Abstract
Our aim was to evaluate the prevalence of exercise-induced bronchoconstriction (EIB) in elite football players and assess subsequent impact of therapy on airway health and exercise performance.
97 male professional football players completed an airway health assessment with a eucapnic voluntary hyperpnoea (EVH) challenge to diagnose EIB. Players demonstrating a positive result (EVH+) were prescribed inhaler therapy depending on severity, including inhaled corticosteroids and inhaled short-acting β2-agonists, and underwent repeat assessment after 9 weeks of treatment. Eight players (EVH+ n=3, EVH− n=5) completed a peak oxygen uptake (V′O2peak) test at initial and follow-up assessment.
Out of the 97 players, 27 (28%) demonstrated a positive EVH result. Of these, 10 had no prior history (37%) of EIB or asthma. EVH outcome was not predictable by respiratory symptoms. Seven (24%) of the 27 EVH+ players attended follow-up and demonstrated improved post-challenge spirometry (forced expiratory volume in 1 s pre-test −22.9±15.4%, post-test −9.0±1.6%; p=0.018). At follow-up V′O2peak improved by 3.4±2.9 mL·kg−1·min−1 in EVH+ players compared to 0.1±2.3 mL·kg−1·min−1 in EVH− players. Magnitude of inference analysis indicated treatment was possibly beneficial (74%) for exercise capacity.
Elite football players have a high EIB prevalence. Treatment with inhaler therapy reduces EIB severity.
Short abstract
Screening and treating EIB in elite football players improves airway health and may improve exercise performance http://ow.ly/ngE530iWGJh
Introduction
Exercise-induced bronchoconstriction (EIB) is now recognised to be highly prevalent in some groups of elite athletes [1, 2] and can impair their health and exercise performance [3–6]. The nature of elite-level football suggests that EIB may be relevant and pose a particular risk to this group of athletes. Specifically, elite football players regularly sustain high ventilation rates [7] from a young age [8], while often training and competing in an asthmogenic environment, i.e. in cold air, high pollen and areas of high pollution. Despite this, little is currently known regarding the nature of EIB in professional football players.
Previous studies suggest that football players are often misdiagnosed or incorrectly labelled as having EIB or asthma [9], highlighting that respiratory symptoms are poorly predictive in the diagnosis of EIB and therefore limit the accuracy of a symptom-based approach to diagnosis [10]. However, there have been no prospective studies evaluating the prevalence of EIB, or indeed the benefits of treating EIB in this group of elite athletes.
It is recognised that EIB has deleterious effects on the efficiency of gas exchange [4], and therefore can impair performance. Indeed, Stensrud et al. [5] report impaired oxygen uptake in individuals with EIB, and an improvement in peak oxygen uptake (V′O2peak) was reported in players of Australian Rules football following the initiation of treatment for previously undetected EIB [6].
Football clubs have a duty of care for their players; to protect their health while at the same time optimising their performance. There are rigorous medical guidelines in place which include screening players for certain medical conditions (e.g. cardiac abnormalities) [11]; however, clubs do not routinely assess players for EIB. Some authors have called for screening for EIB to be implemented [12, 13]; however, before a screening programme can be put in place, a number of stringent criteria must be met, including demonstrating prevalence, ability to detect the condition of interest and an understanding of the impact of intervention in the population of interest [14, 15].
The aim of this work was to address these deficiencies by using robust objective tests to provide a comprehensive assessment of the impact of EIB in professional football players by: 1) determining the prevalence of EIB in elite footballers; 2) assessing the impact of appropriate therapy on airway inflammation and EIB control; and 3) investigating the effect of treating players with EIB on exercise performance. In order to achieve this aim with a standardised and widely accepted approach, we utilised the bronchoprovocation methodology of eucapnic voluntary hyperpnoea (EVH) [16] testing to establish the presence of airway dysfunction and EIB. We hypothesised that EIB would be highly prevalent and that initiation of standard asthma therapy would be beneficial for airway health, as assessed by physiological measures of airway hyperreactivity and inflammation, and potentially for exercise performance.
Methods
Study population
Male professional football players from the English Football Premier League, Championship and League One were invited to participate in a detailed respiratory health assessment, as a component of their preseason medical examination in July, prior to their preseason training period.
