Abstract
Exposure at the World Trade Center (WTC) terrorist collapse site on September 11, 2001 has been associated with increased cancer risk, though observational studies have identified very few cases of head and neck cancer (HNC) in exposed individuals. Eighty seven patients were identified who presented to our institution with HNC diagnosed from 2002–2017 who reported WTC exposure. The annual number and proportion of WTC-exposed HNC patients has been steadily increasing since 2002, with most cancers developing >10 years following the event. Furthermore, WTC-exposed patients with human papillomavirus (HPV)-positive OPC experienced significantly inferior outcomes compared to non-WTC exposed patients with HPV+ OPC (disease free survival 80.1% vs 65.6% at 4 years, p=0.04). This single institution study cannot establish evidence of exposure-mediated causation but higher recurrence rates in the WTC-exposed HPV+ OPC population suggest a treatment refractory tumor biology and possible exposure synergism with HPV-mediated oncogenesis.
Introduction
In the aftermath of the World Trade Center (WTC) terrorist attacks, over 100,000 people were exposed to respirable toxins resulting in numerous adverse health consequences [1]. The dust cloud that formed following the building collapse contained many known carcinogens [2] and exposure at the collapse site has been linked to an increase in overall cancer risk [1, 3–5]. However, observational studies have identified very few cases of head and neck cancer (HNC) in this population [1, 3, 4], despite direct exposure of the aerodigestive mucosa and possible associations of inhaled toxins and HNC. Importantly, studies have assessed cancers that were diagnosed within 7–10 years of follow-up, despite a high likelihood of longer latency for solid tumor development. Furthermore, treatment outcomes have never been described in cancer patients with WTC exposure. Herein, we describe the oncologic characteristics and disease outcomes of WTC-exposed patients with HNC who have presented to our institution.
Methods
With institutional review board approval, we queried our institutional medical records for all patients diagnosed with HNC from 1/1/2002 to 3/1/2017 (excluding skin, melanoma, lymphoma, and thyroid cancers) who reported exposure at the WTC site on or after 9/11/2001 or involvement in the rescue/cleanup effort. WTC exposure was elicited from documentation in the electronic medical record (EMR) chart. Specifically, HNC patients whose EMR chart contained terms associated with the world trade center (“WTC”, “world trade center”, “9/11”, “ground zero”, etc.) were individually reviewed to document the type of exposure that the patient experienced. In most cases, the nature of the exposure and the patient’s role in the events of 9/11 were clearly documented. 10 patients (12%) did not have sufficient details of exposure to be specifically classified and were listed as “exposure not otherwise specified”.
For patients with oropharyngeal carcinoma (OPC), human papillomavirus (HPV) positivity was defined by positivity of p16 immunohistochemistry and/or HPV in-situ hybridization. p16 IHC was considered positive when strong and diffuse nuclear and cytoplasmic staining was observed in ≥70% of tumor cells [6]. HPV-ISH was performed with a probe for HPV types 16, 18, 31, 33 and 51; HPV-ISH positivity was defined as any positive staining. Cases were defined as HPV/p16- with a negative p16 IHC or HPV-ISH. Cases were defined as HPV unknown if neither p16 IHC nor HPV-ISH information were available. Outcomes for patients with HPV+ OPC and WTC exposure (median follow-up 32.8 months) were compared to cohorts of HPV+ and HPV- OPC patients without WTC exposure (median follow-up 53.0 months) who were treated at our institution with definitive chemoradiation from 2002–2013 [7]. The locoregional control (LRC, defined as freedom from recurrence at site of primary tumor and/or cervical lymph nodes), disease free survival (DFS, defined as survival without any recurrence or death), freedom from distant metastasis (FFDM, defined as any recurrence in an organ outside of the head and neck) and overall survival (OS) were estimated from the time of diagnosis using the Kaplan-Meier method and compared between groups with log-rank testing. Cox regression analysis was used for multivariate analysis. Statistical significance for all analyses was 2-sided and used a 5% significance level (P < .05). Statistical analyses were performed with SPSS (SPSS version 24, IBM).
