Abstract
[Purpose] Few studies on the transverse arch (TA) in the forefoot have been conducted. The forefoot is where pains occur most frequently and is related to walking and balance; hence, paying attention to TA is vital. However, the relationship between TA and foot muscles has not been investigated. Therefore, this study aims to investigate muscles related to TA. [Subjects and Methods] Nineteen healthy young males were included. Measurements of their feet, excluding one foot with recent foot pain (n=37), were obtained. The height of TA (TAH) was measured in two ways: during 10% and 90% loading of body weight. The cross-sectional area and thickness of five muscles were measured: flexor digitorum longus, peroneus longus and brevis, flexor hallucis brevis, flexor digitorum brevis (FDB) and abductor hallucis (ABH). All measurements were performed with an ultrasound device. [Results] FDB and ABH were correlated with TAH during 10% and 90% loading after removing the effect of body mass index and age. The greater FDB and ABH, the higher TAH. [Conclusion] As FDB becomes larger, the second, third and fourth metatarsal heads are raised more. Furthermore, the height of the first metatarsal head is lowered by a larger ABH. These mechanisms may increase TAH.
Key words: Transverse arch, Alignment, Muscle
INTRODUCTION
The foot is the only part of the human body that is in contact with the ground during typical activities of daily living and is thus considered one of the most important parts. The function of the foot depends on its morphological structure and the shape of the longitudinal and transverse arches1). These arches in the foot have a characteristic structure. The arch structure enhances energy sufficiency by enabling the accumulation and release of mechanical energy generated during a loading action, such as walking and running1, 2). There are three arches in the foot: medial longitudinal arch (MLA), lateral longitudinal arch (LLA), and transverse arch (TA). MLA and LLA are constructed on the sagittal plane from the rearfoot to the forefoot, and TA is formed on the coronal plane in the forefoot. Although few studies on TA have conducted, some studies reported the importance of the forefoot. That foot pain occurs most frequently in the forefoot3, 4), and that forefoot bones may be related to superior sprint performance5). Therefore, the forefoot, particularly the TA, is the part to be paid attention.
The TA consists of five metatarsal heads. Several previous studies measured the TA on the surface, such as foot width measurement, or using 3-dimensional foot scanner, and few studies measured the TA with an ultrasound (US) device, which could confirm the position of the bone6, 7). US imaging could be highly usable in various situations and has the advantages of non invasiveness, low cost, and high portability compared with other imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI). According to Kondo et al., static measurement of the TA during loading is related to a change in the ankle plantar moment and hip joint extension angle during gait6). Weijers et al. reported that the soft tissue of the forefoot changes during loading, which in turn has an important function in shock absorption8). These studies showed the importance of foot structure and function during loading. Since it is difficult to evaluate forefoot structure in the weightbearing position with other imaging techniques, such as CT and MRI, US imaging was used in this study.
Numerous studies on the relationship between MLA and muscles have been conducted. Angin et al. showed that people who have high MLA have larger flexor digitorum longus (FDL) and flexor hallucis longus, and have smaller peroneus longus and brevis (PER), flexor hallucis brevis (FHB) and flexor digitorum brevis (FDB)9). Gray et al. and Mann et al. reported that various intrinsic foot muscles, such as the abductor hallucis (ABH), FDB, and interosseous muscle, contribute to MLA stabilization during propulsion10, 11) and presented different views on the relationship between muscles and MLA, and their studies showed that both intrinsic and extrinsic muscles may be related to the foot arch. For the TA, the previous studies reported that the TA is related to walking and balance6, 12) and to medial tibial stress syndrome13). However, no investigations about the muscles constituting the TA have been conducted. Similar to MLA, the TA is presumed to be related to muscle structure. Therefore, in this study, both the intrinsic and extrinsic muscles were focused and evaluated with their correlation with the TA.
Morphological evaluation of muscles often includes the determination of muscle cross-sectional area (CSA) and thickness. Muscle morphology measurement (CSA, muscle thickness) has been shown to be indicative of muscle performance, including strength, thereby providing a surrogate measure of mechanical function14). In this study, FDL, PER, FHB, FDB and ABH were selected as representative of the intrinsic and extrinsic muscles because a more accurate estimation of muscles by US and calculation of muscle CSA and thickness can be achieved.
