Abstract
Estradiol modulates the rewarding and reinforcing properties of cocaine in females, including an increase in selection of cocaine over alternative reinforcers. However, the effects of estradiol on male cocaine self-administration behavior are less studied despite equivalent levels of estradiol in the brains of adult males and females, estradiol effects on motivated behaviors in males that share underlying neural substrates with cocaine reinforcement as well as expression of estrogen receptors in the male brain. Therefore, we sought to characterize the effects of estradiol in males on choice between concurrently-available cocaine and food reinforcement as well as responding for cocaine or food in isolation. Male castrated rats (n=46) were treated daily with estradiol benzoate (EB) (5ug/0.1, S.C.) or vehicle (peanut oil) throughout operant acquisition of cocaine (1 mg/kg, IV; FI20 sec) and food (3x45 mg; FI20 sec) responding, choice during concurrent access and cocaine and food reinforcement under progressive ratio (PR) schedules. EB increased cocaine choice, both in terms of percent of trials on which cocaine was selected and the proportion of rats exhibiting a cocaine preference as well as increased cocaine, but not food, intake under PR. Additionally, within the EB treated group, cocaine-preferring rats exhibited enhanced acquisition of cocaine, but not food, reinforcement whereas no acquisition differences were observed across preferences in the vehicle treated group. These findings demonstrate that estradiol increases cocaine choice in males similarly to what is observed in females.
Keywords: Estrogen, Cocaine, Self-Administration, Choice, Addiction, Sex Hormones
Cocaine use disorder exacts devastating consequences on the afflicted individual and profound costs on society as a whole. The individual often experiences health, social, and legal consequences, with mortality as a far too common end point (McGinnis & Foege, 1999; Pouletty, 2002). These consequences pose a burden on society in the form of elevated health care costs, lost productivity and increased crime rates (McGinnis & Foege, 1999; Pouletty, 2002). Although many individuals use drugs of abuse, such as cocaine, only a minority go on to develop drug use disorders and it remains unclear why specific individuals are resistant or vulnerable to addiction (Cantin et al., 2010; Kerstetter et al., 2012; Perry, Westenbroek, & Becker, 2013b). Cocaine addiction is considered a neuropsychiatric condition involving dysregulated and deleterious use (Volkow, Fowler, & Wang, 2003) and the choice to take cocaine at the expense of other, normally rewarding, alternatives is an important dimension of cocaine abuse (American Psychiatric Association, 2003). Choice procedures, in which subjects can choose between concurrently available cocaine or alternative reinforcement, are sensitive to individual differences and offer a unique window into this specific aspect of addiction vulnerability (Cantin et al., 2010; Kerstetter et al., 2012; Perry et al., 2013b).
The propensity to choose cocaine over a concurrently available reinforcer is sex- and hormone-dependent. The proportion of cocaine-preferring rats as well as the average proportion of cocaine choice is greater in females compared to males (Kerstetter et al., 2012; Perry et al., 2013b; Perry, Westenbroek, Jagannathan, & Becker, 2015). Further, the sex difference in the propensity to select cocaine reinforcement over an alternative is abolished by gonadectomy and restored by the administration of estradiol (Kerstetter et al., 2012). These findings are congruent with both clinical reports indicating that females have higher addiction vulnerability and with preclinical studies reporting estradiol-modulated sex differences in cocaine-related behaviors (Hu & Becker, 2008; Jackson, Robinson, & Becker, 2005; Kerstetter et al., 2012; Kosten, Gawin, Kosten, & Rounsaville, 1993; Larson, Anker, Gliddon, Fons, & Carroll, 2007; Lynch, Roth, Mickelberg, & Carroll, 2001; Ramoa, Doyle, Naim, & Lynch, 2013; Robbins, Ehrman, Childress, & O’Brien, 1999; Russo et al., 2003; Zhao & Becker, 2010). Taken together, these data identify estradiol as a modulator of the reinforcing and rewarding properties of cocaine, including effects on behavior allocation, in females.
In contrast, the effects of estradiol on male cocaine-related behavior are largely understudied. In contrast to blood levels, levels of estradiol in most brain regions are equivalent in males and females (Barker & Galea, 2009; Konkle & McCarthy, 2011). Male brains express estrogen receptors and the many effects of testosterone on the brain, particularly motivational effects, are in part mediated by conversion to estradiol (Celec, Ostatníková, & Hodosy, 2015; Tetel & Pfaff, 2010). Testosterone has been shown to modulate acute cocaine locomotion and sensitization in males (Chen, Osterhaus, McKerchar, & Fowler, 2003; Chin et al., 2002; Long, Dennis, Russell, Benson, & Wilson, 1994; Martínez-Sanchis, Aragon, & Salvador, 2002; Martínez-Sanchis et al., 2002; Menéndez-Delmestre & Segarra, 2011; Minerly et al., 2008, 2010). Although the effect of targeted stimulation of estrogen receptors alone on cocaine locomotion in males is not reported, both estradiol and the estrogen receptor modulator raloxine potentiate amphetamine-induced locomotion (Menniti & Baum, 1981; Purves-Tyson et al., 2015). Thus, the male central nervous system is responsive to estradiol and the addiction relevant effects of estradiol may be similar across sexes.
Despite findings of estradiol modulation of psychostimulant-induced locomotion in males, estradiol has been reported to not impact cocaine-taking behavior. Estradiol enhances the acquisition of cocaine self-administration (Jackson et al., 2005; Perry, Westenbroek, & Becker, 2013a) as well as intake under fixed- or progressive-ratio schedules of reinforcement (Perry et al., 2013a; Ramoa et al., 2013) in females but not males. However, there are no studies on the impact of estradiol in males on concurrent reinforcement. This procedure uniquely assesses the allocation of behavior, which is not fully recapitulated during single reinforcement procedures and is central to addiction processes (Ahmed, 2010). Accordingly, we sought to investigate the effects of estradiol on gonadectomized males, in an identical fashion as used in ovariectomized females (Kerstetter et al., 2012). In addition to cocaine choice, we also assessed responding under a PR schedule for cocaine-only or food-only reinforcement.