Results
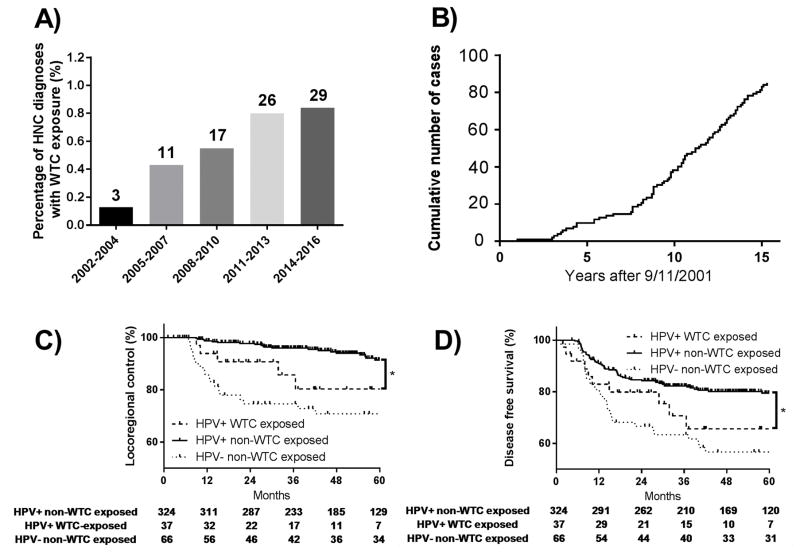
Eighty-seven patients were identified with HNC diagnosed from 2002–2017 who reported WTC exposure (Table). The annual number and proportion of WTC-exposed HNC patients has been increasing since 2002 (Figure 1 panels A,B); the median time from 9/11/2001 to HNC diagnosis was 10.5 years. Among all HNC patients with WTC-exposure, the median follow-up time was 42.1 months and 45.2 months in surviving patients. Among patients with HPV+ OPC, the median follow-up in the non-exposed group was 53.0 months while the median follow-up in the WTC exposed group was 32.8 months.
Table.
Head and Neck Cancers after World Trade Center Exposure: Patient and Tumor Characteristics
| Total Head and Neck Cancers (N=87) | HPV/p16+ oropharyngeal cancers (N=38) | |||
|---|---|---|---|---|
| Gender | ||||
| Male | 83 | 95% | 37 | 97% |
| Female | 4 | 5% | 1 | 3% |
| Age at diagnosis | ||||
| Median | 55 | 58 | ||
| Range | 35–79 | 40–79 | ||
| Smoking | ||||
| 0 pack years | 43 | 49% | 20 | 53% |
| 1–10 pack years | 16 | 18% | 8 | 21% |
| >10 pack years | 28 | 32% | 10 | 26% |
| Primary site | ||||
| Oropharynx | 49 | 56% | 38 | 100% |
| HPV/p16-positive | 38 | 44% | 38 | 100% |
| HPV/p16-negative | 6 | 7% | ||
| HPV/p16 unknown | 5 | 6% | ||
| Nasopharynx | 5 | 6% | ||
| Larynx | 13 | 15% | ||
| Hypopharynx | 2 | 2% | ||
| Oral cavity | 11 | 13% | ||
| Unknown primary | 5 | 6% | ||
| Salivary duct | 1 | 1% | ||
| Sinonasal | 1 | 1% | ||
| Histology | ||||
| Squamous cell carcinoma | 83 | 95% | 38 | 100% |
| Neuroendocrine carcinoma | 1 | 1% | ||
| Adenoid cystic carcinoma | 1 | 1% | ||
| NUT midline carcinoma | 1 | 1% | ||
| Salivary duct carcinoma | 1 | 1% | ||
| AJCC stage | ||||
| I | 12 | 14% | 1 | 3% |
| II | 6 | 7% | 0 | 0% |
| III | 3 | 3% | 4 | 11% |
| IV | 66 | 76% | 33 | 87% |
| M category | ||||
| M0 | 86 | 99% | 38 | 100% |
| M1 | 1 | 1% | 0 | 0% |
| Exposure | ||||
| Occupational exposure | 61 | 70% | 25 | 66% |
| Police officers | 21 | 24% | 6 | 16% |
| Firefighters | 15 | 17% | 9 | 24% |
| Construction workers/electricians | 16 | 18% | 5 | 13% |
| Emergency medical technicians/volunteers | 4 | 5% | 1 | 3% |
| Terrorist task force/government employees | 2 | 2% | 1 | 3% |
| Other occupational exposure | 3 | 3% | 3 | 8% |
| Non-occupational exposure | 26 | 30% | 13 | 34% |
| World trade center occupants | 9 | 10% | 3 | 8% |
| Proximal exposure | 4 | 5% | 3 | 8% |
| Nearby resident/worker | 3 | 3% | 1 | 3% |
| Exposure not otherwise specified | 10 | 12% | 6 | 16% |
| Definitive treatment | ||||
| Surgical resection | 2 | 5% | ||
| Resection followed by adjuvant radiotherapy | 3 | 8% | ||
| Definitive chemoradiation | 29 | 76% | ||
| Induction chemotherapy followed by chemoradiation | 3 | 8% | ||
| Treated at outside institution* | 1 | 3% | ||
Patients treated at an outside institution were not included in the outcomes analysis.
