Abstract
[Purpose] Most of rehabilitation programmes for Anterior Cruciate Ligament (ACL) injury focus on quadriceps-hamstrings activation imbalances and less is known about kinetically linked muscles. This study investigated electromyographic activity of selected trunk, core, and thigh muscles during common rehabilitation exercises for ACL injury. [Subjects and Methods] Twelve active female volunteers participated in this cross-sectional laboratory study. Surface EMG was used to compare activation of eight trunk, hip/core, and lower limb muscles: Erector Spinae (ES), Rectus Abdominis (RA), Gluteus Maximus (GM), Vastus Lateralis (VL), Rectus Femoris (RF), Vastus Medialis (VM), Biceps Femoris (BF), and Semitendinosus (ST) during Forward Lunge, Double Leg Raise, Glute Bridge, Sit-Up, and Squat. [Results] Forward lunge produced significantly higher activation in the VM (61.1 ± 19.4), VL (59.2 ± 12.9), and RF (32.0 ± 2.6). Double leg raise generated highest activity in the RF (26.6 ± 2.8) and RA (43.3 ± 4.4); and Glute Bridge in the GM (44.5 ± 19.0) and BF (22.4 ± 4.3). Sit-up produced the highest activation in the RF (36.6 ± 4.7) followed by RA (18.9 ± 3.8). Squat produced a higher activation in VL (55.0 ± 12.9), VM (51.5 ± 18.2), and ES (40.4 ± 18.3). [Conclusion] This study provide further evidence for developing training programmes for ACL injury prevention and rehabilitation. A combination of exercises to reinstate quadriceps-hamstrings activation balance and enhance core stability is recommended.
Key words: Electromyography, Activation balance, Neuromuscular function
INTRODUCTION
The anterior cruciate ligament (ACL) is a common site for sport injury often occurring during a non-contact twisting movement such as pivoting once slowing down or landing1). ACL injury involves 20% of all sports-related knee injuries with an annual incidence of 81 injuries per 100,000 people leading to functional deficits and knee joint instability during sporting activities2). Female athletes are 5.4 to 7.8 times more likely to sustain ACL injury than male athletes3). Increased Q angle and higher joint laxity in females have been linked to abnormal knee kinematics by means of inward rotation of the tibia and placing high levels of stress on the ACL4). In terms of neuromuscular characteristics, imbalanced hamstring-to-quadriceps strength and activation ratios have been suggested as potential risk factors in female athletes5, 6).
Electromyography (EMG) is widely used in the field of sports medicine for investigating potential alterations in the muscle activation patterns in pathologic conditions in order to facilitate the development of evidence-based training and rehabilitation programmes. EMG has however produced conflicting reports regarding ACL injuries: “Quadriceps impairment, as assessed by EMG, has been reported by some researchers while others have reported no impairment7,8,9). Likewise for hamstrings, despite reports of increased activity in ACL injury, some others found no difference in their activity between patients and controls7,8,9). Recently, gathering knowledge suggests that altered quadriceps and hamstrings activation in ACL injury may only exist in the presence of knee instability as part of an adaptation strategy to support joint stability i.e. inhibited quadriceps activity with concurrent increased activity of both quadriceps and hamstrings9).
Deficits within the muscular function (strength and activation) are commonly associated with knee instability in ACL injury leading to the altered joint biomechanics and development of aberrant movement patterns. Quadriceps weakness, in particular, has been associated with these alterations and strength deficits reported between 5–30%5, 10). It has been reported that disproportionate activity of the quadriceps and hamstring muscles results in muscle imbalance and increased strain over the ACL. Appropriate hamstrings activity is essential to counterbalance quadriceps contraction in order to constrain anterior displacement of the tibia5, 11). Hence, many ACL injury prevention and rehabilitation programmes attempt to reinstate quadriceps-hamstrings activation balance12).
In addition to quadriceps and hamstrings, kinetically and functionally linked muscles such as gluteus maximus, rectus abdominis, and erector spinae may also be affected following ACL injury as a result of coping strategies. The gluteus maximus contributes greatly to the core stability, postural alignments, and functional abilities essential for normal gait. Weakness of this muscle may lead to abnormal gait cycle and affect the movement mechanics at both hip and knee joints13, 14). It is suggested that gluteus muscle weakness contributes to ACL injury due to increased hip internal rotation and adduction as well as the dynamic knee valgus movements which in turn place additional stress on the knee joint1, 5). Rectus abdominis and erector spinae contribute to the core stability and to the controlling of trunk posture during whole-body sports activities15). It has been reported that enhanced activity of rectus abdominis and erector spinae during stability-enhancing exercise programmes leads to significant enhancement in cooperative spine/core muscle activity and stability16). Furthermore, both rectus abdominis and erector spinae contribute to the normal gait by generating and controlling the motion between the trunk and pelvis17, 18). Hence sufficient activation of these muscles is important in decreasing body’s vertical displacement (involving knees) and producing a smoother trajectory for the centre of mass during the gait cycle17).
