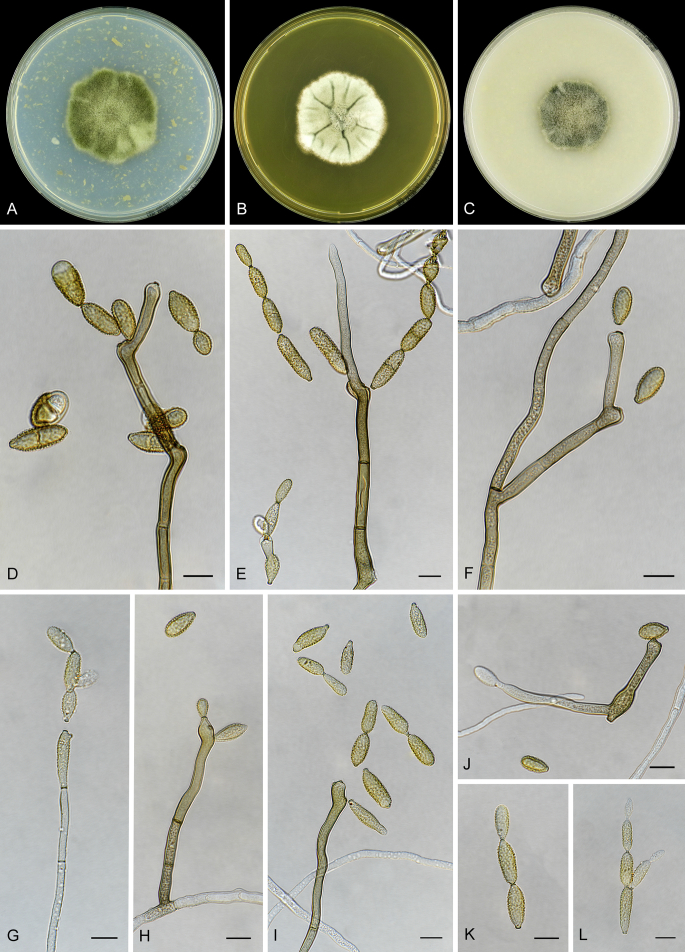
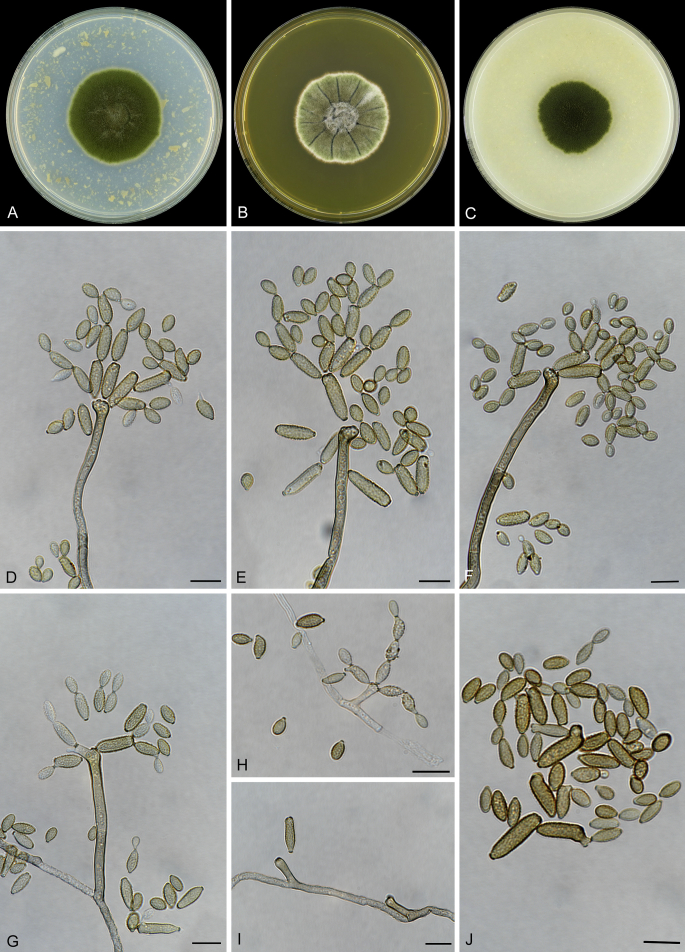
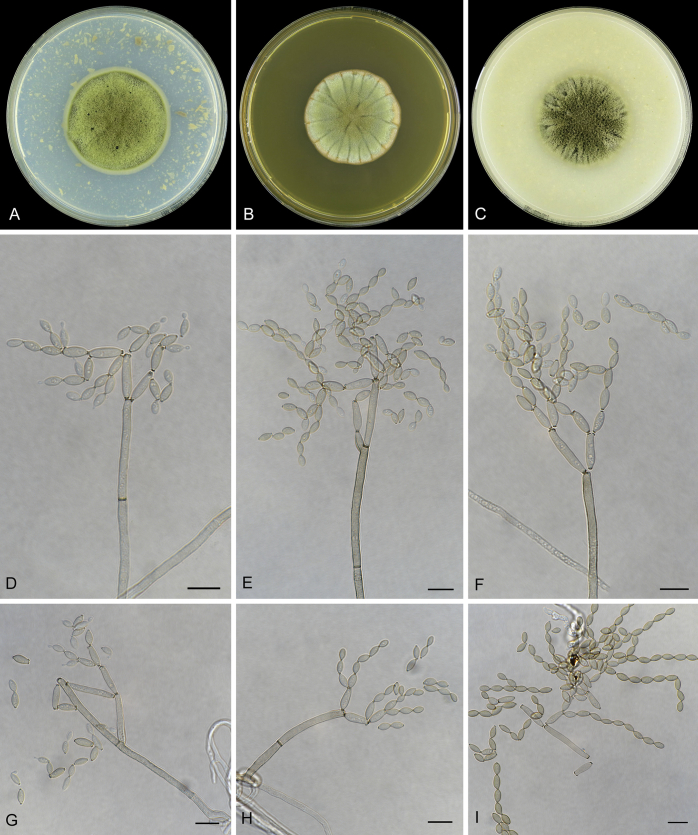
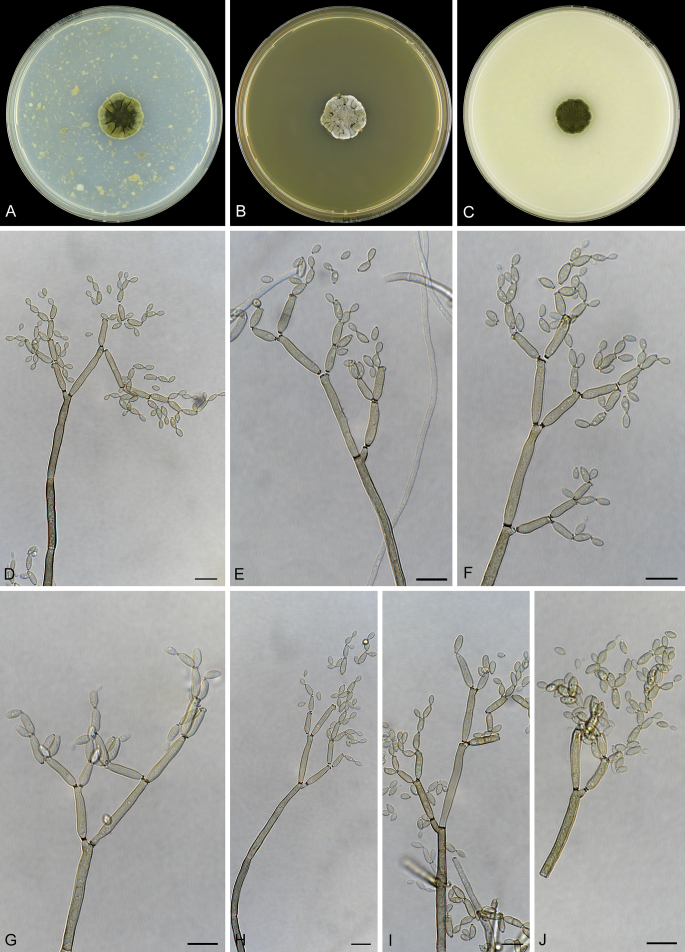
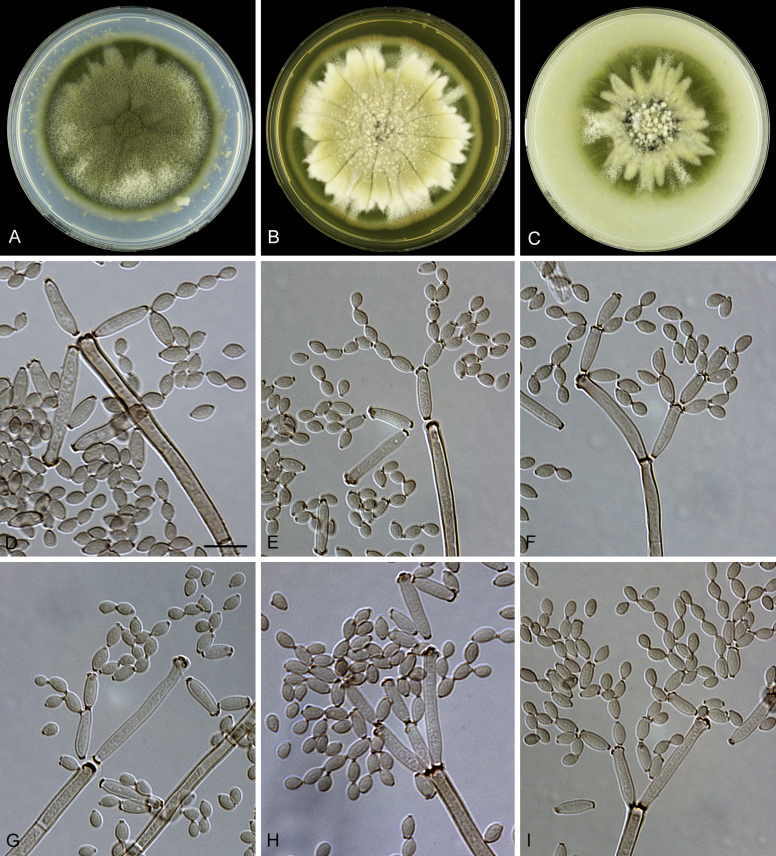
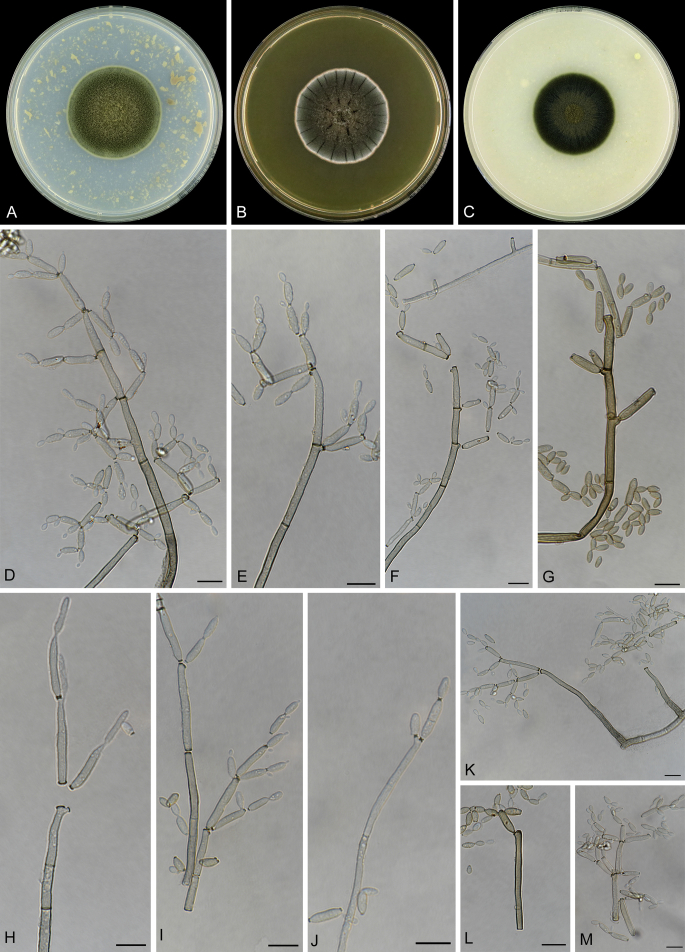
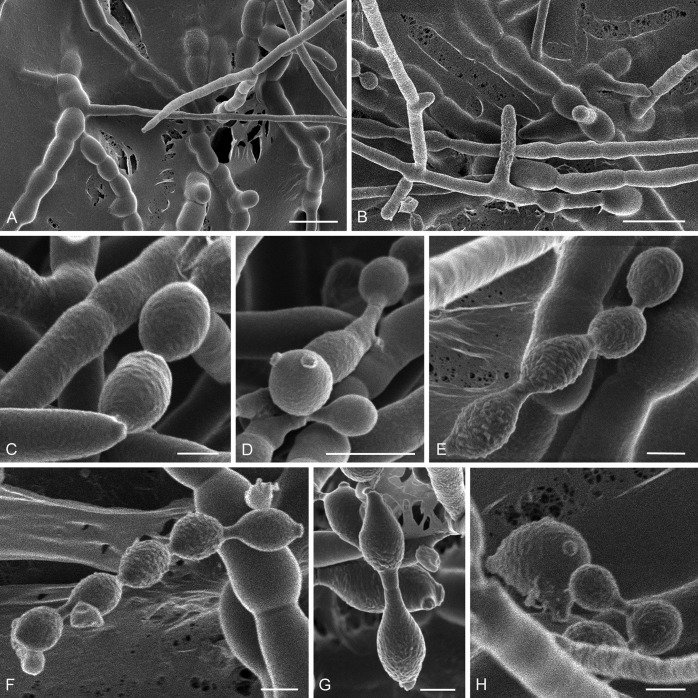
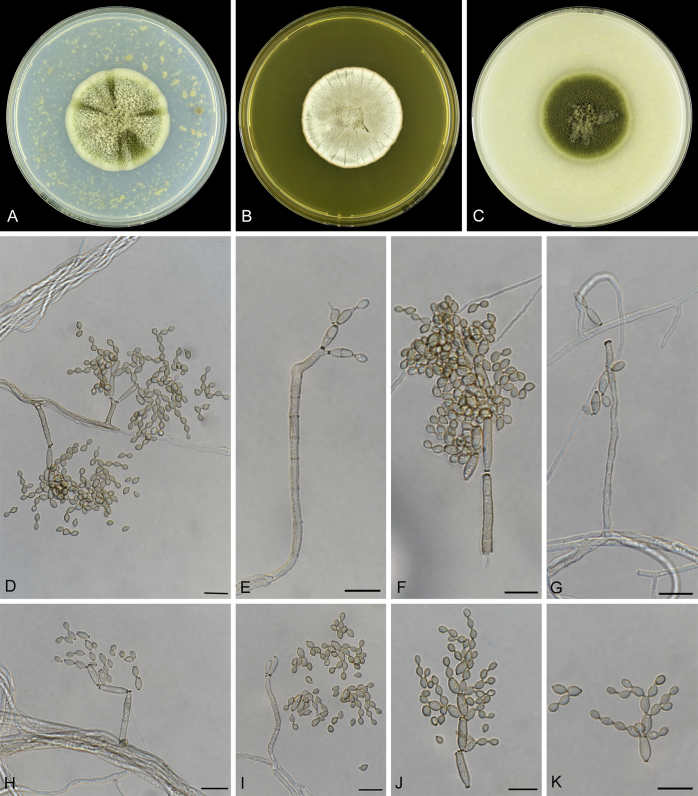
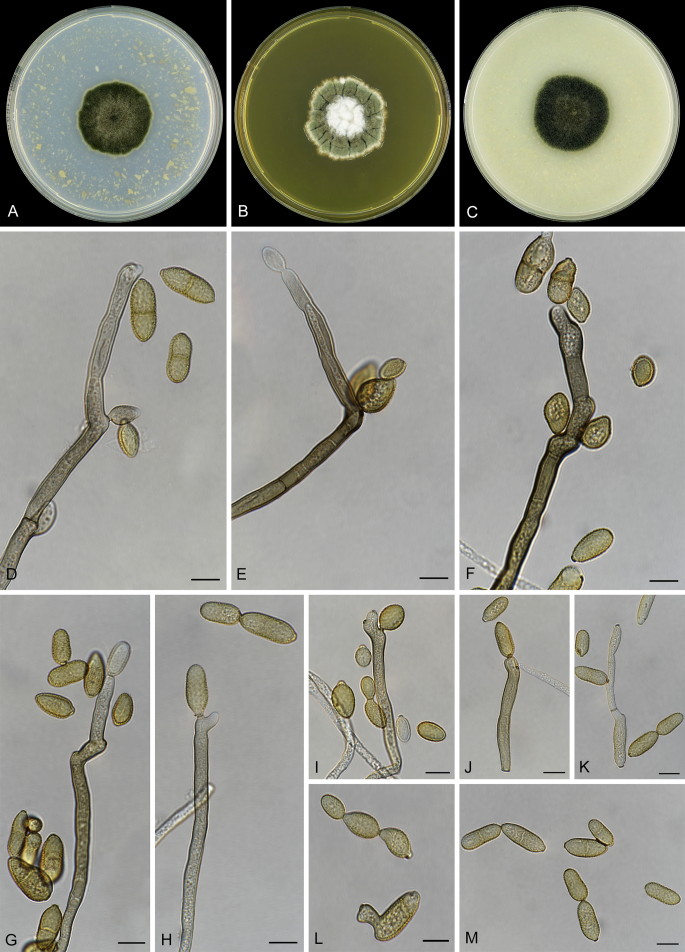
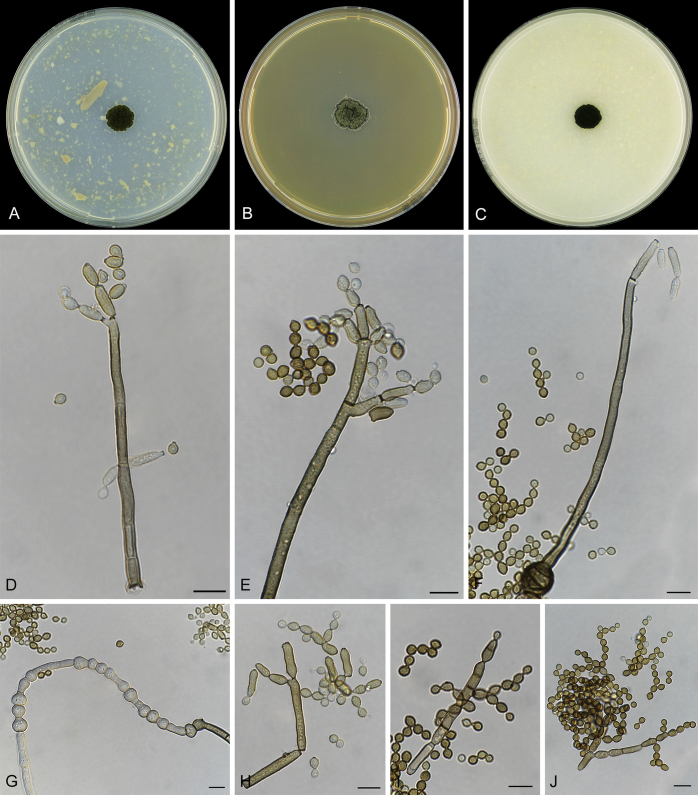
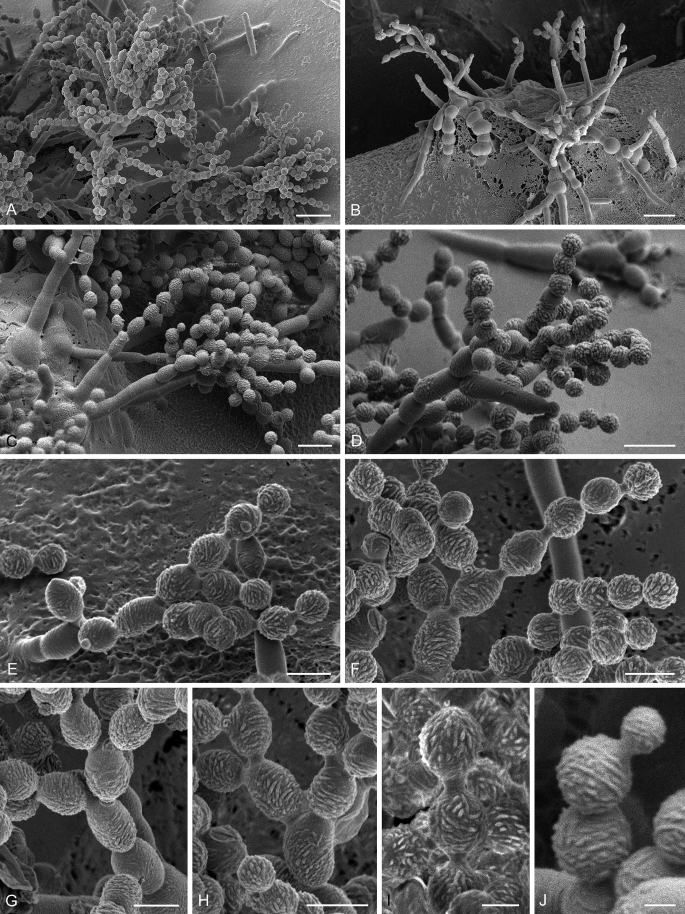
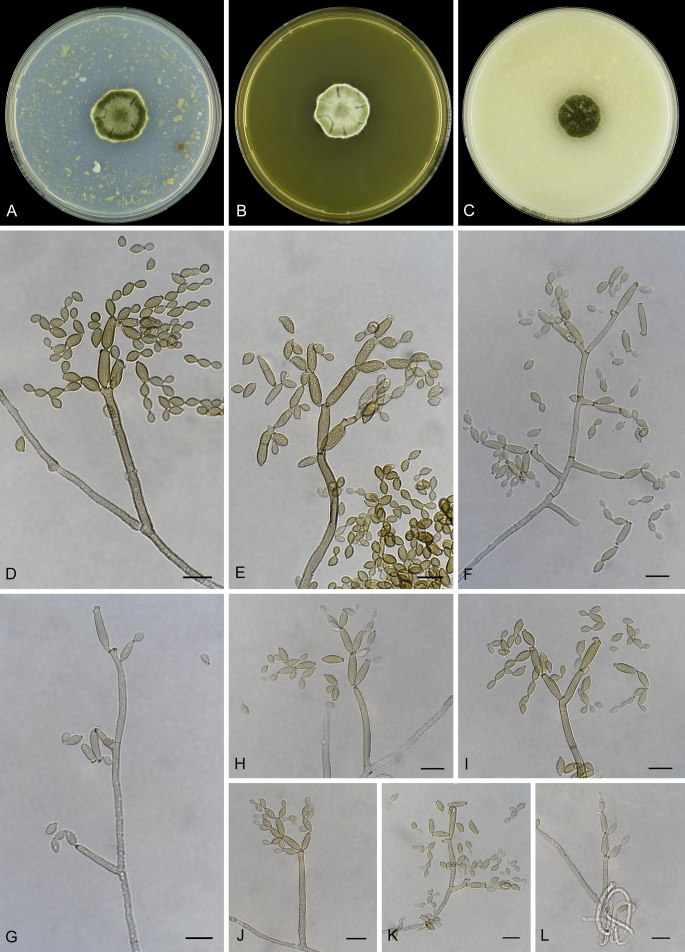
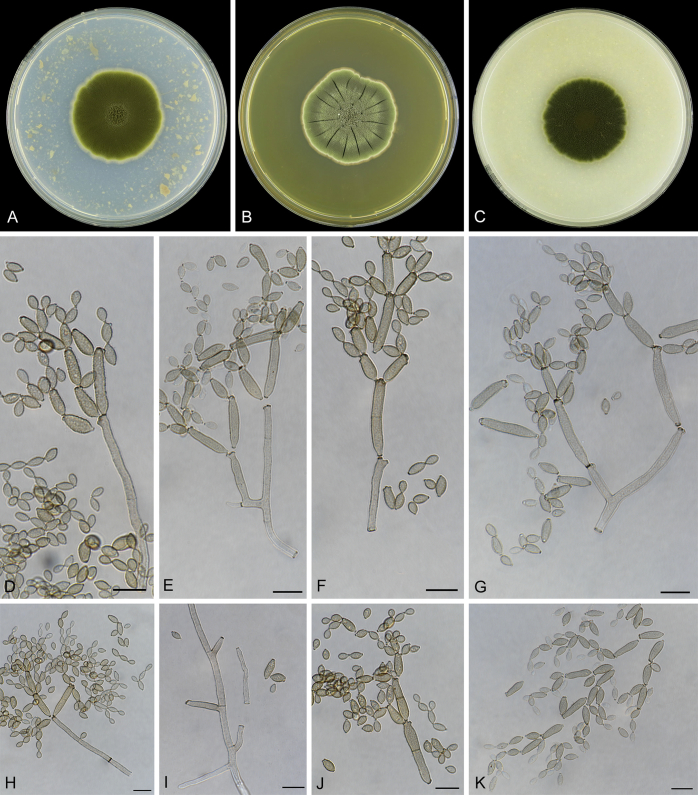
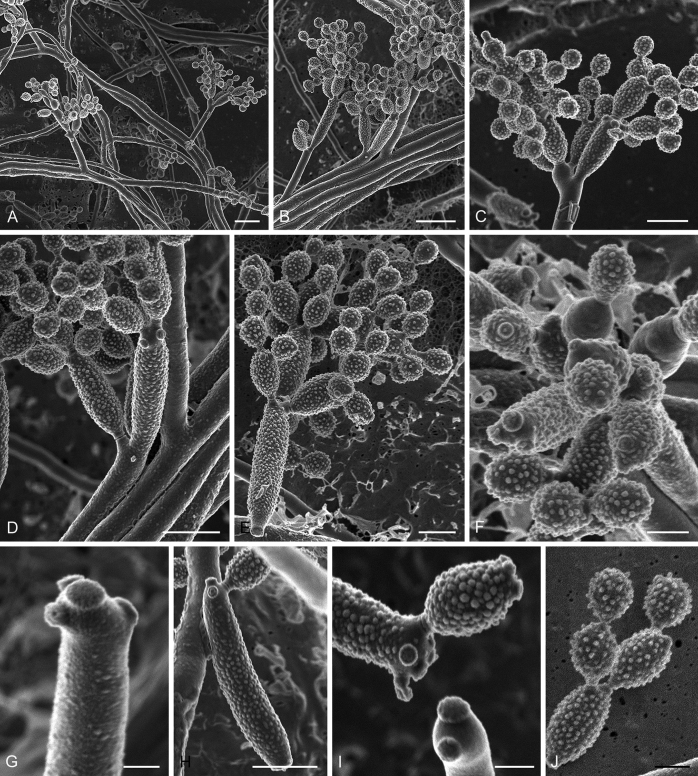
