Abstract
Standard chemotherapy for women with advanced or recurrent cervical cancer involves a combination of paclitaxel, platinum, and bevacizumab. However, for patients who experience anaphylaxis in response to paclitaxel or platinum, have permanent peripheral neuropathy, or develop early recurrence or progressive disease during first-line chemotherapy, the development of a non-taxane non-platinum regimen is mandatory. Clinical trials using anti-angiogenic treatment demonstrated favorable outcomes in cases of highly vascularized cervical cancer. Metronomic chemotherapy has been considered an anti-angiogenic treatment, although its use in combination with bevacizumab has not been studied in cervical cancer. We treated four patients with recurrent cervical cancer with 50 mg of oral cyclophosphamide daily and 15 mg/kg of intravenous bevacizumab every 3 weeks (CFA-BEV). One patient experienced disease progression after 4 months, whereas the other three patients continued the regimen until their last follow-up at 13, 14, and 15 months, respectively. One patient suffered from grade 3 neutropenia; however, no grade 2 or higher non-hematological toxicities were observed. These cases demonstrate the use of CFA-BEV with minimal toxicity and expected anti-cancer activity and indicate that this regimen should be considered for second-line chemotherapy in advanced recurrent cervical cancer.
Keywords: Cervical cancer, Metronomic chemotherapy, Bevacizumab
Highlights
-
•
Development of non-taxane, non-platinum regimen is warranted in the second line treatment of recurrent cervical cancer.
-
•
Clinical trials using anti-angiogenetic drugs showed better outcomes in cases of highly vascularized cervical cancer.
-
•
Metronomic chemotherapy has been shown to inhibit angiogenesis.
-
•
This is the first case report of metronomic chemotherapy with bevacizumab in cervical cancer.
1. Introduction
Platinum-based chemotherapy has been a standard treatment for advanced or recurrent cervical cancer, and a combination regimen of paclitaxel, platinum, and bevacizumab (TP-Bev) is widely recognized as the current standard treatment (Tewari et al., 2017). However, some patients suffer from anaphylaxis after taxane- or platinum-based therapy. There is no standard treatment for progressive disease that develops during or shortly after platinum-based chemotherapy (Takekuma et al., 2017). Development of a non-taxane, non‑platinum regimen for recurrent cervical cancer is therefore warranted.
Clinical trials using anti-angiogenic drugs, including bevacizumab and cediranib, for treatment of highly vascularized cervical cancer have demonstrated high efficacy (Symonds et al., 2015). Metronomic chemotherapy—which is described as frequent administration of low-dose chemotherapeutic drugs despite the maximum tolerated dose (MTD) therapy—has also demonstrated high clinical efficacy and only mild toxicities in patients with various cancers, which is likely due to inhibition of angiogenesis (Mpekris et al., 2017). The combination of metronomic chemotherapy with bevacizumab has been reported in several cancer types. For ovarian cancer, there have been six studies that have reported the results of a regimen including 50 mg of oral cyclophosphamide (CFA) daily and 10 mg/kg of intravenous bevacizumab (BEV) repeated every 2 weeks. Alagkiozidis et al. reported 2 cases with endometrial cancer that were treated with 50 mg of oral CFA daily and 15 mg/kg of BEV repeated every 3 weeks. The two patients had a progression-free survival (PFS) of greater than 16 months, with prolonged quality of life (Alagkiozidis et al., 2015).
We treated four patients with recurrent cervical cancer with 50 mg of oral CFA daily and 15 mg/kg of BEV repeated every 3 weeks (CFA-BEV). Among three patients (cases 1, 2, and 4) who received salvage chemotherapy, case 2 had a PFS of 4.2 months and cases 1 and 4 continued treatment for more than 13 months without disease progression. One patient (case 3) continued the regimen as maintenance chemotherapy for more than 13 months after radiotherapy for metastases in the pubic region and surrounding soft tissues (Table 1). Written informed consent was obtained from all patients to publish their data in this case report.
Table 1.