This study aims to determine the muscle that is related to the TA using an US device.
SUBJECTS AND METHODS
Nineteen males (age, 23.9 ± 1.6 years; body mass index (BMI), 21.9 ± 1.3) participated in this study, and measurements of their feet, excluding one foot with recent foot pain, were obtained (n=37). All participants were university students or graduate students and provided written informed consent. This study was in accordance with the current local guideline and the Declaration of Helsinki, and was approved by the Ethical Committee for Human Experiments of Kyoto University (R0645-2).
In this study, TAH of the foot region, the CSA and thickness of the five muscles that may be related to TAH were measured. TAH was evaluated using a weightbearing plantar ultrasound imaging device (WPUID) (Fig. 1), as previously described15). An US diagnostic device (Noblus, Hitachi Aloka Medical, Tokyo, Japan) was placed in WPUID. Briefly, participants placed one foot on a solid gel block for US evaluation. The other foot was placed on a digital weighting scale to adjust the weightbearing rate of each foot. In this study, US images were obtained when subjects bore 10% and 90% of their weight. At the time of measuring the 10% loading position, the subject was instructed that 90% of the body weight was applied to the weight scale. In the measurement at 90% load, conversely, the subject was instructed that the 10% load would be applied to the weight scale. The device acquires images using B-mode US with a frequency of 9.0 MHz during weightbearing. US evaluation of forefoot structure in the coronal plane showed good agreement with CT and repeatability based on two ultrasonograms15). Moreover, US images were obtained at positions where the following four points were confirmed: each of the lowest points of the epiphysis of the medial sesamoid bone (MS), the lateral sesamoid bone (LS), the second metatarsal bone (2MT) and the fifth metatarsal head (5MTH). The US images were transferred to a computer and analyzed using ImageJ software (National Institutes of Health). The length of the line perpendicular (LP) to the line passing through both the MS and 5MTH as well as the length between MS and 5MTH (LM5) were identified. TAH was calculated as follows: LP/LM5 × 100.
Fig. 1.

Weight-bearing plantar ultrasound-imaging device.
One forefoot (right foot in this case) was placed on a solid gel block for ultrasound evaluation and the other foot was placed on a digital weighing scale. The scale is used to measure the body weight on the foot to measure on the opposite side.
The CSA and thickness of the foot muscles were measured with an US diagnostic device that is similar to that used in TAH measurement. The US images of PER, FDL, FHB, FDB, and ABH were obtained using the same device. CSA was defined as the area of the cross-section of a structure perpendicular to its longitudinal dimension. Thickness of the structure was defined as the distance between its aponeuroses16). US images of the foot muscles on both feet of each subjects were obtained.
Moreover, the details of probe position for measuring each muscle (FDL, PER, FHB, FDB and ABH) followed the method described by Crofts et al17). The CSA of FDL was determined on a transverse line drawn at the middle between the medial tibial plateau and the inferior border of the medial malleolus on the medio-posterior aspect of the tibia. The CSA of PER was obtained at a line located the center between the fibular head and the inferior border of the lateral malleolus. The CSA of FHB was measured in a perpendicular direction along the shaft of the first metatarsal at the thickest portion of the muscle. The CSA of FDB was scanned in a perpendicular direction along a line from the medial tubercle of the calcaneus to the third toe at the thickest portion of the muscle. The CSA of ABH was obtained on a scanning line perpendicular to the long axis of the foot at the anterior aspect of the medial malleolus. The muscle thickness of all muscles was scanned rotated by 90 degrees at the position of each CSA. Each subject was in the prone position for FHB and FDB scanning and in the supine position for ABH, FDL, and PER scanning. All muscles were scanned with the ankle joint in the neutral position. All measurement images were transferred to a computer and analyzed using ImageJ software (National Institutes of Health).
All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). To identify normality, the Shapiro-Wilk test was employed. The correlations between TAH with 10% or 90% loading and the CSA and thickness of each of the five muscles were determined using the Pearson correlation analysis. Furthermore, partial correlation analysis was performed to remove the effect of BMI and age from the correlation estimates. A p value less than 0.05 was considered statistically significant.