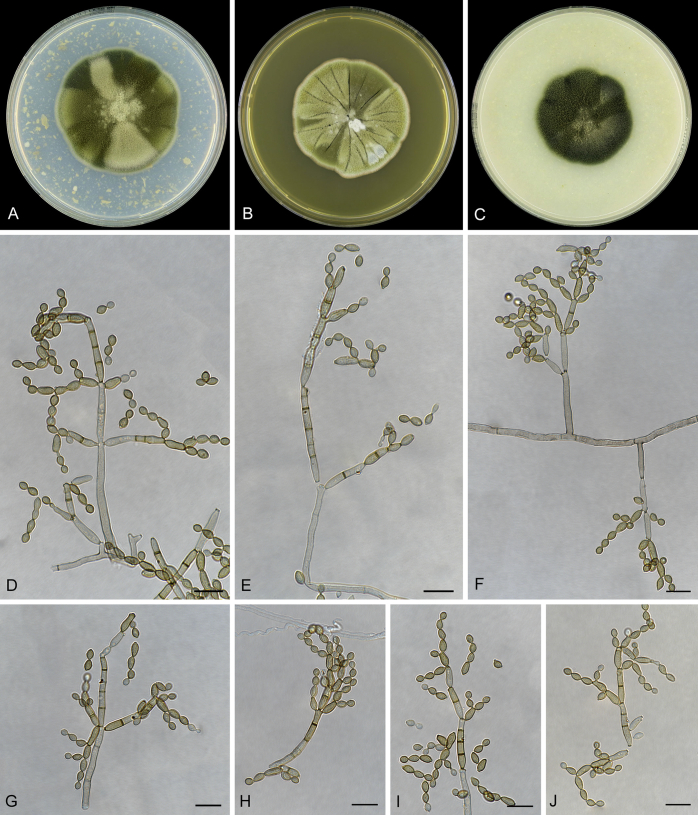
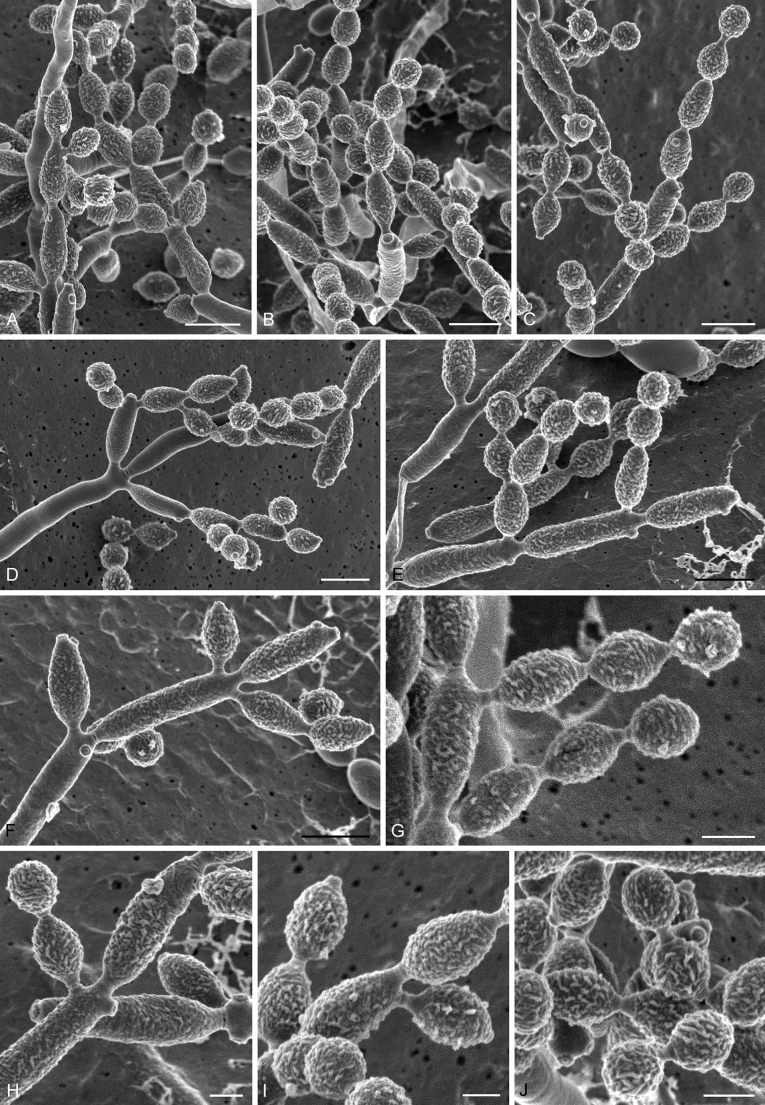
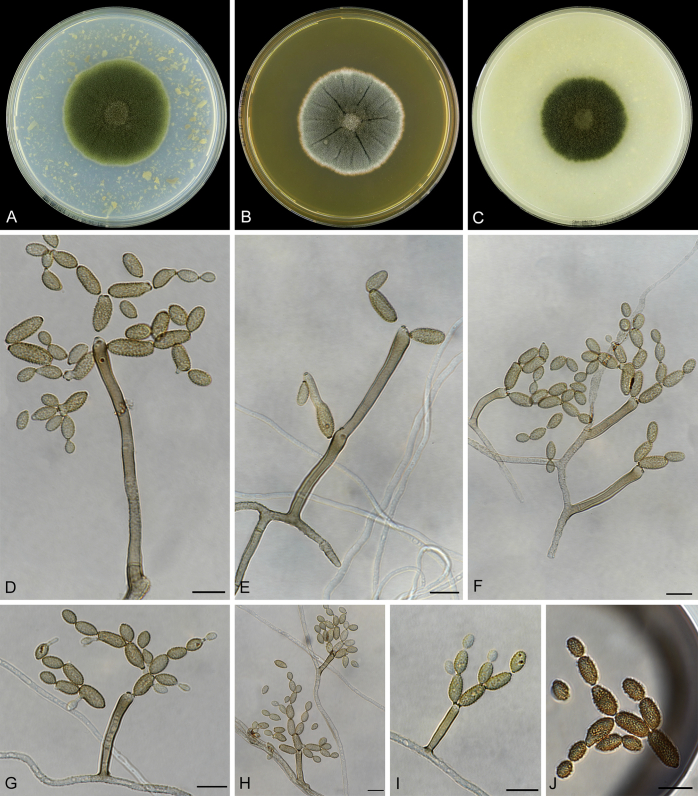
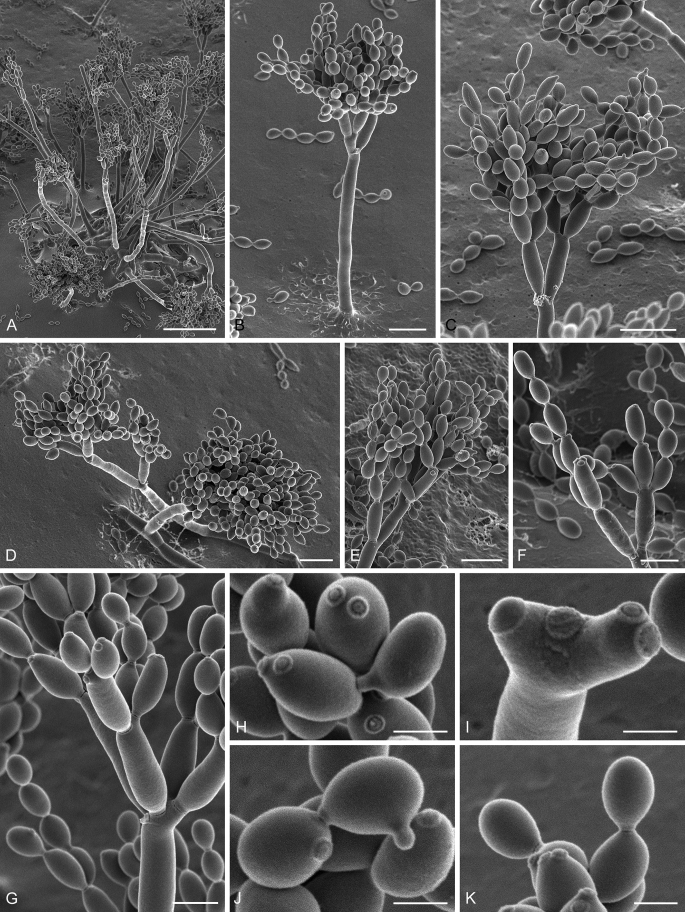
Abstract
As part of a worldwide survey of the indoor mycobiota about 520 new Cladosporium isolates from indoor environments mainly collected in China, Europe, New Zealand, North America and South Africa were investigated by using a polyphasic approach to determine their species identity. All Cladosporium species occurring in indoor environments are fully described and illustrated. Fourty-six Cladosporium