Abstract
Complete genome sequence of Gluconacetobacter xylinus CGMCC 2955 for fine control of bacterial cellulose (BC) synthesis is presented here. The genome, at 3,563,314 bp, was found to contain 3,193 predicted genes without gaps. There are four BC synthase operons (bcs), among which only bcsI is structurally complete, comprising bcsA, bcsB, bcsC, and bcsD. Genes encoding key enzymes in glycolytic, pentose phosphate, and BC biosynthetic pathways and in the tricarboxylic acid cycle were identified. G. xylinus CGMCC 2955 has a complete glycolytic pathway because sequence data analysis revealed that this strain possesses a phosphofructokinase (pfk)-encoding gene, which is absent in most BC-producing strains. Furthermore, combined with our previous results, the data on metabolism of various carbon sources (monosaccharide, ethanol, and acetate) and their regulatory mechanism of action on BC production were explained. Regulation of BC synthase (Bcs) is another effective method for precise control of BC biosynthesis, and cyclic diguanylate (c-di-GMP) is the key activator of BcsA–BcsB subunit of Bcs. The quorum sensing (QS) system was found to positively regulate phosphodiesterase, which decomposed c-di-GMP. Thus, in this study, we demonstrated the presence of QS in G. xylinus CGMCC 2955 and proposed a possible regulatory mechanism of QS action on BC production.
Introduction
Bacterial cellulose (BC), naturally produced by several species of Acetobacter, is a strong and ultra-pure form of cellulose1. It has the same chemical structure as plant cellulose but is devoid of hemicellulose and lignin2. Until now, many researchers have endeavoured to control BC production to realise its potential applications. Most applications have been implemented by optimizing the carbon source and culture conditions3,4. Additionally, functionalisation or modification of BC has mainly been achieved via chemical or mechanical modifications of the cellulose matrix5,6. Nowadays, synthetic biology enables model microorganisms to be easily reprogrammed with modular DNA code to perform a variety of new tasks for useful purposes7. This approach may allow for more finely tuned control over BC synthesis by gene regulation. Nonetheless, only a few attempts at genetic engineering have been made for BC-producing bacteria, and the toolkit for genetic modification has hardly been described8. Thus, it is essential for researchers to gain genomic insights into various BC-producing strains.
So far, several BC-producing Gluconacetobacter (Komagataeibacter) species have been sequenced, and the genomes of K. xylinus E259, K. medellinensis NBRC 3288 (ref.10), K. nataicola RZS0111, and Gluconobacter oxydans DSM 3504 were sequenced completely. Gluconacetobacter xylinus (formerly Acetobacter xylinum) is one of the most commonly studied species because of its ability to produce relatively large amounts of BC from a wide range of carbon and nitrogen sources in liquid culture3,4,12 and has become the most popular strain so far for manufacturing of various materials, including paper13, food packaging14, medical materials15, and cell culture16. It is difficult to obtain high productivity by means of G. xylinus in a large-scale fermentation system owing to its low yield under agitated culture. Nonetheless, BC secreted by G. xylinus has unique properties including high quality in terms of mechanical strength, water-holding capacity, crystallinity, biodegradability, and biocompatibility17. G. xylinus CGMCC 2955 originates from the fermentation substrates of vinegar18. It has been studied as a model organism for BC production for decades and has been successfully applied to production of wound dressings19,20. In the current study, we present a complete genome sequence of G. xylinus CGMCC 2955 to provide background information for genetic engineering so that precise control of BC biosynthesis can be achieved on the basis of the metabolic pathway that we proposed previously3. A comparison of the arrangement and composition of BC synthase operons (bcs) with those of other BC-producing strains was performed. Furthermore, the relation between the bcs operon and quorum sensing (QS) system – a widely studied system for population regulation – is discussed.
Results
Sequencing showed that the genome is 3,563,314 bp long with GC content of 63.29%. One scaffold was obtained without gaps (Table 1). Gene prediction and annotation of the G. xylinus CGMCC 2955 genome resulted in 3,193 predicted genes. Amongst 117 non-coding RNAs, there are 45 rRNAs, 57 tRNAs, and 15 other RNAs. In the repeat DNAs, simple repeats turned out to be the most abundant repeat families, accounting for 0.51% of the G. xylinus CGMCC 2955 genome, followed by small RNA, accounting for 0.20% of the genome. Non–long terminal repeats were also identified in the genome and contain 13 short interspersed nuclear elements and nine long interspersed nuclear elements. Besides, six DNA transposons (five DNA/hAT-Ac specimens and one DNA/TcMar-Tigger) were detected. No long terminal repeats or satellites were observed.
Table 1.
General features of G. xylinus CGMCC 2955 genome.
| Genome size (bp) | 3,563,314 |
| Gap N (bp) | 0 |
| GC content (%) | 63.29 |
| Total genes | 3,139 |
| Non-coding RNA | 117 |
| rRNA | 45 |
| tRNA | 57 |
| others | 15 |
| Repeat DNAs | |
| SINEs | 13 |
| LINEs | 9 |
| LTR | 0 |
| DNA elements | 6 |
| Small RNA | 41 |
| Satellites | 0 |
| Simple repeats | 409 |
| Low complexity | 38 |
SINEs: short interspersed elements.
LINEs: long interspersed nuclear elements.
LTR: long terminal repeat.
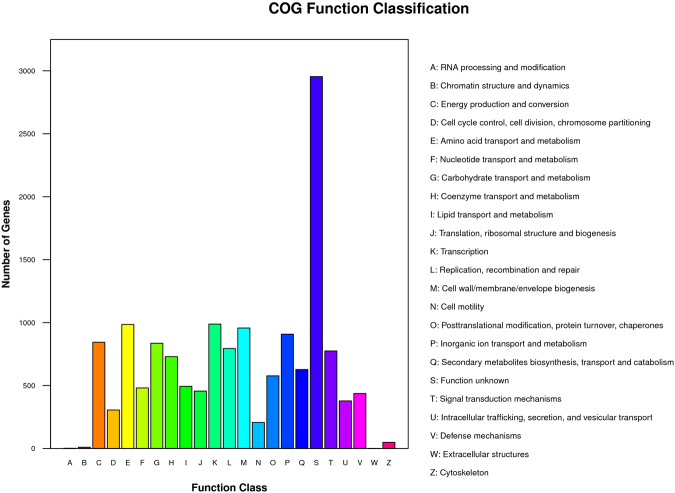
The cluster of orthologous groups (COG) function-based classification of G. xylinus CGMCC 2955 genes revealed 23 functional groups, in which 19.96% of the genes are functionally uncharacterised (Fig. 1). The second and third largest functional groups were ‘transcription’ and ‘amino acid transport and metabolism’; they consisted of 988 and 985 genes, respectively.
Figure 1.