RESULTS
The average CSA and thickness of each muscle, LP, LM5, and TAH are shown in Table 1. The Pearson correlation and partial correlation coefficients between TAH during 10% or 90% loading and the CSA and thickness of each of the five muscles are shown in Tables 2 and 3. The CSA of FDB (10%: r=0.56, 90%: r=0.49) and thickness of FDB (10%: r=0.42, 90%: r=0.33) and ABH (10%: r=0.42, 90%: r=0.42) were significantly correlated with TAH at both 10% and 90% loading. Furthermore, after removing the effect of BMI and age, the associations between TAH and the CSA of FDB (10%: r=0.58, 90%: r=0.51) and between TAH and the thickness of FDB (10%: r=0.45, 90%: r=0.37) and ABH (10%: r=0.42, 90%: r=0.43) remained highly significant.
Table 1. The average of CSA, thickness, LP, LM5 and TAH.
| FDL | CSA (mm2) | 162.9 ± 33.2 |
| Thickness (mm) | 15.9 ± 2.9 | |
| PER | CSA | 496.6 ± 78.7 |
| Thickness | 17.3 ± 2.8 | |
| FHB | CSA | 184.5 ± 46.2 |
| Thickness | 16.1 ± 2.0 | |
| FDB | CSA | 226.6 ± 48.8 |
| Thicknesss | 10.0 ± 1.9 | |
| ABH | CSA | 248.8 ± 46.9 |
| Thickness | 12.7 ± 1.6 | |
| During 10% loading | LM5 (mm) | 72.7 ± 2.4 |
| LP (mm) | 9.0 ± 3.4 | |
| TAH | 12.4 ± 4.7 | |
| During 90% loading | LM5 | 74.9 ± 2.3 |
| LP | 9.2 ± 3.2 | |
| TAH | 12.4 ± 4.5 | |
Data presented as mean ± standard deviation.
FDL: flexor digitorum longus; PER: peroneus longus and brevis; FHB: flexor hallucis brevis; FDB: flexor digitorum brevis; ABH: abductor halluces; LM5: length between medial sesamoid bone and 5th metatarsal heads; LP: length of the line perpendicular to LM5; TAH: transverse arch height.
Table 2. The correlations coefficients between TAH and muscles.
| Pearson correlation coefficients (r) | ||
|---|---|---|
| TAH during 10% loading | ||
| Thickness | CSA | |
| FDL | −0.14 | 0.227 |
| PER | 0.097 | 0.162 |
| FHB | 0.289 | 0.202 |
| FDB | 0.424** | 0.564** |
| ABH | 0.415* | 0.147 |
| TAH during 90% loading | ||
| Thickness | CSA | |
| FDL | 0.08 | 0.095 |
| PER | −0.054 | 0.06 |
| FHB | 0.257 | 0.143 |
| FDB | 0.326* | 0.485** |
| ABH | 0.423** | 0.241 |
Data presented as mean ± standard deviation.
*p<0.05, **p<0.01.
TAH: transverse arch height; FDL: flexor digitorum longus; PER: peroneus longus and brevis; FHB: flexor hallucis brevis; FDB: flexor digitorum brevis; ABH: abductor halluces; CSA: cross-sectional area.
Table 3. Partial correlation coefficients between TAH and muscles.
| Partial correlation coefficients with the effect of BMI and age removed (r) | ||
|---|---|---|
| TAH during 10% loading | ||
| Thickness | CSA | |
| FDL | 0.012 | 0.232 |
| PER | 0.159 | 0.217 |
| FHB | 0.298 | 0.217 |
| FDB | 0.448** | 0.577** |
| ABH | 0.42* | 0.154 |
| TAH during 90% loading | ||
| Thickness | CSA | |
| FDL | 0.138 | 0.107 |
| PER | 0.04 | 0.148 |
| FHB | 0.268 | 0.161 |
| FDB | 0.368* | 0.514** |
| ABH | 0.434** | 0.264 |
Data presented as mean ± standard deviation.
