Abstract
[Purpose] The aim of this study was to test the hypothesis that Lee Silverman Voice Treatment-BIG decreases the negative impact of hypokinesia on dual task performance in persons with Parkinson’s disease. [Subjects and Methods] The records of 114 patients with Parkinson’s admitted to outpatient rehabilitation at a suburban hospital were reviewed. Demographics and data for 8 outcome measures were extracted for subjects that completed 14 of 16 sessions of BIG. 93 of these subjects had records of pre and post-test Timed Up and Go, Timed Up and Go Motor, and Timed Up and Go Cognitive scores. Average age was 68.4 years (SD=10.6) and average disease duration was 4.9 years (SD=5.3). [Results] Subjects demonstrated statistically significant improvements for Timed Up and Go (3.3 SD=4.5), Timed Up and Go Motor (4.4 SD=5.8) and Timed Up and Go Cognitive (4.7 SD=5.4). Concurrent motor and cognitive performance remained stable. Dual task cost decreased at a statistically significant level for Timed Up and Go Cognitive (7% SD=31%) but not Motor (4% SD=32%). [Conclusion] These findings suggest that cueing strategies associated with LSVT BIG become internalized and decrease the negative impact of hypokinesia on mobility and cognitive performance while performing two tasks simultaneously in persons with Parkinson’s.
Key words: Parkinson’s disease, LSVT BIG, Dual task
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disorder affecting the basal ganglia and causing the degeneration of dopaminergic neurons1) resulting in bradykinesia, resting tremor, rigidity of muscles, and impaired posture2). Impairments in limb function, gait, balance, speech, mobility, and activities of daily living significantly affect the quality of life in people with PD. While people with PD perceive their movement as normal, they often present with abnormally small amplitude movement3). Several treatment approaches to address this movement disorder including visual, auditory and self-cuing are associated with normalizing movement amplitude temporarily, but no definitive long term solutions have been developed to date4). Even with dopaminergic medication, gait and balance impairments persist, contributing to increased fall risk5, 6). In one systematic review, it was reported that an average of 60.5% of people with PD reported at least one fall, and 39% reported recurrent falls7). Physical therapy is an integral part of PD treatment, focusing on gait training, balance training, and fall prevention as hallmark interventions5, 8). Lee Silverman Voice Treatment (LSVT) BIG® is an exercise and self-cuing based physical or occupational therapy treatment derived from the speech treatment, LSVT LOUD® which is utilized in the treatment of speech deficits including hypophonia. LSVT LOUD has demonstrated short-term and 2 year long-term retention of improvements in loudness and is now considered an evidence-based treatment in people with PD3, 9). LSVT BIG is designed to decrease the impact of hypokinesia on the functional mobility of people with PD by encouraging patients to move using powerful, large amplitude movements during progressive, high intensity training10). The goal of LSVT BIG is to restore normal movement amplitude by recalibrating the patient’s perception of movement execution3). One unique aspect of LSVT BIG’s treatment protocol is its emphasis on key principles associated with motor learning, including high intensity, salience, multiple repetitions, and progressive complexity11, 12). These principles may help to facilitate activity dependent motor learning and neuroplasticity to enhance the generalizability and automaticity of movement12). This emphasis maximizes a client’s ability to develop, consolidate, and generalize self-cuing skills to the point that they transfer into more effective real world function13, 14). Previous studies have suggested that LSVT BIG improves gait speed, mobility, TUG performance, and other functional measures1,2,3, 10).
An overwhelming majority of people with PD demonstrate a deterioration of their functional ability while performing two (dual) or more tasks simultaneously8). Dual task conditions exacerbate gait impairments and result in added mobility deficits including reduced gait speed and decreased coordination15). It has been suggested that people with PD may adopt a strategy that focuses a majority of their attention on the concurrent cognitive task and less attention to the mobility task during dual task activities, thus increasing fall risk16). Several interventions have been proposed to improve dual task performance, such as rhythmic auditory cueing, attentional strategies, treadmill training, and task specific training5, 6, 17,18,19,20,21). LSVT BIG may be another viable intervention to improve dual task walking and mobility. The existing literature examining LSVT BIG suggests that this approach to treatment has a positive impact on PD related movement disorders including hypokinesia and bradykinesia while performing single tasks12). We hypothesize that these decreases in the negative impact of movement disorders should transfer to tasks performed in dual task conditions if the cueing strategies used in LSVT BIG are learned to the point that they do not require substantial conscious attention. While LSVT BIG has not been specifically designed to address dual task abilities, we believe that the emphasis on learning to use the internal cueing strategies taught during LSVT BIG treatment to the point that they are used automatically serves to facilitate their incorporation during the performance of two concurrent tasks. However, there are no published studies examining the impact of LSVT BIG treatment on dual task performance. To initiate the testing of this overarching hypothesis, this retrospective cohort study was performed to examine the initial hypothesis that LSVT BIG intervention may decrease the negative impact of performing a second task on the speed of mobility in persons with PD.
