Abstract
It is becoming increasingly clear that the genetic background of mice and rats, even in inbred strains, can have a profound influence on measures of seizure susceptibility and epilepsy. These differences can be capitalized upon through genetic mapping studies to reveal genes important for seizures and epilepsy. However, strain background and particularly mixed genetic backgrounds of transgenic animals need careful consideration in both the selection of strains and in the interpretation of results and conclusions. For instance, mice with targeted deletions of genes involved in epilepsy can have profoundly disparate phenotypes depending on the background strain. In this review, we discuss findings related to how this genetic heterogeneity has and can be utilized in the epilepsy field to reveal novel insights into seizures and epilepsy. Moreover, we discuss how caution is needed in regards to rodent strain or even animal vendor choice, and how this can significantly influence seizure and epilepsy parameters in unexpected ways. This is particularly critical in decisions regarding the strain of choice used in generating mice with targeted deletions of genes. Finally, we discuss the role of environment (at vendor and/or laboratory) and epigenetic factors for inter- and intrastrain differences and how such differences can affect the expression of seizures and the animals’ performance in behavioral tests that often accompany acute and chronic seizure testing.
Keywords: genetic heterogeneity, inbred rodent strains, genetic background effects, genetic mapping
1. Introduction
Similar to humans, genetic background plays an important role in modulating both seizure susceptibility and its neuropathological consequences in rodent models of seizures or epilepsy, a factor which has received inadequate consideration in prior studies [1]. Outbred strains of mice (e.g., Swiss, NMRI or CD-1) or rats (e.g., Wistar or Sprague-Dawley (SD)) have been widely used in models of seizures or epilepsy, but such outbred strains can increase seizure variability with a high intrastrain phenotypic variation due to genetic heterogeneity [2,3]. Genetic divergence between outbred subpopulations may arise from a number of mechanisms, including natural selection, mutation, unconscious experimenter selection, and genetic drift [2,3]. Such intrastrain differences may be an important reason for discrepancies between studies from different laboratories using outbred strains. However, outbred strains of rodents can have important contributions to experimental design, since in many ways they can model the genetic diversity observed in the human population. But the genetic variance imparted by the use on outbred strains also needs to be factored into the experimental interpretations, particularly as it may relate to the understanding of single genes effects being studied (i.e., targeted deletions of genes). Furthermore, apart from genetics, intrastrain and interstrain differences in models of seizures or epilepsy can also be due to the environmental conditions under which the animals are bred and maintained [4].
It is generally assumed that using inbred strains of mice or rats minimizes the effect of intrastrain differences because of genetic homogeneity within inbred strains. However, the inbreeding that makes an inbred strain so useful can also result in genetic divergence between differing strains. This genetic divergence is often unaccounted for in experiments, but may be a confounding factor when comparing studies that have utilized different inbred strains [1]. Inbred mouse strains may contain hidden or “quiet” mutations, that have no discernable effect, but which may be uncovered during behavioral phenotyping [5,6]. Inbred strains are also subject to new mutations and to genetic drift during breeding [5]. Careful attention is needed in rodent colony management to prevent or limit genetic drift by reintroducing the parental strain systematically into breeding programs. Furthermore, variation of the environmental conditions under which the animals are bred and reared at a specific vendor may have marked effects on animal behavior once the rodents are used for experiments, not to mention variabilities from University animal facility to animal facility. In addition, genetically similar mice from different commercial vendors may exhibit differences in their gut microbiota composition, which can exert profound variation in animal models [7,8].
When discussing strain effects on expression of seizures and epilepsy, it is important to consider the commonly misapplied distinction of wild-type rodents [9]. Multiple studies have demonstrated that some inbred strains of so-called wild-type mice, even if they never show spontaneous seizures, can be much more easily induced to have seizures than other wild-type strains. Clearly, mice are only wild-type with respect to a specific genetic locus and according to a specific user-defined assay for the structure or function of that locus in a controlled laboratory environment [9]. Every “wild-type” mouse carries multiple genetic differences (mutations or polymorphisms) that distinguish it from mice of other strains, although only some of these differences produce phenotypes that are obvious to a scientist, and many fewer that are relevant to phenotypes related to seizures and epilepsy. Nonetheless, a large body of evidence has accrued to document the strong influence of genetic variation on susceptibility to seizures or epilepsy, especially in rodent models.
In this review, it is not possible to discuss all the innumerous studies that have demonstrated inter- and intrastrain strain effects on expression of seizures and epilepsy in laboratory mice and rats. Rather, we will illustrate the impact of such strain effects by reviewing a series of experiments that were performed by our groups in the last ~25 years in a variety of models of seizures and epilepsy. Furthermore, some important studies from other groups will be highlighted. This review will not deal with rodent models of genetic epilepsies, such as spontaneously occurring mutants or genetically engineered rodents (including knockout and transgenic strains or lines) that are widely used as models of epilepsy. Our aim is to emphasize that even in the absence of engineered mutations, different inbred strains, and even substrains of the same inbred strain or sublines of the same substrain, can vary drastically in their susceptibility to induction of seizures or epilepsy. However, these genetic differences present opportunities to identify biological factors (i.e., genes) that are relevant to elucidating mechanisms of seizures and epilepsy. Thus, the use of different strain backgrounds, when studying epilepsy mutations, enhances the modeling of epilepsy as a complex genetic disease [10] and facilitates insight into the pathophysiology of epilepsy and for potential treatments. Another important issue that we will discuss is that, in addition to genetic inter- and intrastrain differences in rodents, differences in housing and handling of the animals, both at the vendor and in the laboratory, may have a marked impact on the expression of seizures and associated behavioral alterations. Importantly, some of these factors may have effects that are strain-specific. Lastly, we will briefly discuss age and sex differences in the expression of seizures and epilepsy in rodents and how this may affect inter- and intrastrain differences, whereas we will not discuss species (mouse vs. rat) differences that are of documented importance when interpreting data from rodent models of seizures or epilepsy.
2. Intrastrain differences in mice and rats
As described in the introduction, genetic intrastrain differences can occur in both outbred and inbred strains of mice and rats. Furthermore, epigenetic differences may occur in response to the environment under which the animals are born and raised (see section 6).
For generating seizure or epilepsy models in rats, outbred strains such as Wistar or SD are often used. Outbred rat strains are known to be genetically heterogeneous populations with a high intrastrain phenotypic variation [2,3,11,12]. In contrast to inbred strains, outbred strains (or stocks) are “genetically undefined” in the sense that each animal is genetically unique and the alleles it carries are unknown until they are analyzed [3]. Like other outbred strains, SD and Wistar rats are randomly outbred; hence allelic variations can occur across separate colonies. Thus, stock names such as SD or Wistar can be misleading because stocks with the same name from different breeders may have different genetic and/or phenotypic characteristics [3]. The phenotypic characteristics of an outbred stock can change relatively fast as a result of random genetic drift in gene frequency, selective breeding (say for large litter size, body weight, blood pressure, etc.) and/or genetic contamination, which may go undetected because genetic quality control is rarely done [3]. As a consequence, outbred rat strains from different vendors may have little in common with each other besides their names and similarities in pelage [2,11]. Lack of standards and the unreliability of stock names hinder scientific reproducibility [3]. Genetic divergence between outbred subpopulations may arise from a number of processes, including mutation, natural selection, unconscious selection, and random genetic drift [13]. By far the most common source of genetic divergence among outbred subpopulations is random genetic drift, especially in small isolated populations. Genetic drift over time, and the resultant genetic divergence between colonies, is the inevitable result of breeding stocks and strains in isolation without reintroduction of founder stocks to the breeding program. This may also occur within the same large subsidiary of a breeder when subpopulations of outbred rodents are maintained in different barriers or breeding colonies over the long-term, eventually resulting in genetic drift and genetic divergence between barriers [14]. In addition to germline genetic differences between outbred SD or Wistar rats from different vendors or locations of the same vendor, intrastrain differences can also be due to housing and handling conditions at the vendor during development of the animals through presumed epigenetic modifications or early life stress effects on brain development [4,15-19].
To minimize inbreeding, Charles River Laboratories have adopted the International Genetic Standardization (IGS) Program for SD and Wistar rats. The aim of the IGS Program is to standardize multiple production colonies of these animals that are geographically separated. This insures that each colony has the same range of genetic variation. This is accomplished by monitoring heterozygosity and actively managing genetic drift that both could otherwise lead to colony divergence in outbred strains [13]. However, to our knowledge, other vendors have not adopted similar programs in rats (Jackson Laboratory has established a Genetic Stability Program (GSP) for mice). In any event, investigators need to consider the issue of intrastrain differences when planning and performing a study, and preferably use rats from the same stock and barrier for all experiments within a study. Furthermore, intrastrain differences may form an important bias when comparing results from different laboratories. On the other hand, once characterized, such differences provide a chance to study the impact of genetic and environmental factors on seizure susceptibility, epileptogenesis, and the relationship between behavior and epilepsy and vice versa.
The potential problems associated with outbred stocks of rats also apply to outbred mice [20]. However, genetic and epigenetic differences may also occur between inbred lines of mice from different vendors or different barriers of the same vendor, as shown by our experiments with pilocarpine in the C57BL/6 (B6) mouse strain [21], the most widely used inbred mouse strain in biomedical research. There continues to be an implicit assumption that B6 substrains can be used interchangeably [22]. However, molecular genetic studies indicate simple sequence-length polymorphisms, single-nucleotide polymorphisms (SNPs), and copy- number variants among B6 substrains may contribute to phenotypic differences [22,23]. Multiple branches of the B6 lineage arose in the early 1950s and have been maintained as separate breeding colonies since that time; two branches in particular are denoted as C57BL/6J (“J” for The Jackson Laboratory or JAX) and C57BL/6N (“N” for National Institutes of Health) [24]. Isolation and genetic drift of these colonies has resulted in the emergence of genetically distinct substrains [22,25]. Furthermore, B6J and B6N strains obtained from different breeders and even from different barriers of the same breeder may differ in their genotypes and phenotypes [21-23,25]. In addition to such substrain differences in inbred mouse strains, new mutations in sublines of inbred mice have been described previously for epilepsy, for example, within the B6J substrain of B6 mice [26] and in the C3H/HeJ strain [27]. Large breeding colonies at vendors are historically organized in the form of sub-colonies. Here, the possibility exists that a spontaneous mutation occurs in a founder animal and becomes fixed in the resulting sub-colony. Although this may be a rare event, once discovered, it offers a unique possibility to identify new susceptibility genes that can often result in human gene identification (reviewed in Davisson et al. [28]).
2.1 Intrastrain differences in the pilocarpine model of status epilepticus and temporal lobe epilepsy in mice
We became interested in intrastrain differences in the B6 mouse strain when establishing the pilocarpine model of temporal lobe epilepsy (TLE) in B6 mice [21]. In this model, the cholinergic, muscarinic agonist pilocarpine is administered systemically (i.p. or s.c.) to induce status epilepticus (SE), which after a seizure-free latent period, is followed by development of epilepsy with spontaneous recurrent seizures (SRS) and hippocampal degeneration [29]. Peripherally acting anticholinergics such as methyl-scopolamine are administered before pilocarpine to reduce the peripheral cholinomimetic adverse effects of the convulsant. Borges et al. [30] reported that B6 mice from the Jackson Laboratory (B6J) responded with a markedly higher mortality to systemic administration of pilocarpine than a substrain of B6 mice obtained from Charles River (both substrains were injected with methyl- scopolamine and terbutaline 15–30 min prior to pilocarpine to minimize peripheral side effects of pilocarpine). In view of the popularity of the pilocarpine model in epilepsy research, and the fact that this convulsant is increasingly used to induce SE and epilepsy in mice, we directly compared the response of three different B6 substrains (B6JOla; B6NHsd; B6NCrl) from two suppliers (Harlan [now Envigo] and Charles River) to pilocarpine [21]. In B6JOla and B6NHsd mice, only a small percentage of mice developed SE independently of whether pilocarpine was administered at high bolus doses or with a ramping-up dose protocol, but mortality was high (despite pretreatment with methyl-scopolamine to reduce peripheral cholinomimetic effects of pilocarpine). The reverse was true in B6NCrl mice, in which a high percentage of mice developed SE, but mortality was much lower compared to the other substrains. However, in subsequent experiments with B6NCrl mice, striking differences in SE induction and mortality were found in sublines of this substrain coming from different barrier rooms of the same vendor (Charles River). In B6NCrl mice from Barrier #8, administration of pilocarpine resulted in a high percentage of mice developing SE, but mortality was low, whereas the opposite was found in B6NCrl mice from four other barriers of the same vendor. The analysis of F1 mice from a cross of female Barrier #8 pilocarpine-susceptible mice with resistant male mice from another barrier (#9) showed that male F1 mice were significantly more sensitive to pilocarpine than the resistant parental male mice, whereas female F1 mice were not significantly different from resistant Barrier #9 females. These observations strongly indicated X-chromosome-linked genetic variation as the cause of the observed phenotypic alterations [21]. To our knowledge, this was the first report which demonstrates that not only the specific B6 substrain, but also sublines derived from the same substrain, may markedly differ in their response to convulsants such as pilocarpine. It further illustrates that the breeding/housing environment in which mice are maintained can have effects in regards to seizure responsivity (possibly through epigenetic mechanisms), assuming that these vendors are managing genetic drift properly in their mouse populations.
There is one potential caveat in interpreting effects of mortality following pilocarpine treatment that is of the peripheral effects of pilocarpine. It is accepted as true that systemic effects induced by high convulsant doses of pilocarpine are sufficiently antagonized by pretreatment with the peripherally-acting muscarinic antagonist methyl-scopolamine [29]. This approach is meant to minimize the contribution of systemic cholinergic activation in favor of CNS effects. However, scarce information is available in support of this assumption. For example, at the doses of pilocarpine used to induce SE, a significant muscarinic blockade by methyl-scopolamine (typically given at 0.5-1 mg/kg) is virtually impossible [31]. Therefore, the differences in mortality observed between substrains of B6 mice may relate at least in part to the effect of pilocarpine peripherally altering cardiovascular or cardiopulmonary physiology, thus affecting mortality. However, this still illustrates our caution that choice of B6 substrain can have important ramifications with seizure-inducing drug effects, and obvious autonomic differences between substrains. It is also important to note that increasing the dose of methyl-scopolamin (i.e., 10 mg/kg in mice and 20 mg/kg in rats, s.c.) can prevent induction of SE by pilocarpine [29].
In a subsequent study [32], we generated a new B6NCrl subline that is highly susceptible to the effects of pilocarpine by crossing female B6NCrl Barrier #8 mice with male F1 hybrids (B6NCrl Barrier #8 × B6NCrl). Further sister-brother matings of the resulting N2 generation resulted in a highly pilocarpine-sensitive F3 generation. Similar to B6NCrl Barrier #8 mice, mice from the F3 generation were significantly more susceptible to SE induction than any other B6 substrain, including B6J mice, which were particularly insensitive to SE induction (Fig. 1A). It is important to note that pilocarpine induced convulsive seizures in the majority of mice, independent of substrain or subline, but the percentage of mice progressing from isolated convulsive seizures to SE differed markedly. Interestingly, SE-associated mortality was negatively correlated with incidence of SE across B6 substrains (Fig. 1B).
Fig. 1.
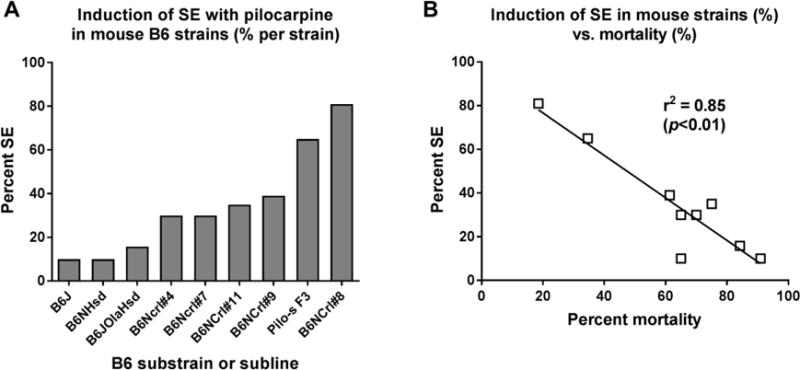
Differences in sensitivity to pilocarpine in various C57BL/6 (B6) substrains and sublines. All data were generated by a ramping-up dosing protocol with i.p. administration of 100 mg/kg pilocarpine every 20 min until onset of SE. All mice were pretreated with methylscopolamine to prevent peripheral cholinomimetic effects of pilocarpine. In case that no SE was induced, the maximum number of repeated injections was restricted to about 10, because mice died in individual convulsive seizures without reaching SE. “A” illustrates the percentage of mice per substrain or subline in which pilocarpine induced SE. Pilo-s F3 and B6NCrl#8 mice exhibited a significantly higher sensitivity to SE induction by pilocarpine than any other B6 substrain or subline. In “B” the SE-associated mortality (in % of mice per substrain) is correlated with percent SE induction in the respective B6 substrains and sublines, resulting in a significant negative correlation between the two endpoints. In A and B, the following substrains and sublines of B6 mice are shown: B6J, C57BL/6J mice from the Jackson Laboratory (JAX); B6NHsd, C57BL/6NHsd from Harlan (Harlan-Winkelmann; B6JOlaHsd, C57BL/6JOlaHsd mice from Harlan-Winkelmann; B6NCrl, C57BL/6NCrl mice from 4 different barriers (#4, #7, #8, and #9) from Charles River; Pilo-s F3, a pilocarpine-sensitive B6NCrl subline obtained by crossing female B6NCrl#8 mice with male F1 hybrids (B6NCrl#8 × B6NCrl); further sister-brother mating of the resulting F2 generation generated a highly susceptible F3 generation. Data are from Müller et al. [21] and Bankstahl et al. [32].
Borges et al. [30] suggested that the pilocarpine dose needed to induce SE is closer to the lethal dose in B6J (JAX) than in B6NCrl (Charles River) mice, thus explaining the low percentage of JAX mice that developed SE. We consider this an unlikely explanation, because in our experiments with the ramping-up dosing protocol, B6J (and other substrains) typically died ~2 h following the first injection of pilocarpine, whereas SE typically started <2 h in the different B6 substrains and sublines [21,32]. Consequently, B6 substrain or subline differences, as observed in our studies with pilocarpine, may further our understanding of basic processes that are involved in the evolution of single seizures to a self-sustaining SE and SE-associated mortality, and provide new opportunities for interventions.
In contrast to the marked inter-subline differences in susceptibility to induction of SE, B6 sublines did not differ in their long-term consequences of SE (i.e., development of spontaneous seizures and neurodegeneration in the hippocampus), although hippocampal damage was much less severe than previously reported for other mouse strains [32]. It has been repeatedly reported in the literature that it is difficult, if not impossible, to induce SE without high mortality in B6 mice using systemic administration of convulsants such as pilocarpine or kainate, thus critically restricting the use of this mouse strain and B6-based transgenic mice in convulsant-induced epilepsy models [21,30,33-36]. However, our experiments demonstrate that pilocarpine-sensitive B6 mice can be obtained by selective breeding, using different B6 sublines. This indicates that sensitivity to pilocarpine-induced SE in B6 mice is genetically modulated, thus offering the possibility to identify the genes and pathways involved in susceptibility to SE. In the future, we will continue to search for genetic loci underlying the high SE susceptibility of B6NCrl Barrier #8 mice and the pilocarpine- sensitive filial generations obtained by cross-breeding with this B6 subline.
