Abstract
Nivolumab for the treatment of advanced nonsmall cell lung cancer (NSCLC) evaluated in phase III trials showed 50% progression at first evaluation, but better overall survival (OS), suggesting regained efficacy of treatments given thereafter. We aimed to evaluate the efficacy of nivolumab and of next treatment received after nivolumab progression in patients with advanced NSCLC.
Our multicentre retrospective study included all patients receiving nivolumab between January and December 2015. The primary end-point was progression-free survival (PFS) of treatment given after nivolumab.
The 303 patients had the following characteristics: median age 63 years, 69% males, 92% smokers, 67% performance status 0–1 and 61% adenocarcinoma. Nivolumab was given as second-line treatment in 40% of patients. With 13.7 months of median follow-up, nivolumab PFS and OS were 2.6 and 11.3 months, respectively. At the cut-off analysis 18% were controlled under nivolumab, 14% were deceased and 5% were lost to follow-up under nivolumab. Among the 191 (63%) patients eligible for post-nivolumab (PN) treatment, 115 (38%) received further treatment and were characterised by better performance status (p=0.028) and by receiving more injections of nivolumab (p=0.001). Global PN-OS and PN-PFS were 5.2 and 2.8 months, respectively. Drugs most frequently used after nivolumab were gemcitabine (23%), docetaxel (22%) and erlotinib (16%), with median PFS of 2.8, 2.7 and 2.0 months, respectively.
Nivolumab produced similar efficacy as in phase III trials, although patients received nivolumab later and had worse performance status. 38% received treatment after nivolumab progression with efficacy comparable to historical second-line trials.
Short abstract
Efficacy of nivolumab in nonsmall cell lung cancer http://ow.ly/k2uX30iGZbY
Introduction
Nivolumab is a fully human IgG4 programmed death (PD)-1 immune checkpoint inhibitor (ICI) antibody that disrupts PD-1 mediated signalling. Nivolumab is authorised in the second-line setting for the treatment of advanced nonsmall cell lung cancer (NSCLC) for both squamous and nonsquamous histology in patients having progressed during or after a first-line of treatment with a platinum-based doublet chemotherapy regimen. In the two pivotal CheckMate trials [1, 2] comparing nivolumab to docetaxel in the second-line setting, nivolumab was found to bring longer overall survival (OS) in both squamous and nonsquamous advanced NSCLC. However, longer progression-free survival (PFS) was only found in squamous cell carcinoma and 50% of patients presented with progression at first evaluation under nivolumab. These results suggest that the prolonged OS observed after nivolumab exposure could be due to a better efficacy of treatments received after nivolumab progression in the overall population. In this context, efficacy of the next chemotherapy received after other ICIs has been reported. In the OAK phase 3 trial [3], NSCLC patients treated with atezolizumab in the second- or third-line setting showed a median OS of 8.8 months (CI 95% 6.0–12.1 months) in those receiving chemotherapy beyond disease progression. The 18-month survival rate was 20% and 9%, respectively, in patients who did or did not receive chemotherapy after progression. Similarly, an update of the KEYNOTE 024 study [4] evaluating the efficacy of pembrolizumab compared to chemotherapy in PD ligand (PD-L)1-positive patients (PD-L1 expression on tumour cells determined by immunohistochemistry >50%) in the first-line setting showed that PFS2 (combined PFS of the first- and second-line treatment) was significantly longer in the immunotherapy followed by chemotherapy sequence than in the chemotherapy followed by immunotherapy sequence (18.3 versus 8.4 months, p<0.01).
The aim of this study was to evaluate the efficacy of the first line of treatment received after nivolumab progression in a large cohort of patients with advanced NSCLC receiving nivolumab in the “real-world” setting of the French expanded access programme. In addition, the efficacy of nivolumab in this cohort is reported.
Materials and methods
Study population
We performed a multicentre retrospective study in nine thoracic oncology centres from the Paris (France) area. All consecutive patients who had received at least one injection of nivolumab for the treatment of NSCLC in 2015 were identified from hospital charts and pharmacy records by the investigators. The patients all received nivolumab during the French expanded access programme (temporary authorisation for use (ATU)), before definitive authorisation, between the January 1, 2015 and December 31, 2015. The median follow-up was 14.6 months.
Data collection
Medical records were reviewed (AC, JC) and data on clinical and pathological features as well as treatment history extracted retrospectively. Information concerning molecular analyses were collected, especially regarding epidermal growth factor receptor (EGFR), KRAS and BRAF mutations as well as anaplastic lymphoma kinase (ALK) rearrangement. In addition, PD-L1 immunohistochemistry (IHC) was collected. IHC was performed at the discretion of each centre using different methods, and was not reviewed centrally.
