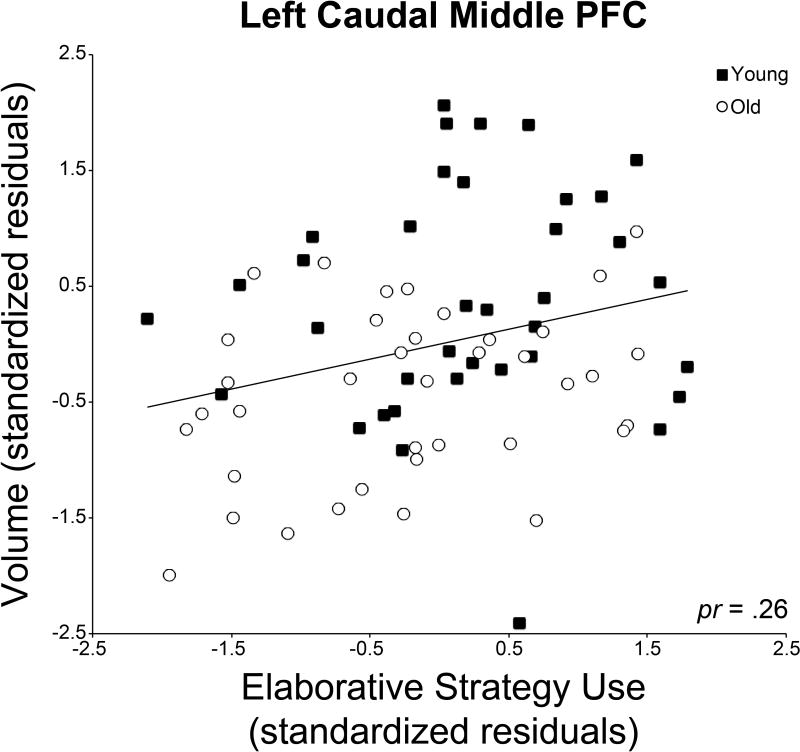
Analyses of the relationships between regional gray matter volumes, age group, and self-initiated elaborative encoding strategy use (Table 3) revealed that age group was significantly negatively correlated with gray matter volume in all ROIs except for the left medial orbital frontal exploratory region. Elaborative strategy composite scores were significantly positively correlated with gray matter volume in the left caudal middle frontal hypothesis-driven ROI (Figure 2). Gray matter volume in this region was also significantly positively correlated with recognition memory performance (pr = 0.28, p = 0.008; Supplementary Table 1).
Table 3.
Partial correlations between prefrontal regional gray matter volumes, age group, and self-initiated use of elaborative encoding strategies.
| Age Group |
Elaborative Strategy Composite |
|||
|---|---|---|---|---|
| Region | pra | p | pra | p |
| Hypothesis-Driven | ||||
| Left caudal middle | −.45# | .000 | .26# | .013 |
| Right rostral middle | −.57# | .000 | .15 | .097 |
| Left inferior | −.58# | .000 | .05 | .338 |
| Exploratory | ||||
| Left superior | −.70* | .000 | .08 | .245 |
| Left rostral middle | −.65* | .000 | .17 | .072 |
| Left medial orbital | −.09 | .212 | −.09 | .228 |
| Left lateral orbital | −.59* | .000 | .10 | .211 |
| Right superior | −.64* | .000 | .14 | .123 |
| Right caudal middle | −.41* | .000 | .23 | .023 |
| Right inferior | −.72* | .000 | .21 | .035 |
| Right medial orbital | −.54* | .000 | .03 | .388 |
| Right lateral orbital | −.62* | .000 | .16 | .088 |
Partial correlation coefficient controlling for sex and years of education.
Indicates significant partial correlation in hypothesis-driven ROI.
Indicates significant partial correlation in exploratory ROI after FDR multiple comparisons correction.
Fig. 2.
Correlation between left caudal middle frontal gray matter volume and self-initiated elaborative encoding strategies. Gray matter volume was significantly positively correlated with self-initiated use of elaborative encoding strategies in left caudal middle frontal cortex. Data are standardized residuals controlling for sex and years of education. PFC = prefrontal cortex.
