Abstract
Children who experience severe early life stress show persistent deficits in many aspects of cognitive and social adaptation. Early stress might be associated with these broad changes in functioning because it impairs general learning mechanisms. To explore this possibility, we examined whether individuals who experienced abusive caregiving in childhood had difficulties with instrumental learning and/or cognitive flexibility as adolescents. Fifty‐three 14–17‐year‐old adolescents (31 exposed to high levels of childhood stress, 22 control) completed an fMRI task that required them to first learn associations in the environment and then update those pairings. Adolescents with histories of early life stress eventually learned to pair stimuli with both positive and negative outcomes, but did so more slowly than their peers. Furthermore, these stress‐exposed adolescents showed markedly impaired cognitive flexibility; they were less able than their peers to update those pairings when the contingencies changed. These learning problems were reflected in abnormal activity in learning‐ and attention‐related brain circuitry. Both altered patterns of learning and neural activation were associated with the severity of lifetime stress that the adolescents had experienced. Taken together, the results of this experiment suggest that basic learning processes are impaired in adolescents exposed to early life stress. These general learning mechanisms may help explain the emergence of social problems observed in these individuals.
RESEARCH HIGHLIGHTS
Adolescents who experienced early life stress and controls were scanned with fMRI as they completed an instrumental learning task.
Stress‐exposed adolescents learned associations of rewards and punishments more slowly than typically developing peers and showed profound deficits in reversing learned stimulus–response associations.
Brain activity in the putamen and anterior cingulate cortex during the learning task were associated with the degree of lifetime stress adolescents had experienced.
This study demonstrates that basic learning mechanisms are altered in stress‐exposed youth.
1. INTRODUCTION
Each year, more than 6 million children in the United States are referred to Child Protective Services for abuse or neglect (Fang, Brown, Florence, & Mercy, 2012). Part of the profound public health burden of such early stressful experiences is that these children begin to develop persistent deficits in a wide range of socio‐emotional and cognitive processes over their lives (Hart & Rubia, 2012). For example, children who have experienced severe early stressors, such as child abuse, are likely to have difficulties with peer relationships, as well as deficits in the cognitive processes underlying social judgment and decision‐making (dePrince, Weinzierl, & Combs, 2009; Kim‐Spoon, Cicchetti, & Rogosch, 2012; Mueller et al., 2010; Pollak et al., 2010). These sequelae of early stress exposure may lead to problems processing and integrating information from the environment, which allows individuals to rapidly and seamlessly adjust their behavior as needed (Leitzke & Pollak, 2016). Because social cues are dynamic, processing this information requires a high level of cognitive flexibility. For example, a social partner can quickly transition from being amused to annoyed during an interaction; understanding and responding to such a change would lead to a positive outcome. Here, we explore the possibility that children who experienced early and chronic stress show deficits in quickly learning and flexibly updating contextual cues, which may play a role in the development of maladaptive social processing.
1.1. Learning and social function
Basic learning mechanisms appear to be integral to social functioning in humans throughout the lifespan. Associative learning is the ability to link specific events or stimuli to other events or stimuli. In a recent study (Reeb‐Sutherland, Levitt, & Fox, 2012), associative learning at 1 month of age predicted social behaviors and face‐evoked neural activity later in infancy. In adults, too, efficient detection of stimulus–response contingencies during a learning task involving social rewards predicted social ability (Heerey, 2014). Social learning also appears to depend on the same associative mechanisms and neural circuitry that facilitate reward‐based learning (Behrens, Hunt, Woolrich, & Rushworth, 2008). For example, the ability to change behavior based on positive or negative facial expressions has been linked to activity in regions that also facilitate learning in non‐social contexts, such as orbitofrontal and anterior cingulate cortices (Kringelbach & Rolls, 2003). Thus, there is evidence to suggest that basic learning mechanisms might underlie social problems in certain populations, including children exposed to severe stress.
1.2. Effects of early life stress on learning
In this study, we examined a group of children exposed to high levels of early stress, with documented child maltreatment as a way of operationalizing severe stress in early life. Although these children likely experienced multiple sources of stress, one feature most often noted about abusive families is the physical harm and threat that children experience (Bick & Nelson, 2016; Pollak, 2015). It is known that heightened anxiety and threat adversely affect learning and attention (Eysenck, Santos, Derakshan, & Calvo, 2007). An additional factor is that caregivers in these families are often inconsistent in their responses to a child's behavior—sometimes responding in normative ways to their children, and other times becoming either extremely reactive or, conversely, unresponsive to their children (Milner & Robertson, 1989). This type of inconsistency can make it challenging for children to learn environmental contingencies. A third potential factor is that parents in these high‐risk families provide poor emotional signaling to their children, producing unclear facial and vocal emotional expressions (Shackman et al., 2011). Thus, although these parents may often be experiencing high levels of emotion, they may not convey their feelings in ways that are readily discernable for their children. Together, these factors create a very challenging learning environment for children, with input that may be too complex, too inconsistent, or too poorly signaled to support efficient learning. These circumstances may disrupt children's developing abilities to reliably associate their own and others’ emotions and behaviors (Hanson et al., 2017; Perlman, Kalish, & Pollak, 2008).
In addition to these specific consequences of abuse and unresponsive caregiving, children who are abused or neglected also tend to experience significant economic stress (Brooks‐Gunn & Markman, 2005). Poverty and/or low socioeconomic status (SES) have been associated with deficits in executive function and disrupted attention‐related neural processing (Kishiyama, Boyce, Jimenez, Perry, & Knight, 2009; Stevens, Lauinger, & Neville, 2009). However, many studies that have examined effects of SES on neurocognitive development have not examined parenting quality (for review, see Hackman, Farah, & Meaney, 2010). Several that did found that responsive parenting mitigated many of the neurocognitive sequelae associated with low SES (Farah et al., 2008; NICHD, 2005). Therefore, some reported effects of SES might actually be due to a combination of low SES and other risk factors such as parental neglect and/or abuse. This notion is consistent with a cumulative risk model, which emphasizes the importance of the additive effect of all the different stressors a child experiences (Evans, Li, & Whipple, 2013).
Both low SES and other types of early stress appear to target brain regions involved in evaluating and responding to positive and negative feedback, which may be central to the link between learning and social behavior problems. The neural circuitry involved in learning the associations between key stimuli and their contingencies includes the ventral and dorsal striatum, orbital frontal cortex (OFC; Galvan et al., 2005), prefrontal cortex (PFC), and anterior cingulate cortex (ACC) (Berridge & Robinson, 2003; see Mead, Beauchaine, & Shannon, 2010, for a review). A number of these brain regions are affected by early stress exposure. For example, early life stress has been associated with smaller prefrontal volumes in children, which could impair the abilities to regulate emotions, represent abstract gain and loss information, and change stimulus–response associations (Hanson et al., 2010; Hodel et al., 2015). Early stress exposure is also related to development of the hippocampus and amygdala (Hanson et al., 2015), circuitry necessary for distinguishing between safe and aversive cues in the environment (McLaughlin et al., 2016). Finally, abnormal structure and function of the ACC, which lies at the interface of cortical and limbic regions, and facilitates conflict detection and error monitoring, has also been observed in abused individuals (Kelly et al., 2013; Lim et al., 2015; Pechtel & Pizzagalli, 2013). In combination, these findings motivated our exploration of children's learning from both positive and negative cues as underlying the behavioral problems frequently observed in stress‐exposed children.
Functional neuroimaging and behavioral studies also suggest abnormal reward processing in individuals exposed to early stress, including physical abuse (Dillon et al., 2009; Goff et al., 2013; Hanson, Hariri, & Williamson, 2015; Mehta et al., 2010; Weller & Fisher, 2013). Reward processing is a key component of associative learning. An illustrative study examined incentive‐based learning using a Wheel of Fortune task. Here, abused children and adolescents, unlike controls, did not respond to differences in reward probability, failing to increase their response speed as the chance of winning increased (Guyer et al., 2006). This finding suggests that the children were unable to acquire or effectively use information about differing reward probabilities. In addition, studies of risk‐taking (using a Balloon Analogue Risk task [BART]) found reduced exploration behavior in neglected (Loman, Johnson, Quevedo, Lafavor, & Gunnar, 2014) and abused adolescents (Sujan, Humphreys, Ray, & Lee, 2014). These stress‐exposed children failed to engage in exploratory behavior that could have yielded larger rewards. Lack of exploration would also inhibit children's ability to efficiently learn contingencies in their environment because they would limit their range of potential input and information available. Although one study reported increased exploration among stress‐exposed adolescents during a probabilistic learning task (Hanson et al., 2017), as a whole, previous work suggests that children who experienced early stress might engage in less exploration when immediate rewards are at stake.