Both closed and open kinetic chain exercises (CKC and OKC, respectively) are commonly recommended for rehabilitation of ACL injury with a primary focus on restoring normal range of motion and strengthening selected lower extremity and core muscle groups and reducing anterior-posterior (A-P) tibial displacement. Due to weight-bearing nature of CKC exercises a compressive joint load is produced which in turn forces the articular surfaces together in order to eliminate anteroposterior displacement of the tibia relative to the femur8). It is suggested that CKC exercises are more effective in enhancing knee arthrokinematics than OKC exercises by means of producing a smaller magnitude of anterior tibial translation and enhance activation of lower extremity muscles (hamstrings-quadriceps in particular) to support knee stability8, 19). Hence, it is important to explore exercises with an optimal effect on the restoration of hamstrings-quadriceps activation balance.
While the majority of ACL injury prevention and rehabilitation programmes aim to concurrently activate hamstrings and quadriceps to constrain tibial translation, there is limited data on the activity of selected core and trunk muscles during such exercises as the majority of previous studies primarily examined lower extremity (i.e. thigh) muscle activations. With a kinetic chain approach, the present study aimed to investigate activity of selected lower extremity, hip/core, and trunk muscles contributing to the knee joint stability and mobility during five commonly prescribed exercises for ACL injury to provide rationalised evidence-based recommendations. Considering that commonly used therapeutic exercises would have different impact on core and lower extremity muscle activations, study aimed to identify exercises that support balanced activations.
SUBJECTS AND METHODS
The study aimed to determine the EMG activation of eight muscles of thigh, core/hip, and trunk during common lower extremity exercises in order to provide further knowledge for the development of training, injury prevention, and rehabilitation strategies particularly in athletes at high risk of ACL injuries. The percent of maximum voluntary isometric contractions (MVCs) for each muscle was determined and compared across the five exercises using repeated-measures analysis of variance (ANOVA) in order to determine whether exercise condition had a significant effect on mean activity of each muscle tested.
Twelve healthy and physically active female participants with no history of lower extremity or back problems participated in this study in a university research laboratory. Those with a history of lower extremity and low back pain or surgery, neurological disorders, and severe systematic diseases were excluded from the study. The mean age, height, weight and BMI of participants were; 20.10 ± 1.10 years, 165.90 ± 4.77 cm, 63.50 ± 6.22 kg, and 23.06 ± 2.17, respectively. The study received ethical approval from the Institutional Review Board and all participants gave written informed consent prior to partaking in the experiments.
Five common exercises were performed by each subject; Squat, Sit-Up, Forward Lunge, Glute Bridge, and Double Leg Rise. Subjects performed 10 repetitions of each exercise of 60 beats per minute on a metronome. Participants received instructions as how to perform each exercise:
1) Forward Lunge: Participants stood with their feet near each other and hands on their hips, a forward step (with dominant limb) was taken in the sagittal plane and lowering into 90° of hip and knee flexion while the trunk was maintained in an upright position; 2) Squat: Participants stood with feet shoulder-width apart. Hip, knees and ankles were flexed in a squatting motion until reaching 90° of knee flexion (parallel to the horizontal). Participants were instructed to keep their chest up, weight over the heels and not to allow their knees fall into a valgus position; 3) Sit-Up: In a supine position on the floor with flexed knees, participants lifted their torso up to approximately 45° at which their torso was in a V position with thighs and then lowered the torso back to the starting position in a controlled manner guided by the metronome; 4) Glute Bridge: Participants laid supine with both knees flexed to 90° and feet flat on the floor. Hips were raised off the floor (pushing through the heels) until a straight line was made between their shoulders and knees. Subjects then lowered their hips back to the starting position in a controlled manor; 5) Double Leg Raise: In a supine position with hands by sides or under gluteus (whichever was preferred) and keeping knees in extended position, participants slowly raised both legs until a hip flexion angle of up to 75° and held the contraction before lowering both legs according to the metronome.
