Abstract
Circulating vitamin B6 levels have been found to be inversely associated with lung cancer. Most studies have focused on the B6 form pyridoxal 5′-phosphate (PLP), a direct biomarker influenced by inflammation and other factors. Using a functional B6 marker allows further investigation of the potential role of vitamin B6 status in the pathogenesis of lung cancer. We prospectively evaluated the association of the functional marker of vitamin B6 status, the 3-hydroxykynurenine:xanthurenic acid ratio (HK:XA), with risk of lung cancer in a nested case-control study consisting of 5,364 matched case control pairs from the Lung Cancer Cohort Consortium (LC3). We used conditional logistic regression to evaluate the association between HK:XA and lung cancer, and random effect models to combine results from different cohorts and regions. High levels of HK:XA, indicating impaired functional B6 status, were associated with an increased risk of lung cancer, the odds ratio comparing the fourth and the first quartiles (OR 4th vs 1st) was 1.25 [95% confidence interval, 1.10-1.41]. Stratified analyses indicated that this association was primarily driven by cases diagnosed with squamous cell carcinoma. Notably, the risk associated with HK:XA was approximately 50% higher in groups with a high relative frequency of squamous cell carcinoma, i.e. men, former and current smokers. This risk of squamous cell carcinoma was present in both men and women regardless of smoking status.
Keywords: Pyridoxal 5′-phosphate, Functional vitamin B6 marker, 3-hydroxykynurenine:xanthurenic acid, Lung cancer cohort consortium
Introduction
Lung cancer is the most common cause of cancer related death, contributing to almost 20% of all cancer deaths worldwide1. The four major histological types of lung cancer are adenocarcinomas, squamous cell carcinomas, large cell carcinomas, and small cell carcinomas. The most important risk factor for lung cancer is smoking, but the strength of the association depends on the type of lung cancer2. Some lung cancer types like small cell and squamous cell carcinomas occur almost exclusively due to smoking, while others, like adenocarcinomas, also occur frequently in non-smokers2.
Vitamin B6 may play a role in carcinogenesis, since it is involved in DNA synthesis, methylation, and repair3, chromosomal stability4 and oxidative stress5. Indeed, circulating B6 measured as pyridoxal 5′-phosphate (PLP) was found to be inversely associated with lung cancer risk in two earlier case control studies, nested in prospective cohorts6,7, but in a recent analysis within the Lung Cancer Cohort Consortium (LC3), vitamin B6 was found to be only marginally associated with cancer risk in former and current smoking men8.
However, circulating levels of the vitamin B6 measure used in these papers, the widely used PLP, are influenced by factors other than vitamin B6 status. These factors include inflammation, alkaline phosphatase activity, low serum albumin and renal function9, and reduce the usefulness of PLP as a marker of vitamin B6 status.
A recently established functional marker of vitamin B6 status is the ratio of circulating levels of two metabolites in the kynurenine pathway of tryptophan metabolism, 3-hydroxykynurenine (HK) and xanthurenic acid (XA), i.e. HK:XA [4]. The conversion of HK to XA is catalyzed by the PLP-dependent enzyme kynurenine aminotransferase, while the formation of HK does not require PLP10. The substrate-product ratio HK:XA has been shown to increase in B6 deficient individuals and reduced to normal levels after supplementation with B610.
Given the drawbacks of PLP as a marker of vitamin B6 status, the aim of the present study was to use the functional vitamin B6 marker HK:XA to further investigate the role of vitamin B6 status as a predictor of lung cancer risk. The study used data from over 5,000 cases-controls pairs from the Lung Cancer Cohort Consortium (LC3), nested within 20 prospective cohorts from the USA, Europe, Asia and Australia.
Methods
Study population
All prospective cohort studies within the National Cancer Institute (NCI) Cancer Consortium were invited to participate in the study. Twenty cohorts, from USA (11 cohorts), Europe (total of 4 cohorts from Norway, Sweden, and Finland), Asia (4 cohorts consisting of Chinese populations residing in Shanghai and Singapore) and Australia (1 cohort), fulfilled the inclusion criteria (having cryopreserved baseline plasma or serum samples, and being members of the US National Cancer Institute (NCI) Cohort Consortium in 2009) and accepted to participate. Details on design of the cohorts and their follow-up procedures have been previously published8.
Selection of cases and controls
Lung cancer cases were defined on the basis of the International Classification of Diseases for Oncology, Second Edition (ICD-O-2) and included invasive cancers coded as C34.0-C34-9. From the 11,399 incident lung cancer cases with pre-diagnostic blood samples, 5,545 cases were selected by oversampling never and former smoking cases. For each case, one control was randomly chosen from risk-sets consisting of all cohort members alive and free of cancer (except non-melanoma skin cancer) at the time of diagnosis of the index case. Matching criteria were cohort, sex, date of blood collection, and date of birth. Controls were also matched by smoking status at time of blood collection in 5 categories; never smokers, short and long term quitters among former smokers (<10 years, ≥10 years since quitting), and light and heavy smokers among current smokers (< 15, ≥15 cigarettes per day). In total, 5,364 lung cancer case-control pairs were eligible for inclusion after excluding cases who were not correctly matched on smoking status (n=124), who had insufficient plasma sample volume for analysis of biomarkers (n=42), or had a revised date of diagnosis prior to blood draw (n=13)8.