Relatedly, stress‐exposed individuals also show abnormal brain function while learning cues about punishment or differentiating positive and negative feedback. For example, in an inhibition task, abused adolescents showed more ACC activation than controls when they made errors (Lim et al., 2015), perhaps reflecting increased distress, which could undermine reward learning (Cavanaugh, Frank, & Allen, 2011). Women who were sexually abused also showed less neural differentiation between correct and incorrect responses during reinforcement learning, as well as increased ACC activation (Pechtel & Pizzagalli, 2013). In sum, individuals exposed to early stress show reduced sensitivity to reward, engage in behaviors that provide them with less exposure to positive and negative feedback that would facilitate learning when salient rewards and punishments are at stake, and show increased neural reactivity to punishment. All of these features could reasonably be related to difficulty with instrumental learning, a specific type of associative learning that involves mapping stimulus–response contingencies. Inabilities to learn these features of the social environment could in turn lead to behavioral problems. However, previous literature has not addressed whether instrumental learning is indeed impaired in stress‐exposed individuals.
In addition to disrupting the ability to learn from positive and negative feedback, early stress appears to impair cognitive flexibility, which is necessary to alter one's behavior in response to changing circumstances. Early adversity has been linked to lower cognitive flexibility relative to controls in preschool‐age children who were in foster care (Lewis‐Morrarty, Dozier, Bernard, Terracciano, & Moore, 2012) or who had been adopted from institutions (Hostinar, Stellern, Schaefer, Carlson, & Gunnar, 2012). Diminished cognitive flexibility has also been observed in neglected adolescents (Bauer, Hanson, Pierson, Davidson, & Pollak, 2009). However, all these studies used standardized “cold” executive function assessments that involved no extra incentives. In contrast, “hot” executive function tasks involve affective and/or reward‐related components. No previous study to our knowledge has examined cognitive flexibility in stress‐exposed adolescents in the context of incentive‐based reversal learning, which unlike standard executive function tasks, requires updating associations of reward and punishment value. We predicted that the affect‐laden nature of reversal learning would expose more profound deficits in cognitive flexibility among stress‐exposed adolescents than are found in standard executive function assessments.
1.3. Current study
The current study examines whether adolescents who experienced early stress have difficulties learning stimulus–response contingencies and flexibly updating these associations. This type of instrumental learning is a primary mechanism through which individuals can learn from outcomes that contain affective value and adjust adaptive behaviors. We examined two aspects of learning. First, we tested whether stress‐exposed adolescents were as able as their peers to efficiently acquire stimulus–response associations from rewards and punishments. Second, based on evidence that children who experienced early life stress might have particular difficulty changing pre‐potent responses (Mueller et al., 2010), we examined these individuals’ cognitive flexibility. That is, once an adolescent had learned a stimulus–response contingency, we tested the extent to which they were able to update and adjust their behavior when these contingencies were changed (e.g., a stimulus that was linked to reward became linked to punishment, or vice versa).
We predicted that stress‐exposed youth would have difficulty learning the pairings relative to controls. Beyond this initial group difference, however, we were especially interested in what these adolescents would do once they had learned an association. We expected that the stress‐exposed adolescents would have deficits in reversing or flexibly updating previously learned associations. We reasoned that such a finding would be consistent with the hypothesis that these individuals show a deficit in flexibly changing behavior, above and beyond general learning difficulties. To specify the neural mechanisms affected in these youth, we examined brain regions involved in associative learning using a whole‐brain analysis. Based on previous literature, we hypothesized that regions such as the PFC, ACC, and dorsal striatum (i.e., caudate, putamen) would be affected by early stress. Another goal of this study was to examine dose‐dependent effects of stressful life experiences on the neural correlates of these instrumental learning and cognitive flexibility processes. A few studies (Cohen et al., 2006; Hanson et al., 2016; Mueller et al., 2010) have examined cumulative effects of stress on brain structure and function, but not on the learning processes we assessed here. In the event that main effects of group or task emerged in predicted regions, we planned to explore whether deficits in learning performance were reliably associated with the degree of lifetime stress adolescents had experienced.
2. METHOD
2.1. Participants
Fifty‐three adolescents ages 14–17 were recruited from the Madison metropolitan area to participate. To recruit a sample of children who had experienced severe adversity, we targeted adolescents with documented physical abuse through the Child Protective Services division of the local Department of Human Services. This group is subsequently referred to as the “early stress” group. Non‐abused children were recruited through local advertisements. Children in the early stress group (N = 31, 12 females) had been exposed to physical abuse according to DHS reports. Children in the comparison group (N = 22, 10 females) had no exposure to maltreatment according to both DHS reports and parent responses to the Conflict Tactics Scale Parent‐Child Version (PC‐CTS; Straus, Hambey, Finkelhor, Moore, & Runyan, 1998), which quantifies the occurrence of disciplinary practices that cause distress or fear in children. Four of these participants were excluded due to missing behavioral data or not completing the task. For fMRI analyses, data from participants (n = 5) with excessive motion were omitted. This left 22 stress‐exposed and 22 control adolescents in the imaging analysis. Parents of all participants provided permission for us to access Child Protective Services Records from Dane County, Wisconsin. All parents and participants gave informed consent/assent for the study and the university IRB approved all procedures. The groups did not significantly differ on age, sex ratio, psychoactive medication use, or working memory (as indicated by Spatial Span on the CANTAB). Spatial Span, an aspect of working memory, is a measure that taps generalized cognitive ability but unlike traditional IQ tests, does not overlap with demands of the experimental learning task. Groups differed on socioeconomic status (SES), measured using the Hollingshead index of social position (Hollingshead, 1975) so we controlled for this variable in analyses. Demographic characteristics of each group are described in Table 1.
Table 1.
Characteristics of early stress and control groups
| Healthy control (n = 22) | Early stress (n = 29) | Comparison statistic | |
|---|---|---|---|
| Age: M (SD) | 14.95 (.91) | 14.78 (.85) | F(1, 49) = .75 |
| Spatial Span (CANTAB) | 6.75 (1.19) | 7.35 (1.18) | F(1, 43) = 2.79 |
| Sex | X2(1) = .085 | ||
| Male | 12 (54.5%) | 17 (58.6%) | |
| Female | 10 (45.5%) | 12 (41.4%) | |
| Ethnicity | X2(6) = 13.63a | ||
| White | 14 (63.6%) | 8 (27.6%) | |
| African American | 1 (4.5%) | 9 (31.0%) | |
| Asian | 0 | 1 (3.4%) | |
| Native American | 0 | 1 (3.4%) | |
| Mixed | 1 (4.5%) | 2 (6.9%) | |
| Other | 2 (9.1%) | 0 | |
| Did not answer | 4 (18.2%) | 8 (27.6%) | |
| Psychoactive medication | 1 (4.5%) | 4 (14.3%) | X2(1) = 1.30 |
| Socioeconomic status | 45.18 (12.92) | 27.96 (12.73) | F(1, 49) = 22.25a |
| Lifetime adversity rating | 2.5 (1.38) | 4.79 (2.5) | F(1, 49) = 14.45a |
Indicates group difference, p < .05.
2.2. Measures
2.2.1. Stress exposure
Adolescents and their parents each separately completed portions of the Youth Life Stress Interview (YLSI; Rudolph et al., 2000) to elicit information about the participants’ exposure to severe negative life events and circumstances. Trained interviewers used semi‐structured questions to assess the context of the event (e.g., timing, duration, objective consequences). Data from these interviews were then evaluated by an independent team of three to seven raters who provided a consensual rating on a 10‐point scale reflecting an overall level of cumulative life stress. The following examples illustrate the kinds of experiences children in this study described that were associated with each score. A life stress score of 1 was given to a child whose pet was hit by a car, but the pet was not seriously injured. A score of 5 was given to a child who was placed in foster care early in life and then experienced multiple placements between families; during this time the child's biological parent, with whom the child maintained a relationship, died. A score of 7.5 was given to a child whose parent and sibling both had serious, chronic medical and mental health problems; long‐term instability in parental employment; severe inter‐parental marital conflict resulting in parental separation; and extensive incarceration of one of the child's parents. A score of 10 was given to a child who was homeless; had several close family members die unexpectedly; and had physically violent parents, resulting in separation of the child from the family. This measure demonstrates high reliability (average intraclass correlations = .88–.93; Rudolph & Flynn, 2007; Rudolph et al., 2000).