Materials and Methods
Subjects
Castrated male (n=48) Sprague-Dawley rats (Charles-River, Wilmington, MA) weighing 275–300 g at time of arrival served as subjects. Castration was performed by the vendor prior to shipping and occurred approximately 3 weeks prior to behavioral testing. Rats were individually housed in a temperature- and humidity-controlled vivarium on a 12-h light–dark cycle and were allowed to habituate to the colony for 1 week prior to manipulations. Rats were restricted to 22 g of rat chow per day (Harlan, Indianapolis, IN); All rats were maintained on ad libitum water access. All procedures were approved by the University of California at Santa Barbara Institutional Animal Care and Use Committee and conducted in accordance with the National Institute of Health (NIH) Guide for Care and Use of Laboratory Animals (National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011).
Castration and Catheter Surgery
All rats underwent castration (performed by the vendor) 2 weeks before catheter implantation. All rats received surgery for implantation of an indwelling jugular catheter as previously described (Kerstetter, Aguilar, Parrish, & Kippin, 2008). Chronic indwelling catheters were constructed using a bent steel cannula with a screw-type connector (Plastics One, Roanoke, VA), silastic tubing (10 cm, i.d. 0.64mm, o.d. 1.19 mm; Dow Corning, Midland, MI), polypropylene mesh (Atrium Medical, Hudson, NH), and cranioplastic cement. The catheter was inserted into the right jugular vein, secured to surrounding tissue, and then ran subcutaneously to exit posterior to the shoulder blades. All rats were allowed a minimum of 5 days to recover from surgery before operant training. Following surgery, catheters were flushed before operant sessions with entamycin (0.2mg/0.1 ml) dissolved in 0.9% physiological saline. After operant sessions, catheters were flushed with 0.1 ml of heparin (6.0 IU/0.1ml prepared in 0.9% physiological saline, i.v.) and 0.1 ml ceflazolin ( 10 mg/0.1 ml prepared in 0.9% physiological saline , i.v.), as a prophylactic measure against microbial infection and to extend catheter patency. Catheter patency was verified by infusing 0.10 methohexital sodium (10 mg/ml i.v.; Eli Lilly, Indianapolis, IN), which produces a rapid loss of muscle tone when administered intravenously. Failed catheters were replaced one time with 1 week of recovery prior to resuming testing. Upon a second failure the rat was removed from the study (this resulted in the loss of 4 rats from study).
Estrogen Treatments
Rats received daily subcutaneous (s.c.) injections of peanut oil (n=22, vehicle, 0.1 ml) or estradiol-benzoate (EB) (n=23, EB; 5 μg/0.1 ml peanut oil) 30 min before operant sessions. This dose of EB was selected because it has been shown to enhance cocaine choice in ovariectomized female rats (Kerstetter et al., 2012). Vehicle and EB treatment began on the first day of operant training.
Operant Procedures
The operant chambers were equipped with two retractable levers, a stimulus light above each lever, a food pellet dispenser between the levers and swivel to suspend infusion tubing (ANL-926, Med Associates). Rats were trained on a Fixed Interval: 20 s (FI 20-s) schedule to lever press on the right lever for food (3x45 mg pellets; Noyes, Lancaster, NH) and on the left lever for cocaine (1 mg/kg, IV, cocaine hydrochloride; National Institute on Drug Abuse, Research Triangle Park, NC). Acquisition sessions lasted until the rat earned a total of 25 reinforcers or 6h elapsed. Rats had to earn at least 20 reinforcers during a training session to count towards a criterion day, and rats needed 5 consecutive criterion sessions for each reinforcer to complete acquisition. At the start of each session, the rat’s catheter was connected to a liquid swivel (Instech, Plymouth Meeting, PA) via polyethylene 20 tubing that was encased in a steel spring leash (Plastics One), and the swivel was suspended above the operant conditioning chamber and connected to an infusion pump (Model PHM- 100, Med Associates). For training sessions, only one lever was extended (i.e., only cocaine or food available), and rats were trained to press the lever under a Fixed Interval: 20 s (FI 20-s) schedule of reinforcement. During food training sessions, responses on the right lever resulted in the delivery of three 45 mg grain food pellets into the food pellet dispenser, and a 5-s presentation of the white stimulus light above the food lever. During cocaine training sessions, responses on the left lever resulted in a cocaine infusion that involved a 4-s activation of the infusion pump and a 5-s presentation of the white stimulus light above the cocaine lever. Cocaine hydrochloride was dissolved in saline, filtered using a 0.45- mm ultracleaning filter unit (Fisher Scientific), and delivered at a dose of 1.0mg/kg per 0.10 ml infusion. This cocaine dose was selected in order to match the dose used to reveal an effect of EB on female cocaine choice (Kerstetter et al., 2012). Rats had to earn at least 20 reinforcers during a training session to count towards a criterion day, and rats needed 5 consecutive criterion sessions for each reinforcer to complete acquisition. Accordingly, the training procedures ensured that rats had equal experience in obtaining food and cocaine reinforcement before choice sessions.
After acquisition, five concurrent reinforcement sessions (FI 20-s) were conducted, during which both levers were extended, allowing the rat to select either food or cocaine. It was noted that rats responded during the 20-s period when responses were not reinforced, so after the completion of concurrent reinforcement sessions, five discrete trial sessions were conducted under the same conditions as the concurrent reinforcement sessions, with the exception that following a response on either lever, both levers were retracted to prevent non-reinforced responding and returned after an inter-trial interval (ITI) of 20 s (i.e. discrete trials), thus minimizing perseveration of response patterns. Concurrent and discrete trials lasted until rats earned 25 reinforcers or 3 hours elapsed.