Signal acquisition, processing and analysis were performed using a wireless TeleMyo 2400 G2 Telemetry System (Noraxon Inc., AZ, USA) with synchronised video recording. The bipolar self-adhesive Ag/AgCL surface electrodes (Noraxon Inc., AZ, USA), with a 20 mm inter-electrode distance, were placed in parallel to the muscle fibre orientation20). EMG signals were collected from eight muscles on the dominant side: Vastus Lateralis (VL: two-thirds of the thigh length from the greater trochanter on the lateral side of the thigh), Rectus Femoris (RF: midway between the anterior inferior iliac spine and the patella on the anterior side of the thigh), Vastus Medialis (VM: three-fourths of the thigh length from the anterior inferior iliac spine on the medial side of the thigh), Semitendinosus (ST: midway between the ischial tuberosity and the medial condyle of the femur on the posterior side of the thigh Iliac crest of the right leg), Biceps Femoris (BF: midway between the ischial tuberosity and the lateral condyle of the femur on the posterior side of the thi), Gluteus Maximus (GM: 50% on the line between the sacral vertebrae and the greater trochanter), Erector Spinae (ES: three centimetres lateral to the L3 spinous process), and Rectus Abdominis (RA: above the anterior superior iliac spine)21, 22).
EMG signals from 10 exercise cycles were differentially amplified (Common Mode Rejection Ratio-CMRR >100 dB; input impedance >100 Mohm; gain 500 dB), digitized at a sampling rate of 3,000 Hz and band‐pass filtered at 10–500 Hz. This was followed by full-wave rectification and smoothing at 100 ms to determine EMG amplitudes by means of root mean square (RMS). Exercise EMG amplitudes were then normalized to the EMG during MVIC for individual muscles: VL- in the sitting position with 90° hip flexion and 90° knee flexion and resistance applied to the distal leg just above the ankle during knee extension; RF- in a sitting position with extended knee and resistance applied to the anterior part of the ankle directed toward the knee flexion; VM- in the sitting position with the knee flexed be- tween 45° to 60° and resistance applied just above the ankle; ST- in a prone position with 90° knee flexion and resistance applied to the posterior part of the ankle in the direction of the knee extension; BF- in a prone position with knee flexion at 45° and resistance applied above the ankle; GM- in the prone position, with the knee flexed to 90° and the hip extended and resistance applied above the knee; ES- in a prone position and resistance applied across the posterior deltoids to resist spinal extension; and RA- a partial curl-up with the feet secured and resistance applied at the shoulders. The maximum EMG signal amplitude (RMS) during the MVIC of each muscle represented 100% muscle activity. The muscle activity recorded during the exercises was then expressed as a percentage of the MVIC and the average amplitudes from 10 exercise repetitions were taken into analysis.
A one-way repeated measures analysis of variance (ANOVA) was applied to determine whether exercise conditions had a statistically significant effect on mean EMG activity (%MVIC) of each muscle tested (within exercise differences). Significance was set at the 0.05. A Bonferroni post‐hoc test was then applied for the comparative pair‐wise analysis of mean normalized EMG (% MVIC) to detect significant differences in the activation of muscles when differences were observed. SPSS (IBM Corp. Released 2013. IBM SPSS Statistics, Version 22.0, NY, USA) was used for statistical analysis.
RESULTS
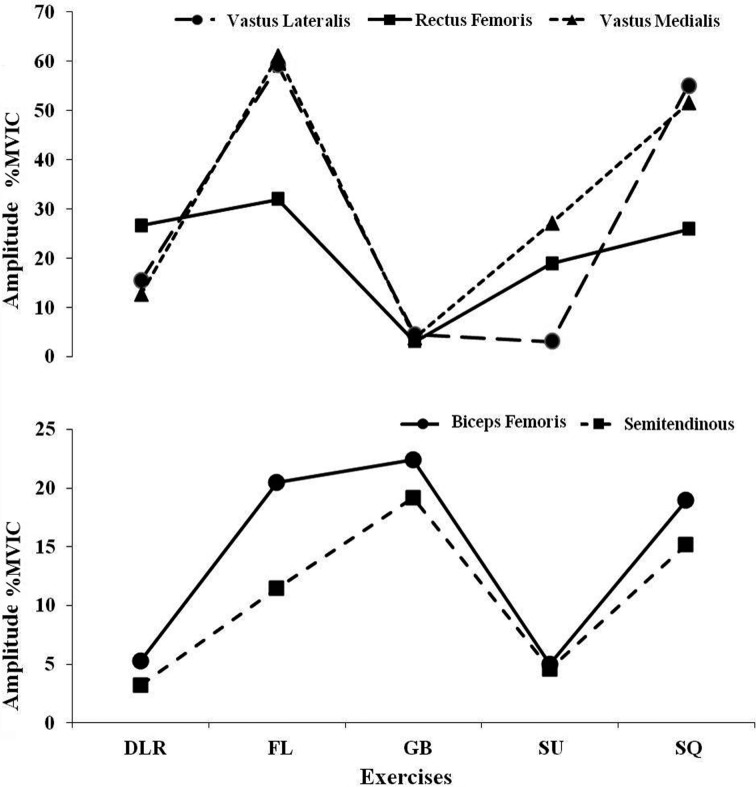
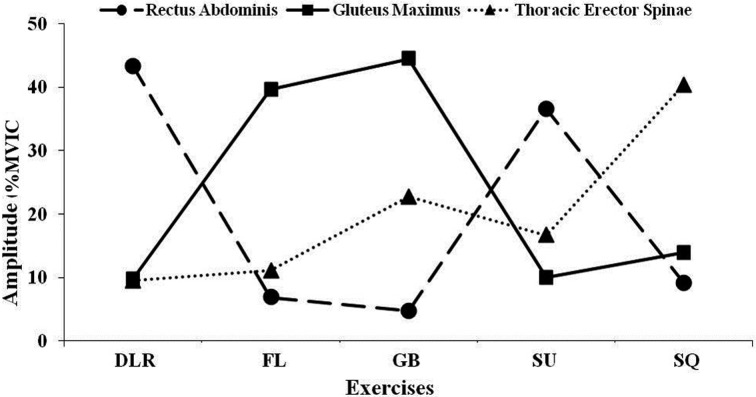
Table 1 and Figs. 1, 2 summarise and compare the mean activation of muscles during exercises:
Table 1. Comparison of mean (± SEM) muscle activation (%MVIC) for individual muscles during five rehabilitation exercises.