Description of each case.
| Case | Histology | Primary treatment | Treatment after recurrence | Baseline of CFA-BEV |
Response of CFA-BEV |
AEs of CFA-BEV CTCAE ver 4.0 |
Current status | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | PS | Moore criteria |
PFS (months) |
Non-hematologic |
Hematologic |
||||||
| Score | Risk | ≥ grade 2 | ≥ grade 3 | ||||||||
| 1 | SCC | RH+CCRT | RT, anaphylaxis to platinum, UFT, TAE, RFA | 73 | 0 | 1 | low | 15.2* | No | Grade 3 neutropenia | AWD |
| 2 | Adeno-SCC | CCRT | Surgery, RT | 47 | 0 | 1 | low | 4.2 | No | No | AWD |
| 3 | SCC | RH | RT, surgery, anaphylaxis to platinum | 75 | 1 | NA | 12.8* | No | No | NED | |
| 4 | SCC | RH+CCRT | Progression during platinum, RT, UFT | 47 | 0 | 2 | intermediate | 13.8* | No | No | NED |
Moore criteria included black race, performance status 1, pelvic disease, prior cisplatin, and a progression-free interval <365 days. CFA-BEV, oral cyclophosphamide and bevacizumab; AEs, adverse events; CTCAE, Common Terminology Criteria for Adverse Events; PS, performance status; PFS, progression free survival; SCC, squamous cell carcinoma; RH, radical hysterectomy; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; UFT, tegafur/uracil; TAE, trans-arterial embolization; RFA, radiofrequency ablation; NA, not available; AWD, alive with disease; NED, no evidence of disease.
Denotes patients who continued to receive CFA-BEV until their last follow-up.
2. Case reports
2.1. Case 1
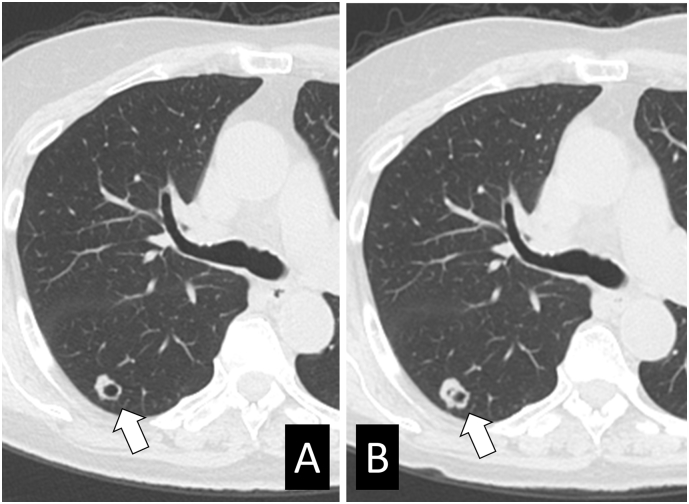
A 66-year-old woman was diagnosed with stage IB1 squamous cell carcinoma and underwent radical hysterectomy. The surgical specimen revealed pelvic lymph node metastases. Adjuvant concurrent chemoradiotherapy with weekly administration of 40 mg/m2 cisplatin for 5 cycles was administered. Five years after first-line treatment, the patient was diagnosed with metastases in the left axillary lymph nodes. Following surgical resection, she received adjuvant radiotherapy to the field and received triweekly administration of 80 mg/m2 nedaplatin for 2 cycles. She discontinued nedaplatin because of anaphylaxis and was administered tegafur/uracil for 7 months. At the age of 73 years, she was diagnosed with a solitary liver metastasis and multiple lung metastases. After transcatheter arterial chemoembolization and radiofrequency ablation for local control of liver metastasis, she continued to receive CFA-BEV. Computed tomography (CT) was conducted 5.2, 9.2, 11.3, and 14.8 months after treatment and revealed that her lung disease had stabilized (Fig. 1). During CFA-BEV treatment, she developed grade 3 neutropenia (950 cells/mm3) and grade 1 fatigue, and refrained from chemotherapy for one month after 10 months of treatment.
Fig. 1.
Stable disease of lung metastases during CFA-BEV treatment in case 1.