Cluster of orthologous groups (COG) classification of protein functions.
Carbon source metabolism
A total of 50 genes of transporters, 13 genes of symporters, and 50 genes of permeases were identified in the genome of G. xylinus CGMCC 2955. They are responsible for the transport of sugars (e.g. glucose, arabinose, and galactose), sugar acids (e.g. gluconate, α-ketoglutarate, and galactonate), amino acids, spermidines, vitamin B12, phosphate, metal ions, and lipopolysaccharide.
G. xylinus CGMCC 2955 is capable of producing BC from several carbon sources3. Generally, glucose (Glc) is metabolised through the Embden–Meyerhof–Parnas (EMP) pathway, which has proven to be incomplete in most BC-producing stains because of the lack of a gene encoding phosphofructokinase (PFK; EC 2.7.1.11)11,21,22. Of note, according to the gene sequence analysis, the G. xylinus CGMCC 2955 genome possesses a gene encoding phosphofructokinase B (pfkB, CT154_09360), while there is no gene encoding phosphofructokinase A (pfkA). The other two key enzymes are glucokinase (GK) and pyruvate kinase (PK), whose encoding gene locus tags are CT154_11525 and CT154_15810, respectively. The pentose phosphate pathway (PPP) is another effective glucose metabolic pathway in G. xylinus. In the G. xylinus CGMCC 2955 genome, genes encoding glucose-6-phosphate dehydrogenase (6pgd, CT154_14710), glucose-6-phosphate 1-dehydrogenase (6pgd-1, CT154_14205 and CT154_05345), 6-phosphogluconolactonase (6pgl, CT154_14190), 6-phosphogluconate dehydrogenase (6pgad, CT154_14210), ribose-5-phosphate isomerase A (rpi A, CT154_14185), ribulose-phosphate 3-epimerase (rpe, CT154_13290), transketolase (tkt, CT154_07695, CT154_08455, CT154_12910, and CT154_14220), and transaldolase (tsd, CT154_07690 and CT154_14215) were identified. Gluconic acid (GlcA) is an important by-product of BC production in G. xylinus3,23. It was found to be produced from glucose by glucose dehydrogenase (gd, CT154_01250 and CT154_06665) and can be converted to 6-phosphogluconate (GlcA6P) by gluconokinase (GntK). There are two genes encoding GntK (CT154_14180 and CT154_03305), and the latter was hypothesised to encode a thermosensitive GntK. The tricarboxylic acid cycle (TCA) has three key enzymes – citrate synthase (CT154_06205), isocitrate dehydrogenase (CT154_11710 and CT154_00570), and 2-oxoglutarate dehydrogenase (CT154_14680) – and the genes coding for these enzymes are all present in G. xylinus CGMCC 2955. Fructose (FRU) and glycerol (GLY) can considerably contribute to a BC yield just as glucose can3. Via catalysis by fructokinase (frk, CT154_06355), fructose was found to be converted to fructose-6-phosphate (F6P). When glycerol was utilised for BC production, it was firstly converted to glycerol 3-phosphate (GLY3P) by glycerol kinase (glyk, CT154_10145 and CT154_10550), and then was transformed into dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP) by glycerol-3-phosphate dehydrogenase (G3PD) and triosephosphate isomerase (TPI), entering the EMP pathway and PPP. Their encoding gene locus tags were found to be CT154_10150 and CT154_05740, respectively.
When ethanol (ETH) or acetate (AC) served as a sole carbon source for BC production, as shown in Fig. 2, both contributed to a BC production of 0.4 g/l. Glucose was then added as the main carbon source, and either ethanol or acetate were used as supplementary carbon sources. As compared to glucose as the sole carbon source, the ethanol and acetate supplementation increased the BC yield to 1.26-fold and 1.31-fold, respectively. When ethanol is added as the supplementary carbon source, Acetobacter xylinum BPR 3001 A can synthesise BC with a higher yield24. In BPR 3001 A, ethanol is first converted to acetaldehyde by alcohol dehydrogenase (ADH), and then acetaldehyde is converted to acetate under the action of aldehyde dehydrogenase (ALDH). After that, acetyl-coenzyme A (ACCoA) is produced from acetate. In the genome of G. xylinus CGMCC 2955, the gene sequences of Adh (CT154_02385, CT154_08135, CT154_09950, CT154_11925, and CT154_13530) and Aldh (CT154_00245, CT154_02130, CT154_06670, and CT154_12865) were found to be available. In Escherichia coli, acetate can be utilised as a sole carbon source in the phosphotransacetylase-acetate kinase pathway (Pta-Ack), in which phosphate acetyltransferase (PATs) and acetate kinase (ACK) are required25, encoded by CT154_12395 and CT154_15860 in G. xylinus CGMCC 2955.
Figure 2.
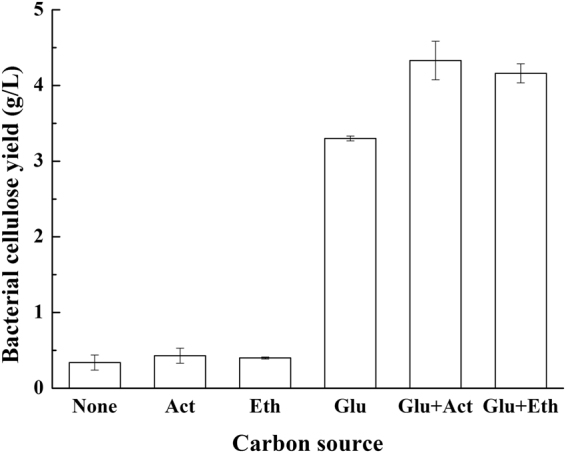
BC production from ethanol and acetate as the sole or supplementary carbon sources. (None: no carbon source, Act: acetate, Eth: ethanol, Glu: glucose).
Energy metabolism
The BC yield enhancement by ethanol and acetate supplementation has been reported to be related to adenosine triphosphate (ATP) production24. There are two patterns for ATP production: substrate level phosphate production through glucose metabolism and oxidative phosphate production via a reduced form of nicotinamide-adenine dinucleotide (NADH) (or reduced flavin adenine dinucleotide, FADH2) generated by glucose metabolism and transferred to electron acceptors. Glucose metabolism is the main energy source of G. xylinus CGMCC 2955.