| Exercises | VL | RF | VM | BF | ST | RA | GM | ES |
|---|---|---|---|---|---|---|---|---|
| Double Leg Raise | 15.4 ± 4.3 | 26.6 ± 2.8*c | 12.6 ± 3.6 | 5.3 ± 1.3 | 3.2 ± 0.9 | 43.3 ± 4.4*bce | 9.8 ± 4.3 | 9.5 ± 2.2 |
| Forward Lunge | 59.2 ± 12.9*acd | 32.0 ± 2.6*cd | 61.1 ± 19.4*ac | 20.5 ± 6.4*ad | 11.5 ± 3.7 | 6.9 ± 0.9 | 39.7 ± 17.2 | 11.1 ± 1.6 |
| Glute Bridge | 4.5 ± 1.7 | 3.1 ± 0.7 | 3.9 ± 1.1 | 22.4 ± 4.3*ad | 19.2 ± 3.2*a | 4.8 ± 0.8 | 44.5 ± 19.0*ad | 22.8 ± 2.9 |
| Sit-Up | 3.1 ± 0.6 | 18.9 ± 3.8*c | 27.2 ± 22.4 | 5.0 ± 1.2 | 4.6 ± 1.0 | 36.6 ± 4.7*bce | 10.0 ± 2.9 | 16.7 ± 3.7 |
| Squat | 55.0 ± 12.9*acd | 25.9 ± 3.6*c | 51.5 ± 18.2*c | 19.0 ± 8.1 | 15.2 ± 10.3 | 9.2 ± 5.4 | 13.9 ± 1.5 | 40.4 ± 18.3*ab |
VL: Vastus Lateralis; RF: Rectus Femoris; VM: Vastus Medialis; BF: Bicep Femoris; ST: Semitendinosus; RA: Rectus Abdominis; GM: Gluteus Maximus; ES: Erector Spinae.
*p<0.05.
a: Significantly higher than Double Leg Raise; b: Significantly higher than Forward Lunge; c: Significantly higher than Glute Bridge; d: Significantly higher than Sit-Up; e: Significantly higher than Squat.
Fig. 1.
Mean normalized EMG activity (%EMGmax) for the lower extremity muscles during exercises. DLR: Double Leg Raise; FL: Forward Lunge; GB: Glute Bridge; SU: Sit-Up; SQ: Squat.
Fig. 2.
Mean normalized EMG activity (%EMGmax) for the core/trunk muscles during exercises. DLR: Double Leg Raise; FL: Forward Lunge; GB: Glute Bridge; SU: Sit-Up; SQ: Squat.
Forward lunge created significantly higher muscle activation in the VL compared to double leg raise, glute bridge, and sit-up (p<0.001). The RF activation was also considerably higher than glute bridge and sit-up (p<0.001). Forward lunge generated significantly higher activity in the VM compared to double leg raise and glute bridge (p<0.05); and in the BF compared to double leg raise and sit-up (p<0.05). Squat was associated with a significantly higher activation in the VL compared to double leg raise, glute bride and sit-up (p<0.001). The RF and VM both were activated significantly higher than glute bridge (p<0.05) and p<0.001, respectively); and ES had a significantly higher activation compared to double leg raise and forward lunge (p<0.05). Glute Bridge produced significantly higher activation in BF and GM compared to double leg raise and sit-up (p<0.05); and in the ST compared to double leg raise (p<0.05). Sit-Up generated markedly higher activation in the RA (p<0.001) compared to forward lunge, glute bridge, and squat; and in the RF compared to glute bridge (p<0.05). Double Leg Raise was associated with a significantly higher activation in the RA than in forward lunge, glute bridges and squat (p<0.001); and there was a markedly in the RF compared to the glute bridge (p<0.05).