Nivolumab OS (nivoOS) was measured from initiation of nivolumab (date of first injection) to death. Post-nivolumab OS (post-nivoOS) was measured from initiation of post-nivolumab treatment to death. Nivolumab PFS (nivoPFS) and PFS of the first line of treatment received after nivolumab progression (post-nivoPFS) were measured from initiation of nivolumab (date of first injection) or initiation of post-nivolumab treatment (date of first administration) to progressive disease according to classical RECIST (response evaluation criteria in solid tumors) version 1.1 criteria or death. RECIST version 1.1 was used as it was in phase III trials [1, 2]. In all cases, if death had not occurred at the defined cut-off date, patients were censored. Patients lost during follow-up were censored on the date of last follow-up.
End-points
The primary end-point was the evaluation of post-nivoOS and post-nivoPFS. The secondary end-point was the efficacy of nivolumab in the real-world setting evaluated by nivoOS and nivoPFS.
Statistical analysis
Descriptive analyses comparing patients treated beyond progression and others used Chi-squared and Fisher's tests for categorical variables and t-tests and Mann–Whitney tests for continuous variables according to their distribution. The Kaplan–Meier method was used to estimate OS and PFS. Multivariate analysis was performed using Cox proportional hazards model. Data analysis was computed using XLSTAT version 19.4 (Addinsoft, Bordeaux, France).
Ethical considerations
Participating centres were responsible for patient consent and institutional approval. All contributors were trained in good clinical practice. The study was purely an academic collaboration and was not funded by industry.
Results
Overall population
Characteristics of patients and treatments
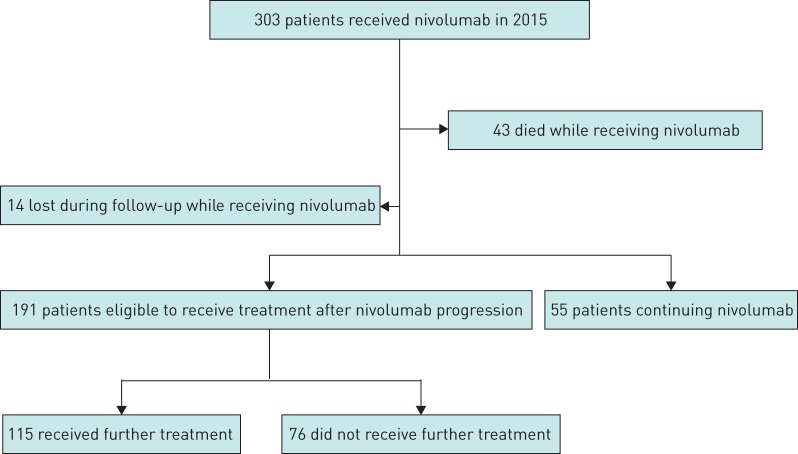
We included 303 patients in the cohort study (figure 1). The baseline clinical and pathological characteristics of these patients are summarised in table 1. The median (range) age at nivolumab initiation was 65 (17–91) years. Most patients were male (69%), current or former smokers (92%) with advanced disease at diagnosis (90%). 30 (10%) patients had limited disease at diagnosis, of which 26 (86%) had surgery and thus presented with post-operative recurrence. The four remaining patients were not operable due to limiting lung function or other comorbidities and progressed from limited to advanced disease. The most frequent histology on initial biopsy was adenocarcinoma (61%). The other histological subtypes were squamous cell carcinoma (28%) and other histologies (11%). 10 (3%) patients had EGFR mutation, 50 (17%) had KRAS mutation, two (1%) had ALK rearrangement, seven (2%) had a BRAF mutation and 219 (72%) were wild-type.
FIGURE 1.
Study flow chart.
TABLE 1.