SUBJECTS AND METHODS
The Institutional Review Board of JFK Johnson Rehabilitation Institute, where 100% of the studied patient encounters occurred, deemed this study exempt from human subjects consideration because all data utilized were collected as part of the patients’ routine clinical care. Criteria for referral to the LSVT BIG treatment program included: 1) diagnosis of PD, 2) written referral from their physician, 3) ambulatory with or without an assistive device, and 4) stable on PD medications. Contraindications for LSVT BIG intervention included: 1) unstable blood pressure, 2) osteoporosis, and/or 3) other comorbidities that influenced their ability to engage in physical activity for 1 hour. Inclusion criteria for data analysis included: 1) completion of at least 14 of the 16 scheduled LSVT BIG treatment sessions and 2) medical records that included pre- treatment and post-treatment scores for the Timed Up and Go Test (TUG), the TUG motor test (TUG MOTOR), and the TUG cognitive test (TUG COG). The records of 114 patients diagnosed with PD who participated in the LSVT BIG program at JFK Johnson Rehabilitation Institute, an outpatient rehabilitation department of a suburban hospital, were reviewed for this retrospective cohort study. The data of 93 subjects that met all inclusion criteria for treatment and data analysis were extracted (54 males, 39 females; mean age, 68.4 ± 10.6; mean PD duration, 4.9 ± 5.3 years).
The treating physical therapist evaluated each patient prior to the start of LSVT BIG intervention and again after completion of the last treatment. Data was collected for the TUG, TUG MOTOR, and TUG COG outcome measures. The TUG has been previously established as valid and reliable tool to assess patients with PD both within and between practitioners22,23,24). For the TUG, participants were required to stand up from a seated position in a chair, walk 3 meters at their optimal walking speed, turn around, walk back to the chair and sit down. The time taken to complete the task was measured with a stopwatch25). This test has been demonstrated to be valid and reliable in persons with PD to detect change and indicate fall risk26) and an established cutoff score of greater than 11.5 seconds has been indicative of increased risk for falls in persons with PD27). For the TUG MOTOR, participants completed the TUG described above while simultaneously carrying a cup of water28). The time taken to complete the task has been suggested to correlate to the level of functional mobility29). For the TUG COG, participants completed the TUG while simultaneously reciting the days of the week in backwards order30, 31). This approach, which has been validated for persons with PD30), deviates from the original testing protocol described by Shumway Cook and Woollcott, who recommended that clients be instructed to count backwards by threes or to recite alternate letters of the alphabet aloud29,30,31,32).
The LSVT BIG intervention has previously been described in detail11, 12). It consists of 16, one hour treatment sessions (4 sessions per week for 4 weeks) in clinic, one on one with an LSVT BIG certified therapist, and one to two home practice sessions per day lasting 15−20 minutes each. During all activities, patients are instructed to perform large amplitude movements with near maximal effort and the most efficient biomechanics available. Initially, when smaller amplitude or lower effort movements are observed the therapist immediately cues the patient to increase, or the activity is modified. As treatments progress, participants are encouraged to self-monitor their effort and amplitude with the goal of completing this accurately and independently11).
The first part of each clinic session and all home practice sessions incorporates seven standardized whole-body movements which combine stepping, weight shifting and reaching movements in multiple directions as well as sustained holds. The second portion of each session includes five simple every day functional activities (e.g. sit to stand, rolling in bed) that the patient has identified as needing improvement. This is followed by walking with larger amplitude movements and posture. Finally whole, multi-step task practice follows. These tasks are complex functional activities that are directly related to the patient’s goals and scaled to the patient’s current ability level. They are progressed over the course of treatment by increasing task complexity or difficulty or by adding a concurrent task.