Biochemical analyses
Analysis of all serum or plasma samples was performed in the Bevital A/S laboratory (http://www.bevital.no) in Bergen, Norway. Concentrations of HK, XA, PLP and cotinine, a marker of recent nicotine exposure11 were determined using a liquid chromatography–tandem MS assay12, and C-reactive protein (CRP) was analysed by immuno-MALDI-MS13 in batches of 86 samples. Quality control procedures included 6 calibration plasma, 2 control plasma, and 1 blank sample (water) in each batch. All blood samples were stored at −80°C or lower until analysis and cases and their matched controls were analyzed together within the same batches in random order, with laboratory staff blinded to case-control status. Further details on the biochemical analyses have been published elsewere8.
Statistical analysis
We used conditional logistic regression (conditioning on individual case sets) to calculate the odds ratios (OR) with 95% confidence intervals (CI) for lung cancer according to levels of HK:XA. The analysis was adjusted for smoking intensity using quartiles of cotinine concentrations based on the distribution of cotinine among current smokers. We performed analyses within each cohort, comparing the fourth to the first quartile (OR 4th vs 1st) of HK:XA. Results were combined for each region (United States [USA], Europe, Asia, Australia), and for the overall study population by using random effects models. Heterogeneity across subgroups was quantitatively assessed by the Q-test and I2 index14.
We further performed stratified analyses for sex, smoking category (never, former, and current smokers), histology of lung cancer (by HK:XA tertiles), and time between blood sample collection and diagnosis. Due to the large differences in vitamin status between regions15, quartiles (or tertiles) of concentrations for each biomarker were based on the distribution among controls by region. We additionally used conditional logistic regression for calculating the odds ratio for lung cancer across quartiles by region and for the total population, using the first quartile as reference. Quartiles were included as a continuous variable to calculate p for trend.
In supplementary analysis stratified by histology in addition to smoking status or sex we included HK:XA as a continuous variable, using the base-2 logarithm (log2) of the biomarker in a conditional logistic regression model. Estimates from this model may be interpreted as the relative risk associated with a doubling in circulating biomarker concentration. Partial Spearman correlations adjusted for age and sex were used to describe the association between HK:XA and PLP, and both biomarkers with cotinine. All statistical analyses were conducted using R 3.2.2 for Macintosh16. The package “survival”17 was used for conditional logistic regression, and package “metafor” for forestplots18.
Results
Study population
The final study population included 5,364 lung cancer cases and 5,364 matched controls, with a median age of 62 years at blood sample collection (Table 1). Median time between blood draw and lung cancer diagnosis was 6.3 years. Of the total study population, 46% of the participants were women. At baseline, nearly half of the participants were current smokers, and one fourth were former, and never smokers, respectively (Table 1). Due to different inclusion criteria in the original cohorts, five cohorts (Health Professionals Follow-up Study, Physicians Health Study, ATBC, The Shanghai Cohort Study and The Shanghai Mens’ Health Study) included only men, and five cohorts (WHI, NYUWHS, WHS, NHS and SWHS) only women (Figure 1). The prevalence of smoking also differed substaintially between cohorts (Figure 1).
Table 1.
Baseline and clinical characteristics of study participants overall and according to region1
| Overall
|
Asian cohorts
|
Australian cohort
|
European cohorts
|
USA cohorts
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls (n=5364) |
Cases (n=5364) |