Progressive Ratio
After completion of concurrent and discrete reinforcement sessions, three cocaine-only and three food-only progressive ratio (PR) sessions were conducted with the food and cocaine sessions alternating daily with only one lever extended (right for food, left for cocaine). Completion of the required ratio resulted in the delivery of 3 food pellets or 0.1 mL of 1mg/kg cocaine. The PR response requirements were adapted from (Richardson & Roberts, 1996) and failure to earn a reinforcer within 30 minutes resulted in termination of the session.
Ad Libitum Feeding
Following completion of concurrent and discrete choice and PR testing, the rats were switched to ad libitum (ad lib) feeding. Five discrete trials were conducted under ad lib feeding and then a subset (n= 14, EB, n=15, vehicle) were re-tested on PR schedules (3 sessions each for cocaine and food reinforcement).
Hormone Treatment Reversal
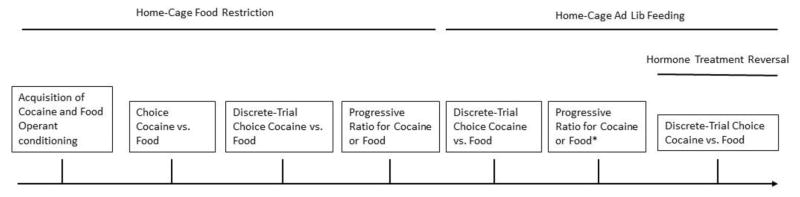
Following ad lib testing, hormone treatment was reversed for all rats (vehicle to EB or EB to vehicle). Rats were maintained an ad lib feeding during this period. Ten daily discrete choice sessions were completed. In order to allow for hormone washout, the last 5 sessions were analyzed. See Figure 1 for a timeline of experimental procedures.
Figure 1.
Timeline of experimental procedures.
* only a subset of rats were subjected to these tests.
Data analyses
Reinforcement acquisition was assessed by Student’s t-test. Mixed analysis of variance (ANOVA) were used to assess concurrent/discrete choice sessions and effects of the switch from restricted to ad lib feeding and hormone treatment reversal. Proportions of cocaine preferring animals (75% or greater cocaine choice average in last four sessions) and proportion of cocaine selection by trial during discrete choice were assessed by chi-square tests for between group analysis and by McNemar tests for within group analysis where appropriate. The relationship between discrete choice behavior and PR testing was assessed by Pearson’s correlation. The first session of concurrent, discrete and PR trial sessions were not included in analysis in order to exclude behavior that was reflective of adjustment to new schedules or conditions and the data for the remainder of the sessions were collapsed for each subject. For hormone treatment reversal, the first 5 sessions were excluded to allow for latent effects of hormone treatment/removal. The level of statistical significance for all comparisons was 0.05.
Results
Acquisition of Food and Cocaine Reinforcement
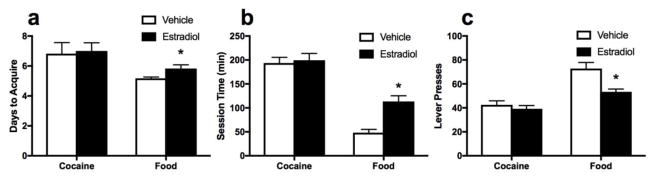
An effect of hormone treatment was found for number of days to meet criterion for food reinforcement [t(44)=2.59, p= .016, see Figure 2a] with EB rats taking longer to meet criterion for training. An effect of hormone treatment was also found for time required to complete food sessions [t(44)=2.95, p=0.005, see Figure 2b], with EB rats taking longer to complete sessions and for food lever responses [t(44)=3.45, p=.002, see Figure 2b], with EB rats responding less. No effects of hormone treatment were detected for number of days to meet criterion for cocaine reinforcement (see Figure 2a), time to complete cocaine sessions (see Figure 2b) or cocaine lever pressing (see Figure 2c).
Figure 2.
Impact of EB on cocaine and food acquisition. (a) Days to acquire cocaine and food self-administration. EB rats took longer to acquire food self-administration. No differences were found for cocaine acquisition. (b) Time to complete acquisition sessions (collapsed over five criterion days). EB rats took longer to complete food sessions; no differences were found for cocaine sessions. (c) Lever presses during acquisition sessions (collapsed over five criterion days). EB rats pressed less in food sessions; no differences were found for cocaine sessions.
Concurrent Reinforcement
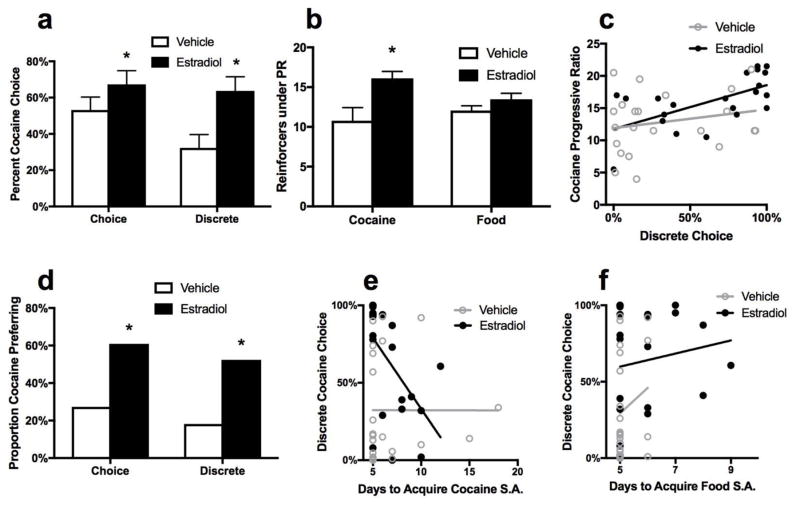
The percent cocaine reinforcers selected (cocaine reinforcers/(cocaine reinforcers+food reinforcers)*100) during concurrent reinforcement was impacted by EB treatment. A mixed ANOVA, with non-discrete versus discrete trial types as a within factor and hormone treatment as a between factor, revealed a main effect of test type [F(1, 41) = 16.38, p < 0.001] and a main effect of hormone treatment [F(1, 41) = 7.02, p < 0.001] with EB subjects choosing cocaine on a greater number of trials regardless of trial type (see Figure 3a) but no interaction of hormone treatment with choice test type was detected (F < 1.0, p > 0.05). Selection of cocaine decreased in both groups from non-discrete to discrete trials.
