Abstract
Patients with multiply relapsed or refractory chronic lymphocytic leukemia (CLL) have a poor prognosis. Chimeric antigen receptor (CAR)–modified T cells targeting CD19 have the potential to improve on the low complete response rates with conventional therapies by inducing sustained remissions in patients with refractory B cell malignancies. We previously reported preliminary results on three patients with refractory CLL. We report the mature results from our initial trial using CAR-modified T cells to treat 14 patients with relapsed and refractory CLL. Autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector were infused into patients with relapsed/refractory CLL at doses of 0.14 × 108 to 11 × 108 CTL019 cells (median, 1.6 × 108 cells). Patients were monitored for toxicity, response, expansion, and persistence of circulating CTL019 T cells. The overall response rate in these heavily pretreated CLL patients was 8 of 14 (57%), with 4 complete remissions (CR) and 4 partial remissions (PR). The in vivo expansion of the CAR T cells correlated with clinical responses, and the CAR T cells persisted and remained functional beyond 4 years in the first two patients achieving CR. No patient in CR has relapsed. All responding patients developed B cell aplasia and experienced cytokine release syndrome, coincident with T cell proliferation. Minimal residual disease was not detectable in patients who achieved CR, suggesting that disease eradication may be possible in some patients with advanced CLL.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most common type of adult leukemia. The natural history of CLL is quite variable with overall survival ranging from 2 to more than 20 years. Although many effective treatment options are available, CLL remains incurable with conventional therapies, and disease progression is inevitable. Whereas some patients may be cured with allogeneic stem cell transplantation, this is associated with extensive morbidity and mortality, and many patients with CLL are not eligible for transplant therapies (1). Patients with multiply relapsed or refractory CLL have a poor prognosis, and overall survival is typically determined by the duration of their previous progression-free interval (2).
Targeting CD19 with chimeric antigen receptor (CAR)–modified T cells is a rational approach to treating CLL and other B cell malignancies. CARs are genetically engineered molecules that combine a single-chain variable fragment (scFv) domain of a targeting antibody with intracellular signaling and costimulatory domains. Previous attempts to use CAR-modified T cells to target malignancy were limited in part by inadequate in vivo expansion and minimal persistence (3, 4) or by toxicity related to off-tumor targeting of normal tissue expressing the antigen (5). CTL019 (previously known as CART19) are modified T cells expressing a CAR that combines the anti-CD19 scFv with the CD3ζ signaling domain and a costimulatory signal provided by the 4-1BB (CD137) domain; in murine models, T cells modified with this CAR experience long-term persistence and induce potent antitumor activity (6, 7). Because CD19 expression is restricted to normal and malignant B cells and B cell precursors but is not expressed on other cells, toxicity from targeting normal cells is limited.
In a small number of patients, we and others have shown previously that CAR-modified T cells that target CD19 can induce durable complete remissions (CRs) for patients with relapsed or refractory CLL (7–9), acute lymphoblastic leukemia (ALL) (10–12), and non-Hodgkin’s lymphoma (NHL) (9). We now report mature results from a pilot clinical trial of CTL019 in 14 patients with CLL. We show that CTL019 cells undergo robust in vivo expansion, exhibit long-term functional persistence, and induce deep molecular remissions in some patients with relapsed and refractory CLL and that sustained remission is associated with durable persistence and function of CAR T cells.
RESULTS
Patient characteristics
Twenty-three patients with CLL were enrolled in the study, with 14 patients infused with at least one dose of CTL019. A consort diagram is provided in fig. S1. Of the nine patients who did not get treated, three died from complications of disease before infusion, four withdrew consent, and two were screen failures. The characteristics of the 14 treated patients are shown in Table 1. There were 12 male and 2 female patients with a median age of 66 years (range, 51 to 78 years). Patients had received a median of 5 previous therapies (range, 1 to 11), and eight patients had chromosome 17p (Tp53) deletion. One subject (09) had undergone two previous autologous stem cell transplants and one subject (25) had progressed on previous ibrutinib. All patients had active disease at the time of CTL019 cell infusion. From the 10 patients with bone marrow biopsies available for review, lymphocytes comprised a median of 87% (range, 40 to 95%) of the bone marrow cellularity before lymphodepleting chemotherapy. Lymphodepleting chemotherapy was fludarabine/cyclophosphamide in three patients, pentostatin/cyclophosphamide in five, and bendamustine in six. Rituximab was not included.
Table 1.
Summary of patient baseline characteristics (N = 14).
| Characteristics | Statistics, n (%) |
|---|---|
| N | 14 |
| Age at infusion (years) | |
| Mean (SD) | 66.9 (8.1) |
| Median (range) | 66 (51–78) |
| Gender | |
| Male | 12 (85) |
| Female | 2 (14) |
| No. of previous therapies | |
| Mean (SD) | 5.3 (2.8) |
| Median (range) | 5 (1–11) |
| P53 or 17p deletion | |
| No | 8 (57) |
| Yes | 6 (43) |
| IGHV mutation | |
| No | 9 (64) |
| Yes | 4 (29) |
| Unknown | 1 (7) |
| Lymphocyte-depleting chemotherapy | |
| Bendamustine | 6 (43) |
| Fludarabine/cyclophosphamide | 3 (21) |
| Pentostatin/cyclophosphamide | 5 (36) |
| Lymphocytes in bone marrow at enrollment (%)* | |
| Mean (SD) | 79.5 (17.9) |
| Median (range) | 87.5 (40–95) |
| Rai stage | |
| 1 | 5 (36) |
| 4 | 9 (64) |
| Binet stage | |
| A | 1 (7) |
| B | 4 (29) |
| C | 9 (64) |
Data not available for four subjects.
CTL019 cell manufacturing feasibility and product characteristics
Product characteristics are outlined in table S1. All but one infused patient met the target manufactured dose range of 1.5 × 107 to 5 × 109 total nucleated cells (TNCs). Patients received a median of 7.5 × 108 (range, 1.7 × 108 to 50 × 108) TNCs. CTL019 cells refer to the transduced, CAR-expressing T cells; the median transduction efficiency of the 14 manufactured products was 20.1% (range, 4.7 to 39.2%) and the total CTL019 dose infused was a median of 1.6 × 108 cells (range, 0.14 × 108 to 11 × 108) infused over days 0, 1, and 2. Eight (57%) patients completed the full 3-day split-dose regimen; three patients received only one fraction (10% of the manufactured dose) and three patients received only two fractions (total of 40% of the manufactured dose) after developing fevers within 24 hours of CTL019 infusion, accounting in part for the variability in cell dose. The total infused product contained a median of 81% (range, 68 to 95%) of CD4-positive cells and a median of 20% (range, 4.6 to 34%) of CD8-positive cells.
Products from 12 patients were tested for reactivity against CD19-positive targets using functional flow cytometry, measuring degranulation as a measure of cytolytic activity, and the intracellular accumulation of cytokines. All 12 products showed cytolytic activity against the CD19 targets and produced high levels of most cytokines, indicating that the CTL019 cells generated for this patient cohort expressed a functional CAR (fig. S2).