Study design
All players attended for a detailed respiratory assessment, including screening for EIB using EVH challenge [17] (part 1). Subsequently, players with EIB were treated for 9 weeks, after which they had a follow-up respiratory assessment (part 2). A subgroup of players completed maximal exercise testing at both baseline and follow-up (part 3). All players were free from illness in the 2 weeks prior to assessment, and were requested to avoid exercise and caffeine for ≥4 h before assessment. Ethical approval for the study was obtained from the University of Kent School of Sport and Exercise Sciences Research ethics committee (144-2014_2015) and participants provided written consent.
Part 1: baseline assessment
Players completed a detailed respiratory assessment and initially completed a questionnaire to determine their medical history and evaluate presence of respiratory symptoms. Players previously prescribed medication for asthma/EIB (n=7) were asked to withhold treatment at the time of assessment, in accordance with guideline recommendations [1].
Respiratory assessment
Airway inflammation was assessed by determining exhaled nitric oxide fraction (FeNO) (NIOX VERO; Aerocrine, Uppsala, Sweden) [18] and lung function was assessed by maximal flow volume spirometry (Spiro-USB and MicroLab; CareFusion, Hoechberg, Germany) [19].
An EVH challenge was then conducted, as described previously [1]. In brief, players were required to inspire medical-grade air (21% oxygen, 5% carbon dioxide and 74% nitrogen with <2% humidity) for 6 min at a target ventilation rate of 85% maximal voluntary ventilation (MVV). Target MVV was calculated as 30× forced expiratory volume in 1 s (FEV1) [20] and minute ventilation was recorded. Maximal flow volume loops were measured in duplicate at 3, 5, 7, 10 and 15 min post-EVH, with the flow loop with the best FEV1 accepted at each time point. A test was considered positive for EIB if FEV1 fell by ≥10% from baseline at two consecutive time points. At this point, 4×100 µg of inhaled salbutamol was administered using a metered dose inhaler and maximal flow volume loops were recorded 15 min post-inhalation.
Severity of EIB was classified as mild, moderate or severe, dependent on the fall in FEV1 post-EVH (10–<25%, 25–<40% and ≥40%, respectively) [21]. Players with a positive EVH challenge (EVH+) test were then prescribed medication by their team physician, in accordance with recommendations [22], with a spacing device and inhaler technique advice [23].
Treatment
Medication was prescribed according to EIB severity; players with mild EIB were prescribed a daily inhaled corticosteroid (ICS), in conjunction with an inhaled short-acting β2-agonist (SABA), as needed. Those with moderate EIB were prescribed a combination inhaler containing ICS and a long-acting β2-agonist, with a SABA as needed. Finally, players with severe EIB were prescribed the combination inhaler with the addition of a daily montelukast and a SABA as required.
Part 2: follow-up assessment
Players continued with their usual preseason training and no change was made to training load. After 9 weeks, EVH+ players underwent a further respiratory assessment (as detailed earlier).
Part 3: performance assessment
A subgroup of players (n=8) completed a V′O2peak test on a motorised treadmill with simultaneous gas analysis (Oxycon Pro; Jaeger, Hoechberg, Germany). Initial running speed was set at 11 km·h−1 and a gradient of 1%. Speed increased 1 km·h−1 every 3 min to 16 km·h−1, at which point the gradient was increased by 1% every minute until volitional exhaustion. V′O2peak was determined as the single highest 30-s average in V′O2.
Statistical analysis
Data are presented as mean±sd unless otherwise stated. Players were grouped according to the result of their EVH challenge (EVH+, EVH−). Group differences and pre- to post- data were analysed using independent or paired t-tests or the non-parametric equivalent and symptom data were analysed using Chi-squared analysis and Fisher's exact test. Given the small number of subjects completing performance assessment, magnitude-base inference analysis was utilised, using the spreadsheet from Hopkins [24] to assess the clinical impact of detection and treatment of EIB. All other analyses were conducted using SPSS software (version 23; SPSS, Chicago, IL, USA) with significance accepted at p<0.05.
Results
98 players (age 24±4 years, height 183±7 cm, mass 80.3±7.2 kg) completed the initial respiratory assessment; however, one player was subsequently excluded due to his inability to perform reliable spirometry (table 1). 17 players reported a previous diagnosis of asthma and/or EIB, of whom only seven were currently prescribed medication. 16 players reported troublesome respiratory symptoms, including cough, wheeze and dyspnoea at a frequency of 5%, 3% and 8%, respectively.