Under aerobic conditions, oxygen is the electron acceptor from NADH and FADH2 through the electron transport chain. Under these conditions, energy production is mainly based on oxidative phosphorylation, supplemented by substrate level phosphorylation26. Under anaerobic culture conditions, nitrate acts as the electron acceptor in Enterobacter sp. FY-07, which is able to produce BC with a high yield under anaerobic conditions. It is reduced to nitrite by accepting the electrons transferred through the electron transport chain, and nitrite is then reduced to ammonia27. Nitrate reductase and nitrite reductase are involved in this process, and in the present study, only a nitrite reductase gene (CT154_11275) was identified in the G. xylinus CGMCC 2955 genome. Moreover, genes encoding ADH (CT154_02385, CT154_05910, CT154_08135, CT154_09950, CT154_11925, and CT154_13530) and lactate dehydrogenase (ldh, CT154_01275 and CT154_10640) were identified in the G. xylinus CGMCC 2955 genome. They are responsible for the production of NADH when oxygen supply is deficient. This finding suggested that G. xylinus CGMCC 2955 has the potential to produce sufficient energy in hypoxic environments.
BC synthesis
In G. xylinus, glycolytic intermediate glucose-6-phosphate is the origin of substrate synthesis for BC production. It is isomerised to glucose-1-phosphate (G1P) by phosphoglucomutase (PGM; encoded by CT154_06830), which then reacts with UTP, forming uridine-5′-phosphate-α-D-glucose (UDPG) under the action of UDP-glucose pyrophosphorylase (UGPase; encoded by CT154_06835)28. Finally, cellulose is synthesised by a BC synthase (Bcs) complex containing subunits BcsA, BcsB, BcsC, and BcsD, which are encoded by three (bcsAB, bcsC, and bcsD) or four (bcsA, bcsB, bcsC, and bcsD) genes11,29.
The Bcs complex spans the outer and inner cell membranes to synthesise and extrude glucan chains, which are then assembled into fibrils that are further assembled into a ribbon30. BcsA is the catalytic subunit of the Bcs complex28,31. BcsB is an auxiliary subunit that interacts with BcsA via its C-terminal transmembrane helix and regulates BC synthesis by interacting with cyclic diguanylate (c-di-GMP)32. The BcsA–BcsB complex is sufficient for BC synthesis and translocation31. BcsC is believed to play important roles in cell membrane pore formation for BC secretion32. BcsD may control the crystallisation of cellulose into nanofibrils5. There can be one or several bcs operons in a single organism, and the sequence identity of genes encoding the same subunits may not be that high. Moreover, the composition and arrangement of bcs operons are diverse in the same or two different organisms. Additionally, among the bcs operons in the same genome, only one or some of the operons perform key functions in BC synthesis33.
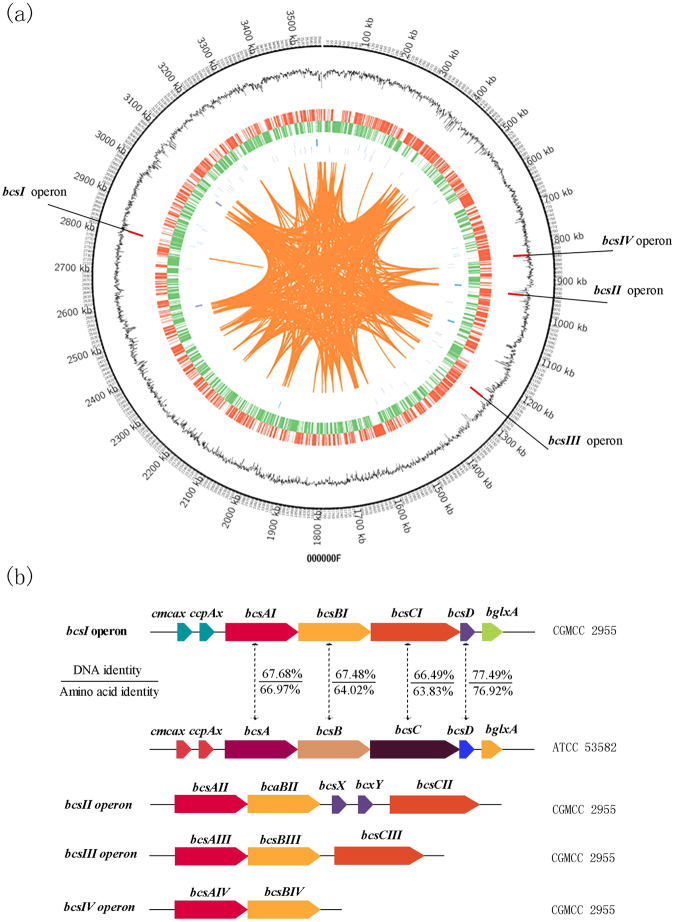
In the G. xylinus CGMCC 2955 genome, a total of four bcs operons were identified. As shown in Fig. 3, their compositions were different among the operons. Operon bcs I was the only structurally complete one, containing bcsA (CT154_12695), bcsB (CT154_12690), bcsC (CT154_12685), and bcsD (CT154_12680). In addition to the above genes, cmcax (CT154_12705) and ccpax (CT154_12700) were identified upstream, and the bglxA (CT154_12675) gene was located downstream in operon bcs I. Gene cmcax encodes endo-β-1,4-glucanase (CMCax). When antibodies to recombinant CMCax are added to the culture medium, the formation of cellulose fibre is severely inhibited34. The CcpAx protein (also known as ORF-2), encoded by ccpax, functions as a mediator of protein–protein interactions and is important for localisation of the Bcs complex to the cell membrane. The bglxa gene encodes a β-glucosidase. CMCax and β-glucosidase both can hydrolyse tangled glucan chains when there is a failure in chain arrangement and are both crucial for BC synthesis35. Operons bcs II and bcs III consist of bcsA, bcsB, and bcsC, where BcsA and BcsB are encoded by one gene. Operon bcs II (bcsA, bcsB, and bcsC) was found to be encoded by CT154_04230 and CT154_04245, with bcsX (CT154_04235) and bcsY (CT154_04240) in the middle of bcsB and bcsC. The products of bcsX and bcsY have not yet been characterised although Bcs Y was predicted to function as a transacylase, participating in the production of acetyl cellulose or similarly modified polysaccharides36. Operon bcs III (bcsA, bcsB, and bcsC) turned out to be encoded by CT154_05660 and CT154_05665. Operon bcs IV uniquely contains only bcsA and bcsB (CT154_03760).
Figure 3.
An overview of the G. xylinus CGMCC 2955 genome. (a) The circle represents (from outside in): circle 1, genome; circle 2, G + C content; circle 3, genes on forward (red) and reverse (green) strands; circle 4, distribution of genes encoding non-coding RNAs; circle 5, long terminal repeats in the genome. (b) The arrangement of bcs operons and sequence alignment of the bcs I operon and G. xylinus ATCC 53582 bcs operon.