Patient outcomes: Response and survival
Response was determined based on the International Workshop Group on CLL response criteria (13). In addition, deep sequencing analysis of the immunoglobulin heavy chain (IGH) locus was performed for each patient and used for disease detection in blood and bone marrow over time. The best overall response and clinical outcomes are summarized in Table 2. The median follow-up for all 14 treated patients is 19 months (range, 6 to 53 months). Eight of 14 [57%; exact 95% confidence interval (CI), 29 to 82%) patients responded. Four patients (29%) achieved CR. No patient with CR has relapsed with median duration of response of 40 months (range, 21 to 53 months from time of infusion). Remissions included eradication of minimal residual disease (MRD) as assessed through deep sequencing analysis which failed to identify the presence of CLL clones in the blood or bone marrow from subjects 01, 02, and 09 by month 1 and from subject 10 by month 3 [Table 3, table S2, and (8)]. Three of these patients remain alive with no evidence of recurrent disease 28 to 53 months after CTL019 infusion. Subject 09 was in CR and underwent removal of basal cell carcinoma from his lower leg 15 months after CTL019 infusion; the surgical site became infected leading to lethal pseudomonas sepsis and ecthyma gangrenosum 21 months after CTL019 infusion.
Table 2. Treatment and clinical characteristics of subjects (N = 14).
DLBCL, diffuse large B cell lymphoma; NED, no evidence of disease; NR, no response. MRD tested by deep sequencing analysis as described in Materials and Methods.
| ID | Total T cells infused (× 108) | Total CTL019 cells infused (× 108) | Peak CTL019 expansion (% of CD3+ cells) | Best overall response | Last follow-up or progression (months) | Comments, current status |
|---|---|---|---|---|---|---|
| 01 | 50 | 11.3 | N/A† | CR | 53 | MRD-negative; progression-free |
| 02 | 3.0 | 0.142 | N/A† | CR | 52 | MRD-negative; progression-free |
| 03 | 25.5 | 5.86 | N/A† | PR | 5 | Progression, 5 months; died of disease, 27 months |
| 05 | 10.0 | 3.92 | 14.1 | PR | 13 | Progression, 13 months; alive with disease, 36 months |
| 06 | 3.0 | 0.646 | 0.2 | NR | 1 | Died of disease, 8 months |
| 07 | 1.7 | 0.172 | 0.3 | NR | 1 | Died of complications from bone marrow transplant, 9 months |
| 09 | 5.0 | 1.70 | 81.9 | CR | 21 | MRD-negative; progression-free, 21 months; died of infection |
| 10 | 30.0 | 5.61 | 34.3 | CR | 28 | Bulky adenopathy (11 cm); MRD-negative; progression-free |
| 12 | 5.0 | 1.18 | 18.3 | PR | 6 | Bulky adenopathy (9 cm); died of pulmonary embolus |
| 14 | 18.0 | 1.56 | <0.1 | NR | 7 | Alive with disease, 26 months |
| 17 | 4.2 | 1.03 | 1.6 | NR | 10 | Alive with disease, 18 months |
| 18 | 50.0 | 2.77 | 0.2 | NR | 4 | Alive with disease, 17 months |
| 22 | 5.0 | 0.864 | 34.9 | PR | 10 | Bulky adenopathy (9 cm); progressed 10 months with transformed CD19-dim DLBCL; died of disease at 10 months |
| 25 | 20.0 | 2.71 | 2.6 | NR | 3 | Alive with disease, 16 months |
CTL019 refers to transduced, CAR-modified T cells.
Subjects 01, 02, and 03 were not evaluated for early expansion by flow cytometry because an antibody to detect CTL019 cells was not available at the time of their treatment.
Table 3. IGH deep sequencing analysis of blood and bone marrow shows eradication of CLL and B cells for subjects 01 and 02.
BM, bone marrow; PB, peripheral blood; Mo, month; Yr, year.
| Patient UPCC04409 no. | Sample type | Time point | Cell equivalents sequenced | Total reads of IGH | Total unique IGH reads | Tumor clone reads | CLL clone (% of total) |
|---|---|---|---|---|---|---|---|
| 01 | PB | Baseline | 408,579 | 48 | 407,592 | 99.76 | |
| Mo 6 | 285,305 | 7362 | 0 | 0.00 | |||
| Yr 1 | 41 | 12 | 0 | 0.00 | |||
| Yr 3 | 298,667 | 174 | 6 | 0 | 0.00 | ||
| Yr 3.5 | 350,171 | 123 | 8 | 0 | 0.00 | ||
| BM | Mo 1 | 408,838 | 179 | 3 | 0 | 0.00 | |
| Mo 6 | 202,535 | 4451 | 0 | 0.00 | |||
| Mo 12 | 18,506 | 231 | 0 | 0.00 | |||
| Mo 24 | 88 | 2 | 0 | 0.00 | |||
| 02 | PB | Baseline | 1,385,340 | 4544 | 1,285,862 | 92.82 | |
| Mo 6 | 25,041 | 38 | 0 | 0.00 | |||
| Mo 32 | 317,714 | 88 | 8 | 0 | 0.00 | ||
| Yr 3 | 346,057 | 160 | 8 | 0 | 0.00 | ||
| Yr 4 | 308,419 | 212 | 10 | 0 | 0.00 | ||
| BM | Yr 1 | 5 | 2 | 0 | 0.00 | ||
| Yr 2 | 601 | 25 | 0 | 0.00 |
Four subjects (03, 05, 12, and 22) (29%) achieved a partial response (PR) within the first month of CTL019 infusion. The median duration of response in the four patients achieving PR was 7 months (range, 5 to 13 months). In two of these patients (03 and 05), there was improvement in lymphocytosis, marrow involvement, and adenopathy, but both patients progressed 4 months after the infusion. Subject 12 entered treatment with extensive marrow infiltration, transfusion dependence, and diffuse bulky adenopathy and achieved PR. After infusion, he had a 2-log CTL019 expansion with complete clearance of CLL from his blood and marrow and >50% reduction in his adenopathy. The patient died of complications from a massive pulmonary embolus 6 months after T cell infusion with no evidence of CLL in the blood or marrow. At autopsy however, sampling of residual enlarged abdominal lymph nodes showed residual infiltration with CD19+ CLL. Subject 22 also had extensive marrow infiltration, circulating disease, and bulky adenopathy at baseline. After CTL019 infusion, he achieved PR with complete clearance of CLL from the blood and marrow with >50% reduction in adenopathy by 3 months and sustained at 6 months. Nine months after infusion, he presented with rapidly growing adenopathy; lymph node biopsy showed diffuse large B cell lymphoma arising from CLL (Richter transformation) with cells that were CD19-dim. Marrow biopsy showed about 15% involvement with CLL and large transformed-appearing lymphocytes. At the time of transformation and relapse, no CTL019 cells were detected either in his blood, marrow, or lymph node biopsy specimen by flow cytometry though CTL019 cells were detected by quantitative polymerase chain reaction (qPCR) in the blood and bone marrow at the time of progression.
Six subjects (43%) had no response and all six progressed within 1 to 9 months (median, 4 months) of CTL019 therapy. T cell expansion was limited in all six of these subjects (median, 420 copies/μg; range, 6.5 to 13,876, or 0.2% of the total CD3+ T cells). CLL cells from these patients were not resistant to lysis in vitro. Two of these subjects have died; one from complications of CLL and infection 8 months after CTL019, and one from complications of allogeneic stem cell transplantation performed 9 months after CTL019 and after two additional therapies (ofatumumab and CHOP) for his CLL. The other four subjects are alive 9 to 15 months after CTL019, all receiving alternative therapies.
