Abstract
Pair bonding is generally linked to monogamous mating systems, where the reproductive benefits of extended mate guarding and/or of bi-parental care are considered key adaptive functions. However, in some species, including coral reef butterflyfishes (f. Chaetodonitidae), pair bonding occurs in sexually immature and homosexual partners, and in the absence of parental care, suggesting there must be non-reproductive adaptive benefits of pair bonding. Here, we examined whether pair bonding butterflyfishes cooperate in defense of food, conferring direct benefits to one or both partners. We found that pairs of Chaetodon lunulatus and C. baronessa use contrasting cooperative strategies. In C. lunulatus, both partners mutually defend their territory, while in C. baronessa, males prioritize territory defence; conferring improvements in feeding and energy reserves in both sexes relative to solitary counterparts. We further demonstrate that partner fidelity contributes to this function by showing that re-pairing invokes intra-pair conflict and inhibits cooperatively-derived feeding benefits, and that partner endurance is required for these costs to abate. Overall, our results suggest that in butterflyfishes, pair bonding enhances cooperative defense of prey resources, ultimately benefiting both partners by improving food resource acquisition and energy reserves.
Introduction
Pair bonding, a selective pro-social and enduring affiliation between two individuals that is maintained beyond reproduction1, has independently evolved numerous times across the animal kingdom2–4. Pair bonding is generally associated with monogamous mating (mammals:5, birds:6, reptiles:7, amphibians:8, marine fishes:4) where it has been hypothesised to be advantageous due to reproductive benefits of extended mate-guarding9,10 and/or bi-parental care11. However, the presence of pair bonding between sexually immature12 and homosexual13,14 partners indicates that the benefits of pairing extend beyond those of reproduction.
Aside from mate-guarding and bi-parental care, pair bonding might be attributed to the benefits of social assistance during ecological processes that are directly conferred to one or both partners15–17. One such process may be cooperative defense of high value resources; such as food, shelter, or nesting sites18,19; by one or both partners. In heterosexual pairs, resources are often defended primarily or exclusively by males (sensu male-prioritized “division of labor”20 or “resource brokering”21), with benefits presumably related to increased mating access to22 or fecundity of23 females, or to other resources/services that are partitioned by females (e.g., burrow maintenance)20,24. Alternatively, resources may be mutually defended, or “co-defended” by male and female partners12,22, presumably because both partners directly benefit from sharing this responsibility22.
This cooperative or assisted resource defense hypothesis (ARDH) for pair bonding makes several fundamental predictions. Male-prioritized defense expects that males primarily defend resources within a territory4,18,22 wherein females are unable to maintain a territory alone and/or directly benefit from male’s assistance4. Alternatively, mutual resource defense predicts that both partners mutually defend resources within a territory4,18,22,24, wherein both are unable to maintain a territory alone and/or directly benefit from each other’s assistance4,24. Although the role of assisted resource defense (ARD) in promoting pair bonding has received less research attention than that of mate-guarding or bi-parental care, in situ observations and explicit tests of these predictions have supported the ARDH for pair bonding across a wide range of taxa (Supplementary Table S1) (but see25,26).
Butterflyfishes of the genus Chaetodon are ideal model taxa for testing the ARDH for pair bonding. Among the ~91 species within the genus, at least 59 reportedly pair bond (data sourced from12,27–30. Heterosexual pairs of at least some species (mainly, Chaetodon lunulatus) are monogamous31,32 and display mate-guarding12,33. However, same-sexed1,13,34, reproductively immature16,34 and reproductively inactive12,35 pairing also occurs. Moreover, butterflyfishes do not provide parental care31,36,37. At least 24 Chaetodon species feed predominantly (≥80%), if not exclusively, on a diet that is temporally and spatially stable, and therefore economically defendable (i.e., coral)33,38,39. There is, however, considerable variation in the level of dietary specialization among corallivorous butterflyfishes that is related to interspecific dominance over feeding sites, such that obligate corallivores dominate territorial disputes over feeding generalists40. Pairing in corallivorous butterflyfishes is suggested to arise from the need for assisted defense of coral prey against con- and heterospecifics in order to better invest in feeding and energy reserves12,41,42. Although pair bonding butterflyfishes are presumed to have very high levels of partner fidelity (up to 7 yrs) (Supplementary Table S2), the ecological basis of pair bond fidelity among these organisms remains unknown.
The overall aim of this study was to test whether pair bonding in two species of common coral-feeding butterflyfishes (C. lunulatus and C. baronessa) may be attributed to benefits of assisted defense of dietary resources, and whether pair bond endurance enhances the effectiveness of assisted resource defense. Specifically, we aimed to test ARDH predictions that either: i) males primarily defend a feeding territory, and females benefit from male assistance by improved investment in feeding and energy reserves, or ii) both partners mutually defend their feeding territory and benefit from each other’s assistance by improved investment in feeding and energy reserves. If so, then finally, we tested the prediction that iii) pair bond endurance reduces intra-pair conflict and/or promotes assisted territory defense and/or energy reserves.
Methods
Study location and model species
This study was conducted on snorkel at adjacent sheltered reefs of Lizard Island, located on the northern Great Barrier Reef, Australia (14°40′S, 145°27′E). To minimize effects of reproductive activity, sampling was conducted January–March (2014) at haphazard times between 0830–1730 h, which are times outside of peak reproductive activity of coral reef fishes at the study location. Sampling was conducted on the two most locally abundant coral-feeding and pair bonding butterflyfishes, C. lunulatus and C. baronessa (Fig. 1). Only individuals that were within 80% of the asymptotic size for the species (C. lunulatus: >64 mm standard length (SL); C. baronessa: >61 mm SL), and therefore likely to be reproductively mature16 were considered. Both species are territorial40 and are predominantly found in long-term, heterosexual partnerships1,43,44. This study was conducted using Great Barrier Reef Marine Park Authority permits: G10/33239.1, G13/35909.1, G14/37213.1; James Cook University General Fisheries permit: 170251. It was approved by James Cook University Animal Ethics committee (approval # A1874), and performed in accordance with relevant guidelines and regulations.
Figure 1.
Chaetodon lunulatus (a) and C. baronessa (b) as model species of pair bonding butterflyfishes used in the current study. At the study location, Lizard Island (GBR), these species are territorial coral feeding specialists that display enduring pair bonds. Pictures are of focal pairs used in this study, taken by J. P. N.