TABLE 1.
Player characteristics and baseline respiratory assessment data for the 97 players who performed the baseline eucapnic voluntary hyperpnoea (EVH) challenge
| All players | EVH+ | EVH− | p-value EVH+ versus EVH− | |
| Subjects | 97 | 27 | 70 | |
| Age years | 24±4 | 24±4 | 24±4 | 0.56 |
| Height cm | 182.6±6.8 | 183.0±6.7 | 182.5±6.9 | 0.77 |
| Weight kg | 80.3±7.2 | 80.7±6.3 | 80.2±7.5 | 0.73 |
| FeNO ppb | 21.0 (24.6) | 36.0 (63.0) | 18.8 (17.3) | <0.01# |
| FEV1 L | 4.71±0.65 | 4.51±0.55 | 4.78±0.68 | 0.07 |
| FVC L | 5.67±0.76 | 5.68±0.64 | 5.67±0.81 | 0.95 |
| PEF L·min−1 | 630.0 (135.0) | 616.0 (102.0) | 635.0 (148.8) | 0.29# |
| FEV1/FVC | 82.7±6.8 | 79.3±8.0 | 84.1±5.8 | 0.01 |
| MVV during EVH % predicted | 77.68±11.76 | 83.07±10.88 | 75.54±11.48 | <0.01 |
| Fall FEV1 from EVH % | −8 (5.5) | −13 (10.0) | −6 (4.0) | <0.01# |
Data are presented as n, mean±sd or median (interquartile range), unless otherwise stated. FeNO: exhaled nitric oxide fraction; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; PEF: peak expiratory flow; MVV: maximal voluntary ventilation. #: data analysed using Mann–Whitney U-tests.
Part 1: baseline assessment
Pulmonary function, inflammation and EIB
Lung function was normal in all players at the baseline assessment, with no evidence of significant airflow obstruction (FEV1 >70% predicted). In 39 (40%) players FeNO was raised above normal (>25 ppb), with 19 (20%) players having a value >50 ppb (table 1).
A positive EVH result was found in 27 (28%) players, of which the majority (n=21, 78%) were classified as having a mild response and fewer having a moderate (n=4, 15%) or severe (n=2, 7%) response (figure 1). All EVH+ players demonstrated a subsequent improvement in FEV1 following administration of bronchodilator treatment (change in FEV1 20.6±11.6%, range 6–40%; p<0.001). There was a weak inverse relationship between FeNO and percentage fall in FEV1 post-EVH (rs −0.24, p=0.02), i.e. the greater the FeNO the larger the reduction in lung function post-EVH.
FIGURE 1.
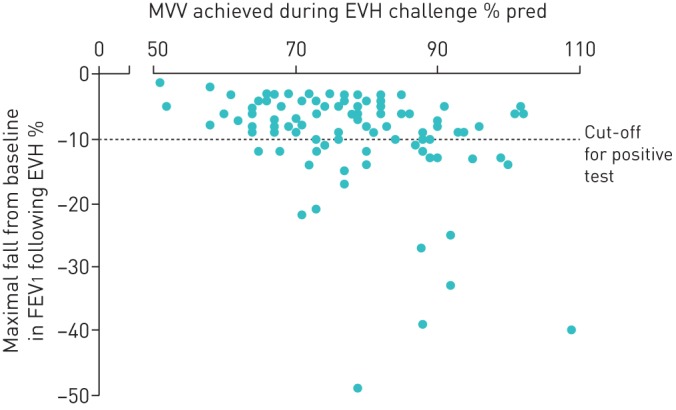
Maximum fall in forced expiratory volume in 1 s (FEV1) following eucapnic voluntary hyperpnoea (EVH) challenge
Relationship between EVH result and prior diagnosis and symptoms
Only 10 (37%) of the EVH+ players reported a history of asthma and/or EIB and four (15%) of those were currently prescribed asthma medication. Although respiratory symptoms were reported frequently, there was no relationship between the presence of symptoms and likelihood of a positive EVH result (table 2). Moreover, the most severe EVH result (49% reduction in FEV1) was observed in a player with no prior history of airways disease or current respiratory symptoms.