Sequence alignment and analysis of bcsA and bcsB in the four operons indicated that the identity was very low between them (Table 2). Operon bcs I was found to be structurally the same as the sole bcs operon in G. xylinus ATCC 53582. The sequence alignment of each gene is presented in Fig. 3b. It suggests that bcsA in bcs I (bcsAI) genes in G. xylinus CGMCC 2955 and G. xylinus ATCC 53582 share 67.68% and 66.97% nucleotide sequence identity and amino acid sequence identity, respectively. For bcsBI, the respective identity values were 67.48% and 64.02%. For bcsCI, the respective identity values were 66.49% and 63.83%. For bcsDI, the respective identity levels were 77.49% and 76.92%. There is only one bcs operon in G. xylinus ATCC 53582. The high identity of operon bcs I sequences between G. xylinus CGMCC 2955 and G. xylinus ATCC 53582, and its complete structure suggested that bcs I may be deeply involved in BC synthesis in G. xylinus CGMCC 2955. These results provide a foundation for genetic manipulation for research regarding which bcs operon is essential for BC synthesis in G. xylinus CGMCC 2955.
Table 2.
sequence alignment of bcs A-B in four bcs operons of G. xylinus CGMCC 2955.
| bcs I A-B | bcs II A-B | bcs III A-B | bcs IV A-B | |
|---|---|---|---|---|
| bcs I A-B | 100 | 57.31 | 53.36 | 56.79 |
| bcs II A-B | 57.31 | 100 | 56.99 | 67.87 |
| bcs III A-B | 53.36 | 56.99 | 100 | 55.87 |
| bcs IV A-B | 56.79 | 67.87 | 55.87 | 100 |
Regulation of BC synthesis by c-di-GMP and by the QS system
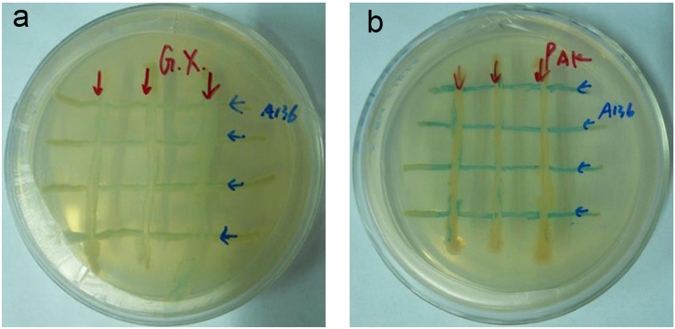
The BcsA–BcsB complex in Bcs is activated by c-di-GMP37. The latter is synthesised from guanosine triphosphate (GTP) by diguanylate cyclase (DGC) and is degraded by two phosphodiesterases (PDEs), thereby regenerating 5′-guanosine monophosphate (5′-GMP; that can be used for GTP synthesis) and forming a biosynthetic and degradative pathway: the c-di-GMP cycle2,38. In the G. xylinus CGMCC 2955 genome, there is only one dgc gene (CT154_08975), while there are several pde genes, including CT154_00885, CT154_01735 – CT154_01745, CT154_08940, CT154_08945, CT154_08975, and CT154_14645. Besides, CT154_05705 and CT154_14650 are hypothesised to encode DGC/PDE. These genes together control intracellular c-di-GMP levels. In Gluconacetobacter intermedius, pde expression was shown to be positively regulated by the GinI–GinR QS system39,40. The presence of a QS system can be determined by detection of chemical signals called autoinducers41. These signalling molecules are produced from common metabolites such as fatty acids, anthranilate, and S-adenosylmethionine42–44. In the current study, the presence of signalling molecules was identified by a vertical streaking agar well diffusion method. Agrobacterium tumefaciens A136 allows for detection of a broad range of acyl-homoserine lactones (AHLs) with acyl chain lengths from 4 to 12 carbons as well as 3-unsubstituted AHLs45. As shown in Fig. 4, the A. tumefaciens A136 biosensor turned blue in (a) and (b) indicating that G. xylinus CGMCC 2955 and Pseudomonas aeruginosa PAK both synthesise a QS AHL autoinducer signalling molecule (Fig. 4a,b)46. This finding revealed that G. xylinus CGMCC 2955 is capable of producing AHLs, and the latter are usually produced by the LuxI–LuxR QS system in gram-negative bacteria47. Genes luxI and luxR are normally homologs of ginI and ginR. Nevertheless, only the luxR (CT154_10285) gene was identified in G. xylinus CGMCC 2955.
Figure 4.
The reported vertical streaking method with bacterial biosensors. (a) G. xylinus CGMCC 2955. (b) P. aeruginosa PAK (positive control).
Discussion
To control the production of BC for different purposes, researchers manipulate the cell culture process. The carbon source was reported to be one of the most effective factors that contribute to differences in BC production and structural characteristics12,48,49. Zhong et al. revealed that G. xylinus CGMCC 2955 can produce BC from various carbon sources, including glucose, fructose, and glycerol3. Distinct BC network structures were obtained from these carbon sources, resulting from different metabolic processes, including BC production and cell growth rates. The metabolic network necessary to study the metabolism of different carbon sources has already been built3. In the present study, genome sequence analysis revealed the metabolic characteristics of G. xylinus CGMCC 2955. Most BC-producing strains do not possess the EMP pathway because they lack PFK activity50. Nevertheless, genome sequence analysis indicated that G. xylinus CGMCC 2955 has pfkB (CT154_09360), which encodes PFK II51. PFK I and II both convert fructose-6-phosphate (F6P) to fructose-1,6-diphosphate (F16P). In E. coli, ~90% of the activity is attributed to PFK I, a well-known allosteric enzyme. The remaining activity is PFK II52. Therefore, in contrast to other BC-producing microorganisms, G. xylinus CGMCC 2955 has a complete EMP pathway. Moreover, ~33% of glucose enters the PPP and 17% entered the TCA cycle in G. xylinus CGMCC 29553. In the genome of G. xylinus CGMCC 2955, all genes encoding enzymes of the PPP were found, confirming that glucose could be metabolised by the PPP. Besides, flux analysis suggested that 92.85% of glucose is fluxed into gluconic acid3. Liu et al. speculated that gluconic acid can serve as a second carbon source when glucose is exhausted in G. xylinus CGMCC 295523. The presence of gluconate kinase–encoding gene gntK (CT154_14180, CT154_03305) – coupled with a flux analysis that indicated that more than a half of gluconic acid (52.83%) was converted to glucose-6-phosphate – suggested that gluconic acid may be utilised as a carbon source for cell growth and BC production through the PPP. This finding is consistent with our previous report showing that dry cell weight and the BC yield increased 2.55- and 14.17-fold, respectively, when gluconic acid was provided as the sole carbon source as opposed to no carbon source53.