A hierarchical linear regression analysis explored whether age group moderated the significant positive relationship between gray matter volume and self-initiated use of elaborative encoding strategies in left caudal middle frontal cortex to explore whether this relationship was present in both younger and older adults. The elaborative strategy composite score was the dependent variable. The first step of the model which contained sex and years of education was not significant (ΔR2 = 0.028, F(2,73) = 1.07, p = 0.349). The second step of the model which contained age group and gray matter volume was significant (ΔR2 = 0.081, F(2,71) = 3.21, p = 0.046). Importantly, the last step of the model which contained an age group x volume interaction term was not significant (ΔR2 = 0.020, F(1,70) = 1.58, p = 0.214). This indicates that age group did not significantly moderate the positive relationship between gray matter volume and self-initiated use of elaborative encoding strategies in left caudal middle frontal cortex, which suggests that larger gray matter volume in this region is associated with greater use of elaborative encoding strategies in both younger and older adults.
3.3 Prefrontal mediation of the age effect on self-initiated elaborative encoding strategies
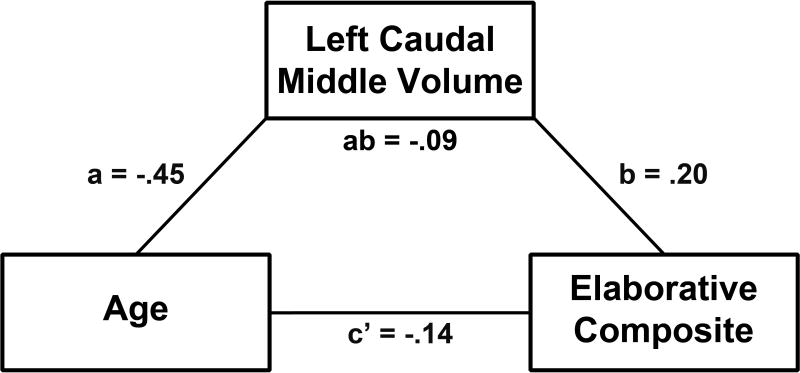
A mediation analysis examined whether prefrontal regional gray matter volume in the left caudal middle frontal hypothesis-driven ROI made a significant contribution to the age group effect on self-initiated elaborative encoding strategies (see mediation model in Figure 3). The 95% bias-corrected confidence interval for the indirect effect of age group on elaborative encoding strategy use mediated by gray matter volume did not contain zero, indicating that it was significant (PE = −0.09, SE = 0.06, Lower = −0.228, Upper = −0.002). The direct effect of age group on elaborative encoding strategy use was not significant when controlling for gray matter volume (Direct Coefficient = −0.14, SE = 0.13, p = 0.260). This pattern of results demonstrates that gray matter volume in left caudal middle frontal cortex is a significant mediator of the age effect on self-initiated elaborative encoding strategies.
Fig. 3.
Left caudal middle frontal gray matter volume mediates the age effect on self-initiated elaborative encoding strategies. Mediation model of the relationships between age group, left caudal middle frontal gray matter volume, and elaborative strategy composite scores. The indirect effect of age group on self-initiated elaborative strategy use mediated by gray matter volume was significant. The direct effect of age group on self-initiated elaborative strategy use controlling for gray matter volume was not significant. a = The effect of age group on gray matter volume. b = The effect of gray matter volume on self-initiated elaborative strategy use controlling for the effect of age group. ab = The indirect effect of age group on self-initiated elaborative strategy use mediated by gray matter volume. c’ = The direct effect of age group on self-initiated elaborative strategy use controlling for gray matter volume. PFC = prefrontal cortex.
4. Discussion
This study investigated whether age-associated reductions in prefrontal gray matter volume are one of the mechanisms of age-associated declines in elaborative encoding strategies by examining whether prefrontal regional gray matter volumes mediated the effect of age on self-initiated use of elaborative encoding strategies. Gray matter volume in the hypothesis-driven left caudal middle frontal ROI was significantly positively correlated with the overall frequency of self-initiated elaborative encoding strategies and recognition memory performance, suggesting that the region supports self-initiated use of multiple elaborative encoding strategies. Gray matter volume in the left caudal middle frontal ROI was also significantly negatively correlated with age group and mediated the age effect on self-initiated elaborative encoding strategies. To our knowledge, this is the first study to find a significant association between the effect of age on prefrontal regional gray matter volume and the effect of age on self-initiated use of elaborative memory strategies specifically during encoding. Therefore, these results substantially increase support for the proposal that age-associated declines in prefrontal integrity make a significant contribution to age-associated declines in self-initiated elaborative encoding strategies.