TABLE 2.
Respiratory symptoms reported by players
| No symptoms | ≥1 symptom | Coughing | Excessive mucus production | Chest tightness | Difficulty breathing | Wheezing | |
| EVH+ | 19 (70) | 8 (30) | 1 (4) | 1 (4) | 2 (7) | 4 (15) | 1 (4) |
| EVH− | 60 (89) | 8 (11) | 4 (6) | 1 (1) | 2 (3) | 4 (6) | 2 (3) |
| p-value | 0.06 | 0.06 | 1.00 | 0.50 | 0.32 | 0.22 | 1.00 |
Data are presented as n (%), unless otherwise stated. n=95; n=2 did not complete questionnaires. EVH: eucapnic voluntary hyperpnoea.
Part 2: follow-up assessment
After 9 weeks, only 11 of the 27 (41%) EVH+ players attended for the follow-up visit. Reasons for not attending included club not permitting time for the medical team to schedule follow-up, not believing it was necessary, away for international competition, injured, and not wanting to repeat the EVH test. A further four players were excluded from follow-up analysis due to them not taking medication as prescribed.
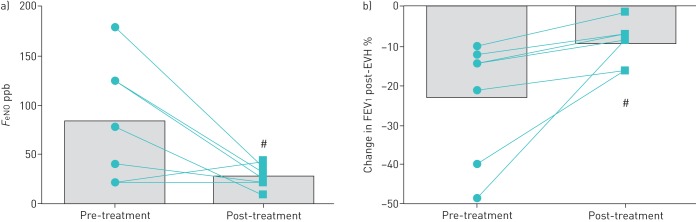
No difference was seen in resting FEV1 between baseline and follow-up visits (table 3); however, FeNO was lower at follow-up (p=0.043) (table 3, figure 2a). In addition, there was a reduction (p=0.02) in the percentage fall in FEV1 following EVH and this was apparent despite players achieving a similar total ventilation during the challenge tests (table 3, figure 2b). Moreover, all but two players had a negative EVH test at the second visit (i.e. fall in FEV1 <10% post-challenge). Despite these findings, two (29%) of the seven players reported an increase in symptoms.
TABLE 3.
Differences before and after treatment in eucapnic voluntary hyperpnoea (EVH)+ players on medication
| Pre-treatment | Post-treatment | p-value | |
| FeNO ppb | 85.1±60.7 | 27.5±10.91 | 0.04 |
| Baseline FEV1 L | 4.41±0.55 | 4.25±0.32 | 0.23 |
| MVV during EVH % predicted | 85.29±13.61 | 87.81±13.58 | 0.38 |
| Fall in FEV1 post-EVH % | 14 (28.0) | 8 (9.0) | 0.02# |
Data are presented as mean±sd or median (interquartile range), unless otherwise stated. Bold type represents statistical significance. FeNO: exhaled nitric oxide; FEV1: forced expiratory volume in 1 s; MVV: maximal voluntary ventilation. #: data analysed using sign exact tests.
FIGURE 2.
a) Exhaled nitric oxide fraction (FeNO) before and after 9 weeks of treatment; b) percentage change in forced expiratory volume in 1 s (FEV1) following eucapnic voluntary hyperpnoea (EVH) challenge before and after 9 weeks of treatment. Data are presented as mean and individual values. #: p<0.05 versus pre-treatment.
Part 3: performance assessment
All treated EVH+ players (n=3) showed an improvement in V′O2peak (pre 50.60±5.65 mL·kg−1·min−1, post 53.98±2.80 mL·kg−1·min−1) (figure 3); however, the magnitude of this change was not significant (p=0.184). Three EVH− players had a decrease in V′O2peak and two demonstrated an improvement (pre 53.06±2.14 mL·kg−1·min−1, post 53.15±2.38 mL·kg−1·min−1). No significant difference was found in the change in V′O2peak between the two groups (p=0.13); EVH+ players had an improvement of 3.38±2.93 mL·kg−1·min−1 and EVH− players improved by 0.09±2.30 mL·kg−1·min−1; however, the effect of treatment on maximal exercise capacity, from magnitude-base inference analysis, was found to be possibly beneficial (74.1%).
FIGURE 3.