Leukemia response to CTL019 was assessed using IGH deep sequencing in all 14 patients (Table 3 and table S2) and four patterns were identified: (i) CTL019 infusion was followed by eradication of MRD with rapid and persistent disappearance of the leukemic clone (subjects 01, 02, and 09). Peripheral blood DNA from subjects 01, 02, and 09 was sequenced up to 4, 2, and 1 year, respectively, after infusion, and the leukemic clone was undetectable in all cases; (ii) CTL019 infusion resulted in a delayed complete eradication of MRD with molecular remission determined by deep sequencing 3 months after infusion; (iii) Some patients responded with a transient complete (subject 22) or incomplete (subjects 03, 05, 12, 17, and 25) disappearance of the leukemic clone; (iv) CTL019 did not result in any decrease of the number of IGH reads or leukemic clone in subjects 06, 07, 14, and 18.
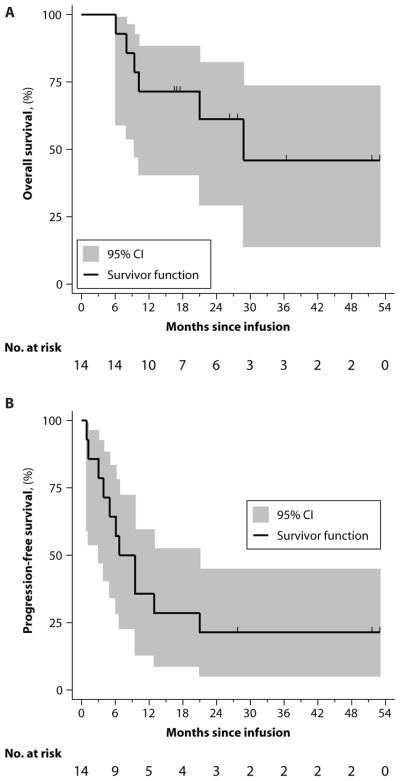
The estimated median overall survival for the 14 treated and evaluable subjects was 29 months, and the 18-month overall survival was 71% (95% CI, 40.6 to 88.2%). The estimated median progression-free survival (PFS) for treated and evaluable patients was 7 months, and the 18-month PFS was 28.6% (95% CI, 8.8 to 52.4%) (Fig. 1 and table S6). Whereas one subject in CR died 21 months after infusion because of complications related to infection, no patient who achieved CR progressed. All other patients ultimately progressed or died of disease-related complications 1 to 10 months after infusion.
Fig. 1. Overall survival and PFS.
(A and B) Kaplan-Meier curves for overall survival (A) and PFS (B) were estimated for the 14 subjects who received therapy. Shaded region indicates 95% CI. Number of subjects at risk is presented in a 3-month interval. Vertical tick marks indicate time points with censored data. (Source data, table S6).
As shown in fig. S1, 23 patients were screened for this study and 9 were not treated. Five patients did not proceed from screening to enrollment because they either declined participation or failed the screening. Therefore, a total of 18 patients were enrolled in the study and 4 were not treated because of rapid disease progression and/or death after enrollment, before therapy (n = 3), or they decided to withdraw consent in favor of alternative therapies (n = 1). Because a delay between enrollment and start of therapy is unique to these types of cell therapy approaches, we performed an analysis of PFS of all 18 enrolled subjects. The median PFS from the date that eligibility was confirmed for all 18 subjects was 9 months (95% CI, 3 to 13.6).
There was no statistically significant association between response and patient age, number of previous therapies, stage at enrollment, deletion of chromosome 17p, IGH variable (IGHV) mutation status, or CTL019 cell dose (all P >0.05, table S3). Because all patients had extensive disease, it was not possible to determine an association between disease burden and response. We also analyzed CD19 expression levels on CLL cells before infusion, and there was no difference in CD19 mean fluorescence intensity between responders and nonresponders, suggesting that target density did not predict response.
CTL019 in vivo expansion and persistence
Persistence and quantitation of CTL019 cells (transduced and CAR-expressing) were performed by flow cytometry to identify T cells expressing the anti-CD19 CAR and by qPCR to identify gene-modified T cells. The degree of expansion and the duration of persistence were correlated to response (Fig. 2 and table S7). The expansion of CTL019 cells was coincident with the development of cytokine release syndrome (CRS) in most cases (described below), and CAR T cells expanded to high levels in all responding patients.
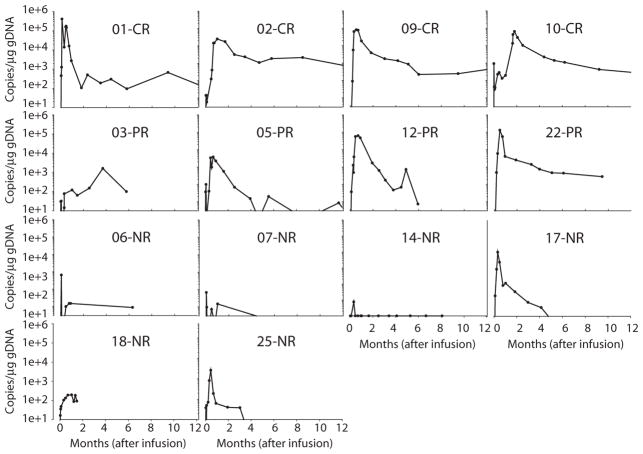
Fig. 2. CTL019 expansion by qPCR in the first 12 months.
Peripheral blood CTL019 expansion was measured as copies per microgram of genomic DNA by qPCR in 14 subjects. Values below the quantitative limit of detection (<25 copies/μg DNA) are shown with open circles. (Source data, table S7).
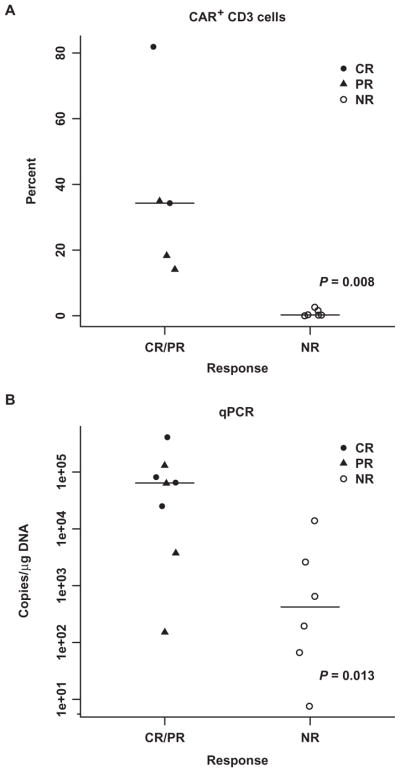
Among the four patients achieving CR, CTL019 peak expansion was 25,070 to 409,645 (median, 73,237) copies/μg by qPCR; for the subjects that had expansion measured by flow cytometry, the peak percentage of CD3+ cells that were positive for CTL019 was 34.3 and 81.9% of CD3+ in peripheral blood mononuclear cells (PBMCs). Expansion was less robust in four patients achieving PR (range, 1607 to 130,258; median, 33,453 copies/μg) by qPCR or a median of 18.3% of the CD3+ population. Expansion was minimal in patients who did not achieve a response (range, 6.5 to 13,876; median, 420 copies/μg) by qPCR or a median of 0.2% of the total CD3+ population by flow cytometry. The peak value of CTL019 was statistically associated with response (Fig. 3 and table S8) (P = 0.013 by qPCR; P = 0.008 by flow cytometry).
Fig. 3. Association between peak CTL019 expansion and response.
(A and B) Peak CTL019 expansion in the first 3 months measured by flow cytometry (A) in 11 subjects and qPCR (B) in 14 subjects. Flow cytometry detection was not available for subjects 01, 02, and 03 because an antibody to detect CTL019 cells was not available at the time of their treatment. Wilcoxon rank-sum P values are provided. (Source data, table S8).
