Abstract
Host quality is critical for parasites. The common cuckoo Cuculus canorus is a generalist avian brood parasite, but individual females show strong preference for a specific host species. Here, we use three extensive datasets to investigate different host characteristics determining cuckoo host selection at the species level: (i) 1871 population-specific parasitism rates collected across Europe; (ii) 14 K cases of parasitism in the United Kingdom; and (iii) 16 K cases of parasitism in Germany, with data collected during the period 1735–2013. We find highly consistent effects of the different host species traits across our three datasets: the cuckoo prefers passerine host species of intermediate size that breed in grass- or shrubland and that feed their nestlings with insects, and avoids species that nest in cavities. Based on these results, we construct a novel host suitability index for all passerine species breeding in Europe, and show that host species known to have a corresponding cuckoo host race (gens) rank among the most suitable hosts in Europe. The distribution of our suitability index shows that host species cannot be classified as suitable or not but rather range within a continuum of suitability.
Introduction
Parasites are strongly dependent on host suitability for successful reproduction. Some parasites may prosper using a broad range of hosts, while others specialise on one particular host species1. For generalist parasites, it is very important to select host species that maximize their fitness. Studies on host species preferences show variable degrees of preference in different systems (e.g.2–4), and significant proportions of parasite host range is currently unknown in many systems5. Disentangling the characteristics determining host use in parasites is important for understanding host-parasite coevolution and the evolution of host-specific adaptations in their ongoing arms races, but also for understanding emerging diseases and invasion success of pathogens exploiting crops, domestic animals and human beings. Knowledge of the importance of various host life history traits for successful parasite utilization would for instance allow predictions concerning the host potential for species in geographical areas into which parasites may expand, either in relation to climate change scenarios or in a biological invasion framework6,7.
Avian brood parasites exploit the parental care of their avian hosts, often at the expense of the hosts’ own reproductive output. The common cuckoo Cuculus canorus is a generalist avian brood parasite that is widely distributed throughout Eurasia and is known to have utilized at least 125 passerine species in Europe alone8–10. Individual female cuckoos, however, are considered host-specific and preferentially lay in nests of one, or a few, host species, which then incubate their eggs and raise the parasitic chick11–18. Individual cuckoos can therefore be classified into host races, termed gentes, of which several can be recognized based on their egg phenotype, and which often mimics the egg phenotype of their host19–21, but not always10. More than 200 passerine species breed in Europe22 but interestingly, fewer than 20 cuckoo gentes have so far been described within this area8–10,23,24, implying that many potential hosts are not regularly used by cuckoos10,25–29. Female cuckoos belonging to a specific gens may sometimes lay eggs in the nests of hosts other than the target species11,30, and probing of new hosts and host switching is likely to be an important mechanism for the evolution of new gentes12,25,31–34. The use of a particular host species may therefore change through time; several passerine species show strong anti-parasite adaptations without being parasitized at present, indicating that they have been used as cuckoo hosts in the past35–40.
Data on cuckoo parasitism clearly show that host use varies among habitats26,41,42. In addition, parasitism within a particular host population is not random, but depends on specific variables related to nest structure and placement, phenology and host behaviour, quality and density24,43–55. The relative importance and generality of each specific characteristic that makes a potential host species prone to parasitism is, however, not well known. Such information is pivotal for understanding the potential host range of parasite species.
Many factors have been suggested to affect the suitability of passerine species as cuckoo hosts: diet, nest placement, habitat structure, abundance, duration of egg and nestling period, body size (as a proxy of egg size), nest size and timing of their breeding season9,27,34,35,41,56–63. Glue and Murray64 added “tolerance of the host to disturbance and egg mimicry” as another important attribute of a suitable host.
However, few studies have analysed the factors explaining variation in host use by the cuckoo by using individual-level data across host species. Soler et al.65 found that cuckoo parasitism in UK was more prominent in potential host species with a relatively short nestling period, host species building open nests, and host species with large populations. However, host species with large populations were not more prone to parasitism in southern Finland13. Soler et al.66 found that cuckoo gentes with highly mimetic eggs were most often found in hosts with large population sizes and with low spatial variation in abundance across countries, suggesting that large host populations are important for stable long-term co-evolutionary interactions.
Here, we present three unique datasets with one dataset on 1871 population-specific parasitism rates across European passerines and two datasets on 14 K and 16 K host species-specific records of parasitism in UK and Germany, respectively. These two geographical entities are ideal for analysis of host use because of long-term collections of data on parasitism available from museum collections, literature and various ringing and nest record schemes. This approach allows us to evaluate the generality of the results by determining whether the same factors are identified as important predictors of parasitism in the three independent datasets. We investigate seven specific host life-history traits (nestling food, adult body size, nest placement, habitat, overlap in breeding period with the cuckoo, nest cup depth, and nest height above ground) that may all influence suitability as cuckoo hosts among passerine birds as suggested by previous studies. Finally, we use the population-specific parasitism rates to calculate a host suitability index and predict the suitability of all passerine bird species breeding in Europe.
Results
Models based on the two independent datasets describing the number of parasitism cases in Germany and the UK and the dataset with parasitism rates across Europe show remarkably similar results: (i) species that nest in cavities are used less frequently than those with other nest locations (Fig. 1a and Table 1); (ii) species breeding in forest and rocky areas are used less than species breeding in other habitats (Fig. 1b, Tables 1 and 2); (iii) species feeding their nestlings with plant material are used less frequently than species feeding their nestlings with insects, although not significantly so in the UK (Fig. 1c, Tables 1 and 2); (iv) species with either larger or smaller body size are used less than species with intermediate sizes (Fig. 1d, Tables 1 and 2); and (v) species with smaller population sizes have fewer parasitism events than species with larger populations both in the UK and Germany (Table 1). However, from the current analyses we are not able to tell if there is a deviation from what would be expected if hosts are being used at random as expected from population size. Nest height, nest depth and overlap in breeding period do not affect parasitism in any of the three datasets (Tables 1 and 2). We also note that results are qualitatively and quantitatively very similar if we include all populations regardless of number of nests or if we exclude all populations with less than ten nests from our analysis.
Figure 1.
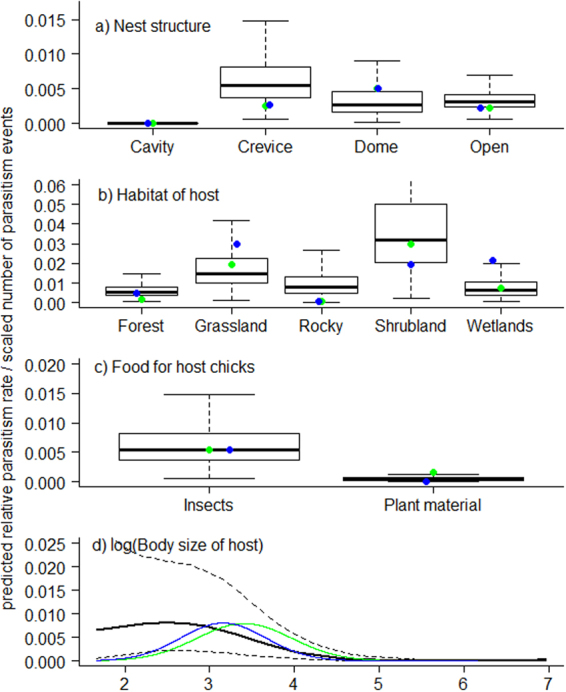
Predicted effects of ecological variables on population-specific parasitism rates (black) and number of parasitism events by cuckoos in UK (green) and Germany (blue) based on (a) nest structure (b) habitat of host (c) food type the host provide for its young and (d) the body size of the host. Note that values for number of parasitism cases in UK and Germany are scaled for easier comparison with parasitism rates and are not originally on the scale of the y-axis. Box blots show the mean predicted relative parasitism rate, outer box show the first and third quantile and the dotted lines show the 95% credible limits. See Materials and Methods for further explanation on how these predicted values were obtained.