Clinical and pathological baseline characteristics (all patients)
| Subjects | 303 |
| Age at nivolumab initiation years | 65 (17–91) |
| Sex | |
| Male | 208 (69) |
| Female | 95 (31) |
| Smoking history | |
| Never-smoker | 23 (8) |
| Current or ex-smoker | 278 (92) |
| Missing data | 2 (1) |
| Histology on initial biopsy | |
| Adenocarcinoma | 186 (61) |
| Squamous cell carcinoma | 86 (28) |
| Giant cell carcinoma | 20 (7) |
| Sarcomatoid carcinoma | 4 (1) |
| Adenosquamous carcinoma | 3 (1) |
| Muco-epidermoid carcinoma | 2 (1) |
| Undifferentiated EBV-linked carcinoma | 1 (<1) |
| Missing | 1 (<1) |
| Mutational status | |
| EGFR | 10 (3) |
| KRAS | 50 (17) |
| ALK | 2 (1) |
| BRAF | 7 (2) |
| Wild-type | 219 (72) |
| Other | 15 (5) |
| Stage at diagnosis | |
| Stage I–II | 30 (10) |
| Post-operative recurrence | 26 (87) |
| Stage III–IV | 273 (90) |
| Lines of therapy before nivolumab | 2 (0–9) |
| 0 | 1 (<1) |
| 1 | 120 (40) |
| 2 | 88 (29) |
| ≥3 | 94 (31) |
| Performance status at nivolumab initiation | |
| 0 | 59 (19) |
| 1 | 145 (48) |
| 2 | 60 (20) |
| 3 | 7 (2) |
| 4 | 2 (1) |
| Missing | 30 (10) |
| Metastatic sites at nivolumab initiation | |
| Lung | 120 (40) |
| Pleura | 88 (29) |
| CNS | 62 (20) |
| Liver | 48 (16) |
| Adrenal glands | 54 (18) |
| Bone | 78 (26) |
| PD-L1 status determined by IHC | 120 (40) |
| Positive (>1%) | 31 (10) |
| Negative | 32 (11) |
| Not performed | 240 (79) |
Data are presented as n, median (range) or n (%). EBV: Epstein–Barr virus; EGFR: epidermal growth factor receptor; ALK: anaplastic lymphoma kinase; CNS: central nervous system; PD-L: programmed death ligand; IHC: immunohistochemistry.
At nivolumab initiation, performance status was as follows. 0–1: 204 (67%) patients; 2: 60 (20%) patients; and 3–4: nine (3%) patients. The most frequent metastatic sites were the lungs and pleura in 120 (40%) patients and 88 (29%) patients, respectively; 62 (20%) patients had central nervous system metastasis. 120 (40%) patients received nivolumab after receiving one line of treatment and 88 (29%) patients received nivolumab in the third-line setting. The 94 (31%) remaining patients received nivolumab later. One patient received nivolumab in the first-line setting for compassionate reasons. On average, the patients had received 2.2 lines of treatment before receiving nivolumab with a median (range) of 2 (0–9). Only 21% of patients had PD-L1 status determined by IHC. The European Medicines Agency authorisation of nivolumab states that nivolumab is indicated for the treatment of locally advanced or metastatic NSCLC after prior chemotherapy in adults. The prescription is not conditioned by PD-L1 testing, explaining the small number of patients with PD-L1 IHC testing.
Patient outcomes and nivolumab OS and PFS
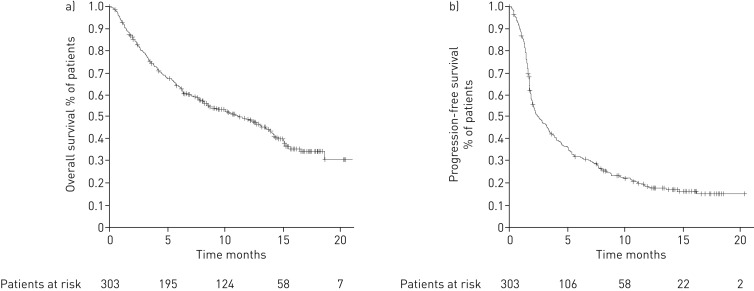
At the time of analysis 55 (18%) patients were controlled under nivolumab, 43 (14%) were deceased and 14 (5%) were lost to follow-up while receiving nivolumab; 191 (63%) had progressed under nivolumab and were eligible for further treatment, of which 115 (38%) received further treatment and 76 (25%) did not. Median (95% CI) nivoPFS and nivoOS for the overall population were 2.6 (2.1–3.5) months and 11.3 (8.5–13.8) months, respectively. The Kaplan–Meier survival curves are detailed in figure 2. In the subgroup of patients with squamous histology (n=86, 28%), median (95% CI) nivoPFS was 2.9 months (2.1–4.6) months and nivoOS was 8.5 months (6.3–13.5) months. In the subgroup of patients with adenocarcinoma histology (n=186, 61%), median (95% CI) nivoPFS was 2.3 (1.9–3.5) months and nivoOS was 12.1 (8.1–15.1) months.
FIGURE 2.