Gray matter volume in the right rostral middle frontal ROI was not significantly positively correlated with the overall frequency of self-initiated elaborative encoding strategies as hypothesized. Gray matter volume in the exploratory right caudal middle frontal ROI was positively correlated with the overall frequency of self-initiated elaborative encoding strategies, but the correlation did not survive FDR correction. This pattern of results could indicate that right middle frontal cortex supports self-initiated use of elaborative encoding strategies, but that the present study did not have a large enough sample size to detect a relationship between gray matter volume in this region and the overall frequency of self-initiated elaborative encoding strategies that met our statistical significance criterion. This pattern of results could also indicate that a subregion of right middle frontal cortex supports self-initiated use of elaborative encoding strategies, but the subregion does not follow the anatomical boundaries of the right rostral or caudal middle frontal ROIs defined by Freesurfer.
Prior functional neuroimaging research suggests that bilateral middle frontal cortex may support self-initiated use of elaborative encoding strategies by performing relational processing during encoding. For example, Blumenfeld and colleagues (2011) had younger adults form interactive visual images of the object referents of pairs of unrelated concrete nouns. Bilateral activity in the middle frontal gyrus during performance of this relational encoding task predicted participants’ subsequent associative recognition of the word pairs. Fletcher and colleagues (1998) found that brain activity was greater in the left middle frontal gyrus during intentional encoding when participants were required to process the meaning of words in relationship to each other in order to identify the categories present in a list compared to when participants were told what categories would be present in a list before encoding. In addition, training younger adults to use a method of loci mnemonic (Bower, 1970), which requires the formation of relationships between items (e.g., words or objects) and visualized locations during encoding, has also been shown to increase brain activity bilaterally in the middle frontal gyrus (Kondo et al., 2005; Nyberg et al., 2003). The results of these studies, in combination with the current findings, suggest that age differences in self-initiated use of elaborative encoding strategies may be driven in part by age differences in relational processing during encoding.
We hypothesized that gray matter volume in left inferior prefrontal cortex would mediate the effect of age on self-initiated use of elaborative encoding strategies in this study based on the results of prior research that suggested that there are positive relationships between use of elaborative semantic strategies and gray matter volume and brain activity in this region. For example, in a previous structural neuroimaging study we found that gray matter volume in left inferior prefrontal cortex mediated the age effect on semantic clustering during free recall (Kirchhoff et al., 2014). We also found that training older adults to use semantic encoding strategies led to changes in brain activity in left inferior prefrontal cortex during verbal unsupported intentional encoding that were positively correlated with training-related changes in memory performance and self-initiated use of a sentence generation encoding strategy in a prior fMRI study (Kirchhoff et al., 2012). Positive correlations have also been reported between gray matter volume (Matsui et al., 2008) and brain activity during verbal unsupported intentional encoding (Savage et al., 2001) in left inferior prefrontal cortex and semantic clustering during free recall in younger adults. However, contrary to our hypothesis, gray matter volume in left inferior prefrontal cortex did not mediate the effect of age on overall self-initiated use of elaborative encoding strategies in this study. Therefore, left inferior prefrontal cortex may selectively support self-initiated use of encoding strategies that require controlled semantic processing (e.g., semantic categorization and sentence generation) but not those that require minimal or relatively automatic semantic processing (e.g., visual imagery and personal relevance). Consistent with this possibility, prior research has suggested that a portion of left inferior prefrontal cortex may play a central role in controlled semantic retrieval (Badre et al., 2005; Gold et al., 2006).
Currently, relatively little is known regarding whether there are age differences in the regions of prefrontal cortex that support self-initiated elaborative memory strategies. In a prior study (Kirchhoff et al., 2014), we found that age did not moderate the positive associations between prefrontal regional gray matter volumes and semantic clustering during free recall, demonstrating that the relationships between gray matter volumes and self-initiated memory strategy use during encoding and/or retrieval were the same in both younger and older adults. In the present study, age group also did not moderate the positive association between left caudal middle frontal gray matter volume and overall self-initiated use of elaborative encoding strategies, suggesting that larger gray matter volume in left caudal middle frontal cortex is associated with greater use of elaborative encoding strategies in both younger and older adults. Overall, the results of our research to date suggest that the same regions of prefrontal cortex may support self-initiated elaborative memory strategy use across the lifespan.