Figure 1. Head and neck cancers in patients with exposure at the World Trade Center (WTC) site.
The number of new head and neck cancer diagnoses following exposure at the WTC as a fraction of the total number of head and neck cancer patients treated at our institution by time period since 2001 are shown in (A) with the number of head and neck cancer diagnoses for each time interval indicated above each bar. The cumulative number of head and neck cancer cases since 2001 is shown in (B). WTC exposed patients with human papillomavirus (HPV) associated oropharyngeal carcinoma demonstrate significantly inferior locoregional control (C) and disease free survival (D) compared to a similar cohort of patients without WTC exposure. Locoregional control and disease free survival were not significantly different between patients with HPV-negative oropharyngeal carcinoma without exposure vs. patients with HPV-positive oropharyngeal carcinoma and exposure at the WTC site.
Thirty eight patients presented with HPV+ OPC, 37 of whom underwent definitive treatment at our center. WTC-exposed patients with HPV+ OPC demonstrated inferior LRC and DFS compared to non-WTC exposed patients with HPV+ OPC (WTC-exposed: 48-month LRC 80.3%, DFS 65.6%; non-exposed: LRC 94.0%, DFS 80.1%, p=0.03 and p=0.04, Figure 1 panels C,D). After adjustment for age, smoking history (>10 pack-years), T-category and N-category, the differences in LRC and DFS between these groups remained (hazard ratio 2.72 (95%CI 1.03–7.19, p=0.04) for LRC and 2.07 (95%CI 1.08–3.96, p=0.03) for DFS). Rates of FFDM and OS were similar in WTC-exposed and non-exposed HPV+ OPC patients (WTC-exposed: 48-month FFDM 83.2%, OS 83.3%; non-exposed: FFDM 86.4%, OS 89.3%, p=0.29 and p=0.86). No significant differences in LRC or DFS were observed between HPV+ WTC-exposed OPC patients and HPV- non-exposed OPC patients (p=0.20 and p=0.38).
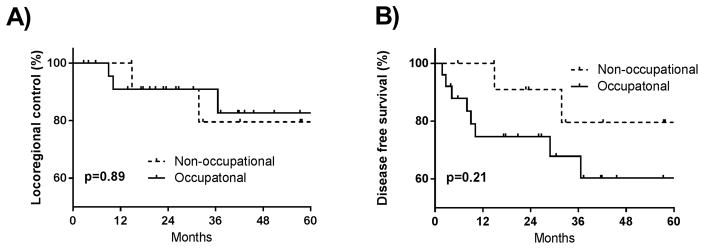
We performed additional comparative analysis of outcomes between patients with HPV+ OPC who experienced occupational (n=25) versus non-occupational exposure (n=13). We found no significant difference in LRC (p=0.89) and a non-significant trend towards inferior DFS in patients with occupational exposure (p=0.21, figure 2).
Figure 2.
Disease outcomes in patients with HPV+ oropharynx cancer according to exposure type. There was no statistically significant difference in terms of (A) locoregional control, (p=0.89) or (B) disease-free survival (p=0.21) in patients with occupational versus non-occupational exposure.