2.2 Intrastrain differences in the pilocarpine model of status epilepticus and temporal lobe epilepsy in rats
Similar to mice, intrastrain differences may affect the outcome in this model in rats. In a retrospective study, Portelli et al. [18] evaluated the consequences of intrahippocampal pilocarpine administration in male Wistar rats that were either purchased from two breeding locations of Charles River Laboratories (Germany [CRL1, CRL2] and France [CRL HAN]), or obtained from Harlan Laboratories in the Netherlands (HAR HAN). Following intrahippocampal administration of 10 mM pilocarpine, CRL1 rats were significantly more prone to seizures than the CRL2 and HAR HAN rats, while the CRL HAN rats showed similar seizure susceptibility as the CRL1 rats [18]. The latter study prompted us to compare the consequences of systemic administration of pilocarpine (following pretreatment with lithium) in SD rats from different breeders (Harlan, Charles River [CRL], Taconic) as well as different breeding locations of the same breeder (Harlan-Winkelmann [HW] in Germany vs. Harlan Laboratories [HL] in the Netherlands) [37]. Pilocarpine was administered by a ramp- up dosing protocol that allows determining inter-individual differences in susceptibility to the convulsant. Marked intrastrain differences in induction of SE and its long-term consequences were found (Table 1). SD rats from HW were significantly more sensitive to SE induction than all other SD substrains. The lowest rate of SE induction was obtained in SD rats from Taconic. The majority of SD rats from different vendors developed SRS after SE except SD rats from HL. CRL-SD rats markedly differed in basal behavior and SE-induced behavioral alterations compared to other SD substrains.
Table 1.
Effects of intra-strain differences in Wistar and Sprague-Dawley rats on seizure threshold, induction of SE, and development of kindling or epilepsy in three models of temporal lobe epilepsy. N.d. = not determined.
| Rat strain | Amygdala kindling (Honndorf et al. [40]) |
BLA-SE (Langer et al. [19]) |
Lithium/pilocarpin SE (Brandt et al. [37]) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre- kindling ADT |
Post- kindling ADT |
ADD to stage 5 |
SE induction |
SE type III |
SRS | Hippocampal damage |
SE induction |
SRS | Hippocampal damage |
|
| Wistar | ||||||||||
| 1. Harlan-Winkelmann (Hsdcpb:WU) | No intra-strain differences | No intra-strain differences | Not different from 3 | 56/77 (73%) |
23/77 (30%) |
6/6 (100%) |
n.d. | 116/170 (68%) |
37/41 (90%) |
n.d. |
| 2. Charles River (Crl:WI (Han)) | No intra-strain differences | No intra-strain differences | Significantly higher vs. 1 and 3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 3. Janvier (RiHan:WI) | No intra-strain differences | No intra-strain differences | Not different from 1 | 5/7 (71%) |
0/7 (0%) |
1/1 (100%) |
n.d. | n.d. | n.d. | n.d. |
| 4. Taconic (HanTac:WH) | n.d. | n.d. | n.d. | 6/12 (50%) |
0/121 (0%) |
2/2 (100%) |
n.d. | 0/172 (0%) |
n.d. | n.d. |
| Sprague-Dawley (SD) | ||||||||||
| 1. Harlan-Winkelmann (Hsd:SD) | n.d. | n.d. | n.d. | 236/255 (93%) |
145/255 (57%) |
75/81 (93%) |
+ | 34/46 (74%) |
7/7 (100%) |
+ |
| 2. Harlan NL (Hsd:SD) | n.d. | n.d. | n.d. | 48/49 (98%) |
32/49 (65%) |
10/14 (71%) |
+ | 7/183 (39%) |
3/7 (43%) |
+ |
| 3. Charles River (Crl:CD (SD)) | n.d. | n.d. | n.d. | 30/37 (81%) |
14/374 (38%) |
9/205 (45%) |
− | 10/293 (34%) |
11/13 (85%) |
+ |
| 4. Janvier (RjHan:SD) | n.d. | n.d. | 7/114 (64%) |
6/11 (55%) |
3/5 (60%) |
± | n.d. | n.d. | n.d. | |
| 5. Taconic (NTac:SD) | n.d. | n.d. | n.d. | 10/10 (100%) |
5/10 (50%) |
8/9 (89%) |
± | n.d. | n.d. | n.d. |
P<0,05 vs. 1 (Wistar);
P<0.0001 vs. 1 (Wistar);
P<0.05 vs. 1 (SD);
P<0.01 vs. 1 (SD);
P<0.0001 vs. 1 (SD)
In order to determine whether outbred SD rats from Taconic are generally less susceptible to SE induction by pilocarpine, we used the ramp-up dosing protocol also in Wistar rats (HanTac:WH) purchased from this vendor (Table 1). In 17 rats used for pilocarpine experiments, SE could not be induced in any rat [37]. This sharply distinguished this Wistar substrain from Wistar rats from Harlan-Winkelmann (Wistar Unilever) that we had previously used for several pilocarpine experiments, resulting in an overall incidence of SE of 68% of a group of 170 rats by the ramp-up protocol [37].
2.3 Interstrain differences in a rat model of temporal lobe epilepsy resulting from sustained stimulation of the basolateral amygdala
In addition to SE induction by chemical convulsants, such as pilocarpine or kainate, electrical induction of SE via depth electrodes in amygdala or hippocampus in rats is widely used as a model of brain insult with subsequent development of neurodegeneration, SRS, and cognitive and behavioral alterations resembling the long-term consequences of epileptogenic brain insults in humans [38]. When inducing SE by sustained electrical stimulation of the basolateral nucleus of the amygdala (BLA), SD rats were more sensitive to SE induction than Wistar rats [39]. This prompted us to compare SE induction by BLA stimulation and its long- term consequences in SD and Wistar rats from different breeders (Harlan, Charles River, Janvier, Taconic) as well as different breeding locations of the same breeder (Harlan- Winkelmann in Germany vs. Harlan in the Netherlands) [19]. Several marked inter- and intrastrain differences in induction of SE and its long-term consequences were found (Table 1). Wistar rats from different vendors were all strikingly less sensitive to SE induction than SD rats from Harlan-Winkelmann or Harlan. Within the SD strain, SD rats from Charles River exhibited markedly lower sensitivity to SE induction than all other groups of SD rats. The majority of SD rats from different vendors developed SRS after SE except SD rats from Charles River. The latter rats also markedly differed in basal behavior, SE-induced behavioral alterations and neurodegeneration from other SD substrains. These marked inter- and intrastrain differences provide an interesting tool to study the impact of genetic and environmental factors on seizure susceptibility, epileptogenesis, and the relationship between behavior and epilepsy, and vice versa.
2.4 Intrastrain differences in the amygdala kindling model of temporal lobe epilepsy in rats
We examined whether adult female Wistar rats from different breeders vary in response to electrical amygdala stimulation in the amygdala-kindling model of TLE [40]. Female Wistar rats from three different commercial breeders (Harlan-Winkelmann [HsdCpb:WU], Charles River [Crl:WI(Han)], and Janvier [RjHan:WI]) were kindled by daily electrical stimulation of the right BLA as a model of TLE. Wistar rats from Charles River exhibited significantly increased cumulative motor seizure duration and cumulative ADD until seizure generalization compared to Wistar rats from Harlan-Winkelmann and Janvier (Table 1). In other words, Wistar rats from Charles River showed a longer time of focal seizures until generalization, reflecting a slower epileptogenesis. Initial afterdischarge threshold (ADT) did not differ significantly between Wistar rats from the three breeders, indicating that baseline seizure susceptibility is not different between the three groups, but that there are clear differences in the plasticity related to kindling.
Racine et al. [41] combined the amygdala kindling model with selective-breeding procedures to develop two new lines (or strains) of rats that are kindling-prone or kindling- resistant. The selection of these strains was based on their rates of amygdala kindling. From a parent population of Long Evans hooded and Wistar rats, the males and females that showed the fastest and slowest amygdala kindling rates were selected and bred. Similar selection procedures continued through F11, although there was little or no overlap in the distribution of kindling rates for the two new strains (FAST and SLOW) by F6. Examination of both local and propagating seizure profiles of the new strains from F6 to F10 revealed that the FAST and SLOW rats had similar amygdala ADTs and associated afterdischarge durations (ADDs). Also, the convulsion profiles of the stage-5 responses were similar, although the severity was greater in the FAST rats. Clearly the selection was not based on local mechanisms controlling the threshold for amygdala afterdischarge (AD) evocation, but rather for the spread of AD from the focus and the recruitment of other structures, ultimately triggering convulsive seizures.
In subsequent studies, it was shown that the FAST and SLOW rat lines were associated with different neurological, electrophysiological and behavioral features [42]. Behaviorally, the FAST rats appear much like humans with attention deficit hyperactivity disorder (ADHD), showing easy distraction, hyperactivity and impulsivity, compared to SLOW rats. More recently, Sharma et al. [43] aimed to investigate neuroanatomical differences between these strains that may be associated with a differential vulnerability towards kindling and behavioral comorbidities. Behavioral testing demonstrated hyperactivity, impulsivity, and polydipsia differences in FAST versus SLOW rats, consistent with an ASD (autism spectrum disorder)/ADHD-like phenotype. Magnetic resonance imaging analysis identified brain structural differences in FAST compared with SLOW rats, including increased volume of the cerebrum, corpus callosum, third ventricle, and posterior inferior cerebellum, with decreased volume of the anterior cerebellar vermis. Stereological measurements of histological slices indicated significantly larger white matter layer volume, reduced number of Purkinje cells, and smaller molecular layer volume in the cerebellum in FAST versus SLOW rats. These findings provided evidence of structural differences between the brains of FAST and SLOW rats that may be mechanistically related to their differential vulnerability to kindling and associated comorbid ASD/ADHD-like behaviors.
2.5 Intrastrain differences in drug response in the amygdala kindling, BLA, and pilocarpine models of temporal lobe epilepsy in rats
In view of the unresolved problem of drug resistant seizures in about a third of patients with epilepsy [44], we became interested in intrastrain differences in drug response in the amygdala kindling model. When repeatedly treating large groups of amygdala-kindled Wistar outbred rats from the same breeder with phenytoin and measuring the effect on ADT, a subset of rats always responded with an ADT increase (responders), whereas another subset never responded (non-responders) [45]. Phenytoin resistance in non-responders extended to other anti-seizure drugs and was not due to pharmacokinetic differences (drug plasma levels at time of ADT determination) between responders and non-responders. By directly comparing the responder and non-responder subgroups, a variety of differences, including genetic factors, were found that may explain the drug resistance in the non-responders [46]. Thus, these data demonstrate that individual animals within a group of outbred rats may markedly differ in a disease model, thus replicating the intra-individual variability in human diseases. Such inter- individual variability in drug response was not found when kindled inbred strains of rats were used [47].
A similar phenomenon of striking inter-individual differences in drug responses in a model of TLE in an outbred rat strain was found in the BLA model in SD rats [48]. When prolonged treatment with phenobarbital was used to suppress SRS in this model, responders and non-responders to this treatment could be selected [48]. This experiment was repeated several times in a prospective manner, resulting in about 30% non-responders in all experiments, which is similar to the 30% of drug-resistant patients in epilepsy. Similar to the phenytoin experiments in kindled rats, phenobarbital resistance in non-responders extended to other anti-seizure drugs and was not due to pharmacokinetic differences between responders and non-responders. By directly comparing the responder and non-responder subgroups in the BLA model, a variety of differences were found that were similar to the risk factors of drug resistant epilepsy in patients, including high seizure density before onset of treatment, hippocampal damage, and behavioral abnormalities [46].
More recently, we found that phenobarbital responders and non-responders can also be selected from the pilocarpine model of TLE in SD rats [49]. Collectively, these findings from three models of TLE demonstrate that, similar to epilepsy patients, epileptic rats differ in their response to anti-seizure drugs, which is most likely due to as yet unknown genetic factors.
Drug responders and non-responders in such models are a unique resource for the investigation of mechanisms for drug resistance in epilepsy, particularly because pathophysiological processes in drug-resistant rats can be directly compared with those of rats which reproducibly respond to the same drug [50]. By using phenytoin-responding and nonresponding kindled rats and phenobarbital-responding and nonresponding epileptic rats of the BLA model, we discovered various differences between responders and non-responders, which, however, partly differed between the two models [50]. The only consistent difference in both models was higher expression of the drug efflux transporter P-glycoprotein at the blood-brain barrier in the epileptic focus, which would indicate that despite similar plasma levels the anti-seizure drug does not reach sufficiently high levels at its brain target(s) [50].
2.6 Intrastrain differences in Theiler’s virus mouse model of encephalitis-induced temporal lobe epilepsy
This model will be discussed in more detail in section 3.9. Recently, we compared the Theiler’s virus model of TLE in two B6 substrains, B6J (the strain used in the initial observations of Theiler’s murine encephalomyelitis virus (TMEV)-induced seizures by Libbey et al. [51] and Stewart et al. [52]) and B6JOlaHsd (B6H) [53]. As a result of genetic drift of B6H mice, since their separation from the Jackson breeding line, B6H and B6J mice are known to be different in many genotypic and phenotypic aspects, including a deletion of the alpha-synuclein locus in B6H mice, which encodes a presynaptic telencephalic protein that has been implicated in the etiology of Parkinson and Alzheimer diseases [23,54,55]. Following infection with the DA strain of TMEV, no differences in incidence of early and late seizures or hippocampal damage were observed between B6H and B6J mice, but some mouse substrain differences were observed with the BeAn strain of TMEV [53].
3. Interstrain differences in the expression of seizures and epilepsy in mice
Inbred strains of rodents have been used for many decades in biomedical research as a means to reduce experimental variability and enhance scientific reproducibility, particularly with regards to studies that are conducted by different research teams in different laboratories. With the creation of the DBA/1 and DBA/2 inbred mouse strains in the early 1900s, and the generation of additional families of inbred strains, including C3H, CBA and B6, several decades later, the biomedical research community gained access to an important and still- growing resource that now, through advances in genetic technology, computational genetics and bioinformatics, is being exploited to elucidate molecular mechanisms underlying quantitative traits.
A number of characteristics of the mouse species enhance its value with regards to the dissection of quantitative traits. These characteristics include intrinsic factors such as size and breeding habits as well as extrinsic factors such as availability of genome sequence and gene expression datasets. For example, at the present time, full genome sequence data for over 30 different inbred mouse strains is freely available online [56,57]. Access to specific genetically-defined strains of inbred mice by researchers across the globe now permits rapid advancement of scientific discovery in the realm of phenotype and genotype associations, and promises to help elucidate the pathophysiology of many common human illnesses.
To date, inbred mouse strains have been most useful in studying seizures and epilepsy because most forms of epilepsy are viewed as complex traits, that is, diseases that develop due to the influence of multiple genetic factors interacting with factors in the environment. Given this perspective, there is recognized value in conducting surveys of mouse inbred strains in order to define differences in seizure or epilepsy-related phenotypes which may ultimately be tractable to genetic dissection. Herein lies the power of using inbred strain surveys in that it limits genetic variability within the seizure model in the hopes of mapping candidate genetic loci. As documented by examples in Section 5, inbred mouse strain differences in seizure phenotypes are leading to the identification of genes and gene variants that are relevant to the etiology and potentially the treatment of human epilepsy.
3.1 Interstrain differences in mouse models of acute seizures and seizure susceptibility
Effects of mouse strain diversity on seizure threshold and susceptibility to acute, experimentally-induced seizures have been well documented for many years. Strain differences were initially characterized using seizure paradigms involving physical stimuli, such as high-intensity sound waves [58] or electric shock [59]. These studies were soon followed by others that involved the use of convulsant drugs [60]. Subsequently, many different types of physical and chemical stimuli have been applied to study seizure responses in different strains of mice. It is notable that the earliest studies of mouse strain differences in response to stimuli that cause acute seizures focused on comparing two strains, B6 and DBA/2. Overall, these studies documented that B6 mice are relatively resistant to experimental seizures, whereas in contrast, DBA/2 mice are relatively susceptible (reviewed in Ferraro [61]). Although there are exceptions to this generalization for certain seizure paradigms [62], this perspective served as an impetus to perform genetic mapping studies in these strains and to analyze potential biological factors that underlie observed strain differences. Many other strains have been studied; however, not as comprehensively as B6 and DBA mice.
Rodent models of acute seizures have long been the mainstay method for evaluating new anticonvulsant (anti-seizure) medications. Although a number of different seizure paradigms have been used, those involving determination of electrical seizure threshold and/or susceptibility to the convulsant effects of the compound pentylenetetrazol (PTZ) have traditionally been most utilized [63]. Since then, many mouse strain comparisons have been conducted using alternative seizure paradigms for inducing acute seizures. Below we highlight key studies that have compared inbred mouse strain differences in susceptibility to acutely-induced experimental seizures.
3.1.1 Physically-induced seizures
Physical stimuli of various types can induce seizures and have been used to compare the susceptibility of different inbred mouse strains. As noted above, one of the earliest published scientific studies on differences in seizure susceptibility between mouse strains involved the use of sound-induced (i.e., audiogenic) seizures. Such work documented that DBA/2 mice are significantly more susceptible to fatal audiogenic seizures compared to B6 mice [58]. Interestingly, susceptibility to audiogenic seizures in DBA/2 mice is observed primarily before the onset of sexual maturation or in young mice (although this could be related to this strains susceptibility to progressive hearing loss); however, there is a significant correlation between susceptibility to audiogenic seizures and the threshold for electroshock seizures at a later age [64]. Conversely, this relationship is not observed between susceptibility to audiogenic and hyperthermia-induced seizures [65], but this may be the result of developmental changes as well.
Mouse strain differences in the threshold for electrically-induced focal, generalized and maximal seizures have not been comprehensively studied; however, several published reports have shed some light on this issue [66-68]. Although important methodological differences exist between these studies with regard to parameters of stimulus delivery including the use of corneal [68] versus auricular [66,67] electrodes, a close correspondence between strain responses is apparent, particularly in the characterization of B6 mice as resistant and DBA mice as susceptible.
Another means of inducing acute seizures through physical stimulation involves elevation of body temperature. Such paradigms are intended as models of febrile seizures in humans, an important concept since febrile seizures in childhood may increase the risk for developing epilepsy later in life [69]. Relative to other models, published mouse strain comparisons for hyperthermia-induced seizures in mice are limited in number. One notable study compared seizure responses to hyperthermia in 6 inbred strains, and in contrast to results obtained in other seizure models, reported B6 as the most susceptible strain and DBA as most resistant [62]. This line of study suggests that a distinct set of genetic variants influences susceptibility to hyperthermia-induced seizures, relative to seizures induced by sound or electrical stimulation. However, caution is needed in comparing any strain difference studies in regards to seizures and epilepsy, since such differences in susceptibility may be related to the animal’s physical responsivity mechanisms to the stimuli (i.e., different responses to body/brain changes with increased body temperature between strains), and not necessarily genetic differences mediating seizure responses.
The EL strain of mice is somewhat unique in the expression of focal seizures that can be induced by simple handling or more reliably by rhythmic stimulation on a moving platform [70]. The absence of this phenotype in other inbred strains facilitated its genetic dissection into multiple quantitative trait loci (QTL), each exerting a partial influence over seizure susceptibility in this model [71,72].
3.1.2 Chemically-induced seizures
A wide variety of chemical agents have been utilized for comparing acute seizure susceptibility in different mouse strains, including traditional chemoconvulsants such as PTZ [73], picrotoxin [74], bicuculline [75], 3-mercaptopropionic acid [76], beta-carbolines [77,78], flurothyl [76], methionine sulfoximine [79], soman [80], kainic acid [81], isoniazid [60], and pilocarpine [82], as well as other drugs that are known to produce seizures clinically such as carbapenems [83], monobactams [84], anesthetic drugs [85], caffeine [77], cocaine [86], and nicotine [87]. Early studies established the relative susceptibility of DBA strains to chemoconvulsants compared to B6 strains [33], although DBA was reported to be one of the most resistant strains to nicotine-induced seizures [87]. Another notable study examined genetic correlations between strain differences in seizure response to 9 different chemoconvulsants including many of those listed above [34]. In addition to seizure expression, cell death in response to an episode of chemically-induced status epilepticus is also influenced by strain variation in mice [35,88].