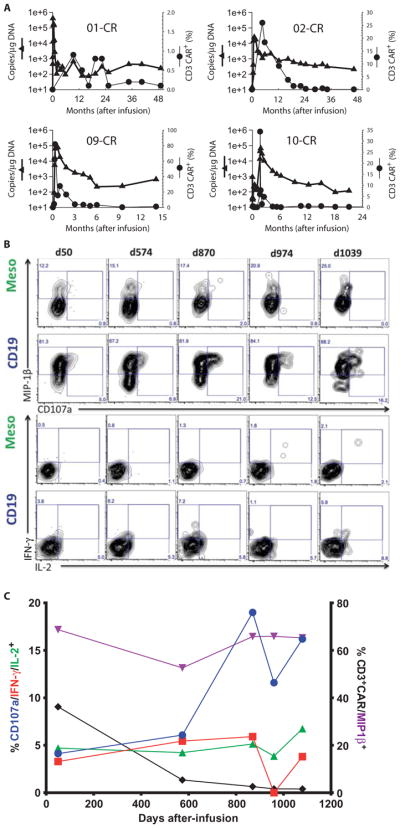
Long-term persistence of CTL019 cells has been detected by means of flow cytometry or qPCR to be 14 to 49 months in the four patients who achieved CRs (Fig. 4A and table S9). All four patients had ongoing B cell aplasia at the time last tested for persisting CTL019 cells, suggesting that the persisting cells remain functional by inhibiting the development of CD19+ B cells. Furthermore, to directly show that persisting CTL019 cells were functional, CAR-modified T cells were isolated from subject 02 almost 3 years after infusion; the CTL019 cells retained immediate and specific reactivity against CD19+ targets, highlighting that at least some of these cells remain functional without exhaustion for years after infusion (Fig. 4, B and C and table S10).
Fig. 4. Long-term persistence of CTL019 cells and poly-functionality in patients achieving CR.
(A) Long-term persistence of CTL019 cells by flow cytometry (solid circle) and qPCR (solid triangle) beyond 12 months from infusion. All observed values were above the limit of detection by flow cytometry (0.1%) and above the limit of quantification by qPCR (<25 copies/μg DNA). (B and C) Purified PBMCs from patient UPCC04409-02 from the indicated postinfusion time points were stimulated for 6 hours with APC-expressing CD19 or control antigen (mesothelin) and examined for de-granulation (CD107a) and cytokine protein expression. (B) Contour plots displaying CD107a versus MIP-1β (top two rows) or IL-2 versus IFN-γ expression (bottom rows) in CD8+ CAR+ T cells stimulated with K562 cells expressing control antigen (mesothelin) or CD19. (C) Summary graph of functional response of CAR19+ CD8 T cells upon stimulation with CD19+ target cells over time. Data are plotted from (B) and are depicted as the background-subtracted frequency of this subset. (Source data, tables S9 and S10).
Toxicity
CTL019 infusions were well tolerated. Infusional toxicities were infrequent and mild (grade <2) and included primarily low-grade fevers and chills. No patient had a significant reaction during CTL019 infusion. The most frequent related events were associated with complications of neutropenia (including fevers) and delayed CRS, which correlated with in vivo CTL019 expansion. Two cases of tumor lysis syndrome were noted. One patient died in remission 21 months after T cell infusion, after developing overwhelming ecthyma gangrenosum from a pseudomonas wound infection from a skin biopsy site.
Cytokine release and macrophage activation syndrome
Symptoms from CRS ranged from mild to severe and were frequently self-limiting. CRS presenting symptoms included fever, myalgia, and nausea, and in severe cases, escalated to hypotension, capillary leak, and hypoxia. CTCAE (Common Terminology Criteria for Adverse Events) version 3.0 captures CRS as an acute infusional toxicity but is inadequate to describe delayed CRS that may occur days after the administration of CTL019. Therefore, we devised a novel grading scale for CRS (table S4, A and B). In addition, during CRS, we also noted concurrent cytopenia, coagulopathy, histologic macrophage activation highlighted by CD163 staining, and hemophagocytosis, as noted in one patient with a marrow biopsy at peak of CRS, and patients with CRS had marked elevations in ferritin, C-reactive protein (CRP), and soluble interleukin-2 receptor (sIL-2R), findings consistent with macrophage activation syndrome (MAS) or hemophagocytic lymphohistiocytosis (HLH).
Nine patients developed CRS 1 to 14 days (median, 7 days) after CTL019 infusion, including one grade 1, two grade 2, two grade 3, and four grade 4. CRS required intervention with anti-cytokine directed therapy in five patients a median of 9.5 days after infusion (range, 9 to 55 days), and four patients required an intensive care unit (ICU) level of care for complications related to CRS, such as hypotension and hypoxia, with a median length of ICU stay of 6 days (range, 4 to 9 days).
We previously noted marked elevations in IL-6 associated with severe CRS after CTL019 infusions in a patient with ALL and rapid reversal with the IL-6 receptor–blocking antibody tocilizumab (14). Before this observation, one patient (subject 03) received corticosteroids to successfully reverse CRS. For subsequent patients, tocilizumab was incorporated into the management of severe CRS for CLL. Four patients received tocilizumab (with the addition of steroids in two patients), which resulted in rapid defervescence, stabilization of blood pressure, and rapid improvement in biochemical abnormalities associated with macrophage activation (ferritin and CRP). An example of the biochemical response of ferritin to tocilizumab is illustrated in fig. S3.
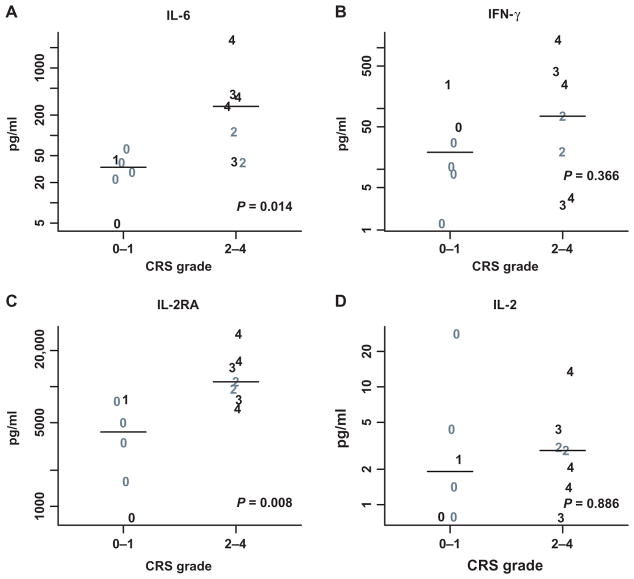
There was higher peak expansion of CTL019 associated with CRS, and CRS was associated with clinical response (P < 0.05) as shown in fig. S4. Compared to patients with no or mild CRS (grade 0 to 1), patients with significant CRS (grade 2 to 4) had higher peak levels of IL-6 (median peak, 269 versus 34 pg/ml; P = 0.014). Levels of sIL-2R were higher in patients with grade 2 to 4 CRS (median peak, 10,962 versus 4177 pg/ml; P =0.008), whereas no statistically significant differences were found in serum interferon-γ (IFN-γ) or IL-2 (Fig. 5 and table S11). The data were limited in the month after the infusion for ferritin (eight subjects) and CRP (five subjects), which prevented a definitive analysis of their association with CRS grade. Patients with grade 2 to 4 CRS had higher peak levels of ferritin (median, 21,502 versus 1980) and C-reactive protein (median, 160 versus 42) than patients with grade 0 to 1 CRS; because of the small number of patient samples available, detailed statistical analysis was not performed, but this limited data set suggests that ferritin and/or CRP may serve as useful biomarkers for CRS activity and response to treatment. There was no disease or patient-specific characteristics that predicted the development of CRS including age, previous therapy, genetic risk profile, or cell dose (table S5).