Table 1.
Factors explaining variation in cuckoo parasitism rates among passerine species in Europe.
| Estimate | SE | p | |
|---|---|---|---|
| Intercept | −16.416 | 4.428 | 0.000 |
| log(nest.depth + 1) | −0.206 | 0.954 | 0.829 |
| Crevice vs. Cavity | 7.047 | 1.230 | 0.000 |
| Dome vs. Cavity | 6.405 | 1.329 | 0.000 |
| Open vs. Cavity | 6.517 | 1.174 | 0.000 |
| log(Nest height + 1) | 0.001 | 0.095 | 0.988 |
| Grassland vs. Forest | 1.003 | 0.604 | 0.097 |
| Rocky areas vs. Forest | 0.380 | 0.980 | 0.698 |
| Shrubland vs. Forest | 1.757 | 0.462 | 0.000 |
| Wetlands vs. Forest | 0.158 | 0.660 | 0.811 |
| Plant material vs. Invertebrates | −2.694 | 0.678 | 0.000 |
| log(Female body size) | 3.556 | 2.360 | 0.132 |
| log(Female body size2) | −0.689 | 0.347 | 0.047 |
| Breeding overlap | 0.001 | 0.013 | 0.901 |
Parameter estimates are from a binomial regression model of population-specific parasitism rates with species identity included as random factor. Estimates in bold have p-values below 0.05 and are considered significant. See Materials and Methods for details regarding each factor.
Table 2.
Ecological factors explaining variation in cuckoo parasitism among passerine species in UK and Germany.
| Count model coefficients | UK | Germany | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | p | Estimate | SE | p | |
| Intercept | −20.030 | 4.742 | 0.000 | −27.758 | 6.054 | 0.000 |
| log (nest.depth + 1) | −0.330 | 0.885 | 0.709 | −0.995 | 1.707 | 0.560 |
| Crevice vs. Cavity | 4.707 | 0.730 | 0.000 | 3.750 | 1.147 | 0.001 |
| Dome vs. Cavity | 5.405 | 1.140 | 0.000 | 5.033 | 1.228 | 0.000 |
| Open vs. Cavity | 4.505 | 0.777 | 0.000 | 3.766 | 1.070 | 0.000 |
| log(Population size + 1) | 0.283 | 0.067 | 0.000 | 0.524 | 0.090 | 0.000 |
| log(Nest height + 1) | −0.041 | 0.131 | 0.756 | 0.257 | 0.168 | 0.125 |
| Grassland vs. Forest | 1.105 | 0.575 | 0.055 | 1.770 | 0.868 | 0.041 |
| Rocky areas vs. Forest | −1.228 | 1.272 | 0.334 | −1.764 | 1.172 | 0.132 |
| Shrubland vs. Forest | 1.596 | 0.508 | 0.002 | 1.933 | 0.816 | 0.018 |
| Wetlands vs. Forest | 3.074 | 0.752 | 0.000 | 3.177 | 1.163 | 0.006 |
| Plant material vs. Invertebrates | −1.194 | 0.624 | 0.056 | −4.326 | 0.907 | 0.000 |
| Breeding overlap | −0.008 | 0.022 | 0.721 | 0.023 | 0.025 | 0.345 |
| log(Female body size) | 10.625 | 2.487 | 0.000 | 13.683 | 3.207 | 0.000 |
| log(Female body size2) | −1.544 | 0.367 | 0.000 | −2.146 | 0.465 | 0.000 |
| Log(theta) | −0.291 | 0.224 | 0.194 | −0.971 | 0.283 | 0.001 |
| Zero hurdle model coefficients | ||||||
| Intercept | −5.696 | 14.438 | 0.693 | −20.092 | 7.837 | 0.010 |
| log(nest.depth + 1) | −1.939 | 2.110 | 0.358 | 1.144 | 1.095 | 0.296 |
| Crevice vs. Cavity | 4.252 | 2.353 | 0.071 | 3.650 | 1.610 | 0.023 |
| Dome vs. Cavity | 0.069 | 2.703 | 0.980 | 3.972 | 1.870 | 0.034 |
| Open vs. Cavity | 5.489 | 3.157 | 0.082 | 4.148 | 1.562 | 0.008 |
| log(Population size + 1) | 0.895 | 0.385 | 0.020 | 0.543 | 0.143 | 0.000 |
| log(Nest height + 1) | −1.359 | 1.313 | 0.301 | 0.027 | 0.209 | 0.896 |
| Grassland vs. Forest | 9.059 | 5.601 | 0.106 | 3.307 | 2.933 | 0.260 |
| Rocky areas vs. Forest | −0.144 | 2.264 | 0.949 | −1.540 | 1.450 | 0.288 |
| Shrubland vs. Forest | 12.633 | 6.433 | 0.050 | 0.391 | 0.870 | 0.653 |
| Wetlands vs. Forest | −4.319 | 2.932 | 0.141 | −0.199 | 1.316 | 0.880 |
| Plant material vs. Invertebrates | 16.245 | 4479.8 | 0.997 | −2.481 | 1.146 | 0.030 |
| Breeding overlap | 0.078 | 0.063 | 0.213 | −0.029 | 0.029 | 0.319 |
| log(Female body size) | 3.798 | 5.730 | 0.508 | 7.897 | 3.925 | 0.044 |
| log(Female body size2) | −0.919 | 0.853 | 0.281 | −1.249 | 0.573 | 0.029 |
The estimates presented are from hurdle regression models with two components: a truncated negative binomial component for the positive counts (Count model coefficients) and a binomial component for the zero vs positive counts (Zero hurdle model coefficients). Estimates in bold have p-values below 0.05 and are considered significant. See Materials and Methods for details regarding each factor.
The distribution of our host suitability index calculated for all European passerine species does not show a clear bimodal separation between suitable and unsuitable host species, but rather a continuum from low to high suitability (electronic supplementary material, Table S1 and Fig. 2). However, all species with a recognized corresponding cuckoo gens are ranked towards the high suitability end of the index (Fig. 2). Furthermore, the host suitability index, which is based on the model of parasitism rates across Europe, shows a strong correlation with the number of parasitism events in both Germany and the UK (Fig. 3a,b).
Figure 2.