Discussion
In summary, the number of patients presenting to our institution with HNC following exposure at the WTC site has been increasing, with most cancers developing >10 years following September 11, 2001. This may be due in part to aging of the population, increasing incidence of HPV-associated OPC, increased referral since establishment of the WTC health program, or reduction in cost of cancer care under new reimbursement policies for WTC associated illnesses. The limitations of this analysis include a lack of quantified exposure details, non-uniform treatment in the WTC-exposed cohort, and inability to account for other occupational exposures. The single institution nature of the study is also significantly limiting as the population of patients that present to MSKCC may not be representative of the general population. As a tertiary care hospital, the severity of illness of patients at MSKCC may be distinct from patients who present to other centers. Nonetheless, our comparison of outcomes is performed on a group of patients who were similarly treated at our institution which may eliminate some confounding.
The follow-up of the WTC exposed patients in this cohort is significantly shorter than the follow-up of patients without exposure (32.8 vs. 53.0 months) which could potentially affect the outcomes comparisons. However, our analysis demonstrates inferior outcomes in the WTC exposed cohort even with a shorter follow-up period. If any effect of bias were present due to shorter follow-up in the exposed cohort it would be expected to result in fewer events and non-inferior outcomes. Furthermore, for patients with HPV+ OPC, the vast majority of recurrence events occur within the first 24 months following treatment [6], a time period that is well captured in this study.
The mechanism underlying our observation that outcomes are poorer among HPV+ patients with WTC exposure remains to be elucidated and presently remains unknown though there are a number of viable hypotheses. It has become well established that smoking confers a higher risk for development of HPV-associated cancers including cervical cancer and HPV+ HNC [8, 9]. Within HPV+ HNC, it is also noted that environmental exposures, such as cigarette smoking, are associated with poorer outcomes following treatment [6, 10]. Furthermore, air pollutants other than cigarette smoke have also been linked to HPV-associated cervical dysplasia [11]. While the specific compound or compounds within the WTC dust that may have resulted in an increased virulence of cancers in those exposed has not been identified, it is possible that WTC exposure results in worse outcomes analogously to how cigarette smoking is also associated with treatment resistance.
Analysis of the WTC dust did reveal a significantly elevated concentration of polycyclic aromatic hydrocarbons (PAHs) that arose from building material remnants, jet fuel from the airplanes and collapse of the WTC towers [2]. PAHs have been linked to causation of multiple cancer types and through their ability to form DNA damaging adducts, they have been directly associated with mutations in the TP53 gene [12] which are known to confer resistance to therapy and are enriched in HPV+ recurrent/metastatic cases [13]. In this way, DNA damage and mutagenesis conferred by inhaled toxins may synergize with HPV-mediated inhibition of apoptosis and cell cycle disruption to result in formation of particularly aggressive cancers. PAHs have also been found to increased viral load of HPV and are implicated in carcinogenesis of other virally mediated cancers [14, 15].. It will be important to characterize the mutational profile of the tumors in this cohort, as well as other WTC cancer tissue banks, to identify genetic and genomic causes of treatment resistance. While we do not know whether PAHs or another substance are responsible for the possible increase in cancer risk and poorer outcomes that are observed, there are many plausible cooperative mechanisms between toxic environmental exposures and HPV-mediated carcinogenesis that may result in resistance to therapy.
As an additional possibility, the toxic substances found in WTC dust may have adversely affected the immune systems of those exposed. While most adults will experience HPV infection at some point, only a minority will go on to harbor chronic infection and a further minority will develop head and neck cancer. In this way, immunity plays a critical role in the development of HPV-associated cancers in particular. It has recently also become evident that WTC exposure is associated with autoimmune disease and possible immune dysregulation [16]. As a result, it is possible that systemic immune effects of WTC exposure may synergize with HPV mediated carcinogenesis. Additionally, altered immunity and rheumatological disease have been linked to an inferior response to chemoradiation therapy for HPV+ OPC [17].
This study cannot estimate true incidence or establish definitive evidence of exposure-mediated causation; however, higher recurrence rates in the WTC-exposed HPV+ OPC population suggest a potentially more aggressive biology. The limited number of patients did not provide sufficient power to detect differences in outcomes between patients with occupational versus non-occupational exposures though a non-statistically significant trend towards inferior DFS was observed among patients with occupational exposure and HPV+ disease. It will be critical to reevaluate these findings with a larger multi-institutional analysis and more granular details of individual patient exposure. Further studies of toxicology associated with WTC dust, genomic characterization and epidemiological studies with follow-up extending >10 years are needed to better characterize this unique and growing population.
Novelty and Impact statement.