Kaplan–Meier survival curves showing nivolumab a) overall survival and b) progression-free survival in all patients.
Post-nivolumab population
Characteristics of patients and treatments
We defined a population of patients eligible to receive treatment after nivolumab progression. This population excluded patients who died while receiving nivolumab, patients who had not progressed under nivolumab or patients lost to follow-up while receiving nivolumab. 191 (63%) patients were eligible for post-nivolumab treatment; of them, 115 received treatment after nivolumab progression (figure 1). In table 2, we compare the characteristics of eligible patients who received chemotherapy after nivolumab progression and those who did not.
TABLE 2.
Clinical and pathological baseline characteristics of patients eligible for post-nivolumab treatment
| Did not receive further chemotherapy | Received further chemotherapy | p-value | |
| Patients | 76 (40) | 115 (60) | |
| Age at nivolumab initiation | 65.5 (34–86) | 63 (17–84) | 0.11 |
| Sex | 0.78 | ||
| Male | 52 (68) | 76 (66) | |
| Female | 24 (32) | 39 (34) | |
| Smoking history | 0.69 | ||
| Never-smoker | 6 (8) | 10 (9) | |
| Current or ex-smoker | 68 (89) | 104 (90) | |
| Missing data | 2 (3) | 1 (1) | |
| Histology on initial biopsy | 0.91 | ||
| Adenocarcinoma | 46 (61) | 72 (63) | |
| Squamous cell carcinoma | 22 (29) | 30 (26) | |
| Other | 8 (11) | 13 (11) | |
| Stage at diagnosis | 0.57 | ||
| I–II | 7 (9) | 8 (7) | |
| III–IV | 69 (91) | 107 (93) | |
| Lines of therapy before nivolumab | 0.68 | ||
| 0 | 1 (1) | 0 (0) | |
| 1 | 30 (39) | 43 (37) | |
| 2 | 23 (30) | 34 (30) | |
| ≥3 | 22 (29) | 38 (33) | |
| Performance status at nivolumab initiation | 0.028 | ||
| 0 | 10 (13) | 28 (24) | |
| 1 | 35 (46) | 61 (53) | |
| 2 | 21 (28) | 15 (13) | |
| 3 | 1 (1) | 0 (0) | |
| 4 | 0 (0) | 0 (0) | |
| Missing | 9 (12) | 11 (10) | |
| Metastatic sites at nivolumab initiation | 0.49 | ||
| Lung | 31 (41) | 48 (42) | |
| Pleura | 27 (36) | 36 (31) | |
| CNS | 13 (17) | 20 (17) | |
| Liver | 17 (22) | 13 (11) | |
| Adrenal glands | 19 (25) | 15 (13) | |
| Bone | 21 (28) | 26 (23) | |
| Number of nivolumab injections before progression | 5 | 8 | 0.001 |
| Performance status at first tumour evaluation | <0.0001 | ||
| 0 | 0 (0) | 23 (20) | |
| 1 | 20 (26) | 41 (36) | |
| 2 | 19 (25) | 21 (18) | |
| 3 | 16 (21) | 7 (6) | |
| 4 | 7 (9) | 0 (0) | |
| Missing | 14 (18) | 23 (20) | |
Data are presented as n (%), median (range) or median, unless otherwise stated. n=191. CNS: central nervous system. Bold type represents statistical significance.
Patients who did not receive treatment after nivolumab progression had worse performance status at nivolumab initiation than those who did (p=0.028), were slightly older (median age 65.5 years versus 63.0 years; p=0.11) and had received fewer injections of nivolumab (median 5.0 versus 8.0, p=0.001). In addition, the group of patients who did not receive chemotherapy after nivolumab progression had worse performance status at first nivolumab evaluation than those who did (p<0.0001). However, they had received the same number of treatment regimens before receiving nivolumab (p=0.68).
Further treatment after nivolumab progression
Details of the different drug regimens and drugs used are given in table 3. Multiple drug regimens were used in the post-nivolumab setting. The most frequent drugs used, alone or in combination, were gemcitabine in 27 (23%) patients, docetaxel in 25 (22%) patients and erlotinib in 18 (16%) patients. Of note, an EGFR mutation was identified in the tumours of eight (7%) patients who received further treatment after nivolumab progression. Two of these patients received erlotinib after nivolumab progression; one as monotherapy and the other in combination with paclitaxel. In all, 18 different drugs were used after nivolumab progression, some alone and others in combination, resulting in a total of 25 different drug regimens.
TABLE 3.