Images from computed tomography performed before (A) and 14.8 months after (B) the initiation of CFA-BEV treatment. The arrow shows the maximum size of the lung metastases (diameter, 10 mm).
2.2. Case 2
A 40-year-old woman was diagnosed with stage IIIB adenosquamous cell carcinoma. A computed tomography (CT) scan revealed pelvic and paraaortic lymph node metastases. She received extended-field radiotherapy with 80 mg/m2 of nedaplatin every 3 weeks for 5 cycles. After 30 months, she underwent a thoracoscopic pulmonary upper lobectomy for right solitary lung metastasis. After 14 months, she received radiotherapy for Virchow metastasis. After 29 months, at the age of 47 years, she was diagnosed with multiple lymph node and lung metastases on 18F-fluoro-deoxyglucose (18-FDG) positron emission tomography (PET)-CT. She displayed no symptoms and rejected taxane-based chemotherapy owing to its side effect of alopecia. She was treated with CFA-BEV. After 2 months, PET-CT showed stable disease. She discontinued treatment because of onset of cough caused by progressive lung metastasis after 4.2 months of treatment. Following this, she agreed to receive a regimen of paclitaxel, carboplatin and bevacizumab, which resulted in partial response of lung metastases.
2.3. Case 3
A 66-year-old woman was diagnosed with stage IB1 squamous cell carcinoma. She received radical hysterectomy. Two years later, she received high-dose-rate intracavitary radiotherapy because of sustained evidence of the presence of malignant cells (class V) on vaginal wall cytology. After 6 months, she was diagnosed with left inguinal lymph node metastases; these were resected. Six months later, a recurrence in the anterior vaginal orifice and left inguinal lymph node metastases were identified. Resection of the urethra and vaginal wall, suprapubic cystostomy and left inguinal lymph node resection were conducted. Four years later, she experienced pelvic recurrence, therefore pelvic exenteration with urostomy, and colonostomy was performed. Eight months later, metastases in the pubic symphysis and the surrounding soft tissues were identified, for which concurrent chemoradiotherapy with nedaplatin was administered. She discontinued nedaplatin after 3 cycles because of anaphylaxis, by which point she was 75 years old. The patient continued to receive CFA-BEV. No evidence of recurrence was detected on CT after 11.9 months of treatment.
2.4. Case 4
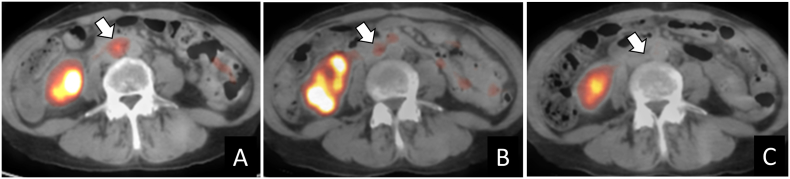
A 45-year-old woman was transferred to our hospital because of massive vaginal bleeding. A cervical tumor 7 cm in diameter was identified, and stage IB2 squamous cell carcinoma was diagnosed. Following transarterial chemoembolization with 50 mg of cisplatin, she underwent a radical hysterectomy which resulted in incomplete lymph node resection because of dense adhesion to the vessels. She received pelvic radiotherapy with nedaplatin for 6 cycles. One month after the last cycle of nedaplatin, PET-CT scan revealed paraaortic and mediastinal lymph node metastases, for which additional irradiation was performed. After 12 months, at the age of 47 years, a PET-CT scan revealed recurrence of the paraaortic lymph node metastases. She continued to receive CFA-BEV. PET-CT scanning was conducted at 5.1, 9.9, and 13.8 months after treatment and revealed complete remission (Fig. 2).
Fig. 2.
Decreased 18F-fluoro-deoxyglucose uptake of paraaortic lymph node during CFA-BEV treatment in case 4. The short axis was 8.7 mm in diameter, and the maximum standard uptake value (SUVmax) was 2.7 (A) before CFA-BEV initiation. After 5.1 months, the SUVmax decreased to 2.4 (B); no abnormal uptake was detected 9.9 months (C) and 13.8 months after treatment. The arrow indicates the paraaortic lymph node.