When ethanol or acetate were used as the sole carbon source, neither of them could contribute to a considerable BC yield (Fig. 2). On the other hand, the genes encoding the enzymes of the Pta-Ack pathway are all present in G. xylinus CGMCC 2955. When G. xylinus CGMCC 2955 was incubated in a glucose-containing culture medium, both ethanol and acetate contributed to the increased BC yield. These results support other reports24. We believe that ethanol or acetate cannot be utilised as a substrate for BC production owing to the absence of a precursor for BC synthesis. Nevertheless, ethanol or acetate functioned as an energy source for ATP generation through the TCA cycle. Therefore, it was inferred that the promotion of BC production by supplementary ethanol or acetate can be attributed to ATP generation, which accelerated the BC synthesis pathway by inhibiting glucose-6-phosphate dehydrogenase activity24. Furthermore, the relation between ATP production and BC synthesis was recently studied in Enterobacter sp. FY-07, which is capable of synthesizing BC under both aerobic and anaerobic culture conditions27. The BC biosynthesis process in Enterobacter sp. FY-07 was found to be the same as that of G. xylinus. It manifested a high yield of BC even under anaerobic conditions, suggesting that oxygen was not directly involved in the BC synthesis reaction. Meanwhile, ATP was found to be necessary to activate glucose to UDPG, which is also essential for c-di-GMP biosynthesis. Our recent work indicates that G. xylinus CGMCC 2955 can produce more BC during hypoxia than under atmospheric and oxygen-enriched culturing conditions53. Further research on the effect that oxygen and energy generation have on BC production is a possible next project.
The Bcs complex is the key enzyme in BC synthesis. Four bcs operons were identified in the G. xylinus CGMCC 2955 genome. Up to three bcs operons in other BC-producing species have been reported (G. xylinus ATCC 23769 and G. hansenii ATCC 53582)10,54,55, where a high cellulose synthase copy number generally indicates contributions to high BC productivity8. Our phylogenetic analysis indicates that bcs II and bcs IV were the most closely related (Table 2) and possibly arose from duplication and subsequent translocation. With this information, we can study the function of different bcs operons, e.g. investigate whether operon bcs I, the only structurally complete bcs operon, functions alone. If the genes encoding Bcs subunits were deleted in bcs I, will other bcs operons act as candidate genes?
c-di-GMP, an activator of the BcsA–BcsB complex, is synthesised from GTP by DGC and is degraded by PDE2. In G. intermedius, the expression of pde was found to be positively regulated by the GinI–GinR QS system39,40. QS is a microbial cell-to-cell communication process that allows a group of bacterial cells to regulate their gene expression in unison, which is important for implementation of group behaviours such as bioluminescence56, virulence57, phenotypic heterogeneity58, genetic exchange59, and bacterial pathogenicity60. QS systems have been identified in both gram-negative and gram-positive bacterial species61. The LuxI–LuxR-type system is common among gram-negative QS bacteria, in which LuxR serves as both the cytoplasmic signalling molecule’s receptor and the transcriptional activator of the lux operon’s QS system, whereas LuxI works as the signalling molecule’s synthase47,62,63. In this study, Fig. 4 illustrates the presence of AHLs in G. xylinus CGMCC 2955. AHLs were synthesised by the LuxR–LuxI system. When the AHL signal reaches a threshold concentration, it is bound by LuxR, and this complex activates the transcription of a specific operon. Nonetheless, in the G. xylinus CGMCC 2955 genome, only luxR (CT154_10285) was identified. The absence of luxI suggests that the signalling molecule may be produced by other pathways instead of luxI or that the identity of the luxI sequence in G. xylinus CGMCC 2955 with that in other microorganisms is too low for identification of luxI.
The regulation of the QS system by PDE involves the 89 aa protein GinA (target protein) in G. intermedius, where the production of GinA is induced by the QS system. A putative c-di-GMP phosphodiesterase, pde was shown to be GinA inducible and is involved in the repression of oxidative fermentation by the GinI–GinR QS system40. Therefore, because luxI and luxR are homologs of ginI and ginR, and c-di-GMP is an activator of the BcsA–BcsB subunit, we speculated that BC biosynthesis may be regulated by QS by controlling c-di-GMP levels. Bacteria generally depend on QS to regulate cellular processes that are essential for survival and adaptation to changing environments. Although it is still unclear why microorganisms produce BC in nature, BC has been shown to confer access to sufficient oxygen at the liquid–gas interface and high resistance to UV irradiation64. Therefore, there is a possibility that QS participates in the BC synthesis. Various methods have been applied to manipulate the biosynthesis of BC, but little attention has been paid to the regulation of QS during BC production. Nonetheless, it has been successfully applied to the production of biofilms65. The BC-regulatory mechanism involved in the relations among QS, c-di-GMP, and BC is worthy of further study. The regulatory mechanism of action on BC production presented in this study is summarised in Fig. 5.
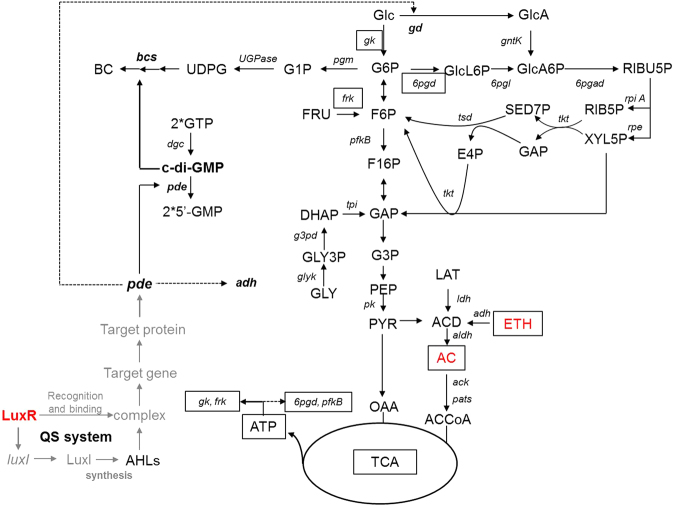
Figure 5.