species are treated of which 16 species are introduced as new. A key for the most common Cladosporium species isolated from indoor environments is provided. Cladosporium halotolerans proved to be the most frequently isolated Cladosporium species indoors.
Key words: Indoor molds, New species, Phylogeny, Taxonomy, 16 new taxa
Taxonomic novelties: New species: Cladosporium aerium Bensch & Samson, C. coloradense Bensch & Samson, C. domesticum Bensch & Samson, C. europaeum Bensch & Samson, C. needhamense Bensch & Samson, C. neerlandicum Bensch & Samson, C. neolangeronii Bensch & Samson, C. parahalotolerans Bensch & Samson, C. parasubtilissimum Bensch & Samson, C. pulvericola Bensch & Samson, C. sinense Bensch & Samson, C. sloanii Bensch & Samson, C. uwebraunianum Bensch & Samson, C. vicinum Bensch & Samson, C. westerdijkiae Bensch & Samson, C. wyomingense Bensch & Samson
Introduction
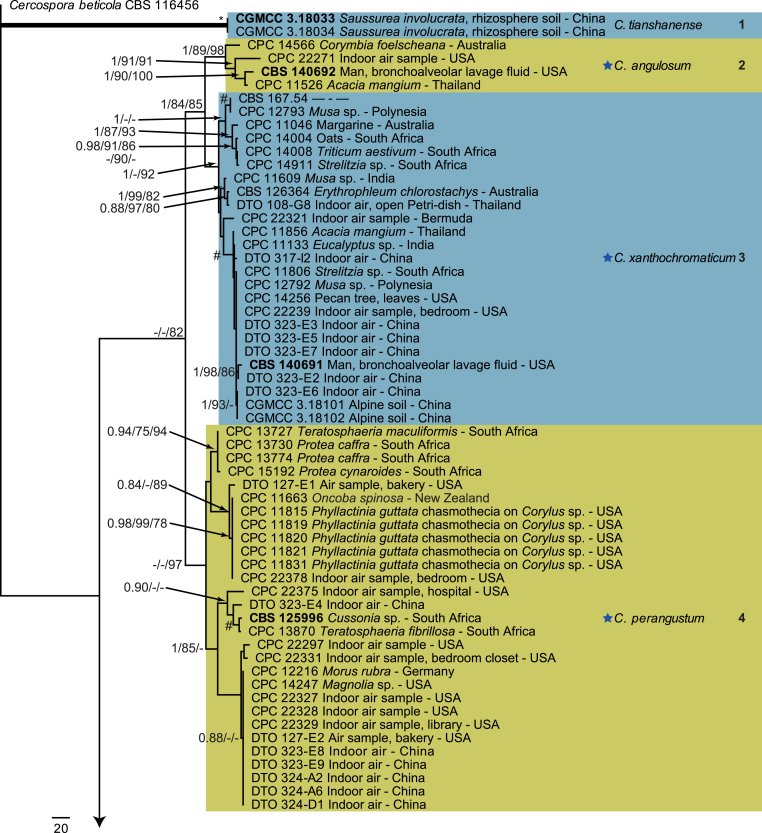
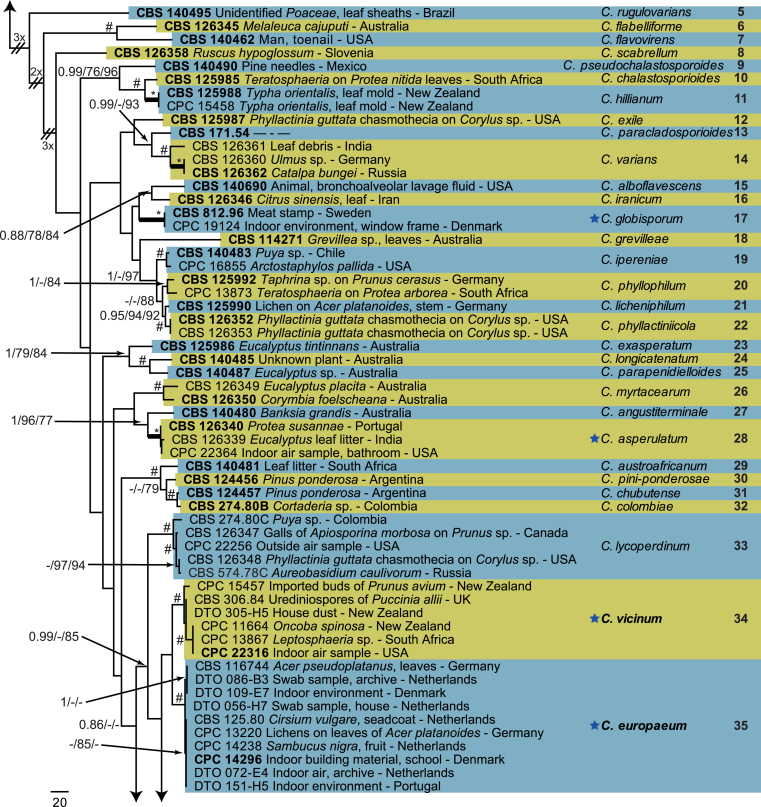
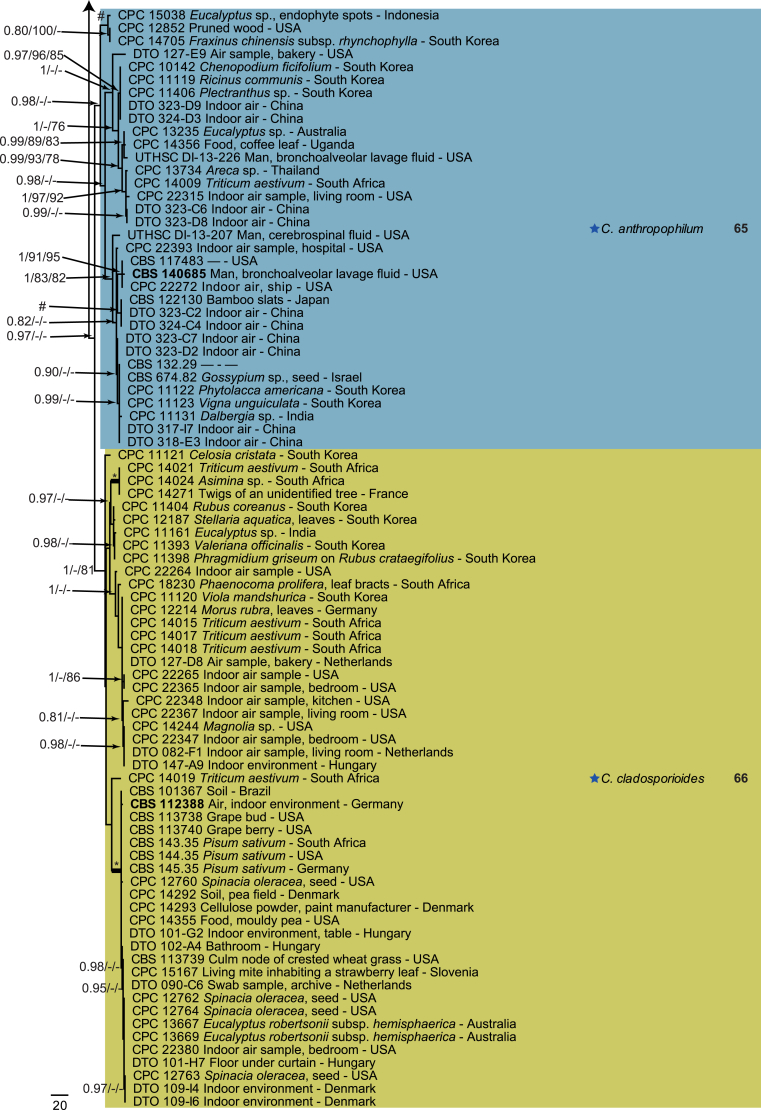
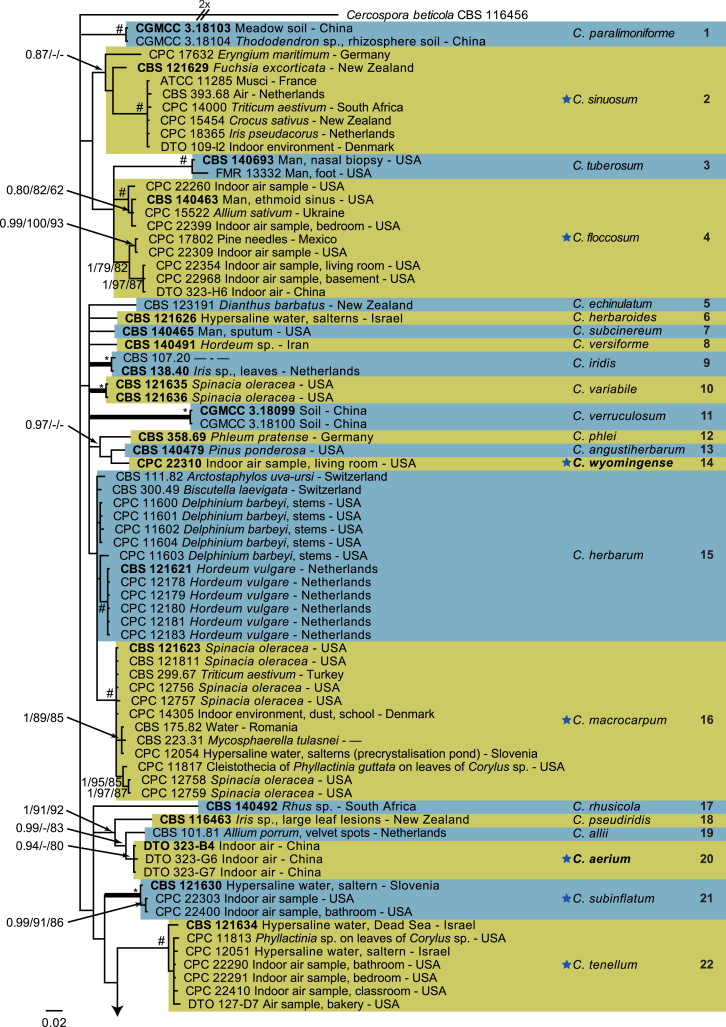
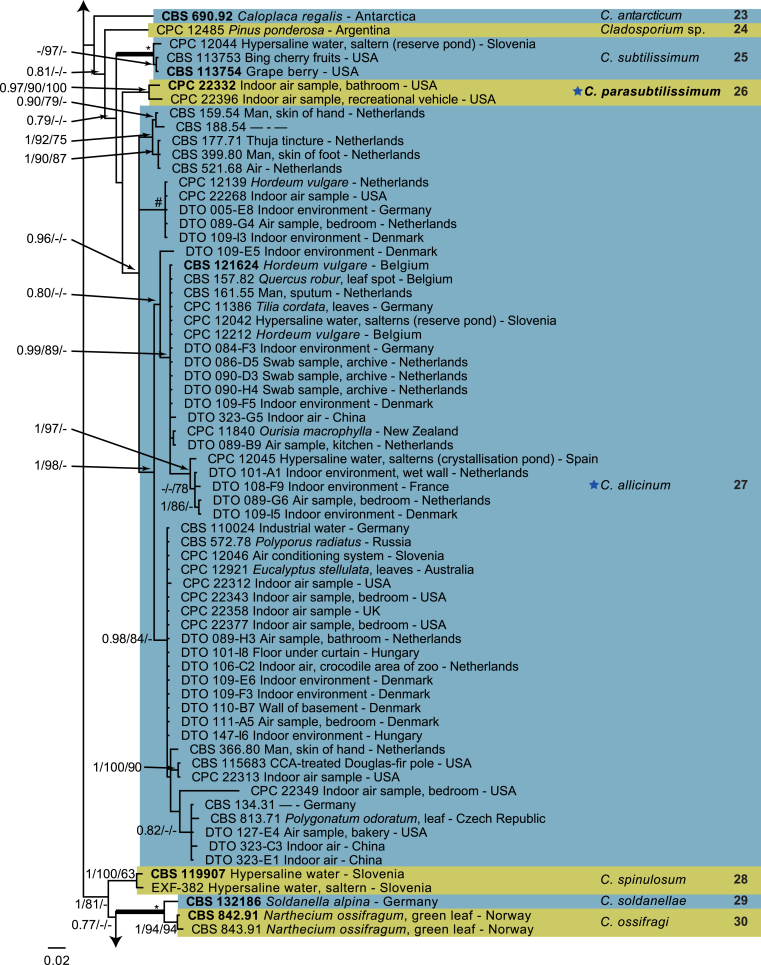
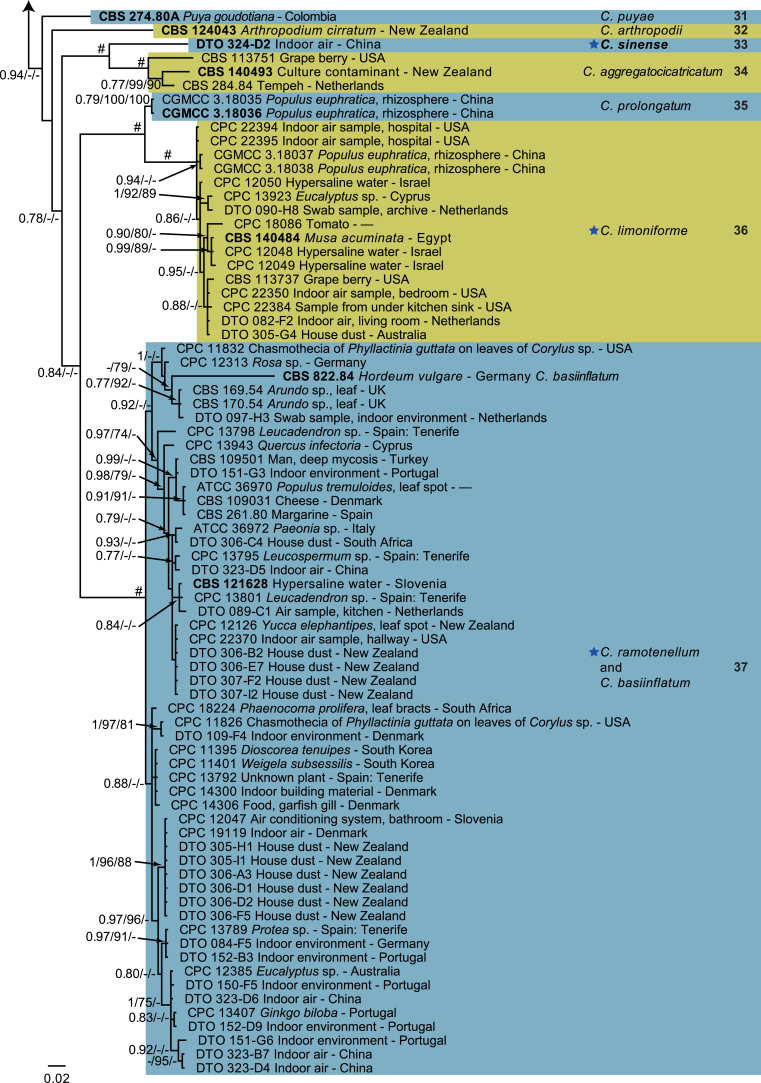
