Abstract
Background: To optimize the success of insecticide-based malaria control intervention, knowledge of the distribution of Anopheles gambiae species and insecticide resistance mechanisms is necessary. This paper reported an updated data on pyrethroids/DDT resistance in the An. gambiae s.l population from Togo.
Methods: From December 2013 to April 2015, females of indoor-resting An. gambiae s.l were captured in three locations belonging to three different ecological zones. Resistance to DDT, permethrin and deltamethrin was screened in F1 progeny of collected mosquitoes using WHO susceptibility tests. The identification of species of An. gambiae complex and the detection of kdr and ace.1 R allele were carried out using DNA-based molecular techniques.
Results: An. gambiae from Kovié and Nangbéto were highly resistant to DDT and permethrin with mortalities rate ranging from 0.83% to 1.58% for DDT and zero to 8.54% for permethrin. Mosquitoes collected in Nangbéto displayed 81.53% mortality with deltamethrin. An. coluzzii and An. gambiae s.s were found in sympatry in Nangbéto and Mango . The allelic frequency of L1014F was high, ranging from 66 to 100% in both An. coluzzii and An. gambiae s.s. For the first time we detected the L1014S allele in both An. coluzzii and An. gambiae s.s. from Togo at the frequency ranging from 5% to 13% in all the sites. The kdr N1575Y was present at various frequencies in both species ranging from 10% to 45%. Both An. gambiae s.s. and An. coluzzii shared the ace1 R mutation in all investigated sites with allelic frequency ranging from 4% to 16%.
Conclusion: These results showed that multiple mutations are involved in insecticides resistance in An. gambiae populations from Togo including the kdr L1014F, L1014S, and N1575Y and ace.1 R G119S mutations.
Keywords: Insecticides resistance, Anopheles gambiae s.s., Anopheles coluzzii, kdr mutation, Ace.1R mutation, Togo.
Introduction
Despite a reported decline in infection and mortality, malaria remains the fourth leading cause mortality in children under-five in the sub-region 1. In Togo, the malaria control strategy is based on universal access to Long Lasting Insecticidal Nets (LLINs) as recommended by the World Health Organization (WHO). However, malaria vectors were found resistant to all insecticide classes used in public health interventions in West Africa. Mosquito resistance to insecticides stands as a serious obstacle to the effectiveness of LLINs. Since 2010, reports of pyrethroids and dichlorodiphenyltrichloroethane (DDT) resistance have been widespread 2– 6 with further reports of carbamate resistance 7– 9. The resistance to pyrethroids/DDT is conferred by two main physiological mechanisms including metabolic resistance and the target site insensitivity 10. The target site resistance remains the most studied to date, a result of the easier means of its assessment 11. In Anopheles gambiae complex, kdr gene mutations, including the substitutions of leucine to phenylalanine (L1014F) and leucine to serine (L1014S), are the two mutations involved in the target site resistance 12, 13. These two target site mutations are largely distributed across the African Continent, yet differences in the allelic frequency have been reported between An. gambiae s.l. species and between breeding sites 3, 14, 15. In several studies, clear associations has been shown between DDT/pyrethroids resistance and the presence of kdr mutations 16. Recently, a new mutation named N1575Y has emerged within the linker between domains III-IV of the voltage gate sodium channel (VGSC) in An. gambiae s.s., An coluzzii and An. arabiensis 17. Studies have demonstrated the appearance of the N1575Y mutation as an additional resistance mechanism that appears with the L1014F kdr mutation. N1575Y mutation is therefore being suggested as a secondary selective sweep, associated with resistance to pyrethroids/DDT in the West African region 17, 18. The development of new resistance mechanisms among Anopheles populations highlights a failure in pyrethroids-based control strategies, and could jeopardize the mosquito control efforts 19. It is therefore seminal to establish the epidemiological consequences of pyrethroids resistance, and develop new intervention strategies for the management of insecticides resistance in West Africa.
Monitoring the molecular and physiological markers of pyrethroids resistance has significant advantages for the management of insecticides resistance. In Togo, despite the huge investments in LLINs, information is still lacking on the routine monitoring of insecticides resistance in malaria vectors. An entomological survey was carried out since 2009 in two localities of the Southern coastal region (Lomé and Kovié) and revealed the involvement of kdr L1014F in insecticides resistance 20. Additional knowledge on the mechanisms involved in insecticides resistance is needed across the country, as this is seminal to design new strategies for insecticides resistance management. We therefore attempted in this study to investigate the distribution of the kdr L1014F, L1014S and N1575Y alleles and ace.1 R mutation in three localities across the country using WHO bioassays and advanced molecular methods.