Figure 3.
(a) Percent cocaine choice during concurrent and discrete trials. EB rats chose cocaine significantly more than vehicle rats. No difference was found for concurrent trials. (b) Reinforcers earned during cocaine and food PR. EB rats earned significantly more reinforcers in cocaine PR. No difference was found for food PR. (c) Correlation between cocaine choice in discrete trials and cocaine PR, within each treatment group. A significant, positive correlation was found within the EB group but not the vehicle group. (d) Proportion of cocaine-preferring rats in concurrent and discrete trials. The EB group had significantly greater proportion of cocaine-preferring rats in concurrent and discrete trials. (e) Correlation between days to acquire cocaine self-administration and percent cocaine choice during discrete choice trials. A significant, negative correlation was found for the estradiol group but not the vehicle group. (f) Correlation between days to acquire food self-administration and percent cocaine choice during discrete choice trials. No significant correlations were found for the estradiol group or the vehicle group.
Similarly, a greater proportion of the EB group was cocaine-preferring (selected cocaine 75% or more of trials) than the vehicle group in non-discrete [χ2 (1, N=45) = 5.14, P = 0.023] and discrete trials [χ2 (1, N=43)=5.53, P=0.019], with a 2.9-fold greater proportion of cocaine preferring subjects in the EB group in discrete trials (Figure 3d).
As EB treatment and food appetite may interact to facilitate satiation within choice sessions and cause within-session transitions between reinforcers, trial by trial choices were analyzed for EB and vehicle groups during the fifth session of discrete choice under food restriction. The proportion of rats choosing cocaine in the first trial was greater in the EB group [χ2 (1, N=41) = 5.24, P=0.022], (proportion cocaine choice on trial 1, EB 33.3%, vehicle 5.0%). Thus, at the outset of the session, EB rats show a greater propensity to select cocaine over food as compared to vehicle rats. Across subsequent trials during the session, both groups increased the frequency of cocaine choices. A 2-way ANOVA, with trial as a within subjects factor and hormone treatment as a between subjects factor, revealed a main effect of trial F(24, 936) = 7.89, p<0.000) and hormone treatment F(1, 39) = 6.67, p=0.014) but no interaction with the EB group exhibiting greater cocaine choice than the vehicle group across all trials (data not shown).
In order to examine the impact of home-cage food availability on cocaine choice, rats were given ad lib access to food in their home-cage during continued testing on discrete choice sessions. Comparison of food restricted and ad lib discrete cocaine choice in EB and vehicle groups indicated an impact of hormone treatment (see Table 1); mixed factors ANOVA revealed a main effect of hormone treatment [F(1, 41) = 5.60, p= .023], with EB rats taking more cocaine reinforcers but no main effect of feeding status or interaction was detected (Fs < 1.0, ps > 0.05). Similarly, a McNemar test to compare paired proportions found no effect of feeding status (see Table 1).
Table 1.
Choice, preference and progressive ratio under food restriction, ad libidum food access and hormone treatment reversal.
| Percent Cocaine Choice (mean ± SEM) | Proportion Cocaine Preferring | Cocaine PR (mean ± SEM) | Food PR (mean ± SEM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Food Restricted | Ad Lib Food | Hormone Reversal | Food Restricted | Ad Lib Food | Hormone Reversal | Food Restricted | Ad Lib Food | Food Restricted | Ad Lib Food | |
|
|
||||||||||
| Vehicle | 32.3 ± 7.1 | 32.4% ± 6.1 | 43.2% ± 8.2 | 18.2% | 13.6% | *40.0% | 12.6 ± 1.3 | 10.8 ± 1.7 | 12.6 ± 0.8 | *10.7 ± 0.7 |
| EB | 63.7% ± 7.8 | 49.7% ± 9.1 | 44.8% ± 9.2 | 52.4% | 42.9% | 40.0% | 15.3 ± 1.1 | 14.9 ± 1.1 | 13.2 ± 0.9 | *14.8 ± 1.0 |
= p<0.05 for within subjects comparison.
PR means under food restriction were calculated from the subset of subjects tested for ad lib PR.
We next determined the impact of reversing hormone treatments on the established patterns of reinforcement selection by ceasing and initiating EB treatments in the previous EB and vehicle groups, respectively. Immediate prior responding on discrete choice (last 4 days under ad lib) was taken as baseline and compared to responding under hormone reversal in the same conditions (the last 5 days). Although the choice of cocaine tended to increase during the switch from vehicle to EB, no significant effects of hormone reversal were detected (ps > 0.05; see Table 1). However, examination of preference types in each condition revealed a significant increase in the proportion cocaine-preferring rats in the vehicle to EB group [χ2 (1, N=20)=4.00, P=0.046; see Table 1] but no significant change in the EB to vehicle group (McNemar test, p > 0.1; see Table 1).