Drugs and drug regimens used
| Patients | |
| Drug regimens (n=25) | |
| Docetaxel | 24 (21) |
| Paclitaxel | 9 (8) |
| Erlotinib | 17 (15) |
| Vemurafenib | 1 (1) |
| Gemcitabine | 26 (23) |
| Vinorelbine | 8 (7) |
| Pemetrexed | 6 (5) |
| Afatinib | 3 (3) |
| Abemaciclib | 1 (1) |
| Platin docetaxel | 1 (1) |
| Platin pemetrexed | 4 (3) |
| Platin gemcitabine | 1 (1) |
| Platin paclitaxel | 1 (1) |
| Platin paclitaxel bevacizumab | 1 (1) |
| Platin vinorelbine | 1 (1) |
| Platin etoposide | 1 (1) |
| Paclitaxel trastuzumab | 1 (1) |
| Vinorelbin trastuzumab | 1 (1) |
| Abemaciclib necitumumab | 1 (1) |
| Pemetrexed bevacizumab | 1 (1) |
| Paclitaxel bevacizumab | 2 (2) |
| Pertuzumab afatinib | 1 (1) |
| Paclitaxel erlotinib | 1 (1) |
| Cytarabine | 1 (1) |
| Everolimus | 1 (1) |
| Drugs (n=18) | |
| Docetaxel | 25 (22) |
| Paclitaxel | 15 (13) |
| Erlotinib | 18 (16) |
| Vemurafenib | 1 (1) |
| Gemcitabine | 27 (23) |
| Vinorelbine | 10 (9) |
| Pemetrexed | 11 (10) |
| Afatinib | 4 (3) |
| Abemaciclib | 2 (2) |
| Trastuzumab | 2 (2) |
| Necitumumab | 1 (1) |
| Bevacizumab | 4 (3) |
| Vemurafenib | 1 (1) |
| Pertuzumab | 1 (1) |
| Etoposide | 1 (1) |
| Platin | 10 (9) |
| Everolimus | 1 (1) |
| Cytarabine | 1 (1) |
Data are presented as n (%), unless otherwise stated.
Patient outcomes, OS and PFS of the first line of treatment received after nivolumab progression
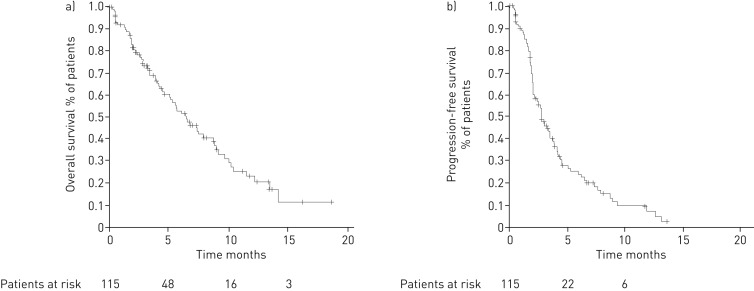
Of the 115 patients who received next-line treatment after nivolumab progression, 66 were deceased at the time of analysis (57%), seven (6%) were lost during follow-up and 27 (23%) were continuing post-nivolumab treatment, while 15 (13%) were alive without treatment. Median (95% CI) post-nivoOS was 5.2 (4.1–7.3) months. In the group of patients receiving treatment after nivolumab progression, median post-nivoOS was 6.4 (5.1–8.8) months (figure 3), but it was 2.2 (1.6–3.0) months in patients who had progressed under nivolumab without receiving further treatment (p<0.0001) (online supplementary figure S1). At the time of cut-off, the survival rate was 43% in patients who received chemotherapy after progression (49 out of 115) and 28% (21 out of 76) in patients who did not receive chemotherapy after progression (p=0.035).
FIGURE 3.
Kaplan–Meier survival curves showing a) overall survival and b) progression-free survival in patients receiving post-nivolumab treatment.
Multivariate analysis was performed to identify factors associated with longer OS in patients eligible for post-nivolumab treatment. We found that the number of injections of nivolumab (p=0.036) and receiving post-nivolumab treatment (p<0.0001) were associated with longer post-nivoOS (table 4).
TABLE 4.
Multivariate analysis of factors associated with post-nivolumab overall survival
| Multivariate analysis | ||
| Hazard ratio (95% CI) | p-value | |
| Sex (male versus female) | 0.92 (0.62–1.36) | 0.67 |
| Number of injections of nivolumab | 0.95 (0.00–0.997) | 0.036 |
| Performance status at nivolumab initiation (0–1 versus 2–4) | 0.999 (0.60–1.65) | 0.998 |
| Performance status at first tumour evaluation (0–1 versus 2–4) | 1.60 (0.97–2.65) | 0.065 |
| Post-nivolumab treatment (yes versus no) | 2.55 (1.68–3.89) | <0.0001 |
| Histology (squamous versus nonsquamous) | 1.47 (0.96–2.27) | 0.079 |
Bold type represents statistical significance.