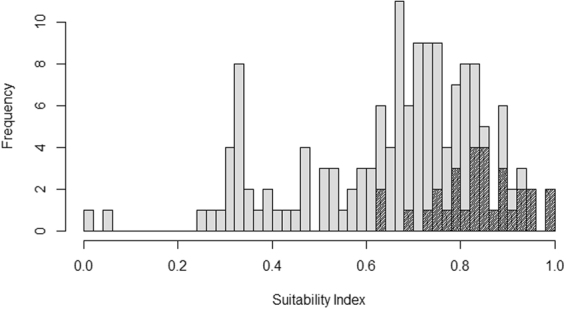
Frequency plot of the host suitability index for passerine birds in Europe. Hatched bars = species with a corresponding cuckoo host race (gens) light grey bars include all passerine species in Europe.
Figure 3.
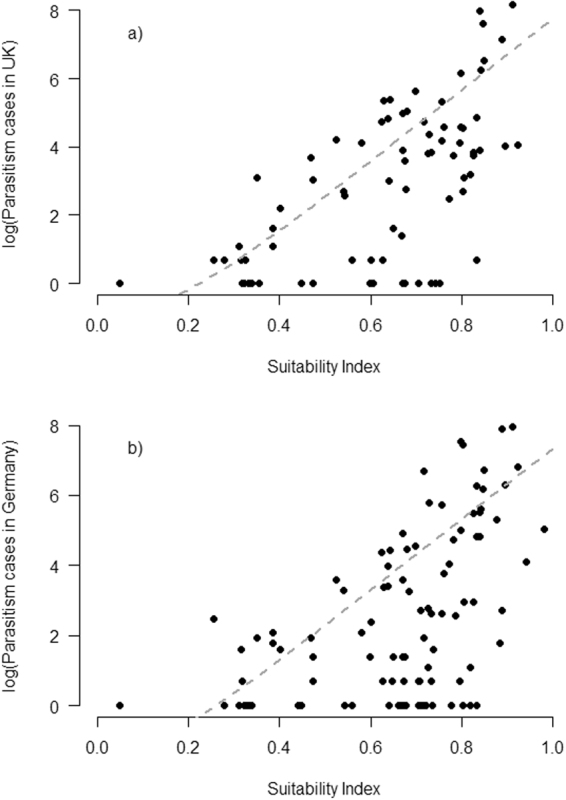
Fit between our host suitability index based on European population-specific parasitism rates and number of parasitism events recorded in (a) UK (R2 = 0.35) and (b) Germany (R2 = 0.28). The grey dotted lines are predicted number of records for increasing suitability along the x-axis. This line is based on predictions from hurdle models (see main text), including only the suitability index as explanatory variable and recorded parasitism events as response variable in each of the two countries. Only species that are recorded in the specific country are included.
Discussion
Species vary in their quality as hosts for parasites, as manifested through host-specific variation in parasite reproductive success3,67. Such variation in parasite success is also evident in avian brood-parasite systems68 and brood parasites should selectively target hosts that maximize the probability of successful fledging of the parasitic chick. Since there is pronounced variation in utilization among potential hosts, selection by cuckoos is clearly not random25,26,28. We find highly similar effects of the different ecological host traits on cuckoo parasitism in our three independent datasets. According to our results, the cuckoo prefers host species of intermediate size that feed their nestlings with insects, and tends to avoid species that nests in cavities or breed in forest or rocky areas, but we find little effect of nest height, nest depth and breeding overlap. Importantly, there is no single variable explaining host use by cuckoos, but rather a combination of variables that together influence parasitism rates.
Host body size is clearly important for host selection by cuckoos. Intermediate-sized passerines are in general parasitized at higher rates than smaller or larger species. Use of the smallest passerines may be hampered by inefficient incubation of the parasitic egg and inadequate provisioning of the parasitic chick. The largest passerines may be avoided for the same reasons, and in addition large host nests, eggs and chicks may render it difficult for the cuckoo chick to evict potential competitors. However, nest cup depth was not an important predictor of cuckoo parasitism in our analyses, despite a deeper or steeper-sided nest tending to render eviction more difficult62,69. Experiments have shown that cuckoo chicks growing up together with host chicks suffer significantly lower probability of survival than when raised alone69–71, but see72. It is possible that the combined effect of larger eggs and nest steep-sidedness would render the largest passerines unsuitable as cuckoo hosts.
The cuckoo chick is dependent on invertebrate food, and seed eating species have therefore been considered unsuitable as cuckoo hosts e.g.10. Nevertheless, species like greenfinch Carduelis chloris and linnet C. cannabina rank among the 10 most commonly used hosts in UK, and there was no significant effect of nestling food on parasitism in UK (Table 1). Greenfinches have been able to raise cuckoo chicks73, but this observation alone is not sufficient to conclude that they are high quality hosts, as we do not know the condition of the fledgling cuckoos and hence the likelihood of recruitment of cuckoos raised by greenfinches. Furthermore, none out of 20 cuckoo eggs in linnet nests recorded in the BTO Nest Record Scheme resulted in successfully fledged cuckoo chicks25. In contrast to UK, our data disclose that few cuckoo eggs have been found in this abundant species in Germany. Hence, the most plausible explanation for the relatively high use of seed eaters in UK is “mislaid” eggs by cuckoos belonging to other tribes. The dunnock Prunella modularis, one of the favourite hosts in UK but less so in Germany, often nests in similar habitats like linnets and greenfinches, and with similar nests and nest sites. Hence, we agree with Davies10 that dunnock cuckoos are most likely responsible for many of the parasitic eggs ending up in finch nests.
Species nesting in cavities often have small entrance holes and deep nests74. The small entrance hole poses great problems for the female cuckoo attempting to successfully place her egg into the nest cup74 and, even if she succeeds, her chick may grow too big to escape and become trapped inside. Chicks may also struggle to evict competing eggs and nestlings. Cavity nesters are therefore regarded as unsuitable hosts35,71,75, a prediction confirmed by our results.
Habitat showed a strong relationship with the number of parasitism events in all three datasets. Species breeding in shrubland and grassland were preferred by the cuckoo whereas species breeding in forests and rocky habitats were largely avoided. Wetland species were utilized relatively frequently in Germany, but not in the two other datasets. The cuckoo is dependent on high vantage points from where it can search for available host nests58, which may render species breeding in rocky areas unsuitable in most cases. Moreover, most of the species in UK and Germany breeding in rocky areas have small population sizes/densities, potentially making it more difficult for cuckoos to maintain a viable population. Many potential host species breeding in forest habitat are cavity breeders (like tits, Paridae) and of larger size (like thrushes, Turdidae), which may explain the relatively less use of forest breeding species than those breeding in other habitats. Wetland breeding species are apparently more used in Germany than in UK, which seems to be due to utilization of great reed warblers Acrocephalus arundinaceus in Germany, a species that is absent from UK.
Cuckoos are dependent on the ability to synchronise the timing of their breeding with that of their hosts. However, our analyses did not provide a significant effect of overlap in breeding season between the potential host species and the cuckoo. The reason for this could simply be that most passerine species overlap in duration of breeding season, and those with a small overlap in breeding season are generally large species or cavity breeders and therefore not suitable anyway.