Coordination and competitor aggression between male and female partners
To test whether pairs displayed either male-prioritized or mutual territory defence, we conducted in situ observations on naturally occurring paired and solitary individuals. Fishes were haphazardly encountered, approached from 2–4 m, and given 3-min to acclimate to observer presence. Following the acclimation period, social system was estimated during a 5-min observation. Pair bonded individuals were identified as displaying coordinated swimming exclusively with another conspecific, whereas solitary individuals were identified as displaying no coordinated swimming with another conspecific. For pair bonds, each individual was then identified using unique body markings (as per32) and assigned an identity number used for ongoing behavioral observations and sexing. Following, levels of coordination and competitor aggression were measured during a further 6-min observation. Level of coordination was measured in pair bonded and solitary individuals, and determined by recording the presence or absence of coordinated swimming every 10-sec. Coordination, defined as the synchronisation of individuals’ movements in space and time45, was considered as the focal fish being positioned within a 2-m distance from another conspecific whilst being faced within a 315-45° angle relative to the faced position of the other conspecific (designated as 0°) (Fig. 2). Coordination was visually estimated after practicing accuracy on dummy fishes prior to the study. Level of aggression toward competitors was measured in male and female partners of pairs, and determined by quantifying the total number of aggressive acts (i.e., staring, chasing, fleeing, encircling, and head down, tail-up displays) expressed (see46 for detailed descriptions). Only aggression towards other butterflyfishes was measured, because for most butterflyfish, territorial competition is intra-familial47. After each observation, both partners of pairs were collected by spearing through the dorsal musculature and sacrificed in an ice slurry for sex determination. A one-way ANOVA was used to compare the level of pair coordination between C. lunulatus and C. baronessa. Coordination data was square-root transformed prior to analysis to improve normality of residual variance. For each species, a paired t-test was used to compare rate of aggressive acts between males and females within pairs.
Figure 2.
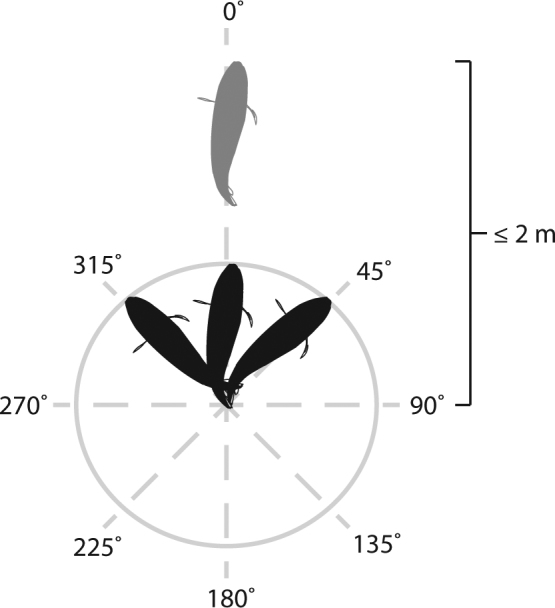
Coordinated swimming examined in pair bonded and solitary butterflyfish. Coordinated swimming by focal fish (black) was defined as being positioned within a 2-m distance from another conspecific (grey) whilst being faced within a 315-45° angle relative to the faced position of another conspecific (designated as 0°).
Paired vs. solitary individuals: Competitor aggression and feeding bites
To test whether one or both sexes benefit from pairing through reduced competitor aggression or increased feeding rates, we measured and compared these variables between naturally occurring paired and solitary individuals of both sexes. Individuals were considered pair bonded and solitary using the criteria previously described. After establishing their social status and undergoing 3-min acclimation to observer presence, focal individuals underwent a single 6-min observation to record: i) total feeding bite rate, determined by the number of bites taken on any coral ii) total feeding bites on preferred coral types (for C. baronessa only, since this species is a particularly specialized coral feeder, favoring Acropora hyacinthus, A. florida, and Pocillopora damicornis48,49), and iii) total rates of aggression toward neighboring butterflyfish. Rates of aggression may be affected by the local abundance of competitors (independent of levels of aggression exhibited by focal individuals), which was higher in paired than solitary fish. Therefore, in order to account for this potential confound, the number of aggressive acts recorded during replicate observations was standardized to per butterflyfish present within the immediate vicinity (3 m) of the fish’s feeding territory. Immediately following, butterflyfishes were collected by spearing through the dorsal musculature and sacrificed in an ice slurry for sex determination and energy reserves analysis. For each sex of each species, rates of aggression were compared between social conditions using non-parametric Mann-Whitney U tests, due to non-normal distribution of residual variance. For each species, feeding bite rate on total coral was compared between social conditions using a factorial ANOVA (with sex and social condition as fixed factors). For each sex of C. baronessa, a non-parametric Mann-Whitney U test was used to compare feeding bite rate on preferred coral between social conditions, due to non-normal distribution of residual variance.
Enduring vs. new pairs: Intra-pair relations, and per capita competitor aggression and feeding bites
We used a partner removal-replacement experiment to examine whether pair bond endurance reduces territory defense or increases feeding of paired individuals by promoting cooperative territory defense and/or reducing intra-pair conflict. Both partners of naturally occurring pair bonds of C. lunulatus (n = 9) and C. baronessa (n = 10) were identified and monitored through time using unique body markings (as per32), which were photographed and printed on water-proof paper to assist observers (see Supplementary Fig. S2 for example photographs). Pairs were assumed to have been enduring, based on previous research showing a high level of partner endurance in these species at the study location44. Prior to experimentation, one individual from each pair was haphazardly chosen as the focal individual for the experiment. To identify the focal individual and its partners throughout the experiment, a photograph of both lateral sides of their body was taken, from which a unique body markings were recognized and used44. Behavioral expression of the focal individual while with its original partner was measured throughout an 8-min observation, for 5 consecutive days. Prior to each observation, the focal individual and its partner were allowed to acclimate to observer presence for 3-mins (as described above). During each observation, i) time spent coordinated swimming with partner, ii) aggression towards partner, iii) aggression per competitor, and iv) feeding bites of the focal individual were recorded using the methods previously described. Immediately following observations conducted over 5 consecutive days, the partner of the focal individual was removed via spearing and sacrificed in an ice slurry for sex determination and energy reserve analysis. All focal individuals had re-paired with a new partner within 18 hours of partner removal, as determined by identification methods previously described. We then conducted the same behavioral observations for a further 7 (C. lunulatus) or 9 (C. baronessa) consecutive days. After experimentation, the focal individual and its new partner were collected by spearing through the dorsal musculature and sacrificed in an ice slurry to determine the sex of both individuals and energy reserve of the focal individual’s new partner. Temporal changes in time spent coordinated swimming with partner, aggression towards partner, aggression per competitor, and feeding bites were analyzed using multivariate analysis of variance (MANOVA) followed by univariate Tukey’s post hoc tests to identify between-group differences, with results displayed using canonical discriminant analysis (CDA)50,51.
Solitary vs. newly paired vs. enduringly paired individuals: Differences in liver hepatocyte vacuolation
To assess changes in energy reserves in association with pairing and partner endurance, we compared liver hepatocyte vacuole density between individuals who (i) naturally occurred in solitude (from in situ observation study), (ii) were in new pair bonds (C. lunulatus: 7 day old partnerships; C. baronessa: 9 day old partnerships) and (iii) were in naturally occurring enduring pair bonds (latter two conditions were acquired from individuals from partner removal experiment). Whole livers were dissected and fixed in 4% phosphate-buffered formalin (PBF). Fixed liver tissues were then dehydrated in a graded ethanol series and embedded in paraffin wax blocks. Tissues were sectioned at 5 µm, mounted onto glass slides, and stained using Mayer’s hematoxylin and eosin to emphasize hepatocyte vacuoles. Hepatocyte vacuole density was quantified using a Weibel eyepiece to record the proportion of points (out of 121) that intersected with hepatocyte vacuoles when viewed at X 40 magnification. Three estimates of hepatocyte vacuolation were taken for each of 3 cross sections, totaling 9 replicate estimates per fish liver, following52. In both species, differences in the percentage of liver hepatocyte vacuolation between solitary, newly paired, and enduringly paired fish were analyzed using a non-parametric Kruskal-Wallis one-way ANOVA53, due to non-normality in residual variance. Variation in hepatocyte vacuolation could not be analyzed for each sex separately, due to small sample sizes. Tukey and Kramer (Nemenyi) post hoc tests were used to identify differences between social condition means.