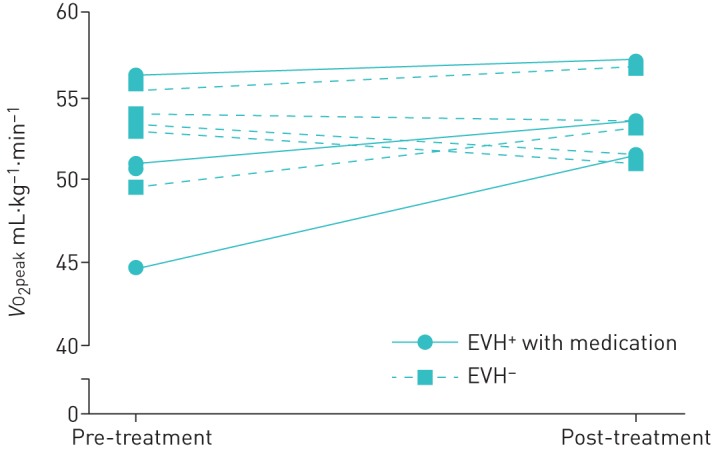
Peak oxygen uptake (V′O2peak) before and following 9 weeks of treatment. EVH: eucapnic voluntary hyperpnoea.
Discussion
In a cohort of elite football players completing a preseason medical screening we found a high prevalence of both respiratory symptoms and airway dysfunction. Indeed, by employing a widely accepted bronchoprovocation methodology [17], namely EVH testing, we found that approximately one-third of this prospectively screened cohort of elite football players had evidence of EIB. Moreover, subsequent treatment with appropriate therapy was associated with a clear attenuation in airway dysfunction and improvement in markers of airway inflammation and hyperreactivity. In addition, all treated players demonstrated an improvement in peak exercise capacity.
The high prevalence of EIB in elite footballers observed in this study concurs with previous smaller-scale studies that have reported a prevalence of between 29% [25] and 51% [9] in this group of athletes. Dickinson et al. [25] included only a small sample of one elite football team (n=21), while Ansley et al. [9] studied a highly selected population of football players using inhaler therapy. Thus, this is the first study to truly assess prevalence in a prospective “medical screening”-type scenario across several elite football teams.
The study findings are consistent with other studies demonstrating a heightened prevalence of airway dysfunction in running-based sports [1]. In addition, they highlight again the poor predictive value of symptoms in the diagnosis of EIB [10, 26]. Indeed “undetected” EIB was found in 63% of players, and no single symptom or combination of symptoms was predictive of the presence of EIB. Accordingly, it is concerning that there appears to be a high proportion of professional footballers currently training and playing with undiagnosed and uncontrolled EIB. Moreover, in keeping with our previous study [9], we found evidence of players with a prior diagnosis of EIB/asthma that was incorrect and not supported by the objective test findings. Indeed 71% of players with a prior diagnosis and treatment plan were either over- or under-medicated.
In addition, it is apparent that respiratory symptoms are not useful in predicting the airway response following treatment [27]. Specifically, we found that despite a reduction in the fall in FEV1 post-EVH challenge, almost one-third of players with EIB reported an increase in symptoms at the follow-up assessment. This is in keeping with the findings of Simpson et al. [28], who reported that half the athletes assessed reported at least one ongoing respiratory symptom despite their fall in FEV1 post-EVH being blunted by the use of inhaled terbutaline. Indeed, in this study 28% had a higher symptom score when this fall was blunted. These findings act to emphasise the poor relationship between the presence of respiratory symptoms and airway dysfunction and highlight our current limited understanding of the pathophysiological mechanisms underpinning airway-centric symptoms in athletes [29]. Furthermore, it serves to promote the importance of other conditions, such as exercise-induced laryngeal obstruction, which may coexist with EIB and cause symptoms [30, 31].
In the current study, we repeated objective testing 9 weeks after EIB therapy was initiated to ensure adequate control of EIB [17]. Data from this follow-up assessment reveals that standard asthma therapy can improve EIB and airway inflammation. Although treatment was recommended for all 27 EVH+ athletes, only seven took the medication as prescribed. This represents an attrition rate of 74%, which suggests a degree of resistance to football players engaging in therapy for this condition. Certainly, anecdotally, EIB often appears to be discounted as medically relevant by medical staff in football squads and viewed as of secondary importance to cardiac screening [32]. Hopefully, our findings challenge this presumption and provide the supporting basis to educate both players and team clinicians regarding the importance of EIB in elite football players. Our work acts to address some of the concerns regarding screening athletes for airway dysfunction [15] and certainly suggests that focus on airway health is important in football players, given the work suggesting that EIB in endurance athletes could be considered akin to an occupational lung disease [33].