The metabolic and regulatory network of G. xylinus CGMCC 2955. Framed means regulation by ethanol and acetate addition, and boldfacing denotes regulation by the QS system. Gray means needing further examination. A solid line indicates that gene expression, metabolite synthesis, or metabolic pathway was activated; a dashed line means they were inhibited. (Abbreviations of genes. 6pgad: 6-phosphogluconate dehydrogenase; 6pgd: glucose-6-phosphate dehydrogenase; 6pgl: 6-phosphogluconolactonase; ack: acetate kinase; adh: alcohol dehydrogenase; aldh: aldehyde dehydrogenase; bcs: bacterial cellulose synthase; dgc: diguanylate cyclase; frk: fructokinase; g3pd: glycerol-3-phosphate dehydrogenase; gd: glucose dehydrogenase; gk: glucokinase; glyk: glycerol kinase; gntK: gluconokinase; ldh: lactate dehydrogenase; pats: phosphate acetyltransferase; pde: phosphodiesterase; pfkB: phosphofructokinase B; pgm: phosphoglucomutase; pk: pyruvate kinase; rpe: ribulose-phosphate 3-epimerase; tkt: transketolase; tpi: triosephosphate isomerase; tsd: transaldolase; UGPase: UDP-glucose pyrophosphorylase), (Abbreviations of metabolites. AC: acetate; ACCOA: acetyl-coenzyme A; ACD: acetaldehyde; BC: bacterial cellulose; c-di-GMP: cyclic diguanylate; DHAP: dihydroxyacetone phosphate; E4P: erythrose 4-phosphate; ETH: ethanol; F16P: fructose-1,6-diphosphate; F6P: fructose 6-phosphate; FRU: fructose; G1P: glucose 1-phosphate; G3P: glyceraldehyde-3-phosphate; G6P: glucose 6-phosphate; GAP: glyceraldehyde-3-phosphate; Glc: glucose; GlcA: gluconate; GlcA6P: 6-phosphogluconate; GlcL6P: 6-phosphogluconolactone; GLY: glycerol; GLY3P: glycerol 3-phosphate; GMP: guanosine monophosphate; GTP: guanosine triphosphate; LAT: lactate; OAA: oxaloacetate; PEP: phosphoenol pyruvate; PYR: pyruvate; RIB5P: ribose-5-phosphate; RIBU5P: ribulose-5-phosphate; SED7P: sedoheptulose 7-phosphate; UDPG: uridine-5′-phosphate-α-D-glucose; XYL5P: xylulose-5-phosphate).
Materials and Methods
Cell culture and chromosomal DNA extraction
G. xylinus CGMCC 2955 was isolated from 16 solid fermentation substrates of vinegar by Tianjin University of Science and Technology18. After cultivation on a solid medium (25 g/l glucose [0.83 (mol carbon)/l], 10 g/l peptone, 7.5 g/l yeast extract, and 10 g/l Na2HPO4) at 30 °C for 3 days, cells in the medium were washed with normal saline and centrifuged at 4000 rpm for 5 min (Eppendorf 5804 R). The cell pellets were subjected to DNA extraction. A TAKARA DNA extraction kit was employed for DNA extraction. DNA quality was evaluated on a BASIC biospectrometer (Eppendorf).
DNA sequencing and assembly
The genome of G. xylinus CGMCC 2955 was sequenced at Genewiz Biotechnology Co., Ltd. (Suzhou, China) by means of a PacBio RS II DNA sequencing 9 K library. Single-molecule real-time sequencing (SMRT) allows for highly precise sequencing, with accuracy of more than 99.999% (QV50) and is not affected by GC and AT content. Sequences were assembled according to principles similar to those of the first-generation sequencing technology. Consequently, a single scaffold was assembled in the SMRT Link software (version 4.1). The genomic fine drawing was completed by analysing bioinformatic means after quality control procedures.
Effects of acetate and ethanol on BC production
G. xylinus CGMCC 2955 was cultured as previously reported3. When acetate or ethanol was supplied as the sole carbon source, the culture media had the following composition: 0.43 (mol C)/l carbon source, 10 g/l peptone, 7.5 g/l yeast extract, and 10 g/l Na2HPO4. The initial pH was adjusted to 6.0. The same culture medium without addition of a carbon source served as the control. For supplementary ethanol or acetate experiments, 0.43 (mol C)/l ethanol or acetate was added to the glucose culture medium. BC was harvested after static culture at 30 °C for 10 days. Before weighing, BC was processed as reported elsewhere3. The dry weight was recorded for each pellicle at room temperature.
Nucleotide sequence accession numbers
The complete sequences of G. xylinus CGMCC 2955 analysed in this study can be found in the NCBI GenBank (http://www.ncbi.nlm.nih.gov) under accession No. CP024644.
Biological detection of signalling molecule AHLs
The presence of AHLs in G. xylinus CGMCC 2955 was determined in an agar well-diffusion assay and β-galactosidase activity assay based on a biosensor46. This is a traditional and powerful method for investigating QS systems in gram-negative bacteria. The AHLs were detected by a vertical streaking agar well diffusion assay with the AHL biosensor.
G. xylinus CGMCC 2955 was streaked on Luria Bertani (LB) agar containing 40 μg/ml 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-Gal) perpendicular to A. tumefaciens A136, with P. aeruginosa PAK serving as the positive control and A. tumefaciens A136 alone serving as the negative control. AHL production was detected as the production of a blue pigment by A. tumefaciens A136. The AHL biosensor, carrying TraR-regulated traI-lacZ fusion genes, can produce a blue pigment in the presence of X-Gal in response to exogenous AHLs.
Data availability statement
All data generated or analysed during this study are included in this published article.
Ethical statement
This article does not contain any experiments on human participants or animals that were performed by any of the authors.
Acknowledgements
The authors would like to express their appreciation to professor Robert J.C. McLean at Texas A&M University for supplying A. tumefaciens A136 and for financial support from the National Natural Science Foundation of China (grants No. 21576212 and No. 31470610), Natural Science Foundation of Tianjin (grant No. 15JCZDJC32600), and Innovation Foundation for Doctoral Dissertation of Tianjin University of Science and Technology (grant No. 2016001).