Controls (n=1775) |
Cases (n=1775) |
Controls (n=354) |
Cases (n=354) |
Controls (n=835) |
Cases (n=835) |
Controls (n=2400) |
Cases (n=2400) |
|
| Characteristics | ||||||||||
| Age2 (years) | 62 (47–75) | 62 (47–75) | 62 (46–74) | 62 (46–74) | 61 (45–67) | 61 (45–67) | 60 (45–71) | 60 (45–71) | 64 (48–78) | 64 (48–78) |
| Sex | ||||||||||
| Men | 2908 (54%) | 2908 (54%) | 1229 (69%) | 1229 (69%) | 213 (60%) | 213 (60%) | 475 (57%) | 475 (57%) | 991 (41%) | 991 (41%) |
| Women | 2456 (46%) | 2456 (46%) | 546 (31%) | 546 (31%) | 141 (40%) | 141 (40%) | 360 (43%) | 360 (43%) | 1409 (59%) | 1409 (59%) |
| Smoker | ||||||||||
| Never | 1327 (25%) | 1327 (25%) | 602 (34%) | 602 (34%) | 49 (14%) | 49 (14%) | 107 (13%) | 107 (13%) | 569 (24%) | 569 (24%) |
| Former | 1518 (28%) | 1518 (28%) | 176 (10%) | 176 (10%) | 145 (41%) | 145 (41%) | 190 (23%) | 190 (23%) | 1007 (42%) | 1007 (42%) |
| Current | 2519 (47%) | 2519 (47%) | 997 (56%) | 997 (56%) | 160 (45%) | 160 (45%) | 538 (64%) | 538 (64%) | 824 (34%) | 824 (34%) |
| Biomarkers | ||||||||||
| HK:XA | 2.98 (1.39–7.70) | 3.13 (1.44–8.49) | 3.01 (1.52–7.09) | 3.10 (1.58–7.98) | 2.88 (1.45–6.68) | 3.00 (1.34–7.55) | 3.08 (1.58–7.08) | 3.28(1.64–9.13) | 2.93 (1.27–8.24) | 3.13 (1.29–8.98) |
| HK (nmol/L) | 36.6 (20.3–70.6) | 37.1 (20.1–74.5) | 38.7 (21.9–81.6) | 39.6 (22.4 –85.4) | 36.0 (22.1–65.8) | 37.8 (21.3–69.3) | 37.2 (21.6–63.9) | 38.3 (23.1–67.2) | 34.8 (18.9–65.2) | 34.7 (18.2–66.2) |
| XA (nmol/L) | 12.4 (4.6–29.1) | 11.9 (4.3–28.7) | 13.5 (5.4–29.9) | 13.0 (5.1 –29.2) | 12.4 (4.9–26.5) | 12.9 (5.2–29.4) | 11.9 (5.1–26.5) | 11.4 (4.5–27.1) | 11.8 (4.2–29.4) | 11.1 (3.9–29.1) |
| PLP (nmol/L) | 37.1 (13.9–197) | 35.1 (12.5–204) | 30.8 (12.3–118) | 28.9 (11.0–114) | 31.3 (14.3–110) | 31.3 (14.2–207) | 30.9 (13.1–101) | 28.1 (12.5–104) | 49.9 (16.4–271) | 47.6 (15.2–266) |
| Clinical characteristics | ||||||||||
| Age at diagnosis (years) | 69.8 (53.6–82.0) | 69 (52–80) | 70 (56–78) | 68 (53–81) | 70 (55–83) | |||||
| Time to diagnosis3 (years) | 6.3 (1.0–16.0) | 5.8 (0.7–16.5) | 9.7 (1.3–16.2) | 10.0 (1.8–16.1) | 5.2 (1–15.5) | |||||
| Histology | ||||||||||
| Large cell carcinoma | 174 (3%) | 16 (1%) | 31 (9%) | 15 (2%) | 112 (5%) | |||||
| Small cell carcinoma | 492 (9%) | 99 (6%) | 47 (13%) | 103 (12%) | 245 (10%) | |||||
| Squamous cell carcinoma | 836 (16%) | 319 (18%) | 67 (19%) | 162 (19%) | 291 (12%) | |||||
| Adenocarcinoma | 2056 (39%) | 615 (35%) | 153 (43%) | 260 (31%) | 1034 (43%) | |||||
| Missing/Unknown | 1806 (34%) | 726 (41%) | 56 (16%) | 295 (19%) | 735 (31%) | |||||
Characteristics are presented as n (%) for discrete variables and median (5th, 95th percentile) for continuous variables
At blood collection
Time from blood draw to diagnosis
HK:XA, 3-hydroxykynurenine:xanthurenic acid; PLP, pyridoxal 5′-phosphate
Figure 1.
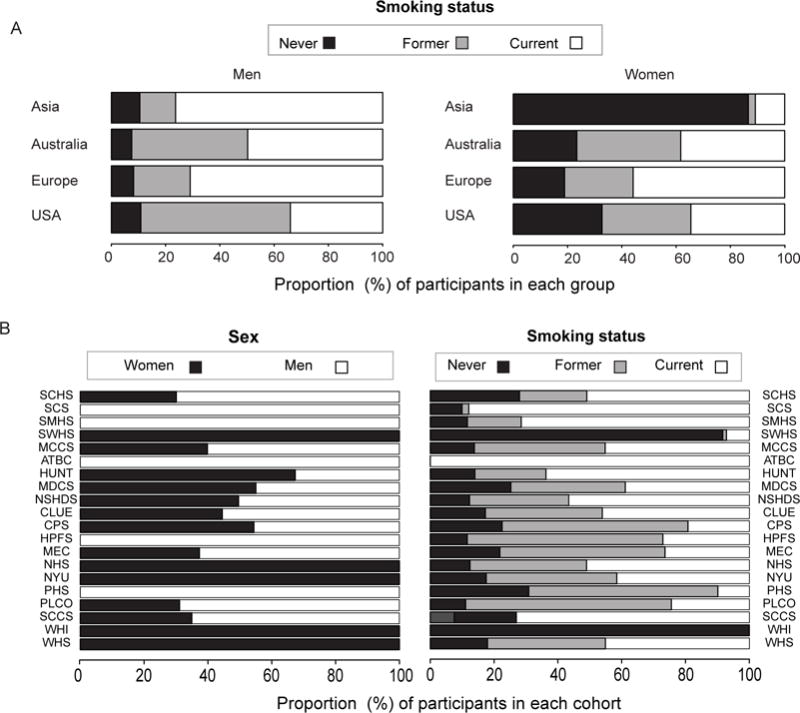
Panel A. Distribution of smoking status stratified by sex in the different regions.Panel B. Distribution of smoking status and sex in the different cohorts.ATBC, The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CLUE, The Campaign Against Cancer and Stroke (CLUE I) and the Campaign Against Cancer and Heart Disease (CLUE II); CPS-II, The American Cancer Society Cancer Prevention Study-II Nutrition Cohort; HPFS, Health Professionals Follow-up Study; HUNT, The Nord-Trøndelag Health Study; MCCS, The Melbourne Collaborative Cohort Study; MDCS, The Malmö Diet and Cancer Study; MEC, The Multiethnic Cohort; NHS, The Nurses’ Health Study; NSHDS, The Northern Sweden Health and Disease Study Cohort; NYUWHS, The New York University Women’s Health Study; PHS, Physicians’ Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; SCCS, The Southern Community Cohort Study; SCHS, The Singapore Chinese Health Study; SCS, The Shanghai Cohort Study; SMHS, The Shanghai Men’s Health Study; SWHS, The Shanghai Women’s Health Study; WHI, The Women’s Health Initiative; WHS, Women’s Health Study.