2.2.2. General cognitive ability
Participants completed the spatial span length (SSP) task from the Cambridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition, Cambridge, UK) to assess individual differences in cognitive abilities not tied to associative learning. This was done to address the possibility that stress‐exposed children might show deficits in working memory that would result in poorer performance on the learning task. The CANTAB is computerized for standardized administration and does not require verbal responses. Raw performance data were z‐transformed based on norms for each subject's age and gender. CANTAB data were not collected on three participants due to a mechanical problem. No differences emerged between stress‐exposed children and controls on spatial span length (p > .1).
2.2.3. Instrumental learning task
Adolescents completed an instrumental learning task while undergoing an fMRI scan (Finger, Mitchell, Jones, & Blair, 2008; see Figure 1). Task parameters were exactly the same as in Finger, Mitchell et al. (2008). Each block consisted of 12 images, six with reward (a button press resulted in a reward of 100 points) and six with punishment (a button press resulted in a loss of 100 points). Within each block, each image was presented once in a randomized order. After eight blocks of this acquisition condition (a total of 96 trials), these associations switched for three of the positive images and three of the negative images for the remaining eight blocks of the run. This set of 96 trials formed the reversal condition. Trials in which the stimulus changed association from reward to punishment, or vice versa, are referred to as “switch” trials. Participants completed four runs, each of which lasted 10 minutes and 18 seconds. Each run involved a new set of 12 images. Trials began with the presentation for 1100 ms of one of these 12 stimuli. If no response was made, a fixation cross was presented in place of reward or punishment. The feedback phase (reward, punishment or fixation) lasted for 1000 ms, followed by a 200 ms fixation cross before the next trial began. Four Fixation trials (2300 ms) were randomly interspersed within each block, and served as the implicit baseline. The participant's goal was to win as many points as possible by learning to respond to images linked to reward and refrain from responding to the images linked to punishment.
Figure 1.
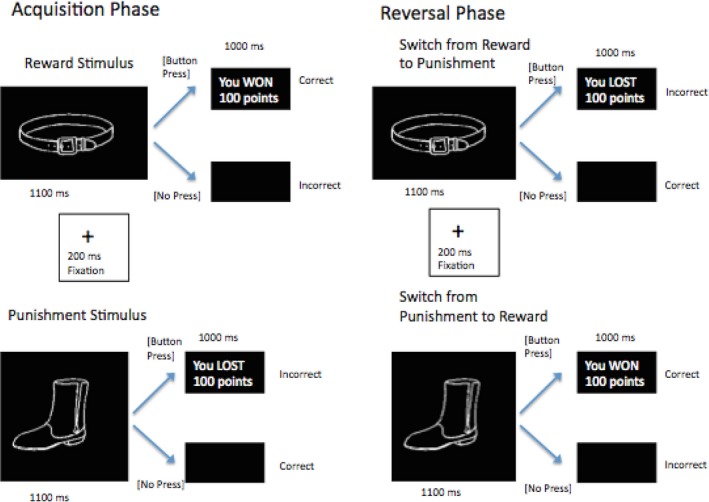
Schematic of the learning task. During the acquisition condition (left), a button press to a given image was followed by either reward (top left) or punishment (bottom left). Correct responses were those that resulted in reward. During the reversal condition (right), half of the images switched their association
Before the main task, participants completed two practice blocks outside the scanner. These blocks contained eight images, which were different from those in the main task outside the scanner. A 3.0 T GE scanner (General Electric Healthcare, Waukesha, WI) was used. Four versions of the task were developed to counterbalance the reinforcements associated with each of the stimuli. The task version and run order were randomized across participants.
2.2.4. Behavioral analysis
The acquisition and reversal conditions of the task were analyzed separately. For acquisition, all adolescents were included in the analysis (29 early stress, 22 control). For reversal, adolescents who did not perform above chance level (50%) on punishment trials in the acquisition condition were excluded (three early stress and one control), leaving 26 early stress and 21 control adolescents. We analyzed the data using two mixed factors ANOVAs, one for acquisition and one for reversal, with time (early vs. late) × stimulus type (reward vs. punishment) as within‐subjects factors and group (early stress vs. control) as a between‐subjects factor. For the time factor, block 1 was classified as early and blocks 2–8 were classified as late (see Finger, Mitchell et al., 2008). In the reversal condition, only switch trials were analyzed because non‐switch trials involved no cognitive flexibility demands.
To examine whether groups differed more in acquisition or in reversal learning, we ran a condition (acquisition vs. reversal) × stimulus type (reward vs. punishment) × group (early stress vs. control) ANOVA, excluding adolescents who performed below chance during acquisition. Given group differences in SES, we followed up all analyses adding SES as a covariate.
2.2.5. Imaging acquisition and analysis
The fMRI data were acquired on 3.0T GE Discovery, BOLD EPI, 2.0 mm slices, 1.5 mm gap, axial slices, TE: 20 ms, TR: 2300 ms, FA = 60°, FOV: 224, 64 × 64. Preprocessing and fMRI analyses were conducted using AFNI software, version 16.0.14 (Cox, 1996). Standard preprocessing steps were implemented with afni_proc.py; these steps included removing pre‐steady‐state TRs (n = 4) from the beginning of each run, slice timing, co‐registration, smoothing to 5 mm full‐width half maximum (FWHM), spatial normalizing to standard Talairach space, and resampling, which resulted in 3.5 mm3 voxels. Individual‐level regression analyses were carried out with AFNI's 3dDeconvolve function, which automatically flags regressors with medium (r > .40) to high (r > .76) levels of collinearity. Sixteen types of task‐specific events were convolved with a gamma hemodynamic response function (HRF). An additional six regressors modeled motion residuals (corresponding to translation and rotation in each of the Montreal Neurological Institute, or MNI, x, y, z directions), and four regressors modeled low‐frequency baseline drift. This analysis produced a β coefficient and t statistic for each voxel and regressor. We generated whole‐brain percentage signal‐change maps by dividing signal intensity at each voxel by the mean voxel intensity, and multiplying by 100. Temporally adjacent TRs with a Euclidian‐norm motion derivative greater than 1.5 mm were censored. Participants (n = 53) with censoring rates of 30% or more TRs were omitted from analysis. Four additional participants were excluded due to missing behavioral data or not completing the task. This left 22 stress‐exposed and 22 control adolescents in the imaging analysis. Total motion did not differ between the remaining control (M = .015 mm) and stress‐exposed adolescents (M = .016 mm).
AFNI's 3dANOVA software (Chen, Adleman, Saad, Leibenluft, & Cox, 2014) was used to conduct a repeated measures ANOVA with group (early stress, control) as a between‐participants factor and stimulus type (reward, punishment) as a within‐participants factor. Rewarded and punishment trials were contrasted against implicit baseline. We did not compare brain activity for early versus late trials because this analysis would be underpowered due to the small number of events. Acquisition and reversal conditions were analyzed separately. All events were included in these models; thus inferences were based on results from the highest‐order interactions, with significance set using an overall false detection probability based on 10,000 Monte Carlo simulations calculated by AFNI's 3dClustSim using the new ACF function which assumes a non‐Gaussian distribution of noise and is therefore less prone to false positives than older methods (Cox, Chen, Glen, Reynolds, & Taylor, 2017). This resulted in a mean estimated spatial correlation of 4.22 mm × 13.31 mm × 11.45 mm FWHM. In brief, this approach creates multiple simulated null datasets from which a distribution of cluster sizes corresponding to a desired corrected p‐value can be determined (using AFNI's 3dClustStim). An initial (uncorrected) statistical threshold of p < .005 was chosen. Based on this threshold, the number of comparisons in our imaging volume, and the smoothness of our imaging data (as measured by 3dFWHMx), a minimum cluster size of 34 voxels (417 mm3) was required to have a corrected p ≤ .05.
To facilitate interpretation and interrogate factors driving interactions, we conducted post‐hoc analyses in SPSS on extracted mean signal changes from significant clusters that were hypothesized to be active during the learning task.