Sex determination
The sex of focal fish was determined histologically. Gonads were removed and fixed in formaldehyde-acetic acid-calcium chloride (FACC) for at least 1 week. Thereafter, gonads were dehydrated in a graded alcohol series, cleared in xylene, embedded in paraplast, sectioned transversely (7 µm thick), and stained with hematoxylin and eosin. Sections were examined under a compound microscope (400 × magnification) for the presence of sperm (male) or oocytes (female)16.
Results
Coordination and competitor aggression between male and female partners
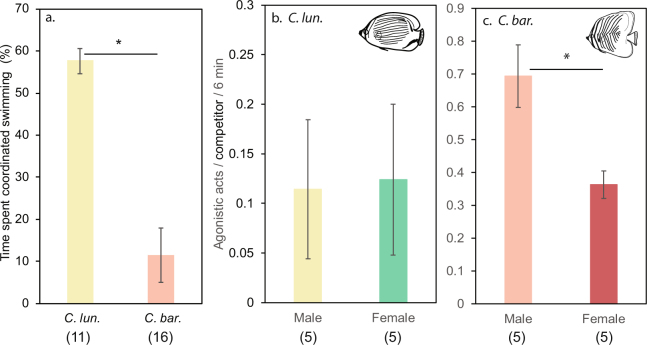
The two study species exhibited contrasting modes of cooperative or assisted territory defense. In both species, solitary individuals displayed no level of coordinated swimming with another conspecific. Pairs of C. lunulatus spent most of their time (56%) swimming with coordination throughout their feeding territory (Fig. 3a; see Supplementary Video S1 for example). When encountering neighboring butterflyfishes, both partners displayed equal levels of aggressive acts (t4 = 0.097, p = 0.93; Fig. 3b), suggesting that there is mutual assistance in territory defense. By contrast, C. baronessa partners spent notably less time (10%) swimming with coordination than C. lunulatus (F1,25 = 40.04, p = 0.00) (Fig. 3a). In general, males tended to move over large distances within and along the boundaries of their territory whilst continuously foraging, whereas females tended to restrict movement to areas of their preferred coral (if available) whilst continuously foraging on the outcrop. When territorial disputes occurred, C. baronessa males exerted 48% higher levels of aggression than females (t4 = 3.05, p = 0.04; Fig. 3c), suggesting that in this species, territory defense is male-prioritized.
Figure 3.
Patterns of (a) pair coordination and aggression towards competitors between male and female partners of (b) C. lunulatus and (c) C. baronessa. Data are represented as the mean ± SE; asterisks indicate statistically significant differences between treatment groups (ANOVA, p < 0.05). Sample sizes are listed below each treatment group.
Paired vs. solitary individuals: Competitor aggression and feeding bites
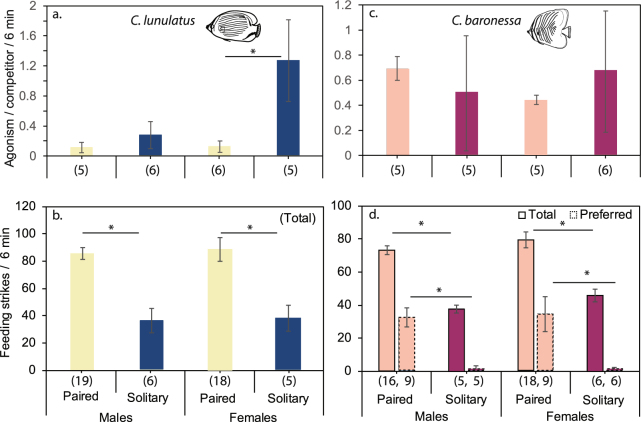
Across study sites, naturally occurring pairs of both species were common, whereas singletons were rare. A higher abundance of neighboring butterflyfish surrounding paired individual’s territories (in C. lunulatus by ~36%, in C. baronessa by ~75%) was found, suggesting that they had more neighboring competitors than solitary counterparts. For each sex of each species, after standardizing aggression to per competitor present, there was no significant difference in rates of aggression between paired and solitary individuals (C. lunulatus males: z = −0.21, p = 0.93; C. baronessa males: z = −0.53, p = 0.64; females: z = −0.00, p = 1.00), except for in female C. lunulatus, where paired females exerted 71% less aggression per competitor than single females (z = −2.11, p = 0.05; Fig. 4a,c).
Figure 4.
Differences in (a,c) aggression towards competitors and (b,d) bite rates between paired and solitary C. lunulatus and C. baronessa individuals. Total feeding strikes refer to the number of bites taken on any coral, whereas preferred feeding strikes (only measured in C. baronessa) refer to number of feeding bites taken on preferred coral types (i.e., Acropora hyacinthus, A. florida, and Pocillopora damicornis). Data are represented as the mean ± SE; asterisks indicate statistically significant differences between treatment groups (ANOVA or Mann-Whitney U, p < 0.05). Sample sizes are listed below each treatment group.
In both sexes of both species, paired individuals had higher feeding bite rates than solitary counterparts (C. lunulatus: total coral bites: F1,44 = 28.57, p = 0.00; C. baronessa: total coral bites: F1,40 = 28.91, p = 0.00, preferred coral bites: z = −2.42, p = 0.01 (males), z = −2.88, p = 0.00 (females), Fig. 4b,d). In C. lunulatus, single males took 36.83 ± 8.87 SE bites per 6-min, whereas paired males took 86.21 ± 4.28 SE bites (~57% more); and single females took 37.40 ± 9.41 SE bites per 6-min, whereas paired females took 88.67 ± 8.56 SE bites (approx. 58% more). Consistently, in C. baronessa, single females took 46.00 ± 3.21 SE total coral bites per 6-min, among which 1.17 ± 1.17 SE bites were on preferred coral; whereas paired females took 79.67 ± 4.91 SE total coral bites per 6-min, among which 33.33 ± 10.98 SE bites were on preferred coral (~43% more total coral bites, and ~96% more preferred coral bites). Similarly, single males took 37.40 ± 3.93 SE bites per 6-min (1.4 ± 1.16 SE bites on preferred coral), whereas paired males took 73.13 ± 4.86 SE bites per 6-min (32.44 ± 10.54 SE bites on preferred coral), equating to ~49% more bites on coral and ~96% more bites on preferred corals.