*p<0.05, **p<0.01.
TAH: transverse arch height; FDL: flexor digitorum longus; PER: peroneus longus and brevi; FHB: flexor hallucis brevis; FDB: flexor digitorum brevis; ABH: abductor halluces; CSA: cross-sectional area.
DISCUSSION
This study was the first study to investigate the association between TAH and the muscles around the ankle joint. The most important finding in our study was that subjects who had a high TAH during both 10% and 90% loading had larger FDB and ABH. For MLA, a previous study reported that larger extrinsic muscles may reflect compensatory activities to maintain the shape of the MLA9). However, for the TA, the result of our study suggested that larger intrinsic muscles keep TAH than extrinsic muscles.
FDB is a fusiform muscle originating from the medial calcaneal process and the plantar aponeurosis. At its distal aspect, it is divided into four parts, which gives rise to four tendons that insert into the lateral four toes on the plantar surface of the intermediate phalanx18). Anatomical muscle CSA and muscle volume are valuable predictors of muscular strength and power output19,20,21). Considering the origin and insertion of FDB, the power to flex the proximal interphalangeal (PIP) joint of the lateral four toes should increase as the FDB grows larger. In this study, TAH was measured in the standing position. Thus, the action of the measured muscle was similar to a closed-chain action, and it is expected that the height of the metatarsal head increases as the flexor angle of the PIP joint increases (Fig. 2). Further, some studies on cadaver feet showed that the muscular slip for the fifth toe, or for the fifth and fourth toes is much smaller and the tendons are thinner than that for the other toes18, 22, 23). Therefore, the flexor force applied to the PIP joint of the second, third, and fourth toes could be greater, thereby increasing the height of the second, third, and fourth metatarsal heads, respectively; the height of the first and fifth metatarsal heads did not increase. This finding could explain the higher TA in individuals with large FDB.
Fig. 2.
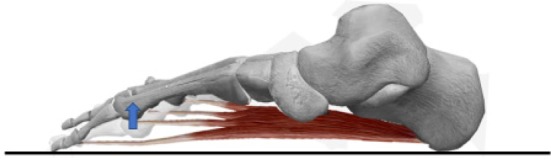
The skeleton of the foot, excluding the hallux and medial cuneiform, and FDB from the inside on the sagittal plane.
Deformation of lateral four metatarsal bones may occur as the muscular strength of FDB increases. FDB may make lateral four metatarsal heads height high.
FDB: flexor digitorum brevis.
Additionally, FDB has two functions: the first is to assist FDL in maintaining stability of the lesser digits against the ground during propulsion and the second is to stabilize the intermediate phalanx of each toe posteriorly against the proximal phalanx, and the proximal phalanx against its respective metatarsal head18). These functions are also related to balance ability and walking. Okai et al. reported that FDB has a vital contribution to postural control24). Moreover, Green and Brekke, and Hughes et al. described FDB as a stance phase muscle of walking25, 26). All previous studies agreed that FDB is active from heel lift through toe off, to maintain the digits in the rectus position, thereby obtaining the necessary stability during the propulsive phase of gait. Moreover, these studies and this study may support the finding that TAH is related to balance ability and walking6, 12).
ABH, which is located medial to the first metatarsal, originates from the medial process of the calcaneal tuberosity and inserts into the medial aspect of the proximal phalanx and sesamoid bone27, 28). ABH is described not only as abductor but also as flexor of the metatarsophalangeal (MTP) joint of the hallux. Further, ABH prevents abnormal transverse plane motion29). ABH has the same insertion, which is proximal to proximal phalanx, and the same action to flex the MTP joint as those of FHB. Thus, ABH and FHB work to lower the height of the proximal phalanx and first metatarsal head during the closed-chain action (Fig. 3). However, differences between the two muscles were noted. Kura et al. showed that ABH has the largest CSA among the intrinsic muscles30). ABH, FDB, and abductor digiti minimi muscles are described in layer one and FHB is described in layer three31, 32). Kelly et al. revealed that individual activation of ABH and FDB is sufficient to produce forces large enough to induce angular metatarsals displacements (flexion and adduction)33). Apparently, these previous studies showed that the flexion muscle force applied to the MTP joint by ABH is greater than that by FHB. In this study, TAH was defined by the medial sesamoid bone attached to first metatarsal head, the fifth metatarsal head and the second metatarsal head. Therefore, it is speculated that ABH lowers the height of the first metatarsal head, thereby increasing TAH. In addition, previous studies showed that ABH also relates to hallux valgus and flat foot9, 34), and it is observed that ABH is related to several kinds of foot deformations.