A key driving factor in elite sport is the impact of any intervention (e.g. screening or new treatment) on athlete performance, and in addition to improving airway health, treating EIB may be beneficial for performance. Studying performance impact at the elite level is clearly complex, with multiple potential confounding factors and very few footballers completed this component of our study for the above-mentioned reasons, meaning this section of our data is difficult to interpret. Although not statistically significant, EVH+ players had mean increase in V′O2peak of 7.2%, whereas EVH− players had only a 0.24% increase over the same time period, with the observed increases in V′O2peak bringing the performance of the EVH+ players in line with EVH− players. As previously stated, due to the small sample size, these results should be interpreted with caution and future research should aim to follow this up with an adequately powered study. Similar findings have been reported in sports with similar demands. Brukner et al. [6] found that players of Australian Rules football with newly diagnosed EIB had a significant improvement (9%) in the V′O2peak following 6 weeks of treatment compared to controls. In addition, Spiteri et al. [34] demonstrated that appropriately medicating elite rugby players with previously undiagnosed EIB improved their performance in a rugby-specific aerobic exercise challenge by 8% over the course of 12 weeks compared to 6% in the control group; however, this was not a significant finding. It has been hypothesised that this impact on performance may be due in part to bronchoconstriction during and following exercise resulting in reduced alveolar ventilation and efficiency of alveolar-to-arterial blood oxygen exchange [4].
Methodological considerations
A key limitation of this study is that our findings represent the result of a cross-sectional assessment. Specifically, players were not studied in a prospective randomised placebo-controlled fashion and thus our results have to be interpreted in view of their pragmatic limitations. Due to the elite nature of the football players involved, clubs would not allow us to withhold medication from those who needed it, or provide a placebo medication to those who did not. Moreover, players are subject to antidoping regulations and need to know exactly what they are taking at all times. In addition, we recognise that diagnosis of EIB from a one-off test is not entirely robust [35]; however, due to time restraints imposed by the clubs and the large numbers of players to screen, it would not have been practical to perform multiple tests. Our report replicates how a screening programme would work in “real-life”, and is similar to the way football players are currently screened for cardiac conditions.
During the EVH test, the %MVV achieved was significantly lower among EVH− players than among EVH+ players; however, both groups achieved a MVV >60% which is required for an adequate test [36], and we think it is most unlikely that the mean difference of 8% between groups would explain the major findings of this study.
The study was initially powered based on ∼30% of players being found to have evidence of EIB. Initial power calculations showed that 15 EVH+ players were required to detect a 10% change in FEV1 in follow-up assessments. To accommodate for an anticipated high dropout rate in this population, we deliberately over-recruited. However, even with a sample size of 97 players, the high dropout rate meant that our study was under-powered.
As discussed, it was difficult for us to follow-up EVH+ players and clearly it would have been desirable to significantly increase the number of players undergoing repeat assessment, including performance assessment. It may be possible to reduce the attrition rate by working with subelite cohorts or academy players; however, this limits potential generalisation to the elite population. Players and medical staff at clubs are under huge pressures from clubs and it is hard for them to allocate time for follow-up testing. At the follow-up assessment, it became evident that despite support from coaching and medical staff, many players had decided not to take the inhalers, and there was some confusion among these players regarding what they should be taking and when. Miller et al. [37] highlights the importance of educating coaching staff in the management of asthma and this may be an area which needs to be addressed when establishing a screening programme. For a screening programme to be effective in elite-level football, time needs to be invested in educating players about EIB and its treatment. Although there is a lack of evidence in the athletic population, it is clear that educational interventions can have a positive effect on clinical patients’ asthma management [38]. Therefore, it is vital to have the cooperation of players at the start of the treatment.