DISCUSSION
Findings of this study provide further evidence, by means of muscle activation and strengthening, for optimal prescription of training and rehabilitation exercises in athletes with ACL injury. EMG signal amplitude has been shown to have both linear and non-linear relationship with the force produced by the muscle23, 24) and hence, has been widely used to underpin potential rehabilitation exercises by means of enhancing strength, endurance, and stability. It is generally accepted that for strength gains to occur muscle activation should reach the 40% MVC threshold during therapeutic exercises in order to accomplish strengthening adaptation25, 26) as such the greater the muscle activation the greater the gains27). The use of exercises with moderate activity, which fail to reach the threshold for strength gains, may instead be used as a high repetition exercise to enhance muscle endurance26, 28).
One of the strategic aims of the current ACL rehabilitation programmes is to correct aberrant muscle activation patterns of the lower extremity muscles, the quadriceps and hamstrings in particular, following ACL-injury and ACL-reconstruction. These altered activations have been linked to compensatory adaptations in response to arthrogenic muscle inhibition in the quadriceps and/or muscle strength deficits associated with ACL injury (both pre-operatively and following ACL reconstruction)10, 29).
While the quadriceps function is key for appropriate positioning of the body’s centre of mass during communal athletic movements such as running, jump, landings, and cutting manoeuvres30) strong uncontrolled quadriceps forces can lead to anterior translation of the tibia and increase the risk of ACL injury31). It is noted that training the quadriceps disproportionately to the hamstrings may impair hamstrings activation, reduce joint stability, increase anterior tibial translation in response to strong quadriceps forces and potentially increase the incidence of ACL injury30). Hence prescribed exercises should have elements of hamstrings training to counterbalance quadriceps activation (restore hamstrings-to-quadriceps activation balance) and support knee ligamentous function in maintaining joint stability and balancing articular surface load distribution31).
In addition to the lower extremity muscles, the majority of sporting movements also place large demands on trunk/core musculature as they control the body’s centre of mass in response to the forces generated from distal body parts32, 33). As a result, training of trunk/core muscles should be considered in the training and rehabilitation programmes in order to restore core strength and stability, improve lower extremity alignment, enhance landing quality, and reduce risk of ACL injury by reducing valgus force to the knee32, 34, 35). Hence, in addition to hamstrings and quadriceps muscles, the present study also measured activity of selected trunk and core muscles, which may directly or indirectly contribute to the knee joint alignment, stability, and mobility, during some commonly prescribed exercises to further support evidence-based training and exercise prescription for the prevention and rehabilitation of ACL injury.
The present study found significant differences in the mean activation (i.e. an estimate of exercise intensity) of all muscles across the five exercises indicating their contributions to the potential effectiveness of these rehabilitative exercises. With regard to activation of quadriceps (VL, VM, and RF), forward lunge (32–61%MVIC) and squat (25–55%MVIC) appeared to be the optimal exercises and may be considered for enhancing strength and function of this muscle group. This is consistent with that of Ebben et al.36) who reported the highest activation of RF, VL, and VM during squat and forward lunge. Bryanton et al.37) investigated the impact of squat depth on relative muscular effort (RME) and reported increased RME of Knee and hip extensors with greater squat depth. Ayotte et al.25) investigated activation of VM during several weight-bearing exercises and reported considerable activation (sufficient for muscle strengthening) during the wall squat. Ekstrom et al.28) reported activation levels greater than 45% MVIC in the VM during lunge exercises and recommended it for strengthening of the muscle. Pincivero et al.38) also found very high levels of EMG activity for VM and VL during the lunge exercise (150% to 175%MVIC). In terms of different squat exercises, Contreras et al.39) measured mean and peak EMG amplitude of the GM, BF, and VL during front, full, and parallel squats in resistance-trained females and reported similar EMG activity of muscles.
Regarding hamstrings, the forward lunge and glute bridge produced considerably higher activation of BF and ST compared to other exercises supporting their integration into the training and rehabilitation programmes for enhancing hamstring function, endurance in particular. It has been shown that hamstrings and ACL act synergistically to limit anterior tibial translation during quadriceps contraction. It has also been suggested that in the presence of ACL injury concurrent increase in hamstring activity and quadriceps inhibition may happen as part of adaptation strategy to resume functional stability40). In a study of BF and ST muscle activation during forward lunge, Pincivero et al.38) demonstrated a significant increase in BF activation while no significant increase was found for ST. Ekstrom et al.28) investigated EMG activity of hamstrings during glute bridge exercise and reported a similar activation level of that to the present study. Begalle et al.5) reported a very high quadriceps-hamstrings ratio during the lunge exercises. Considering high activity of quadriceps during forward lunge and associated detrimental increase in quadriceps–hamstring activation ratio, the glute bridge may be more advantageous for facilitating a more balanced activation. Farrokhi et al.41) investigated the effect of changes in trunk position on lower limb muscle activity during forward lunge exercises and found that performing a lunge with a forwarded trunk increased the GM and BF activity compared to when it performed with extended trunk.