Relationship Between Acquisition and Discrete Choice
The relationships between days to acquire cocaine and food self-administration and discrete choice were assessed by Pearson’s correlation both across and within treatment groups. A significant, negative correlation was found between days to acquire cocaine self-administration and discrete choice for the EB group (r=−0.54, p=0.011) but not for the vehicle group (r=0.00, p=1.0) or for treatment groups collapsed (r=−0.19, p=0.224) (see Figure 3e). No significant correlations were detected for days to acquire food self-administration and discrete choice for EB (r=0.15, p=0.524), vehicle (r=0.19, p=0.319) or treatment groups collapsed (r=0.28, p=0.07) (see Figure 3f). Further investigation of these correlations by planned orthogonal comparisons confirmed that the cocaine preferring subgroup (selected cocaine 75% or more of trials) took significantly fewer days to acquire cocaine self-administration than did non-preferring rats (less than 75% discrete cocaine choice) in the EB condition [t(19)=4.41, p<0.001; mean ± SEM for cocaine-preferring, 5.2 ± 0.2, for non-preferring, 8.6 ± 0.8] but not the VEH condition (p > 0.05; mean ± SEM for cocaine-preferring 6.8 ± 1.1, for non-preferring, 6.8 ± 0.9).
Progressive Ratio
The effect of hormone treatment on cocaine and food PR was assessed by Student’s t-test. The EB-treated group earned more reinforcers during cocaine PR in restricted food-access conditions [t(41)=2.45, p=0.019, see Figure 3b]. No effect of EB on food PR was found.
A subset of rats were again tested on PR after switching to ad lib home-cage food access. The effect of this switch was assessed by mixed 2-way ANOVA with home-cage food access as a within subjects and hormone treatment as a between subjects factor for cocaine and food PR. For cocaine PR, no significant effects were detected. For food PR, a food access by hormone treatment interaction [F(1, 27)=14.47, p=0.001] and a main effect of hormone treatment [F(1, 27)=4.68, p=0.04] were found. Paired-samples t-test indicated that both groups shift food PR reinforcers earned from restricted to ad lib conditions, but in opposite directions. The EB group increased food reinforcers earned [t(13)=3.63, p=0.003] and the vehicle group decreased reinforcers earned [t(14)=2.38, p=0.032] (see Table 1).
Relationship between Choice and Progressive Ratio
Across hormone conditions, a positive correlation between percent cocaine choice under a discrete trial schedule and the number of cocaine reinforcers earned under PR was detected (r = 0.48, p = 0.001). However, when data were assessed within each hormone treatment group, a significant correlation was detected in EB rats (r = 0.60, p = 0.004), but not vehicle rats (r = 0.21, p = 0.343; see Figure 3c). No significant correlations were detected between discrete cocaine choice and PR food reinforcers earned (data not shown). Furthermore, a positive correlation was detected between total cocaine infusions earned up to PR cocaine testing (i.e. combined intake for acquisition, choice and discrete trials) and PR cocaine reinforcers earned within the EB group (r = 0.587, p = 0.005), but not the vehicle group r = 0.250, p = 0.263 (data not shown).
Discussion
The present study indicates that chronic treatment with estradiol (EB) increased cocaine choice in gonadectomized males. Relative to vehicle controls, rats treated with EB selected cocaine over food reinforcement more frequently and a greater proportion of EB treated rats exhibited a preference for cocaine over food. The cocaine-preferring rats in the EB condition also exhibited faster acquisition of cocaine-, but not food-, reinforcement whereas no difference in cocaine acquisition was observed in cocaine- vs food-preferring rats in the vehicle condition nor was preference type in either hormone condition related to acquisition of food reinforced responding. Further, EB-treated rats exhibited increased motivation to obtain cocaine, but not food, as measured by progressive ratio schedules. Taken together, the present findings, along with those in females (Kerstetter et al., 2012), indicate that the effects of estradiol on cocaine preference, as well as some aspects of cocaine reinforcement, generalize between males and females. Notably, for both males and females, the dose of estradiol employed here and the vast majority of studies of cocaine-related behaviors is likely to produce supraphysiological levels of the hormone in relevant brain regions (Barker & Galea, 2009), thus, future studies focusing on the role of EB at physiological level and manipulation of steroid synthesizing enzymes would bolster our knowledge of the potential contribution of these mechanisms to individual differences in cocaine reinforcement. Nevertheless, the present data indicate that the response to EB on cocaine reinforcement is specific or more pronounced in a subpopulation of males and similar to the impact of EB on cocaine choice in females (Kerstetter et al., 2012).
In castrated males, EB treatment increased the selection of cocaine reinforcement over food reinforcement as measured in two ways. First, EB drove a majority of castrated males to take greater than 75 percent of their reinforcers as cocaine, in comparison to a minority of cocaine preferring rats in the vehicle control group. Second, the percent of trials on which cocaine reinforcement was selected over food was also greater in the EB group. Notably, cocaine choice dropped in both groups when levers were retracted between reinforcer availability (i.e. under discrete trial procedures), however, this drop was greater in control group (53.2% to 32.3%) relative to the EB group (67.3% to 63.3%) which is consistent with data in intact males (Kerstetter et al., 2012). Previous work indicated that intact female rats display a higher cocaine preference relative to males, and this sex difference is largely driven by estradiol (Kerstetter et al., 2012). Thus, estradiol has the same effect on the selection of cocaine over a competing reinforcer in both male and female rats following gonadectomy.