3. Discussion
The mainstay of treatment for recurrent cervical cancer is to maintain the quality of life, and prolonged survival is expected by administration of bevacizumab, especially in high-risk women. The four patients presented in this case report had no cancer-associated symptoms before the initiation of CFA-BEV therapy. Therefore, metronomic chemotherapy with bevacizumab was considered because it has the advantage of prolonged tumor stabilization with mild toxicities, rather than the rapid and short-term tumor regression responses caused by standard MTD therapy.
Metronomic chemotherapy has been most extensively studied in breast cancer (Liu et al., 2017), where, in combination with bevacizumab, it has demonstrated potent anti-tumor activity with only mild toxicities. Metronomic weekly administration of paclitaxel has been shown to have anti-angiogenic activity. In a randomized clinical trial in which a combination of chemotherapy and bevacizumab was used to treat platinum-resistant ovarian cancer, weekly administration of paclitaxel had the highest efficacy compared with other drugs, presumably because of the synergistic effect of weekly dosing of paclitaxel and bevacizumab on angiogenesis (Pujade-Lauraine et al., 2014). The combination of low dose oral cyclophosphamide and bevacizumab presumably had a synergic anti-angiogenic effect on recurrent cervical cancer in this study, as it did on ovarian cancer in previous studies.
Although metronomic chemotherapy was initially considered to target angiogenesis, recent studies have shown other anti-tumor mechanisms targeting stem-like cells (CSCs) and the immune system (Kareva, 2017). Metronomic chemotherapy in combination with an anti-angiogenic drug showed a reduction in CSCs in rat glioma (Folkins et al., 2007). Metronomic cyclophosphamide therapy reduced CD4 + CD25+ regulatory T cells and activated cytotoxic NK and CD8+ T cells (Ghiringhelli et al., 2007), although immunotherapy alone using ipilimumab or pembrolizumab showed limited activity against cervical cancer (Frenel et al., 2017; Lheureux et al., 2017).
A non-platinum-based regimen for recurrent cervical cancer could comprise topotecan, paclitaxel, and bevacizumab (TP-BEV); however, the toxicities would be greater than those experienced with CFA-BEV. In the GOG-0240 trial, a regimen of topotecan-paclitaxel with or without bevacizumab resulted in grade 3–4 neutropenia in 64% of cases and grade 2–3 sensory neuropathy in 18% (Tewari et al., 2017). Garcia et al. treated 70 patients with recurrent ovarian cancer with CFA-BEV, and only one (1.4%) patient developed grade 3 neutropenia (Garcia et al., 2008). Patients with low and intermediate risk, according to the Moore criteria (Moore et al., 2010), and those administered bevacizumab-containing chemotherapy doublets in the GOG-0240 trial had a PFS of 10.9 and 7.9 months, respectively (Tewari et al., 2015). In our report, cases 1 and 4 had similar risks and had a PFS of over 13 months, confirmed by CT and PET-CT, respectively. CFA-BEV could be a promising potential treatment for patients who experience recurrent disease shortly after platinum treatment, anaphylaxis in response to paclitaxel or platinum, or peripheral neuropathy. In addition, the application of this therapy regimen to asymptomatic patients with minor or dormant recurrent disease should be further investigated.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- Alagkiozidis I., Lozano M., Devraj M., Lee Y.C., Abulafia O. Metronomic cyclophosphamide with bevacizumab provides disease stabilization in patients with advanced uterine cancer. Gynecol. Oncol. Rep. 2015;12:23–26. doi: 10.1016/j.gore.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkins C., Man S., Xu P., Shaked Y., Hicklin D.J., Kerbel R.S. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- Frenel J.S., Le Tourneau C., O'Neil B., Ott P.A., Piha Paul S.A., Gomez-Roca C., van Brummelen E.M.J., Rugo H.S., Thomas S., Saraf S., Rangwala R., Varga A. Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: Results From the Phase Ib KEYNOTE-028 Trial. J. Clin. Oncol. 2017;35:4035–4041. doi: 10.1200/JCO.2017.74.5471. [DOI] [PubMed] [Google Scholar]