Conclusion
In conclusion, this study revealed that approximately one-third of a cohort of elite football players appear to have EIB when screened for respiratory dysfunction at a preseason medical assessment, which in the majority of cases was not predictable by symptom-based assessment. Treatment of screen-detected EIB+ players resulted in improved airway inflammation and reduced airway hyperresponsiveness. Overall, the findings support the use of improved diagnostic testing for airway dysfunction in elite football players and highlight the importance of educating both players and football medical staff regarding this condition.
Acknowledgements
The authors are grateful to the football club players and medical staff for participating in and contributing to the study. We also like to thank Irisz Levai, Will Gowers, Tim Wheeler and Sarah Coakley (School of Sport and Exercise Sciences, University of Kent, Chatham, UK) for their assistance with data collection.
Footnotes
Conflict of interest: None declared.
References
- 1.Dickinson J, McConnell A, Whyte G. Diagnosis of exercise-induced bronchoconstriction: eucapnic voluntary hyperpnoea challenges identify previously undiagnosed elite athletes with exercise-induced bronchoconstriction. Br J Sports Med 2011; 45: 1126–1131. [DOI] [PubMed] [Google Scholar]
- 2.Levai IK, Hull JH, Loosemore M, et al. Environmental influence on the prevalence and pattern of airway dysfunction in elite athletes. Respirology 2016; 21: 1391–1396. [DOI] [PubMed] [Google Scholar]
- 3.Becker JM, Rogers J, Rossini G, et al. Asthma deaths during sports: report of a 7-year experience. J Allergy Clin Immunol 2004; 113: 264–267. [DOI] [PubMed] [Google Scholar]
- 4.Haverkamp HC, Dempsey JA, Pegelow DF, et al. Treatment of airway inflammation improves exercise pulmonary gas exchange and performance in asthmatic subjects. J Allergy Clin Immunol 2007; 120: 39–47. [DOI] [PubMed] [Google Scholar]
- 5.Stensrud T, Berntsen S, Carlsen KH. Exercise capacity and exercise-induced bronchoconstriction (EIB) in a cold environment. Respir Med 2007; 101: 1529–1536. [DOI] [PubMed] [Google Scholar]
- 6.Brukner P, Holzer K, Davies L, et al. The impact of exercise-induced bronchoconstriction on exercise performance. Med Sci Sport Exerc 2007; 39: S30. [Google Scholar]
- 7.Bangsbo J. Energy demands in competitive soccer. J Sports Sci 1994; 12: S5–S12. [PubMed] [Google Scholar]
- 8.Read PJ, Oliver JL, De Ste Croix MBA, et al. The scientific foundations and associated injury risks of early soccer specialisation. J Sports Sci 2016; 34: 2295–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ansley L, Kippelen P, Dickinson J, et al. Misdiagnosis of exercise-induced bronchoconstriction in professional soccer players. Allergy 2012; 67: 390–395. [DOI] [PubMed] [Google Scholar]
- 10.Rundell KW, Im J, Mayers LB, et al. Self-reported symptoms and exercise-induced asthma in the elite athlete. Med Sci Sports Exerc 2001; 33: 208–213. [DOI] [PubMed] [Google Scholar]
- 11.UEFA. UEFA Medical Regulations Nyon, UEFA, 2014. www.uefa.com/MultimediaFiles/Download/Tech/uefaorg/General/02/11/35/23/2113523_DOWNLOAD.pdf [Google Scholar]
- 12.Dickinson J, Whyte G, McConnell A, et al. Impact of changes in the IOC-MC asthma criteria: a British perspective. Thorax 2005; 60: 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holzer K, Brukner P. Screening of athletes for exercise-induced bronchoconstriction. Clin J Sport Med 2004; 14: 134–138. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JMG, Jungner G. Principles and Practice of Screening for Disease Geneva, World Health Organization, 1968. http://apps.who.int/iris/bitstream/10665/37650/17/WHO_PHP_34.pdf [Google Scholar]
- 15.Hull JHK, Ansley L, Garrod R, et al. Exercise-induced bronchoconstriction in athletes – should we screen? Med Sci Sports Exerc 2007; 39: 2117–2124. [DOI] [PubMed] [Google Scholar]
- 16.Hull JH, Ansley L, Price OJ, et al. Eucapnic voluntary hyperpnea: gold standard for diagnosing exercise-induced bronchoconstriction in athletes? Sport Med 2016; 46: 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiler JM, Brannan JD, Randolph CC, et al. Exercise-induced bronchoconstriction update – 2016. J Allergy Clin Immunol 2016; 138: 1292–1295. [DOI] [PubMed] [Google Scholar]