Enduring vs. new pairs: Intra-pair relations, and per capita competitor aggression and feeding bites
Costs of establishing new partnerships
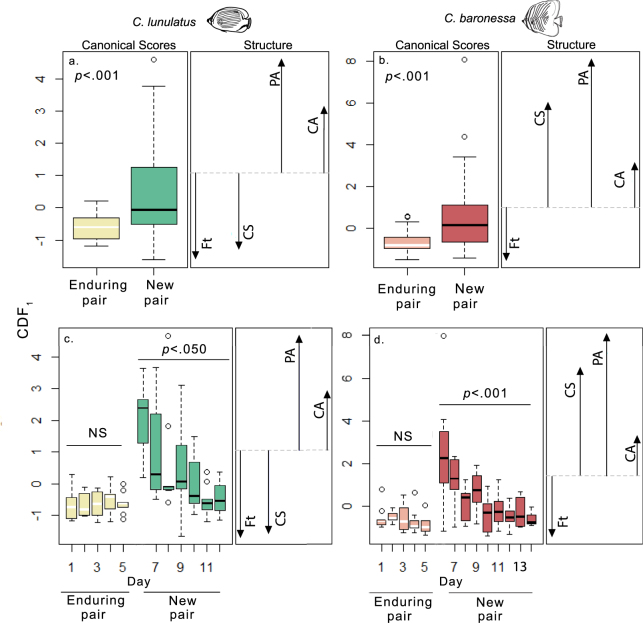
Throughout the 5 consecutive days leading up to partner removal, all focal individuals maintained their same partner and territory, and their activity profiles (union of pair swimming, within-pair aggression rate, competitor aggression rate, and feeding bite rate) remained unchanged (C. lunulatus: Hotelling’s trace = 0.35, df = 16, p = 0.70; C. baronessa: Hotelling’s trace = 0.22, df = 12, p = 0.86; Fig. 4c,d canonical score plots; see Supplementary Fig S1 for behaviour specific profile plots). Within 18 hours of removing their original partner, all focal individuals had kept their same territory, where they had re-paired with a new partner. After re-pairing, the activity profile of fishes dramatically changed (C. lunulatus: Hotelling’s trace = 0.23, df = 1, p = 0.00; C. baronessa: Hotelling’s trace = 0.29, df = 1, p = 0.00; Fig. 4a,b canonical score plots). This change was mostly attributed to higher within-pair aggression, and to a lesser extent altered coordinated swimming (C. lunulatus: reduced coordinated swimming; C. baronessa: increased coordinated swimming), higher aggression per neighbouring competitor, and lower total feeding bites than when they were in their enduring partnership (Fig. 5a,b canonical structure plots; see Supplementary Video S2 for example of day 1 of new partnership).
Figure 5.
Changes in intra-pair relations, aggression towards competitors, and feeding bites in response to (a,b) re-pairing, and (c,d) subsequent endurance of new pairs throughout several days. Means of standardized canonical scores of the first canonical discriminant function (CDF1) are represented by box and whisker plots. Structure vectors show the relative strength (length of the vector relative to length of other vectors) and direction (+ or −) of the correlation between each contributing response variable and the canonical discriminant function. MANOVA p-value for change in activity profile in response to relationship phase (a,b) or day (c,d) is shown in the corner. (a,b) In both species, re-pairing with a new partner increases intra-pair aggression (PA). Concurrently, it (a) reduces coordinated swimming (CS) in C. lunulatus (n = 9), and (b) increases coordinated swimming in C. baronessa (n = 10). (a,b) These changes in intra-pair relations are associated with increased competitor aggression (CA) and a reduction in total feeding bites (Ft). (c,d) However, as new pairs endure, intra-pair relations recover along with recovered losses in competitor aggression and feeding bite efficiency.
Recovery with new partnership endurance
After re-pairing, focal individuals maintained association with their new partner throughout the remainder of the study (C. lunulatus, seven days; C. baronessa, nine days), except for one C. lunulatus individual, who underwent a second re-pairing 3 days after its original partner was removed. As new pairs endured, focal individuals’ activity profiles significantly changed (C. lunulatus: Hotelling’s trace = 0.77, df = 24, p = 0.03; C. baronessa: Hotelling’s trace = 1.25, df = 32, p = 0.00; Fig. 5c,d canonical score plots; see Supplementary Fig S1 for behaviour specific profile plots). This change was mostly attributed to a reduction in intra-pair aggression, and to a lesser extent to adjusted coordinated swimming (C. lunulatus: increased; C. baronessa: decreased), reduced aggression per competitor, and increased feeding bites (Fig. 5c,d canonical structure plots). Within 4 days of forming a new partnership, activity profiles recovered to levels displayed by original pairs (Supplementary Tables S3 and S4; Fig. 5c,d canonical structure plots). (For behaviour-specific effect and post hoc analyses, see Supplementary Table S5.)
Energy reserves of solitary vs. newly paired vs. enduringly paired individuals
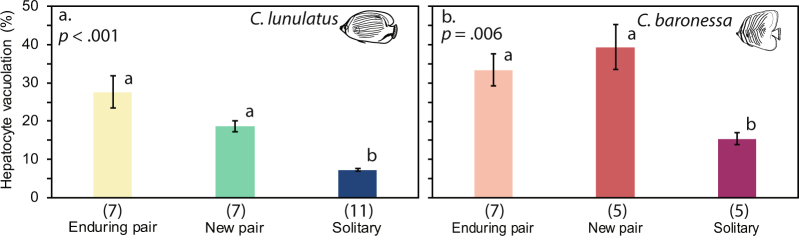
For both C. baronessa and C. lunulatus, liver hepatocyte vacuole density varied significantly with social condition (C. lunulatus: Kruskal-Wallis = 19.39, df = 2, p = 0.00; C. baronessa: Kruskal-Wallis = 10.27, df = 2, p = 0.01). While there was no difference in liver vacuole density between individuals that were in enduring and relatively new (i.e., that persisted for 7–9 days) partnerships, paired individuals had greater hepatocyte vacuolation than solitary counterparts (Fig. 6a,b).
Figure 6.
Variation in liver hepatocyte vacuole density among individuals in enduring partnerships, new partnerships (7-9-day persistence), and living in solitude. (a) C. lunulatus; (b) C. baronessa. Data are represented as the mean ± SE. Kruskal-Wallis p value is shown in top corners, while groups not sharing the same letter are significantly different [Tukey and Kramer (Nemenyi) post-hoc analysis at p < 0.05]. Sample sizes are listed below each treatment group.
Discussion
In this study, we provide field-based observational evidence for the ARDH for pair bonding in two species of butterflyfishes. We show that pairs of C. baronessa and C. lunulatus appear to exhibit alternative modes of assisted territory defense (male-prioritized and mutual defense, respectively) that is associated with increased feeding bites relative to solitary counterparts. We furthermore provide the first evidence that this feeding bite advantage translates into significant gains in energy reserves in butterflyfishes. Finally, this is one of the first studies to examine why organisms that exhibit ARD have long-term partnerships, providing experimental evidence that partner endurance plays a critical role by inhibiting conflict and promoting ARD between partners.