Nest height above ground was also not a significant predictor of cuckoo parasitism in our analyses. This is contrary to the findings of Martín-Vivaldi et al.76, who suggested that cuckoos have difficulties finding host nests on the ground. They found lower egg rejection in ground-nesting passerines and hence concluded that ground-nesters are rarely used by cuckoos. Several cuckoo gentes, however, utilize hosts breeding on the ground. In the UK for instance, ground-nesting meadow pipits Anthus pratensis are among the most common hosts41. Moksnes and Røskaft9 mention Anthus, yellow wagtail, white wagtail, blue and Emberiza cuckoo egg morphs being found in ground-nesters. Previous within-species analyses are also in line with our findings; nest height was not a predictor of parasitism in marsh Acrocephalus palustris and reed warblers A. scirpaceus24,77.
There is considerable interspecific variation in the ability of hosts to recognize and reject cuckoo eggs e.g.35,37, which may influence our estimation of parasitism rates. In host species with well-developed egg rejection abilities, a poor mimic may be removed before its presence could be detected in the nest. This plausible scenario may lead to an underestimation of relative parasitism in such species compared to hosts that are poor rejecters. The data available in the present study, except those eggs stored at museums, do not allow us to assess egg mimicry since there is no description of egg appearance of either host or parasite in most sources. Variation in egg rejection among species may therefore blur the apparent suitability of different species over time. This could potentially make it harder for us to detect the factors important for host suitability, but is unlikely to contribute to false positive effects in our analyses. While some suitable hosts may not have been identified and hence misclassified in our suitability index, there is no reason to suspect an equivalent bias towards detection of parasitism in unsuitable hosts, because rejection behaviour is likely to have been selected for due to historic parasitism.
Our host suitability index based on population-specific parasitism rates correlated well with the number of parasitism events both in Germany and UK. In both countries, we also observed that few species were used more than would be expected by their suitability. On the other hand, quite a few species are being used less than predicted purely by the host suitability index. Although one should always be careful in the interpretation of variables based on estimates from statistical analyses, this bias suggests that the factors we have investigated may together act to modulate the suitability of species as cuckoo hosts. There may also be other limitations that we have not been able to detect with the current dataset, however, such as local variation in population sizes and rejection ability. Despite these possible caveats, the strong correlation between the host suitability index and the number of parasitism events suggest that it is useful as a species level index of host suitability among European passerines. When we then look at the distribution of this index among species (Fig. 2), it becomes clear that it would be too simplistic to regard species being either suitable or not as hosts for the cuckoo, but rather that the various species show various degrees of suitability. The host species with a corresponding cuckoo gens (classified based on egg mimicry) are all, as expected, placed among the most suitable hosts. According to the index there are a fair number of additional species that appear to be suitable for parasitism, but apparently without any gens attached to them. There are several possible explanations for this pattern. Firstly, some of these species only have very small population sizes in Europe, rendering them unsuitable as cuckoo hosts here but not necessarily in areas where they are more abundant. Little buntings Emberiza pusilla and Blyth’s reed warblers Acrocephalus dumetorum, for instance, are regularly parasitized in parts of Russia by cuckoos laying mimetic eggs78,79. Secondly, host use in some areas of Europe is poorly known, especially the southern and eastern parts. Hence, gentes that are still unknown to us may exist, such as cuckoos targeting those Sylvia warblers with a southern distribution (e.g.80,81). Thirdly, some cuckoo gentes, e.g. the dunnock gens, do not mimic the eggs of their hosts10. Hence, a classification based on egg appearance alone would result in missing some of the existing cuckoo gentes (e.g.82). Finally, as stated above, even though we have included many factors of importance for cuckoo host selection in constructing the host suitability index, there may still be others.
In many systems with generalist parasites, like ecto- or endo-parasites, the parasite is limited by dispersal between species. This is clearly not the case for the cuckoo and most other brood parasites. The bitterling Rohdeus sericeus is a parasitic fish that shares many of the same attributes as avian brood parasites and a similar pattern emerges for their host use. Investigations of four of their potential mussel hosts (Anodonta anatine, A. cygnea, Unio pictorum and U. tumidus) reveal differential suitability of the different host species, with the most suitable host offering twice as high survival for embryos as compared to the least suitable species of the four, and the two other species offer intermediate survival probabilities3. Furthermore, the bitterling prefers the four different hosts in the exact same order as their suitability4,83. The brown-headed cowbird Molothrus ater is, like the common cuckoo, a generalist brood parasite. In a study of nests of 34 potential host species, 18 were parasitized by cowbirds and a large range of parasitism frequencies were observed84. This suggests that even though a range of hosts can be used by generalist brood parasites, they are used in different frequencies according to factors that affect their suitability. In general, the nature of suitability indices will depend on parasite requirements. In the present analyses, we have selected host characteristics that have been hypothesized to explain variation in host use by the common cuckoo. For other parasites, there may be additional host traits that could be of importance in this sense (e.g. intraspecific variation in size and morphology and interspecific variation in coloniality85), and obviously the suitability index would also depend on the level of host specialization of the parasite.
In this study, relying on three novel large datasets, we have disclosed characteristics of potential hosts that may be important for cuckoo host selection at the species level. Host body size, nest structure, habitat and food type for the chicks are all important predictors of cuckoo parasitism, either independently or in combination. The same set of predictors explained variation in host use both in Germany and the UK, even though the actual species used varied somewhat between the two countries. Our findings offer a basis for more thorough analyses of temporal and spatial variation in cuckoo host use. We have shown that the relative importance of a suite of host characteristics on parasite utilization can be modelled statistically by using data from a subset of hosts in specific geographical areas. The outputs from such exercises can then be used to construct host suitability indices on a larger geographical scale for a larger set of species with unknown status as hosts, but where data on life history traits can be retrieved. Our results also demonstrate that potential cuckoo hosts should not distinctly be considered suitable or unsuitable, but rather be placed on a suitability continuum, with the majority of species located towards the more suitable end. We may predict similar patterns in other generalist parasites: many host traits going into their suitability index and similar distributions for the suitability of potential host species. More generally, such suitability indices may be valuable for predicting the potential for host use (current and future) in a whole range of host-parasite systems. This may be increasingly important for understanding species interactions in a world where both parasites and their potential hosts may have to shift their ranges due to climate change or human induced alterations of landscapes.
Ethics
The data used in this study were entirely retrieved from the literature, museum collection, databases, etc.
Material and Methods
Population-specific parasitism rates and cases of parasitism (cuckoo egg or chick) were obtained through various literature search, resulting in data ranging from the period 1735–2013 with the majority of cases from 1850 onwards, originating from more than 7,000 publications meticulously browsed by BGS (ISI Web of Science and Biodiversity Heritage Library, Google Scholar and the Natural History Museum library in Tring, UK, communication with British and German ornithologists, ringing and nest record schemes, museum egg collections and unpublished notes or reports stored in libraries and museums).