In addition to increased cocaine choice, EB treatment also demonstrated increased reinforcer efficacy in castrated males as measured by reinforcers earned under a progressive ratio schedule of reinforcement which is commonly used to index motivation to obtain a reinforcer (Richardson & Roberts, 1996). As PR testing occurred after choice testing, it is likely that the greater cocaine infusions earned by the EB rats in choice testing may account for the increased cocaine motivation observed. Elevated cocaine break points under PR schedules has been reported for intact male and female rats after cocaine/food choice experience (Perry et al., 2013a, 2013b, 2015). Correlational analysis indicated that cocaine intake during the choice procedure, and total cocaine infusions earned up to PR cocaine testing, predicts reinforcers earned under PR testing. However, this was only true within the estradiol group, indicating that cocaine experience was not predictive of PR reinforcers earned in the control animals. This EB-specific relationship suggests that the correlation is driven by responders and non-responders to EB, rather than the correlation being simply a consequence of greater cocaine experience. Chronic EB in males has been reported to have no affect on acquisition of responding or level of intake of cocaine under a FR schedule as it does in females (Jackson et al., 2005; Perry et al., 2013a). However, correlational analysis indicated acquisition of cocaine is predictive of cocaine choice within the EB group (Figure 3e). This relationship was not observed in the vehicle group indicating the lack of a general relation between cocaine reinforcement acquisition and cocaine choice, nor was there predictive value of food reinforcement acquisition and cocaine choice (Figure 3f). These results suggest that EB enhances motivation for cocaine in a subset of “sensitive” rats that produces effects that persist across acquisition, choice and PR testing. Such individual differences in the response to EB are likely obscured when analysis is done at the group level (see e.g. Figure 2a versus 3e) and may explain the apparent discrepancy between the presently observed EB-induced increase in PR responding (Figure 3b) and the negative findings previously reported (Perry et al., 2013a). Thus, the motivation for responding for cocaine reinforcement appears to be increased by estradiol in both a subset of male rats that are readily identified under concurrent access.
Conversely, the selection of cocaine under concurrent access may also be sensitive to changes in responding for food or food appetite/motivation. Estradiol is known to decrease food intake in males and females (Dubuc, 1985; Eckel, 2011; Kuchár, Mozes, Boda, & Koppel, 1982; Simpkins et al., 1988) as well as promotes taste aversions (reviewed in Asarian & Geary, 2013). Furthermore, food PR breakpoints decrease in females during proestrous/estrous, a time in which endogenous estradiol levels are high (Feltenstein & See, 2007; Perry et al., 2013b, 2015) and when responding for cocaine is elevated (Feltenstein & See, 2007; Kerstetter et al., 2008; Lynch, 2008; Roberts, Bennett, & Vickers, 1989). Gonadectomy does not affect acquisition of food self-administration or food breakpoints under PR in females (Heinsbroek, van Haaren, Zantvoord, & van de Poll, 1987; van Hest, van Haaren, & van de Poll, 1988) leaving the precise relationship between estradiol and operant responding for food, including potential generalization between sexes, unclear. Consistent with the EB suppression of ad lib food intake, the rats in the EB condition overall took more days to meet criteria for food acquisition, pressed less frequently during food training sessions and took longer to finish these sessions compared to those in the vehicle condition, indicating suppressed motivation for food whereas there were no overall differences for cocaine acquisition. However, food reinforcers earned under PR schedules during homecage food restriction, as well as the ad lib availability of food, suggests that EB did not suppress motivation for food reinforcement whereas EB did increase cocaine reinforcers earned under PR schedules. Furthermore, satiation may facilitate transition from food choice within daily sessions but this effect did not interact with EB treatment as the EB group demonstrated greater cocaine choice on the first trial and across all subsequent trials of the discrete choice session. The parameters of food reinforcement acquisition also failed to predict individual differences in the preference for cocaine or food during concurrent reinforcement in either of the hormone treatment conditions. Thus, there appears to be limited predictive value of food intake or responding for food reinforcement for behavioral allocation between food and cocaine under concurrent access. Single reinforcer procedures may not have a simple or linear relationship with the allocation of behavior assessed under concurrent access and, as such, choice procedures appear to identify distinct factors of relevance to cocaine abuse and addiction (Ahmed, 2010).
EB appears to have effects on cocaine acquisition that are predictive of cocaine choice in a sub-population of rats. This raises the possibility that exposure to EB during acquisition is required for the effects of EB later in concurrent access. Furthermore, it is not clear if ongoing EB exposure, after administration during acquisition and initial concurrent access, is required to maintain cocaine preference. In order to assess these possibilities, the treatments were reversed (vehicle group switched to EB, EB group switched to vehicle) and rats were exposed to further discrete choice trials. For rats initially trained and tested in the vehicle condition, the addition of EB significantly increased the proportion of rats exhibiting a cocaine preference (from 14% to 40%) and tended to increase average proportion of trials on which cocaine was selected (from 32% to 43% of trials). These findings suggest that choice preference, after concurrent access exposure, remains sensitive to the addition of EB treatment. Conversely, cessation of hormone treatment in the rats initially trained and tested with EB failed to impact cocaine preferences indicating that EB is not required to maintain established cocaine preferences. Although, greater than 10 days of hormone treatment cessation may be required to allow for a reduction of EB effects. In total, these findings are consistent with the notion that there is a unidirectional transition to a drug preferring state (Perry et al., 2013b; 2015) which is modulated by EB, however, given that under all conditions only subsets of rats are cocaine-preferring, the interrelations between steroids and other individual factors in determining cocaine preferences is still in need of exploration.
The finding that estradiol increases cocaine choice and motivation in males is supported indirectly by existing well-established estrogen-dependent motivational systems in the male brain. Male and female appetitive and consummatory sexual behaviors are dependent on activation of estrogen receptor within the medial preoptic area (MPOA) which are upstream of mesocorticolimbic dopamine circuits with cell bodies in the ventral tegmental area (reviewed by Will et al., 2014; Paredes, 2003). This dopamine system is widely implicated in both the behavioral effects of addiction to drugs of abuse, including cocaine (Koob & Volkow, 2016; Everitt & Robbins, 2013; Wise & Morales, 2010; Di Chiara, 1999; Robinson & Berridge, 1993) and cocaine induces higher levels of dopamine in the accumbens in cocaine-preferring males and females as compared to non-preferring rats (Perry et al., 2015). Several studies by Becker and colleagues have demonstrated estradiol in females modulates dopamine release in the striatum by acting on medium spinal neurons within these regions (reviewed in Yoest et al., 2014) with preliminary experiments suggesting a somewhat more limited effects in males (Yoest et al., 2016). More recently, Dominguez and colleagues have demonstrated that MPOA regulates cocaine-induced locomotor responses in male rats (Will et al., 2016) and cocaine-induced conditioned place preferences in female rats (Tobiansky et al., 2013). Further, administration of estradiol into the MPOA enhances cocaine-induced increases in dopamine within the nucleus accumbens (Tobiansky et al., 2016) as well as cocaine-induced conditioned place preferences (Robison et al., 2016). Thus, the MPOA or striatal terminal of the mesocorticolimbic systems are likely candidates in the ability of estradiol to modulate cocaine choice in male and female rats.