3. RESULTS
3.1. Is early stress exposure associated with impaired learning?
To examine potential effects on adolescents’ acquisition of stimulus contingencies, we first analyzed the rate of participants’ learning (i.e., performance in early vs. late blocks) during the acquisition condition. These data, shown in Figure 2, show an expected effect of time on acquisition, F(1, 49) = 314.33, p < .001: participants responded less accurately in block 1 and then improved across time in blocks 2–8. In addition, a trial type × time interaction, F(1, 49) = 303.01, p < .001, indicated that participants improved over time for punishment trials, but did not improve over time for reward trials. This pattern was expected, because on this instrumental learning task, the optimal strategy is to respond to all stimuli initially in order to learn whether they are linked to reward or punishment. The challenge is to learn which stimuli should elicit withholding a response.
Figure 2.
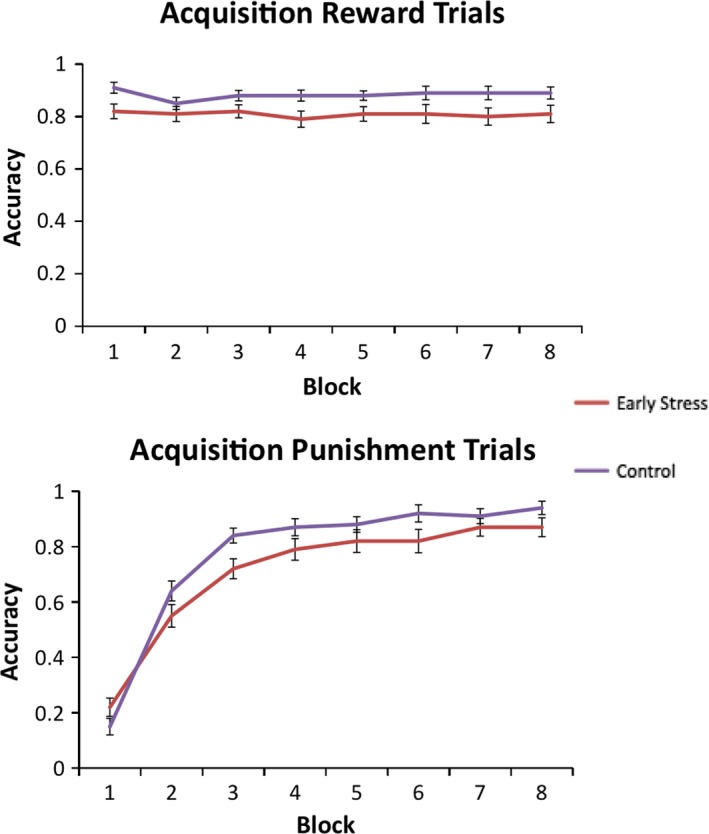
Accuracy in each block for the early stress and control groups during acquisition for images associated with reward (top) and with punishment (bottom). Error bars show ± 1 SE
There was also a significant main effect of group, showing that stress‐exposed adolescents responded less accurately throughout the task, F(1, 49) = 7.5, p < .001. But this effect was qualified by a trial type × time × group interaction, F(1, 49) = 5.38, p < .03. Simple effects tests clarified that (1) the early stress group did not respond to reward stimuli as frequently as controls throughout the task, F(1, 49) = 4.8, p < .04, and (2) for punishment cues, stress‐exposed participants both responded marginally less accurately, F(1, 49) = 3.12, p = .08, and learned more slowly than their peers, as indicated by a significant group × time interaction for punishment trials only, F(1, 49) = 5.44, p < .03. When we controlled for SES, all main effects held (time, F(1, 47) = 18.55, p < .001; trial type, F(1, 47) = 9.29, p < .005), including the main effect of group, F(1, 47) = 3.97, p = .05. However the trial type × time × group interaction was no longer significant, p > .02.
3.2. Is early stress associated with impaired flexibility in learning?
We were especially interested in whether early life stress would be associated with an individual's ability to update a learned association. Results for the reversal condition (in which half of previously rewarded stimuli are punished and half of previously punished stimuli are rewarded) are shown in Figure 3. There was again a main effect of time, F(1, 45) = 325.9, p < .001, indicating poorer initial accuracy in block 1. In addition, a significant trial type × time interaction, F(1, 45) = 5.36, p < .03, showed that participants improved more rapidly on trials that switched from punishment to reward than on trials that switched from reward to punishment.
Figure 3.
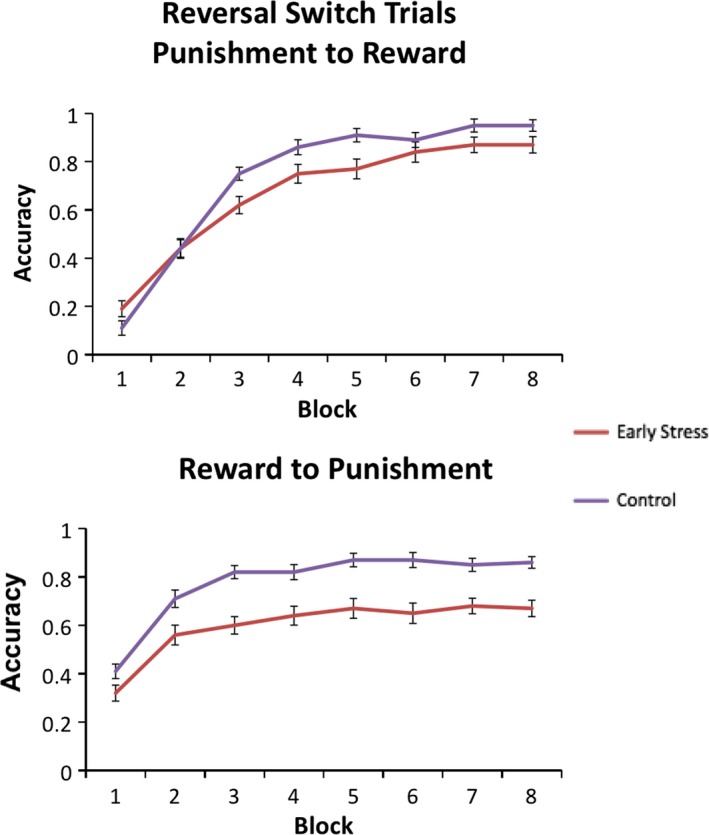
Accuracy in each block for the early stress and control groups during reversal for images that switched their association from punishment to reward (top) and reward to punishment (bottom). Error bars show ± 1 SE
As predicted, a main effect of group, F(1, 45) = 4.98, p = .03, reflected that stress‐exposed participants had greater difficulty learning the new contingencies. A group × time interaction, F(1, 45) = 5.45, p < .03, indicated that adolescents with early stress exposure did not improve their accuracy over time to the same extent as controls (i.e., groups performed equally in block 1 (p > .8), but the control group performed more accurately in subsequent blocks, F(1, 47) = 12.58, p < .001. All of these effects held when controlling for SES (time, F(1, 47) = 17.98, p < .001; time × group, F(1, 47) = 3.45, p < .07; group, F(1, 47) = 5.01, p = .03).
3.3. Is cognitive flexibility more impaired than associative learning in stress‐exposed adolescents?
Finally, to further test the role of cognitive flexibility we determined whether group differences were more pronounced in the reversal condition. To do so, we ran a 2 (condition: acquisition vs. reversal) × 2 (trial type) × 2 (group) ANOVA on blocks 2–8. A main effect of condition, F(1, 45) = 41.07, p < .001, indicated that all participants responded less accurately during the reversal condition. In addition to a main effect of group, F(1, 45) = 12.37, p = .001, there was a condition × group interaction, F(1, 45) = 6.40, p <.02, which reflects that early stress and control groups differed more in reversal, F(1, 47) = 12.58, p < .001, than in acquisition, F(1, 47) = 6.53, p < .02. In other words, stress‐exposed adolescents showed more pronounced learning problems in the reversal condition. All effects remained significant when we controlled for SES (condition, F(1, 45) = 3.46, p < .07; condition × group, F(1, 45) = 7.73, p < .01; group, F(1, 45) = 5.69, p = .02).
3.4. Neural mechanisms associated with learning performance
Next, we examined neural circuitry differences between the groups to better understand the basis of their learning performance. Given this study's focus on neural responses to positive and negative feedback, we tested only trials in which participants made a response (correct hits on reward trials and punished errors on avoidance trials), to control for motor activity. We did not examine time as a factor, given the small number of trials, so that we could obtain a strong signal to noise ratio for the imaging analyses. Finally, we conducted two 2 (group) × 2 (trial type) ANOVAs, one for acquisition and one for reversal, to compare group differences in each type of trial.