Fig. 5. Peak cytokines values over the first month after infusion.
(A to D) Peak values for IL-6 (A), IFN-γ (B), IL-2 receptor antagonist (IL-2RA) (C), and IL-2 (D) by CRS grades are displayed. Gray symbols represent nonresponders; black symbols are those who achieved a complete or partial remission. Individual values are plotted with a symbol that reflects their CRS grade (1 to 4). Wilcoxon rank-sum P values are also provided. Cytokine values were missing for one grade 4 subject who achieved CR (n = 13). (Source data, table S11).
All four recipients of tocilizumab for CRS had ongoing T cell expansion at the time of tocilizumab infusion. After tocilizumab, there was marked improvement in symptoms, although in at least two cases, T cell proliferation peaked several days after administration, suggesting that tocilizumab may not fully inhibit ongoing proliferation. Because all patients had persisting cells, it is clear that tocilizumab did not affect long-term T cell survival.
Concurrent with CRS were six recorded neurologic events in five patients that included grade 1 or 2 hallucinations, confusion, or delirium typically associated with high fevers, ICU care, or medication use. There was one case of grade 4 confusion lasting 2 days and attributed at least in part to T cell therapy.
B cell aplasia and hypogammaglobulinemia
B cell aplasia and hypogammaglobulinemia were common. B cells became undetectable in all patients achieving CR and in two of the four patients achieving PR. Patients who did not respond to CTL019 cells did not develop B cell aplasia. Hypogammaglobulinemia was managed with intravenous immunoglobulin repletion dosed according to immunoglobulin levels. B cell aplasia was sustained for 4 years in the initial patients, further indicating that persisting CTL019 cells remain functional.
DISCUSSION
The use of CAR-modified T cells to target cancer has been studied for more than 20 years, but clinical success was initially limited by poor in vivo expansion and the lack of persistence of the genetically modified T cells (3, 5, 15–17).In a preliminary report, we found that CTL019 had clinical activity in three patients with CLL (7, 8). We now report an overall response rate of 8 of 18 enrolled patients, that these responses were durable, and that no patient in CR has relapsed. Eradication of the malignant clone was sustained without further therapy. Responses were associated with high levels of CTL019 cell expansion and long-term persistence and B cell aplasia for years after infusion.
We have not identified any pretreatment demographic or disease-related factors to predict which patients are most likely to respond, though the study was not powered to investigate these associations. The numbers of patients treated remain limited, but there was no distinction between responders and nonresponders in patient age, previous therapies, or genetic risk factors. There was no difference in the level of CD19 expression measured by mean fluorescence intensity between responders and nonresponders. There was no association between T cell dose and response, and there were no obvious characteristics of the manufactured cell product that predicted response, such as phenotype, in vitro activity against CD19-positive targets, or premanufacturing telomere length. However, we did determine that the level of T cell expansion in vivo correlated with response. In addition, CLL cells from non-responders were susceptible to lysis in vitro, supporting the hypothesis that resistance is due to poor T cell amplification in vivo.
Similar second-generation CARs have shown remarkable activity against CD19+ lymphoma (9), as well as B cell ALL, with complete response rates between 66 and 90% reported in adult and pediatric ALL patients (10–12). In some ALL patients, there is also a high level of in vivo expansion reported, eradication of large volume leukemia burdens, and, in some cases, long-term functional persistence of the CAR-modified T cells (11). It is not known why the incidence of complete response varies between diseases. At our institution, manufacturing of CTL019 cells is identical for use with both CLL and ALL patients. Patients with CLL tend to be significantly older than ALL patients treated in these studies, and previous treatment regimens are different for patients with CLL and ALL. It is possible that the functional abilities of the autologous T cells are different between CLL and ALL patients (18). It is also likely that tumor environments differ in patients with CLL versus ALL. Many mechanisms that lead to local immunologic inhibition have been identified in patients with CLL including the inability of T cells to synapse appropriately with leukemia cells, the production of immunosuppressive cytokines within the microenvironment, and possible overexpression of immune checkpoint inhibitors that all serve to attenuate and block an immune response (19, 20). This finding suggests that strategies combining CTL019 with immune checkpoint inhibitors or other methods to stimulate T cell recognition of tumor cells would be appropriate.
The most serious toxicity associated with CAR T cell therapy has been CRS. CRS is a systemic inflammatory response that is due to high levels of inflammatory cytokines that are associated with T cell activation and proliferation. CRS may be mild and self-limiting with manifestations that may include fever, myalgia, arthralgia, anorexia, and fatigue. CRS can progress with severe and life-threatening complications that include persistent markedly elevated temperatures, hypotension, capillary leak, hypoxia, cytopenia, and co-agulopathy. Renal insufficiency likely related to hypotension can be seen. The CTCAE grading scale for CRS captures only acute infusional toxicities and was therefore not appropriate or adequate to capture CRS occurring after CTL019 therapy. We and others have developed a CRS grading scale to better reflect clinical parameters seen with CAR T cell–induced CRS (21).
Patients with CRS also had clinical and biochemical manifestations consistent with HLH or MAS. There were clinical and biochemical changes of MAS with evidence of hemophagocytosis, disseminated intravascular coagulation, and cytopenia, similar to our experience in patients with ALL (14). Hemophagocytosis and staining for the macrophage activation marker CD163 were observed at least on one bone marrow specimen taken at the peak of CRS, and patients had marked elevations in ferritin, CRP, and sIL-2R, a hallmark of MAS/HLH. The peak elevation of ferritin, CRP, and sIL-2R was significantly higher in patients with grade 2 to 4 CRS compared to patients with grade 0 to 1 CRS and was correlated with severity of CRS. These biochemical changes were useful diagnostic markers but were not used to determine the need for intervention for CRS; treatment of CRS was based on clinical manifestations alone. Others also observed that CRP was elevated in CRS in ALL patients and suggested that elevated CRP would predict for development of CRS (10). Although the data set is limited, it suggests that ferritin and/or CRP may serve as useful biomarkers for CRS activity and response to treatment without the need for less commonly available assays such as IL-6 levels or measurement of CAR-modified T cells.
Neurologic events directly related to the CAR T cells were not common. There were six neurologic events in five patients that included reversible grade 1 or 2 hallucinations, confusion, or delirium typically associated with high fevers, often ICU care, and narcotic or other medication use. There was one case of reversible grade 4 confusion lasting 2 days that was concurrent with grade 3 CRS and associated with high fevers, ICU care, and sedative and narcotic use. Computerized tomography scanning in this patient during the event was unremarkable. Other studies have identified more severe neurologic complications related to CAR T cell therapy, particularly in ALL and NHL patients, which included more severe obtundation, encephalopathy, and seizures (9–11).
The etiology of neurologic toxicity in this setting is not known, but we speculate that it is related to T cell activation and perhaps cytokine elevation. It is unclear whether toxicity may be different in CLL compared to ALL or NHL; the limited neurologic toxicity seen in our patients may simply be due to the small number of patients treated who developed CRS. CAR T cells are frequently found in the cerebrospinal fluid of patients with or without neurologic symptoms, suggesting they are not direct mediators of neurologic toxicity. Central nervous system toxicity has also been associated with other T cell–engaging therapies such as blinatumomab (22, 23), supporting the concept that T cell activation leads to these neurologic events (12).