Determinants of HK:XA within the LC3
HK:XA varied somewhat across regions, (median values ranging from 2.88 to 3.28 among controls) with the lowest level among Australian controls and the highest among Europeans. Larger variations were observed for plasma PLP, with the highest concentrations in the controls from US cohorts (median 49.9 nmol/L) and the lowest concentrations among the European cases, at 28.1 nmol/L. We observed an inverse relation between HK:XA and PLP (Spearman rho =−0.37), while smoking was essentially not associated with HK:XA (rho =0.11), but was inversely related to plasma PLP (rho =−0.30) (all p<0.001).
HK:XA and lung cancer
Random effects models were used to investigate the relation of HK:XA with risk of lung cancer across geographic regions because the heterogeneity by cohort varied significantly across the geographic regions (Supplemental Table S1). Overall, high levels of HK:XA (4th vs. 1st quartile) were associated with a 25% increased risk for lung cancer (Figure 2). However, results differed across regions with positive associations observed in Europe, with an odds ratio comparing the fourth and the first quartiles (95% confidence interval) of 1.43 (1.06, 1.95), and the USA 1.31 (1.05, 1.62), but no association in Asia or Australia (Figure 2). Results were similar when using quartiles based on the distribution of each region, instead of cohort specific cut-offs (Supplemental Table S2).
Figure 2.
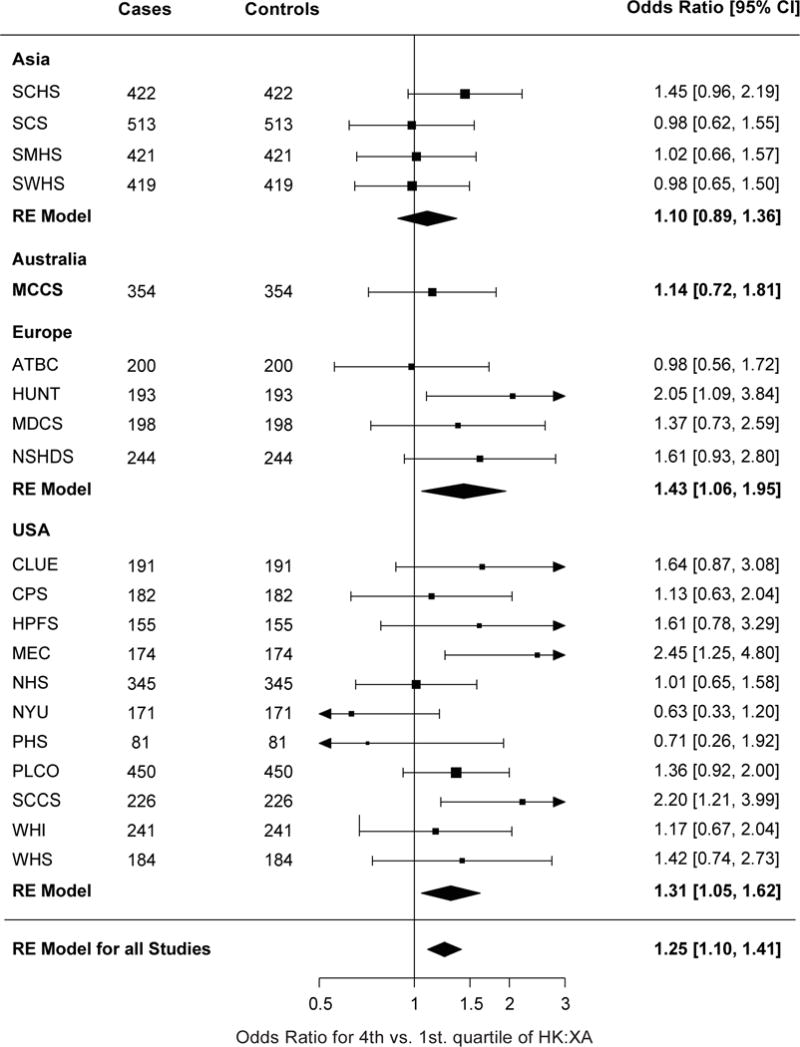
Forestplot showing odds ratios for lung cancer comparing the fourth to the first quartile of HK:XAConditional logistic regression was performed for each cohort and was adjusted for smoking intensity using quartiles of cotinine among current smokers. Cases and controls were matched on age, sex, and smoking status. Results were combined using random effect models for each region and in all studies combined. HK:XA, 3-hydroxykynurenine:xanthurenic acid.