3.1.3 Antiseizure drugs
Mouse strain differences have also been reported with regard to the anti-seizure effects of a wide variety of drugs, including benzodiazepines [89,90], phenobarbital [91], valproic acid [91], delta-aminolevulenic acid [92], MK-801 [93], levetiracetam [94], phenytoin [94], and neurosteroids [95]. These studies utilized diverse paradigms to induce acute seizures, but all documented significant effects of genetic background on measured responses. It is possible that some of these strain differences are due to differences in anti-seizure drug pharmacokinetic parameters [96], a factor that is sometimes overlooked but that may have important implications with regard to the interpretation of results. Overall, the results of studies published to date suggest that the anti-seizure effects of most standard drugs currently used to treat epilepsy vary across different inbred mouse strains, and interestingly this phenomenon is also observed in the human epilepsy population [97]. This variation may ultimately be useful in identifying genes that influence these responses. Such discoveries in mice would allow investigation of similar relationships in patients with epilepsy and possibly lead to biomarkers for prediction of therapeutic response.
3.2 Intrastrain differences in the 6-Hz psychomotor seizure model of partial epilepsy in mice
The 6-Hz focal seizure model is considered a useful tool for the discovery of anti- seizure compounds with potential activity against therapy-resistant epilepsy [98]. In this test, “psychomotor” seizures are induced via corneal stimulation (6 Hz, 0.2 ms rectangular pulse width, 3 s duration) in normal (non-epileptic) mice at supra-threshold current intensities of 32 or 44 mA. In the studies of the White group, adult male CF-1 outbred mice have been used [98]. In these mice, seizure became resistant to phenytoin and other drugs at a stimulation intensity of 44 mA. However, a recent study of Leclercq and Kaminski [94] showed that treatment resistance of 6-Hz seizures should be interpreted with strain and experimental conditions in mind. While CF-1 and B6 mice were resistant to phenytoin when stimulated at 44 mA, phenytoin suppressed 6-Hz seizures in NMRI mice at this stimulus intensity. NMRI mice were also more sensitive to the anti-seizure effects of levetiracetam in this model [94]. The authors concluded that strain differences, much like human genetic differences, may explain why some mice and patients respond to a given treatment and others do not.
3.3 Mouse strain differences in occurrence of spike-wave discharges
Laboratory mouse strains often serve as ‘normal’ controls for neurological and other studies, but as shown for many phenotypes, they are not necessarily wild-type, as they segregate natural genetic variants that either predispose to disease or cause it outright [99]. For instance, when screening 27 widely used inbred strains for the presence of spike-wave discharges (SWDs) in cortical EEG, occasional or frequent periods of bilateral synchronous 6-8 Hz SWD activity occurred in 9 of these strains, A/J, AKR/J, C3H/HeJ, C57BLKS/J, CBA/J, DBA/1J, DBA/2J, NOR/LtJ, SM/J [99]. This activity was associated by behavioral arrest and was suppressed by the anti-absence drug, ethosuximide, indicating that it represents epileptic absence-like activity (Letts et al., 2014). In the 18 other mouse strains (B6J, B6NJ, C57BL/10J, C57L/J, FVB/NJ, BALB/cByJ, BTBR<+>tf/J, I/LnJ, LP/J, MRL/LpJ, NOD/ShiLtJ, NZB/B1NJ, NZW/LacJ, P/J, PL/J, SJL/j, SWR/J, 129/SvlmJ), such SWD activity was not observed [99]. Knowledge of paroxysmal EEG activity in otherwise “normal” mice that are used as experimental controls is important because it may interfere with epilepsy or seizure models using such mouse strains. In outbred mouse strains such as NMRI or Swiss, no SWDs have been observed [100-102].
3.4 Interstrain differences in the pilocarpine model of temporal lobe epilepsy in mice
As in many other models of seizures and epilepsy, B6 mice are generally less susceptible to the convulsive effect and neurotoxicity of pilocarpine or other convulsant stimuli than most other mouse strains [34-36,48,66,103], which hampers the use of B6 mice and B6-based transgenic mice in epilepsy research. As shown by our intra-strain B6 studies (see section 2.1), this is particularly true for B6J (JAX) mice, in which, even when using a ramping-up dose protocol for pilocarpine, SE induction is only possible in about 10% of the mice (Fig. 2A). A large percentage of B6J animals only exhibited single seizures, often terminating in death, resulting in high mortality despite the pretreatment with methyl- scopolamine (Fig. 2B). The doses of pilocarpine to induce single seizures in B6J mice were much higher (500-900 mg/kg i.p.) compared to other B6 substrains or other strains of mice [32]. For instance, in the DBA/2J and A/J mouse strains, doses of 250-300 mg/kg pilocarpine induced SE in the majority of animals and mortality was low, particularly in DBA/2J mice [82].
Fig. 2.

C57BL substrain differences in flurothyl generalized seizure thresholds and in their final flurothyl-induced seizure phenotypes (following 8 seizures, a 28-day incubation phase, and a final flurothyl challenge). A) The latency to a generalized clonic-forebrain seizure (generalized seizure threshold (GST)) on each seizure trial was determined for 5 C57BL substrains by exposure to 10% flurothyl during eight induction trials followed by a 28-day rest period and a single flurothyl retest. B) While none of the 6NJ and KSJ mice expressed a more complex forebrain→brainstem seizure on flurothyl rechallenge, 25% of 10SNJ mice, 25% of 10J mice, and 80% of 6J mice did express a more complex forebrain→brainstem seizure. Forebrain→brainstem seizures are seizures in which the mouse has a generalized forebrain-clonic seizure that rapidly and uninterruptedly progresses into a seizure with tonic- brainstem components to the seizure. These differences in GST and seizure phenotype between these closely related strains of C57BL mice demonstrate the power of the genetic background on seizure measurements. Data are modified from Kadiyala et al. [116].
Schauwecker [88] characterized neuronal pathologies after pilocarpine-induced SE in eight inbred strains of mice focusing on the hippocampus. As in our studies, a ramping-up dose protocol for pilocarpine was used. Although somewhat surprising, and in contrast to prior studies of mouse strain seizure response to pilocarpine [82], no significant differences in seizure latency or duration to pilocarpine were observed among the inbred strains, although all mice were from the same source (Jackson Laboratory); however, a significant difference in susceptibility to the neuropathological consequences of pilocarpine-induced SE was observed. Of the eight genetically diverse mouse strains screened for pilocarpine-induced SE (A/J, AKR/J, BALB/cByJ, BALB/cJ, CBA/J, C3H/HeJ, B6J, DBA/2J, FVB/NJ, SJL/J and 129T2/SvJ), only two strains, BALB/cJ and BALB/cByJ were resistant seizure-induced cell death [88]. These results are somewhat in contrast to the findings of Mohajeri et al. [104], who reported that hippocampal neurons in B6 mice were most resistant to cell death, whereas they were highly vulnerable in FVB/N mice.
3.5 Interstrain differences in the kainate model of temporal lobe epilepsy in mice
As with systemic administration of pilocarpine, systemic administration of kainate is a standard model of TLE [4]. Schauwecker and Stewart [35] reported that certain strains of mice, including strains that are commonly used for gene targeting/transgenic studies, do not exhibit excitotoxic cell death after kainate-induced seizures. Kainate produced excitotoxic cell death in the CA3 and CA1 subfields of the hippocampus in 129/SvEMS and FVB/N mice, in the same pattern as described in rats. B6 and BALB/c mice exhibited excitotoxic cell death only at very high doses of kainate, and then only in a very restricted area, although again the strains were reported to exhibit comparable seizures. Subsequent experiments with ionotropic glutamate receptor agonists in B6 and FVB/N mice provided further evidence that susceptibility to excitotoxin-induced cell death is highly strain-dependent and is kainate- and NMDA-receptor-dependent [105]. Results of phenotypic analysis suggested that the difference in susceptibility between these two strains is conferred by a single dominant gene on chromosome 18 [106]. However, none of these studies examined the effect of mouse strain on kainate-induced seizures or development of epilepsy with SRS in detail, leaving open the possibility that cell death was related to the cumulative amount of seizure activity.
In a study by the Schwartzkroin group, the impact of genetic background on mouse strain seizure susceptibility, seizure phenotype, mortality, and hippocampal histopathology was evaluated with a subcutaneous kainate multiple injection protocol [107]. Five mouse strains were tested: B6J, 129/SvJ, C3HeB/FeJ, 129/SvEms, and a mixed genetic background strain (129/SvJ × B6J). When injected with kainate, C3HeB/FeJ and B6J strains were resistant to cell death and synaptic reorganization despite severe behavioral seizures, while 129/SvEms mice developed marked pyramidal cell loss and mossy fiber sprouting despite limited seizure activity. The mixed background 129/SvJ × B6J group exhibited features of both parental strains. In the mouse strains tested, the duration and severity of seizure activity was not predictive of subsequent hippocampal pyramidal cell death and/or synaptic reorganization. Unlike rats, mice exhibiting prolonged high-grade kainate-induced seizure activity did not develop subsequent spontaneous behavioral seizures [107].
3.6 Interstrain differences in the intrahippocampal kainate model of temporal lobe epilepsy in mice
The intrahippocampal kainate mouse model of mesial TLE is increasingly used in the search for anti-seizure and anti-epileptogenic drugs [50]. Induction of SE by unilateral intrahippocampal injection of kainate is associated with almost no mortality, and most mice develop ipsilateral hippocampal damage, highly frequent non-convulsive electrographic seizures, and less frequent convulsive seizures after SE. For this model, both inbred (B6, FVB/N) and outbred (Swiss, NMRI) mouse strains have been used without any obvious inter- strain differences. However, we recently reported that the latent period after kainate-induced SE before onset of SRS varies as a function of mouse strain and sex [101]. A clear seizure- free latent period was only observed in male NMRI mice, but not in female NMRI, B6 or FVB/N mice. In a study by the Pitkänen group, using continuous (24/7) video/EEG monitoring after SE in male B6 mice and defining a seizure by an electrographic event of at least 10 sec, the latent period to the first spontaneous seizure ranged from 1-6 days with a median of 2 days, so that the authors concluded that the mice developed epilepsy without any remarkable latency period [106]. This was substantiated in a subsequent study by the same group [107], in which the first generalized convulsive seizure was observed 1-2 days after intrahippocampal injection of kainate in male B6 mice. Together, these data suggest that mouse strain rather than sex explains our findings on strain differences in the latent period of the intrahippocampal kainate model [101]. Lastly, further support for a lack of a latent period in mice comes from studies utilizing 8 repeated flurothyl-induced seizures, in which both B6J and DBA2/J mice develop SRS with no latent period [110, 111].
In a subsequent study, we found that the mouse strain also affected the occurrence of different types of spontaneous electrographic seizures and their pharmacological sensitivity in this model [102]. The typical electrographic seizures observed in epileptic mice are high- voltage sharp waves (HVSWs) and hippocampal paroxysmal discharges (HPDs) [100-102]. Epileptic FVB/N mice predominantly exhibited frequent HVSWs, but only infrequent HPDs, whereas NMRI mice exhibited both HVSWs and HPDs. NMRI mice were more sensitive to the anti-seizure effect of carbamazepine than FVB/N mice [102].
3.7 Interstrain differences in the electrical kindling model of temporal lobe epilepsy in mice
To our knowledge, Leech and McIntyre [112] were the first to describe differences in electrical kindling rates in inbred mouse strains. In a subsequent study [113], olfactory bulb kindling rates were studied in two inbred strains of genetically seizure-prone mice (DBA/2 and EL) and in three non-epileptic inbred strains (B6, ddY, and C3H/He). The seizure sensitive DBA/2 strain required the fewest number of stimulations to reach stage 5 seizures, whereas C3H/He mice required the most stimulations to reach stage 5 seizures. Kindling rates for B6 and ddY strains were intermediate, and the kindling rate for each strain was significantly different from that of the other strains. These findings showed that the seizure- susceptible EL mouse kindles more rapidly than the genetically similar ddY control mice and suggested that an inherited seizure susceptibility gene(s) can produce accelerated kindling rates. B6 mice kindled more rapidly than EL mice, however, suggesting that genetic factors other than those that influence seizure susceptibility are of primary importance in the determination of kindling rate [113].
3.8 Interstrain differences in the flurothyl kindling mouse model of primary generalized epilepsies
With flurothyl kindling, adult mice are exposed to the inhalant chemoconvulsant, flurothyl (GABAA antagonist) infused into a closed chamber. The latency to a generalized seizure, identified by a loss of postural control when sufficient drug is inhaled, serves as a measure of generalized seizure threshold (GST). At the start of the generalized clonic seizure, the chamber top is removed, allowing seizure recovery. Seizures are repeated daily for 8-days and most mice express have generalized clonic-forebrain seizures during this phase. During this induction phase, B6J mice will have decreases in their GST (kindling) that plateaus around trials 4-5. After a 28-day incubation-phase when mice are left in their home cage, they are retested with flurothyl and GST and seizure behavior are scored a final time. On rechallenge, over 80% of B6J mice have seizures that begin as a generalized clonic-forebrain seizure that transition into tonic-brainstem seizures upon retest. Such seizures are described as forebrain→brainstem seizures to denote their ictal propagation. As an alternative to electrical kindling, which utilizes depth electrodes, this flurothyl kindling model is a non-invasive method of epileptogenesis [114]. Papandrea et al. [115] investigated flurothyl kindling in five inbred mouse strains (DBA/2J, B6J, 129S1/SvImJ, BALB/cJ, and C3H/HeJ). The five strains of mice demonstrated inter-strain differences in initial flurothyl-induced generalized seizure threshold (GST), decreases in GST across seizure exposures (kindling), and differences in the types of behavioral seizures observed. Since many of the seizure characteristics that were examined in the flurothyl kindling model were dissociable between B6J and DBA/2J mice, these strains were analyzed in detail. Unlike B6J mice, DBA/2J mice had a lower initial GST on the first flurothyl exposure, did not demonstrate a decrease in GST with repeated flurothyl exposures (did not kindle), nor did they show a change in seizure phenotype upon a flurothyl rechallenge following a 1 month incubation period (did not express a forebrain→brainstem seizure as B6J mice do) [115]. Surprisingly, B6J X DBA/2J F1-hybrids had an initial GST on the first flurothyl trial and a GST decrease across flurothyl exposures similar to what was observed in B6J mice, but these F1-hybrids did not undergo the alteration in behavioral seizure phenotype that is observed in B6J mice. These F1-hybrid data demonstrate that initial GST, decreases in GST across flurothyl trials (kindling), and the change in seizure phenotype after a 28-day incubation period differ from the parental strains, suggesting that these phenotypes are controlled by independent genetic loci [115]. In fact, we have now completed an inbred stain survey of over 70 mouse strains, further confirming that the phenotypes observed with repeated flurothyl exposures are influenced by genetic factors and have led to the identification of genetic loci controlling these seizure traits (manuscript under review).
To further demonstrate the sensitivity of seizure traits in inbred mouse strains, we examined flurothyl kindling in closely related mice of the C57BL lineage (C57BL/10J, C57BL/10SNJ, C57BL/6J, C57BL/6NJ and C57BLKS/J) revealed significant diversity in the seizure characteristics observed with repeated flurothyl exposure (Fig. 2) [116]. Such differences further highlight the importance of genetic background in assessing the effects of genes in preclinical epilepsy models.
The repeated flurothyl seizure model also permits analysis of myoclonus (sudden involuntary shock-like movements of the head and body). Similar to the generalized seizure characteristics observed in inbred mice, myoclonus also demonstrated evidence for genetic heterogeneity among inbred mouse strains [117].
Recently,the repeated flurothyl seizure model has been shown to produce SRS in both B6J and DBA2/J mice without an obvious latent period and without hippocampal cell death [110,111]. These SRS could be blocked with valproate, but not with phenytoin [110]. Interestingly, the spontaneous seizures observed in B6J mice following flurothyl kindling remitted without any treatment after 1 month, but did not remit in DBA/2J mice; however B6J mice tended to have more spontaneous seizures than DBA/2J mice [110,111]. Not only does this implicate a genetic influence on whether seizures remit with flurothyl kindling, but also allows for the possibility that differing inbred strains of mice have differential spontaneous seizure characteristics that are amenable to genetic mapping.
Lastly, genetic studies in rodents taking advantage of interstrain variability have been critical for the discovery of seizure susceptibility loci. However, genetic analyses of epilepsy phenotypes in mice from diverse background strains have been predominantly performed through assays examining baseline seizure threshold. With the repeated flurothyl seizure model, we followed seizure susceptibility in >70 inbred genetic mouse reference populations over 36 days. Importantly, our baseline seizure threshold differences mapped to the well- characterized seizure susceptibility locus on chromosome 1 [67,118]. However, with repeated seizures the influence of this seizure susceptibility locus on chromosome 1 diminished with additional flurothyl-induced seizures and decreases in generalized seizure threshold. Administration of eight seizures followed by an incubation period revealed novel associations on chromosome 4 which are further being refined [119]. Such an approach demonstrates that novel genetic associations can become apparent with repeated seizures that would not otherwise be found with single seizures. Thus, inducing repeated seizures allowed for the observation of shifts in genetic associations to novel genomic regions containing genes suitable for facilitating epileptogenesis [119].
3.9 Interstrain differences in Theiler’s virus mouse model of encephalitis-induced temporal lobe epilepsy
Infections of the brain are among the most common risk factors for seizures and acquired epilepsy, but until recently no rodent models for infection-induced epilepsy were available, possibly because most infectious agents are associated with high mortality in rodents [120]. One exception is a recently developed mouse model of virus encephalitis that mirrors many of the features of viral encephalitis-induced epilepsy in humans [121]. In this model, in which encephalitis is induced by intracerebral infection with Theiler’s murine encephalomyelitis virus (TMEV) in B6 mice, mice survive the infection and about 50% of them develop early and late seizures and hippocampal damage [120,121]. The late seizures are not persistent effects of the virus (the virus is rapidly cleared from the brain of B6 mice following infection), but this model leads to a permanent epilepsy, as is so often the case in humans surviving virus-induced encephalitis [120]. However, if the virus is inoculated in another mouse strain, SJL/J mice, which in contrast to B6 are T cell defective [122], the TMEV virus persists and induces inflammatory demyelination with oligodendrocyte apoptosis and axonal degeneration in the white matter of the spinal cord (a widely used model of multiple sclerosis [123]), demonstrating a striking mouse strain-specific SRS response to the virus.
3.10 Interstrain differences in the expression of sudden unexpected death in epilepsy (SUDEP)
SUDEP is a multi-factorial outcome, which can take the lives of those with epilepsy without warning [124]. Preventing SUDEP in humans is problematic given its unpredictable nature, but progress has been made by characterizing mechanisms causing seizure-related death in genetically engineered mice. There are a few genes known to contribute to SUDEP, of which include dominant truncating mutations in SCN1A that are found in many individuals with Dravet syndrome [125]. Given that Dravet syndrome can sometimes be inherited from a mildly affected parent with the same mutation, this clearly indicates additional factors that can modify the effects of SCN1A [126]. In the mouse model of heterozygous Scn1a-mediated Dravet syndrome, Scn1a+/− mice present with spontaneous seizures and premature lethality [127,128]. Originally, these Scn1a+/− mice were on a mixed genetic background. However, the phenotypic severity observed in Scn1a+/− mice was strongly dependent on background strain. Scn1a+/− mice on a 129S6/SvEvTac background showed no overt phenotype, whereas in a C57BL/6J × 129S6/SvEvTac F1background, these mice demonstrated spontaneous seizures and early lethality [126]. Subsequent mapping and congenic analyses of Dravet survival modifier loci (Dsm) have implicated differential expression of Gabra2 as a potential modifier in the lethality observed in Dravet syndrome [129]. This work further highlights that seizure occurrence and outcomes (i.e., SUDEP) can be modified depending on the choice of genetic background, but appropriately designed crosses can be utilized to successfully identify modifying genes.