It has previously been proposed that, where ARD drives pairing, males nonetheless take-on the greatest burden of defense54. Although this is largely consistent with our results for C. baronessa, our findings for C. lunulatus contribute to a growing body of literature indicating that males and females may contribute equally to resource defense, because both may equally benefit from each other’s assistance (Supplementary Table S1). Partners frequently swam with coordination while foraging throughout their territory. When encountering neighboring butterflyfishes, aggression was generally passive, consistent with some other butterflyfishes39,42. Presumably, the function of pair swimming in butterflyfish pair bonds may be akin to duetting in bird pair bonds, in that it conspicuously advertises territory occupancy, thereby avoiding usurpation attempts by neighbors12. Notably, when territorial aggression did occur, it was exerted by both partners mutually. For both sexes, this ostensive co-defense in paired individuals was associated with an improved feeding bite rate (by ~58%), and energy reserves (by 69%, as indicated by hepatocyte vacuolation) relative to solitary counterparts. Consistently, in C. chrysurus (=paucifasciatus), male-female partners continuously travel closely together throughout their territory, mutually engage in territory defense, and both partners have higher feeding rates than solitary individuals12.
In contrast to C. lunulatus, territory defense by pairs of C. baronessa appeared to be male-prioritized. Partners frequently traveled independently from each other, spending only ~10% of their time swimming with coordination. For most time (~90%) males independently patrolled larger areas within and along the boundaries of territories, exerting ~48% more aggression towards neighboring butterflyfishes than females. This seemed to allow females to mainly focus on foraging, notably in a more restricted area within the territory that contained a dominant assemblage of preferred coral (e.g., A. hyacinthus or A. florida). In association, paired females bit 43% more total coral, and 96% more preferred coral then their solitary counterparts. Moreover, and probably because of increased food bites, liver lipid reserves in paired individuals were higher by ~57% than solitary individuals, though no distinction was made between males versus females. Among species that pair for assisted resource defense purposes, male-prioritized defense is the most commonly reported mode of assistance (Supplementary Table S1), where it has been attributed to supporting female food consumption in birds55,56 and butterflyfishes33, presumably enabling the male to share his mate’s subsequent increased fecundity33,56. This form of sexual division-of-labor is thought to occur when female egg production is especially costly, and disproportionally more male assistance is required for females to build the energy reserves needed for egg production33,57. Egg production in female C. baronessa may be particularly energetically costly, thereby favoring male-prioritized over mutual defense. Although some of C. baronessa’s preferred diet, Acropora corals, provide the best energetic return among coral families (after accounting for feeding efficiency)58,59, they nonetheless exhibit higher feeding rates on coral tissue per day than C. lunulatus and other congeners59, indicating that perhaps they have a relatively low energetic absorption efficiency. To ascertain this possibility, analysis of energetic absorption efficiency (relative to other corallivorous species) would be required. Interestingly, despite pair bonded male C. baronessa exerting more territory defense effort than females, pair bonded males also conferred feeding benefits, as they bit 49% more total, and 96% more preferred coral than their solitary counterparts. This might suggest that in addition to presumably sharing in female’s increased fecundity, pairing males might also directly confer an advantage to food consumption, due to the (albeit relatively little) territorial defense assistance provided by females.
In previous studies of pair bonding butterflyfishes, ARD led to increased feeding rates by reducing the time needed for territory defense, thereby providing more time to invest in feeding12,41. In our study, since pair bonded C. lunulatus exhibited mutual ARD, we expected that both males and females would confer reductions in territory defense; and since pair bonded C. baronessa exhibited male-prioritized ARD, we expected that males would be burdened with increased territory defense while females would benefit from reductions in this task. This expectation was met for female C. lunulatus, as pair bonders exhibiting mutual ARD and increased feeding also conferred a reduction in territory defense relative to solitary, non-assisting counterparts. While the same pattern was found for male C. lunulatus; this was highly statistically insignificant. Likewise, in accord with expectation for pair bonded C. baronessa, there was a pattern for territory aggression to increase for males and decrease for females relative to solitary counterparts; however, this was also highly insignificant. Importantly, rates of aggression tended to be highly variable for solitary individuals. This, coupled with low sample sizes, limited power to detect potential differences in aggression between paired and solitary individuals. Alternatively, ARD may have enabled pairs to establish territories with greater food availability, thereby providing feeding and energy reserve benefits independently of reduced per capita territory defense. It may also be argued that rather than ARD, increased feeding and energy reserves might be consequent of other function(s) of pairing (e.g., increased predator vigilance)16,60. Finally, it is conceivable that higher food consumption and energy reserves promoted pair bonding. Nevertheless, a causal effect of ARD on improved feeding experimentally shown in other pair bonding butterflyfish species12,61 further supports the idea that it also exist in species of the current study. To be certain, however, this should now be confirmed experimentally.
Although species that pair for ARD display long-term partner fidelity, reasons for this are almost wholly unknown1,22,33,37,61–70 (Supplementary Table S2). In the current study, we show that within 18 hours of removing their original partner, all remaining fish had kept their same territory, wherein they had re-paired with a new partner. This indicates strong territory fidelity. It moreover suggests that while there is strong pressure to be paired, this is not due to partner/mate scarcity. There are, however, definite benefits of pair bond endurance. Experimentally inducing new partnerships caused an immediate and marked decline in partner relations, although these were relatively short-lived. This decline was mostly attributed to increased intra-pair aggression, primarily from the widower towards the new partner, and to a lesser extent to reduced expression of species-specific modes of ARD, as indicated by decreased pair swimming in C. lunulatus, and increased pair swimming in C. baronessa. This decline in partner relations appeared to initiate heightened territorial activity with neighbouring butterflyfish pairs. Subsequently, newly paired individuals suffered from having to shift investment from feeding to territory defence. As new partnerships subsequently endured, however, these intra- and inter-pair disruptions abated, and incurred costs to individual territory defence-feeding budgets recovered accordingly. Similarly, it has been shown (in wood louse, Hemilepistus reaumuri) that widowed individuals with established territories will initially aggressively resist the elicitation to form new partnerships prior to conceding20. It has also been shown (in barnacle geese, Branta leucopsis23,56) that pair bonds of longer duration monopolize higher quality feeding territories, ostensibly through enhanced cooperation, and this is further linked to improvements in life-time reproductive success. Overall, our results suggest that partner fidelity is exhibited in Chaetodon butterflyfishes because it plays a critical role in promoting assisted resource defence, and inhibits intra- and inter-pair conflict, ultimately conferring feeding investment gains. Although we found no evidence that this translates into energy reserve gains, this may be consequent of limited sample size and/or sampling after new pairs had already endured for 7–9 days, when they displayed fully-recovered behavioral and energetic profiles. Indeed, in fishes, liver hepatocyte vacuole density has been shown to respond rapidly to changes in feeding (i.e., within 5–8 days)71,72. To ascertain this, energy reserves should be re-sampled in more individuals and on a shorter time-scale throughout the development of new partnerships. Of course, there may be other reasons for long-term partner fidelity among pair bonding species that exhibit ARD. These might include partners experiencing a delay in the time at which their services are reciprocated4. For example, if male assistance is based on increasing female feeding to share her improved fecundity, then males may remain with females across reproductive periods if there is a time-lag between enhanced female feeding and egg production4. Partner fidelity may also be attributed mutual site-attachment to the feeding territory, which may arise if it new territories are scarce or competitively costly to acquire19,39.