Author Contributions
Miao Liu and Lingpu Liu contributed to the annotation of the genome and analysed the genomic information; Miao Liu wrote the first draft of the manuscript; Shiru Jia and Cheng Zhong contributed genomic analysis and manuscript writing suggestions; Siqi Li and Yang Zou substantially participated in most experiments as well as manuscript revisions.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koyama M, Helbert W, Imai T, Sugiyama J, Henrissat B. Parallel-up structure evidences the molecular directionality during biosynthesis of bacterial cellulose. Proc. Natl. Acad. Sci. USA. 1997;94:9091–9095. doi: 10.1073/pnas.94.17.9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross P, Mayer R, Benziman M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991;55:35–58. doi: 10.1128/mr.55.1.35-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong C, et al. Metabolic flux analysis of Gluconacetobacter xylinus for bacterial cellulose production. Appl. Microbiol. Biotechnol. 2013;97:6189–6199. doi: 10.1007/s00253-013-4908-8. [DOI] [PubMed] [Google Scholar]
- 4.Hong F, Qiu K. An alternative carbon source from konjac powder for enhancing production of bacterial cellulose in static cultures by a model strain Acetobacter aceti subsp. xylinus ATCC 23770. Carbohydr. Polym. 2008;72:545–549. doi: 10.1016/j.carbpol.2007.09.015. [DOI] [Google Scholar]
- 5.Lee KY, Buldum G, Mantalaris A, Bismarck A. More than meets the eye in bacterial cellulose: biosynthesis, bioprocessing, and applications in advanced fiber composites. Macromol. Biosci. 2014;14:10–32. doi: 10.1002/mabi.201300298. [DOI] [PubMed] [Google Scholar]
- 6.Hu W, Chen S, Yang J, Li Z, Wang H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014;101:1043–1060. doi: 10.1016/j.carbpol.2013.09.102. [DOI] [PubMed] [Google Scholar]
- 7.Cameron DE, Bashor CJ, Collins JJ. A brief history of synthetic biology. Nat. Rev. Microbiol. 2014;12:381–390. doi: 10.1038/nrmicro3239. [DOI] [PubMed] [Google Scholar]
- 8.Florea M, et al. Engineering control of bacterial cellulose production using a genetic toolkit and a new cellulose-producing strain. Proc. Natl. Acad. Sci. USA. 2016;113:E3431–E3440. doi: 10.1073/pnas.1522985113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubiak K, et al. Complete genome sequence of Gluconacetobacter xylinus E25 strain–valuable and effective producer of bacterial nanocellulose. J. Biotechnol. 2014;176:18–19. doi: 10.1016/j.jbiotec.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Ogino H, et al. Complete genome sequence of NBRC 3288, a unique cellulose-nonproducing strain of Gluconacetobacter xylinus isolated from vinegar. J. Biotechnol. 2011;193:6997–6998. doi: 10.1128/JB.06158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, et al. Complete genome sequence of the cellulose-producing strain Komagataeibacter nataicola RZS01. Sci. Rep. 2017;7:4431. doi: 10.1038/s41598-017-04589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung HI, et al. Influence of glycerol on production and structural-physical properties of cellulose from Acetobacter sp V6 cultured in shake flasks. Bioresour. Technol. 2010;101:3602–3608. doi: 10.1016/j.biortech.2009.12.111. [DOI] [PubMed] [Google Scholar]
- 13.Shah J, Brown RM., Jr. Towards electronic paper displays made from microbial cellulose. Appl. Microbiol. Biotechnol. 2005;66:352–355. doi: 10.1007/s00253-004-1756-6. [DOI] [PubMed] [Google Scholar]
- 14.Fabra MJ, López-Rubio A, Ambrosio-Martín J, Lagaron JM. Improving the barrier properties of thermoplastic corn starch-based films containing bacterial cellulose nanowhiskers by means of PHA electrospun coatings of interest in food packaging. Food Hydrocoll. 2016;61:261–268. doi: 10.1016/j.foodhyd.2016.05.025. [DOI] [Google Scholar]
- 15.Czaja W, Krystynowicz A, Bielecki S, B R., Jr. Microbial cellulose–the natural power to heal wounds. Biomaterials. 2006;27:145–151. doi: 10.1016/j.biomaterials.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Yin N, et al. Bacterial cellulose as a substrate for microbial cell culture. Appl. Environ. Microbiol. 2014;80:1926–1932. doi: 10.1128/AEM.03452-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iguchi M, Yamanaka S, Budhiono A. Bacterial cellulose-a masterpiece of nature’s arts. J. Mater. Sci. 2000;35:261–270. doi: 10.1023/A:1004775229149. [DOI] [Google Scholar]
- 18.Liu M, et al. Metabolic investigation in Gluconacetobacter xylinus and its bacterial cellulose production under a direct current electric field. Front. Microbiol. 2016;7:331. doi: 10.3389/fmicb.2016.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang XN, et al. Improvement of antimicrobial activity of graphene oxide/bacterial cellulose nanocomposites through the electrostatic modification. Carbohydr. Polym. 2016;136:1152–1160. doi: 10.1016/j.carbpol.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Zhu H, et al. Characterization of bacteriostatic sausage casing: A composite of bacterial cellulose embedded with ɛ-polylysine. Food Sci. Biotechnol. 2010;19:1479–1484. doi: 10.1007/s10068-010-0211-y. [DOI] [Google Scholar]
- 21.Prust C, et al. Complete genome sequence of the acetic acid bacterium Gluconobacter oxydans. Nat. Biotechnol. 2005;23:195–200. doi: 10.1038/nbt1062. [DOI] [PubMed] [Google Scholar]
- 22.Illeghems K, De VL, Weckx S. Complete genome sequence and comparative analysis of Acetobacter pasteurianus 386B, a strain well-adapted to the cocoa bean fermentation ecosystem. BMC Genomics. 2013;14:526. doi: 10.1186/1471-2164-14-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu M, et al. Metabolomic profiling coupled with metabolic network reveals differences in Gluconacetobacter xylinus from static and agitated cultures. Biochem. Eng. J. 2015;101:85–98. doi: 10.1016/j.bej.2015.05.002. [DOI] [Google Scholar]
- 24.Naritomi T, Kouda T, Yano H, Yoshinaga F. Effect of ethanol on bacterial cellulose production from fructose in continuous culture. J. Ferment. Bioeng. 1998;85:598–603. doi: 10.1016/S0922-338X(98)80012-3. [DOI] [Google Scholar]
- 25.Wanner BL, Wilmesriesenberg MR. Involvement of phosphotransacetylase, acetate kinase, and acetyl phosphate synthesis in control of the phosphate regulon in Escherichia coli. J. Bacteriol. 1992;174:2124–2130. doi: 10.1128/jb.174.7.2124-2130.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingledew WJ, Poole RK. The respiratory chains of Escherichia coli. Microbiol. Rev. 1984;48:222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji K, et al. Bacterial cellulose synthesis mechanism of facultative anaerobe Enterobacter sp. FY-07. Sci. Rep. 2016;6:21863. doi: 10.1038/srep21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai T, Sun S, Horikawa Y, Wada M, Sugiyama J. Functional reconstitution of cellulose synthase in Escherichia coli. Biomacromolecules. 2014;15:4206–4213. doi: 10.1021/bm501217g. [DOI] [PubMed] [Google Scholar]
- 29.Kawano S, et al. Cloning of cellulose synthesis related genes from Acetobacter xylinum ATCC23769 and ATCC53582: comparison of cellulose synthetic ability between strains. DNA Res. 2002;9:149–156. doi: 10.1093/dnares/9.5.149. [DOI] [PubMed] [Google Scholar]