As stated above, subjects were also instructed to engage in ten, 15–20 minute bouts of a home exercise program each week (one home exercise bout each treatment day and two bouts on the other three days). These home exercise sessions included the standardized exercises, functional component tasks, walking and a daily carryover assignment designed to emphasize the use of LSVT BIG strategies in daily activities12).
All statistical analyses were conducted using Minitab 18™. TUG, TUG MOTOR, and TUG COG scores preceding and following LSVT BIG treatment were compared using paired t-tests. Statistical significance was accepted for values of p<0.05. Dual task cost (DTC) was calculated by dividing the difference between TUG MOTOR or TUG COG times and the TUG time collected the same day and then dividing by the TUG score (TUG Cog − TUG) / TUG33). For further analyses, TUG and TUG COG change scores were z-normalized to eliminate the impact of score magnitude on interpretation of relative change between these two constructs. Dual task cost is a measure of the impact of performing a second task on performance of a reference task. We chose to consider this measure because the ability to perform two tasks simultaneously is impaired and correlated with quality of life as well as safety in persons with PD8). It is particularly useful when comparing patients with a wide variety of mobility levels because it normalizes the impact of the second task with baseline mobility levels15). This flexibility was useful considering the heterogeneity of initial TUG performances demonstrated by our sample.
RESULTS
Demographic data and outcome measures at baseline and after LSVT BIG intervention are reported in Table 1. Changes in TUG, TUG COG, and TUG MOTOR were all statistically significant (p<0.001) with moderate effect sizes (Hedges g= 0.59, 0.55, and 0.55 respectively). The mean TUG score was reduced from 13.2 ± 6.9 seconds at baseline to 9.94 ± 3.53 seconds (p<0.001) after LSVT BIG intervention. The mean TUG COG time was reduced from 16.3 ± 10.5 seconds at baseline to 11.5 ± 6.1 seconds (p<0.001) after LSVT BIG intervention, and the TUG MOTOR time was similarly reduced from 16.0 ± 9.8 seconds at baseline to 11.7 ± 5.1 seconds (p<0.001) after LSVT BIG intervention.
Table 1. Demographic data and functional outcome measure scores, at baseline and post LSVT BIG treatment.
| Pre LSVT BIG treatment n=93 | Post LSVT BIG treatment | Effect size (Hedge’s g) | |
|---|---|---|---|
| Age (years) | 68.4 ± 10.6 | — | — |
| Gender (M/F) | 54/39 | — | — |
| Duration of PD (years) | 4.9 ± 5.3 | — | — |
| Falls in the past 6 months (%) | 17.2% | — | — |
| TUG test (seconds) | 13.2 ± 6.9 | 9.94 ± 3.53** | 0.59 (0.40–0.78) |
| TUG Cognitive test (seconds) | 16.3 ± 10.5 | 11.5 ± 6.1** | 0.55 (0.42–0.72) |
| TUG Motor test (seconds) | 16.0 ± 9.8 | 11.7 ± 5.1** | 0.55 (0.38–0.72) |
| TUG Cog cost | 21.6% | 14.9%* | |
| TUG Motor cost | 21.8% | 17.8% |
Values represent mean ± SD.
*Statistically significant difference (p<0.05).
**Statistically significant difference (p<0.001).
TUG COG cost reflects the degree to which the cognitive task of reciting the days of the week backwards caused a deterioration of TUG performance. This value was significantly improved from 21.6% to 14.9% after LSVT BIG intervention (p<0.05). TUG MOTOR cost reflects the degree to which the motor task of carrying a cup of water caused a deterioration of TUG performance and was calculated using the formula (TUG MOTOR −TUG) / TUG34). TUG MOTOR cost was improved from 21.8% to 17.8%; however, the value was not statistically significant. Greater than 10% dual task cost has been associated with decreased mobility and decreased functional ability35, 36). After LSVT BIG intervention, the number of subjects who had>10% dual task cost was significantly decreased for both the motor and cognitive tasks by 44.44% and 44.23%, respectively (p<0.001) as depicted in Fig. 1.
Fig. 1.