The CRS was self-limited in four patients and required intervention for hemodynamic instability including hypoxia and hypotension in five patients. One patient had a severe CRS that responded to high-dose glucocorticoid therapy. This patient had a dramatic though partial response to CTL019 and had loss of detectable T cells after steroid 2therapy. Before treating subsequent patients with CLL, we observed a marked elevation in IL-6 in a pediatric patient with ALL and severe CRS (14). This patient had received tocilizumab, an anti–IL-6 receptor antagonist, and showed a rapid and marked improvement of severe CRS, without loss of T cells. Therefore, in subsequent patients, tocilizumab was incorporated into the management of CRS. Future studies may further determine predictors for CRS, the optimal timing of tocilizumab, the role for alternative anti-cytokine therapy, and whether or not CRS can be managed preemptively. Our preliminary data suggest that T cell expansion can occur even after symptoms improve with tocilizumab, at least in some patients (data not shown). In the case of subject 12, peak expansion was identified 5 days after tocilizumab, though testing was not done daily. In another case (subject 22), initial expansion peaked the day after tocilizumab, decreased, and then increased again on day 28. Both patients responded. Whether these cells remain functional after tocilizumab is not yet known. We hope to collect a larger data set in the future to determine the impact of tocilizumab and other IL-6 antagonists on T cell proliferation and subsequent activity. Another limitation of our current data is that it remains unknown whether IL-6 antagonists could be administered pro-phylactically to prevent CRS, and related to this, whether that could adversely affect clinical antitumor responses.
All patients who achieved CR developed B cell aplasia and hypogammaglobulinemia managed with intravenous immunoglobulin repletion. One patient, who was in remission, died 21 months after T cell infusion from complications of ecthyma gangrenosum developing after a pseudomonas wound infection. He had been receiving intravenous immunoglobulin for hypogammaglobulinemia; whether B cell aplasia and hypogammaglobulinemia contributed to his death is unclear, but we advocate immunoglobulin repletion for all patients with hypogammaglobulinemia after CAR T cell therapy.
We attempted to identify patients for enrollment who were at high risk of death from CLL without effective treatment. All 10 patients who failed to achieve CR ultimately progressed or died 1 to 10 months after infusion, a testament to the advanced stage and high-risk nature of these patients. Although there is exciting activity from many of the newer agents for CLL, complete responses with these therapies are unusual, and subsequent progression is anticipated (24); there remains an important need for newer and more potent therapies. Notably, our data show that a one-time therapy with CTL019 cells can induce deep and sustained remissions in some patients. It remains to be determined whether treating patients earlier in the course of their disease with CTL019 or combining CTL019 with other therapies such as immunomodulation will improve clinical responses and long-term outcomes.
The long-term durability of the cells beyond 4 years is not yet known; the number of patients with long-term follow-up is limited, and we do not know if loss of CTL019 cells would result in recurrence of CLL. In our experience in CLL and ALL, persistence has correlated with ongoing leukemia-free survival. Ultimately, longer follow-up of additional patients will be needed to determine if CTL019 can result in complete eradication of CLL and whether CAR T cells can be eliminated using various strategies (25). A general limitation to this approach is that adequate T cells must be collected from patients who are heavily pretreated, and it may take 2 to 3 weeks to manufacture the CTL019 cells. Patients must be clinically stable during this time to receive therapy, and a number of patients considered for treatment may not be able to receive therapy as highlighted in fig. S1.
The ability of CTL019 to eradicate the leukemic clone in a subset of patients indicates that deep remissions were achieved, which sets CTL019 apart from other targeted agents such as ibrutinib and idelalisib. Eradication of MRD only occurs consistently with allogeneic stem cell transplantation (26), suggesting that this may be the only modality capable of curing CLL, though nonrelapse mortality remains high (1). Whether CTL019 will ultimately cure CLL remains to be determined with longer follow-up. A dose optimization study of CTL019 in CLL is currently ongoing, and continued study to define factors that determine response is warranted.
MATERIALS AND METHODS
Study design and oversight
Adults aged 18 years or older with CD19+ CLL with relapsed or persistent disease after at least two previous treatment regimens were eligible. Eligibility also required there be less than 2 years between at least second-line chemotherapy and progression, with patients either ineligible for or having declined allogeneic stem cell transplantation. Patients with high-risk disease manifested by deletion of chromosome 17p or with TP53 mutation were eligible if they failed to achieve CR to initial therapy or progressed within 2 years of one previous regimen. After tumor restaging, peripheral blood T cells for CTL019 manufacturing were collected by apheresis. Subjects were given a single course of conventional chemotherapy for lymphodepletion, designed to end about 4 days before infusion. Lymphodepleting chemotherapy included standard doses of commonly accepted regimens for CLL and was chosen at the discretion of the treating physician. The primary objectives of this pilot study were to determine the safety of this therapy and the feasibility to manufacture CTL019 cells from patient apheresis products. Secondary objectives included evaluation of function and persistence of the infused genetically modified T cells, response rate, patient survival, and other correlative end points. The protocol was approved by the Institutional Review Board of the University of Pennsylvania, the U.S. Food and Drug Administration (FDA), and the Recombinant DNA Advisory Committee and was conducted under an FDA-approved Investigational New Drug Application. All patients gave informed consent in accordance with the Declaration of Helsinki. This study was registered at ClinicalTrials.gov (identifier NCT01029366).
Follow-up is as of December 31, 2014. All the authors discussed and interpreted the study results and vouch for the data and analyses. The source data for all figures are available in the Supplementary Materials (tables S6 to S11).
Vector production
The CD19-BB-z transgene (GeMCRIS 0607-793) was designed and constructed as described (27). A lentiviral vector was produced according to current manufacturing practices with a three-plasmid production approach at either Lentigen Corporation (28) or Children’s Hospital of Philadelphia.
T cell collection and generation of CTL019 cells
Autologous T cells were collected by leukapheresis and were stimulated with paramagnetic polystyrene beads coated with anti-CD3 and anti-CD28 monoclonal antibodies (mAbs) (29, 30). Cells were transduced with a lentiviral vector encoding anti-CD19 scFv linked to 4-1BB and CD3-z signaling domains as described (31) and were expanded ex vivo for 10 to 12 days. The median transfection efficiency was 20.1% (range, 4.7 to 39.2%). Release criteria have been previously described (8).
Response assessment
Disease response assessments were done at protocol-defined time points and were based on the International Workshop Group on CLL response criteria (13) with the incorporation of independent radiology review. In addition, investigational assessments for MRD using deep sequencing analysis were performed (see below).
Correlative studies
Sample processing, flow cytometry, cytokine and cytokine receptor analysis, and qPCR analyses were performed as described (11). In addition, the following studies were performed.
Next generation sequencing of immunoglobulin heavy chain rearrangements
Genomic DNA was extracted from the manufactured cell products and postinfusion peripheral blood using the iPrep (Invitrogen). The third complementarity-determining region of the IGH locus was amplified and deep-sequenced using primers specific for the variable and joining gene segments (Adaptive Biotechnologies). Sequence data were examined using immunoSEQ. For each patient, the leukemic clone was identified in the baseline sample, and the response of this clone to CTL019 was assessed in follow-up marrow and/or peripheral blood specimens. For each specimen, the total and unique number of productive reads was determined, and the frequency of the leukemic clone in each specimen was calculated.