How and why might partner fidelity promote ARD and inhibit intra-pair conflict in these species? Perhaps partner fidelity improves ARD through partner familiarity. Indeed, it has been shown in fishes that cooperation with specific partners stabilizes over time, because individuals are more cooperative with familiar partners73. The mechanism(s) for this may be unique to the species-specific mode of assistance. For C. lunulatus, partners appear to work together simultaneously to provide mutual assistance, and as such, familiarity may facilitate learning and accurate prediction of partner behavior (e.g., chosen defense route or routine), thereby fine-tuning pair-wise coordination74–77. For C. baronessa, partners appear to exhibit male-prioritized assistance in exchange for sequentially reciprocated partitioning of services/resources by females (i.e., direct reciprocity), and as such, partner familiarity may allow individuals to learn which “partner control mechanism” is best suited to stabilize cooperation, based on the tendency of partners to reciprocate (or cheat) in the past78,79. Upon new pair formation, partner familiarity (and therefore effective cooperation, and cooperatively derived feeding benefits) takes several days to develop; however, the cost of food sharing is immediately incurred. Hence, until cooperative relations develop, the costs (food sharing) likely outweigh the benefits (maximizing feeding investment) of pairing, causing territory holders to aggressively resist new partner elicitation.
Energy acquisition is fundamental to growth, reproduction, and maintenance for all animals80. However, corallivorous butterflyfishes rely almost exclusively on a diet of hard coral81, which is a relatively nutrient poor, but abundant resource34,38. Consequently, both sexes are energy maximizers, feeding almost continuously33,39,41. Foraging is therefore constrained by time spent on other activities, including territory defense. As such, attributes that alleviate time constraints on foraging are likely to directly benefit individual fitness33. This study suggests that for C. lunulatus and C. baronessa, pair bonded partners use territorial defense assistance to increase their feeding of coral food and energy reserves. We further show that partner fidelity plays a critical role in this function by inhibiting intra-and inter-pair conflict and promoting territorial defense assistance between partners, providing an ecological advantage to pair formation and fidelity in these species. Whether this translates into an adaptive advantage should now be addressed by undertaking long-term monitoring studies to discern whether long-term pair bonding also confers improved survivorship and/or life-time fitness56.
Electronic supplementary material
Acknowledgements
We thank Manuela Giammusso, Patrick Smallhorn-West, Andrés Jácome, and staff at Lizard Island Research Station for field assistance. We acknowledge the fishes that were used in the current study. This research was supported by an ARC research grant to M.S.P and S.P.W.
Author Contributions
J.P.N., S.P.W. and M.S.P. designed the study; J.P.N., D.J.C. and K.J.N. collected the data; J.P.N., S.P.W. and K.J.N. analyzed the data, J.P.N. wrote the manuscript, and all authors contributed to reviewing and editing the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-24412-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jessica P. Nowicki, Email: jnowicki@stanford.edu
Morgan S. Pratchett, Email: morgan.pratchett@jcu.edu.au
References
- 1.Nowicki, J. P., O’Connell, L. A., Walker, S. P., Coker, D. J. & Pratchett, M. S. Variation in social systems within Chaetodon butterflyfishes, with special reference to pair bonding. PloS ONE, 13(4): e0194465. 10.1371/journal.pone.0194465 (2018). [DOI] [PMC free article] [PubMed]
- 2.Bull CM. Monogamy in lizards. Behavioural Processes. 2000;51:7–20. doi: 10.1016/S0376-6357(00)00115-7. [DOI] [PubMed] [Google Scholar]
- 3.Reichard, U. H. & Boesch, C. Monogamy: mating strategies and partnerships in birds, humans and other mammals. (Cambridge University Press, 2003).
- 4.Whiteman EA, Cote IM. Monogamy in marine fishes. Biological Reviews. 2004;79:351–375. doi: 10.1017/S1464793103006304. [DOI] [PubMed] [Google Scholar]
- 5.Clutton-Brock T, Isvaran K. Paternity loss in contrasting mammalian societies. Biology Letters. 2006;2:513–516. doi: 10.1098/rsbl.2006.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith SC, Owens IPF, Thuman KA. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Molecular Ecology. 2002;11:2195–2212. doi: 10.1046/j.1365-294X.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- 7.Uller T, Olsson M. Multiple paternity in reptiles: patterns and processes. Molecular Ecology. 2008;17:2566–2580. doi: 10.1111/j.1365-294X.2008.03772.x. [DOI] [PubMed] [Google Scholar]
- 8.Brown JL, Morales V, Summers K. A key ecological trait drove the evolution of biparental care and monogamy in an amphibian. American Naturalist. 2010;175:436–446. doi: 10.1086/650727. [DOI] [PubMed] [Google Scholar]
- 9.Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- 10.Lukas D, Clutton-Brock TH. The evolution of social monogamy in mammals. Science. 2013;341:526–530. doi: 10.1126/science.1238677. [DOI] [PubMed] [Google Scholar]
- 11.Wittenberger JF, Tilson RL. The evolution of monogamy: hypotheses and evidence. Annual Review of Ecology and Systematics. 1980;11:197–232. doi: 10.1146/annurev.es.11.110180.001213. [DOI] [Google Scholar]
- 12.Fricke HW. Pair swimming and mutual partner guarding in monogamous butterflyfish (pisces, chaetodontidae) - a joint advertisement for territory. Ethology. 1986;73:307–333. doi: 10.1111/j.1439-0310.1986.tb00812.x. [DOI] [Google Scholar]
- 13.Gore MA. The effect of a flexible spacing system on the social organization of a coral reef fish. Chaetodon capistratus. Behaviour. 1983;85:118–145. doi: 10.1163/156853983X00066. [DOI] [Google Scholar]
- 14.Bagemihl, B. Biological exuberance: animal homosexuality and natural diversity. (Macmillan, 1999).
- 15.Black, J. M. In Partnerships in birds. The study of monogamy (ed J. M. Black) (Oxford University Press, 1996).
- 16.Pratchett MS, Pradjakusuma OA, Jones GP. Is there a reproductive basis to solitary living versus pair-formation in coral reef fishes? Coral Reefs. 2006;25:85–92. doi: 10.1007/s00338-005-0081-6. [DOI] [Google Scholar]
- 17.Brandl SJ, Bellwood DR. Pair-formation in coral reef fishes: an ecological perspective. Oceanography and Marine Biology: An Annual Review. 2014;52:1–79. [Google Scholar]
- 18.Rutberg AT. The evolution of monogamy in primates. Journal of Theoretical Biology. 1983;104:93–112. doi: 10.1016/0022-5193(83)90403-4. [DOI] [PubMed] [Google Scholar]
- 19.Wilson, E. O. Sociobiology. (Harvard University Press, 2000).