Methods
Study sites
The study was carried out in three rural sites from different ecological zones of Togo ( Figure 1, Table 1). Togo is a coastal country located in West Africa, with a population of ~7 million inhabitants. The country has two tropical climates: the Sudanian in the North, characterized by one rainy season and one dry season; and the subequatorial in the South, characterized by two dry seasons (from December to March and from August to September) and two rainy seasons (from April to July and from October to November). According to Ahadji-Dabla et al. 21, the Republic of Togo covers five ecological zones: the plains zone in the North (zone I), the mountains zone in the North (zone II), the Central plains zone (zone III), the Mounts Togo meridional zone (zone IV), and the Southern coastal zone (zone V). Table 1 describes the characteristics and geographic coordinates (GPS) of the investigated sites.
Figure 1. Map of Togo showing the study sites.
Table 1. Characteristics of study sites.
| Localities | Main agriculture
practices |
Ecological
zones |
Periods of
collection |
Geographic coordinates | |
|---|---|---|---|---|---|
| Kovié | Rice, vegetables | (Coastal) | December 2013 | N 06°20.305' | 001°07.425' E |
| Nangbéto | Cereals, tubers | (Forest) | April 2015 | N 07°25.802' | 001°26.822' E |
| Mango | Cereals, cotton | (Savannah) | April 2015 | N 10°21’17.9’’ | 0°28’21.7’’E |
Mosquito collection and rearing
Mosquito sampling was conducted from December 2013 to April 2015 using electric aspirators. In each study sites, the households were randomly selected for mosquito aspirations. Verbal and written consents of the household heads were sought prior to insect collection in their houses. Indoor resting blood fed adult female Anopheles mosquitoes (F0), were captured between 06.00 and 10.00 am, kept in cool boxes and brought to the insectary of the AgroEcoHealth Platform of the International Institute of Tropical Agriculture (IITA-Benin). A forced-egg laying method was used to induce the females to lay eggs as previously described 22. The egg batches were then allowed to hatch in a small paper cup and later transferred to larvae bowls for rearing as previously described 22, 23.
Insecticide susceptibility test
F1 female progeny of wild An. gambiae and laboratory susceptible Kisumu strain aged 3–5 days were exposed to impregnated papers at diagnostic concentrations of insecticides according to WHO protocol 24. Insecticide papers were obtained from the WHO reference centre at the Vector Control Research Unit, University Sains Malaysia. The impregnated papers included 4% DDT, 0.75% permethrin and 0.05% deltamethrin. Briefly, for each tested insecticide, batches of 20–25 unfed females were exposed to an impregnated paper for 60 min, after which they were transferred into tubes containing untreated papers and placed under observation at 25°C and 80% relative humidity (RH) with 10% sugar solution. Mortality rate was recorded 24h post-exposure. Tube tests containing untreated papers were run in parallel as a control.
Species identification, kdr L1014F, L1014S, N1575Y and ace.1 R genotyping
Genomic DNA from respectively 88, 70 and 58 females (F0) collected in Kovié, Nangbéto and Mango of bioassay control was individually extracted using the Livak DNA extraction method 25. The species of An. gambiae mosquitoes were identified using polymerase chain reaction techniques. The kdr mutations L1014F, L1014S and N1575Y were screened using TaqMan real time PCR assays as previously described 17, 26. The presence of G119S-Ace1 allele was also screened in An. gambiae populations as describe by Bass et al. 27.
Statistical analysis
The resistance profile of An. gambiae s.l. was determined using WHO criteria 28:
Mortality rate > 98% = susceptible mosquito population
Mortality rate between 90–98% = suspected resistance in the mosquito population
Mortality rate < 90% = resistant mosquito population
According to Hardy-Weinberg equilibrium using the Had2know online statistical software, calculated genotype frequencies of L1014F, L1014S, N1575Y and G119S were confirmed and compared between An. coluzzii and An. gambiae s.s. with Chi-square test.
Results
Species composition of An. gambiae s.l.