The present study demonstrates for the first time that gonadal hormones modulate the propensity to prefer cocaine over food reinforcement in males. The effects of estradiol on cocaine preference were preceded by enhanced acquisition of cocaine, but not food, reinforcement and were paralleled by greater motivation to obtain cocaine, but only in the subset of EB-treated rats that exhibited preference for cocaine over food reinforcement. Conversely, although in lower proportion, some vehicle-treated individuals also exhibited preferences for cocaine over food reinforcement, however, no clear relations to parameters of food or cocaine reinforcement were evident. Thus, there appears to be marked individual differences mediating the effects of estradiol that may be useful in identifying neurobiological factors mediating addiction vulnerability.
Highlights.
Estrogen modulates cocaine reinforcement in male rats
Estrogen increases choice of cocaine over food in concurrent reinforcement
Estrogen increases cocaine intake under progressive ratio
Estrogen effects on acquisition may predict later cocaine choice
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH. Validation crisis in animal models of drug addiction: Beyond non- disordered drug use toward drug addiction. Neuroscience & Biobehavioral Reviews. 2010;35(2):172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5 2003. [Google Scholar]
- Asarian L, Geary N. Sex differences in the physiology of eating. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2013;305(11):R1215–R1267. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Galea LAM. Sex and regional differences in estradiol content in the prefrontal cortex, amygdala and hippocampus of adult male and female rats. General and Comparative Endocrinology. 2009;164(1):77–84. doi: 10.1016/j.ygcen.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, … Ahmed SH. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PloS One. 2010;5(7):e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celec P, Ostatníková D, Hodosy J. On the effects of testosterone on brain behavioral functions. Frontiers in Neuroscience. 2015:9. doi: 10.3389/fnins.2015.00012. [DOI] [PMC free article] [PubMed]
- Chen R, Osterhaus G, McKerchar T, Fowler SC. The role of exogenous testosterone in cocaine-induced behavioral sensitization and plasmalemmal or vesicular dopamine uptake in castrated rats. Neuroscience Letters. 2003;351(3):161–164. doi: 10.1016/j.neulet.2003.07.018. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HBK, Burrell S, Lu D, Jenab S, … Quiñones-Jenab V. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Research. 2002;945(1):123–130. doi: 10.1016/s0006-8993(02)02807-x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. European journal of pharmacology. 1999;375(1):13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Dubuc PU. Proceedings of the Society for Experimental Biology and Medicine. 3. Vol. 180. Society for Experimental Biology and Medicine; New York, N.Y: 1985. Effects of estrogen on food intake, body weight, and temperature of male and female obese mice; pp. 468–473. [DOI] [PubMed] [Google Scholar]
- Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiology & Behavior. 2011;104(4):517–524. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neuroscience & Biobehavioral Reviews. 2013;37(9):1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug and Alcohol Dependence. 2007;89(2–3):183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek RPW, van Haaren F, Zantvoord F, van de Poll NE. Sex differences in response rates during random ratio acquisition: Effects of gonadectomy. Physiology & Behavior. 1987;39(2):269–272. doi: 10.1016/0031-9384(87)90020-5. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug and Alcohol Dependence. 2008;94(1–3):56–62. doi: 10.1016/j.drugalcdep.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex Differences and Hormonal Influences on Acquisition of Cocaine Self-Administration in Rats. Neuropsychopharmacology. 2005;31(1):129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology. 2008;198(1):63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2012;37(12):2605–2614. doi: 10.1038/npp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. Journal of Substance Abuse Treatment. 1993;10(1):63–66. doi: 10.1016/0740-5472(93)90100-G. [DOI] [PubMed] [Google Scholar]
- Kuchár S, Mozes S, Boda K, Koppel J. The effect of androgen and estrogen on food intake and body weight in rats--age dependency. Endokrinologie. 1982;80(3):294–298. [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Experimental and Clinical Psychopharmacology. 2007;15(5):461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Long SF, Dennis LA, Russell RK, Benson KA, Wilson MC. Testosterone implantation reduces the motor effects of cocaine. Behavioural Pharmacology. 1994;5(1):103–106. doi: 10.1097/00008877-199402000-00012. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197(2):237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacology Biochemistry and Behavior. 2001;68(4):641–646. doi: 10.1016/S0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Martínez-Sanchis S, Aragon CMG, Salvador A. Cocaine-induced locomotor activity is enhanced by exogenous testosterone. Physiology & Behavior. 2002;76(4–5):605–609. doi: 10.1016/s0031-9384(02)00764-3. [DOI] [PubMed] [Google Scholar]
- McGinnis JM, Foege WH. Mortality and Morbidity Attributable to Use of Addictive Substances in the United States. Proceedings of the Association of American Physicians. 1999;111(2):109–118. doi: 10.1046/j.1525-1381.1999.09256.x. [DOI] [PubMed] [Google Scholar]
- Menéndez-Delmestre R, Segarra AC. Testosterone is essential for cocaine sensitization in male rats. Physiology & Behavior. 2011;102(1):96–104. doi: 10.1016/j.physbeh.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti FS, Baum MJ. Differential effects of estrogen and androgen on locomotor activity induced in castrated male rats by amphetamine, a novel environment, or apomorphine. Brain Research. 1981;216(1):89–107. doi: 10.1016/0006-8993(81)91280-4. [DOI] [PubMed] [Google Scholar]