In the most frequently used drugs, median (95% CI) post-nivoOS was as follows: gemcitabine 7.5 (3.0–13.4) months, docetaxel 6.8 (5.2–11.5) months, and erlotinib 2.7 (1.8–5.1) months. The survival curves for each drug are detailed in online supplementary figure S2.
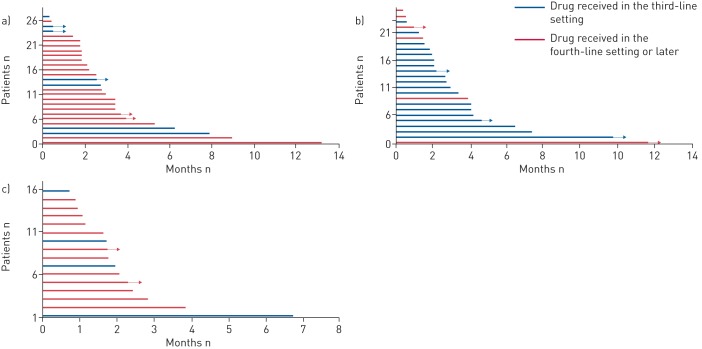
Median (95% CI) post-nivoPFS was 2.8 (2.1–3.4) months (figure 3). In the most frequently used drugs, median (95% CI) post-nivoPFS was as follows: gemcitabine 2.8 (2.1–3.4) months, docetaxel 2.7 (2.0–4.1) months and erlotinib 2.0 (1.6–2.8) months. Of note, 25% of patients receiving gemcitabine and docetaxel had treatment duration of >5 months and >4 months, respectively. The Kaplan–Meier survival curves for each drug are detailed in online supplementary figure S3. Individual PFS of post-nivolumab treatment for each patient are shown in figure 4 for docetaxel, gemcitabine and erlotinib.
FIGURE 4.
Swimmer plots showing post-nivolumab progression-free survival for the three main drugs studied according to the line of treatment in which they were received. a) Gemcitabine; b) docetaxel; c) erlotinib.
Discussion
The effect of chemotherapy received after nivolumab progression has been evaluated recently. In one study the response to chemotherapy was compared before and after nivolumab treatment [5]; another study reported the objective response rate of chemotherapy received after nivolumab progression [6]. However, these studies were monocentric and performed in small cohorts of patients. Our study is the largest to show that chemotherapy received after nivolumab progression performs in a similar fashion as in historical studies [7–10], even though patients received chemotherapy in a further line of treatment than in historical studies.
In the CheckMate trials [1, 2], nivolumab showed a 21% and a 19% 1-year survival rate in squamous advanced NSCLC and nonsquamous advanced NSCLC, respectively. Patients receiving first-line ICIs will experience progressive disease in 60% of cases and those receiving second-line ICIs will do so in 80% of cases. After disease progression, further treatment is often suggested.
A simplistic view would be to assume that patients who are able to receive several lines of treatment intrinsically have a better prognosis. Another explanation could be that exposure to immunotherapy has an effect on the tumour biology. It has been shown that chemotherapy increases tumour immunity by inducing immunogenic cell death, thus releasing tumour antigens and danger-associated molecular patterns. Additionally, chemotherapy acts by disrupting strategies that tumours use to evade immune recognition. It can upregulate costimulatory molecules or downregulate co-inhibitory ones, enhancing the strength of effector T-cell activity [11]. Preclinical studies have evaluated the combination of chemoimmunotherapy with encouraging results in strategies combining chemotherapy followed by immunotherapy, but also immunotherapy followed by chemotherapy [12, 13], which did not always translate into clinical benefit [14–16]. ICIs have a prolonged tissue half-life [17, 18] producing a remaining effect on immune memory cells which might be boosted by the ulterior line of chemotherapy with its own particular immunological effects.