3.4.1. Acquisition condition
Significant clusters of activation for the acquisition condition are shown in Table 2. A main effect of trial type indicated that participants showed greater activation during punished errors than correct hits in the cerebellum, thalamus, and midbrain.
Table 2.
Clusters showing significant activation during acquisition. Only contrasts that yielded significant clusters are listed
| Punishment > Reward (All) | Cluster size (mm3) | MNI | ||
|---|---|---|---|---|
| x | y | z | ||
| Midbrain/Thalamus/Cerebellum | 10,265 | −8 | −72 | −23 |
| Punishment > Reward (Early Stress Group) | Cluster size (mm3) | MNI | ||
|---|---|---|---|---|
| x | y | z | ||
| Left cerebellum | 2,033 | −29 | −71 | −32 |
| Right culmen | 1,347 | 2 | −35 | −6 |
| Left lingual gyrus | 968 | −17 | −89 | −12 |
| Right cerebellum | 857 | 18 | −81 | −27 |
3.4.2. Reversal condition
We restricted analyses of fMRI data in the reversal condition to trials that switched contingencies (rewarded → punished; punished → rewarded). A main effect of trial type indicated several significant clusters of activation, which are shown in Table 3. Participants showed greater activation in right anterior cingulate, putamen, and left cerebellum during punished reversal errors than during correct hits. There were also significant group × trial type interactions: During incorrect trials that switched from reward to punishment (punished reversal errors), controls showed greater activation than the early stress group in a number of attention‐related regions, including bilateral middle frontal gyri, cuneus, and cerebellum (see Figure 4). During correct hits, no significant group activation differences were detected.
Table 3.
Clusters showing significant activation during reversal learning (switch trials). Only contrasts that yielded significant clusters are listed
| Punishment Trials > Baseline, Controls > Early Stress | Cluster Size (mm3) | MNI | ||
|---|---|---|---|---|
| x | y | z | ||
| Left Middle Frontal Gyrus | 625 | −48 | 22 | 38 |
| Right Middle Frontal Gyrus | 588 | 46 | 30 | 32 |
| Right Cuneus | 514 | 13 | −93 | 16 |
| Right Cerebellum | 955 | 31 | −59 | −39 |
| Punishment > Reward (All) | Cluster Size (mm3) | MNI | ||
|---|---|---|---|---|
| x | y | z | ||
| Right Anterior Cingulate | 845 | 3 | 41 | 19 |
| Left Cerebellum | 502 | −1 | −52 | −52 |
| Right Putamen | 1,017 | 20 | 22 | −1 |
| Punishment > Reward, Early Stress | Cluster Size (mm3) | MNI | ||
|---|---|---|---|---|
| x | y | z | ||
| Left Anterior Cingulate | 674 | −2 | 42 | 13 |
| Punishment > Reward, Controls | Cluster Size (mm3) | MNI | ||
|---|---|---|---|---|
| x | y | z | ||
| Right Lingual Gyrus | 502 | 19 | −93 | −11 |
| All Switch Trials > Baseline, Controls > Early Stress | Cluster Size (mm3) | MNI | ||
|---|---|---|---|---|
| x | y | z | ||
| Right Cerebellum | 2,070 | 33 | −61 | −43 |
| Right Cerebellar Vermis | 1,421 | 1 | −68 | −42 |
| Left Middle Frontal Gyrus | 1,384 | −49 | 16 | 40 |
| Right Superior Frontal Gyrus | 1,053 | 34 | 57 | 21 |
| Right Middle Frontal Gyrus | 968 | 45 | 21 | 37 |
| Left Inferior Frontal Gyrus | 723 | −48 | 23 | 2 |
| Right Culmen | 698 | 18 | −43 | −28 |
| Right Fusiform/Middle Occipital Gyrus | 2,866 | 20 | −89 | 13 |
Figure 4.
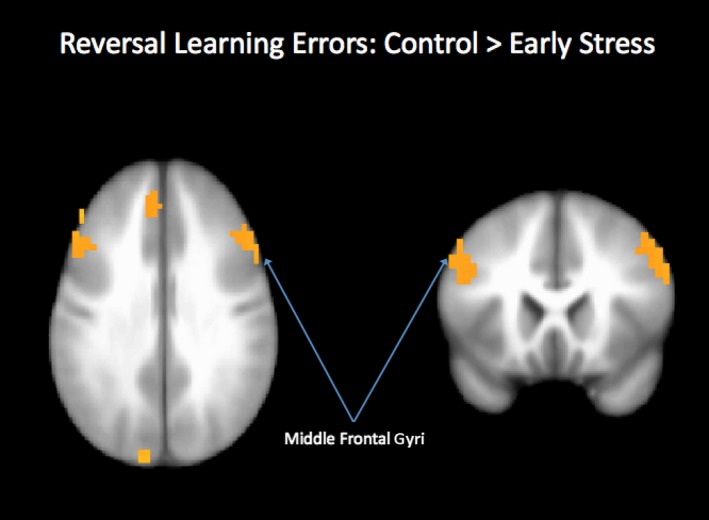
Regions showing a group by condition interaction during reversal learning. Regions showing significant activation for the contrast of Controls > Early Stress, punished reversal errors > baseline included bilateral middle frontal gyri
We extracted signal change values for a priori regions of interest from task‐based activation results that were significant in the full sample to articulate group × trial type interactions: These a priori regions included the right ACC and putamen. By using a task‐based cluster to explore group differences in these ROIs, we avoid conducting a circular analysis. These regions showed greater activation to punishment than to reward in both groups (putamen: F(1, 42) = 21.92, p < .001; ACC: F(1, 42) = 19.74, p < .001), but no group differences emerged. We confirmed these findings using the anatomical ROIs from the Talairach‐Tournoux (1988) atlas (see online supplement).
3.5. Is neural activation related to learning performance?
We next examined the extent to which learning performance was related to mean voxel activation in the putamen, and ACC in the early stress and control groups separately (given group differences in performance). For learning performance, we calculated the average accuracy in blocks 2–8 (given expected poor performance in block 1) for each type of trial in the acquisition and reversal conditions. No significant associations emerged among controls. However, among stress‐exposed adolescents, ACC activation during reward trials was positively associated with reversal learning performance (see Table 4). The overall summary of these findings is that adolescents who experienced early stress tended to show activation to reward that was associated with better performance.
Table 4.
Associations (Pearson R) between neural activation (mean voxel value) and behavioral performance in the early stress group
| fMRI condition | Region | Correlation |
|---|---|---|
| Reversal, Reward | Putamen | .318 |
| Reversal, Punishment | Putamen | −.172 |
| Reversal, Reward | ACC | .517* |
| Reversal, Punishment | ACC | −.137 |
*p < .05; **p < .01; *** p < .001. Correlations between putamen/anterior cingulate activation and behavior were examined for reversal only, because these regions were not active during acquisition.
3.6. Is learning performance related to individual differences in stress exposure?
Finally, we examined the extent to which both learning performance and brain activity in the putamen and ACC were linked to the degree of lifetime stress participants had experienced. We examined brain activation in the putamen and anterior cingulate clusters that were differentially activated by trial type among the full sample. These brain regions have previously been linked to instrumental and associative learning (Berridge & Robinson, 2003; Williams & Eskandar, 2006). We used Pearson correlations with the youth life stress interview on both the full sample and in the early stress group alone, controlling for SES.
Both instrumental learning performance and brain activation during learning were correlated with lifetime stress (see Table 5). Individuals who had experienced more lifetime stress showed poorer performance in avoidance learning during acquisition, and less cognitive flexibility during the reversal condition. In addition, higher lifetime stress was associated with reduced reward‐related activation in the right putamen and ACC during reversal learning. Furthermore, these correlations were also significant when analyses were restricted to the early stress group alone, and were significant when using mean signal change from anatomical ROIs (see Supplementary Table 1). Correlations between activation in these regions during punished errors were not correlated with lifetime stress.
Table 5.