- Garcia A.A., Hirte H., Fleming G., Yang D., Tsao-Wei D.D., Roman L., Groshen S., Swenson S., Markland F., Gandara D., Scudder S., Morgan R., Chen H., Lenz H.-J., Oza A.M. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret hospital phase II consortia. J. Clin. Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F., Menard C., Puig P.E., Ladoire S., Roux S., Martin F., Solary E., Le Cesne A., Zitvogel L., Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareva I. A combination of immune checkpoint inhibition with metronomic chemotherapy as a way of targeting therapy-resistant cancer cells. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18102134. (pii: E2134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lheureux S., Butler M.O., Clarke B., Cristea M.C., Martin L.P., Tonkin K., Fleming G.F., Tinker A.V., Hirte H.W., Tsoref D., Mackay H., Dhani N.C., Ghatage P., Weberpals J., Welch S., Pham N.A., Motta V., Sotov V., Wang L., Karakasis K., Udagani S., Kamel Reid S., Streicher H.Z., Shaw P., Oza A.M. Association of Ipilimumab with Safety and Antitumor Activity in Women with Metastatic or Recurrent Human Papillomavirus-Related Cervical Carcinoma. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gu F., Liang J., Dai X., Wan C., Hong X., Zhang K., Liu L. The efficacy and toxicity profile of metronomic chemotherapy for metastatic breast cancer: a meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D.H., Tian C., Monk B.J., Long H.J., Omura G.A., Bloss J.D. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a gynecologic oncology group study. Gynecol. Oncol. 2010;116:44–49. doi: 10.1016/j.ygyno.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpekris F., Baish J.W., Stylianopoulos T., Jain R.K. Role of vascular normalization in benefit from metronomic chemotherapy. Proc. Natl. Acad. Sci. 2017;114:1994–1999. doi: 10.1073/pnas.1700340114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujade-Lauraine E., Hilpert F., Weber B., Reuss A., Poveda A., Kristensen G., Sorio R., Vergote I., Witteveen P., Bamias A., Pereira D., Wimberger P., Oaknin A., Mirza M.R., Follana P., Bollag D., Ray-Coquard I. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- Symonds R.P., Gourley C., Davidson S., Carty K., McCartney E., Rai D., Banerjee S., Jackson D., Lord R., McCormack M., Hudson E., Reed N., Flubacher M., Jankowska P., Powell M., Dive C., West C.M.L., Paul J. Cediranib combined with carboplatin and paclitaxel in patients with metastatic or recurrent cervical cancer (CIRCCa): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:1515–1524. doi: 10.1016/S1470-2045(15)00220-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekuma M., Mori K., Iida T., Kurihara K., Saitou M., Tokunaga H., Kawana K., Ikeda M., Satoh T., Saito T., Miyagi E., Nagai Y., Furusawa A., Kawano Y., Kawano K., Tabata T., Ota Y., Hayase R., Mikami M., Sugiyama T. The concept of platinum sensitivity could be applied to recurrent cervical cancer: a multi-institutional retrospective study from the Japanese gynecologic oncology group. Cancer Chemother. Pharmacol. 2017;80:697–705. doi: 10.1007/s00280-017-3402-x. [DOI] [PubMed] [Google Scholar]
- Tewari K.S., Sill M.W., Monk B.J., Penson R.T., Long H.J., III, Poveda A., Landrum L.M., Leitao M.M., Brown J., Reid T.J., Michael H.E., Moore D.H. Prospective validation of pooled prognostic factors in women with advanced cervical cancer treated with chemotherapy with/without bevacizumab: NRG oncology/GOG study. Clin. Cancer Res. 2015;21:5480–5487. doi: 10.1158/1078-0432.CCR-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K.S., Sill M.W., Long H.J., 3rd, Penson R.T., Huang H., Ramondetta L.M., Landrum L.M., Oaknin A., Reid T.J., Leitao M.M., Michael H.E., Monk B.J. Bevacizumab plus low-dose metronomic oral cyclophosphamide in heavily pretreated patients with recurrent ovarian cancer. N. Engl. J. Med. 2017;370:734–743. [Google Scholar]