Emerging data supporting the important role of the muscles acting upon the hip joint, GM in particular, during athletic movements has led to an increasing number of EMG studies aiming to identify optimal training and rehabilitation exercises for athletes with lower extremity injuries25, 28). Furthermore, a significant muscle activation deficit has been reported in patients undergoing ACL reconstruction compared to healthy controls42). In the present study GM was activated the greatest during glute bridge (44%MVIC) and forward lunge (40%MVIC) supporting their effective contribution to enhancing core strength and stability. In an EMG study of common exercises, Ekstrom et al.28) reported markedly increased GM activity (36%MVIC) during forward lunge compared to several other exercises and a moderately increased activity during glute bridge (25%MVIC). Some other studies, have however reported significant increase in GM activity during squat-based exercises25, 43).
The greatest muscle activity for RA and ES was produced during the double leg raise (43%MVIC) and squat (40%MVIC), respectively. While many sporting performances require efficient contribution from the core and trunk muscles for maintaining correct posture and establishing core stability, there is limited data on the activity of RA and ES during lower extremity exercises. As part of lower extremity training and rehabilitation programme, it is critical to maintain the appropriate activity levels and strength of these muscles to enable optimal function and reduce the risk of re-injury. In their study of various core and lower extremity rehabilitation exercises, Ekstrom et al.28) and Comfort at al.44) reported the highest RA activity during prone bridge (40%MVIC and 0.454 Root Mean Square-RMS[V], respectively). These studies did not however include the double leg raise exercise. Comfort et al.44) reported a significant increase in the ES activity during front squat (1.010 RMS[V]) suggesting that such dynamic exercise may be beneficial for strengthening the muscle. The study however did not report normalised EMG activity data that would allow direct comparisons.
While performing exercises in different planes may influence the kinetics and activation of the muscles, the forward lunge was only measured with the trunk in one plane and squat only to 90° of parallel flexion. Furthermore, study included only two exercises for the true RA activity. Considering emerging evidence to support the employment of more dynamic and criteria-based progression exercises following ACL reconstruction45, 46), some exercises included in the present study such as sit-up and double leg raise may have limited effect on ACL rehabilitation.
Findings of this study provide further evidence for optimal prescription of training and rehabilitation exercises in athletes with ACL injury. In terms of muscle activation, study demonstrated that the forward lunge and squat are the best exercises for the quadriceps; the glute bridge and forward lunge for the GM and hamstrings; double leg raise and sit-up for the RA; and squat for the ES. These exercises may be recommended for enhancing muscle activation patterns and muscle endurance. In terms of strengthening (reaching 40%MVIC for strength gain), we recommend squat and forward lunge for the quadriceps, Glute Bridge for the GM, double leg raise for the RA, and squat for the ES. Clinical outcome studies on the efficacy of these exercises in enhancing lower extremity function and in athletes with ACL injury are needed to further support their integration into training and rehabilitation plans.
Conflict of interest
None.