This study had a relatively modest sample size, which may have limited our power to detect all of the age-associated regional declines in prefrontal gray matter volume that contribute to age-associated declines in self-initiated use of elaborative encoding strategies. This study also lacked an adult lifespan sample, and as a result, mediation analyses were conducted on cross-sectional data with only two age groups which limits our ability to make strong claims regarding the causality of the relationship between the age effect on left caudal middle frontal gray matter volume and the age effect on self-initiated elaborative encoding strategies. Statistical simulations run by Lindenberger and colleagues (2011) have suggested that mediation analyses of cross-sectional data in aging research can both overestimate and underestimate the true relationships between longitudinal changes in variables. Therefore, future research should be conducted to further investigate the contribution of age differences in prefrontal regional gray matter integrity to age differences in self-initiated use of elaborative encoding strategies within a larger, longitudinal adult lifespan sample. However, a significant potential limitation of longitudinal studies of self-initiated encoding strategy use is that initial strategy self-reports could alter subsequent strategy use, which would significantly hinder the ability of longitudinal studies to investigate age-associated changes in truly self-initiated use of encoding strategies. Therefore, both cross-sectional and longitudinal research is necessary to investigate the contribution of age-associated declines in prefrontal integrity to age-associated declines in self-initiated elaborative encoding strategies.
Another limitation of this study is that the unsupported intentional encoding task was performed inside of an MRI scanner. This may have altered participants’ encoding strategy use and memory performance relative to what would have occurred in a non-scanning laboratory environment, especially for older adults (Gutchess & Park, 2006). However, prior purely behavioral studies have reported significantly less frequent self-initiated elaborative encoding strategy use (Hertzog et al., 1998; Naveh-Benjamin et al., 2007; Verhaeghen & Marcoen, 1994) and worse memory performance in older relative to younger adults (Hultsch et al., 1990; Sanders et al., 1980), consistent with the results of the present study.
The present study used retrospective item-by-item strategy self-reports because providing descriptions of common encoding strategies before encoding has been shown to increase older adults’ use of elaborative strategies (Dunlosky & Hertzog, 2001), strongly suggesting that having younger and older adults report the strategies used to learn words during encoding would alter their strategy use. A potential limitation of retrospective item-by-item strategy self-reports are that they rely heavily on participants’ memory for the strategy(ies) used to encode individual words following a several minute delay. Strategy report accuracy could be diminished by participants forgetting the actual strategies used, rationalizing their memory performance, and/or attempting to comply with the perceived aims of the experimenter. It is possible that participants made “no strategy” responses to some words for which they did use a strategy during encoding but then subsequently forgot which strategy they used, therefore underestimating the true proportion of trials for which they used a strategy but forgot which one that they used. There could also be age differences in the accuracy of strategy self-reports. However, prior fMRI research from our lab that used retrospective encoding strategy self-reports with a similar delay between stimulus encoding and the strategy reports demonstrated that individual differences in prefrontal brain activity during encoding were significantly positively correlated with individual differences in retrospective elaborative encoding strategy self-reports in young adults (Kirchhoff & Buckner, 2006). We have also found that training older adults to use semantic encoding strategies led to changes in brain activity in left inferior prefrontal cortex during verbal unsupported intentional encoding that were positively correlated with training-related changes in self-reported use of a sentence generation encoding strategy in a prior fMRI study that also used retrospective encoding strategy reports with a comparable delay between stimulus encoding and the strategy reports (Kirchhoff et al., 2012). In the present study, left caudal middle frontal gray matter volume was significantly positively correlated with self-reported elaborative encoding strategy use frequency. Age group did not moderate this relationship, indicating that the strategy self-reports had a consistent association with brain structure in both younger and older adults. Furthermore, there were no age differences in forgot strategy reports, and the frequency of forgot strategy reports was very low for both age groups. Altogether, these findings indicate that the retrospective item-by-item strategy self-reports used in this study were an accurate, although imperfect, measure of both younger and older adults’ self-initiated use of encoding strategies.
5. Conclusions
Gray matter volume in left caudal middle frontal cortex was positively associated with the overall frequency of self-initiated elaborative encoding strategies. Age group did not moderate this relationship, suggesting that left caudal middle frontal cortex supports self-initiated use of multiple elaborative encoding strategies in both younger and older adults. Gray matter volume in left caudal middle frontal cortex also mediated the age group effect on self-initiated elaborative encoding strategies. Given that this was the first study to examine the relationships between prefrontal regional gray matter volumes and elaborative strategy use specifically during encoding, the results of this study substantially increase support for the proposal that age-associated declines in prefrontal integrity make a significant contribution to age-associated declines in self-initiated elaborative encoding strategies.
Supplementary Material
Highlights.