4. Interstrain differences in the expression of seizures and epilepsy in rats
Similar to mice, differing rat strains have a marked effect on expression of seizures and epilepsy in a variety of models [1,10,50]. In most studies on seizures and epilepsy in rats, outbred strains such as Wistar or SD are used, which increases variability of the data because of the intrastrain genetic differences in such outbred animals (see section 2).
4.1 Interstrain differences in the pilocarpine model of temporal lobe epilepsy in rats
As in mice (see section 2.1), all studies with pilocarpine in rats used pretreatment with a peripherally acting anticholinergic drugs such as methyl-scopolamine to minimize the peripheral cholinomimetic adverse effects of the convulsant. Most studies on the pilocarpine model of TLE have been carried out in Wistar rats [29]. Results from SD rats have not shown major differences in the development of behavioral and electrographic alterations [130,131], while ameliorating the mortality rates [29]. In a recent study in which SE was induced in female Wistar and SD rats by lithium-pilocarpine [37], Wistar and SD rats obtained from the same breeder (Harlan-Winkelmann) in Germany did not differ in SE induction (68 vs. 74%) or percent of rats developing SRS (90 vs. 100%)(Table 1), whereas SD rats from other breeders were less sensitive (see section 2.2). Pilocarpine effects were more pronounced in Long-Evans rats, in which a higher mortality rate, larger damage to the hippocampus and a behavioral outcome worse than in Wistar rats was found [132]. However, Xu et al. [133] found no differences in SE induction or severity by pilocarpine and subsequent development of SRS between Wistar and Long-Evans rats.
4.2 Interstrain differences in the electrical kindling model of temporal lobe epilepsy in rats
The amygdala kindling model in rats is one of the most widely used models of TLE and the only chronic epilepsy model that has been validated clinically [50,134]. In most studies on kindling in rats, outbred strains such as Wistar have been used. Racine et al. [135] reported that Wistar rats required a significantly higher number of stimulations to fully kindle than did SD rats or Royal Victoria Hooded rats. We compared rates of amygdala kindling development in two outbred (SD, Wistar) and five inbred (Lewis, Fischer 344, ACI, Wistar- Kyoto, Brown Norway) rat strains, including several strains which have not been kindled before [136]. We were particularly interested to learn which parts of the stepwise progression of kindling differ among these strains. Furthermore, the sensitivity of the BLA to electrical stimulation was determined before and after kindling. Once-daily electrical stimulation of the BLA resulted in marked differences in kindling rates between the 7 strains, with SD and Brown-Norway rats requiring the lowest number of stimulations to reach fully kindled (stage 5) seizures, and Lewis rats requiring the highest number of stimulations (Fig. 3A). In contrast to the significant strain differences in number of stimulations required to reach the fully kindled state, total (cumulative) ADD to reach stage 5 did not significantly differ among strains (Fig. 3B), substantiating that cumulative ADD is the principal factor in the acquisition of kindled seizures (although Fischer 344 and Lewis rats showed a trend for higher cumulative ADD than other strains; cf., Fig. 3B). Nevertheless, when analyzing whether the two estimates of kindling rate are correlated, a significant correlation was obtained (Fig. 3C). Post-kindling ADT varied significantly among strains, but only 3 of the 7 strains showed a decrease of ADT compared to pre-kindling values. When the stepwise progression of kindling was evaluated, pronounced interstrain differences were observed in the time spent in the initial phase of kindling (i.e., stage 1 seizures), both in terms of stimulations and cumulative ADD, indicating that variations in kindling rates were predominantly due to the time needed to progress from stage 1 to subsequent stages of the kindling process. These data indicate that inbred rat strains offer a potentially valuable resource for dissecting the underlying genetic basis for phenotypic differences in epileptogenesis as assessed by electrical kindling.
Fig. 3.

Kindling rate in seven outbred and inbred rat strains. Rats were kindled by once daily stimulation of the basolateral amygdala with a stimulus intensity of 500 μA for 1 sec. A: Number of daily stimulations to first fully kindled stage 5 seizure. ANOVA on ranks indicated a highly significant differences between strains (P<0.001). B: Cumulative afterdischarge duration (ADD) to to first fully kindled stage 5 seizure. ANOVA on ranks indicated no significant differences between strains (P=0.09), although F344 and Lewis rats showed a trend for high ADD. C: Correlation between the two estimates of kindling rate shown in A and B. A significant linear correlation (r2 = 0.6282, P = 0.0335) was calculated between the two estimates of kindling rate. Data are from Löscher et al. [136]. Abbreviations: BN, Brown Norway; SD, Sprague-Dawley; WK, Wistar Kyoto; F344, Fischer 344.
4.3 Interstrain differences in a rat model of temporal lobe epilepsy resulting from sustained stimulation of the basolateral amygdala
As described in section 2.3, electrical induction of SE in rats via a depth electrode in the BLA produces a brain insult with subsequent development of neurodegeneration, SRS, and cognitive and behavioral alterations resembling the long-term consequences of epileptogenic brain insults in humans [38]. When inducing SE by sustained electrical stimulation of the BLA, SD rats were more sensitive to SE induction than Wistar rats [39]. As shown in Table 1, when comparing Wistar and SD rats from the same breeder (Harlan- Winkelmann) in Germany, SE could be induced in 56/77 (73%) Wistar rats vs. 236/255 (93%) SD rats (P<0.0001). Development of SRS after SE was similar in both strains (Table 1).
4.4 Rat strain differences in occurrence of spike-wave discharges or other EEG alterations
In adult Wistar and Long-Evans rats, short-lasting EEG episodes of medium-voltage 5-9 Hz (mean = 6 Hz) cortical oscillations, which were distinguishable from sleep spindles in internal frequency, duration, morphology, and moment of occurrence, were described as normal physiological EEG activity [137,138]. Extensive analysis in Wistar rats has shown that 5–9 Hz oscillations alone generally do not lead to seizure activity; however, epileptic SWD activity may emerge following induced or natural genetic alterations, such as in the GAERS (Genetic Absence Epilepsy Rat from Strasbourg) Wistar rat [137]. As observed in mouse strains, several outbred and inbred rat strains exhibit bilaterally symmetrical SWDs (8-11 Hz; sometimes also termed high-voltage rhythmic spike [HVRS] discharges, high-voltage cortical oscillations, or electrocortical polyspiking [139-142]) in the cortical EEG. These EEG alterations are associated with sudden arrest of ongoing behavior (immobility) involving occasional facial/whisker twitching, and can be suppressed by ethosuximide, thus behaving as absence-like seizure activity [143-146]. Such SWDs may be age-dependent in a specific strain of rats (e.g., they were not observed in cortical EEG recordings of the pre-adult male SD rats used for induction of traumatic brain injury by D’Ambrosio et al. [147]).
In addition to low-frequency 5-9 Hz oscillations or SWDs, high frequency oscillations (HFOs) at frequencies in the range of 30–600 Hz occur in several cortical and subcortical brain areas of rats [148]. For instance, large amplitude local field potentials (‘sharp waves’; SPWs) occur irregularly in the hippocampal CA1 stratum radiatum of normal rats when the animal has minimal or no interaction with its environment, such as immobility, consummatory behaviors or slow wave sleep [148]. SPWs reflect the depolarization of the apical dendrites of CA1 and CA3 pyramidal cells, due to the synchronous bursting of CA3 pyramidal cells. Such physiological population patterns in the brain are characterized by their strict bounds of both duration and synchrony [148]. This organization is in sharp contrast to epileptic patterns in which scale-free behavior dominates and, therefore event magnitudes vary by several orders of magnitude [148-150]. The distinction between normal and pathologic HFOs (or any other type of oscillation in the cortical or subcortical EEG) can only be made by directly comparing epileptic animals with sham controls. In addition, the lesion associated with implantation of a depth electrode into the limbic system may induce pro- epileptogenic brain alterations, including blood-brain barrier disruption, chronic inflammation, decreases in seizure threshold, and epileptiform discharges in the hippocampus [50,151,152], so that it is important to include adequate sham controls when studying video- EEG alterations in rodent models of acquired epilepsy.
5. Dissection of genes that may underlie differences in expression of seizures or epilepsy
Classical, forward genetic mapping studies of seizures and epilepsy-related phenotypes have taken advantage of a number of the strain differences discussed in Section 3 and have led to the identification of a diverse set of genes that may be useful to consider in regard to elucidating biological mechanisms of seizures and epilepsy and developing new therapies for epilepsy patients. In general, epilepsy-related genes identified using inbred mice are difficult to validate because, like other complex trait genes, they have only a partial effect on determination of the phenotype, and sometimes this effect is weak or modest. Translation of putative epilepsy-related mouse genes to human epilepsy patients is one criterion that is viewed as helping to establish the biological significance of mouse gene variants. Below we review examples where the identification of gene variants associated with seizure susceptibility or other epilepsy-related phenotypes in mice is accompanied by validation in human epilepsy populations.
5.1 Signal-recognition-particle assembly 9 (Srp9)
Quantitative trait loci (QTL) mapping experiments were used to dissect the genetic factors that mediate mouse strain differences in susceptibility to seizures induced by hyperthermia [62]. These studies led to the identification of Srp9 as a major underlying factor [153]. Signal-recognition-particle (SRP) assembly has a crucial role in targeting secretory proteins to the rough endoplasmic reticulum membrane. SRP9 together with SRP14 and the Alu portion of the SRP RNA, constitutes the elongation arrest domain of SRP. The complex of SRP9 and SRP14 is required for SRP RNA binding. Mice with reduced Srp9 expression and febrile seizure susceptibility exhibited reduced hippocampal AMPA and NMDA currents and downregulation of neuronal Srp9 reduced surface expression of the AMPA receptor subunit, GluA1 [153]. In the same study, it was shown that individuals with mesial TLE (mTLE) that had antecedent febrile seizures had higher SRP9 expression than patients without febrile seizures and that SRP9 promoter SNP rs12403575(G/A) was genetically associated with febrile seizures and mTLE [153]. These results identify SRP9 as a novel febrile seizure susceptibility gene and indicate that SRP9 conveys its effects through endoplasmic reticulum- dependent trafficking of membrane proteins, such as glutamate receptor subunits. This discovery may provide new leads for early diagnosis and treatment of children with complex febrile seizures at risk for mTLE.
5.2 Potassium voltage-gated channel subfamily J member 10 (Kcnj10)
A gene that has been proposed as underlying a major portion of differences in seizure susceptibility between B6 and DBA mice is Kcnj10. This gene was first mapped as a major chromosome 1 QTL in several studies including one involving kainic acid seizure susceptibility [120], another involving PTZ seizure susceptibility [154] and a third involving maximal electroshock seizure (MES) threshold [155]. These QTL studies were followed by congenic strain studies in which the chromosome 1 QTL was confirmed and the Kcnj10 variant, Thr262Ser, was proposed as causative [67,156]. The gene was shown by genetic association to harbor common variants that influence susceptibility to sporadic forms of human epilepsy [157]; a discovery that was confirmed by several laboratories [158-161]. The KCNJ10 gene was also subsequently shown to harbor rare variants that eliminate or that are highly detrimental to the function of the KCNJ10 protein product (Kir4.1), which cause major medical syndromes that include epilepsy as one component of a constellation of other abnormalities including ataxia, deafness and renal dysfunction [162,163]. Kir4.1 channels are responsible for potassium buffering in glial cells in the brain; a critical function as sustained elevation (or depression) of extracellular potassium levels in the brain can result in neuronal hyperexcitability and may contribute to initiation of a seizure. Thus, the identification of Kcnj10 as a seizure susceptibility gene in mice, and then an epilepsy gene in humans, suggests a novel target and mechanism for the development of new epilepsy therapies.
5.3 Multiple PDZ domain crumbs cell polarity complex component (Mpdz)
QTL mapping studies based on the robust difference in susceptibility to ethanol withdrawal seizures between B6 and DBA mice led to the discovery that Mpdz plays an important role in controlling neuronal hyperexcitability [164]. The protein encoded by the Mpdz gene has multiple PDZ domains, which are hallmarks of protein-protein interactions. It has been reported to interact with serotonin HTR2C receptors as well as NMDA receptors and may affect synaptic plasticity in excitatory synapses. QTL-follow-up studies with congenic strains led to the identification of regulatory sequence differences in Mpdz between B6 and DBA mice that impacted gene expression levels and correlated with ethanol withdrawal seizure susceptibility [165]. The role of Mpdz in this model was validated using knockout and transgenic approaches [166]. Although translation of these findings to human epilepsy has not been reported, genetic association studies suggest that MPDZ variation in humans may influence the risk for the development of alcohol use disorder [167].
5.4 Cholinergic receptor genes
A relationship between cholinergic neurotransmission and seizures or epilepsy has long been established. Drugs that interact with nicotinic or muscarinic receptors are able to elicit seizures in animal models as well as humans. Human genetic studies have discovered that variation in genes that encode cholinergic receptor subunits influence the risk for epilepsy [168-171]. Mutations in the genes encoding for CHRNA4 or CHRNB2 subunits of the neuronal nicotinic acetylcholine receptor are found specifically in familial nocturnal frontal lobe epilepsy [172,173]. Mouse strain surveys on acute seizure response to nicotine [166] led to studies of C3H and DBA strains and the discovery of an association between seizure response to nicotine and the density of alpha-bungarotoxin binding sites in the brain [174]. Evidence has also been reported to support a role for both the Chrna4 and Chrna6 gene variants in differences between mouse strains in susceptibility to nicotine-induced seizures [175,176]. This body of work supports continued efforts to develop new ways to modulate cholinergic neurotransmission for treating epilepsy.
5.5 Galanin Receptor 1 (Galr1)
Neurotransmission involving the neuropeptide galanin has been implicated in the control of seizure activity by numerous lines of research. Early work showing that exogenous galanin injected into the brain of rats could suppress chemically-kindled seizures [177] led ultimately to the creation of galanin receptor knockout mice which were shown to exhibit spontaneous seizures [178]. Subsequent creation of galanin receptor 2 (Galr2) knockout mice and demonstration that they have normal seizure susceptibility [179] indicated that the anti-seizure effects of galanin and galanin receptor agonists are mediated via Galr1. Interestingly, work using inbred mouse strains suggested that genetic polymorphisms affecting brain Galr1 expression underlie strain differences in excitotoxic cell death [180] and further supported the concept of Galr1 as an epilepsy susceptibility gene. The relevance of galanin to human epilepsy was recently confirmed through the identification of a galanin gene mutation determined as causative for temporal lobe epilepsy in a pair of monozygotic twins [181].
6. Role of environment (at vendor and/or laboratory) and epigenetic factors for inter- and intra-strain differences
In addition to genetic differences in mice and rat strains, various other confounding variables can affect the expression of seizures or epilepsy (Fig. 4). These factors can lead to troublesome variability in rodent studies, and should be carefully considered in designing and interpreting in vivo experiments [6,182-184].
Fig. 4.

Confounding variables at animal vendor and in the laboratory that may affect the experimental outcome of rodent studies on seizure expression, drug activities or behavior. Several of the environmental variables shown may induce epigenetic changes. For more details see text and Brown et al. [182] and Schellinck et al. [6].
A very impressive example of the magnitude of the problem was reported by Crabbe et al. in 1999 [185]. In this study, possible confounding influences of the laboratory environment were studied in mice of both sexes of several inbred strains (A/J, BALB/cByJ, B6J, DBA/2J, 129/Sv-ter, and 129/SvEvTac) and one null mutant by simultaneous testing in three laboratories on a battery of six behaviors. Each mouse was given the same order of tests (Day 1: locomotor activity in an open field; Day 2: an anxiety test, exploration of two enclosed and two open arms of an elevated plus maze; Day 3: walking and balancing on a rotating rod; Day 4: learning to swim to a visible platform; Day 5: locomotor activation after cocaine injection; Days 6 to 11: preference for drinking ethanol versus tap water). Inbred mice were obtained from the same breeders (Jackson Laboratory or Taconic Farms). Apparatus, test protocols, and many environmental variables were rigorously equated. Furthermore, because there is evidence that behavior differs depending on whether mice are obtained from a commercial vendor or are bred “in house” [186,187], animals bred locally were included in the study. Mice either shipped (5 weeks before testing) or bred locally were compared at the same age (77 days) starting at the same time (08:30 to 09:00 h local time on 20 April 1998) in all three labs. Despite these efforts to equate laboratory environments, significant and, in some cases, large effects of site were found for nearly all variables. Furthermore, the pattern of strain differences varied substantially among the sites for several tests. Sex differences were only occasionally detected, and there were almost no effects of shipping animals before testing. The authors concluded that experiments characterizing behaviors of inbred mice or mutants may yield results that are idiosyncratic to a particular laboratory.
In addition to variables in the laboratory environment, housing and handling conditions at the breeder/vendor or in an Institution’s animal facility may markedly influence the later behavior of rodents in a laboratory [4](Fig. 4). Environmental variables, such as those shown in Fig. 4, can have epigenetic effects on behavioral phenotypes, i.e., effects on chromatin regulation and DNA methylation [6]. Thus, two animals of the same genotype reared under different conditions may show different phenotypes. Because the rearing environment may have long-term epigenetic effects on the brain and behavior, the rearing environment of a rodent may not only affect their behavior, but that of their F1 and F2 generations [6]. Thus, the behavioral phenotype of an individual mouse or rat is the product of a complex environment–epigenetic–genome interaction [6].
Table 2 illustrates housing and handling conditions of two outbred rat strains (Wistar and SD) at five different vendors to illustrate typical differences in such variables. One may argue that although such variables may affect complex behaviors of rodents in sophisticated test batteries, they are unlikely to affect robust manipulations such as induction of seizures. However, although electrical kindling is a robust manipulation, differences in housing conditions of rodents have been described to influence the kindling process [188,189], although data are not consistent. Thus, Young et al. [189] reported that environmental enrichment facilitates amygdala kindling, but reduces kindling-induced fear in male rats, while Auvergne et al. [188] reported that enriched environment results in delayed kindling epileptogenesis and increased neurogenesis in adult rats. In another epilepsy model involving transgenic mice harboring a missense mutation of the sodium channel Scn2a [190], it was found that environmental enrichment from birth reduces spontaneous seizures and neuronal damage. The beneficial effects of environmental enrichment against seizures and seizure- induced cell death were initially shown in kainate–treated rats [191]. Indeed, based on its known effects on neuronal plasticity, environmental enrichment has been proposed as a possible strategy for preventing or treating chronic TLE [192]. Decades of research using environmental enrichment has demonstrated beneficial effects on brain and behavior in both wild-type and genetically modified rodent models, relative to standard-housed littermate controls [193]. Environmental enrichment, including environmental and cognitive stimulation with inanimate objects and opportunities for physical exercise, reduces the stress and anxiety associated with housing of rodents in social isolation. In turn, traditional housing without enrichment, particularly when animals are singly housed as used in many laboratories for models of epilepsy with chronically EEG electrode-implanted rodents, will produce stress and anxiety. It is long known that environmental or social stressors can increase seizure susceptibility in rodents (and patients) [194]. So these factors alone may have a marked effect on the expression of seizures in rodent models.
Table 2.