REFERENCES
- 1.Lorenz D, Reiman M: The role and implementation of eccentric training in athletic rehabilitation: tendinopathy, hamstring strains, and ACL reconstruction. Int J Sports Phys Ther, 2011, 6: 27–44. [PMC free article] [PubMed] [Google Scholar]
- 2.Frobell RB, Lohmander LS, Roos EM: The challenge of recruiting patients with anterior cruciate ligament injury of the knee into a randomized clinical trial comparing surgical and non-surgical treatment. Contemp Clin Trials, 2007, 28: 295–302. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi H, Kanamura T, Koshida S, et al. : Mechanisms of the anterior cruciate ligament injury in sports activities: a twenty-year clinical research of 1,700 athletes. J Sports Sci Med, 2010, 9: 669–675. [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis T: Anterior cruciate ligament injury in female athletes: why are women so vulnerable?: Literature review. Physiotherapy, 2000, 86: 464–472. [Google Scholar]
- 5.Begalle RL, Distefano LJ, Blackburn T, et al. : Quadriceps and hamstrings coactivation during common therapeutic exercises. J Athl Train, 2012, 47: 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewett TE: Neuromuscular and hormonal factors associated with knee injuries in female athletes. Strategies for intervention. Sports Med, 2000, 29: 313–327. [DOI] [PubMed] [Google Scholar]
- 7.Knoll Z, Kocsis L, Kiss RM: Gait patterns before and after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc, 2004, 12: 7–14. [DOI] [PubMed] [Google Scholar]
- 8.Kvist J, Gillquist J: Anterior positioning of tibia during motion after anterior cruciate ligament injury. Med Sci Sports Exerc, 2001, 33: 1063–1072. [DOI] [PubMed] [Google Scholar]
- 9.Shanbehzadeh S, Mohseni Bandpei MA, Ehsani F: Knee muscle activity during gait in patients with anterior cruciate ligament injury: a systematic review of electromyographic studies. Knee Surg Sports Traumatol Arthrosc, 2017, 25: 1432–1442. [DOI] [PubMed] [Google Scholar]
- 10.de Jong SN, van Caspel DR, van Haeff MJ, et al. : Functional assessment and muscle strength before and after reconstruction of chronic anterior cruciate ligament lesions. Arthroscopy, 2007, 23: 21–28, 28.e1–28.e3. [DOI] [PubMed] [Google Scholar]
- 11.Hewett TE, Zazulak BT, Myer GD, et al. : A review of electromyographic activation levels, timing differences, and increased anterior cruciate ligament injury incidence in female athletes. Br J Sports Med, 2005, 39: 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappas E, Nightingale EJ, Simic M, et al. : Do exercises used in injury prevention programmes modify cutting task biomechanics? A systematic review with meta-analysis. Br J Sports Med, 2015, 49: 673–680. [DOI] [PubMed] [Google Scholar]
- 13.Dingenen B, Janssens L, Claes S, et al. : Lower extremity muscle activation onset times during the transition from double-leg stance to single-leg stance in anterior cruciate ligament reconstructed subjects. Clin Biomech (Bristol, Avon), 2016, 35: 116–123. [DOI] [PubMed] [Google Scholar]
- 14.Huxel Bliven KC, Anderson BE: Core stability training for injury prevention. Sports Health, 2013, 5: 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall PW, Murphy BA: Core stability exercises on and off a Swiss ball. Arch Phys Med Rehabil, 2005, 86: 242–249. [DOI] [PubMed] [Google Scholar]
- 16.Yu SH, Park SD: The effects of core stability strength exercise on muscle activity and trunk impairment scale in stroke patients. J Exerc Rehabil, 2013, 9: 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White SG, McNair PJ: Abdominal and erector spinae muscle activity during gait: the use of cluster analysis to identify patterns of activity. Clin Biomech (Bristol, Avon), 2002, 17: 177–184. [DOI] [PubMed] [Google Scholar]
- 18.Wirth K, Hartmann H, Mickel C, et al. : Core stability in athletes: a critical analysis of current guidelines. Sports Med, 2017, 47: 401–414. [DOI] [PubMed] [Google Scholar]
- 19.Saka T: Principles of postoperative anterior cruciate ligament rehabilitation. World J Orthop, 2014, 5: 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermens HJ, Freriks B, Disselhorst-Klug C, et al. : Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol, 2000, 10: 361–374. [DOI] [PubMed] [Google Scholar]
- 21.Cram JR, Kasman GS, Holtz J: Electrode Placement. Gaithersburg: Aspen, 1998. [Google Scholar]
- 22.Rainoldi A, Melchiorri G, Caruso I: A method for positioning electrodes during surface EMG recordings in lower limb muscles. J Neurosci Methods, 2004, 134: 37–43. [DOI] [PubMed] [Google Scholar]
- 23.Alkner BA, Tesch PA, Berg HE: Quadriceps EMG/force relationship in knee extension and leg press. Med Sci Sports Exerc, 2000, 32: 459–463. [DOI] [PubMed] [Google Scholar]
- 24.Saito A, Akima H: Knee joint angle affects EMG-force relationship in the vastus intermedius muscle. J Electromyogr Kinesiol, 2013, 23: 1406–1412. [DOI] [PubMed] [Google Scholar]