Firstly, we investigated 1871 population-specific parasitism rates from 139 passerine species, collected across Europe (10.5061/dryad.9r0n681). We only included parasitism rates based on a minimum of five nests (including parasitized and non-parasitized nests). Although we find five nests to be an appropriate cut-off for the number of nests needed to qualify as a population in these analyses, the number is not based on previous knowledge. We have therefore also undertaken the analyses with (1) all data included regardless of sample size, and (2) populations with ten or more nests included.
Secondly, we investigated 16,515 cases of parasitism from 100 passerine species in Germany and 14,507 cases of parasitism from 78 passerine species in UK (10.5061/dryad.9r0n681). One potential bias using these data is that parasitism rates are generally overestimated because populations that are likely to be parasitized are also more likely to be investigated for parasitism. However, our main question does not relate to actual parasitism rates, but rather to how host life-history traits affect relative parasitism rates, and we have no reason to believe that parasitism rates are overestimated relatively more for species with specific ecological characteristics.
The dataset on population-specific parasitism rates contains 2696 cases from UK and 2660 cases from Germany that are also included in the “cases of parasitism” datasets from UK and Germany. On the other hand, the dataset on population-specific parasitism rates includes additional cases from UK and Germany, where parasitism rates were reported to be zero (these are of course not included in the “cases of parasitism” datasets).
We selected the following variables as predictors of variation in parasitism between species:
Nest cup depth: Inner height of nest cup from bottom to rim (cm)86;
Nest structure: Classification of main nest structure. Four categories: (a) open, (b) crevice, (c) dome, or (d) cavity87.
Nest height above ground: Mean height of nest above ground or water (cm)87.
Habitat: Classification of main breeding habitat. Five categories: (a) wetlands, (b) shrubland, (c) forest, (d) grassland, or (e) rocky areas22,87.
Diet: Classification of main food source brought by host parents. Two categories; (a) animal or (b) plant material87.
Body size: Mean female body size (g)87.
Breeding overlap: Number of days that overlap with cuckoo breeding period. Breeding periods were extracted from87.
Population size: Number of breeding pairs in Germany and UK (mean estimate from BirdLife International22).
Using the dataset on population-specific parasitism rates in Europe, we ran a binomial generalised linear mixed effects model with counts of parasitized and unparasitised nests as response variable, using the glmer function in the lme4 package88 in R89. In this mixed model, we included species as a random effect. We have chosen not to include any phylogenetic effects in our analysis, because we assume that cuckoo host preferences in Europe were established after most passerine species evolved, and, therefore, do not expect cuckoo parasitism rates to be affected by the phylogeny of the species, but only by their actual trait values. Closely related species may have similar parasitism rates, but we believe that is due to similarities in their ecology rather in their phylogenetic history. We analysed predictor variables 1–7 listed above and additionally included the square of bird size to allow for a non-linear relationship, as we expected that species could potentially be both too small and too large to be suitable as hosts for the cuckoo. Generally, fixed factors were not markedly correlated, but nest height and habitat type grassland showed a correlation of 0.38, while body size and domed nests showed a correlation of 0.31. All other correlations had absolute values below 0.3.
Data from the UK and Germany were analysed separately. In each country we ran a hurdle regression model using the pscl package90,91 in R. Hurdle models are well suited to handle datasets with excess zeros, such as ours, since we have many records of host species with no parasitism. In Hurdle models, two different components are estimated: (i) a truncated count component and (ii) a hurdle component. The latter component estimates the zero vs. larger counts as a binomial process, while the former component excludes the zeros and models all the positive counts of parasitism. In our case a negative binomial distribution fitted the first component best. All predictor variables, 1–7 listed above, were part of both the binomial and the negative binomial components of the model and the model structure was the same in both cases.
In these hurdle models for UK and Germany, each passerine species breeding in the given country was included as a data point in our model. In each case, our response variable was the number of parasitized nests recorded. In these datasets we do not know the number of non-parasitized nests in the populations where these parasitism events were recorded. Therefore, we also included population size of each species in the given country (variable 8 listed above) to account for the uneven availability of the different species. All continuous predictor variables were log-transformed except overlap in breeding period. To create figures illustrating the significant effects, new models were run without non-significant predictor variables. To determine the model predictions from the hurdle models for UK and Germany, we used the predict function in R. We used the response predictions and kept all other variables constant whilst varying the focus variable within its observed range. For graphical purposes, these estimates were then scaled so that the highest estimate was the same as the highest estimate for predicted parasitism rates to allow comparison of patterns between the otherwise incomparable model estimates (rates vs numbers).
To determine the model predictions for the parasitism rates across Europe, we used the sim function in the arm package92 in R to simulate the posterior distributions of the parameters in our fitted model. From these distributions, average effect sizes and credibility intervals for different parameter combinations were calculated and used to make the figures. We chose the parasitism rates as reference values in our figures because these have an intuitive biological meaning while the predictions from the hurdle models provide a less obvious meaning biologically.
Next, we used the model predictions from the binomial model of parasitism rates as a species-specific index and a measure of suitability of each species as a cuckoo host. This index was scaled so that the most suitable species had an index of 1 and the least suitable species have an index of 0. Furthermore, we report the distribution of this host suitability index calculated for all European passerines (electronic supplementary material, Table S1). By separating between species where a corresponding cuckoo gens has been described based on egg characters8–10,23,24, we disclose how well our index relates to the number of parasitism cases found in the independent datasets for Germany and UK, and hence validates that these numbers do reflect parasitism rates.
Electronic supplementary material
Table S1. Suitability index of all European passerine species
Acknowledgements
We want to express our gratitude for the generosity and service mindedness of staff at various institutions, ornithologists and others (10.5061/dryad.9r0n681). Special thanks to Douglas Russell and Alison Harding at NHM, Tring, UK for providing access to egg collections and library. We are grateful to the thousands of volunteers who provide data for the BTO Nest Record Scheme, which is funded through the BTO/JNCC partnership. We are also grateful to Subhash Lele, Ivar Herfindal, Otso Ovaskainen, and the BEEhive for discussions about statistics and our results. We want to thank three anonymous reviewers for their insightful and constructive comments that significantly improved the quality of the manuscript. The project was developed during a stay at the Centre for Advanced Study at the Norwegian Academy of Sciences and Letters. B.G.S., I.I.R. and F.F. were funded by the Research Council of Norway (218144).
Author Contributions
B.G.S., F.F. and I.I.R. conceived the ideas and designed methodology; B.G.S. collected the data with aid from A.P.M., E.R., A.M., D.I.L. and K.S.H.; I.I.R., F.F. and B.G.S. analysed the data; B.G.S. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-24627-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Combes, C. Parasitism. The ecology and evolution of intimate interactions. (The University of Chicago Press, 2001).