Housing and handling conditions of two outbred rat strains (Wistar and Sprague-Dawley) in breeding facilities at five different vendors (Harlan- Winkelmann, Harlan, Charles River, Janvier, and Taconic) and consequences for behavioral testing in the Loscher lab. Note that only Janvier performed daily handling habituation, while all other vendors handled the rats only shortly once to twice per week during cage cleaning. Data are from Langer et al. [19] and Honndorf et al. [40]. All data are from female rats (which were housed without males once arriving in the Loscher lab). See Theilmann et al. [217] for experiments in male rats, which resulted in similar findings. Abbreviations: n.t., not tested.
| Sprague-Dawley | Wistar | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Harlan- Winkelmann (HW) |
Harlan | Charles River (CR) |
Janvier | Taconic | Harlan- Winkelmann (HW) |
Charles River (CR) | Janvier | Taconic | |
| Housing and handling conditions at vendor | |||||||||
| Number of barriers | One | One to two | Two (closed every 5 years) | Several | One | Several | One | ||
| Type of cage | Transparent | Non transparent | Transparent | Non transparent | Transparent | Transparent | Transparent | Non-transparent | Transparent |
| Environmental enrichment in cages | Yes (tissue, PVC channels) | Yes (tissue, PVC channels) | No | No | Yes (tissue, wood, rat shelters) | Yes (tissue) | No | No | Yes (tissue, wood, rat shelters) |
| Group caged | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Transfer to new cage | 1/week | 1/week | 2/week | 1/week | 2/week | 1/week | 2/week | 1/week | 2/week |
| Estimating body weight | 3-4/week | 1-2/week | 1-2/week | 1/week | 3-4/week | 1-2/week | 1-2/week | ||
| Socialization to humans (handling to habituate rats to humans) | No | No | No | 6 times per week | No | No | No | 6 times per week | No |
| Male rats housed in the same room | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Light/dark cycle | 12 h/12 h | 12 h/12 h | 12 h/12 h | 12 h/12 h | 12 h/12 h | 12 h/12 h | 12 h/12 h | 12 h/12 h | 12 h/12 h |
| Weaning (days) | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 |
| Behavioral abnormalities during testing in Löscher lab | |||||||||
| 1. Basal (pre-SE or pre-kindling) behavior | |||||||||
| Open field | Less active than other SD substrains; increased anxiety compared to all other SD substrains | More explorative than SD rats from Taconic and CR; least level of anxiety of all SD substrains | Less active than other Wistar substrains; increased anxiety compared to all other Wistar substrains | ||||||
| Elevated plus-maze test | Less active than SD rats from Janvier and Taconic | Less active than SD rats from Janvier and Taconic | Less anxiety-related behavior than other SD substrains | Less active than other Wistar substrains | |||||
| Tests for behavioral hyperexcitability | More excitable than other SD substrains | n.t. | n.t. | n.t. | n.t. | ||||
| 2. Behavior in epileptic rats | n.t. | n.t. | n.t. | n.t. | |||||
| Open field | Increased ambulatory and rearing behavior (not observed in other SD substrains) | Increased anxiety compared to pre-SE (control) behavior (not observed in any other SD substrain) | |||||||
| Elevated plus-maze test | Only SD substrain with increased activity | ||||||||
| Tests for behavioral hyperexcitability | No increased response in pick-up test | No increased response in pick-up test | Increased response in pick-up test | Increased response in pick-up test | |||||
Another more recently discovered confounding variable are differences in gut microbiota composition in rodents from different vendors. For instance, genetically similar inbred strains of mice (B6) maintained by different vendors (Jackson Laboratory and Taconic) have differences in their gut bacterial communities that may affect the outcome of experiments [8]. This is not only relevant for models of infections but, because of the “microbiome-gut-brain-axis”, also for seizure propensity and neurobehavioral testing [7].
7. Role of sex for inter- and intrastrain differences
Sex differences in seizure susceptibility in rodent models have been described in many studies [195-198]. Although robust effects of sex on seizures have been reported, and numerous effects of gonadal steroids on seizure thresholds have been shown throughout the rodent brain, results have differed greatly depending on the method used to induce seizures, how seizures were measured (seizure threshold, latency to seizure onset, seizure severity or duration), species, age, and time of the estrous cycle that was chosen to study females [198]. Furthermore, although the estrous cycle is thought to affect seizure threshold [198,199], thus causing variability when using females, this is not true for all types of seizure threshold in rodents. For instance, PTZ seizure thresholds determined by timed intravenous infusion of this convulsant is not affected by the estrous cycle in rodents, while estrous cycle-dependent changes (and significant differences between females and males) were found for bicuculline seizure thresholds [200,201]. Similarly, the ADT in amygdala kindled female rats did not change significantly during the estrous cycle [202], while small but statistically significant changes were reported for electroshock seizure thresholds (EST) in rats [203]. Studies comparing EST in B6 and DBA mice showed that B6 females have a significantly lower threshold than B6 males; however, EST did not differ between DBA females and males [66]. A larger survey documented a complex pattern of sex differences among inbred mouse strains for end-points associated with electrical seizures [68]. Lastly, sex differences are not observed for repeated flurothyl-induced generalized seizure thresholds indicating that flurothyl may not exhibit sex differences [119].
One important question is whether outbred or intra-strain genetic drift impacts female rodents in the same profound way as in male rodents. Figure 5 illustrates data from experiments in which we determined different types of seizure threshold in male and female NMRI outbred mice from three different breeders as well as in Swiss outbred mice that are closely related to the NMRI strain. Significant intra- and interstrain differences in maximal EST were observed in both male and female mice (Fig. 5A). The maximal EST was significantly lower in NMRI mice from Charles River (Crl) than in NMRI mice from Wiga, independent of sex. The only significant intrastrain sex difference in maximal EST was observed in Swiss mice. With minimal EST, the lowest threshold was observed in Swiss mice of both sexes (Fig. 5B). With PTZ seizure threshold, Swiss mice again exhibited the lowest threshold, but this was only observed in female mice (Fig. 5C). Within each strain or substrain, sex differences were rarely observed with this threshold type. Overall, these data indicate that outbred or intra-strain genetic drift impact female rodents in the same profound way as male rodents.
Fig. 5.

Effect of strain and sex on different types of seizure threshold in mice. Seizure thresholds were determined either in NMRI outbred mice from MØllegard (Ejby, Denmark), Wiga (Sulzfeld, Germany), or Charles River (Crl; Sulzfeld, Germany), or in Swiss mice from Mus Rattus (Brunnthal, Germany). In A, the threshold for maximal electroshock seizures (max EST) is shown. The threshold was determined at the same time in the morning in groups of 20 mice per strain and sex and is indicated as the voltage inducing hind limb extension in 50% of the animals with confidence limits (CI) for 95% probability. Statistical analysis of data by ANOVA indicated significant differences between groups for both male (P = 0.009) and female (P = 0.0094) mice. In B, the threshold for minimal electroshock seizures (min EST) is shown; this type of seizure threshold was not determined in NMRI mice from Crl. The threshold was determined in groups of 20 mice per strain and sex and is indicated as the current inducing clonic activity of the facial musculature, ears, or forelimbs for several seconds in 50% of the animals with CI for 95% probability. Statistical analysis of data by ANOVA indicated significant differences between groups for both male (P<0.0001) and female (P<0.0001) mice. In C, the threshold for clonic seizures induced by PTZ is shown. The threshold was determined in groups of 10 mice and is given as the mean dose (± S.D.) of PTZ causing clonic seizures by i.v. infusion in freely moving animals. Statistical analysis of data by ANOVA indicated significant differences between groups for female (P = 0.0027) but not male mice. In all graphs, significant sex differences within each strain are indicated by circles (oP<0.05; oooP<0.001), whereas significant differences between strains within each sex are indicated by asterisks (*P<0.05; **P<0.01; ***P<0.001). Data are from Löscher et al. [91] and unpublished experiments of W. Löscher.
Epidemiological studies suggest that gender may affect susceptibility to epilepsy and its prognosis; however, only limited research has been dedicated to the impact of gender on susceptibility to acquired epilepsies, so the effect of gender on susceptibility and clinical evolution of acquired epilepsies is largely unknown [204]. The same is true for preclinical research [198,205]. As in many areas of biology [206], most preclinical studies on epileptogenesis have been performed in male rodents, and until recently no study was available that directly compared epileptogenesis in female and male rodents and also in both sexes in different strains of rodents [205;207]. To our knowledge, our recent study in the intrahippocampal kainate model of TLE was the first to evaluate sex differences in epileptogenesis in different rodent strains [208]. As described in section 3.6, a clear seizure- free latent period between kainate-induced SE and onset of SRS was only observed in male NMRI mice, but not in female NMRI, B6 or FVB/N mice [101]. Furthermore, the types of SRS recorded in epileptic mice differed between males and females [101]. Also, the SE induced by intrahippocampal kainate in NMRI mice was more severe in male than female mice, which could have affected the epileptogenic processes induced by SE in both genders [101]. Several previous studies reported that male rodents are more susceptible to the convulsive and neurodegenerative effects of systemic kainate administration than females, and a proconvulsive effect of testosterone and neuroprotective effect of estrogen was demonstrated [208-212]. However, Scharfman and MacLusky [198] reported that the SE induced by kainate was indistinguishable in male and female SD rats, and no significant difference in stages of the estrous cycle was found. In contrast, the latter authors reported sex differences in SE induction by systemic administration of pilocarpine in SD rats.
Another important question is whether there is equivalence between the two sexes as opposed to one sex of rodent possessing inherent advantages or disadvantages for seizure researchers. At least for investigators who apply for funding from the National Institutes of Health, it is imperative to have a solid grasp of sex either as a biological factor or to provide a clear rationale for exclusion of one sex of rodents from pre-clinical study designs [213]. As illustrated by the examples discussed above, there is no general answer to this question, but ideally, both sexes should be incorporated in experiments on seizures or epilepsy. If females are utilized, it is advantageous to be proactive and determine the stage of the estrous cycle on days seizures are administered. This can be done simply through vaginal lavage followed by cresyl violet staining of the sample on microscope slides for vaginal cytological determination of mouse estrous cycle staging [214].
8. Inter- and intra-strain differences affect performance in the behavioral tests that often accompany acute and chronic seizure testing
In addition to inter- and intra-strain effects on expression of seizures or epilepsy, inter- and intra-strain differences in rats and mice may also affect performance of animals in the behavioral tests that often accompany seizure testing, such as tests for anxiety-related behaviors, depression-related behaviors, and cognition and memory. Inter-individual differences in behavior in a given strain may also affect subsequent induction of epilepsy, thus paralleling the association between psychiatric diseases, such as depression or anxiety, and the risk of epilepsy [215]. For instance, Adamec and Shallow [216] investigated the effects of baseline anxiety level, as measured using the elevated plus-maze test, on response to kindling of the right medial amygdala in male Wistar rats. The level of baseline anxiety affected some of the kindling parameters and also affected the consequences of kindling on anxiety.
We tested the hypothesis that adult female Wistar rats from different breeders vary in anxiety-like behavior, seizure susceptibility, and epileptogenesis in the amygdala-kindling model of TLE [40]. In female Wistar rats from three different commercial breeders (Harlan-Winkelmann [HsdCpb:WU], Charles River [Crl:WI(Han)], and Janvier [RjHan:WI]), anxiety- like behavior was monitored in the open field and the elevated plus maze before onset of kindling (Table 2). Wistar rats from Charles River showed lower locomotor activity and higher anxiety-like behavior compared to Wistar rats from Harlan-Winkelmann and Janvier. Female Wistar rats from Harlan-Winkelmann showed the lowest anxiety-like behavior and the highest exploratory behavior. As discussed in section 2.4 and shown in Table 1, Wistar rats from Charles River also differed from the other Wistar substrains in kindling rate (ADD to stage 5).
Apart from genetic variability between outbred stocks, differences in breeding conditions, housing, and handling procedure at the breeders’ facilities are likely to contribute to the behavioral intergroup differences observed in Wistar rats [40](see also section 2.4). As shown in Table 2, housing and handling conditions of the rats used in this study differed in several aspects, comparing the three breeders Harlan-Winkelmann, Charles River, and Janvier.
When we compared male Wistar rats from different breeders in terms of their vulnerability and resilience to chronic mild stress (CMS), Wistar rats from Charles River were the most sensitive substrain to CMS, while Wistar rats from Janvier were resistant to CMS [217]. DNA methylation studies revealed that the behavioral differences between the Wistar substrains are reflected at the epigenetic level [217]. Changes in the activity of genes established through epigenetic alterations occur as a consequence of exposure to environmental adversity, social stress, and traumatic experiences [218]. DNA methylation in particular has thus emerged as a leading candidate biological pathway linking gene- environment interactions to long-term and even multi-generational trajectories in behavioral development, including the vulnerability and resilience to psychopathology [218]. Interestingly, as shown in Table 2, Janvier was the only vendor that performed daily handling habituation during rearing of the Wistar rats, which is a likely explanation of the high resilience of these animals to CMS.
In a subsequent study, we evaluated whether adult female SD rats from different breeders vary in several behavioral tests before and after induction of SE and epileptogenesis by sustained electrical stimulation of the BLA [19]. As in the study with Wistar rats, we obtained details of the breeding conditions from the vendors, housing, and handling procedures (Table 2). Similar experiments were performed with the lithium-pilocarpine model of TLE [37]. As shown in Table 2, only Janvier performed daily handling habituation during rearing of the SD rats, resulting in socialization to humans. As a consequence, SD rats from Janvier were more explorative than SD rats from other breeders in different tests and exhibited the lowest level of anxiety of all SD substrains (Table 2). Following development of epilepsy, increased anxiety occurred in SD rats from Janvier, but not in SD rats from the other vendors [19]. These data indicate that, in addition to genetic intra- and inter-strain differences, variation in housing and handling conditions between different vendors can markedly affect the behavior of animals both before and after induction of epilepsy.
9. Conclusions
While we were not able to discuss all of the studies related to genetic heterogeneity among rodent strains in mediating seizures and epilepsy due to space limitations, it has become clear that the background strain of the rodent utilized as a model, and even the vendor and housing location in the vendor’s barriers, can have a profound effect on seizures. This work highlights the importance of paying particular attention to the strain being utilized and the vendor source, since this may have profound effects on results and interpretations, not only for epilepsy studies, but in the analysis of any phenotypic trait, especially for neurological/behavioral phenotypes. This is particularly critical when it comes to testing mice with targeted deletions of genes important for epilepsy, since if rodents are tested for the effects of a gene deletion in a mixed genetic background, the underlying genetic background differences could be having a role in the effects observed. While such mixed genetic backgrounds are a cause for caution, they can be very informative if closely attended to, since such information could lead to the identification of genetic modifiers of the primary gene under study. Clearly, studies utilizing diverse inbred strains of rodents have and will continue to provide novel insights into genes and modifiers. Through their identification and elucidation of their mechanisms in seizures and epilepsy, it is our hope that these will lead to new treatments or therapies for individuals with epilepsy.
Finally, as advice to minimize variation in experiments on seizures or epilepsy and associated behavioral alterations in mice or rats, we recommend (i) that researchers should know with which exact stock or strain they are working (the scientific nomenclature for a stock or strain provides this information); (ii) that the same stock or strain should be utilized for a series of experiments; (iii) that it should not be switched to another vendor/substrain in the middle of a series of experiments; if this cannot be avoided, for instance because the vendor closed a certain animal barrier, it should be checked whether animals from the new stock behave similarly as the previous animals in a given test paradigm; (iv) it should not be assumed that performance will be the same across different vendor substrains/stocks. A pilot study is ideal to compare performance at the outset of a new project. In addition, as previously recommended [184], in order to increase the reproducibility of data, authors of publications should include full strain information in their manuscripts, whether in the Methods section or in Supplementary Information, and consider discussing how their results may be affected or limited by strain choice and breeding practice. Furthermore, factors such as animal housing, handling, food, lighting and noise conditions, all of which effect behavior and brain chemistry, should be reported in detail, because the key to reproducibility is accurate reporting of these seemingly mundane details, which potentially have large effects [184].
10. Recommendations
Rodent models of seizures and epilepsy should be optimized to address specific research questions. Since, as reviewed in this paper, both genetics and environment can each have a large influence on rodent responses in seizure and epilepsy models, experiments may be designed to study either genetic or environmental influences separately, or to study their interaction. In studies focused on elucidating the impact of factors in the environment, it is imperative that rodents are of the same genetic background. Studies focused on the role of genetics will often involve more than one strain or line, and thus the differences discussed in this review may become relevant. Any study utilizing genetically altered rodents should have controls with a genetic background that is identical or as genetically similar to the mutant strain as possible. The use of stem cells or blastocysts from pure inbred strains to develop new knockout or transgenic rodents may obviate this problem. Results generated from the study of genetic models created on mixed backgrounds are difficult to interpret. Such problems may be minimized by backcrossing genetically unique rodent models created on mixed genetic backgrounds at least 10 generations onto an inbred strain and then to only use littermates as controls. The use of vendor-purchased, parental strain rodents as controls for rodents from knockout or transgenic strains or lines is discouraged. Importantly, if an inbred strain is maintained in an investigator’s animal facility, it is critical to control genetic drift. This may be done in several ways. For genetically unique strains, one approach is through cryopreservation of embryos at the time the strain is developed, with subsequent introduction into the colony of animals derived from these embryos at regular intervals. For commercially-available strains, it is advisable to reintroduce wildtype mice from the vendor into the colony at yearly or otherwise regular intervals. With regard to the latter recommendation, it is important to confirm that the vendor utilizes methods to control genetic drift at the strain production level.
The question remains, what background strain should be used for subsequent testing of seizures and epilepsy in rodents? As mentioned, this is dependent on the research problem being addressed. For example, in studies of knockout models where a specific single-gene defect has a major phenotypic effect, it may be valuable to study the mutation on a genetic background of relative seizure resistance in order to enhance strain viability. On the other hand, for screening potential anti-seizure or anti-epilepsy treatments, it may be more valuable to study treatment effects in a strain that is more readily susceptible to experimental seizures or even a strain that exhibits spontaneous seizures. In all cases, there is value in conducting studies on different strain backgrounds, for comparison, when possible. Unfortunately, there is no general consensus regarding which strains are most valuable for different applications; however, the data reviewed in this paper may serve as a basis to make such decisions.
It is important to consider as well the fact that strain relationships may change based on specific seizure- or epilepsy-related endpoint measurements and that sometimes strains will exhibit diametrically opposite responses such as is the case with B6 and DBA mice in which DBA have a much lower threshold for acute generalized electroshock seizures [66] but a much higher threshold for acute seizures induced by elevating body temperature [62]. Thus, given that different seizure-inducing methodologies and related measurements can be affected by the background strain, it is important to observe the recommendations outlined above.
Concerning the question as to whether it is better to work with inbred strains or outbred strains, again the answer is dependent upon the goals of the experiment. In most cases, within-group variability of response will be minimized by studying inbred rodents. This increases statistical power and facilitates the detection of small effects. On the other hand, responses of outbred rodents may more closely reflect the diversity of human populations and have greater translational potential in certain circumstances.
With regards to the environment, all potential confounding variables should be carefully controlled based on the study. For instance, group housing is a form of environmental enrichment that should be maintained constant during a study, and thus group- housed mice should not be compared to mice that are housed individually. If additional environmental enrichments are added, such as different beddings, housing shacks, toys, etc., these should be present in the control cages as well. If at all possible, the best housing situation in studies of genetically unique mice would be cages that contain a mix of both mutants and littermate controls.
Finally, although not reviewed here in-depth, the variables of age and sex should be considered and carefully controlled in all studies involving rodent models of seizures and epilepsy. Published studies support the concept that both genetic and environmental influences may be modified as a function of rodent age and sex, the latter documented by the female and male rodent differences for seizure endpoints that were reviewed in this paper. The impact of age on seizure and epilepsy models, like the impact of sex, has not been extensively studied and it largely fell outside the purview of this review. However, it is clear that the age of experimental and control subjects should be closely matched within a study. Given the perspective of epilepsy as a neurodevelopmental disease, optimal experimental designs would include evaluation of animals at a range of ages across the life cycle. Such comprehensive approaches are viewed as vital for obtaining the greatest value from rodent models and are to be encouraged as research programs continue to evolve better strategies to understand this common and debilitating neurological disease.