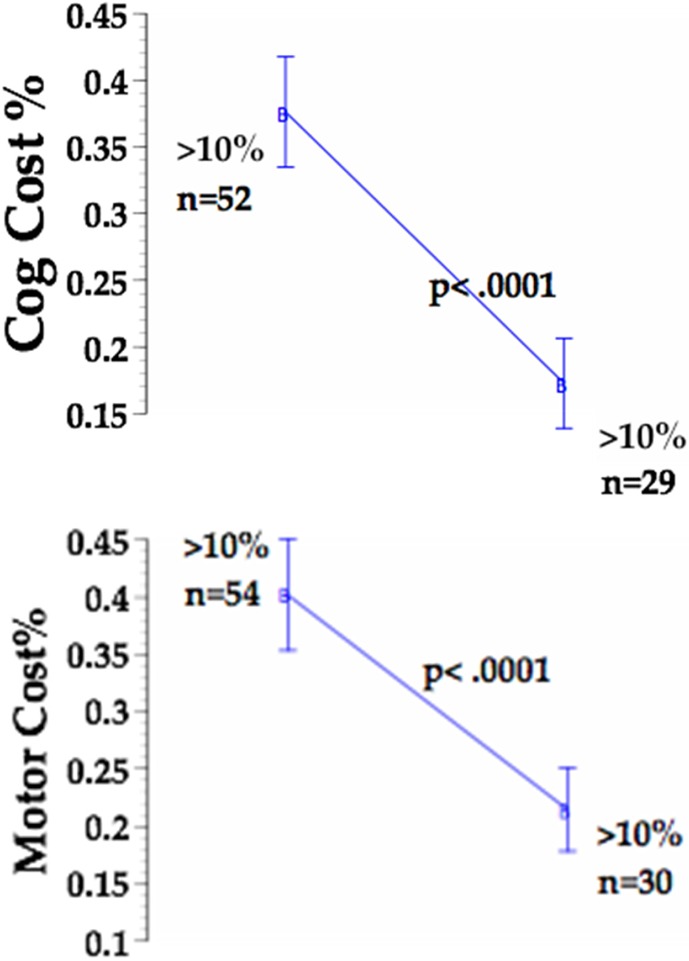
LSVT BIG intervention substantially decreased TUG MOTOR and TUG COG dual task cost in subjects that initially presented with DTC >10%. Note that 24 subjects improved their DTC score to within normal limits.
Dual task interference results are depicted visually in Fig. 2. As a result of LSVT BIG intervention, a reduction in dual task interference is observed. Cognitive performance is relatively stable as evidenced by a large majority of points being on or slightly above the y-axis. The impact of the cognitive task on mobility performance varies a bit more, but the trend toward mobility scores improving relative to baseline is substantial.
Fig. 2.
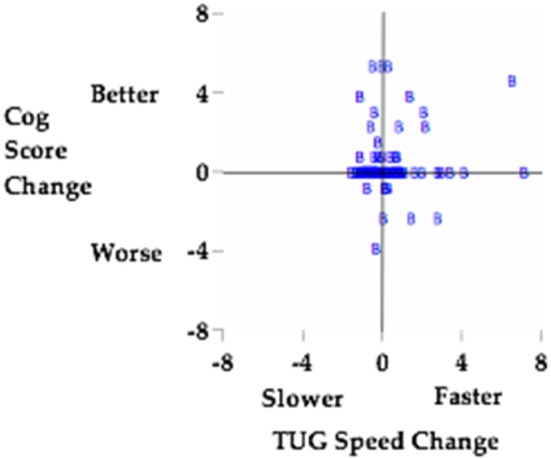
Dual task interference is reduced as a result of LSVT BIG intervention. Change scores are z-normalized to eliminate the impact of change score magnitude27).
DISCUSSION
In previously published studies, LSVT BIG has been suggested to improve gait speed, UPDRS motor scores, TUG performance, endurance, bed mobility, and other functional measures, potentially more so than other exercise programs such as Nordic walking, home exercise programs, and general treadmill training1,2,3, 10, 37). However, little research has been conducted on the impact of LSVT BIG on the ability to dual task. The results of this uncontrolled, retrospective study suggests that LSVT BIG intervention may decrease the negative impact of performing a second task on the speed of mobility in persons with PD. Persons with PD demonstrate improvements in their mobility, speed, and performance of both cognitive and motor dual tasks, as indicated by the significant improvements in TUG, TUG COG, TUG MOTOR, and TUG COG cost following LSVT BIG intervention.