- 20.Eduard VK, Linsenmair C. Pair formation and pair maintenance in the monogamous desert wood louse Hemilepistus reaumuri (Crustacea, Isopoda, Oniscoidea) Zeitschrift fur Tierpsychologie. 1971;29:134–155. doi: 10.1111/j.1439-0310.1971.tb01728.x. [DOI] [PubMed] [Google Scholar]
- 21.Wrangham R. On the evolution of ape social systems. Social Science Information. 1979;18:336–368. doi: 10.1177/053901847901800301. [DOI] [Google Scholar]
- 22.Tecot SR, Singletary B, Eadie E. Why “monogamy” isn’t good enough. American Journal of Primatology. 2016;78:340–354. doi: 10.1002/ajp.22412. [DOI] [PubMed] [Google Scholar]
- 23.Black, J. M., Prop, J. & Larsson, K. The barnacle goose. (Bloomsbury Publishing, 2014).
- 24.Mathews LM. Territorial cooperation and social monogamy: factors affecting intersexual behaviours in pair-living snapping shrimp. Animal Behaviour. 2002;63:767–777. doi: 10.1006/anbe.2001.1976. [DOI] [Google Scholar]
- 25.Gibbon CDF. The adaptive significance of monogamy in the golden‐reumped elephant‐shrew. Journal of Zoology. 1997;242:167–177. doi: 10.1111/j.1469-7998.1997.tb02937.x. [DOI] [Google Scholar]
- 26.Hilgartner R, Fichtel C, Kappeler PM, Zinner D. Determinants of pair-living in red-tailed sportive lemurs (Lepilemur ruficaudatus) Ethology. 2012;118:466–479. doi: 10.1111/j.1439-0310.2012.02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen, G. R., Steene, R. C. & Allen, M. A guide to angelfishes & butterflyfishes. (Odyssey Publishing/Tropical Reef Research, 1998).
- 28.Kuiter, R. H. Butterflyfishes, bannerfishes, and their relatives: a comprehensive guide to Chaetodontidae & Microcanthidae. (Twayne Publishers, 2002).
- 29.Bonaldo R, Krajewski J, Sazima I. Meals for two: foraging activity of the butterflyfish Chaetodon striatus (Perciformes) in southeast Brazil. Brazilian Journal of Biology. 2005;65:211–215. doi: 10.1590/S1519-69842005000200004. [DOI] [PubMed] [Google Scholar]
- 30.Yabuta, S. & Berumen, M. L. In Biology of butterflyfishes (eds M. S. Pratchett, M. L. Berumen, & B. G. Kapoor) Ch. 8, (CRC Press, 2014).
- 31.Neudecker S, Lobel PS. Mating systems of Chaetodontid and Pomacanthid fishes at St. Croix. Ethology. 1982;59:299–318. [Google Scholar]
- 32.Yabuta S. Spawning migrations in the monogamous butterflyfish. Chaetodon trifasciatus. Ichthyological Research. 1997;44:177–182. doi: 10.1007/BF02678695. [DOI] [Google Scholar]
- 33.Hourigan TF. Environmental determinants of butterflyfish social systems. Environmental Biology of Fishes. 1989;25:61–78. doi: 10.1007/BF00002201. [DOI] [Google Scholar]
- 34.Tricas, T. C. Life history, foraging ecology, and territorial behavior of the Hawaiian butterflyfish Chaetodon multicinctus Ph.D. Dissertation thesis, University of Hawaii, Honolulu (1986).
- 35.Yabuta S. Social groupings in 18 species of butterflyfish and pair bond weakening during the nonreproductive season. Ichthyological Research. 2007;54:207–210. doi: 10.1007/s10228-006-0391-x. [DOI] [Google Scholar]
- 36.Fricke H. Behaviour as part of ecological adaptation. Helgoländer Wissenschaftliche Meeresuntersuchungen. 1973;24:120. doi: 10.1007/BF01609505. [DOI] [Google Scholar]
- 37.Driscoll JW, Driscoll JL. Pair behavior and spacing in butterflyfishes (chaetodontidae) Environmental Biology of Fishes. 1988;22:29–37. doi: 10.1007/BF00000542. [DOI] [Google Scholar]
- 38.Tricas T. The economics of foraging in coral-feeding butterflyfishes of Hawaii. Proc. 5th Int. Symp. Coral Reefs. 1985;5:409–414. [Google Scholar]
- 39.Tricas TC. Determinants of feeding territory size in the corallivorous butterflyfish. Chaetodon multicinctus. Animal Behaviour. 1989;37:830–841. doi: 10.1016/0003-3472(89)90067-5. [DOI] [Google Scholar]
- 40.Blowes SA, Pratchett MS, Connolly SR. Heterospecific aggression and dominance in a guild of coral-feeding fishes: the roles of dietary ecology and phylogeny. American Naturalist. 2013;182:157–168. doi: 10.1086/670821. [DOI] [PubMed] [Google Scholar]
- 41.Hourigan, T. F. The behavioral ecology of three species of butterflyfishes (family Chaetodontidae) Ph.D. dissertation thesis, University of Hawaii (1987).
- 42.Roberts CM, Ormond FG. Butterflyfish social behaviour, with special reference to the incidence of territoriality: a review. Environmental Biology of Fishes. 1992;34:79–93. doi: 10.1007/BF00004786. [DOI] [Google Scholar]
- 43.Reese, E. S. Duration of residence by coral reef fishes on home reefs. Copeia, 145-149, 10.2307/1442375 (1973).
- 44.Yabuta S, Kawashima M. Spawning behavior and haremic mating system in the corallivorous butterflyfish, Chaetodon trifascialis, at Kuroshima island, Okinawa. Ichthyological Research. 1997;44:183–188. doi: 10.1007/BF02678696. [DOI] [Google Scholar]
- 45.Herbert-Read JE. Understanding how animal groups achieve coordinated movement. J. Exp. Biol. 2016;219:2971–2983. doi: 10.1242/jeb.129411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yabuta S. Behaviors in agonistic interaction of the butterflyfish (Chaetodon lunulatus) Journal of Ethology. 2000;18:11–15. doi: 10.1007/s101640070018. [DOI] [Google Scholar]
- 47.Wrathall TJ, Roberts CM, Ormond RF. Territoriality in the butterflyfish Chaetodon austriacus. Environmental biology of fishes. 1992;34:305–308. doi: 10.1007/BF00004777. [DOI] [Google Scholar]
- 48.Berumen ML, Pratchett MS, McCormick MI. Within-reef differences in diet and body condition of coral-feeding butterflyfishes (Chaetodontidae) Marine Ecology Progress Series. 2005;287:217–227. doi: 10.3354/meps287217. [DOI] [Google Scholar]
- 49.Lawton RJ, Cole AJ, Berumen ML, Pratchett MS. Geographic variation in resource use by specialist versus generalist butterflyfishes. Ecography. 2012;35:566–576. doi: 10.1111/j.1600-0587.2011.07326.x. [DOI] [Google Scholar]
- 50.Cruz-Castillo J, et al. Applications of canonical discriminant analysis in horticultural research. HortScience. 1994;29:1115–1119. [Google Scholar]
- 51.Cruz‐Castillo J, Lawes G, Woolley D, Ganesh S. Evaluation of rootstock and ‘Hayward’scion effects on field performance of kiwifruit vines using a multivariate analysis technique. New Zealand Journal of Crop and Horticultural Science. 1997;25:273–282. doi: 10.1080/01140671.1997.9514016. [DOI] [Google Scholar]
- 52.Pratchett MS, Wilson SK, Berumen ML, McCormick MI. Sublethal effects of coral bleaching on an obligate coral feeding butterflyfish. Coral Reefs. 2004;23:352–356. doi: 10.1007/s00338-004-0394-x. [DOI] [Google Scholar]
- 53.Siegel, S. & Castellan, N. Nonparametric statistics for the behavioral sciences. NewY) rk: McGraw-Hill (1988).