- 2.Giorgi MS, et al. Causal mechanisms underlying host specificity in bat ectoparasites. Oecologia. 2004;138:648–654. doi: 10.1007/s00442-003-1475-1. [DOI] [PubMed] [Google Scholar]
- 3.Smith C, Reynolds JD, Sutherland WJ, Jurajda P. Adaptive host choice and avoidance of superparasitism in the spawning decisions of bitterling (Rhodeus sericeus) Behav. Ecol. Sociobiol. 2000;48:29–35. doi: 10.1007/s002650000212. [DOI] [Google Scholar]
- 4.Mills SC, Reynolds JD. Host species preferences by bitterling, Rhodeus sericeus, spawning in freshwater mussels and consequences for offspring survival. Anim. Behav. 2002;63:1029–1036. doi: 10.1006/anbe.2001.1988. [DOI] [Google Scholar]
- 5.Dallas, T., Huang, S., Nunn, C., Park, A. W. & Drake, J. M. Estimating parasite host range. Proceedings of the Royal Society B: Biological Sciences284, 10.1098/rspb.2017.1250 (2017). [DOI] [PMC free article] [PubMed]
- 6.Grim, T. & Stokke, B. G. In Biological invasions and animal behaviour (eds Daniel Sol & Judith S. Weis) 133–157 (Cambridge University Press, 2016).
- 7.Becker, D. J., Streicker, D. G. & Altizer, S. Using host species traits to understand the consequences of resource provisioning for host-parasite interactions. J Anim Ecol, 1–16, 10.1111/1365-2656.12765 (2017). [DOI] [PMC free article] [PubMed]
- 8.Wyllie, I. The cuckoo. (Universe Books, 1981).
- 9.Moksnes A, Røskaft E. Egg-morphs and host preference in the common cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. J. Zool. 1995;236:625–648. doi: 10.1111/j.1469-7998.1995.tb02736.x. [DOI] [Google Scholar]
- 10.Davies, N. B. Cuckoos, cowbirds and other cheats. (T & AD Poyser Ltd, 2000).
- 11.Marchetti K, Nakamura H, Gibbs HL. Host-race formation in the common cuckoo. Science. 1998;282:471–472. doi: 10.1126/science.282.5388.471. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs HL, et al. Genetic evidence for female host-specific races of the common cuckoo. Nature. 2000;407:183–186. doi: 10.1038/35025058. [DOI] [PubMed] [Google Scholar]
- 13.Avilés JM, Møller AP. How is host egg mimicry maintained in the cuckoo (Cuculus canorus)? Biol. J. Linnean Soc. 2004;82:57–68. doi: 10.1111/j.1095-8312.2004.00311.x. [DOI] [Google Scholar]
- 14.Skjelseth S, et al. Parentage and host preference in the common cuckoo Cuculus canorus. J. Avian Biol. 2004;35:21–24. doi: 10.1111/j.0908-8857.2004.03219.x. [DOI] [Google Scholar]
- 15.Nakamura H, Miyazawa Y, Kashiwagi K. Behavior of radio-tracked common cuckoo females during the breeding season in Japan. Ornithol. Sci. 2005;4:31–41. doi: 10.2326/osj.4.31. [DOI] [Google Scholar]
- 16.Antonov A, et al. Egg phenotype differentiation in sympatric cuckoo Cuculus canorus gentes. Journal of Evolutionary Biology. 2010;23:1170–1182. doi: 10.1111/j.1420-9101.2010.01982.x. [DOI] [PubMed] [Google Scholar]
- 17.Fossøy F, et al. Genetic differentiation among sympatric cuckoo host races: males matter. Proceedings of the Royal Society B-Biological Sciences. 2011;278:1639–1645. doi: 10.1098/rspb.2010.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fossøy F, et al. Ancient origin and maternal inheritance of blue cuckoo eggs. Nature Communications. 2016;7:10272. doi: 10.1038/ncomms10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooke MDL, Davies NB. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature. 1988;335:630–632. doi: 10.1038/335630a0. [DOI] [Google Scholar]
- 20.Kilner RM. The evolution of egg colour and patterning in birds. Biol. Rev. 2006;81:383–406. doi: 10.1017/S1464793106007044. [DOI] [PubMed] [Google Scholar]
- 21.Stoddard MC, Stevens M. Avian vision and the evolution of egg color mimicry in the common cuckoo. Evolution. 2011;65:2004–2013. doi: 10.1111/j.1558-5646.2011.01262.x. [DOI] [PubMed] [Google Scholar]
- 22.BirdLife International. Birds in Europe: population estimates, trends and conservation status. (BirdLife International, 2004).
- 23.Alvarez F. A gens of cuckoo Cuculus canorus parasitizing rufous bush chat Cercotrichas galactotes. J. Avian Biol. 1994;25:239–243. doi: 10.2307/3677081. [DOI] [Google Scholar]
- 24.Antonov A, Stokke BG, Moksnes A, Røskaft E. Factors influencing the risk of common cuckoo Cuculus canorus parasitism on marsh warblers Acrocephalus palustris. J. Avian Biol. 2007;38:390–393. doi: 10.1111/j.2007.0908-8857.03813.x. [DOI] [Google Scholar]
- 25.Brooke MDL, Davies NB. Recent changes in host usage by cuckoos Cuculus canorus in Britain. Journal of Animal Ecology. 1987;56:873–883. doi: 10.2307/4954. [DOI] [Google Scholar]
- 26.Campobello D, Sealy SG. Avian brood parasitism in a Mediterranean region: hosts and habitat preferences of common cuckoos Cuculus canorus. Bird Study. 2009;56:389–400. doi: 10.1080/00063650903013221. [DOI] [Google Scholar]
- 27.Lack D. Cuckoo hosts in England. Bird Study. 1963;10:185–202. doi: 10.1080/00063656309476050. [DOI] [Google Scholar]
- 28.Moksnes A, Røskaft E. Cuckoo host interactions in Norwegian mountain areas. Ornis Scand. 1987;18:168–172. doi: 10.2307/3676762. [DOI] [Google Scholar]
- 29.Honza M, Moksnes A, Røskaft E, Stokke BG. How are different common cuckoo Cuculus canorus egg morphs maintained? An evaluation of different hypotheses. Ardea. 2001;89:341–352. [Google Scholar]
- 30.Vogl W, Taborsky M, Taborsky B, Teuschl Y, Honza M. Cuckoo females preferentially use specific habitats when searching for host nests. Anim. Behav. 2002;64:843–850. doi: 10.1006/anbe.2003.1967. [DOI] [Google Scholar]