38% of our patients received an anticancer treatment beyond nivolumab progression, similar to the findings of the two pivotal CheckMate studies (42% and 36% in the nonsquamous and squamous studies, respectively). In the OAK study [4], 41% of patients received anticancer treatment, other than atezolizumab continuation, after disease progression. In our study, 43 (37%) patients received nivolumab in the second-line setting and thus received a further treatment in third-line setting. However, up to 63% of our patients received a cancer therapy in the fourth-line setting or later. The median (95% CI) 6.4 (5.1–8.8) months post-nivoOS observed in our retrospective survey suggests that, as long as general condition remains good with performance status ≤2, as patients accept further treatment with an anticipated clinical benefit, chemotherapy remains a valuable therapeutic option beyond nivolumab progression, even if nivolumab has been given later than in the second-line setting. Our results suggest that patients benefiting from nivolumab will also benefit from post-nivolumab chemotherapy, whereas patients who do not respond to nivolumab have less chemosensitive tumours. This could perhaps be explained by the fact that patients who respond to ICIs have a good immunological response, which will eventually get exhausted; chemotherapy then reignites this response by creating an immunological boost. In case of poor initial immunological response this boost is ineffective.
Although next treatments were very heterogeneous, including 18 drugs and 25 regimens, we especially investigated the effects of docetaxel, gemcitabine and erlotinib, the usually recommended second- and third-line regimens for advanced NSCLC before the era of ICIs. In our series, the median PFS for docetaxel was similar to that observed in the TAX317 [7] registration trial evaluating docetaxel in second- and third-line settings, in an all-comer population. In addition, it is similar to the docetaxel arm in the CheckMate studies in the squamous (2.8 (2.1–3.5) months) and nonsquamous histology (4.2 (3.5–4.9) months). The median PFS for gemcitabine, although mostly given as fourth-line treatment or later, was in line with that reported in phase II trials [8, 9]. Interestingly, 25% of patients had a duration of treatment >4 months with docetaxel or gemcitabine. Finally, the median (95% CI) PFS for erlotinib (n=18) was 2.0 (1.6–2.8) months, as compared with the 2.2 months obtained in the BR21 pivotal phase III trial [10] for an unselected population treated in the second- and third-line setting. Although retrospective comparisons across various trials or series are hazardous, our results support the hypothesis of a similar response to anticancer treatments prescribed beyond progression upon second-line nivolumab, at least in all-comer patients. Of note, figure 4 underlines the fact that docetaxel was mostly received in the third-line setting (76%), which is coherent with current guidelines, and that both gemcitabine (74%) and erlotinib (72%) were mostly received in the fourth-line setting or more. Bearing this in mind, the results obtained with erlotinib and especially gemcitabine are encouraging.
There is little or no evidence on which strategy to adopt in the third-line setting in advanced NSCLC. Usually, in cases of maintained performance status and patient acceptance, treating physicians would suggest a regimen of chemotherapy that has not yet been received by the patient, and which presents little cumulative toxicity, while remaining as effective as possible. Our results in the third-line and later remain similar to those observed in the second-line setting. We might have expected to observe worsening results as we progress in the lines of treatment, which is not the case in our study. We thus suggest that patients able to receive post-nivolumab treatment should do so. Docetaxel is recommended as third-line treatment and seems to be as effective as in the second-line setting. Gemcitabine and erlotinib seem to bring encouraging results in terms of survival when given later.
Our patients all received nivolumab in 2015 in the context of the French ATU. It was the first time in France that nivolumab was available outside clinical studies. However, it is interesting to underline that even with 60% of patients receiving nivolumab beyond the second-line setting and 20% with performance status 2, median (95% CI) nivolumab OS (11.3 (8.5–13.8) months) and PFS (2.6 (2.1–3.5) months) were close to those found in the pivotal phase III CheckMate trials [1, 2]. These findings clearly suggest that nivolumab could be used beyond the second-line setting and in more fragilised patients, with the same benefit in terms of survival.
Our study has some limitations. Besides its retrospective and observational design exposing us to survivor treatment selection bias, review of disease response was not independently centralised; this is why survival (OS and PFS) was used, as it offers a more objective evaluation than response rates in this case. Finally, differences in prognosis factors (weight loss, nutritional or inflammatory status) could have led to biases in analysing survivals, deserving prospective studies of post-progression treatment beyond nivolumab and other ICIs. Furthermore, we did not have a control group of patients without nivolumab exposure to compare OS. However, there is little reason to believe that we would achieve much longer survival times than in clinical trials and our results showing the efficacy of nivolumab in the real-world setting remain valid.