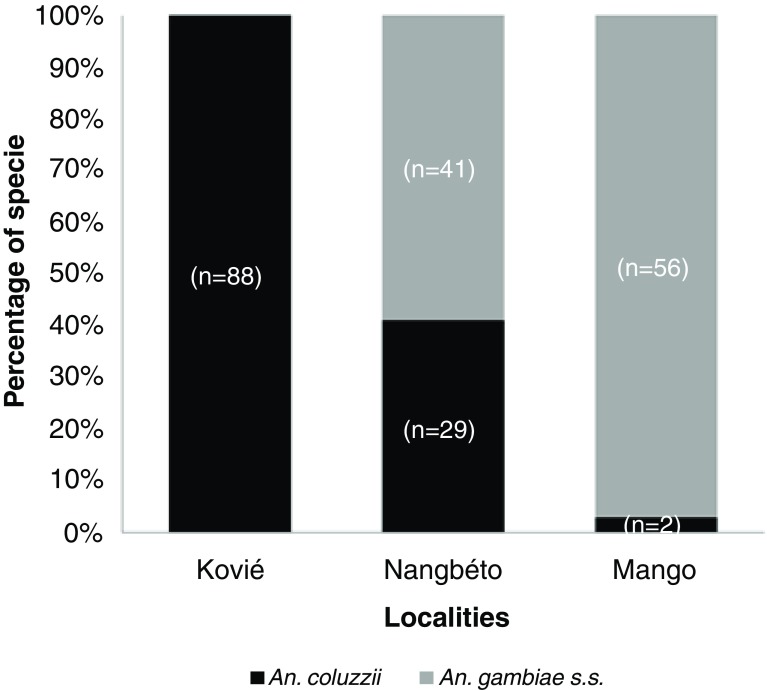
The PCR analysis on the F0 females for identification of sibling species among An. gambiae complex revealed the presence of only two species in the study sites as An. coluzzii and An. gambiae s.s.. Both An. gambiae s.s. and An. coluzzii were found in sympatry in Nangbéto (59% versus 41%) and Mango (97% versus 3%). An. coluzzii was predominant (100%) in Kovié (rice field) ( Figure 2).
Figure 2. Species composition in study sites.
Insecticide resistance status
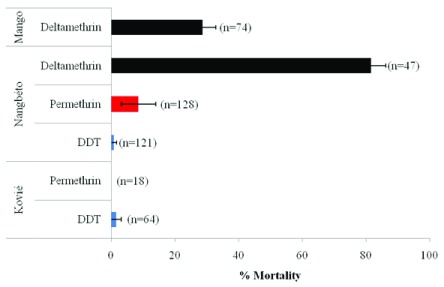
Figure 3 describes the insecticide resistance profile of three An. gambiae s.l populations collected in Togo. The laboratory strain Kisumu exhibited very high susceptibility to the insecticides tested: 99% mortality to 100% mortality to the organochlorine and permethrin respectively, and 99.5% mortality to deltamethrin. In control groups (untreated papers) the mortality rates recorded with the wild An. gambiae populations were below 5% at 24 hours post-exposure.
Figure 3. Insecticide resistance profiles of An. gambiae s.l. in Kovié, Nangbéto and Mango (Error bars are standard error).
An. gambiae population from Kovié and Nangbéto were highly resistant to DDT and permethrin with mortality rates ranging from 0.83% to 1.58% for DDT and from zero to 8.54% for permethrin. Mosquitoes collected in Mango and Nangbéto displayed high resistance to deltamethrin with mortality rates of 28.67% and 81.53% for permethrin and deltamethrin respectively.
Detection of resistance genes
Mosquitoes from each study site were used for kdr and ace.1 R screening. Table 2 presents the distribution of knock-down (L1014F, L1014S and N1575Y) and ace.1 R (G119S) mutations in An. gambiae s.s. and An. coluzzii. The L1014F kdr mutation was found at various allelic frequencies in An. gambiae s.s. and An. coluzzii in the three sites. The 1014F allelic frequency was high in both species ranging from 66% to 100% in An. coluzzii and from 80% to 83.96% in An. gambiae s.s. The 1014S kdr allele was found for the first time in Anopheles mosquitoes from Togo (Kovié and Nangbéto). The allelic frequency of this kdr mutation ranged from 5.17 to 13.46% in both An. gambiae s.s and An. coluzzii. The kdr N1575Y were also detected in the two species with the allelic frequencies ranging from 10.34 to 45.06% in all the sites investigated. ace.1 R mutation was mainly found in An. gambiae s.s with allelic frequency ranging from 4.87 to 16.66%.