Correlations of lifetime stress with learning performance and brain activation (mean voxel value) in the full sample and early stress group only
| Lifetime stress | |
|---|---|
| Brain activation | |
| Putamen, Rewarded trials, Reversal | −.46* (−.53*) |
| ACC, Rewarded trials, Reversal | −.43* (−.43) |
| Behavioral performance | |
| Acquisition, Reward learning | −.13 (−.21) |
| Acquisition, Avoidance learning | −.36* (−.56*) |
| Reversal learning (All switch trials) | −.38* (−.42^) |
*Indicates group difference, ^p < .1; *p < .05. R values in parentheses represent associations in the Early Stress group only.
4. DISCUSSION
In this experiment, we studied individuals who had experienced childhood physical abuse to better understand the role of severe early stress exposure on instrumental learning and cognitive flexibility during adolescence. The goal of this project was to identify one of the potential mechanisms that might explain why these individuals are at heightened risk for a broad range of social, interpersonal, and behavioral problems over the course of their development. Extant research has examined difficulties these individuals encounter with socio‐emotional stimuli and tasks oriented around social processes. However, we aimed to test the idea that general learning mechanisms, rather than specific socio‐emotional processes, might be impacted by early stress. Moreover, we sought to examine both how these individuals initially acquire new information as well as how efficiently they are able to adjust to new information as environmental contingencies are updated. These processes appear to be critical for adaptive navigation of interpersonal situations. In brief, the results of this study indicate that adolescents with histories of early stress were impaired in both instrumental learning and cognitive flexibility. We examined functional brain activity to determine whether there was converging evidence at both behavioral and neural levels, and found that these individuals also showed abnormal activity in learning‐ and attention‐related brain regions. Finally, these altered patterns of behavioral performance and neural activation were linked to the degree of lifetime stress adolescents had experienced.
We tested two primary hypotheses, both of which were supported by the data. First, stress‐exposed adolescents showed impaired instrumental learning relative to controls. These adolescents learned more slowly than controls to inhibit responses to stimuli associated with punishment, showed diminished learning from rewarding feedback, and did not take advantage of their environment to learn stimulus–response contingencies to the same extent as controls. Second, the gap in performance between the early stress and control groups was most striking when the participants had to flexibly update this recently learned information.
To better understand the processes underlying these learning problems we examined activation of brain regions that facilitate instumental learning. Although we also expected to detect overall differences in the caudate, a small cluster in the caudate region did not meet our significance threshold. However, during instrumental learning, stress‐exposed adolescents had greater activation to punished errors as compared to rewarded correct responses in brain regions associated with arousal (midbrain/thalamus), and the cerebellum, which supports some aspects of learning and cognition (Bauer et al., 2009); this was not true for the control youth. These results suggest that early aspects of arousal and attention may result in high reactivity to punishment in stress‐exposed youth; although some reactivity to punishment is expected, too much reactivity could undermine efficient learning (Cavanaugh et al., 2011). Future research should explore the role of these regions in supporting attentional gating, learning, and cognition in both typically developing and stress‐exposed individuals.
When participants had to update recently learned information, we observed robust group differences in frontal brain regions. Stress‐exposed adolescents showed reduced frontal sensitivity to punishment signals. During this “re‐learning”, the early stress group showed less activation than controls after making an error in regions that facilitate attentional control, including bilateral middle frontal gyri. Reduced activation in these frontal regions may be tied to impairments in flexible updating, given their role in facilitating the switching of stimulus–response associations and performance adjustments during cognitive control tasks (Ghahremani, Monterosso, Jentsch, Bilder, & Poldrack, 2010; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004).
Stress‐exposed adolescents also showed different patterns of putamen and ACC activation from controls. First, unlike controls, they showed activation in the left ACC during punished errors, which may reflect frustration and/or emotional distress (Lim et al., 2015). This activation occurred in the pregenual sub‐region of ACC, which is associated with negative affect (Shackman et al., 2011), and has been shown to play a role in regulating emotional responses to stressful events and assigning emotional valence to stimuli (Pizzagalli, 2011). Therefore, stress‐exposed adolescents may experience punishment trials as more aversive and expend more effort to regulate their emotional response to the outcome. Furthermore, adolescents who showed reduced right ACC activation to reward trials during reversal learning had the most difficulty with this cognitive flexibility task. This finding could reflect reduced cognitive engagement during rewarded trials in adolescents who had difficulty switching associations, given the ACC's role in effortful performance and response optimization (Bush et al., 2002). Reduced engagement and attention to reward would lead to reduced learning from reward. This was reflected in the positive association between ACC activation to reward and learning performance.
4.1. Relationship between the concepts of stress exposure and child abuse
In this experiment, we consider the adolescents who experienced physical abuse to represent an “early stress” group. We refer to this group broadly in terms of stress exposure for two reasons. First, these children may have experienced other stressors in addition to abuse that we were not able to accurately measure, including domestic violence, neglect and emotional abuse. Second, although documented physical abuse was used as a way to identify individuals who experienced early stress exposure, the effects on learning and cognitive flexibility that we observed appeared to be dependent upon stress severity. In addition to evaluating differences between abused and nonabused youth, adolescents who experienced many stressful life events showed more difficulty with learning and less neural sensitivity to reward. Even among the early stress group alone, variation in lifetime stress is associated with poorer associative learning and less cognitive flexibility. These associations between childhood stress exposure and putamen and ACC reward responsivity are consistent with other published studies linking the number of adverse childhood experiences (ACEs) to abnormal ACC structure and function (Cohen et al., 2006; Hanson et al., 2016; Mueller et al., 2010). The current data further build on previous findings in linking functional reward‐related brain activity in these regions during a learning task to childhood stress exposure.
4.2. Learning versus flexibility
In this experiment, the stress‐exposed adolescents did not perform as well as controls during acquisition. At first glance, it might not make sense to look at group differences during reversal if one group performed less well on initial learning. However, the initial group differences in acquisition can also mean that the cognitive flexibility deficits in the stress‐exposed participants are especially profound. If these adolescents did not learn the initial stimulus–response pairings as well as controls, the reversal condition should actually be easier for them because they do not need to “un‐learn” these associations. Yet, our high‐stress participants still showed profound deficits in cognitive flexibility. In fact, the group effects on learning deficits in the reversal phase of the task were stronger than those in acquisition, suggesting that the additional demand for cognitive flexibility led to further performance decrements among stress‐exposed adolescents. While further research will be needed to parse the relationship between the effects of multiple cognitive processes, the present data are consistent with the view that the participants who experienced stress exposure in early childhood have distinct difficulties in both associative learning and cognitive flexibility.
There are several ways in which early childhood stress might affect learning abilities. The family environments of children who experience abuse tend to be unstable and unpredictable (Bousha & Twentyman, 1984; Lyons‐Ruth & Block, 1996). We suspect that chronically inconsistent feedback from caregivers makes it difficult for children to learn from positive and negative feedback. In addition, unpredictable harsh feedback might also reduce children's tendency to explore their environment. In fact, the task used in the current experiment required participants to make a response in order to learn whether the image was associated with a reward or a punishment. Unlike controls, stress‐exposed adolescents did not follow the optimal strategy of initially responding to images indiscriminately at the beginning of each acquisition run to explore and gather information. Other recent studies using different tasks have made similar observations that appear to reflect reduced exploration or attenuated tendencies to gather information about an unknown environment among adolescents exposed to early life stress, especially when incentives are at stake (Humphreys et al., 2015). Decreased exploratory behavior during adolescence, or lack of a normative increase in exploration, might have negative impacts on social adjustment (Bhanji & Delgado, 2014; Pfeifer et al., 2011; Telzer, 2016). Furthermore, our results are consistent with a number of studies that show reduced neural sensitivity to reward among individuals exposed to early life stress (Dillon et al., 2009; Guyer et al., 2006; Mehta et al., 2010).
4.3. Limitations
While all of the adolescents in the early stress group experienced verified physical abuse during childhood, there is still some heterogeneity in the timing and duration of childhood maltreatment, and we did not have sufficiently detailed data to examine these individual differences. Relatedly, the abused children's families had lower SES than the controls. This means that the combination of low SES and abuse, rather than abuse alone, may explain differences in learning, cognitive flexibility, and brain function in our early stress group. However, our findings held when controlling for SES, indicating that severe early stressors like physical abuse affect cognitive flexibility over and above the effects of low SES. Future studies should continue to examine interactions between SES/poverty and abuse on children's learning ability, including potential mediating effects of parenting.