The weakest associations were observed in cohorts that included only women. When those cohorts (The Women’s Health Intiative, The New York University Women’s Health Study, Women’s Health Study and Nurses Health Study) were excluded, the association of HK:XA with risk of lung cancer in the USA was similar, 1.41 (1.15, 1.46), to that seen in Europe. Additional adjustment for CRP, a marker for systemic inflammation, did not attenuate the risk association for HK:XA (data not shown).
Analyses stratified by sex and smoking
In analysis stratified by sex the overall association between HK:XA and lung cancer risk was primarily seen among men, with a 50% increased risk of lung cancer when comparing fourth vs. first quartile (Figure 3). No significant association was observed for women, (p heterogeneity = 0.01 and I2 = 62.4%, Supplemental Table S3). Smoking habits differed between sexes, with the proportion of never smokers much higher among women (Figure 1). A similar effect modification was present for smoking categories, with the association between HK:XA and lung cancer limited to current and former smokers (p for heterogeneity =0.18, I2 = 30.1%, Supplemental Table S4) (Figure 4).
Figure 3.
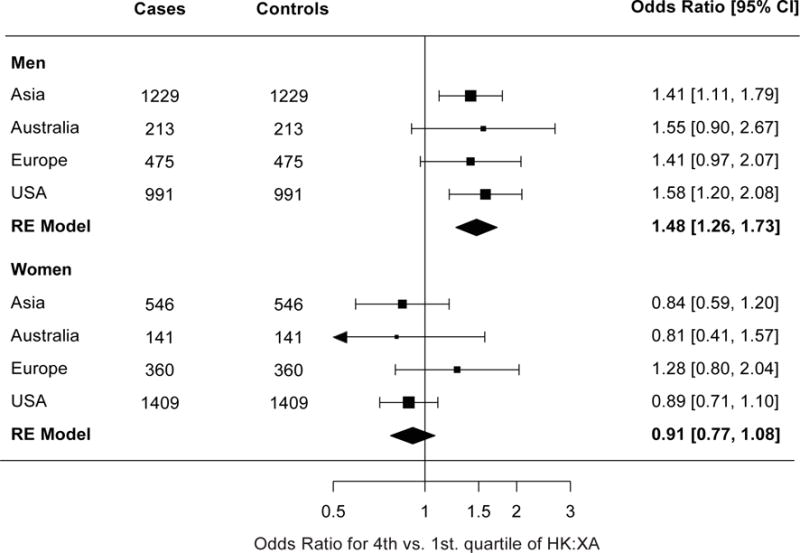
Forestplot showing odds ratios for lung cancer comparing the fourth to the first quartile of HK:XA in the different regions, stratified by gender. Conditional logistic regression was performed for each region and was adjusted for smoking intensity using quartiles of cotinine among current smokers. Cases and controls were matched on age, sex, and smoking status. Results were combined using random effect models. HK:XA, 3-hydroxykynurenine:xanthurenic acid.
Figure 4.
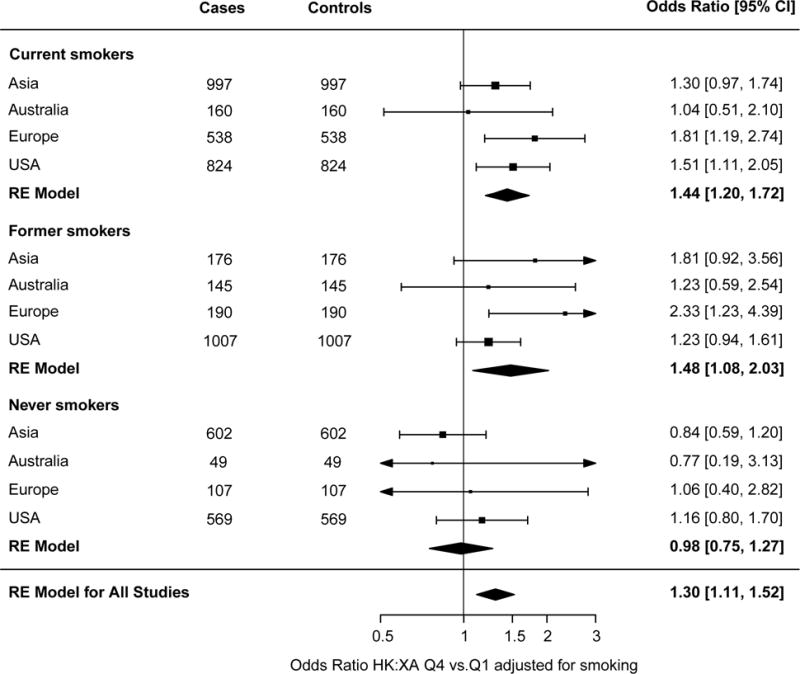
Forestplot showing odds ratios for lung cancer comparing the fourth to the first quartile of HK:XA in the different regions, stratified by smoking status. Conditional logistic regression was performed for each subgroup and among current smokers (adjusted for smoking intensity using quartiles of cotinine among current smokers). Cases and controls were matched for age, sex, and smoking status. Results were combined using random effect models. HK:XA, 3-hydroxykynurenine:xanthurenic acid.