Table 2. Resistant allele frequencies in Anopheles coluzzii and Anopheles gambiae s.s in study sites.
| Localities | Species | N | f (1014F) | N | f (1014S) | N | f (1575Y) | N | f (119S) |
|---|---|---|---|---|---|---|---|---|---|
| Kovié | An. coluzzii | 79 | 67.72%±10.31 | 87 | 5.17%±4.65 | 81 | 45.06%±10.84 | 63 | 16.66%±10.76 |
| Nangbéto | 27 | 66.67%±17.78 | 26 | 13.46%±7.56 | 29 | 10.34%±7.76 | 28 | - | |
| Mango | 2 | 100% | 2 | - | 2 | - | 2 | - | |
| Nangbéto | An. gambiae s.s | 40 | 80%±12.4 | 31 | 6.45%±4.38 | 41 | 17.07%±11.66 | 41 | 4.87%±2.67 |
| Mango | 53 | 83.96%±9.88 | 53 | - | 52 | 11.53%±8.6 | 49 | 7.14%±6.93 |
NB: Allelic frequencies (f) are given in means ± Standard deviation (SD)
Discussion
This study provides an update on current levels of resistance to permethrin and deltamethrin and frequencies of the kdr and ace.1 R mutation in An. coluzzii and An. gambiae s.s in rural areas of Togo.
An. coluzzii and An. gambiae s.s. were the only Anopheles species observed in this study and lived in sympatry at varying frequencies. An. gambiae s.s was the most abundant species at the two cereal cultivation sites of Mango and Nangbéto whereas An. coluzzii is far more predominant in the rice field of Kovié, supporting findings from previous research in Togo and Benin 3, 29. This heterogeneous composition of An. gambiae population observed in these localities could be due to a competitive exclusion between the two subspecies 30. This study provides updated information on the insecticide resistance profile of Anopheles gambiae and the underlying mechanisms involved in rural areas of Togo.
It also provides baseline information on the susceptibility/resistance status of An. coluzzii and An. gambiae s.s in this location to permethrin and deltamethrin, the insecticides used to impregnate the bed nets freely distributed by NMCP of Togo. The WHO bioassay results indicated a high prevalence of resistance to pyrethroids/DDT in all study sites. This suggests that similar selection pressures are acting on these populations. Recently Ahadji-Dabla et al. 20 reported similar observation of resistance in An. gambiae s.l populations to DDT, deltamethrin and permethrin in Lomé. The proportion of An. gambiae surviving to permethrin exposure has increased slightly in Kovié compared with that in previous studies conducted in the same area: In 2009, Ahadji-Dabla et al. 21 reported mortality of 56%, while any mortality was recorded in this study. This observation suggests that pyrethroids resistance had significantly increased in this area over the last five years. It probably indicates that the selection pressure is not high but could change with the intensive and uncontrolled used of chemical and fertilizers in rice production and the mass-distributed of LLINs in this area. Moreover, the implantation of uncontrolled insecticides selling markets in the country especially around rice cultivation areas, contributed to the increase in the resistance 20. This can therefore explain the high frequencies of kdr resistant alleles found in our study areas. The knock-down resistance gene was the main resistance mechanism found in all assessed mosquito populations. The 1575Y allele was found in both An. coluzzii and An. gambiae s.s in all the study sites. The frequencies of this mutation were similar to those found in the nearby countries like Benin 18 and Burkina-Faso 17. This allele was highly distributed in An. coluzzii in Kovié (0.45) as previously reported in the rice fields in Northern Benin 18. However, the prevalence of this mutation has rapidly increased in the West African region suggesting an ongoing strong selection of the L1014F-N1575Y haplotype in this region. The kdr (L1014F) mutation was found at higher frequencies in An. gambiae s.s than An. coluzzii at all sites. These results confirm those of Dabiré et al. 31 who found that An. gambiae s.s showed the highest levels of resistance than An. coluzzii.