It is conceivable that group differences in learning may simply reflect reduced inhibitory control (Hart & Rubia, 2012) in stress‐exposed adolescents. However, the fact that these adolescents showed reduced learning on both types of reversal trials (those that switched from reward to punishment and vice versa) suggests that this deficit is not simply due to reduced inhibitory control. If inhibition were the primary problem, reduced performance would be expected only for stimuli that switched from reward to punishment. Future work may disambiguate the extent to which inhibitory control, learning ability, and cognitive flexibility account for learning impairments in stress‐exposed youth.
4.4. Conclusion
This study demonstrates that early stress exposure alters basic learning processes, including instrumental learning and cognitive flexibility at the behavioral and neural levels. Early life stress, reduced frontal activation, and reduced neural reward responsivity have all been linked to psychopathology, particularly depression (Pizzagalli, 2015; Russo & Nestler, 2013) as well as undermining many aspects of social behavior. Future research should investigate directly how these learning mechanisms may impact social development, and how greater understanding of these processes can be used to guide the development of targeted prevention and intervention programs for high‐risk youth. Our findings suggest that studying basic learning processes in stress‐exposed youth will yield important knowledge about how stress affects brain development and behavior, and in turn, generate interventions to ameliorate the downstream consequences of adverse early environments.
Supporting information
ACKNOWLEDGEMENTS
We thank the children and families who participated in this study, and the research assistants who helped with data collection. J. Hanson is now at the University of Pittsburgh and K. Shannon Bowen is now at Seattle Children's Hospital.
Harms MB, Shannon Bowen KE, Hanson JL, Pollak SD. Instrumental learning and cognitive flexibility processes are impaired in children exposed to early life stress. Dev Sci. 2018;21:e12596 https://doi.org/10.1111/desc.12596
Funding Information
The authors declare no competing financial interests. Funding sources were NIMH MH61285 and NICHD HD03352 to S. Pollak. M. Harms was supported by T32‐MH018931. J. Hanson was supported by F31‐DA028087. K. Shannon Bowen was supported by T‐32 HD07489.
REFERENCES
- Achenbach, T.M. , & Rescorla, L.A. (2001). Manual for the ASEBA School‐Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Bauer, P.M. , Hanson, J.L. , Pierson, R.K. , Davidson, R.J. , & Pollak, S.D. (2009). Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biological Psychiatry, 66, 1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens, T.E. , Hunt, L.T. , Woolrich, M.W. , & Rushworth, M.F. (2008). Associative learning of social value. Nature, 456, 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, K.C. , & Robinson, T.E. (2003). Parsing reward. Trends in Neurosciences, 26, 507–513. [DOI] [PubMed] [Google Scholar]
- Bhanji, J.P. , & Delgado, M.R. (2014). The social brain and reward: Social information processing in the human striatum. Wiley Interdisciplinary Reviews. Cognitive Science, 5, 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick, J. , & Nelson, C.A. (2016). Early adverse experiences and the developing brain. Neuropsychopharmacology Reviews, 41, 177–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousha, D.M. , & Twentyman, C.T. (1984). Mother–child interactional style in abuse, neglect, and control groups: Naturalistic observations in the home. Journal of Abnormal Psychology, 93, 106–114. [DOI] [PubMed] [Google Scholar]
- Brooks‐Gunn, J. , & Markman, L.B. (2005). The contribution of parenting to ethnic and racial gaps in school readiness. Future of Children, 15, 139–168. [DOI] [PubMed] [Google Scholar]
- Bush, G. , Vogt, B.A. , Holmes, J. , Dale, A.M. , Greve, D. , Jenike, M.A. , & Rosen, B.R. (2002). Dorsal anterior cingulate cortex: A role in reward‐based decision making. Proceedings of the National Academy of Sciences, USA, 99, 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh, J.F. , Frank, M.J. , & Allen, J.J.B. (2011). Social stress reactivity alters reward and punishment learning. Social, Cognitive, & Affective Neuroscience, 6, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. , Adleman, N.E. , Saad, Z.S. , Leibenluft, E. , & Cox, R.W. (2014). Applications of multivariate modeling to neuroimaging group analysis: A comprehensive alternative to univariate general linear model. NeuroImage, 99, 571–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R.A., Grieve S., Hoth K.F., Paul R.H., Sweet L., Tate D., … & Williams, L.M. (2006). Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry, 59, 975–982. [DOI] [PubMed] [Google Scholar]
- Cox, R.W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers & Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cox, R.W. , Chen, G. , Glen, D.R. , Reynolds, R.C. , & Taylor, P.A. (2017). fMRI clustering and false‐positive rates. Proceedings of the National Academy of Sciences, USA, 114, E3370–E3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dePrince, A.P. , Weinzierl, K.M. , & Combs, M.D. (2009). Executive function performance and trauma exposure in a community sample of children. Child Abuse and Neglect, 33, 353–361. [DOI] [PubMed] [Google Scholar]
- Dillon, D.G. , Holmes, A.J. , Birk, J.L. , Brooks, N. , Lyons‐Ruth, K. , & Pizzagalli, D.A. (2009). Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry, 66, 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, G.W. , Li, D. , & Whipple, S.S. (2013). Cumulative risk and child development. Psychological Bulletin, 139, 1342–1396. [DOI] [PubMed] [Google Scholar]
- Eysenck, M.W. , Santos, R. , Derakshan, N. , & Calvo, M.G. (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7, 336–353. [DOI] [PubMed] [Google Scholar]
- Fang, X. , Brown, D.S. , Florence, C.S. , & Mercy, J.A. (2012). The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse and Neglect, 36, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah, M.J. , Betancourt, L. , Shera, D.M. , Savage, J.H. , Giannetta, J.M. , Brodsky, & Hurt, H. (2008). Environmental stimulation, parental nurturance and cognitive development in humans. Developmental Science, 11, 793–801. [DOI] [PubMed] [Google Scholar]
- Finger, E.C. , Mitchell, D.G.V. , Jones, M. , & Blair, R.J.R. (2008). Dissociable roles of medial orbital frontal cortex in human operant extinction learning. NeuroImage, 43, 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan, A. , Hare, T.A. , Davidson, M. , Spicer, J. , Glover, G. , & Casey, B.J. (2005). The role of ventral frontostriatal circuitry in reward‐based learning in humans. Journal of Neuroscience, 25, 8650–8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani, D. , Monterosso, J. , Jentsch, J.D. , Bilder, R.M. , & Poldrack, R.A. (2010). Neural components underlying behavioral flexibility in human reversal learning. Cerebral Cortex, 20, 1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff, B. , Gee, D.G. , Telzer, E.H. , Humphreys, K.L. , Gabard‐Durnam, L. , Flannery, J. , & Tottenham, N. (2013). Reduced nucleus accumbens reactivity and adolescent depression following early‐life stress. Journal of Neuroscience, 249, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer, A.E. , Kaufman, J. , Hodgdon, H.B. , Masten, C.L. , Jazbec, S. , Pine, D.S. , & Ernst, M. (2006). Behavioral alterations in reward system function: The role of childhood maltreatment and psychopathology. Journal of the American Academy of Child and Adolescent Psychiatry, 45, 1059–1067. [DOI] [PubMed] [Google Scholar]
- Hackman, D.A. , Farah, M.J. , & Meaney, M.J. (2010). Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience, 11, 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J.L. , Albert, W.D. , Iselin, A.M.R. , Carré, J.M. , Dodge, K.A. , & Hariri, A.R. (2016). Cumulative stress in childhood is associated with blunted reward‐related brain activity in adulthood. Social Cognitive and Affective Neuroscience, 11, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J.L. , Bos, W. , Roeber, B.J. , Rudolph, K.D. , Davidson, R.J. , & Pollak, S.D. (2017). Early adversity and learning: Implications for typical and atypical behavioral development. Journal of Child Psychology and Psychiatry, 58, 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J.L. , Chung, M.K. , Avants, B.B. , Shirtcliff, E.A. , Gee, J.C. , Davidson, J.R.T. , & Pollak, S.D. (2010). Early stress is associated with alterations in the orbitofrontal cortex: a tensor‐based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience, 30, 7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J.L. , Hariri, A.R. , & Williamson, D.E. (2015). Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biological Psychiatry, 78, 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J.L. , Nacewicz, B.M. , Sutterer, M.J. , Cayo, A.A. , Schaefer, S.M. , Rudolph, K.L. , … & Davidson, R.J. (2015). Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry, 77, 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, H. , & Rubia, K. (2012). Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience, 6, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey, E.A. (2014). Learning from social rewards predicts individual differences in self‐reported social ability. Journal of Experimental Psychology: General, 143, 332–339. [DOI] [PubMed] [Google Scholar]
- Hodel, A.S. , Hunt, R.H. , Cowell, R.A. , van den Heuvel, S.E. , Gunnar, M.E. , & Thomas, K.M. (2015). Duration of early adversity and structural brain development in post‐institutionalized adolescents. NeuroImage, 105, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead, A.B. (1975). Four factor index of social status. Unpublished manuscript. Yale University, New Haven, CT.