Table 3 summarizes recommendations to address the major variables most likely to impact the responses of rodents in tests of seizures and epilepsy. Importantly, while these recommendations may appear daunting in regards to the potential confounds of genetic background and environmental factors on seizure phenotypes, it should not dissuade investigators to venture into these areas. Being cognizant of the these issues and considering them in designing experiments, taking into account the points raised in this review, should provide robust and repeatable results important to the field.
Table 3.
Variables that should be controlled to introduce reproducibility and rigor and minimize experimental bias in rodent studies of seizures and epilepsy (see also Fig. 4).
| VARIABLE | COMMENT |
|---|---|
| Environmental | These are factors in the environment that are recognized as having a significant influence on the outcome of seizure tests in rodents |
| Housing | Rodents should be group-housed to the extent possible. Group sizes should be similar between experimental and control animals. Experimental and control animals should be housed in identical caging and maintained in the same room. |
| Enrichment | Cage enrichments should be the same between experimental and control animals. |
| Food/water | The composition and source of food should be identical between experimental and control rodents, as should the source of water. Access to food and water should be identical as well. |
| Litter size | Animals selected to populate experimental and control groups should come from litters that have been culled to the same number of pups. |
| Experimental | These are parameters of seizures that can be quantified as endpoints to compare experimental and control animals and need to be carefully defined for each study. |
| Seizure frequency | This endpoint parameter may be defined as the number of discrete seizure events per unit of time. Seizure events may be defined behaviorally or electrographically. |
| Seizure duration | This endpoint parameter may be defined as the length of a single discrete seizure. Average seizure duration may be calculated for individual animals or groups. |
| Seizure threshold | This endpoint parameter may be calculated based upon stimulus intensity. Intensity is defined as a function of stimulus such as chemoconvulsant dose or electrical current level. |
| Biologic | These parameters include factors that are intrinsic to each organism. |
| Age | Susceptibility to seizures and epilepsy varies over the life cycle for rodents and it is imperative that experimental and control animals be closely matched for age. |
| Sex | Susceptibility to seizures and epilepsy differs between males and females. Ideally, sexes should be tested separately. Alternatively, experimental and control groups should contain the same number of each sex although this latter design may mask sex differences. All female rodents should be tested at the same time in their estrous cycle. |
| Genetic | Naturally-occurring genetic polymorphisms between rodents may impact responses in tests of seizures and epilepsy. |
| Genetic background | DNA polymorphisms, both characterized and uncharacterized, exist between different rodent lines and strains. Study designs should include a breeding strategy to insure that experimental and control groups share the same genetic background. |
| Genetic drift | New mutations may be introduced naturally into a rodent line or strain at any generation. Colony management should include a breeding strategy to maintain the integrity of the genetic background of all rodent lines and strains. |
Highlights.
The relevance of individual genetic background and its role in models of epilepsy is discussed
Strain background of mice and rats critically affects the expression of seizures
This is true for both outbred and inbred strains of mice and rats
In addition, environmental and epigenetic factors can affect seizure expression
Thus, researchers should know with which exact stock or strain of rodents they are working
Acknowledgments
This work was supported, in part, by an NIH/NINDS R01NS064283 grant to RJF. WL’s studies have been supported by grants from the German Research Foundation (Bonn, Germany) and the European Union’s Seventh Framework Programme (FP7) under grant agreements 201380 (EURIPIDES) and 602102 (EPITARGET).
Abbreviations
- AD
afterdischarge
- ADD
afterdischarge duration
- ADHD
attention deficit hyperactivity disorder
- ADT
afterdischarge threshold
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ASD
autism spectrum disorder
- B6
C57BL/6
- BLA
basolateral amygdala
- CNS
central nervous system
- CRL
Charles-River
- EST
electroshock seizure threshold
- GAERS
Genetic Absence Epilepsy Rat from Strasbourg
- GSP
Genetic Stability Program
- GST
generalized seizure threshold
- HAR
Harlan
- HFO
high frequency oscillation
- HL
Harlan Laboratories
- HPD
hippocampal paroxysmal discharge
- HVRS
high-voltage rhythmic spike
- HVSW
high-voltage spike discharge
- HW
Harlan-Winkelmann
- IGS
International Genetic Standardization
- MES
maximal electroshock seizure
- mTLE
mesial temporal lobe epilepsy
- NMDA
N-methyl-D-aspartate
- PDZ
polarity complex component
- PTZ
pentylenetetrazole
- QTL
quantitative trait loci
- SD
Sprague-Dawley
- SE
status epilepticus
- SNP
single-nucleotide polymorphism
- SPWS
sharp waves
- SRP
signal- recognition-particle
- SRS
spontaneous recurrent seizures
- SUDEP
sudden unexplained death in epilepsy
- SWD
spike-wave discharge
- TLE
temporal lobe epilepsy
- TMEV
Theiler’s murine encephalomyelitis virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schauwecker PE. The relevance of individual genetic background and its role in animal models of epilepsy. Epilepsy Res. 2011;97:1–11. doi: 10.1016/j.eplepsyres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Festing MF. Genetic variation in outbred rats and mice and its implications for toxicological screening. J Exp Anim Sci. 1993;35:210–20. [PubMed] [Google Scholar]
- 3.Festing MF. Inbred strains should replace outbred stocks in toxicology, safety testing, and drug development. TOXICOL PATHOL. 2010;38:681–90. doi: 10.1177/0192623310373776. [DOI] [PubMed] [Google Scholar]
- 4.Levesque M, Avoli M, Bernard C. Animal models of temporal lobe epilepsy following systemic chemoconvulsant administration. J Neurosci Methods. 2016;260:45–52. doi: 10.1016/j.jneumeth.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens JC, Banks GT, Festing MF, Fisher EM. Quiet mutations in inbred strains of mice. Trends Mol Med. 2007;13:512–9. doi: 10.1016/j.molmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Schellinck HM. How Many Ways Can Mouse Behavioral Experiments Go Wrong? Confounding Variables in Mouse Models of Neurodegenerative Diseases and How to Control Them. In: Brockmann HJ, editor. Advances in the Study of Behavior. Vol. 41. Burlington: Academic Press; 2010. pp. 255–366. [Google Scholar]
- 7.Burnet PW. Gut bacteria and brain function: the challenges of a growing field. Proc Natl Acad Sci U S A. 2012;109:E175. doi: 10.1073/pnas.1118654109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villarino NF, LeCleir GR, Denny JE, Dearth SP, Harding CL, Sloan SS, et al. Composition of the gut microbiota modulates the severity of malaria. Proc Natl Acad Sci U S A. 2016;113:2235–40. doi: 10.1073/pnas.1504887113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess DL. Transgenic and gene replacement models of epilepsy: targeting ion channel and neurotransmission pathways in mice. In: Pitkänen A, Schwartzkroin PA, Moshé SL, editors. Models of seizures andepilepsy. Burlington: Elsevier Academic Press; 2006. pp. 199–222. [Google Scholar]
- 10.Frankel WN. Genetics of complex neurological disease: challenges and opportunities for modeling epilepsy in mice and rats. Trends Genet. 2009;25:361–7. doi: 10.1016/j.tig.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kacew S, Festing MF. Role of rat strain in the differential sensitivity to pharmaceutical agents and naturally occurring substances. J Toxicol Environment Health. 1996;47:1–30. doi: 10.1080/009841096161960-2840. [DOI] [PubMed] [Google Scholar]
- 12.Yilmazer-Hanke DM. Morphological correlates of emotional and cognitive behaviour: insights from studies on inbred and outbred rodent strains and their crosses. Behav Pharmacol. 2008;19:403–34. doi: 10.1097/FBP.0b013e32830dc0de. [DOI] [PubMed] [Google Scholar]
- 13.White WJ, Lee CS. The development and maintenance of the Crl:CD(SD)IGS BR rat breeding system. In: CD(SD)IGS Study Group, editor. Biological reference data on CD(SD)IGS rats - 1998. Tokyo: Best Printing Co. Ltd.; 1998. pp. 8–14. [Google Scholar]
- 14.Fedrowitz M, Kamino K, Löscher W. Significant differences in the effects of magnetic field exposure on 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in two substrains of Sprague-Dawley rats. Cancer Res. 2004;64:243–51. doi: 10.1158/0008-5472.can-03-2808. [DOI] [PubMed] [Google Scholar]
- 15.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 16.Severino GS, Fossati IA, Padoin MJ, Gomes CM, Trevizan L, Sanvitto GL, et al. Effects of neonatal handling on the behavior and prolactin stress response in male and female rats at various ages and estrous cycle phases of females. Physiol Behav. 2004;81:489–98. doi: 10.1016/j.physbeh.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Burn CC, Deacon RM, Mason GJ. Marked for life? Effects of early cage-cleaning frequency, delivery batch, and identification tail-marking on rat anxiety profiles. Dev Psychobiol. 2008;50:266–77. doi: 10.1002/dev.20279. [DOI] [PubMed] [Google Scholar]
- 18.Portelli J, Aourz N, De Bundel D, Meurs A, Smolders I, Michotte Y, et al. Intrastrain differences in seizure susceptibility, pharmacological response and basal neurochemistry of Wistar rats. Epilepsy Res. 2009;87:234–46. doi: 10.1016/j.eplepsyres.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Langer M, Brandt C, Löscher W. Marked strain and substrain differences in induction of status epilepticus and subsequent development of neurodegeneration, epilepsy, and behavioral alterations in rats. Epilepsy Res. 2011;96:207–24. doi: 10.1016/j.eplepsyres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Festing MF. Evidence should trump intuition by preferring inbred strains to outbred stocks in preclinical research. ILAR J. 2014;55:399–404. doi: 10.1093/ilar/ilu036. [DOI] [PubMed] [Google Scholar]
- 21.Müller CJ, Gröticke I, Hoffmann K, Schughart K, Löscher W. Differences in sensitivity to the convulsant pilocarpine in substrains and sublines of C57BL/6 mice. Genes Brain Behav. 2009;8:481–92. doi: 10.1111/j.1601-183X.2009.00490.x. [DOI] [PubMed] [Google Scholar]
- 22.Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, et al. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J Neurogenet. 2008;22:315–31. doi: 10.1080/01677060802357388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zurita E, Chagoyen M, Cantero M, Alonso R, Gonzalez-Neira A, Lopez-Jimenez A, et al. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011;20:481–9. doi: 10.1007/s11248-010-9403-8. [DOI] [PubMed] [Google Scholar]
- 24.Morse HC. Origins of Inbred Mice. New York: Academic Press; 1978. [Google Scholar]
- 25.Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, et al. Genetic differences among C57BL/6 substrains. Exp Anim. 2009;58:141–9. doi: 10.1538/expanim.58.141. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Beyer BJ, Otto JF, O’Brien TP, Letts VA, White HS, et al. Spontaneous deletion of epilepsy gene orthologs in a mutant mouse with a low electroconvulsive threshold. HUM MOL GENET. 2003;12:975–84. doi: 10.1093/hmg/ddg118. [DOI] [PubMed] [Google Scholar]
- 27.Beyer B, Deleuze C, Letts VA, Mahaffey CL, Boumil RM, Lew TA, et al. Absence seizures in C3H/HeJ and knockout mice caused by mutation of the AMPA receptor subunit Gria4. HUM MOL GENET. 2008;17:1738–49. doi: 10.1093/hmg/ddn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davisson MT, Bergstrom DE, Reinholdt LG, Donahue LR. Discovery Genetics - The History and Future of Spontaneous Mutation Research. Curr Protoc Mouse Biol. 2012;2:103–18. doi: 10.1002/9780470942390.mo110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–57. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, et al. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- 31.Marchi N, Oby E, Batra A, Uva L, De Curtis M, Hernandez N, et al. In vivo and in vitro effects of pilocarpine: relevance to ictogenesis. Epilepsia. 2007;48:1934–46. doi: 10.1111/j.1528-1167.2007.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bankstahl M, Müller CJ, Wilk E, Schughart K, Löscher W. Generation and characterization of pilocarpine-sensitive C57BL/6 mice as a model of temporal lobe epilepsy. Behav Brain Res. 2012;230:182–91. doi: 10.1016/j.bbr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Engstrom FL, Woodbury DM. Seizure susceptibility in DBA and C57 mice: the effects of various convulsants. Epilepsia. 1988;29:389–95. doi: 10.1111/j.1528-1157.1988.tb03736.x. [DOI] [PubMed] [Google Scholar]
- 34.Kosobud AE, Crabbe JC. Genetic correlations among inbred strain sensitivities to convulsions induced by 9 convulsant drugs. Brain Res. 1990;526:8–16. doi: 10.1016/0006-8993(90)90243-5. [DOI] [PubMed] [Google Scholar]
- 35.Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci U S A. 1997;94:4103–8. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLin JP, Steward O. Comparison of seizure phenotype and neurodegeneration induced by systemic kainic acid in inbred, outbred, and hybrid mouse strains. Eur J Neurosci. 2006;24:2191–202. doi: 10.1111/j.1460-9568.2006.05111.x. [DOI] [PubMed] [Google Scholar]
- 37.Brandt C, Bankstahl M, Töllner K, Klee R, Löscher W. The pilocarpine model of temporal lobe epilepsy: Marked intrastrain differences in female Sprague-Dawley rats and the effect of estrous cycle. Epilepsy Behav. 2016;61:141–52. doi: 10.1016/j.yebeh.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Löscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev. 2010;62:668–700. doi: 10.1124/pr.110.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandt C, Glien M, Potschka H, Volk H, Löscher W. Epileptogenesis and neuropathology after different types of status epilepticus induced by prolonged electrical stimulation of the basolateral amygdala in rats. Epilepsy Res. 2003;55:83–103. doi: 10.1016/s0920-1211(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 40.Honndorf S, Lindemann C, Tollner K, Gernert M. Female Wistar rats obtained from different breeders vary in anxiety-like behavior and epileptogenesis. Epilepsy Res. 2011;94:26–38. doi: 10.1016/j.eplepsyres.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Racine RJ, Steingart M, McIntyre DC. Development of kindling-prone and kindling- resistant rats: selective breeding and electrophysiological studies. Epilepsy Res. 1999;35:183–95. doi: 10.1016/s0920-1211(99)00013-3. [DOI] [PubMed] [Google Scholar]
- 42.McIntyre DC, Gilby KL. Genetically seizure-prone or seizure-resistant phenotypes and their associated behavioral comorbidities. Epilepsia. 2007;48(Suppl 9):30–2. doi: 10.1111/j.1528-1167.2007.01398.x. [DOI] [PubMed] [Google Scholar]
- 43.Sharma P, Dedeurwaerdere S, Vandenberg MA, Fang K, Johnston LA, Shultz SR, et al. Neuroanatomical differences in FAST and SLOW rat strains with differential vulnerability to kindling and behavioral comorbidities. Epilepsy Behav. 2016;65:42–8. doi: 10.1016/j.yebeh.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 44.Löscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for antiepileptic drug discovery and development. Nat Rev Drug Discov. 2013;12:757–76. doi: 10.1038/nrd4126. [DOI] [PubMed] [Google Scholar]
- 45.Löscher W, Rundfeldt C. Kindling as a model of drug-resistant partial epilepsy: selection of phenytoin-resistant and nonresistant rats. J Pharmacol Exp Ther. 1991;258:483–9. [PubMed] [Google Scholar]
- 46.Löscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–68. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Cramer S, Ebert U, Löscher W. Characterization of phenytoin-resistant kindled rats, a new model of drug-resistant partial epilepsy: Comparison of inbred strains. Epilepsia. 1998;39:1046–53. doi: 10.1111/j.1528-1157.1998.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 48.Brandt C, Volk HA, Löscher W. Striking differences in individual anticonvulsant response to phenobarbital in rats with spontaneous seizures after status epilepticus. Epilepsia. 2004;45:1488–97. doi: 10.1111/j.0013-9580.2004.16904.x. [DOI] [PubMed] [Google Scholar]
- 49.Bankstahl M, Bankstahl JP, Löscher W. Inter-individual variation in the anticonvulsant effect of phenobarbital in the pilocarpine rat model of temporal lobe epilepsy. Exp Neurol. 2012;234:70–84. doi: 10.1016/j.expneurol.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Löscher W. Fit for purpose application of currently existing animal models in the discovery of novel epilepsy therapies. Epilepsy Res. 2016;126:157–84. doi: 10.1016/j.eplepsyres.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 51.Libbey JE, Kirkman NJ, Smith MC, Tanaka T, Wilcox KS, White HS, et al. Seizures following picornavirus infection. Epilepsia. 2008;49:1066–74. doi: 10.1111/j.1528-1167.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- 52.Stewart KA, Wilcox KS, Fujinami RS, White HS. Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. J Neuropathol Exp Neurol. 2010;69:1210–9. doi: 10.1097/NEN.0b013e3181ffc420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bröer S, Käufer C, Haist V, Li L, Gerhauser I, Anjum M, et al. Brain inflammation, neurodegeneration and seizure development following picornavirus infection markedly differ among virus and mouse strains and substrains. Exp Neurol. 2016;279:57–74. doi: 10.1016/j.expneurol.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Specht CG, Schoepfer R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci. 2001;2:11. doi: 10.1186/1471-2202-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wotjak CT. C57BLack/BOX? The importance of exact mouse strain nomenclature. Trends Genet. 2003;19:183–4. doi: 10.1016/S0168-9525(02)00049-5. [DOI] [PubMed] [Google Scholar]
- 56.Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–94. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doran AG, Wong K, Flint J, Adams DJ, Hunter KW, Keane TM. Deep genome sequencing and variation analysis of 13 inbred mouse strains defines candidate phenotypic alleles, private variation and homozygous truncating mutations. Genome Biol. 2016;17:167. doi: 10.1186/s13059-016-1024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.HALL CS. Genetic differences in fatal audiogenic seizures between two inbred strains of house mice. J Hered. 1947;38:2–6. [PubMed] [Google Scholar]