- 31.Nakamura H. Brood parasitism by the cuckoo Cuculus canorus in Japan and the start of new parasitism on the azure-winged magpie Cyanopica cyana. Japanese Journal of Ornithology. 1990;39:1–18. doi: 10.3838/jjo.39.1. [DOI] [Google Scholar]
- 32.Adamík P, Hušek J, Cepák J. Rapid decline of common cuckoo Cuculus canorus parasitism in red-backed shrikes Lanius collurio. Ardea. 2009;97:17–22. doi: 10.5253/078.097.0103. [DOI] [Google Scholar]
- 33.Takasu F, Moskát C, Muñoz AR, Imanishi S, Nakamura H. Adaptations in the common cuckoo (Cuculus canorus) to host eggs in a multiple-hosts system of brood parasitism. Biol. J. Linnean Soc. 2009;98:291–300. doi: 10.1111/j.1095-8312.2009.01288.x. [DOI] [Google Scholar]
- 34.Møller AP, et al. Rapid change in host use of the common cuckoo Cuculus canorus linked to climate change. Proceedings of the Royal Society B-Biological Sciences. 2011;278:733–738. doi: 10.1098/rspb.2010.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies NB, Brooke MdL. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J. Anim. Ecol. 1989;58:207–224. doi: 10.2307/4995. [DOI] [Google Scholar]
- 36.Davies NB, Brooke MdL. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J. Anim. Ecol. 1989;58:225–236. doi: 10.2307/4996. [DOI] [Google Scholar]
- 37.Moksnes A, et al. Behavioural responses of potential hosts towards artificial cuckoo eggs and dummies. Behaviour. 1991;116:64–89. doi: 10.1163/156853990X00365. [DOI] [Google Scholar]
- 38.Røskaft E, Moksnes A, Stokke BG, Bicik V, Moskat C. Aggression to dummy cuckoos by potential European cuckoo hosts. Behaviour. 2002;139:613–628. doi: 10.1163/15685390260136735. [DOI] [Google Scholar]
- 39.Lovaszi P, Moskat C. Break-down of arms race between the red-backed shrike (Lanius collurio) and common cuckoo (Cuculus canorus) Behaviour. 2004;141:245–262. doi: 10.1163/156853904322890843. [DOI] [Google Scholar]
- 40.Prochazka P, Honza M. Egg discrimination in the yellowhammer. Condor. 2004;106:405–410. doi: 10.1650/7365. [DOI] [Google Scholar]
- 41.Glue D, Morgan R. Cuckoo hosts in British habitats. Bird Study. 1972;19:187–192. doi: 10.1080/00063657209476342. [DOI] [Google Scholar]
- 42.Møller AP, et al. Isolation by time and habitat and coexistence of distinct host races of the common cuckoo. J. Evol. Biol. 2011;24:676–684. doi: 10.1111/j.1420-9101.2010.02202.x. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez F. Proximity of trees facilitates parasitism by cuckoos Cuculus canorus on rufous warblers Cercotrichas galactotes. Ibis. 1993;135:331. doi: 10.1111/j.1474-919X.1993.tb02853.x. [DOI] [Google Scholar]
- 44.Øien IJ, Honza M, Moksnes A, Røskaft E. The risk of parasitism in relation to the distance from reed warbler nests to cuckoo perches. J. Anim. Ecol. 1996;65:147–153. doi: 10.2307/5717. [DOI] [Google Scholar]
- 45.Moskat C, Honza M. Effect of nest and nest site characteristics on the risk of cuckoo Cuculus canorus parasitism in the great reed warbler Acrocephalus arundinaceus. Ecography. 2000;23:335–341. doi: 10.1111/j.1600-0587.2000.tb00289.x. [DOI] [Google Scholar]
- 46.Clarke AL, Øien IJ, Honza M, Moksnes A, Røskaft E. Factors affecting reed warbler risk of brood parasitism by the common cuckoo. Auk. 2001;118:534–538. doi: 10.1642/0004-8038(2001)118[0534:FARWRO]2.0.CO;2. [DOI] [Google Scholar]
- 47.Antonov A, Stokke BG, Moksnes A, Røskaft E. Coevolutionary interactions between common cuckoos and corn buntings. Condor. 2006;108:414–422. doi: 10.1650/0010-5422(2006)108[414:CIBCCA]2.0.CO;2. [DOI] [Google Scholar]
- 48.Avilés JM, et al. Rapid increase in cuckoo egg matching in a recently parasitized reed warbler population. J. Evol. Biol. 2006;19:1901–1910. doi: 10.1111/j.1420-9101.2006.01166.x. [DOI] [PubMed] [Google Scholar]
- 49.Cherry MI, Bennett ATD, Moskat C. Do cuckoos choose nests of great reed warblers on the basis of host egg appearance? J. Evol. Biol. 2007;20:1218–1222. doi: 10.1111/j.1420-9101.2007.01308.x. [DOI] [PubMed] [Google Scholar]
- 50.Munoz AR, Altamirano M, Takasu F, Nakamura H. Nest light environment and the potential risk of common cuckoo (Cuculus canorus) parasitism. Auk. 2007;124:619–627. doi: 10.1642/0004-8038(2007)124[619:NLEATP]2.0.CO;2. [DOI] [Google Scholar]
- 51.Parejo D, Avilés JM. Do avian brood parasites eavesdrop on heterospecific sexual signals revealing host quality? A review of the evidence. Animal Cognition. 2007;10:81–88. doi: 10.1007/s10071-006-0055-2. [DOI] [PubMed] [Google Scholar]
- 52.Polačiková L, Procházka P, Cherry MI, Honza M. Choosing suitable hosts: common cuckoos Cuculus canorus parasitize great reed warblers Acrocephalus arundinaceus of high quality. Evolutionary Ecology. 2009;23:879–891. doi: 10.1007/s10682-008-9278-9. [DOI] [Google Scholar]
- 53.Welbergen JA, Davies NB. Strategic variation in mobbing as a front line of defense against brood parasitism. Curr. Biol. 2009;19:235–240. doi: 10.1016/j.cub.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 54.Honza M, Šulc M, Jelínek V, Požgayová M, Procházka P. Brood parasites lay eggs matching the appearance of host clutches. Proceedings of the Royal Society B-Biological Sciences. 2014;281:20132665. doi: 10.1098/rspb.2013.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jelínek V, Procházka P, Požgayová M, Honza M. Common cuckoos Cuculus canorus change their nest-searching strategy according to the number of available host nests. Ibis. 2014;156:189–197. doi: 10.1111/ibi.12093. [DOI] [Google Scholar]
- 56.Southern, H. N. In Evolution as a process (eds J. Huxley, A. C. Hardy, & E. B. Ford) 219–232 (George Allen & Unwin Ltd, 1958).