Positive functional results have been suggested when using external cueing strategies, cadence matched music, task specific practice (practicing in dual task conditions), and treadmill training to improve gait speed while performing two tasks simultaneously5, 6, 17,18,19). However, the majority of these strategies only demonstrated improvements while the cueing interventions were occurring, which may not be feasible for everyday life. The subjects in this study demonstrated improvements in TUG time during a testing session that occurred at least 24 hours after the last formal LSVT BIG treatment session. These sessions did not include an external cueing strategy applied during testing and did not include encouragement by the examiner to apply the self-cues taught in LSVT BIG. This suggests that the treatment benefits may last at least 24 hours beyond a treatment session. Future studies with longer-term follow-up will be necessary to determine whether the effects of LSVT BIG treatment are more permanent.
For people with PD, a TUG score of greater than 11.5 seconds is indicative of increased risk of falls27). Using this criteria, our subjects’ reduction in the mean TUG score from 13.2 seconds to 9.9 seconds post intervention suggests that LSVT BIG intervention reduces falls risk for people with PD. Mean improvement in TUG score was 3.33 ± 4.4 s, which is smaller than the published minimum detectable changes (MDC) for persons with PD for this measure (3.5 s as described by Huang et al.22) and 4.85 s as described by Dal Bello-Haas et al.38)). It is important to note that the subjects in these two studies were younger and faster than those in our current study. A recent study by Hofheinz applied more stringent sensitivity criteria to determine a cutoff score to identify fallers, suggesting that persons with PD with TUG COG times slower than ten seconds are at a higher risk for falls26). This criteria would indicate that 72 of our subjects were at risk. 70 of these subjects decreased their TUG COG times from pre to post test and 24 at risk subjects demonstrated TUG COG scores below 10 seconds at post-test, further supporting that fall risk had been reduced. Maranhao-Filho et al. found that persons with PD with a difference between a patient’s TUG score and TUG MOTOR score of 4.5 seconds placed them at an increased risk for falls. Based on this criteria, at pre-test nineteen of our subjects were at an increased risk for falls29). Eighteen of these subjects demonstrated decreased differences at post-test and 14 of these subjects decreased their difference scores to, or below, the 4.5 second cutoff score.
Previous studies have presented mixed results regarding attentional strategies similar to LSVT BIG for improving dual task performance8, 17, 39). One explanation for these less than optimal results has been that attentional strategies may add additional cognitive demand to the brain, thereby decreasing the cognitive reserve available for dual tasks8). It has been suggested that the large training volume, consistent increases in training complexity, emphasis on meaningful tasks, and application of LSVT BIG strategies to a wide variety of tasks brings patients to the point that these attentional strategies become automatic12). We would argue that the stable cognitive performances demonstrated by the subjects in this study support the hypothesis that the self-cuing/attentional strategies practiced during the LSVT BIG interventions did not decrease the cognitive reserve available to the subjects and that the dramatically improved motor performances support that these strategies were effective.
It is important to consider the limitations of this study. Due to the retrospective cohort study design, no control group was included; therefore, the improvements in dual task performance cannot be conclusively attributed to the LSVT BIG intervention. The study design also makes it difficult to establish the impairment level of the subjects studied because no standardized measure of disease severity was collected. However, by comparing the cohort’s TUG scores to studies that reported subjects’ TUG score and Hoehn & Yahr (H&Y) scores, it can be inferred that the subjects in this study were similar to those with a H&Y score of 2–340). Although the method used in this study for the TUG COG has been validated for use in older adults31) and in persons with PD30), it is not consistent with the testing protocol described in the original published version of the TUG COG test32). Several subjects encountered a ceiling effect on the cognitive and motor secondary tasks, indicating that the secondary tasks may not have been challenging enough to demonstrate the maximal extent to which dual tasking difficulties impacts their daily life. Lastly, due to the lack of data for motor and cognitive tasks performed without a second mobility task, a direct analysis of the impact of performing a mobility task on cognitive or motor performance cannot be drawn.
Future research should focus on higher-level study designs to draw more definitive conclusions about LSVT BIG intervention. Long term retention of gains should also be investigated to determine if LSVT BIG can help decrease the negative impact of movement disorders on dual task performance in patients with PD well after the patient has completed the 16 LSVT BIG sessions. Finally, it may be noteworthy to investigate the effect LSVT BIG has on freezing of gait (FOG), because those that experience FOG have exacerbated gait impairments when performing a dual task. This study suggests that LSVT BIG treatment may have a positive impact on mobility and cognitive performance while performing two tasks simultaneously in persons with Parkinson’s disease.