- Minerly ACE, Russo SJ, Kemen LM, Nazarian A, Wu HBK, Weierstall KM, … Quinones-Jenab V. Testosterone plays a limited role in cocaine-induced conditioned place preference and locomotor activity in male rats. Ethnicity & Disease. 2008;18(2 Suppl 2):S2-200–4. [PubMed] [Google Scholar]
- Minerly ACE, Wu HBK, Weierstall KM, Niyomchai T, Kemen L, Jenab S, Quinones-Jenab V. Testosterone differentially alters cocaine-induced ambulatory and rearing behavioral responses in adult and adolescent rats. Pharmacology, Biochemistry, and Behavior. 2010;94(3):404–409. doi: 10.1016/j.pbb.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8. Washington (DC): National Academies Press (US); 2011. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK54050/ [PubMed] [Google Scholar]
- Paredes RG. Medial preoptic area/anterior hypothalamus and sexual motivation. Scandinavian journal of psychology. 2003;44(3):203–212. doi: 10.1111/1467-9450.00337. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. Impact of pubertal and adult estradiol treatments on cocaine self-administration. Hormones and Behavior. 2013a;64(4) doi: 10.1016/j.yhbeh.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. The Development of a Preference for Cocaine over Food Identifies Individual Rats with Addiction-Like Behaviors. PLoS ONE. 2013b;8(11):e79465. doi: 10.1371/journal.pone.0079465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Jagannathan L, Becker JB. The Roles of Dopamine and α1-Adrenergic Receptors in Cocaine Preferences in Female and Male Rats. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.116. [DOI] [PMC free article] [PubMed]
- Pouletty P. Drug addictions: towards socially accepted and medically treatable diseases. Nature Reviews Drug Discovery. 2002;1(9):731–736. doi: 10.1038/nrd896. [DOI] [PubMed] [Google Scholar]
- Purves-Tyson TD, Boerrigter D, Allen K, Zavitsanou K, Karl T, Djunaidi V, … Weickert CS. Testosterone attenuates and the selective estrogen receptor modulator, raloxifene, potentiates amphetamine-induced locomotion in male rats. Hormones and Behavior. 2015;70:73–84. doi: 10.1016/j.yhbeh.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Ramoa CP, Doyle SE, Naim DW, Lynch WJ. Estradiol as a Mechanism for Sex Differences in the Development of an Addicted Phenotype following Extended Access Cocaine Self-Administration. Neuropsychopharmacology. 2013;38(9):1698–1705. doi: 10.1038/npp.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66(1):1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug and Alcohol Dependence. 1999;53(3):223–230. doi: 10.1016/S0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SaL, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98(3):408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain research reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robison CL, Martz JR, Will RG, Ray CC, Dominguez JM. Estradiol microinjections to the mPOA increase cocaine-induced conditioned place preference. Program No. 822.02. 2016 Neuroscience Meeting Planner; 2016; San Diego, CA: Society for Neuroscience; 2016. Online. [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quiñones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120(2):523–533. doi: 10.1016/S0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Anderson WR, Dawson R, Seth A, Brewster M, Estes KS, Bodor N. Chronic weight loss in lean and obese rats with a brain-enhanced chemical delivery system for estradiol. Physiology & Behavior. 1988;44(4–5):573–580. doi: 10.1016/0031-9384(88)90321-6. [DOI] [PubMed] [Google Scholar]
- Tetel MJ, Pfaff DW. Contributions of estrogen receptor-α and estrogen receptor-β to the regulation of behavior. Biochimica et Biophysica Acta. 2010;1800(10):1084–1089. doi: 10.1016/j.bbagen.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiansky DJ, Roma PG, Hattori T, Will RG, Nutsch VL, Dominguez JM. The medial preoptic area modulates cocaine-induced activity in female rats. Behavioral neuroscience. 2013;127(2):293. doi: 10.1037/a0031949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiansky DJ, Will RG, Lominac KD, Turner JM, Hattori T, Krishnan K, … Dominguez JM. Estradiol in the preoptic area regulates the dopaminergic response to cocaine in the nucleus accumbens. Neuropsychopharmacology. 2016;41(7):1897–1906. doi: 10.1038/npp.2015.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hest A, van Haaren F, van de Poll NE. The behavior of male and female Wistar rats pressing a lever for food is not affected by sex differences in food motivation. Behavioural Brain Research. 1988;27(3):215–221. doi: 10.1016/0166-4328(88)90118-0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. Journal of Clinical Investigation. 2003;111(10):1444–1451. doi: 10.1172/JCI200318533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will RG, Hull EM, Dominguez JM. Influences of dopamine and glutamate in the medial preoptic area on male sexual behavior. Pharmacology Biochemistry and Behavior. 2014;121:115–123. doi: 10.1016/j.pbb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Wise RA, Morales M. A ventral tegmental CRF–glutamate–dopamine interaction in addiction. Brain research. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoest KE, Cummings JA, Becker JB. Estradiol, dopamine and motivation. Central Nervous System Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Central Nervous System Agents) 2014;14(2):83–89. doi: 10.2174/1871524914666141226103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoest KE, Cummings JA, Aragona BJ, Becker JB. Contribution of estrogen receptor subtypes to the effect of estradiol on cocaine-induced dopamine release in the nucleus accumbens. Program No. 636.21. 2016 Neuroscience Meeting Planner; 2016; San Diego, CA: Society for Neuroscience; 2016. Online. [Google Scholar]
- Zhao W, Becker JB. Sensitization enhances acquisition of cocaine self-administration in female rats: Estradiol further enhances cocaine intake after acquisition. Hormones and Behavior. 2010;58(1):8–12. doi: 10.1016/j.yhbeh.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]