Fig. 3.
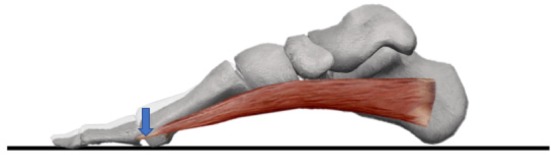
The skeleton of the foot and ABH from the inside on the sagittal plane.
Deformation of first metatarsal bone may occur as the muscular strength of ABH increases. ABH may make the first metatarsal head height low.
ABH: abductor halluces.
No change in TAH during 10% loading and 90% loading was noted; consequently, TAH during 10% and 90% loading did not change the correlation coefficient with the muscles. Although some studies showed that the height of the medial longitudinal arch decreases to absorb shock from the ground when load is applied33), little difference in the TAH between 10% and 90% loading was observed in our study. However, both LP and LM5 were slightly greater in the 90% loading than in the 10% loading. LM5 possibly increased because the TA collapsed because of the load similar to that of the medial longitudinal arch. For LP, muscle action during loading may be related to the change. TAH during 10% loading and 90% loading was measured in the standing position. During 10% loading, the TA was higher because of larger FDB and ABH. Various studies have shown muscles working during loading. Intrinsic foot muscles in human locomotion and postural control, especially ABH, FDB, and quadratus plantae, has increased in size2, 33, 35, 36). Further, Basmajian and Stecko involved incrementally adding weights to the legs of seated subjects and reported that activation of intrinsic muscles increased with increased loading of the foot37). These previous reports showed that FDB and ABH work more effectively and increase LP during 90% loading. Therefore, in the present study, LP and LM 5 during 90% load increased, thereby our study showed no difference in TAH during 10% loading and 90% loading.
This study has some limitations. Firstly, the subjects were only healthy young men from the same university. Some studies demonstrated that men and women have different flexibility of muscles and ligaments38). Hence, these results would not be necessarily the same as those in women; thus, future studies are needed. Furthermore, whether the same results can be obtained in elderly people or children remains unknown, and it is possible to solve problems of the deformity of the feet in future investigation. Secondly, in addition to the muscles investigated, other factors may be related to TAH. Some muscles were not included in our investigation, and bone shape and ligaments may be related to TAH. Previous studies showed that the medial longitudinal arch is affected by plantar aponeurosis and other ligaments9, 39). Therefore, an investigation of the relationship between TAH and ligaments is warranted. Particularly, deep transverse metatarsal ligament, which is a series of four short ligamentous bands that span between the distal ends of adjacent metatarsal bones and intersect with the plantar ligaments of the MTP joint40), runs across and connects all metatarsal heads that construct the TA. Therefore, the relationship between the ligament and TAH should also be investigated.
In conclusion, this study is the first study to investigate the relationship between the TA and muscles, and showed that TAH is related to FDB and ABH. As the TA is related to injuries and performance, the results of this study have an important relevance in the prevention of foot injuries and improvement of performance.
Funding
This research received no specific grant from any funding agency.
Conflict of interest
Authors declare no conflicts of interest associated with this manuscript.