This kdr mutation could be responsible for the high resistance observed to permethrin and deltamethrin in An. gambiae population from Togo. Hence, this is a serious problem for malaria control programs because the currently widespread distributed nets are impregnated by these insecticides. Fortunately, a recent paper published in Benin revealed that insecticide-treated nets provide protection against malaria to children in an area of insecticide resistance in southern Benin 32.
To our knowledge, this study is the first reporting the presence of the L1014S kdr mutation in wild An. gambiae s.l populations from Togo. The L1014S allele was detected in both An. coluzzii and An. gambiae s.s. This allele, originating from East Africa, was recently reported in Benin in An. arabiensis 3 and in An. coluzzii and An. gambiae s.s in Burkina-Faso 33. It is possible that An. gambiae populations carrying the kdr L1014S mutation might have migrated, through active or/and passive ways, from bordering countries (e.g. Benin, Burkina-Faso) due to intense traffic and exchanges between these countries and Togo.
These findings therefore provide strong evidences on the increasing distribution of the kdr mutations among Anopheles mosquitoes across Africa, and could be used as baseline data for proper monitoring of this allele in West African countries. Further research should be implemented to provide knowledge on the geographical distribution of L1014S kdr allele in West Africa, its role in pyrethroids phenotypic resistance, as well as its impact on the efficacy of pyrethroids treated nets.
In the present study, the G119S mutation was identified in all investigated sites at a relatively low frequency. This is in contrast to previous findings that reported a high frequency of ace-1 R mutation in An. gambiae s.l from Lomé 34. This resistant allele was detected in both species with frequencies ranging from 4.8% to 16.66%. The presence of ace-1 R mutations in An. coluzzii and An. gambiae s.s. has already been reported by Weill et al. 35 and Djogbenou et al. 36. The incidence of the G119S mutation in the An. gambiae s.l population from Togo suggests a probable resistance to carbamates and organophosphates insecticides. However, this assertion needs to be proved by WHO toxicological tests 28. We cannot therefore exclude the possibility that besides the four mutations targeted in these study sites, other enzymes and genetic mechanisms could be contributed to the resistance, as suggested by previous studies in Benin 18, 37.
Conclusion
The present study revealed the widespread of kdr 1014F, 1014S and 1575Y as well as the G119 alleles in Togo. For the first time, this gives the evidence of the presence of 1014S kdr allele in wild populations of An. gambiae s.l from Togo where entomological surveys are scanty. Hence confirming the expansion of pyrethroids resistance alleles in Africa. There is therefore a need for regular updating on the current entomological data for appropriate decision making and proper intervention strategies for malaria vector control in this country.
Ethics statement and consent
No ethical clearance was required for this study according to the International Institute of Tropical Agriculture (IITA) Ethical Committee (IITA, 08 P.O. Box 0932, Tri-Postal, Cotonou, Benin). However, consent of the community leaders was sought prior to mosquito larva and adult collections in the community. We explained our study to the communities and household heads. Verbal and written consents of household heads were therefore obtained prior for mosquito collection
Data availability
All data generated and analyzed during this study is included in the published article. Raw data are available from Open Science Framework: Dataset 1. First report of the presence of L1014S Knockdown-resistance mutation in Anopheles gambiae s.s and Anopheles coluzzii from Togo, West Africa, http://doi.org/10.17605/OSF.IO/M3G4P 38
Acknowledgements
We thank all surveyed communities for their cooperation and assistance during the field work. We also appreciate Murielle Soglo for her technical assistance.