A connection between world trade center (WTC) exposure and head and neck cancer (HNC) has never been described. We have identified 87 HNC patients who have reported exposure to the toxic dust clouds at the WTC site following the terrorist attacks on 9/11/01. The annual number of WTC-exposed HNC patients has been steadily increasing, with most diagnosed after 2011. Oncologic outcomes of HNC patients with exposure to the WTC disaster and HPV+ status appear to be worse compared to unexposed controls with HPV+ status.
Acknowledgments
Funding
Funding support was provided by MSK Cancer Center Support Grant (P30 CA008748-50).
No disclosures to report.
References
- 1.Li J, Cone JE, Kahn AR, et al. Association between World Trade Center exposure and excess cancer risk. JAMA. 2012;308(23):2479–88. doi: 10.1001/jama.2012.110980. [DOI] [PubMed] [Google Scholar]
- 2.Lioy PJ, Weisel CP, Millette JR, et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect. 2002;110(7):703–14. doi: 10.1289/ehp.02110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solan S, Wallenstein S, Shapiro M, et al. Cancer incidence in world trade center rescue and recovery workers, 2001–2008. Environ Health Perspect. 2013;121(6):699–704. doi: 10.1289/ehp.1205894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeig-Owens R, Webber MP, Hall CB, et al. Early assessment of cancer outcomes in New York City firefighters after the 9/11 attacks: an observational cohort study. Lancet. 2011;378(9794):898–905. doi: 10.1016/S0140-6736(11)60989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Brackbill RM, Liao TS, et al. Ten-year cancer incidence in rescue/recovery workers and civilians exposed to the September 11, 2001 terrorist attacks on the World Trade Center. Am J Ind Med. 2016;59(9):709–21. doi: 10.1002/ajim.22638. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leeman JE, Li JG, Pei X, et al. Patterns of Treatment Failure and Postrecurrence Outcomes Among Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma After Chemoradiotherapy Using Modern Radiation Techniques. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIntyre-Seltman K, Castle PE, Guido R, et al. Smoking is a risk factor for cervical intraepithelial neoplasia grade 3 among oncogenic human papillomavirus DNA-positive women with equivocal or mildly abnormal cytology. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1165–70. doi: 10.1158/1055-9965.EPI-04-0918. [DOI] [PubMed] [Google Scholar]
- 9.Anantharaman D, Muller DC, Lagiou P, et al. Combined effects of smoking and HPV16 in oropharyngeal cancer. Int J Epidemiol. 2016;45(3):752–61. doi: 10.1093/ije/dyw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheurer ME, Danysh HE, Follen M, et al. Association of traffic-related hazardous air pollutants and cervical dysplasia in an urban multiethnic population: a cross-sectional study. Environ Health. 2014;13(1):52. doi: 10.1186/1476-069X-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu D, Berlin JA, Penning TM, et al. Reactive oxygen species generated by PAH o-quinones cause change-in-function mutations in p53. Chem Res Toxicol. 2002;15(6):832–42. doi: 10.1021/tx010177m. [DOI] [PubMed] [Google Scholar]
- 13.Morris LG, Chandramohan R, West L, et al. The Molecular Landscape of Recurrent and Metastatic Head and Neck Cancers: Insights From a Precision Oncology Sequencing Platform. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu HC, Wang Q, Wang LW, et al. Polycyclic aromatic hydrocarbon- and aflatoxin-albumin adducts, hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Cancer Lett. 2007;252(1):104–14. doi: 10.1016/j.canlet.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam S, Conway MJ, Chen HS, et al. The cigarette smoke carcinogen benzo[a]pyrene enhances human papillomavirus synthesis. J Virol. 2008;82(2):1053–8. doi: 10.1128/JVI.01813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webber MP, Moir W, Zeig-Owens R, et al. Nested case-control study of selected systemic autoimmune diseases in World Trade Center rescue/recovery workers. Arthritis Rheumatol. 2015;67(5):1369–76. doi: 10.1002/art.39059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chera BS, Amdur RJ, Mendenhall W, et al. Beware of deintensification of radiation therapy in patients with p16-positive oropharynx cancer and rheumatological diseases. Pract Radiat Oncol. 2017;7(4):e261–e262. doi: 10.1016/j.prro.2016.12.004. [DOI] [PubMed] [Google Scholar]