Investigated role of PFC in age differences in elaborative encoding strategies
Left caudal middle frontal gray matter volume mediated age effect on strategy use
Suggests PFC contributes to age effects on self-initiated encoding strategies
Acknowledgments
We thank Danielle Kelly, Hannah Novack, Samantha Bueler, Jaya Chandra, Ryan Doan, Lauren Kristich, Jennifer Moser-Keith, Nicole Mitchell, Anish Modi, Samuel Peter, Rachel Rice and Vishal Thakkar for assistance with data collection and/or analysis. We also thank Chris Owen, Karl Friedrichsen, and Chad Sylvester for assistance with FreeSurfer and Tony Buchanan for comments on a prior version of this manuscript. This work was supported by the National Institutes of Health (K01 AG031301 to B.A.K.). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Abbreviations
- CVLT-II
California Verbal Learning Test-II
- FDR
false discovery rate
- MPRAGE
magnetization-prepared rapid gradient echo
- PFC
prefrontal cortex
- ROI
region of interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badre D, Poldrack RA, Paré-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Balota DA, Dolan PO, Duchek JM. Memory changes in healthy older adults. In: Tulving E, Craik F, editors. The Oxford Handbook of Memory. Oxford University; New York: 2000. pp. 395–409. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. 1995;B57:289–300. [Google Scholar]
- Blumenfeld RS, Parks CM, Yonelinas AP, Ranganath C. Putting the pieces together: the role of dorsolateral prefrontal cortex in relational memory encoding. J. Cogn. Neurosci. 2011;23:257–265. doi: 10.1162/jocn.2010.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousfield WA. The occurrence of clustering in the recall of randomly arranged associates. J. Gen. Psychol. 1953;49:229–240. [Google Scholar]
- Bower GH. Analysis of a mnemonic device: modern psychology uncovers the powerful components of an ancient system for improving memory. Am. Sci. 1970;58:496–510. [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Camp CJ, Markley RP, Kramer JJ. Naïve mnemonics: what the “do-nothing” control group does. Am. J. Psychol. 1983;96:503–511. [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: a methodological review and user’s guide. Psychon. Bull. Rev. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Craik FI, Lockhart RS. Levels of processing: a framework for memory research. J. Verbal Learn. Verbal Behav. 1972;11:671–684. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. Second Edition. Pearson Education, Inc; San Antonio: 2000. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devolder PA, Pressley M. Causal attributions and strategy use in relation to memory performance differences in younger and older adults. Appl. Cogn. Psychol. 1992;6:629–642. [Google Scholar]
- Dunlosky J, Hertzog C. Measuring strategy production during associative learning: the relative utility of concurrent versus retrospective reports. Memory Cognit. 2001;29:247–253. doi: 10.3758/bf03194918. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004a;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004b;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. The functional roles of prefrontal cortex in episodic memory: encoding. Brain. 1998;121:1239–1248. doi: 10.1093/brain/121.7.1239. [DOI] [PubMed] [Google Scholar]
- Geiselman RE, Woodward JA, Beatty J. Individual differences in verbal memory performance: a test of alternative information-processing models. J. Exp. Psychol. Gen. 1982;111:109–134. [Google Scholar]
- Gershberg FB, Shimamura AP. Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia. 1995;13:1305–1333. doi: 10.1016/0028-3932(95)00103-a. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH. Dissociation of automatic and strategic lexical-semantics: functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J. Neurosci. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Park DC. fMRI environment can impair memory performance in young and elderly adults. Brain Res. 2006;1099:133–140. doi: 10.1016/j.brainres.2006.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. The Guilford Press; New York, NY: 2013. [Google Scholar]
- Hertzog C, McGuire CL, Lineweaver TT. Aging, attributions, perceived control, and strategy use in a free recall task. Aging Neuropsychol. Cogn. 1998;5:85–106. [Google Scholar]
- Hirst W, Volpe BT. Memory strategies with brain damage. Brain Cogn. 1988;8:379–408. doi: 10.1016/0278-2626(88)90060-7. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA. Ability correlates of memory performance in adulthood and aging. Psychol. Aging. 1990;5:356–368. doi: 10.1037//0882-7974.5.3.356. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation- memory-concentration test of cognitive impairment. Am. J. Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Anderson BA, Barch DM, Jacoby LL. Cognitive and neural effects of semantic encoding strategy training in older adults. Cereb. Cortex. 2012;22:788–799. doi: 10.1093/cercor/bhr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff BA, Buckner RL. Functional-anatomic correlates of individual differences in memory. Neuron. 2006;51:263–274. doi: 10.1016/j.neuron.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Gordon BA, Head D. Prefrontal gray matter volume mediates age effects on memory strategies. Neuroimage. 2014;90:326–334. doi: 10.1016/j.neuroimage.2013.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff BA, Smith SE, Luntz JD. Prefrontal cortex and self-initiated encoding strategy use in healthy younger and older adults. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press; New York: 2013. pp. 567–581. [Google Scholar]