IGHV mutational status
Mutational status of the IGHV region was assessed using genomic DNA extracted from peripheral blood specimens collected before infusion of CTL019 cells (Cancer Genetics Inc.).
Polychromatic flow cytometry
Antibodies
The commercially available antibodies to the following antigens were used: (i) from eBioscience, CD3 fluorescein isothiocyanate (FITC), CD8 phycoerytherin (PE) and cyanin 5.5 PE (Cy5.5PE), CD14 Cy7PE, CD16 Cy7PE, CD5 allophycocyanin (APC), CD19 Cy7PE and APC, CD20 FITC, CD45 PE, CD10 Cy7PE, CD34 Cy7PE, and tumor necrosis factor–α Alexa Fluor 700; (ii) from BioLegend, CD4 Brilliant Violet 605 (BV605) and BV785, CD8 Cy5PE, CD45RO BV570, and IFN-γ BV570; (iii) from Becton Dickinson, CD14 V500, CD3 BV605, IL-2 CF594PE, CD107a Cy5PE, granulocyte-macrophage colony-stimulating factor BV421, and macrophage inflammatory protein (MIP)–1β PE; (iv) from Beckman Coulter, CD27 Cy7PE; (v) from R&D systems, MIP-1α FITC. Alexa Fluor 647–conjugated mAb to detect the CAR19 molecule was described (32). All antibodies used in this study were titrated before use on the respective flow cytometer (see below). Routine assessment of CTL019 expansion and persistence and of CLL cells with a four-color, six-parameter Accuri C6 flow cytometer was performed as described previously (8).
CTL019 anti-CD19 activity
To assess response to CD19+ target cells, the infused product and postinfusion samples were incubated with CD19-expressing K562 cells for 6 hours at 37°C in the presence of monensin, brefeldin A, and anti-CD107a Cy5PE mAb. Mesothelin-expressing K562 cells and PMA (phorbol 12-myristate 13-acetate)/ ionomycin were used as negative and positive controls, respectively. Cells were stained with the dead cell exclusion dye Aqua Blue (Invitrogen), followed by surface staining. Cells were then fixed and permeabilized using Cytofix/Cytoperm and Perm/Wash (BD), followed by staining with anti-cytokine antibodies. Cells were acquired on a special order 18-color, 20-parameter LSR Fortessa equipped with 405-, 488-, 532-, and 628-nm lasers. Data were analyzed using FlowJo (version 10).
CTL019 administration
Lymphodepleting chemotherapy intended for depletion of T lymphocytes was timed to end about 4 days before infusion of CTL019. Cells were administered by intravenous infusion with a 3-day split-dose regimen (10% on day 0, 30% on day 1, and 60% on day 2) with a total targeted dose of 1 × 109 to 5 × 109 CD3+ cells and a minimum acceptable dose of 1.5 × 107 CTL019 cells. Day 1 or 2 infusions were held for fevers or other toxicity. If less than the target dose was available, patients received the total number of manufactured cells as a 3-day split infusion. According to the original protocol design, patients could receive an additional dose of T cells if available on day 10; only one patient (subject 03) received this dose. Subsequently, no additional planned day 10 infusions were given. Subjects were assessed for toxicity planned intervals (a minimum of weekly for 1 month, monthly for 6 months, and then every 3 months) with response assessments performed at 1 month, 3 months, and then every 3 months.
Toxicity grading
Toxicities after CTL019 infusion were graded according to Common Toxicity Criteria (CTC) version 3.0. CTC grading for CRS was designed to grade acute infusional reactions typically seen with antibody therapies and was inadequate to describe the delayed and ongoing CRS from cellular therapy (21); we developed a modified grading scale for CRS designed to describe the severity of the reaction and help identify the need for medical intervention. The revised grading scale for CRS is shown in table S4, A and B.
Statistical analyses
Descriptive statistics were computed for study variables. Data are presented as mean ± SD or median (minimum–maximum) for continuous variables and n (%) for categorical variables. The best overall response rate was computed as the proportion of subjects with CR or PR during the study period along with exact 95% CI. Kaplan-Meier curves and median survival times were estimated for the end points of overall survival and PFS.
Overall survival was defined as the time from the first infusion to death, censored at the earliest date of last follow-up or December 31, 2014. The one exception involved subject 10; he did not respond to initial therapy and received a second dose (60% fraction) of CTL019, and the outcomes for this patient are described from the time of the second infusion course 2 months after the first infusion. Progression was defined by standard criteria (13), and PFS was defined as the time from the first infusion to date of first documented disease progression or death. Duration of response among those that achieved CR and PR was described. For each individual, the percent T cells expressing the anti-CD19 CAR among CD3-positive cells measured by flow cytometry and the number of gene-modified T cells (averaged marking per cell) identified by qPCR were plotted over time to determine the expansion and persistence of CTL019 cells. The level of expansion was determined using the peak (that is, maximum) values, and the detection of CTL019 cells above the lower limit of detection (0.1% for %CD3 CAR+ and 25 copies/μg DNA for qPCR) was used to measure the persistence. Levels of expansion were associated with CRS grade and best overall response, using the Wilcoxon rank-sum test. Cytokine profiles were examined, and differences in the peak values during the first month after infusion for four preselected cytokines (IL-6, IFN-γ, IL2-RA, and IL-2) were compared by CRS grade (0 to 1 versus 2 to 4) using scatterplots and the Wilcoxon rank-sum test. The first month was chosen as the time frame relative to the peak CRS illness.
Statistical comparison between groups, when appropriate, was done using two-sided nonparametric tests (for example, Wilcoxon rank-sum test) at a 0.05 significance level. Statistical analysis was performed using R 3.0.1 (R Development Core Team) or Stata 13.0 (StataCorp).
Supplementary Material
Fig. S1. Consort diagram.
Fig. S2. CTL019 cells are polyfunctional.
Fig. S3. Example of serum ferritin response to tocilizumab in a patient with CRS.
Fig. S4. Association of peak CTL019 expansion with CRS and the response observed within the first 3 months after infusion.
Table S1. Cell dose and product characteristics (N = 14).
Table S2. Tumor burden assessed by IgH deep sequencing analysis of blood and bone marrow of all study subjects.
Table S3. Association of pretreatment characteristics with response.
Table S4A. Penn Grading System for CTL019-associated CRS.
Table S4B. Definition of high-dose vasopressors.
Table S5. Association of pretreatment characteristics with CRS (N = 14).
Table S6. Source data for Fig. 1 (Excel).
Table S7. Source data for Fig. 2 (Excel).
Table S8. Source data for Fig. 3 (Excel).
Table S9. Source data for Fig. 4A (Excel).
Table S10. Source data for Fig. 4C (Excel).
Table S11. Source data for Fig. 5 (Excel).
Acknowledgments
We thank L. Lledo, H. McConville, J. Gilmore, J. Capobianchi, C. Jemison, M. Harvey, H. Difilippo, E. Veloso, and A. Marshall for clinical research assistance; A. Bersenev, A. Lamontagne, A. Malykhin, N. Manvar, M. O’Rourke, M. Suhoski-Davis, M Kalos, and the members of the Clinical Cell and Vaccine Production Facility for cell manufacturing and testing; Y. Mahnke and L. Tian for correlative sciences contributions; J. Finklestein, I. Kulikovskaya, F. Nazimuddin, M. Gupta, E. Pequignot, J.-M. Navenot, F. Chen, H. Parakandi, M. Bogush, Y. Tanner, and N. Kengle for research support; P. Wood, M. Litchman, A. Quintas, and G. Shah from Novartis and S. Maude from the Children’s Hospital of Philadelphia for discussions and protocol support; B. Jena and L. Cooper (MD Anderson Cancer Center) for provision of the CAR anti-idiotype detection reagent; and B. Fuchs and C. Candeloro for assistance in developing our CRS grading scale and defining pressor doses. We would also like to acknowledge the Data and Safety Monitoring Board, the outstanding clinical support provided by our hematology-oncology faculty and nurses, the other critical care faculty and nurses, and our residents and fellows at the Hospital of the University of Pennsylvania. Most importantly, we acknowledge the courageous participation of all the patients in this trial.