Histology of lung cancer
Histology of lung cancer differed according to smoking status, with squamous cell carcinoma being more common among current and former smokers (28% and 20% respectively, compared to 6% among never smokers) and in men compared to women (29% vs. 10%). In analysis according to histology of lung cancer in the overall population, HK:XA was related to an increased risk for squamous cell carcinoma OR (95%CI) 1.42 (1.10, 1.82) for 3rd vs. 1st tertile, but not with other histological types (data not shown).
This association with HK:XA and squamous cell carcinoma was consistently present in subgroup analysis by both sex and smoking status. Specifically, for a continuous log2 model, representing a doubling of HK:XA concentrations, the OR (95%CI) was 1.20 (1.02, 1.41) in men, 1.59 (1.20, 2.10) in women (Supplemental Figure S2). In current smokers the OR (95% CI) was 1.22 (1.02, 1.46), in former smokers 1.37 (1.08, 1.73), and in never smokers 1.59 (0.90, 2.80), even though in this last group the confidence interval was quite wide due to the low number of cases (Supplemental figure S1).
Time to diagnosis
In analysis stratified by time to diagnosis, the association was limited to participants who were diagnosed with lung cancer within 36 months from blood draw, ORlog2 (95%CI) 1.43 (1.27, 1.61) for a doubling in the concentration of HK:XA. No significant association between HK:XA and lung cancer risk was observed for those with a longer time between blood draw and diagnosis (p for heterogeneity <0.001).
Discussion
Main findings
High levels of HK:XA, indicating an impaired functional vitamin B6 status, were associated with an increased risk of lung cancer. In stratified analysis the risk of lung cancer was approximately 50% higher for those in the highest category of HK:XA in men, and in former and current smokers, but not significant in women or never smokers. In analysis stratified by histology HK:XA was associated with an increased risk of squamous cell carcinoma, but not other histological types. When histopathology subtype of lung cancer was considered, a consistent association was found for squamous cell carcinoma regardless sex and smoking status. The lack of association of HK:XA with overall lung cancer among women and never smokers could be at least partly attributed to the low number of cases of squamous cell carcinoma in those strata of the present study population.
Comparison with previous findings
Overall, our findings are in agreement with published results on the B6 vitamer PLP and cancer risk19, even though stronger inverse associations were noted in relation to lung cancer in the EPIC6 and ATBC7 studies. Concordant with the current study, we recently observed an inverse association of PLP with lung cancer risk in LC3, an association that was primarily confined to former and current smoking men8.
We observed a positive association between HK:XA and risk of squamous cell carcinoma, but no significant association with other histological types of lung cancer. This observation is also in line with a previous observation of an inverse association between plasma PLP and risk of cancer primarily classified as squamous cell carcinoma8.
In EPIC an inverse association of PLP on lung cancer was also observed in never smokers, but the number of cases that were never smokers was low (n=96)6, and this results should be viewed with caution.
In a previous cohort study where PLP and HK:XA were simultaneously assessed as predictors of cancer no clear association was found for any of the two markers. However, this study had limited statistical power due to the small number of cases (ncases=85)20.
HK:XA as a marker of vitamin B6 status and predictor of lung cancer
There are consistent reports on plasma PLP as a predictor of cancer in the lungs6, 7 and other organs19. Plasma PLP is the most commonly used marker of vitamin B6 status, but plasma PLP concentrations are reduced by several factors linked to lung cancer carcinogenesis or progression, such as smoking21, inflammation measured as CRP22-24, and increased level of alkaline phosphatase25. On the other hand, inflammation and elevated alkaline phosphatase (ALP) are not associated with impaired vitamin B6 availability in tissues9.
Smoking is associated with lower levels of PLP, and vitamin B6 status gradually improves over years after smoking cessation26. In contrast, smoking shows no or a weak association with the HK:XA ratio10, an observation confirmed in the present study. In the current study, cases and controls were matched for smoking status and we additionally adjusted for smoking intensity, using circulating cotinine concentrations. We cannot exclude residual confounding by smoking, but since the association between HK:XA and lung cancer was also present in former smokers, confounding by smoking is unlikely.
CRP is inversely associated with plasma PLP27, 28 but shows a weak positive association with HK:XA10. After additional adjustment for CRP, the risk estimates of HK:XA and lung cancer remained essentially the same, suggesting no or minor confounding from inflammation. Elevated ALP may reduce PLP through conversion to pyridoxal (PL)9, but HK and XA are not substrates for ALP, and one would not expect any direct effects from ALP on the plasma levels of these metabolites.
Similarly to the findings on PLP in the LC3 study8, the association between HK:XA and lung cancer was stronger among participants with a short time between blood draw and diagnoses.