- 57.Payne RB. The ecology of brood parasitism in birds. Annual Review of Ecology and Systematics. 1977;8:1–28. doi: 10.1146/annurev.es.08.110177.000245. [DOI] [Google Scholar]
- 58.Røskaft E, Moksnes A, Stokke BG, Moskat C, Honza M. The spatial habitat structure of host populations explains the pattern of rejection behavior in hosts and parasitic adaptations in cuckoos. Behav. Ecol. 2002;13:163–168. doi: 10.1093/beheco/13.2.163. [DOI] [Google Scholar]
- 59.Grim T. Cuckoo growth performance in parasitized and unused hosts: not only host size matters. Behav. Ecol. Sociobiol. 2006;60:716–723. doi: 10.1007/s00265-006-0215-z. [DOI] [Google Scholar]
- 60.Saino N, et al. Climate change effects on migration phenology may mismatch brood parasitic cuckoos and their hosts. Biology Letters. 2009;5:539–541. doi: 10.1098/rsbl.2009.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulze-Hagen K, Stokke BG, Birkhead TR. Reproductive biology of the European cuckoo Cuculus canorus: early insights, persistent errors and the acquisition of knowledge. Journal of Ornithology. 2009;150:1–16. doi: 10.1007/s10336-008-0340-8. [DOI] [Google Scholar]
- 62.Grim T, et al. Constraints on host choice: why do parasitic birds rarely exploit some common potential hosts? Journal of Animal Ecology. 2011;80:508–518. doi: 10.1111/j.1365-2656.2010.01798.x. [DOI] [PubMed] [Google Scholar]
- 63.Yang C, et al. Host selection in parasitic birds: are open-cup nesting insectivorous passerines always suitable cuckoo hosts? J. Avian Biol. 2013;44:216–220. doi: 10.1111/j.1600-048X.2013.00123.x. [DOI] [Google Scholar]
- 64.Glue D, Murray E. Cuckoo hosts in Britain. British Trust for Ornithology News. 1984;134:5. [Google Scholar]
- 65.Soler JJ, Møller AP, Soler M. A comparative study of host selection in the European cuckoo Cuculus canorus. Oecologia. 1999;118:265–276. doi: 10.1007/s004420050727. [DOI] [PubMed] [Google Scholar]
- 66.Soler J, Vivaldi M, Møller A. Geographic distribution of suitable hosts explains the evolution of specialized gentes in the European cuckoo Cuculus canorus. BMC Evol. Biol. 2009;9:88. doi: 10.1186/1471-2148-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lootvoet A, et al. Patterns and processes of alternative host use in a generalist parasite: insights from a natural host-parasite interaction. Functional Ecology. 2013;27:1403–1414. doi: 10.1111/1365-2435.12140. [DOI] [Google Scholar]
- 68.Kleven O, et al. Breeding success of common cuckoos Cuculus canorus parasitising four sympatric species of Acrocephalus warblers. J. Avian Biol. 2004;35:394–398. doi: 10.1111/j.0908-8857.2004.03359.x. [DOI] [Google Scholar]
- 69.Grim T, Rutila J, Cassey P, Hauber ME. Experimentally constrained virulence is costly for common cuckoo chicks. Ethology. 2009;115:14–22. doi: 10.1111/j.1439-0310.2008.01574.x. [DOI] [Google Scholar]
- 70.Martín-Gálvez D, Soler M, Soler JJ, Martín-Vivaldi M, Palomino JJ. Food acquisition by common cuckoo chicks in rufous bush robin nests and the advantage of eviction behaviour. Anim. Behav. 2005;70:1313–1321. doi: 10.1016/j.anbehav.2005.03.031. [DOI] [Google Scholar]
- 71.Grim T, Rutila J, Cassey P, Hauber ME. The cost of virulence: an experimental study of egg eviction by brood parasitic chicks. Behav. Ecol. 2009;20:1138–1146. doi: 10.1093/beheco/arp108. [DOI] [Google Scholar]
- 72.Geltsch N, Hauber ME, Anderson MG, Bán M, Moskát C. Competition with a host nestling for parental provisioning imposes recoverable costs on parasitic cuckoo chick’s growth. Behavioural Processes. 2012;90:378–383. doi: 10.1016/j.beproc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 73.Seel DC, Davis PRK. Cuckoos reared by unusual hosts in Britain. Bird Study. 1981;28:242–243. doi: 10.1080/00063658109476729. [DOI] [Google Scholar]
- 74.Rutila J, Latja R, Koskela K. The common cuckoo Cuculus canorus and its cavity nesting host, the redstart Phoenicurus phoenicurus: a peculiar cuckoo-host system? J. Avian Biol. 2002;33:414–419. doi: 10.1034/j.1600-048X.2002.02937.x. [DOI] [Google Scholar]
- 75.Grim T, Samaš P, Procházka P, Rutila J. Are tits really unsuitable hosts for the common cuckoo? Ornis Fennica. 2014;91:166–177. [Google Scholar]
- 76.Martín-Vivaldi M, Soler JJ, Møller AP, Pérez-Contreras T, Soler M. The importance of nest-site and habitat in egg recognition ability of potential hosts of the common cuckoo Cuculus canorus. Ibis. 2013;155:140–155. doi: 10.1111/ibi.12000. [DOI] [Google Scholar]
- 77.Stokke BG, et al. Predictors of resistance to brood parasitism within and among reed warbler populations. Behav. Ecol. 2008;19:612–620. doi: 10.1093/beheco/arn007. [DOI] [Google Scholar]
- 78.Balatsky NN. The common cuckoo (Cuculus canorus L.) – Ecological races of the Blyth’s reed warbler (Acrocephalus dumetorum Blyth.) and the European stonechat (Saxicola torquata L.) near Novosibirsk. Biologicheskie Nauki. 1988;4:57–61. [Google Scholar]
- 79.Balatsky, N. N. & Bachurin, G. N. The cuckoos of the West-Siberian plain. Proceedings of the II Conference of Young Ornithologists of UkraineC, 6–9 (1998).
- 80.Whitehead J. Ornithological notes from Corsica. Ibis. 1885;27:24–48. doi: 10.1111/j.1474-919X.1885.tb06232.x. [DOI] [Google Scholar]
- 81.Chavigny JD, Le Dû R. Note sur l’adaptation des oeufs du coucou de l’Afrique du Nord Cuculus canorus bangsi Oberholser, suivie de quelques observations biologiques. Alauda. 1938;10:91–115. [Google Scholar]
- 82.Edvardsen E, Moksnes A, Røskaft E, Øien IJ, Honza M. Egg mimicry in cuckoos parasitizing four sympatric species of Acrocephalus warblers. Condor. 2001;103:829–837. doi: 10.1650/0010-5422(2001)103[0829:EMICPF]2.0.CO;2. [DOI] [Google Scholar]
- 83.Smith C. Bayesian inference supports the host selection hypothesis in explaining adaptive host specificity by European bitterling. Oecologia. 2017;183:379–389. doi: 10.1007/s00442-016-3780-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strausberger BM, Ashley MV. Community-wide patterns of parasitism of a host “generalist” brood-parasitic cowbird. Oecologia. 1997;112:254–262. doi: 10.1007/s004420050308. [DOI] [PubMed] [Google Scholar]
- 85.Stireman JO, Singer MS. What determines host range in parasitoids? An analysis of a tachinid parasitoid community. Oecologia. 2003;135:629–638. doi: 10.1007/s00442-003-1235-2. [DOI] [PubMed] [Google Scholar]
- 86.Glutz von Blotzheim, U. N. Handbuch der Vögel Mitteleuropas, Band 10–14. (Aula-Verlag, 1985–1997).
- 87.Birdguides L. Birds of the Western Palearctic interactive. 2 edn, (Oxford university press, 2006).
- 88.Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 89.R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/ (2017).
- 90.Zeileis A, Kleiber C, Jackman S. Regression models for count data in R. Journal of Statistical Software. 2008;27:1–25. [Google Scholar]
- 91.pscl: Classes and Methods for R Developed in the Political Science Computational Laboratory, Stanford University. Department of Political Science, Stanford University. Stanford, California. R package version 1.4.9. http://pscl.stanford.edu/ (2017).
- 92.arm: Data analysis using regression and multilevel/hierarchical models. R package version 1.8–4. http://CRAN.R-project.org/package=arm (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Suitability index of all European passerine species