Funding: This study was supported in part by a grant from Novartis and by grants from the Leukemia and Lymphoma Society (Specialized Center of Research Award) and NIH (1R01CA165206 to D.L.P. and C.H.J.; 1K24 CA117879 to D.L.P.); R01CA102646 and R01CA116660 to S.A.G.).
Footnotes
Author contributions: D.L.P. was the primary author of the manuscript; D.L.P. and C.H.J. were involved in primary design, performance, and oversight of the study; W.T.H. and P.A.S. designed and performed the statistical analyses; N.V.F. and A.W.L. were involved with the performance of the study and data analysis; S.F.L., V.G., D.A., Z.Z., M.M., B.L.L., and J.J.M. designed, performed, and/or analyzed the correlative experiments; A.B. reviewed the pathology specimens; K.T.M., A.S., S.A.G., and A.C. contributed to the analyses. All authors contributed to the writing and/ or review of the manuscript. All authors have reviewed and approved the manuscript.
Competing interests: Patents related to the technology described here have been issued in the United States (8,916,381; 8,911,993; 8,906,682; 8,975,071) and are licensed to Novartis.
Data and materials availability: The CAR sequence has been published and is available under a material transfer agreement.
REFERENCES AND NOTES
- 1.Dreger P, Schetelig J, Andersen N, Corradini P, van Gelder M, Gribben J, Kimby E, Michallet M, Moreno C, Stilgenbauer S, Montserrat E. European Research Initiative on CLL (ERIC) and the European Society for Blood and Marrow Transplantation (EBMT), Managing high-risk CLL during transition to a new treatment era: Stem cell transplantation or novel agents? Blood. 2014;124:3841–3849. doi: 10.1182/blood-2014-07-586826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JR. The treatment of relapsed refractory chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:110–118. doi: 10.1182/asheducation-2011.1.110. [DOI] [PubMed] [Google Scholar]
- 3.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JR, DiGiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg JR, Jensen MC. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 5.Lamers CHJ, Sleijfer S, Vulto AG, Kruit WHJ, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: First clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 6.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, Carroll RG, Riley JL, Pastan I, June CH. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen–receptor modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RPT, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, Raffeld M, Feldman S, Lu L, Li YF, Ngo LT, Goy A, Feldman T, Spaner DE, Wang ML, Chen CC, Kranick SM, Nath A, Nathan DAN, Morton KE, Toomey MA, Rosenberg SA. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DCG, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Sustained remissions with chimeric antigen receptor T cells for leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ. International Workshop on Chronic Lymphocytic Leukemia, Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute–Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan DAN, Lanier BJ, Morgan RA, Rosenberg SA. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, Gopal AK, Pagel JM, Lindgren CG, Greenberg PD, Riddell SR, Press OW. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riches JC, Gribben JG. Understanding the immunodeficiency in chronic lymphocytic leukemia: Potential clinical implications. Hematol Oncol Clin North Am. 2013;27:207–235. doi: 10.1016/j.hoc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay AG, Gribben JG. Immune dysfunction in chronic lymphocytic leukemia T cells and lenalidomide as an immunomodulatory drug. Haematologica. 2009;94:1198–1202. doi: 10.3324/haematol.2009.009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsay AG, Johnson AJ, Lee AM, Gorgün G, Le Dieu R, Blum W, Byrd JC, Gribben JG. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118:2427–2437. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, Nichols KE, Suppa EK, Kalos M, Berg RA, Fitzgerald JC, Aplenc R, Gore L, Grupp SA. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, Stelljes M, Schaich M, Degenhard E, Köhne-Volland R, Brüggemann M, Ottmann O, Pfeifer H, Burmeister T, Nagorsen D, Schmidt M, Lutterbuese R, Reinhardt C, Baeuerle PA, Kneba M, Einsele H, Riethmüller G, Hoelzer D, Zugmaier G, Bargou RC. Targeted therapy with the T-cell–engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 24.Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, Spurgeon SE, Kahl BS, Bello C, Webb HK, Johnson DM, Peterman S, Li D, Jahn TM, Lannutti BJ, Ulrich RG, Yu AS, Miller LL, Furman RR. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marktel S, Magnani Z, Ciceri F, Cazzaniga S, Riddell SR, Traversari C, Bordignon C, Bonini C. Immunologic potential of donor lymphocytes expressing a suicide gene for early immune reconstitution after hematopoietic T-cell–depleted stem cell transplantation. Blood. 2003;101:1290–1298. doi: 10.1182/blood-2002-08-2351. [DOI] [PubMed] [Google Scholar]
- 26.Böttcher S, Ritgen M, Dreger P. Allogeneic stem cell transplantation for chronic lymphocytic leukemia: Lessons to be learned from minimal residual disease studies. Blood Rev. 2011;25:91–96. doi: 10.1016/j.blre.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Milone M, Fish J, Carpentito C, Carrol R, Binder G, Teachey D, Samata M, Lakhal M, Gloss B, Danet-Desnoyers G, Campana D, Riley JL, Grupp S, June C. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased anti-leukemic efficacy in vivo. Mol Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 29.Laport GG, Levine BL, Stadtmauer EA, Schuster SJ, Luger SM, Grupp S, Bunin N, Strobl FJ, Cotte J, Zheng Z, Gregson B, Rivers P, Vonderheide RH, Liebowitz DN, Porter DL, June CH. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34+-selected hematopoietic cell transplantation. Blood. 2003;102:2004–2013. doi: 10.1182/blood-2003-01-0095. [DOI] [PubMed] [Google Scholar]
- 30.Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB, June CH. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- 31.Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, Binder GK, Slepushkin V, Lemiale F, Mascola JR, Bushman FD, Dropulic B, June CH. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jena B, Maiti S, Huls H, Singh H, Lee DA, Champlin R, Cooper LJN. Chimeric Antigen receptor (CAR)-specific monoclonal antibody to detect CD19-specific T cells in clinical trials. PLOS One. 2013;8:e57838. doi: 10.1371/journal.pone.0057838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Consort diagram.
Fig. S2. CTL019 cells are polyfunctional.
Fig. S3. Example of serum ferritin response to tocilizumab in a patient with CRS.
Fig. S4. Association of peak CTL019 expansion with CRS and the response observed within the first 3 months after infusion.
Table S1. Cell dose and product characteristics (N = 14).
Table S2. Tumor burden assessed by IgH deep sequencing analysis of blood and bone marrow of all study subjects.
Table S3. Association of pretreatment characteristics with response.
Table S4A. Penn Grading System for CTL019-associated CRS.
Table S4B. Definition of high-dose vasopressors.
Table S5. Association of pretreatment characteristics with CRS (N = 14).
Table S6. Source data for Fig. 1 (Excel).
Table S7. Source data for Fig. 2 (Excel).
Table S8. Source data for Fig. 3 (Excel).
Table S9. Source data for Fig. 4A (Excel).
Table S10. Source data for Fig. 4C (Excel).
Table S11. Source data for Fig. 5 (Excel).