Therefore, it is possible that the observed association between HK:XA and risk may reflect impaired vitamin B6 status due to pre-clinical changes in lung cancer.
Strengths and limitations of the study
The present study is based on a an unprecedented sample of 5,364 pre-diagnostic blood samples from lung cancer cases with comparable control samples recruited in 20 prospective cohorts from around the world. The prospective study design minimizes the risk of reverse causality and selection bias. The use of a centralized laboratory with a stringent quality control protocols and cases and matched controls analyzed together minimizes any technical differential bias, and an overrepresentation of never and former smokers provided adequate power for stratified analysis. By using a functional marker that is largely independent on factors that are related to circulating PLP, we found a clear inverse relation of vitamin B6 status with risk of lung cancer.
There was only one blood sample available for measurement of biomarkers for each participant, so the association between HK:XA and lung cancer may be attenuated due to regression dilution bias. It is possible that depending on the time of the blood draw and the length of study follow-up, the single measurement may not represent the exposure period most relevant for lung cancer development. Lastly, information on the histology of lung cancer was missing for 34 % of the participants.
Conclusions
Our findings provide evidence for an inverse association of functional vitamin B6 status and risk of lung cancer, especially squamous cell carcinoma. This expands our understanding beyond what can be concluded from the modest relation observed for the direct vitamin B6 marker PLP8, circulating levels of which is influenced by factors other than vitamin B6 status, in this same study. It is recommended that future studies strive for a sample size large enough to provide the power necessary for analysis stratified by duration of follow-up, smoking and histology given the potential differences of the role of vitamin B6 in pathogenesis and progression of different histological cancer types.
Supplementary Material
Novelty and Impact.
Low vitamin B6 status, assessed by circulating pyridoxal 5′-phosphate (PLP), has been associated with increased risk of lung cancer. However, factors other than vitamin B6 status may contribute to lower PLP, possibly confounding its association with lung cancer. In the present study we demonstrated, by using a novel functional biomarker of B6 status that impaired functional vitamin B6 status was associated with increased risk of lung cancer, especially squamous cell carcinoma.
Acknowledgments
The Lung Cancer Cohort Consortium (LC3) was supported by NIH/NCI grant 1U01CA155340-01 and Australian National Health and Medical Research Committee grant 1050198. SWHS was/is supported by R37 CA070867 and UM1 CA182910, SMHS by R01 CA082729 and UM1 CA173640 from the U.S. National Cancer Institute. SCCS is supported by R01 CA092447 and U01 CA202979 from the U.S. National Cancer Institute. The Multiethnic Cohort Study was funded in part by grant U01 CA164973. The ATBC Study is supported by the Intramural Research Program of the U.S. National Cancer Institute, National Institutes of Health, and by U.S. Public Health Service contract HHSN261201500005C from the National Cancer Institute, Department of Health and Human Services. CLUE thank the participants and staff for their contributions, as well as the Maryland Cancer Registry, Center for Cancer Surveillance and Control, Department of Health and Mental Hygiene, 201 W. Preston Street, Room 400, Baltimore, MD 21201, http://phpa.dhmh.maryland.gov/cancer, 410-767-4055. CLUE acknowledge the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries of the Centers for Disease Control and Prevention for the funds that support the collection and availability of the cancer registry data. The Prostate Lung Colorectal Ovarian Cancer Screening Trial (PLCO) is supported by contracts from the Division of Cancer Prevention and intramural research funding from the Division of Cancer Epidemiology and Genetics, National Cancer Institute, U.S. National Institutes of Health (NIH), Department of Health and Human Services (DHHS). PLCO was supported by the National Institutes of Health (NIH) grants, UM1CA167552, UM1CA186107, P01CA87969, and R01CA49449. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. CR is supported by CRUK (C18281/A19169) and the Medical Research Council Integrative Epidemiology Unit at the University of Bristol with funds from the MRC (MC_UU_12013/2) and the University of Bristol. The funding organizations had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript.
Abbreviations
- PLP
pyridoxal 5′-phosphate
- HK:XA
3-hydroxykynurenine:xanthurenic acid
- CI
confidence interval
- EPIC
European Prospective Investigation into Cancer and Nutrition
- ATBC
Alpha-Tocopherol, Beta-Carotene Cancer Prevention
- OR
odds ratio
- LC3
Lung Cancer Cohort Consortium
Footnotes
Competing interests
LMB is an employee of Genetech Inc. as of September 16.