- 59.Schlesinger K, Boggan WO, Griek BJ. Pharmacogenetic correlates of pentylenetetrazol and electroconvulsive seizure thresholds in mice. Psychopharmacologia. 1968;13:181–8. doi: 10.1007/BF00401398. [DOI] [PubMed] [Google Scholar]
- 60.Taylor BA. Genetic analysis of susceptibility to isoniazid-induced seizures in mice. Genetics. 1976;83:373–7. doi: 10.1093/genetics/83.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferraro TN. Sixty years in the making: A polygenic mouse model of seizure susceptibility. In: Schwartzkroin PA, editor. Encyclopedia of basic epilepsy research. New York: Academic Press; 2009. pp. 374–81. [Google Scholar]
- 62.van Gassen KL, Hessel EV, Ramakers GM, Notenboom RG, Wolterink-Donselaar IG, Brakkee JH, et al. Characterization of febrile seizures and febrile seizure susceptibility in mouse inbred strains. Genes Brain Behav. 2008;7:578–86. doi: 10.1111/j.1601-183X.2008.00393.x. [DOI] [PubMed] [Google Scholar]
- 63.White HS. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia. 2003;44(Suppl 7):2–8. doi: 10.1046/j.1528-1157.44.s7.10.x. [DOI] [PubMed] [Google Scholar]
- 64.Deckard BS, Lieff B, Schlesinger K, DeFries JC. Developmental patterns of seizure susceptibility in inbred strains of mice. Dev Psychobiol. 1976;9:17–24. doi: 10.1002/dev.420090104. [DOI] [PubMed] [Google Scholar]
- 65.Maxson SC. Febrile convulsions in inbred strains of mice susceptible and resistant to audiogenic seizures. Epilepsia. 1980;21:637–45. doi: 10.1111/j.1528-1157.1980.tb04317.x. [DOI] [PubMed] [Google Scholar]
- 66.Ferraro TN, Golden GT, Snyder R, Laibinis M, Smith GG, Buono RJ, et al. Genetic influences on electrical seizure threshold. Brain Res. 1998;813:207–10. doi: 10.1016/s0006-8993(98)01013-0. [DOI] [PubMed] [Google Scholar]
- 67.Ferraro TN, Golden GT, Smith GG, Martin JF, Lohoff FW, Gieringer TA, et al. Fine mapping of a seizure susceptibility locus on mouse Chromosome 1: nomination of Kcnj10 as a causative gene. Mamm Genome. 2004;15:239–51. doi: 10.1007/s00335-003-2270-3. [DOI] [PubMed] [Google Scholar]
- 68.Frankel WN, Taylor L, Beyer B, Tempel BL, White HS. Electroconvulsive thresholds of inbred mouse strains. GENOMICS. 2001;74:306–12. doi: 10.1006/geno.2001.6564. [DOI] [PubMed] [Google Scholar]
- 69.Dube CM, Brewster AL, Baram TZ. Febrile seizures: mechanisms and relationship to epilepsy. Brain Dev. 2009;31:366–71. doi: 10.1016/j.braindev.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frankel WN, Valenzuela A, Lutz CM, Johnson EW, Dietrich WF, Coffin JM. New seizure frequency QTL and the complex genetics of epilepsy in EL mice. Mamm Genome. 1995;6:830–8. doi: 10.1007/BF00292431. [DOI] [PubMed] [Google Scholar]
- 71.Frankel WN, Johnson EW, Lutz CM. Congenic strains reveal effects of the epilepsy quantitative trait locus, El2, separate from other El loci. Mamm Genome. 1995;6:839–43. doi: 10.1007/BF00292432. [DOI] [PubMed] [Google Scholar]
- 72.Legare ME, Bartlett FS, Frankel WN. A major effect QTL determined by multiple genes in epileptic EL mice. GENOME RES. 2000;10:42–8. [PMC free article] [PubMed] [Google Scholar]
- 73.Kosobud AE, Cross SJ, Crabbe JC. Neural sensitivity to pentylenetetrazol convulsions in inbred and selectively bred mice. Brain Res. 1992;592:122–8. doi: 10.1016/0006-8993(92)91666-3. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz RD, Seale TW, Skolnick P, Paul SM. Differential seizure sensitivities to picrotoxinin in two inbred strains of mice (DBA/2J and BALB/c ByJ): parallel changes in GABA receptor-mediated chloride flux and receptor binding. Brain Res. 1989;481:169–74. doi: 10.1016/0006-8993(89)90499-x. [DOI] [PubMed] [Google Scholar]
- 75.Freund RK, Marley RJ, Wehner JM. Differential sensitivity to bicuculline in three inbred mouse strains. Brain Res Bull. 1987;18:657–62. doi: 10.1016/0361-9230(87)90135-3. [DOI] [PubMed] [Google Scholar]
- 76.Marley RJ, Gaffney D, Wehner JM. Genetic influences on GABA-related seizures. Pharmacol Biochem Behav. 1986;24:665–72. doi: 10.1016/0091-3057(86)90572-1. [DOI] [PubMed] [Google Scholar]
- 77.Seale TW, Carney JM, Rennert OM, Flux M, Skolnick P. Coincidence of seizure susceptibility to caffeine and to the benzodiazepine inverse agonist, DMCM, in SWR and CBA inbred mice. Pharmacol Biochem Behav. 1987;26:381–7. doi: 10.1016/0091-3057(87)90133-x. [DOI] [PubMed] [Google Scholar]
- 78.Desforges C, Venault P, Dodd RH, Chapouthier G, Roubertoux PL. beta-Carboline- induced seizures in mice: genetic analysis. Pharmacol Biochem Behav. 1989;34:733–7. doi: 10.1016/0091-3057(89)90267-0. [DOI] [PubMed] [Google Scholar]
- 79.Bernard-Helary K, Ardourel MY, Hevor T, Cloix JF. In vivo and in vitro glycogenic effects of methionine sulfoximine are different in two inbred strains of mice. Brain Res. 2002;929:147–55. doi: 10.1016/s0006-8993(01)03380-7. [DOI] [PubMed] [Google Scholar]
- 80.Clement JG, Hand BT, Shiloff JD. Differences in the toxicity of soman in various strains of mice. Fundam Appl Toxicol. 1981;1:419–20. doi: 10.1016/s0272-0590(81)80020-6. [DOI] [PubMed] [Google Scholar]
- 81.Ferraro TN, Golden GT, Smith GG, Berrettini WH. Differential susceptibility to seizures induced by systemic kainic acid treatment in mature DBA/2J and C57BL/6J mice. Epilepsia. 1995;36:301–7. doi: 10.1111/j.1528-1157.1995.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 82.Winawer MR, Makarenko N, McCloskey DP, Hintz TM, Nair N, Palmer AA, et al. Acute and chronic responses to the convulsant pilocarpine in DBA/2J and A/J mice. Neuroscience. 2007;149:465–75. doi: 10.1016/j.neuroscience.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Sarro A, Imperatore C, Mastroeni P, De Sarro G. Comparative convulsant potencies of two carbapenem derivatives in C57 and DBA/2 mice. J Pharm Pharmacol. 1995;47:292–6. doi: 10.1111/j.2042-7158.1995.tb05798.x. [DOI] [PubMed] [Google Scholar]
- 84.De Sarro A, Naccari F, Imperatore C, De Sarro GB. Comparative epileptogenic properties of two monobactam derivatives in C57, Swiss and DBA/2 mice. J Antimicrob Chemother. 1996;38:475–84. doi: 10.1093/jac/38.3.475. [DOI] [PubMed] [Google Scholar]
- 85.Sonner JM, Gong D, Li J, Eger EI, Laster MJ. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth Analg. 1999;89:1030–4. doi: 10.1097/00000539-199910000-00039. [DOI] [PubMed] [Google Scholar]
- 86.Marley RJ, Witkin JM, Goldberg SR. Genetic factors influence changes in sensitivity to the convulsant properties of cocaine following chronic treatment. Brain Res. 1991;542:1–7. doi: 10.1016/0006-8993(91)90989-9. [DOI] [PubMed] [Google Scholar]
- 87.Miner LL, Collins AC. Strain comparison of nicotine-induced seizure sensitivity and nicotinic receptors. Pharmacol Biochem Behav. 1989;33:469–75. doi: 10.1016/0091-3057(89)90532-7. [DOI] [PubMed] [Google Scholar]
- 88.Schauwecker PE. Strain differences in seizure-induced cell death following pilocarpine-induced status epilepticus. Neurobiol Dis. 2012;45:297–304. doi: 10.1016/j.nbd.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.File SE. Strain differences in mice in the development of tolerance to the anti- pentylenetetrazole effects of diazepam. Neurosci Lett. 1983;42:95–8. doi: 10.1016/0304-3940(83)90428-7. [DOI] [PubMed] [Google Scholar]
- 90.File SE, Greenblatt DJ, Martin IL, Brown C. Long-lasting anticonvulsant effects of diazepam in different mouse strains: correlations with brain concentrations and receptor occupancy. Psychopharmacology (Berl) 1985;86:137–41. doi: 10.1007/BF00431698. [DOI] [PubMed] [Google Scholar]
- 91.Löscher W, Czuczwar SJ, Wolff GL. AE mice: an inbred mouse strain with interesting features for epilepsy research. Epilepsia. 1986;27:657–64. doi: 10.1111/j.1528-1157.1986.tb03592.x. [DOI] [PubMed] [Google Scholar]
- 92.Wehner JM, Marley RJ. Genetic differences in the effects of delta-aminolevulinic acid on seizure latency in mice. Exp Neurol. 1986;94:280–91. doi: 10.1016/0014-4886(86)90102-0. [DOI] [PubMed] [Google Scholar]
- 93.Deutsch SI, Mastropaolo J, Powell DG, Rosse RB, Bachus SE. Inbred mouse strains differ in their sensitivity to an antiseizure effect of MK-801. Clin Neuropharmacol. 1998;21:255–7. [PubMed] [Google Scholar]
- 94.Leclercq K, Kaminski RM. Genetic background of mice strongly influences treatment resistance in the 6 Hz seizure model. Epilepsia. 2015;56:310–8. doi: 10.1111/epi.12893. [DOI] [PubMed] [Google Scholar]
- 95.Finn DA, Roberts AJ, Lotrich F, Gallaher EJ. Genetic differences in behavioral sensitivity to a neuroactive steroid. J Pharmacol Exp Ther. 1997;280:820–8. [PubMed] [Google Scholar]
- 96.Atlas SA, Zweier JL, Nebert DW. Genetic differences in phenytoin pharmacokinetics. In vivo clearance and in vitro metabolism among inbred strains of mice. Dev Pharmacol Ther. 1980;1:281–304. [PubMed] [Google Scholar]
- 97.Johnson MR, Tan NC, Kwan P, Brodie MJ. Newly diagnosed epilepsy and pharmacogenomics research: a step in the right direction? Epilepsy Behav. 2011;22:3–8. doi: 10.1016/j.yebeh.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 98.Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–28. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- 99.Letts VA, Beyer BJ, Frankel WN. Hidden in plain sight: spike-wave discharges in mouse inbred strains. Genes Brain Behav. 2014;13:519–26. doi: 10.1111/gbb.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Riban V, Bouilleret V, Pham L, Fritschy JM, Marescaux C, Depaulis A. Evolution of hippocampal epileptic activity during the development of hippocampal sclerosis in a mouse model of temporal lobe epilepsy. Neuroscience. 2002;112:101–11. doi: 10.1016/s0306-4522(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 101.Twele F, Töllner K, Brandt C, Löscher W. Significant effects of sex, strain, and anesthesia in the intrahippocampal kainate mouse model of mesial temporal lobe epilepsy. Epilepsy Behav. 2016;55:47–56. doi: 10.1016/j.yebeh.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 102.Twele F, Töllner K, Bankstahl M, Löscher W. The effects of carbamazepine in the intrahippocampal kainate model of temporal lobe epilepsy depend on seizure definition and mouse strain. Epilepsia Open. 2016;1:45–60. doi: 10.1002/epi4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferraro TN, Golden GT, Smith GG, DeMuth D, Buono RJ, Berrettini WH. Mouse strain variation in maximal electroshock seizure threshold. Brain Res. 2002;936:82–6. doi: 10.1016/s0006-8993(02)02565-9. [DOI] [PubMed] [Google Scholar]
- 104.Mohajeri MH, Madani R, Saini K, Lipp HP, Nitsch RM, Wolfer DP. The impact of genetic background on neurodegeneration and behavior in seizured mice. Genes Brain Behav. 2004;3:228–39. doi: 10.1111/j.1601-1848.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- 105.Schauwecker PE. Modulation of cell death by mouse genotype: differential vulnerability to excitatory amino acid-induced lesions. Exp Neurol. 2002;178:219–35. doi: 10.1006/exnr.2002.8038. [DOI] [PubMed] [Google Scholar]
- 106.Schauwecker PE, Williams RW, Santos JB. Genetic control of sensitivity to hippocampal cell death induced by kainic acid: a quantitative trait loci analysis. J Comp Neurol. 2004;477:96–107. doi: 10.1002/cne.20245. [DOI] [PubMed] [Google Scholar]
- 107.McKhann GM, Wenzel HJ, Robbins CA, Sosunov AA, Schwartzkroin PA. Mouse strain differences in kainic acid sensitivity, seizure behavior, mortality, and hippocampal pathology. Neuroscience. 2003;122:551–61. doi: 10.1016/s0306-4522(03)00562-1. [DOI] [PubMed] [Google Scholar]
- 108.Ndode-Ekane XE, Pitkänen A. Urokinase-type plasminogen activator receptor modulates epileptogenesis in mouse model of temporal lobe epilepsy. Mol Neurobiol. 2013;47:914–37. doi: 10.1007/s12035-012-8386-2. [DOI] [PubMed] [Google Scholar]
- 109.Rantala J, Kemppainen S, Ndode-Ekane XE, Lahtinen L, Bolkvadze T, Gurevicius K, et al. Urokinase-type plasminogen activator deficiency has little effect on seizure susceptibility and acquired epilepsy phenotype but reduces spontaneous exploration in mice. Epilepsy Behav. 2015;42:117–28. doi: 10.1016/j.yebeh.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 110.Kadiyala SB, Yannix JQ, Nalwalk JW, Papandrea D, Beyer BS, Herron BJ, et al. Eight Flurothyl-Induced Generalized Seizures Lead to the Rapid Evolution of Spontaneous Seizures in Mice: A Model of Epileptogenesis with Seizure Remission. J Neurosci. 2016;36:7485–96. doi: 10.1523/JNEUROSCI.3232-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kadiyala SB, Ferland RJ. Dissociation of spontaneous seizures and brainstem seizure thresholds in mice exposed to eight flurothyl-induced generalized seizures. Epilepsia Open. 2017;2:48–58. doi: 10.1002/epi4.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leech CK, McIntyre DC. Kindling rates in inbred mice: an analog of learning? Behav Biol. 1976;16:439–44. doi: 10.1016/s0091-6773(76)91603-5. [DOI] [PubMed] [Google Scholar]
- 113.Green RC, Seyfried TN. Kindling susceptibility and genetic seizure predisposition in inbred mice. Epilepsia. 1991;32:22–6. doi: 10.1111/j.1528-1157.1991.tb05605.x. [DOI] [PubMed] [Google Scholar]
- 114.Ferland RJ, Applegate CD. Decreased brainstem seizure thresholds and facilitated seizure propagation in mice exposed to repeated flurothyl-induced generalized forebrain seizures. Epilepsy Res. 1998;30:49–62. doi: 10.1016/s0920-1211(97)00093-4. [DOI] [PubMed] [Google Scholar]
- 115.Papandrea D, Anderson TM, Herron BJ, Ferland RJ. Dissociation of seizure traits in inbred strains of mice using the flurothyl kindling model of epileptogenesis. Exp Neurol. 2009;215:60–8. doi: 10.1016/j.expneurol.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kadiyala SB, Papandrea D, Herron BJ, Ferland RJ. Segregation of seizure traits in C57 black mouse substrains using the repeated-flurothyl model. PLoS One. 2014;9:e90506. doi: 10.1371/journal.pone.0090506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Papandrea D, Kukol WS, Anderson TM, Herron BJ, Ferland RJ. Analysis of flurothyl- induced myoclonus in inbred strains of mice. Epilepsy Res. 2009;87:130–6. doi: 10.1016/j.eplepsyres.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ferraro TN, Golden GT, Smith GG, Schork NJ, St Jean P, Ballas C, et al. Mapping murine loci for seizure response to kainic acid. Mamm Genome. 1997;8:200–8. doi: 10.1007/s003359900389. [DOI] [PubMed] [Google Scholar]
- 119.Ferland RJ, Smith J, Papandrea D, Gracias J, Kadiyala SB, O’Brien B, et al. Multidimensional genetic analysis of repeated seizures in the hybrid mouse diversity panel reveals a novel epileptogenesis susceptibility locus. Genes Genomes Genetics (G3) 2017 doi: 10.1534/g3.117.042234. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vezzani A, Fujinami RS, White HS, Preux PM, Blümcke I, Sander JW, et al. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131:211–34. doi: 10.1007/s00401-015-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Libbey JE, Fujinami RS. Neurotropic viral infections leading to epilepsy: focus on Theiler’s murine encephalomyelitis virus. Future Virol. 2011;6:1339–50. doi: 10.2217/fvl.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hutchings PR, Varey AM, Cooke A. Immunological defects in SJL mice. Immunology. 1986;59:445–50. [PMC free article] [PubMed] [Google Scholar]
- 123.Tsunoda I, Fujinami RS. Neuropathogenesis of Theiler’s murine encephalomyelitis virus infection, an animal model for multiple sclerosis. J Neuroimmune Pharmacol. 2010;5:355–69. doi: 10.1007/s11481-009-9179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Glasscock E. Genomic biomarkers of SUDEP in brain and heart. Epilepsy Behav. 2014;38:172–9. doi: 10.1016/j.yebeh.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dravet C, Oguni H. Dravet syndrome (severe myoclonic epilepsy in infancy) Handb Clin Neurol. 2013;111:627–33. doi: 10.1016/B978-0-444-52891-9.00065-8. [DOI] [PubMed] [Google Scholar]
- 126.Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia. 2010;51:1650–8. doi: 10.1111/j.1528-1167.2010.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. NAT NEUROSCI. 2006;9:1142–9. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- 128.Miller AR, Hawkins NA, McCollom CE, Kearney JA. Mapping genetic modifiers of survival in a mouse model of Dravet syndrome. Genes Brain Behav. 2014;13:163–72. doi: 10.1111/gbb.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hawkins NA, Zachwieja NJ, Miller AR, Anderson LL, Kearney JA. Fine Mapping of a Dravet Syndrome Modifier Locus on Mouse Chromosome 5 and Candidate Gene Analysis by RNA-Seq. PLoS Genet. 2016;12:e1006398. doi: 10.1371/journal.pgen.1006398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Honchar MP, Olney JW, Sherman WR. Systemic cholinergic agents induce seizures and brain damage in lithium-treated rats. Science. 1983;220:323–5. doi: 10.1126/science.6301005. [DOI] [PubMed] [Google Scholar]
- 131.Jope RS, Morrisett RA, Snead OC. Characterization of lithium potentiation of pilocarpine-induced status epilepticus in rats. Exp Neurol. 1986;91:471–80. doi: 10.1016/0014-4886(86)90045-2. [DOI] [PubMed] [Google Scholar]
- 132.Hort J, Brozek G, Komarek V, Langmeier M, Mares P. Interstrain differences in cognitive functions in rats in relation to status epilepticus. Behav Brain Res. 2000;112:77–83. doi: 10.1016/s0166-4328(00)00163-7. [DOI] [PubMed] [Google Scholar]
- 133.Xu B, McIntyre DC, Fahnestock M, Racine RJ. Strain differences affect the induction of status epilepticus and seizure-induced morphological changes. Eur J Neurosci. 2004;20:403–18. doi: 10.1111/j.1460-9568.2004.03489.x. [DOI] [PubMed] [Google Scholar]
- 134.Coulter DA, McIntyre DC, Löscher W. Animal models of limbic epilepsies: what can they tell us? Brain Pathol. 2002;12:240–56. doi: 10.1111/j.1750-3639.2002.tb00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Racine RJ, Burnham WM, Gartner JG, Levitan D. Rates of motor seizure development in rats subjected to electrical brain stimulation: strain and inter-stimulation interval effects. Electroenceph Clin Neurophysiol. 1973;35:553–6. doi: 10.1016/0013-4694(73)90033-3. [DOI] [PubMed] [Google Scholar]
- 136.Löscher W, Cramer S, Ebert U. Differences in kindling development in seven outbred and inbred rat strains. Exp Neurol. 1998;154:551–9. doi: 10.1006/exnr.1998.6948. [DOI] [PubMed] [Google Scholar]
- 137.Pinault D, Vergnes M, Marescaux C. Medium-voltage 5-9-Hz oscillations give rise to spike-and-wave discharges in a genetic model of absence epilepsy: in vivo dual extracellular recording of thalamic relay and reticular neurons. Neuroscience. 2001;105:181–201. doi: 10.1016/s0306-4522(01)00182-8. [DOI] [PubMed] [Google Scholar]