Conclusion
This is one of the first studies to address the question of post-nivolumab treatment strategies ant the largest study evaluating nivolumab in the “real-world” setting. Chemotherapy remains the standard treatment after immunotherapy progression. Future treatment strategies will be built on immunotherapy association, sequential immunotherapy, immunoradiotherapy or other combinations such as immunotherapy and chemotherapy. We found similar median PFS as those observed in historical studies, although our study was in the third-line setting or later, whereas we had second-line setting information at our disposal. Some patients (≈25%) experienced prolonged survival times under chemotherapy. This could be due to a lingering long-term effect of immunotherapy, but we cannot rule out regained chemotherapy efficacy.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
FIGURE S1 Kaplan-Meier survival curves comparing post-NivoOS in patients who received or who did not receive CT after nivolumab progression in the group of patients eligible do post-nivolumab treatment 00120-2017_figureS1
FIGURE S2 Kaplan-Meier survival curves showing post-Nivo0S for gemcitabine, docetaxel and erlotinib 00120-2017_figureS2
FIGURE S3 Kaplan-Meier survival curves showing post-NivoPFS for gemcitabine, docetaxel and erlotinib 00120-2017_figureS3
Footnotes
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: V. Fallet reports receiving nonfinancial support from BMS, Boehringer Ingelheim and Novartis, and personal fees from Lilly, outside the submitted work.
Conflict of interest: C. Chouaid reports receiving grants, personal fees and nonfinancial support from Roche, Amgen, MSD, BMS, Lilly, GSK, AZ, Pfizer and Novartis, outside the submitted work.
Conflict of interest: B. Duchemann reports receiving personal fees and nonfinancial support from BMS and Roche, outside the submitted work.
Conflict of interest: E. Giroux-Leprieur reports receiving grants, personal fees and other support from AZ, Novartis, Roche, BMS and Boehringer Ingelheim, outside the submitted work.
Conflict of interest: J. Cadranel reports receiving personal fees from BMS and Roche, and grants and personal fees from AZ, Pfizer, Lilly, Boehringer Ingelheim and Novartis, outside the submitted work.
References
- 1.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. Progression after the next line of therapy (PFS2) and updated OS among patients (pts) with advanced NSCLC and PD-L1 tumor proportion score (TPS) ≥50% enrolled in KEYNOTE-024. J Clin Oncol 2017; 35: Suppl., 9000. [Google Scholar]
- 5.Park SE, Lee SH, Ahn JS, et al. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol 2018; 13: 106–111. [DOI] [PubMed] [Google Scholar]
- 6.Schvartsman G, Peng SA, Bis G, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer 2017; 112: 90–95. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000; 18: 2095–2103. [DOI] [PubMed] [Google Scholar]
- 8.Crinò L, Mosconi A M, Scagliotti G, et al. Gemcitabine as second-line treatment for advanced non-small cell lung cancer: a phase II trial. J Clin Oncol 1999; 17: 2081–2085. [DOI] [PubMed] [Google Scholar]
- 9.Cho K-H, Song Y-B, Choi I-S, et al. A phase II study of single-agent gemcitabine as second-line treatment in advanced non-small cell lung cancer. Jpn J Clin Oncol 2006; 36: 50–54. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005; 353: 123–132. [DOI] [PubMed] [Google Scholar]
- 11.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 2015; 3: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res 2003; 63: 4490–4496. [PubMed] [Google Scholar]
- 13.Fridlender ZG, Sun J, Singhal S, et al. Chemotherapy delivered after viral immunogene therapy augments anti tumor efficacy via multiple immune-mediated mechanisms. Mol Ther 2010; 18: 1947–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middleton G, Silcocks P, Cox T, et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomized, phase 3 trial. Lancet Oncol 2014; 15: 829–840. [DOI] [PubMed] [Google Scholar]
- 15.Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A phase II trial of safety, efficacy, and immune activation. Ann Surg 2011; 253: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small E, Demkow T, Gerritsen WR, et al. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel vs. docetaxel plus prednisone in symptomatic, castration resistant prostate cancer (CRPC). Presented at the 2009 Genitourinary Cancers Symposium, Orlando, FL, USA; February 26–28, 2009. Abstract 07.
- 17.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015; 21: 4286–4293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
FIGURE S1 Kaplan-Meier survival curves comparing post-NivoOS in patients who received or who did not receive CT after nivolumab progression in the group of patients eligible do post-nivolumab treatment 00120-2017_figureS1
FIGURE S2 Kaplan-Meier survival curves showing post-Nivo0S for gemcitabine, docetaxel and erlotinib 00120-2017_figureS2
FIGURE S3 Kaplan-Meier survival curves showing post-NivoPFS for gemcitabine, docetaxel and erlotinib 00120-2017_figureS3