References
- 1.Ferlay J, S I, Ervik M, Dikshit R, et al. Cancer Incidence and Mortality Worldwide: IARC CancerBase No 11. GLOBOCAN 2012 v1.0. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer. 2001;31:139–48. doi: 10.1016/s0169-5002(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 3.Ames BN. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat Res. 2001;475:7–20. doi: 10.1016/s0027-5107(01)00070-7. [DOI] [PubMed] [Google Scholar]
- 4.Ames BN, Wakimoto P. Are vitamin and mineral deficiencies a major cancer risk? Nat Rev Cancer. 2002;2:694–704. doi: 10.1038/nrc886. [DOI] [PubMed] [Google Scholar]
- 5.Wondrak GT, Jacobson EL. Vitamin B6: Beyond Coenzyme Functions. In: Stanger O, editor. Water Soluble Vitamins: Clinical Research and Future Applicationed. Dordrecht: Springer Netherlands; 2012. pp. 291–300. [DOI] [PubMed] [Google Scholar]
- 6.Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377–85. doi: 10.1001/jama.2010.808. [DOI] [PubMed] [Google Scholar]
- 7.Hartman TJ, Woodson K, Stolzenberg-Solomon R, et al. Association of the B-vitamins pyridoxal 5′-phosphate (B(6)), B(12), and folate with lung cancer risk in older men. Am J Epidemiol. 2001;153:688–94. doi: 10.1093/aje/153.7.688. [DOI] [PubMed] [Google Scholar]
- 8.Fanidi A, Muller D, Yuan JM, et al. Circulating Folate, Vitamin B6 and Methionine in relation to Lung Cancer Risk in the Lung Cancer Cohort Consortium (LC3) Journal of the national cancer institute. 2017 doi: 10.1093/jnci/djx119. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueland PM, Ulvik A, Rios-Avila L, et al. Direct and Functional Biomarkers of Vitamin B6 Status. Annu Rev Nutr. 2015;35:33–70. doi: 10.1146/annurev-nutr-071714-034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulvik A, Theofylaktopoulou D, Midttun O, et al. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am J Clin Nutr. 2013;98:934–40. doi: 10.3945/ajcn.113.064998. [DOI] [PubMed] [Google Scholar]
- 11.Seccareccia F, Zuccaro P, Pacifici R, et al. Serum Cotinine as a Marker of Environmental Tobacco Smoke Exposure in Epidemiological Studies: The Experience of the MATISS Project. Eur J Epidemiol. 2003;18:487–92. doi: 10.1023/a:1024672522802. [DOI] [PubMed] [Google Scholar]
- 12.Midttun Ø, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2009;23:1371–9. doi: 10.1002/rcm.4013. [DOI] [PubMed] [Google Scholar]
- 13.Meyer K, Ueland PM. Targeted Quantification of C-Reactive Protein and Cystatin C and Its Variants by Immuno-MALDI-MS. Anal Chem. 2014;86:5807–14. doi: 10.1021/ac500704y. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Midttun O, Theofylaktopoulou D, McCann A, et al. Circulating concentrations of biomarkers and metabolites related to vitamin status, one-carbon and the kynurenine pathways in US, Nordic, Asian, and Australian populations. Am J Clin Nutr. 2017;105:1314–26. doi: 10.3945/ajcn.116.151241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2105. [Google Scholar]
- 17.T T. A Package for Survival Analysis in S_. version 2.38. 2015 <URL: https://CRAN.R-project.org/package=survival>.
- 18.Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- 19.Mocellin S, Briarava M, Pilati P. Vitamin B6 and Cancer Risk: A Field Synopsis and Meta-Analysis. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw230. [DOI] [PubMed] [Google Scholar]
- 20.Zuo H, Ueland PM, Eussen SJ, et al. Markers of vitamin B6 status and metabolism as predictors of incident cancer: the Hordaland Health Study. Int J Cancer. 2015;136:2932–9. doi: 10.1002/ijc.29345. [DOI] [PubMed] [Google Scholar]
- 21.Doll R, Hill AB. Lung Cancer and Other Causes of Death in Relation to Smoking. Br Med J. 1956;2:1071–81. doi: 10.1136/bmj.2.5001.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiels MS, Katki HA, Hildesheim A, et al. Circulating Inflammation Markers, Risk of Lung Cancer, and Utility for Risk Stratification. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48:155–70. doi: 10.3109/10408363.2011.599831. [DOI] [PubMed] [Google Scholar]
- 24.Bittoni MA, Focht BC, Clinton SK, et al. Prospective evaluation of C-reactive protein, smoking and lung cancer death in the Third National Health and Nutrition Examination Survey. Int J Oncol. 2015;47:1537–44. doi: 10.3892/ijo.2015.3141. [DOI] [PubMed] [Google Scholar]
- 25.Buccheri G, Ferrigno D. Prognostic factors in lung cancer: tables and comments. Eur Respir J. 1994;7:1350–64. doi: 10.1183/09031936.94.07071350. [DOI] [PubMed] [Google Scholar]
- 26.Ulvik A, Ebbing M, Hustad S, et al. Long- and Short-term Effects of Tobacco Smoking on Circulating Concentrations of B Vitamins. Clin Chem. 2010;56:755–63. doi: 10.1373/clinchem.2009.137513. [DOI] [PubMed] [Google Scholar]
- 27.Sakakeeny L, Roubenoff R, Obin M, et al. Plasma Pyridoxal-5-Phosphate Is Inversely Associated with Systemic Markers of Inflammation in a Population of U.S. Adults. J Nutr. 2012;142:1280–5. doi: 10.3945/jn.111.153056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen J, Lai C-Q, Mattei J, et al. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am J Clin Nutr. 2010;91:337–42. doi: 10.3945/ajcn.2009.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