- 138.Wiest MC, Nicolelis MA. Behavioral detection of tactile stimuli during 7-12 Hz cortical oscillations in awake rats. NAT NEUROSCI. 2003;6:913–4. doi: 10.1038/nn1107. [DOI] [PubMed] [Google Scholar]
- 139.Kaplan BJ. The epileptic nature of rodent electrocortical polyspiking is still unproven. Exp Neurol. 1985;88:425–36. doi: 10.1016/0014-4886(85)90204-3. [DOI] [PubMed] [Google Scholar]
- 140.Willoughby JO, Mackenzie L. Nonconvulsive electrocorticographic paroxysms (absence epilepsy) in rat strains. Lab Anim Sci. 1992;42:551–4. [PubMed] [Google Scholar]
- 141.Shaw FZ. Is spontaneous high-voltage rhythmic spike discharge in Long Evans rats an absence-like seizure activity? J Neurophysiol. 2004;91:63–77. doi: 10.1152/jn.00487.2003. [DOI] [PubMed] [Google Scholar]
- 142.Shaw FZ. 7-12 Hz high-voltage rhythmic spike discharges in rats evaluated by antiepileptic drugs and flicker stimulation. J Neurophysiol. 2007;97:238–47. doi: 10.1152/jn.00340.2006. [DOI] [PubMed] [Google Scholar]
- 143.Coenen AML, Drinkenburg WHIM, Inoue M, van Luijtelaar ELJM. Genetic models of absence epilepsy, with emphasis on the WAG/Rij strain of rats. Epilepsy Res. 1992;12:75–86. doi: 10.1016/0920-1211(92)90029-s. [DOI] [PubMed] [Google Scholar]
- 144.Marescaux C, Vergnes M, Depaulis A. Genetic absence epilepsy in rats from Strasbourg - A review. J Neural Transm. 1992;35:37–69. doi: 10.1007/978-3-7091-9206-1_4. [DOI] [PubMed] [Google Scholar]
- 145.Van Luijtelaar G, Onat FY, Gallagher MJ. Animal models of absence epilepsies: what do they model and do sex and sex hormones matter? Neurobiol Dis. 2014;72(Pt B):167–79. doi: 10.1016/j.nbd.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Depaulis A, David O, Charpier S. The genetic absence epilepsy rat from Strasbourg as a model to decipher the neuronal and network mechanisms of generalized idiopathic epilepsies. J Neurosci Methods. 2016;260:159–74. doi: 10.1016/j.jneumeth.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 147.D’Ambrosio R, Hakimian S, Stewart T, Verley DR, Fender JS, Eastman CL, et al. Functional definition of seizure provides new insight into post-traumatic epileptogenesis. Brain. 2009;132:2805–21. doi: 10.1093/brain/awp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Buzsaki G, Silva FL. High frequency oscillations in the intact brain. Prog Neurobiol. 2012;98:241–9. doi: 10.1016/j.pneurobio.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 150.Engel J, Jr, da Silva FL. High-frequency oscillations - where we are and where we need to go. Prog Neurobiol. 2012;98:316–8. doi: 10.1016/j.pneurobio.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 152.Groothuis J, Ramsey NF, Ramakers GM, van der PG. Physiological challenges for intracortical electrodes. Brain Stimul. 2014;7:1–6. doi: 10.1016/j.brs.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 153.Hessel EV, de Wit M, Wolterink-Donselaar IG, Karst H, de Graaff E, van Lith HA, et al. Identification of Srp9 as a febrile seizure susceptibility gene. Ann Clin Transl Neurol. 2014;1:239–50. doi: 10.1002/acn3.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ferraro TN, Golden GT, Smith GG, St Jean P, Schork NJ, Mulholland N, et al. Mapping loci for pentylenetetrazol-induced seizure susceptibility in mice. J Neurosci. 1999;19:6733–9. doi: 10.1523/JNEUROSCI.19-16-06733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ferraro TN, Golden GT, Smith GG, Longman RL, Snyder RL, DeMuth D, et al. Quantitative genetic study of maximal electroshock seizure threshold in mice: evidence for a major seizure susceptibility locus on distal chromosome 1. GENOMICS. 2001;75:35–42. doi: 10.1006/geno.2001.6577. [DOI] [PubMed] [Google Scholar]
- 156.Ferraro TN, Golden GT, Dahl JP, Smith GG, Schwebel CL, MacDonald R, et al. Analysis of a quantitative trait locus for seizure susceptibility in mice using bacterial artificial chromosome-mediated gene transfer. Epilepsia. 2007;48:1667–77. doi: 10.1111/j.1528-1167.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 157.Buono RJ, Lohoff FW, Sander T, Sperling MR, O’Connor MJ, Dlugos DJ, et al. Association between variation in the human KCNJ10 potassium ion channel gene and seizure susceptibility. Epilepsy Res. 2004;58:175–83. doi: 10.1016/j.eplepsyres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 158.Lenzen KP, Heils A, Lorenz S, Hempelmann A, Hofels S, Lohoff FW, et al. Supportive evidence for an allelic association of the human KCNJ10 potassium channel gene with idiopathic generalized epilepsy. Epilepsy Res. 2005;63:113–8. doi: 10.1016/j.eplepsyres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 159.Heuser K, Nagelhus EA, Tauboll E, Indahl U, Berg PR, Lien S, et al. Variants of the genes encoding AQP4 and Kir4.1 are associated with subgroups of patients with temporal lobe epilepsy. Epilepsy Res. 2010;88:55–64. doi: 10.1016/j.eplepsyres.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 160.Phani NM, Acharya S, Xavy S, Bhaskaranand N, Bhat MK, Jain A, et al. Genetic association of KCNJ10 rs1130183 with seizure susceptibility and computational analysis of deleterious non-synonymous SNPs of KCNJ10 gene. Gene. 2014;536:247–53. doi: 10.1016/j.gene.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 161.Guo Y, Yan KP, Qu Q, Qu J, Chen ZG, Song T, et al. Common variants of KCNJ10 are associated with susceptibility and anti-epileptic drug resistance in Chinese genetic generalized epilepsies. PLoS One. 2015;10:e0124896. doi: 10.1371/journal.pone.0124896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–70. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, et al. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A. 2009;106:5842–7. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shirley RL, Walter NA, Reilly MT, Fehr C, Buck KJ. Mpdz is a quantitative trait gene for drug withdrawal seizures. NAT NEUROSCI. 2004;7:699–700. doi: 10.1038/nn1271. [DOI] [PubMed] [Google Scholar]
- 165.Fehr C, Shirley RL, Belknap JK, Crabbe JC, Buck KJ. Congenic mapping of alcohol and pentobarbital withdrawal liability loci to a <1 centimorgan interval of murine chromosome 4: identification of Mpdz as a candidate gene. J Neurosci. 2002;22:3730–8. doi: 10.1523/JNEUROSCI.22-09-03730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Milner LC, Shirley RL, Kozell LB, Walter NA, Kruse LC, Komiyama NH, et al. Novel MPDZ/MUPP1 transgenic and knockdown models confirm Mpdz’s role in ethanol withdrawal and support its role in voluntary ethanol consumption. Addict Biol. 2015;20:143–7. doi: 10.1111/adb.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Karpyak VM, Kim JH, Biernacka JM, Wieben ED, Mrazek DA, Black JL, et al. Sequence variations of the human MPDZ gene and association with alcoholism in subjects with European ancestry. Alcohol Clin Exp Res. 2009;33:712–21. doi: 10.1111/j.1530-0277.2008.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beck C, Moulard B, Steinlein O, Guipponi M, Vallee L, Montpied P, et al. A nonsense mutation in the alpha4 subunit of the nicotinic acetylcholine receptor (CHRNA4) cosegregates with 20q-linked benign neonatal familial convulsions (EBNI) Neurobiol Dis. 1994;1:95–9. doi: 10.1006/nbdi.1994.0012. [DOI] [PubMed] [Google Scholar]
- 169.Chou IC, Lee CC, Huang CC, Wu JY, Tsai JJ, Tsai CH, et al. Association of the neuronal nicotinic acetylcholine receptor subunit alpha4 polymorphisms with febrile convulsions. Epilepsia. 2003;44:1089–93. doi: 10.1046/j.1528-1157.2003.t01-1-44702.x. [DOI] [PubMed] [Google Scholar]
- 170.Rozycka A, Steinborn B, Trzeciak WH. The 1674+11C>T polymorphism of CHRNA4 is associated with juvenile myoclonic epilepsy. Seizure. 2009;18:601–3. doi: 10.1016/j.seizure.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 171.Kevelam SH, Jansen FE, Binsbergen E, Braun KP, Verbeek NE, Lindhout D, et al. Copy number variations in patients with electrical status epilepticus in sleep. J Child Neurol. 2012;27:178–82. doi: 10.1177/0883073811416006. [DOI] [PubMed] [Google Scholar]
- 172.Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, et al. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995;11:201–3. doi: 10.1038/ng1095-201. [DOI] [PubMed] [Google Scholar]
- 173.De Fusco M, Becchetti A, Patrignani A, Annesi G, Gambardella A, Quattrone A, et al. The nicotinic receptor beta 2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat Genet. 2000;26:275–6. doi: 10.1038/81566. [DOI] [PubMed] [Google Scholar]
- 174.Stitzel JA, Blanchette JM, Collins AC. Sensitivity to the seizure-inducing effects of nicotine is associated with strain-specific variants of the alpha 5 and alpha 7 nicotinic receptor subunit genes. J Pharmacol Exp Ther. 1998;284:1104–11. [PubMed] [Google Scholar]
- 175.Stitzel JA, Jimenez M, Marks MJ, Tritto T, Collins AC. Potential role of the alpha4 and alpha6 nicotinic receptor subunits in regulating nicotine-induced seizures. J Pharmacol Exp Ther. 2000;293:67–74. [PubMed] [Google Scholar]
- 176.Fonck C, Nashmi R, Deshpande P, Damaj MI, Marks MJ, Riedel A, et al. Increased sensitivity to agonist-induced seizures, straub tail, and hippocampal theta rhythm in knock-in mice carrying hypersensitive alpha 4 nicotinic receptors. J Neurosci. 2003;23:2582–90. doi: 10.1523/JNEUROSCI.23-07-02582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mazarati AM, Halaszi E, Telegdy G. Anticonvulsive effects of galanin administered into the central nervous system upon the picrotoxin-kindled seizure syndrome in rats. Brain Res. 1992;589:164–6. doi: 10.1016/0006-8993(92)91179-i. [DOI] [PubMed] [Google Scholar]
- 178.Jacoby AS, Hort YJ, Constantinescu G, Shine J, Iismaa TP. Critical role for GALR1 galanin receptor in galanin regulation of neuroendocrine function and seizure activity. Brain Res Mol Brain Res. 2002;107:195–200. doi: 10.1016/s0169-328x(02)00451-5. [DOI] [PubMed] [Google Scholar]
- 179.Gottsch ML, Zeng H, Hohmann JG, Weinshenker D, Clifton DK, Steiner RA. Phenotypic analysis of mice deficient in the type 2 galanin receptor (GALR2) Mol Cell Biol. 2005;25:4804–11. doi: 10.1128/MCB.25.11.4804-4811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kong S, Lorenzana A, Deng Q, McNeill TH, Schauwecker PE. Variation in Galr1 expression determines susceptibility to exocitotoxin-induced cell death in mice. Genes Brain Behav. 2008;7:587–98. doi: 10.1111/j.1601-183X.2008.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Guipponi M, Chentouf A, Webling KE, Freimann K, Crespel A, Nobile C, et al. Galanin pathogenic mutations in temporal lobe epilepsy. HUM MOL GENET. 2015;24:3082–91. doi: 10.1093/hmg/ddv060. [DOI] [PubMed] [Google Scholar]
- 182.Brown RE, Stanford L, Schellinck HM. Developing standardized behavioral tests for knockout and mutant mice. ILAR J. 2000;41:163–74. doi: 10.1093/ilar.41.3.163. [DOI] [PubMed] [Google Scholar]
- 183.Bailey KR, Rustay NR, Crawley JN. Behavioral phenotyping of transgenic and knockout mice: practical concerns and potential pitfalls. ILAR J. 2006;47:124–31. doi: 10.1093/ilar.47.2.124. [DOI] [PubMed] [Google Scholar]
- 184.Editorial. Troublesome variability in mouse studies. NAT NEUROSCI. 2009;12:1075. doi: 10.1038/nn0909-1075. [DOI] [PubMed] [Google Scholar]
- 185.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–2. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 186.Hoorn EJ, McCormick JA, Ellison DH. High tail-cuff blood pressure in mice 1 week after shipping: the need for longer acclimation. Am J Hypertens. 2011;24:534–6. doi: 10.1038/ajh.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Olfe J, Domanska G, Schuett C, Kiank C. Different stress-related phenotypes of BALB/c mice from in-house or vendor: alterations of the sympathetic and HPA axis responsiveness. BMC Physiol. 2010;10:2. doi: 10.1186/1472-6793-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Auvergne R, Lere C, El Bahh B, Arthaud S, Lespinet V, Rougier A, et al. Delayed kindling epileptogenesis and increased neurogenesis in adult rats housed in an enriched environment. Brain Res. 2002;954:277–85. doi: 10.1016/s0006-8993(02)03355-3. [DOI] [PubMed] [Google Scholar]
- 189.Young NA, Wintink AJ, Kalynchuk LE. Environmental enrichment facilitates amygdala kindling but reduces kindling-induced fear in male rats. Behav Neurosci. 2004;118:1128–33. doi: 10.1037/0735-7044.118.5.1128. [DOI] [PubMed] [Google Scholar]
- 190.Manno I, Macchi F, Caleo M, Bozzi Y. Environmental enrichment reduces spontaneous seizures in the Q54 transgenic mouse model of temporal lobe epilepsy. Epilepsia. 2011;52:e113–e117. doi: 10.1111/j.1528-1167.2011.03166.x. [DOI] [PubMed] [Google Scholar]
- 191.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–53. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 192.Dhanushkodi A, Shetty AK. Is exposure to enriched environment beneficial for functional post-lesional recovery in temporal lobe epilepsy? Neurosci Biobehav Rev. 2008;32:657–74. doi: 10.1016/j.neubiorev.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Burrows EL, McOmish CE, Hannan AJ. Gene-environment interactions and construct validity in preclinical models of psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1376–82. doi: 10.1016/j.pnpbp.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 194.Maguire J, Salpekar JA. Stress, seizures, and hypothalamic-pituitary-adrenal axis targets for the treatment of epilepsy. Epilepsy Behav. 2013;26:352–62. doi: 10.1016/j.yebeh.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.McEwen BS, Alves SE, Bulloch K, Weiland NG. Clinically relevant basic science studies of gender differences and sex hormone effects. Psychopharmacol Bull. 1998;34:251–9. [PubMed] [Google Scholar]
- 196.Veliskova J, Desantis KA. Sex and hormonal influences on seizures and epilepsy. Horm Behav. 2013;63:267–77. doi: 10.1016/j.yhbeh.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Galanopoulou AS. Sex and epileptogenesis, introduction to the special issue. Neurobiol Dis. 2014;72(Pt B):123–4. doi: 10.1016/j.nbd.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Scharfman HE, MacLusky NJ. Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol Dis. 2014;72(Pt B):180–92. doi: 10.1016/j.nbd.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Morrell MJ. Hormones and epilepsy through the lifetime. Epilepsia. 1992;33(Suppl 4):S49–S61. doi: 10.1111/j.1528-1157.1992.tb06227.x. [DOI] [PubMed] [Google Scholar]
- 200.Finn DA, Gee KW. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J Pharmacol Exp Ther. 1994;271:164–70. [PubMed] [Google Scholar]
- 201.Rattka M, Brandt C, Löscher W. Do proconvulsants modify or halt epileptogenesis? Pentylenetetrazole is ineffective in two rat models of temporal lobe epilepsy. Eur J Neurosci. 2012;36:2505–2520. doi: 10.1111/j.1460-9568.2012.08143.x. [DOI] [PubMed] [Google Scholar]
- 202.Wahnschaffe U, Löscher W. Lack of changes in seizure susceptibility during the estrous cycle in kindled rats. Epilepsy Res. 1992;13:199–204. doi: 10.1016/0920-1211(92)90053-v. [DOI] [PubMed] [Google Scholar]
- 203.Wooley DE, Timiras PS. Estrous and circadian periodicity and electroshock convulsions in rats. Am J Physiol. 1962;202:379–82. doi: 10.1152/ajplegacy.1962.202.2.379. [DOI] [PubMed] [Google Scholar]
- 204.Perucca P, Camfield P, Camfield C. Does gender influence susceptibility and consequences of acquired epilepsies? Neurobiol Dis. 2014;72(Pt B):125–30. doi: 10.1016/j.nbd.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 205.Pitkänen A, Huusko N, Ndode-Ekane XE, Kyyriainen J, Lipponen A, Lipsanen A, et al. Gender issues in antiepileptogenic treatments. Neurobiol Dis. 2014;72(Pt B):224–32. doi: 10.1016/j.nbd.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 206.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–72. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Löscher W, Hirsch LJ, Schmidt D. The enigma of the latent period in the development of symptomatic acquired epilepsy – Traditional view versus new concepts. Epilepsy Behav. 2015;52:78–92. doi: 10.1016/j.yebeh.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 208.Mejias-Aponte CA, Jimenez-Rivera CA, Segarra AC. Sex differences in models of temporal lobe epilepsy: role of testosterone. Brain Res. 2002;944:210–8. doi: 10.1016/s0006-8993(02)02691-4. [DOI] [PubMed] [Google Scholar]
- 209.Galanopoulou AS, Alm EM, Veliskova J. Estradiol reduces seizure-induced hippocampal injury in ovariectomized female but not in male rats. Neurosci Lett. 2003;342:201–5. doi: 10.1016/s0304-3940(03)00282-9. [DOI] [PubMed] [Google Scholar]
- 210.Zhang XM, Zhu SW, Duan RS, Mohammed AH, Winblad B, Zhu J. Gender differences in susceptibility to kainic acid-induced neurodegeneration in aged C57BL/6 mice. Neurotoxicology. 2008;29:406–12. doi: 10.1016/j.neuro.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 211.Pereno GL, Beltramino CA. Differential role of gonadal hormones on kainic acid- induced neurodegeneration in medial amygdaloid nucleus of female and male rats. Neuroscience. 2009;163:952–63. doi: 10.1016/j.neuroscience.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 212.Pereno GL, Balaszczuk V, Beltramino CA. Effect of sex differences and gonadal hormones on kainic acid-induced neurodegeneration in the bed nucleus of the stria terminalis of the rat. Exp Toxicol Pathol. 2012;64:283–9. doi: 10.1016/j.etp.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 213.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012:e4389. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kanner AM, Mazarati A, Koepp M. Biomarkers of epileptogenesis: psychiatric comorbidities (? Neurotherapeutics. 2014;11:358–72. doi: 10.1007/s13311-014-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Adamec R, Shallow T. Effects of baseline anxiety on response to kindling of the right medial amygdala. Physiol Behav. 2000;70:67–80. doi: 10.1016/s0031-9384(00)00247-x. [DOI] [PubMed] [Google Scholar]
- 217.Theilmann W, Kleimann A, Rhein M, Bleich S, Frieling H, Löscher W, et al. Behavioral differences of male Wistar rats from different vendors in vulnerability and resilience to chronic mild stress are reflected in epigenetic regulation and expression of p11. Brain Res. 2016;1642:505–15. doi: 10.1016/j.brainres.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 218.Roth TL. Epigenetic mechanisms in the development of behavior: advances, challenges, and future promises of a new field. Dev Psychopathol. 2013;25:1279–91. doi: 10.1017/S0954579413000618. [DOI] [PMC free article] [PubMed] [Google Scholar]