Funding Statement
Grant information: This work is supported by the Welcome Trust [099864], [101893].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved, 1 approved with reservations]
References
- 1. World Health Organization: Regional profiles - World Malaria Report 2015.2015;122 Reference Source [Google Scholar]
- 2. Djogbénou L, Pasteur N, Akogbéto M, et al. : Insecticide resistance in the Anopheles gambiae complex in Benin: a nationwide survey. Med Vet Entomol. 2011;25(3):256–67. 10.1111/j.1365-2915.2010.00925.x [DOI] [PubMed] [Google Scholar]
- 3. Djègbè I, Boussari O, Sidick A, et al. : Dynamics of insecticide resistance in malaria vectors in Benin: first evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malar J. 2011;10(1):261. 10.1186/1475-2875-10-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Namountougou M, Simard F, Baldet T, et al. : Multiple Insecticide Resistance in Anopheles gambiae s.l. Populations from Burkina Faso, West Africa. PLoS One. 2012a;7(11):e48412. 10.1371/journal.pone.0048412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chabi J, Baidoo PK, Datsomor AK, et al. : Insecticide susceptibility of natural populations of Anopheles coluzzii and Anopheles gambiae ( sensu stricto) from Okyereko irrigation site, Ghana, West Africa. Parasit Vectors. 2016;9(1):182. 10.1186/s13071-016-1462-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dery DB, Segbaya S, Asoalla V, et al. : Anopheles gambiae (Diptera: Culicidae) Susceptibility to Insecticides and Knockdown Resistance Genes Prior to Introduction of Indoor Residual Spraying in 11 Districts in Ghana. J Med Entomol. 2016;53(6):1403–1409. 10.1093/jme/tjw098 [DOI] [PubMed] [Google Scholar]
- 7. Djogbenou L, Dabiré R, Diabaté A, et al. : Identification and Geographic Distribution of the ACE-1R Mutation in the Malaria Vector Anopheles gambiae in South-Western Burkina Faso, West Africa. Am J Trop Med Hyg. 2008a;78(2):298–302. [PubMed] [Google Scholar]
- 8. Aïkpon R, Agossa F, Ossè R, et al. : Bendiocarb resistance in Anopheles gambiae s.l. populations from Atacora department in Benin, West Africa: a threat for malaria vector control. Parasit Vectors. 2013;6:192. 10.1186/1756-3305-6-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. N'guessan R, Darriet F, Guillet P, et al. : Resistance to carbosulfan in Anopheles gambiae from Ivory Coast, based on reduced sensitivity of acetylcholinesterase. Med Vet Entomol. 2003;17(1):19–25. 10.1046/j.1365-2915.2003.00406.x [DOI] [PubMed] [Google Scholar]
- 10. Hemingway J, Hawkes NJ, McCarroll L, et al. : The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34(7):653–65. 10.1016/j.ibmb.2004.03.018 [DOI] [PubMed] [Google Scholar]
- 11. Lynd A, Ranson H, McCall PJ, et al. : A simplified high-throughput method for pyrethroid knock-down resistance (kdr) detection in Anopheles gambiae. Malar J. 2005;4(1):16. 10.1186/1475-2875-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinez-Torres D, Chandre F, Williamson MS, et al. : Molecular characterization of pyrethroid knockdown resistance ( kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7(2):179–84. 10.1046/j.1365-2583.1998.72062.x [DOI] [PubMed] [Google Scholar]
- 13. Ranson H, Jensen B, Vulule JM, et al. : Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9(5):491–7. 10.1046/j.1365-2583.2000.00209.x [DOI] [PubMed] [Google Scholar]
- 14. Santolamazza F, Calzetta M, Etang J, et al. : Distribution of knock-down resistance mutations in Anopheles gambiae molecular forms in west and west-central Africa. Malar J. 2008;7(1):74. 10.1186/1475-2875-7-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ranson H, Abdallah H, Badolo A, et al. : Insecticide resistance in Anopheles gambiae: Data from the first year of a multi-country study highlight the extent of the problem. Malar J. 2009;8(1):299. 10.1186/1475-2875-8-299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donnelly MJ, Corbel V, Weetman D, et al. : Does kdr genotype predict insecticide-resistance phenotype in mosquitoes? Trends Parasitol. 2009;25(5):213–219. 10.1016/j.pt.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 17. Jones CM, Liyanapathirana M, Agossa FR, et al. : Footprints of positive selection associated with a mutation ( N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc Natl Acad Sci. 2012;109(17):6614–6619. 10.1073/pnas.1201475109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Djègbè I, Agossa FR, Jones CM, et al. : Molecular characterization of DDT resistance in Anopheles gambiae from Benin. Parasit Vectors. 2014;7(1):409. 10.1186/1756-3305-7-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization: Countries and territories affected by malaria in 2010.2012. [Google Scholar]