Conflict of interest
None.
Acknowledgments
The authors would like to acknowledge Pragati Duttaroy, DPT, for her contributions to this study.
REFERENCES
- 1.Janssens J, Malfroid K, Nyffeler T, et al. : Application of LSVT BIG intervention to address gait, balance, bed mobility, and dexterity in people with Parkinson disease: a case series. Phys Ther, 2014, 94: 1014–1023. [DOI] [PubMed] [Google Scholar]
- 2.Millage B, Vesey E, Finkelstein M, et al. : Effect on gait speed, balance, motor symptom rating, and quality of life in those with stage I Parkinson’s disease utilizing LSVT BIG®. Rehabil Res Pract, 2017, 2017: 9871070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebersbach G, Ebersbach A, Edler D, et al. : Comparing exercise in Parkinson’s disease—the Berlin LSVT®BIG study. Mov Disord, 2010, 25: 1902–1908. [DOI] [PubMed] [Google Scholar]
- 4.Morris ME, Iansek R, Matyas TA, et al. : Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain, 1996, 119: 551–568. [DOI] [PubMed] [Google Scholar]
- 5.Brown LA, de Bruin N, Doan JB, et al. : Novel challenges to gait in Parkinson’s disease: the effect of concurrent music in single- and dual-task contexts. Arch Phys Med Rehabil, 2009, 90: 1578–1583. [DOI] [PubMed] [Google Scholar]
- 6.de Bruin N, Doan JB, Turnbull G, et al. : Walking with music is a safe and viable tool for gait training in Parkinson’s disease: the effect of a 13-week feasibility study on single and dual task walking. Parkinsons Dis, 2010, 2010: 483530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen NE, Schwarzel AK, Canning CG: Recurrent falls in Parkinson’s disease: a systematic review. Parkinsons Dis, 2013, 2013: 906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly VE, Eusterbrock AJ, Shumway-Cook A: A review of dual-task walking deficits in people with Parkinson’s disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis, 2012, 2012: 918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farley BG, Koshland GF: Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson’s disease. Exp Brain Res, 2005, 167: 462–467. [DOI] [PubMed] [Google Scholar]
- 10.Ebersbach G, Grust U, Ebersbach A, et al. : Amplitude-oriented exercise in Parkinson’s disease: a randomized study comparing LSVT-BIG and a short training protocol. J Neural Transm (Vienna), 2015, 122: 253–256. [DOI] [PubMed] [Google Scholar]
- 11.LSVT BIG Training and Certification Manual LSVT Global Tuscon AZ, 2016.
- 12.Fox C, Ebersbach G, Ramig L, et al. : LSVT LOUD and LSVT BIG: behavioral treatment programs for speech and body movement in Parkinson disease. Parkinsons Dis, 2012, 2012: 391946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher BE, Petzinger GM, Nixon K, et al. : Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res, 2004, 77: 378–390. [DOI] [PubMed] [Google Scholar]
- 14.Taub E: Harnessing brain plasticity through behavioral techniques to produce new treatments in neurorehabilitation. Am Psychol, 2004, 59: 692–704. [DOI] [PubMed] [Google Scholar]
- 15.Kelly VE, Eusterbrock AJ, Shumway-Cook A: The effects of instructions on dual-task walking and cognitive task performance in people with Parkinson’s disease. Parkinsons Dis, 2012, 2012: 671261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloem BR, de Vries NM, Ebersbach G: Nonpharmacological treatments for patients with Parkinson’s disease. Mov Disord, 2015, 30: 1504–1520. [DOI] [PubMed] [Google Scholar]
- 17.Baker K, Rochester L, Nieuwboer A: The immediate effect of attentional, auditory, and a combined cue strategy on gait during single and dual tasks in Parkinson’s disease. Arch Phys Med Rehabil, 2007, 88: 1593–1600. [DOI] [PubMed] [Google Scholar]
- 18.Mak MK, Yu L, Hui-Chan CW: The immediate effect of a novel audio-visual cueing strategy (simulated traffic lights) on dual-task walking in people with Parkinson’s disease. Eur J Phys Rehabil Med, 2013, 49: 153–159. [PubMed] [Google Scholar]
- 19.Mirelman A, Maidan I, Herman T, et al. : Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease? J Gerontol A Biol Sci Med Sci, 2011, 66: 234–240. [DOI] [PubMed] [Google Scholar]