REFERENCES
- 1.Ker RF, Bennett MB, Bibby SR, et al. : The spring in the arch of the human foot. Nature, 1987, 325: 147–149. [DOI] [PubMed] [Google Scholar]
- 2.Kelly LA, Lichtwark G, Cresswell AG: Active regulation of longitudinal arch compression and recoil during walking and running. J R Soc Interface, 2015, 12: 20141076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorter KJ, Kuyvenhoven MM, de Melker RA: Nontraumatic foot complaints in older people. A population-based survey of risk factors, mobility, and well-being. J Am Podiatr Med Assoc, 2000, 90: 397–402. [DOI] [PubMed] [Google Scholar]
- 4.Menz HB, Tiedemann A, Kwan MM, et al. : Foot pain in community-dwelling older people: an evaluation of the Manchester Foot Pain and Disability Index. Rheumatology (Oxford), 2006, 45: 863–867. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T, Suga T, Otsuka M, et al. : Relationship between the length of the forefoot bones and performance in male sprinters. Scand J Med Sci Sports, 2017, 27: 1673–1680. [DOI] [PubMed] [Google Scholar]
- 6.Kondo T, Muneta T, Fukui T: Evaluation of the relationship between the static measurement of transverse arch flexibility of the forefoot and gait parameters in healthy subjects. J Phys Ther Sci, 2017, 29: 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo S, Hatanaka Y, Naka K, et al. : Flexibility of the transverse arch of the forefoot. J Orthop Surg (Hong Kong), 2014, 22: 46–51. [DOI] [PubMed] [Google Scholar]
- 8.Weijers RE, Walenkamp GH, van Mameren H, et al. : Changes of the soft tissue of the forefoot during loading: a volumetric study. Foot, 2003, 13: 70–75. [Google Scholar]
- 9.Angin S, Crofts G, Mickle KJ, et al. : Ultrasound evaluation of foot muscles and plantar fascia in pes planus. Gait Posture, 2014, 40: 48–52. [DOI] [PubMed] [Google Scholar]
- 10.Gray EG, Basmajian JV: Electromyography and cinematography of leg and foot (“normal” and flat) during walking. Anat Rec, 1968, 161: 1–15. [DOI] [PubMed] [Google Scholar]
- 11.Mann R, Inman VT: Phasic activity of intrinsic muscles of the foot. J Bone Joint Surg Am, 1964, 46: 469–481. [PubMed] [Google Scholar]
- 12.Drzal-Grabiec J, Rachwał M, Trzaskoma Z, et al. : The foot deformity versus postural control in females aged over 65 years. Acta Bioeng Biomech, 2014, 16: 75–82. [PubMed] [Google Scholar]
- 13.Kudo S, Hatanaka Y: Forefoot flexibility and medial tibial stress syndrome. J Orthop Surg (Hong Kong), 2015, 23: 357–360. [DOI] [PubMed] [Google Scholar]
- 14.Kent-Braun JA, Ng AV: Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol 1985, 1999, 87: 22–29. [DOI] [PubMed] [Google Scholar]
- 15.Matsubara K, Matsushita T, Tashiro Y, et al. : Repeatability and agreement of ultrasonography with computed tomography for evaluating forefoot structure in the coronal plane. J Foot Ankle Res, 2017, 10: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narici M: Human skeletal muscle architecture studied in vivo by non-invasive imaging techniques: functional significance and applications. J Electromyogr Kinesiol, 1999, 9: 97–103. [DOI] [PubMed] [Google Scholar]
- 17.Crofts G, Angin S, Mickle KJ, et al. : Reliability of ultrasound for measurement of selected foot structures. Gait Posture, 2014, 39: 35–39. [DOI] [PubMed] [Google Scholar]
- 18.Locke J, Baird SA, Frankis J: Preliminary observations of muscle fibre cross sectional area of flexor digitorum brevis in cadaver feet with and without claw toes. J Foot Ankle Res, 2010, 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikai M, Fukunaga T: Calculation of muscle strength per unit cross-sectional area of human muscle by means of ultrasonic measurement. Int Z Angew Physiol, 1968, 26: 26–32. [DOI] [PubMed] [Google Scholar]