- 18.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels ( FeNO) for clinical applications. Am J Respir Crit Care Med 2011; 184: 602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 20.Argyros G, Roach J, Hurwitz K, et al. Eucapnic voluntary hyperventilation as a bronchoprovocation technique: development of a standardized dosing schedule in asthmatics. Chest 1996; 109: 1520–1524. [DOI] [PubMed] [Google Scholar]
- 21.Anderson SD, Kippelen P. Assessment of EIB: what you need to know to optimize test results. Immunol Allergy Clin North Am 2013; 33: 363–380. [DOI] [PubMed] [Google Scholar]
- 22.Parsons JP, Hallstrand TS, Mastronarde JG, et al. An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med 2013; 187: 1016–1027. [DOI] [PubMed] [Google Scholar]
- 23.van der Palen J, Klein JJ, Kerkhoff AH, et al. Evaluation of the effectiveness of four different inhalers in patients with chronic obstructive pulmonary disease. Thorax 1995; 50: 1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopkins WG. A spreadsheet for deriving a confidence interval, mechanistic inference and clinical inference from a p value. Sportscience 2007; 11: 16–20. [Google Scholar]
- 25.Dickinson J, Drust B, Whyte G, et al. Screening English Premier League football players for exercise-induced bronchoconstriction In: Hiroyuki N, Drust B, Dawson B, eds. Science and Football VII: the Proceedings of the Seventh World Congress on Science and Football. Abingdon: Routledge, 2013; pp. 341–346. [Google Scholar]
- 26.Turcotte H, Langdeau JB, Thibault G, et al. Prevalence of respiratory symptoms in an athlete population. Respir Med 2003; 97: 955–963. [DOI] [PubMed] [Google Scholar]
- 27.Brannan JD, Koskela H, Anderson SD. Monitoring asthma therapy using indirect bronchial provocation tests. Clin Respir J 2007; 1: 3–15. [DOI] [PubMed] [Google Scholar]
- 28.Simpson AJ, Romer LM, Kippelen P. Self-reported symptoms after induced and inhibited bronchoconstriction in athletes. Med Sci Sports Exerc 2015; 47: 2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hull JH, Dickinson JW, Jackson AR. Cough in exercise and athletes. Pulm Pharmacol Ther 2017; 49–55. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen EW, Hull JH, Backer V. High prevalence of exercise-induced laryngeal obstruction in athletes. Med Sci Sports Exerc 2013; 45: 2030–2035. [DOI] [PubMed] [Google Scholar]
- 31.Hall A, Thomas M, Sandhu G, et al. Exercise-induced laryngeal obstruction: a common and overlooked cause of exertional breathlessness. Br J Gen Pract 2016; 66: e683–e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hull JH, Rawlins JC. Screening for cardiac and respiratory problems in elite sport – compare and contrast. Expert Rev Respir Med 2016; 10: 715–717. [DOI] [PubMed] [Google Scholar]
- 33.Price OJ, Ansley L, Menzies-Gow A, et al. Airway dysfunction in elite athletes – an occupational lung disease? Allergy 2013; 68: 1343–1352. [DOI] [PubMed] [Google Scholar]
- 34.Spiteri DB, Dickinson JW, Greenwell J, et al. Impact of exercise-induced bronchoconstriction on athletic performance and airway health in rugby union players. Int Sport J 2014; 15: 333–342. [Google Scholar]
- 35.Price OJ, Ansley L, Hull JH. Diagnosing exercise-induced bronchoconstriction with eucapnic voluntary hyperpnea: is one test enough? J Allergy Clin Immunol Pract 2015; 3: 243–249. [DOI] [PubMed] [Google Scholar]
- 36.Anderson SD, Argyros GJ, Magnussen H, et al. Provocation by eucapnic voluntary hyperpnoea to identify exercise induced bronchoconstriction. Br J Sports Med 2001; 35: 344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller MG, Weiler JM, Baker R, et al. National Athletic Trainers’ Association position statement: management of asthma in athletes. J Athl Train 2005; 40: 224–245. [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson PG, Powell H, Coughlan J, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2003; 1: CD001117. [DOI] [PubMed] [Google Scholar]