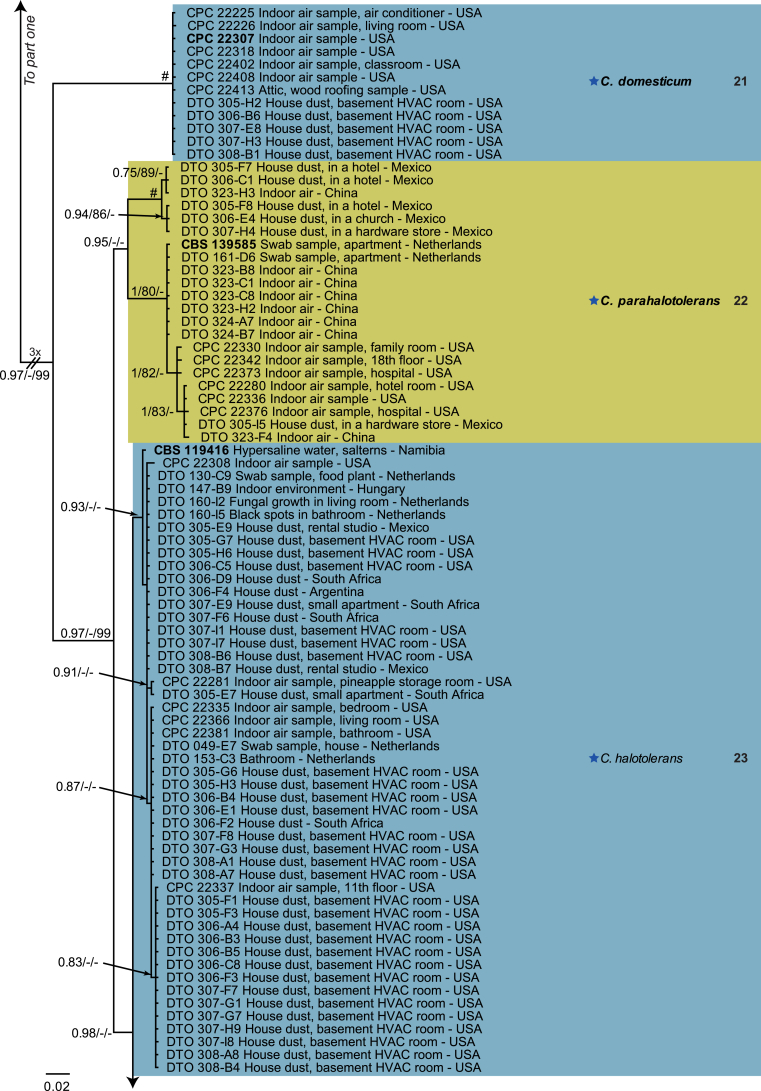
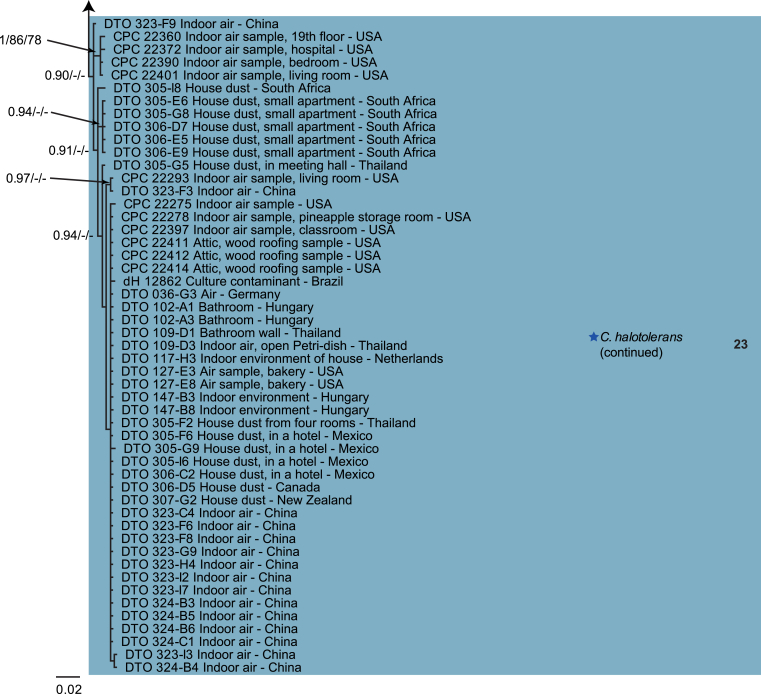
The monophyletic genus Cladosporium residing in the Cladosporiaceae (Dothideomycetes) is well circumscribed by having a unique coronate structure of its conidiogenous loci and conidial hila, consisting of a central convex dome surrounded by a raised periclinal rim (David, 1997, Braun et al., 2003). It has been intensively studied in the last two decades to separate it from cladosporium-like genera (Seifert et al., 2004, Heuchert et al., 2005, Crous et al., 2006, Crous et al., 2007b, Schubert et al., 2007a, Braun et al., 2008, Bezerra et al., 2017, Crous et al., 2017). Three major species complexes are recognised within the genus, mainly based on morphology, and used for practical purposes, viz. the C. herbarum, C. sphaerospermum and C. cladosporioides species complexes. Morphological features describing the three species complexes have been summarised in Bensch et al., 2012, Bensch et al., 2015 and Marin-Felix et al. (2017). Most of the Cladosporium species can be referred to one of the three species complexes based on their morphology. The genus previously encompassed more than 772 names (Dugan et al. 2004) of which only 170 were recognized as true Cladosporium species in a monographic treatment (Bensch et al. 2012). Due to continuous isolations from a range of substrates, collected on continents, this number has increased up to 218 species (Crous et al., 2014, Bensch et al., 2015, Braun et al., 2015, Razafinarivo et al., 2016, Marin-Felix et al., 2017), including several new species isolated from clinical samples in the United States (Sandoval-Denis et al. 2016) and from soil samples in China (Ma et al. 2017).