- 20.Maughan RJ, Watson JS, Weir J: Strength and cross-sectional area of human skeletal muscle. J Physiol, 1983, 338: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shephard RJ, Bouhlel E, Vandewalle H, et al. : Muscle mass as a factor limiting physical work. J Appl Physiol 1985, 1988, 64: 1472–1479. [DOI] [PubMed] [Google Scholar]
- 22.Nathan H, Gloobe H: Flexor digitorum brevis—anatomical variations. Anat Anz, 1974, 135: 295–301. [PubMed] [Google Scholar]
- 23.Yalçin B, Ozan H: Some variations of the musculus flexor digitorum brevis. Anat Sci Int, 2005, 80: 189–192. [DOI] [PubMed] [Google Scholar]
- 24.Okai LA, Kohn AF: Quantifying the contributions of a flexor digitorum brevis muscle on postural stability. Mot Contr, 2015, 19: 161–172. [DOI] [PubMed] [Google Scholar]
- 25.Green DR, Brekke M: Anatomy, biomechanics, and pathomechanics of lesser digital deformities. Clin Podiatr Med Surg, 1996, 13: 179–200. [PubMed] [Google Scholar]
- 26.Hughes J, Clark P, Klenerman L: The importance of the toes in walking. J Bone Joint Surg Br, 1990, 72: 245–251. [DOI] [PubMed] [Google Scholar]
- 27.Agawany AE, Meguid EA: Mode of insertion of the abductor hallucis muscle in human feet and its arterial supply. Folia Morphol (Warsz), 2010, 69: 54–61. [PubMed] [Google Scholar]
- 28.Brenner E: Insertion of the abductor hallucis muscle in feet with and without hallux valgus. Anat Rec, 1999, 254: 429–434. [DOI] [PubMed] [Google Scholar]
- 29.Arinci Incel N, Genç H, Erdem HR, et al. : Muscle imbalance in hallux valgus: an electromyographic study. Am J Phys Med Rehabil, 2003, 82: 345–349. [DOI] [PubMed] [Google Scholar]
- 30.Kura H, Luo ZP, Kitaoka HB, et al. : Quantitative analysis of the intrinsic muscles of the foot. Anat Rec, 1997, 249: 143–151. [DOI] [PubMed] [Google Scholar]
- 31.Mickle KJ, Nester CJ, Crofts G, et al. : Reliability of ultrasound to measure morphology of the toe flexor muscles. J Foot Ankle Res, 2013, 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernhard A, Miller J, Keeler J, et al. : Absence of the fourth tendon of the flexor digitorum brevis muscle: a cadaveric study. Foot Ankle Spec, 2013, 6: 286–289. [DOI] [PubMed] [Google Scholar]
- 33.Kelly LA, Cresswell AG, Racinais S, et al. : Intrinsic foot muscles have the capacity to control deformation of the longitudinal arch. J R Soc Interface, 2014, 11: 20131188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart S, Ellis R, Heath M, et al. : Ultrasonic evaluation of the abductor hallucis muscle in hallux valgus: a cross-sectional observational study. BMC Musculoskelet Disord, 2013, 14: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly LA, Kuitunen S, Racinais S, et al. : Recruitment of the plantar intrinsic foot muscles with increasing postural demand. Clin Biomech (Bristol, Avon), 2012, 27: 46–51. [DOI] [PubMed] [Google Scholar]
- 36.Mulligan EP, Cook PG: Effect of plantar intrinsic muscle training on medial longitudinal arch morphology and dynamic function. Man Ther, 2013, 18: 425–430. [DOI] [PubMed] [Google Scholar]
- 37.Basmajian JV, Stecko G: The role of muscles in arch support of the foot. J Bone Joint Surg Am, 1963, 45: 1184–1190. [PubMed] [Google Scholar]
- 38.Blackburn JT, Bell DR, Norcross MF, et al. : Sex comparison of hamstring structural and material properties. Clin Biomech (Bristol, Avon), 2009, 24: 65–70. [DOI] [PubMed] [Google Scholar]
- 39.Liang J, Yang Y, Yu G, et al. : Deformation and stress distribution of the human foot after plantar ligaments release: a cadaveric study and finite element analysis. Sci China Life Sci, 2011, 54: 267–271. [DOI] [PubMed] [Google Scholar]
- 40.Gu YD, Rong M, Li ZY, et al. : Finite element analysis of deep transverse metatarsal ligaments mechanical response during landing. Adv Mat Res, 2012, 472–475: 2558–2561. [Google Scholar]