- 30.Hu SQ, Cosgrove DJ. Structure of bacterial cellulose synthase subunit D octamer with four inner passageways. Proc. Natl. Acad. Sci. USA. 2010;107:17957–17961. doi: 10.1073/pnas.1000601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omadjela O, et al. BcsA and BcsB form the catalytically active core of bacterial cellulose synthase sufficient for in vitro cellulose synthesis. Proc. Natl. Acad. Sci. USA. 2013;110:17856–17861. doi: 10.1073/pnas.1314063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong HC, et al. Genetic organization of the cellulose synthase operon in Acetobacter xylinum. Proc. Natl. Acad. Sci. USA. 1990;87:8130–8134. doi: 10.1073/pnas.87.20.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Römling U, Galperin MY. Bacterial cellulose biosynthesis: diversity of operons, subunits, products, and functions. Trends Microbiol. 2015;23:545–557. doi: 10.1016/j.tim.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koo HM, Song SH, Yu RP, Yu SK. Evidence that a β-1,4-endoglucanase secreted by Acetobacter xylinum plays an essential role for the formation of cellulose fiber. Biosci. Biotechnol. Biochem. 1998;62:2257–2259. doi: 10.1271/bbb.62.2257. [DOI] [PubMed] [Google Scholar]
- 35.Nakai T, et al. Formation of highly twisted ribbons in a carboxymethylcellulase gene-disrupted strain of a cellulose-producing bacterium. J. Bacteriol. 2013;195:958–964. doi: 10.1128/JB.01473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umeda Y, et al. Cloning of cellulose synthase genes from Acetobacter xylinum JCM 7664: implication of a novel set of cellulose synthase genes. DNA Res. 1999;6:109–115. doi: 10.1093/dnares/6.2.109. [DOI] [PubMed] [Google Scholar]
- 37.Morgan JLW, Mcnamara JT, Zimmer J. Mechanism of activation of bacterial cellulose synthase by cyclic-di-GMP. Nat. Struct. Mol. Biol. 2014;21:489–498. doi: 10.1038/nsmb.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross P, et al. Control of cellulose synthesis Acetobacter xylinum. A unique guanyl oligonucleotide is the immediate activator of the cellulose synthase. Carbohydr. Res. 1986;149:101–117. doi: 10.1016/S0008-6215(00)90372-0. [DOI] [Google Scholar]
- 39.Iida A, Ohnishi Y, Horinouchi S. Control of acetic acid fermentation by quorum sensing via N-acylhomoserine lactones in Gluconacetobacter intermedius. J. Bacteriol. 2008;190:2546–2555. doi: 10.1128/JB.01698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iida A, Ohnishi Y, Horinouchi S. Identification and characterization of target genes of the GinI/GinR quorum-sensing system in Gluconacetobacter intermedius. Microbiology. 2009;155:3021–3032. doi: 10.1099/mic.0.028613-0. [DOI] [PubMed] [Google Scholar]
- 41.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Bio. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 42.Fuqua C, Greenberg EP. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr. Opin. Microbiol. 1998;1:183–189. doi: 10.1016/S1369-5274(98)80009-X. [DOI] [PubMed] [Google Scholar]
- 43.Ryan RP, An SQ, Allan JH, Mccarthy Y, Dow JM. The DSF family of cell–cell signals: an expanding class of bacterial virulence regulators. Plos Pathog. 2015;11:e1004986. doi: 10.1371/journal.ppat.1004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tiaden A, Spirig T, Hilbi H. Bacterial gene regulation by alpha-hydroxyketone signaling. Trends Microbiol. 2010;18:288–297. doi: 10.1016/j.tim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Shaw PD, et al. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeon KM, et al. Quorum sensing: a new biofouling control paradigm in a membrane bioreactor for advanced wastewater treatment. Environ. Sci. Technol. 2009;43:380–385. doi: 10.1021/es8019275. [DOI] [PubMed] [Google Scholar]
- 47.Schaefer AL, et al. E. P. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc. Natl. Acad. Sci. USA. 1996;93:9505–9509. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, et al. Improvement of bacterial cellulose production by manipulating the metabolic pathways in which ethanol and sodium citrate involved. Appl. Microbiol. Biotechnol. 2012;96:1479–1487. doi: 10.1007/s00253-012-4242-6. [DOI] [PubMed] [Google Scholar]
- 49.Mikkelsen D, Flanagan BM, Dykes GA, Gidley MJ. Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J. Appl. Microbiol. 2010;107:576–583. doi: 10.1111/j.1365-2672.2009.04226.x. [DOI] [PubMed] [Google Scholar]
- 50.Schramm M, Gromet Z, Hestrin S. Role of hexose phosphate in synthesis of cellulose by Acetobacter xylinum. Nature. 1957;179:28–29. doi: 10.1038/179028a0. [DOI] [Google Scholar]
- 51.Daldal F. Molecular cloning of the gene for phosphofructokinase-2 of Escherichia coli and the nature of a mutation, pfkB1, causing a high level of the enzyme. J. Mol. Bio. 1983;168:285–305. doi: 10.1016/S0022-2836(83)80019-9. [DOI] [PubMed] [Google Scholar]
- 52.Blangy D, Buc H, Monod J. Kinetics of the allosteric interactions of phosphofructokinase from Escherichia coli. J. Mol. Bio. 1968;31:13–35. doi: 10.1016/0022-2836(68)90051-X. [DOI] [PubMed] [Google Scholar]
- 53.Liu M, et al. Enhanced bacterial cellulose production by Gluconacetobacter xylinus via expression of Vitreoscilla hemoglobin and oxygen tension regulation. Appl. Microbiol. Biotechnol. 2017;102:1155–1165. doi: 10.1007/s00253-017-8680-z. [DOI] [PubMed] [Google Scholar]
- 54.Iyer PR, Geib SM, Catchmark J, Kao T, Ming T. Genome sequence of a cellulose-producing bacterium, Gluconacetobacter hansenii ATCC 23769. J. Bacteriol. 2010;192:4256. doi: 10.1128/JB.00588-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Florea M, Reeve B, Abbott J, Freemont PS, Ellis T. Genome sequence and plasmid transformation of the model high-yield bacterial cellulose producer Gluconacetobacter hansenii ATCC 53582. Sci. Rep. 2016;6:23635. doi: 10.1038/srep23635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nealson KH, Hastings JW. Bacterial bioluminescence: its control and ecological significance. Microbiol. Rev. 1979;43:496–518. doi: 10.1128/mr.43.4.496-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell. 2002;110:303–314. doi: 10.1016/S0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 58.Claudia A, Ursula S, Kirsten J. Single cell analysis of Vibrio harveyi uncovers functional heterogeneity in response to quorum sensing signals. BMC Microbiol. 2012;12:1–10. doi: 10.1186/1471-2180-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Ann. Rev. Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee R, et al. A quorum-sensing antagonist targets both membrane-bound and cytoplasmic receptors and controls bacterial pathogenicity. Mol. Cell. 2009;35:143–153. doi: 10.1016/j.molcel.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hawver LA, Jung SA, Ng W-L. Specificity and complexity in bacterial quorum-sensing systems. FEMS Microbiol. Rev. 2016;40:738–752. doi: 10.1093/femsre/fuw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eberhard A, et al. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 63.Engebrecht J, Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc. Natl. Acad. Sci. USA. 1984;81:4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams WS, Cannon RE. Alternative environmental roles for cellulose produced by Acetobacter xylinum. Appl. Environ. Microbiol. 1989;55:2448–2452. doi: 10.1128/aem.55.10.2448-2452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.