- 54.Fuentes A. Patterns and trends in primate pair bonds. International Journal of Primatology. 2002;23:953–978. doi: 10.1023/A:1019647514080. [DOI] [Google Scholar]
- 55.Boyd H. On encounters between wild White-Fronted geese in winter flocks. Behaviour. 1953;5:85–128. doi: 10.1163/156853953X00069. [DOI] [Google Scholar]
- 56.Black JM. Fitness consequences of long-term pair bonds in barnacle geese: monogamy in the extreme. Behavioral Ecology. 2001;12:640–645. doi: 10.1093/beheco/12.5.640. [DOI] [Google Scholar]
- 57.Lamprecht, J. In The sociobiology of sexual and reproductive strategies (eds A. E. Rasa, C. Vogel, & E Voland) 48–66 (Chapman & Hall, 1989).
- 58.Graham NA. Ecological versatility and the decline of coral feeding fishes following climate driven coral mortality. Marine Biology. 2007;153:119–127. doi: 10.1007/s00227-007-0786-x. [DOI] [Google Scholar]
- 59.Cole A, Lawton R, Pratchett M, Wilson S. Chronic coral consumption by butterflyfishes. Coral Reefs. 2011;30:85–93. doi: 10.1007/s00338-010-0674-6. [DOI] [Google Scholar]
- 60.Brandl SJ, Bellwood DR. Coordinated vigilance provides evidence for direct reciprocity in coral reef fishes. Scientific reports. 2015;5:14556. doi: 10.1038/srep14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hourigan, T. F. The behavioral ecology of three species of butterflyfishes (Family Chaetodontidae) Ph.D. thesis, University of Hawaii, (1987).
- 62.Kotrschal, K., Scheiber, I. B. & Hirschenhauser, K. In Animal behaviour: evolution and mechanisms (ed P. M. Kappeler) 121–148 (Springer, 2010).
- 63.Vaughan TA, Vaughan RP. Seasonality and the behavior of the African yellow-winged bat. Journal of mammalogy. 1986;67:91–102. doi: 10.2307/1381005. [DOI] [Google Scholar]
- 64.Rosell F, Thomsen LR. Sexual dimorphism in territorial scent marking by adult Eurasian beavers (Castor fiber) Journal of chemical ecology. 2006;32:1301–1315. doi: 10.1007/s10886-006-9087-y. [DOI] [PubMed] [Google Scholar]
- 65.Kotrschal, K., Hemetsberger, J. & Weiß, B. M. In Homosexual behaviour in animals. An evolutionary perspective 45–76 (Cambridge University Press, 2006).
- 66.Morley JI, Balshine S. Faithful fish: territory and mate defence favour monogamy in an African cichlid fish. Behavioral Ecology and Sociobiology. 2002;52:326–331. doi: 10.1007/s00265-002-0520-0. [DOI] [Google Scholar]
- 67.Morley JI, Balshine S. Reproductive biology of Eretmodus cyanostictus, a cichlid fish from Lake Tanganyika. Environmental Biology of Fishes. 2003;66:169–179. doi: 10.1023/A:1023610905675. [DOI] [Google Scholar]
- 68.Reese E. How behavior influences community structure of butterflyfishes (family Chaetodontidae) on Pacific coral reefs. Ecol Int Bull. 1991;19:29–41. [Google Scholar]
- 69.Linsenmair KE. Comparative studies on the social behaviour of the desert isopod Hemilepistus reaumuri and of a Porcellio species. Symp. Zool. Soc. London. 1984;53:423–453. [Google Scholar]
- 70.Illes AE. Context of female bias in song repertoire size, singing effort, and singing independence in a cooperatively breeding songbird. Behavioral Ecology and Sociobiology. 2015;69:139–150. doi: 10.1007/s00265-014-1827-3. [DOI] [Google Scholar]
- 71.Green B, McCornick M. Influence of larval feeding history on the body condition of Amphiprion melanopus. J. Fish Biol. 1999;55:1273–1289. doi: 10.1111/j.1095-8649.1999.tb02075.x. [DOI] [Google Scholar]
- 72.Pratchett MS, Gust N, Goby G, Klanten SO. Consumption of coral propagules represents a significant trophic link between corals and reef fish. Coral Reefs. 2001;20:13–17. doi: 10.1007/s003380000113. [DOI] [Google Scholar]
- 73.Granroth-Wilding HM, Magurran AE. Asymmetry in pay-off predicts how familiar individuals respond to one another. Biology letters. 2013;9:20130025. doi: 10.1098/rsbl.2013.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chivers DP, Brown GE, Smith RJF. Familiarity and shoal cohesion in fathead minnows (Pimephales promelas): implications for antipredator behaviour. Canadian Journal of Zoology. 1995;73:955–960. doi: 10.1139/z95-111. [DOI] [Google Scholar]
- 75.Griggio M, Hoi H. An experiment on the function of the long-term pair bond period in the socially monogamous bearded reedling. Animal behaviour. 2011;82:1329–1335. doi: 10.1016/j.anbehav.2011.09.016. [DOI] [Google Scholar]
- 76.Sánchez-Macouzet O, Rodríguez C, Drummond H. Better stay together: pair bond duration increases individual fitness independent of age-related variation. Proceedings of the Royal Society of London B: Biological Sciences. 2014;281:20132843. doi: 10.1098/rspb.2013.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leu ST, Burzacott D, Whiting MJ, Bull CM. Mate familiarity affects pairing behaviour in a long‐term monogamous lizard: evidence from detailed bio‐logging and a 31‐year field study. Ethology. 2015;121:760–768. doi: 10.1111/eth.12390. [DOI] [Google Scholar]
- 78.Gomes CM, Mundry R, Boesch C. Long-term reciprocation of grooming in wild West African chimpanzees. Proceedings of the Royal Society of London B: Biological Sciences. 2009;276:699–706. doi: 10.1098/rspb.2008.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wubs M, Bshary R, Lehmann L. Coevolution between positive reciprocity, punishment, and partner switching in repeated interactions. Proc. R. Soc. B. 2016;283:20160488. doi: 10.1098/rspb.2016.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hughes, R. N. In Behavioural ecology of teleost fishes (ed J Godin, J.) (Oxford University Press, 1997).
- 81.Pratchett MS. Dietary overlap among coral-feeding butterflyfishes (Chaetodontidae) at Lizard Island, northern Great Barrier Reef. Marine Biology. 2005;148:373–382. doi: 10.1007/s00227-005-0084-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.