- 20.O’Shea S, Morris ME, Iansek R: Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks. Phys Ther, 2002, 82: 888–897. [PubMed] [Google Scholar]
- 21.Rochester L, Hetherington V, Jones D, et al. : The effect of external rhythmic cues (auditory and visual) on walking during a functional task in homes of people with Parkinson’s disease. Arch Phys Med Rehabil, 2005, 86: 999–1006. [DOI] [PubMed] [Google Scholar]
- 22.Huang SL, Hsieh CL, Wu RM, et al. : Minimal detectable change of the timed “up & go” test and the dynamic gait index in people with Parkinson disease. Phys Ther, 2011, 91: 114–121. [DOI] [PubMed] [Google Scholar]
- 23.Morris S, Morris ME, Iansek R: Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys Ther, 2001, 81: 810–818. [DOI] [PubMed] [Google Scholar]
- 24.Steffen T, Seney M: Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther, 2008, 88: 733–746. [DOI] [PubMed] [Google Scholar]
- 25.Podsiadlo D, Richardson S: The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc, 1991, 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 26.Hofheinz M, Mibs M: The prognostic validity of the Timed Up and Go Test with a dual task for predicting the risk of falls in the elderly. Gerontol Geriatr Med, 2016, 2: 2333721416637798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nocera JR, Stegemöller EL, Malaty IA, et al. National Parkinson Foundation Quality Improvement Initiative Investigators: Using the Timed Up & Go test in a clinical setting to predict falling in Parkinson’s disease. Arch Phys Med Rehabil, 2013, 94: 1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundin-Olsson L, Nyberg L, Gustafson Y: Attention, frailty, and falls: the effect of a manual task on basic mobility. J Am Geriatr Soc, 1998, 46: 758–761. [DOI] [PubMed] [Google Scholar]
- 29.Maranhão-Filho PA, Maranhão ET, Lima MA, et al. : Rethinking the neurological examination II: dynamic balance assessment. Arq Neuropsiquiatr, 2011, 69: 959–963. [DOI] [PubMed] [Google Scholar]
- 30.Lima LC, Ansai JH, Andrade LP, et al. : The relationship between dual-task and cognitive performance among elderly participants who exercise regularly. Braz J Phys Ther, 2015, 19: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbosa JM, Prates BS, Concalves CF, et al. : Efeito da realizacao simultanea de tarefas cognitivas e motoras no desempenho functional de idosos da comunidade. Fisioter Pesqui, 2008, 15: 374–379. [Google Scholar]
- 32.Shumway-Cook A, Brauer S, Woollacott M: Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther, 2000, 80: 896–903. [PubMed] [Google Scholar]
- 33.Verhaeghen P, Cerella J: Aging, executive control, and attention: a review of meta-analyses. Neurosci Biobehav Rev, 2002, 26: 849–857. [DOI] [PubMed] [Google Scholar]
- 34.Plummer P, Eskes G: Measuring treatment effects on dual-task performance: a framework for research and clinical practice. Front Hum Neurosci, 2015, 9: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montero-Odasso M, Muir SW, Speechley M: Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Arch Phys Med Rehabil, 2012, 93: 293–299. [DOI] [PubMed] [Google Scholar]
- 36.Wajda DA, Motl RW, Sosnoff JJ: Dual task cost of walking is related to fall risk in persons with multiple sclerosis. J Neurol Sci, 2013, 335: 160–163. [DOI] [PubMed] [Google Scholar]
- 37.Ueno T, Sasaki M, Nishijima H, et al. : LSVT-BIG improves UPDRS III scores at 4 weeks in Parkinson’s disease patients with wearing off: a prospective, Open-Label study. Parkinsons Dis, 2017, 2017: 8130140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dal Bello-Haas V, Klassen L, Sheppard MS, et al. : Psychometric properties of activity, self-efficacy, and quality-of-life measures in individuals with Parkinson disease. Physiother Can, 2011, 63: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieuwboer A, Kwakkel G, Rochester L, et al. : Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry, 2007, 78: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell CM, Rowse JL, Ciol MA, et al. : The effect of cognitive demand on Timed Up and Go performance in older adults with and without Parkinson disease. J Neurol Phys Ther, 2003, 27: 2–7. [Google Scholar]