- 20. Ahadji-dabla KM, Ketoh GK, Nyamador WS, et al. : Susceptibility to DDT and pyrethroids, and detection of knockdown resistance mutation in Anopheles gambiae sensu lato in Southern Togo.2014;8:314–323. 10.4314/ijbcs.v8i1.27 [DOI] [Google Scholar]
- 21. Ahadji-dabla KM, Nyamador WS, Amoudji AD, et al. : Susceptibility of a malaria vector Anopheles gambiae s . l (Diptera: Culicidae) to WHO recommended insecticides in Togo (West Africa).2015;3(6):75–79. Reference Source [Google Scholar]
- 22. Morgan JC, Irving H, Okedi LM, et al. : Pyrethroid resistance in an Anopheles funestus population from uganda. PLoS One. 2010;5(7):e11872. 10.1371/journal.pone.0011872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cuamba N, Morgan JC, Irving H, et al. : High level of pyrethroid resistance in an Anopheles funestus population of the chokwe district in mozambique. PLoS One. 2010;5(6):e11010. 10.1371/journal.pone.0011010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization: Tests procedures for insecticide resistance monitoring in malaria vector, bio-efficacy and persistence of insecticides on treated surfaces. WHO/CDS/CPC/MAL/98. 1998;12:43 Reference Source [Google Scholar]
- 25. Livak KJ: Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics. 1984;107(4):611–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bass C, Nikou D, Donnelly MJ, et al. : Detection of knockdown resistance ( kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;6(1):111. 10.1186/1475-2875-6-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bass C, Nikou D, Vontas J, et al. : Development of high-throughput real-time PCR assays for the identification of insensitive acetylcholinesterase (ace–1R) in Anopheles gambiae. Pesticide Biochemistry and Physiology. 2010;96(2):80–85. [Google Scholar]
- 28. WHO (World Health Organization): Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. World Heal Organ Tech Rep Ser. 2013. Reference Source [Google Scholar]
- 29. Ngufor C, Fagbohoun J, Critchley J, et al. : Which intervention is better for malaria vector control: insecticide mixture long-lasting insecticidal nets or standard pyrethroid nets combined with indoor residual spraying? Malar J. 2017;1–9. 10.1186/s12936-017-1987-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wondji C, Frédéric S, Petrarca V, et al. : Species and Populations of the Anopheles gambiae Complex in Cameroon with Special Emphasis on Chromosomal and Molecular Forms of Anopheles gambiae s.s. J Med Entomol. 2005;42(6):998–1005. [DOI] [PubMed] [Google Scholar]
- 31. Dabiré RK, Fagbohoun J, Critchley J, et al. : Population dynamics of Anopheles gambiae s.l. in Bobo-Dioulasso city: bionomics, infection rate and susceptibility to insecticides. Parasit Vectors. 2012;5(1):127. 10.1186/1756-3305-5-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bradley J, Ogouyèmi-Hounto A, Cornélie S, et al. : Insecticide-treated nets provide protection against malaria to children in an area of insecticide resistance in Southern Benin. Malar J. 2017;16(1):225. 10.1186/s12936-017-1873-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Namountougou M, Diabaté A, Etang J, et al. : First report of the L1014S kdr mutation in wild populations of Anopheles gambiae M and S molecular forms in Burkina Faso (West Africa). Acta Trop. 2012b;125(2):123–127. 10.1016/j.actatropica.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 34. Ahadji-dabla KM, Amoudji MD, Dery DB, et al. : Original article Susceptibility to carbamate and organophosphate, and ace 1 allele in Anopheles gambiae s . l. from pyrethroid resistance areas in the city of Lomé , Togo, West Africa.2017;6(2):5843–5847. [Google Scholar]
- 35. Weill M, Malcolm C, Chandre F, et al. : The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol. 2004;13(1):1–7. 10.1111/j.1365-2583.2004.00452.x [DOI] [PubMed] [Google Scholar]
- 36. Djogbénou L, Chandre F, Berthomieu A, et al. : Evidence of introgression of the ace-1 R mutation and of the ace-1 duplication in West African Anopheles gambiae s. s. PLoS One. 2008;3(5):e2172. 10.1371/journal.pone.0002172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Djouaka R, Riveron JM, Yessoufou A, et al. : Multiple insecticide resistance in an infected population of the malaria vector Anopheles funestus in Benin. Parasit Vectors. 2016;9(1):453. 10.1186/s13071-016-1723-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Djouaka R, Akoton R, Tchigossou GM, et al. : Mapping of the Distribution, Plasmodium Infection Rate and Insecticide Susceptibility of Anopheles Funestus in Benin. Open Science Framework.Web.2016. 10.17605/OSF.IO/Y3B8P [DOI] [PMC free article] [PubMed] [Google Scholar]