- 25.Ayotte NW, Stetts DM, Keenan G, et al. : Electromyographical analysis of selected lower extremity muscles during 5 unilateral weight-bearing exercises. J Orthop Sports Phys Ther, 2007, 37: 48–55. [DOI] [PubMed] [Google Scholar]
- 26.Escamilla RF, Lewis C, Bell D, et al. : Core muscle activation during Swiss ball and traditional abdominal exercises. J Orthop Sports Phys Ther, 2010, 40: 265–276. [DOI] [PubMed] [Google Scholar]
- 27.Andersen LL, Magnusson SP, Nielsen M, et al. : Neuromuscular activation in conventional therapeutic exercises and heavy resistance exercises: implications for rehabilitation. Phys Ther, 2006, 86: 683–697. [PubMed] [Google Scholar]
- 28.Ekstrom RA, Donatelli RA, Carp KC: Electromyographic analysis of core trunk, hip, and thigh muscles during 9 rehabilitation exercises. J Orthop Sports Phys Ther, 2007, 37: 754–762. [DOI] [PubMed] [Google Scholar]
- 29.Thomas AC, Villwock M, Wojtys EM, et al. : Lower extremity muscle strength after anterior cruciate ligament injury and reconstruction. J Athl Train, 2013, 48: 610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howell SM: Anterior tibial translation during a maximum quadriceps contraction: is it clinically significant? Am J Sports Med, 1990, 18: 573–578. [DOI] [PubMed] [Google Scholar]
- 31.Baratta R, Solomonow M, Zhou BH, et al. : Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med, 1988, 16: 113–122. [DOI] [PubMed] [Google Scholar]
- 32.Manske RC, Prohaska D, Lucas B: Recent advances following anterior cruciate ligament reconstruction: rehabilitation perspectives: Critical reviews in rehabilitation medicine. Curr Rev Musculoskelet Med, 2012, 5: 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myer GD, Paterno MV, Ford KR, et al. : Neuromuscular training techniques to target deficits before return to sport after anterior cruciate ligament reconstruction. J Strength Cond Res, 2008, 22: 987–1014. [DOI] [PubMed] [Google Scholar]
- 34.Hewett TE, Myer GD, Ford KR, et al. : Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med, 2005, 33: 492–501. [DOI] [PubMed] [Google Scholar]
- 35.Myer GD, Ford KR, Brent JL, et al. : The effects of plyometric vs. dynamic stabilization and balance training on power, balance, and landing force in female athletes. J Strength Cond Res, 2006, 20: 345–353. [DOI] [PubMed] [Google Scholar]
- 36.Ebben WP, Feldmann CR, Dayne A, et al. : Muscle activation during lower body resistance training. Int J Sports Med, 2009, 30: 1–8. [DOI] [PubMed] [Google Scholar]
- 37.Bryanton MA, Kennedy MD, Carey JP, et al. : Effect of squat depth and barbell load on relative muscular effort in squatting. J Strength Cond Res, 2012, 26: 2820–2828. [DOI] [PubMed] [Google Scholar]
- 38.Pincivero DM, Aldworth C, Dickerson T, et al. : Quadriceps-hamstring EMG activity during functional, closed kinetic chain exercise to fatigue. Eur J Appl Physiol, 2000, 81: 504–509. [DOI] [PubMed] [Google Scholar]
- 39.Contreras B, Vigotsky AD, Schoenfeld BJ, et al. : A comparison of gluteus maximus, biceps femoris, and vastus lateralis electromyography amplitude in the parallel, full, and front squat variations in resistance-trained females. J Appl Biomech, 2016, 32: 16–22. [DOI] [PubMed] [Google Scholar]
- 40.Swanik CB, Lephart SM, Giraldo JL, et al. : Reactive muscle firing of anterior cruciate ligament-injured females during functional activities. J Athl Train, 1999, 34: 121–129. [PMC free article] [PubMed] [Google Scholar]
- 41.Farrokhi S, Pollard CD, Souza RB, et al. : Trunk position influences the kinematics, kinetics, and muscle activity of the lead lower extremity during the forward lunge exercise. J Orthop Sports Phys Ther, 2008, 38: 403–409. [DOI] [PubMed] [Google Scholar]
- 42.Harput G, Howard JS, Mattacola C: Comparison of muscle activation levels between healthy individuals and persons who have undergone anterior cruciate ligament reconstruction during different phases of weight-bearing exercises. J Orthop Sports Phys Ther, 2016, 46: 984–992. [DOI] [PubMed] [Google Scholar]
- 43.Blanpied PR: Changes in muscle activation during wall slides and squat-machine exercise. J Sport Rehabil, 1999, 8: 123–134. [Google Scholar]
- 44.Comfort P, Pearson SJ, Mather D: An electromyographical comparison of trunk muscle activity during isometric trunk and dynamic strengthening exercises. J Strength Cond Res, 2011, 25: 149–154. [DOI] [PubMed] [Google Scholar]
- 45.Myer GD, Paterno MV, Ford KR, et al. : Rehabilitation after anterior cruciate ligament reconstruction: criteria-based progression through the return-to-sport phase. J Orthop Sports Phys Ther, 2006, 36: 385–402. [DOI] [PubMed] [Google Scholar]
- 46.Paterno MV: Functional testing, functional training, and criteria for return to play after ACL reconstruction. In: Brotzman SB, Manske RC, eds. Clinical orthopaedic rehabilitation: an evidence-based approach. Elsevier Mosby, 2011, pp 230–233. [Google Scholar]