- Hostinar, C.E. , Stellern, S.A. , Schaefer, C. , Carlson, S.M. , & Gunnar, M.R. (2012). Associations between early life adversity and executive function in children adopted internationally from orphanages. Proceedings of the National Academy of Sciences, USA, 109, 17208–17212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys, K.L. , Lee, S.S. , Telzer, E.H. , Gabard‐Durnam, L.J. , Goff, B. , Flannery, J. , & Tottenham, N. (2015). Exploration‐exploitation strategy is dependent on early experience. Developmental Psychobiology, 57, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, P.A. , Viding, E. , Wallace, G.L. , Schaer, M. , De Brito, S.A. , Robustelli, B. , & McCrory, E.J. (2013). Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: Neural markers of vulnerability? Biological Psychiatry, 74, 845–852. [DOI] [PubMed] [Google Scholar]
- Kim‐Spoon, J.A. , Cicchetti, D. , & Rogosch, F.A. (2012). A longitudinal study of emotion regulation, emotion lability‐negativity, and internalizing symptomatology in maltreated and nonmaltreated children. Child Development, 84, 512–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishiyama, M.M. , Boyce, W.T. , Jimenez, A.M. , Perry, L.M. , & Knight, R.T. (2009). Socioeconomic disparities affect prefrontal function in children. Journal of Cognitive Neuroscience, 21, 1106–1115. [DOI] [PubMed] [Google Scholar]
- Kringelbach, M.L. , & Rolls, E.T. (2003). Neural correlates of rapid reversal learning in a simple model of human social interaction. NeuroImage, 20, 1371–1383. [DOI] [PubMed] [Google Scholar]
- Leitzke, B.T. , & Pollak, S.D. (2016). Developmental changes in the primacy of facial cues for emotion recognition. Developmental Psychology, 52, 572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis‐Morrarty, E. , Dozier, M. , Bernard, K. , Terracciano, S. , & Moore, S. (2012). Cognitive flexibility and theory of mind outcomes among foster children: Preschool follow‐up results of a randomized clinical trial. Journal of Adolescent Health, 51, S17–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, L. , Hart, H. , Mehta, M.A. , Simmons, A. , Mirza, K. , & Rubia, K. (2015). Neural correlates of error processing in young people with a history of severe childhood abuse: An fMRI study. American Journal of Psychiatry, 892, 892–900. [DOI] [PubMed] [Google Scholar]
- Loman, M.M. , Johnson, A.E. , Quevedo, K. , Lafavor, T.L. , & Gunnar, M.R. (2014). Risk‐taking and sensation‐seeking propensity in postinstitutionalized early adolescents. Journal of Child Psychology and Psychiatry, 55, 1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons‐Ruth, K. , & Block, D. (1996). The disturbed caregiving system: Relations among childhood trauma, maternal caregiving, and infant affect and attachment. Infant Mental Health Journal, 17, 257–275. [Google Scholar]
- McLaughlin, K.A. , Sheridan, M.A. , Gold, A.L. , Duys, A. , Lambert, H.K. , Perevill, M. , … & Pine, D.S. (2016). Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology, 41, 1956–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead, H.K. , Beauchaine, T.P. , & Shannon, K.E. (2010). Neurobiological adaptations to violence across development. Development and Psychopathology, 22, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, M.A. , Gore‐Langton, E. , Golembo, N. , Colvert, E. , Williams, S.C. , & Sonuga‐Barke, E. (2010). Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of Cognitive Neuroscience, 22, 2316–2325. [DOI] [PubMed] [Google Scholar]
- Milner, J.S. , & Robertson, K.R. (1989). Inconsistent response patterns and the prediction of child maltreatment. Child Abuse and Neglect, 13, 59–64. [DOI] [PubMed] [Google Scholar]
- Mueller, S.C. , Maheu, F S. , Dozier, M. , Peloso, E. , Mandell, D. , Leibenluft, E. , … & Ernst, M. (2010). Early‐life stress is associated with impairment in cognitive control in adolescence: An fMRI study. Neuropsychologia, 48, 3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Child Health and Human Development Early Child Care Research Network (2005). Duration and developmental timing of poverty and children's cognitive and social development from birth through third grade. Child Development, 76, 795–810. [DOI] [PubMed] [Google Scholar]
- Pechtel, P. , & Pizzagalli, D.A. (2013). Disrupted reinforcement learning and maladaptive behavior in women with a history of childhood sexual abuse. JAMA Psychiatry, 70, 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman, S.B. , Kalish, C.W. , & Pollak, S.D. (2008). The role of maltreatment experience in children's understanding of the antecedents of emotion. Cognition and Emotion, 22, 651–670. [Google Scholar]
- Pfeifer, J.H. , Masten, C.L. , Moore, W.E. , Oswald, T.M. , Mazziotta, J.C. , Iacoboni, M. , & Dapretto, M. (2011). Entering adolescence: Resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron, 69, 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli, D. (2011). Frontocingulate dysfunction in depression: Toward biomarkers of treatment response. Neuropsychopharmacology, 36, 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli, D.A. (2015). Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annual Reviews of Clinical Psychology, 10, 303–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak, S.D. (2015). Multilevel developmental approaches to understanding the effects of child maltreatment: Recent advances and future challenges. Development and Psychopathology, 27, 1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak, S.D. , Nelson, C.A. , Schlaak, M.F. , Roeber, B.J. , Wewerka, S.S. , Wiik, K.L. , … & Gunnar, M.R. (2010). Neurodevelopmental effects of early deprivation in post‐institutionalized children. Child Development, 81, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeb‐Sutherland, B.C. , Levitt, P. , & Fox, N.A. (2012). The predictive nature of individual differences in early associative learning and emerging social behavior. PLoS ONE, 7, e30511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof, K.R. , van den Wildenberg, W.P. , Segalowitz, S.J. , & Carter, C.S. (2004). Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward‐based learning. Brain and Cognition, 56, 129–140. [DOI] [PubMed] [Google Scholar]
- Rudolph, K.D. , & Flynn, M. (2007). Childhood adversity and youth depression: Influence of gender and pubertal status. Developmental Psychopathology, 19, 497–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph, K.D. , Hammen, C. , Burge, D. , Lindberg, N. , Herzberg, D. , & Daley, S.E. (2000). Toward an interpersonal life‐stress model of depression: The developmental context of stress generation. Development and Psychopathology, 12, 215–234. [DOI] [PubMed] [Google Scholar]
- Russo, S.J. , & Nestler, E.J. (2013). The brain reward circuitry in mood disorders. Nature Reviews Neuroscience, 14, 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman, A.J. , Salomons, T.V. , Slagter, H.A. , Fox, A.S. , Winter, J.J. , & Davidson, R.J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12, 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, C. , Lauinger, B. , & Neville, H. (2009). Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: An event‐related brain potential study. Developmental Science, 12, 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus, M.A. , Hambey, S.L. , Finkelhor, D. , Moore, D.W. , & Runyan, D. (1998). Identification of child maltreatment with Parent‐Child Conflict Tactics Scales: Development and psychometric data for a national sample of American parents. Child Abuse and Neglect, 22, 249–270. [DOI] [PubMed] [Google Scholar]
- Sujan, A.C. , Humphreys, K.L. , Ray, L.A. , & Lee, S.S. (2014). Differential association of child abuse with self‐reported versus laboratory‐based impulsivity and risk‐taking in young adulthood. Child Maltreatment, 19, 145–155. [DOI] [PubMed] [Google Scholar]
- Talairach, J. , & Tournoux, P. (1988). Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Telzer, E.H. (2016). Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience, 17, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller, J.A. , & Fisher, P.A. (2013). Decision‐making deficits among maltreated children. Child Maltreatment, 18, 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, Z.M. , & Eskandar, E.N. (2006). Selective enhancement of associative learning by microstimulation of the anterior caudate. Nature Neuroscience, 9, 562–568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