- Kondo Y, Suzuki M, Mugikura S, Abe N, Takahashi S, Iijima T, et al. Changes in brain activation associated with use of a memory strategy: a functional MRI study. Neuroimage. 2005;24:1154–1163. doi: 10.1016/j.neuroimage.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: what’s change got to do with it? Psychol. Aging. 2011;26:34–47. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Lockhart SN, DeCarli C. Structural imaging measures of brain aging. Neuropsychol. Rev. 2014;24:271–289. doi: 10.1007/s11065-014-9268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CJ, Boersma FJ, Cox DL. A classification of associative strategies in paired-associate learning. Psychon. Sci. 1965;3:455–456. [Google Scholar]
- Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A. Correction for head size in brain-imaging measurements. Psychiatry Res. 1993;50:121–139. doi: 10.1016/0925-4927(93)90016-b. [DOI] [PubMed] [Google Scholar]
- Matsui M, Suzuki M, Zhou S, Takahashi T, Kawasaki Y, Yuuki H, Kato K, Kurachi M. The relationship between prefrontal brain volume and characteristics of memory strategy in schizophrenia spectrum disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1854–1862. doi: 10.1016/j.pnpbp.2008.08.018. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Roediger HL, III, McDaniel MA, Balota DA, Hambrick DZ. The relationship between working memory capacity and executive functioning: evidence for a common executive attention construct. Neuropsychology. 2010;24:222–243. doi: 10.1037/a0017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto EC, Balardin JB, Savage CR, Martin MGM, Batistuzzo MC, Amaro E, Jr, Nitrini R. Brain regions supporting verbal memory improvement in healthy older subjects. Arq. Neuropsiquiatr. 2014;72:663–670. doi: 10.1590/0004-282x20140120. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O. The associative memory deficit of older adults: the role of strategy utilization. Psychol. Aging. 2007;22:202–208. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Sandblom J, Jones S, Neely AS, Petersson KM, Ingvar M, et al. Neural correlates of training-related memory improvement in adulthood and aging. Proc. Natl. Acad. Sci. USA. 2003;100:13728–13733. doi: 10.1073/pnas.1735487100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM. Recollective experience, normal aging, and frontal dysfunction. Psychol. Aging. 1992;7:290–298. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb. Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci. Biobehav. Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JTE. The availability and effectiveness of reported mediators in associative learning: a historical review and an experimental investigation. Psychon. Bull. Rev. 1998;5:597–614. [Google Scholar]
- Rowe EJ, Schnore MM. Item concreteness and reported strategies in paired-associate learning as a function of age. J. Gerontol. 1971;26:470–475. doi: 10.1093/geronj/26.4.470. [DOI] [PubMed] [Google Scholar]
- Sanders RE, Murphy MD, Schmitt FA, Walsh KK. Age differences in free recall rehearsal strategies. J. Gerontol. 1980;35:550–558. doi: 10.1093/geronj/35.4.550. [DOI] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Heckers S, Wagner AD, Schacter DL, Alpert NM, Fischman AJ, Rauch SL. Prefrontal regions supporting spontaneous and directed application of verbal learning strategies: evidence from PET. Brain. 2001;124:219–231. doi: 10.1093/brain/124.1.219. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Murphy MD, Sanders RE. Training older adult free recall rehearsal strategies. J. Gerontol. 1981;36:329–337. doi: 10.1093/geronj/36.3.329. [DOI] [PubMed] [Google Scholar]
- Shaughnessy JJ. Memory monitoring accuracy and modification of rehearsal strategies. J. Verbal Learn. Verbal Behav. 1981;20:216–230. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms, and commentary. Oxford University Press; New York, NY: 1991. [Google Scholar]
- Tulving E. Memory and consciousness. Can. Psychol. 1985;26:1–12. [Google Scholar]
- Verhaeghen P, Marcoen A. Production deficiency hypothesis revisited: adult age differences in strategy use as a function of processing resources. Cognition. 1994;1:323–338. [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Weist RM. The role of rehearsal: recopy or reconstruct. J. Verbal Learn. Verbal Behav. 1972;11:440–450. [Google Scholar]
- Wilson M. MRC psycholinguistic database: machine-usable dictionary, version 2.00. Behav. Res. Methods Instrum. Comput. 1988;20:6–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.