However, little is known about which Cladosporium species occur in indoor environments. Besides Aspergillus, Penicillium and Talaromyces (Trichocomaceae, Eurotiomycetes) Cladosporium is considered among the commonest genera found indoors (Flannigan 2001, Visagie et al. 2014), with some species being predominate under ambient conditions.
Cladosporium species are among the most abundant fungi in outdoor and indoor air (Fradkin et al., 1987, Flannigan, 2001, Horner et al., 2004). In fact, C. cladosporioides was reported to be the most predominant fungus in houses in Ontario and Atlanta (Fradkin et al., 1987, Horner et al., 2004) and the most abundant fungus in outdoor air (Fradkin et al. 1987). As the composition of indoor species reflects the composition of outdoor species one would expect to find C. cladosporioides as dominant indoors.
In the present study a multilocus DNA sequence typing approach, employing three loci [the internal transcribed spacers of the rDNA genes (ITS), and partial actin and translation elongation factor 1-alpha gene sequences], as well as morphological examinations and cultural charactersitics were used for the identification and delimitation of more than 500 isolates from indoor environments belonging to the genus Cladosporium.
Material and methods
Isolates
Isolates included in this study were obtained from the culture collection of the Westerdijk Fungal Biodiversity Institute (former CBS-KNAW Fungal Biodiversity Centre; CBS), Utrecht, the Netherlands, from the working collection of Pedro Crous (CPC) and from the working collection of the Applied and Industrial Mycology department (DTO), both housed at the Westerdijk Institute. Isolates were inoculated onto 2 % potato-dextrose agar (PDA), synthetic nutrient-poor agar (SNA), 2 % malt extract agar (MEA), oatmeal agar (OA) (Crous et al. 2009), as well as dichloran 18 % glycerol agar (DG18) and Malt extract + 20 % sucrose (for Cladosporium sloanii sp. nov.) (Samson et al. 2010), and incubated under continuous near-ultraviolet light at 25 °C to promote sporulation. All cultures in this study are maintained at the Westerdijk Institute (Table 1). Nomenclatural novelties and descriptions were deposited in MycoBank (www. mycobank.org; Crous et al. 2004).
Table 1.
Cladosporium isolates treated in the species phylogeny with their Genbank and culture collection accession numbers.
| Species | Species complex | Culture accession number(s)1,2 | Substrate | Country3 | Collector | GenBank accession numbers4 |
||
|---|---|---|---|---|---|---|---|---|
| ITS | tef1 | act | ||||||
| Cercospora beticola | outgroup | CBS 116456; CPC 11557 | Beta vulgaris | Italy | V. Rossi | AY840527 | AY840494 | AY840458 |
| Cladosporium acalyphae | cladosporioides | CBS 125982*; CPC 11625 | Acalypha australis | South Korea | H.D. Shin | HM147994 | HM148235 | HM148481 |
| C. aciculare | sphaerospermum | CBS 140488*; CPC 16547 | Syzygium corynanthum | Australia | P.W. Crous | KT600411 | KT600509 | KT600607 |
| C. aerium sp. nov. | herbarum | CBS 143356*; DTO 323-B4 | Indoor air | China | — | MF472897 | MF473324 | MF473747 |
| DTO 323-G6 | Indoor air | China | — | MF472898 | MF473325 | MF473748 | ||
| DTO 323-G7 | Indoor air | China | — | MF472899 | MF473326 | MF473749 | ||
| C. aggregatocicatricatum | herbarum | CBS 113751 | Grape berry | USA: WA | F.M. Dugan lab | KT600449 | KT600548 | KT600646 |
| CBS 140493*; CPC 14709; ICMP 170869 | Culture contaminant | New Zealand | C.F. Hill | KT600448 | KT600547 | KT600645 | ||
| CBS 284.84 | Tempeh | Netherlands | — | KT600450 | KT600549 | KT600647 | ||
| C. alboflavescens | cladosporioides | CBS 140690*; UTHSC DI-13-225; FMR 13338 | Animal, bronchoalveolar lavage fluid | USA: CA | — | LN834420 | LN834516 | LN834604 |
| C. allicinum | herbarum | CBS 110024 | Industrial water | Germany | — | EF679343 | EF679417 | EF679495 |
| CBS 115683; ATCC 66670; CPC 5101 | CCA-treated Douglas-fir pole | USA: NY | — | AY361959 | EF679418 | AY752193 | ||
| CBS 121624*; CPC 12211 | Hordeum vulgare | Belgium | J.Z. Groenewald | EF679350 | EF679425 | EF679502 | ||
| CBS 139578; DTO 109-I5 | Indoor environment | Denmark | B. Andersen | KP701921 | KP701798 | KP702044 | ||
| CBS 134.31; ATCC 11283; IMI 049632; NCPF 2564 | — | Germany | — | EF679335 | EF679406 | EF679485 | ||
| CBS 157.82 | Quercus robur, leaf spot | Belgium | — | EF679336 | EF679407 | EF679486 | ||
| CBS 159.54; ATCC 36948 | Man, skin of hand | Netherlands | — | EF679337 | EF679408 | EF679487 | ||
| CBS 161.55 | Man, sputum | Netherlands | — | EF679338 | EF679409 | EF679488 | ||
| CBS 177.71; JCM 11500 | Thuja tincture | Netherlands | — | EF679339 | EF679410 | EF679489 | ||
| CBS 188.54; ATCC 11290; IMI 049638; STE-U 3686 | — | — | — | AY251077 | EF679411 | EF679490 | ||
| CBS 366.80 | Man, skin of hand | Netherlands | St. Barbara Ziekenhuis Geleen | EF679340 | EF679412 | EF679491 | ||
| CBS 399.80 | Man, skin of foot | Netherlands | St. Barbara Ziekenhuis Geleen | AJ244227 | EF679413 | EF679492 | ||
| CBS 521.68 | Air | Netherlands | — | EF679341 | EF679414 | EF679493 | ||
| CBS 572.78; VKM F-405 | Polyporus radiatus | Russia | — | DQ289799 | EF679415 | DQ289866 | ||
| CBS 813.71 | Polygonatum odoratum, leaf | Czech Republic | — | EF679342 | EF679416 | EF679494 | ||
| CPC 11386 | Tilia cordata, leaves | Germany | K. Schubert | EF679344 | EF679419 | EF679496 | ||
| CPC 11840 | Ourisia macrophylla | EF679345 | EF679420 | EF679497 | ||||
| CPC 12042; EXF-389 | Hypersaline water, salterns (reserve pond) | Slovenia | P. Zalar | EF679346 | EF679421 | EF679498 | ||
| CPC 12045; EXF-594 | Hypersaline water, salterns (crystallisation pond) | Spain | New Zealand | A. Blouin | EF679422 | EF679499 | ||
| CPC 12046; EXF-680 | Air conditioning system | Slovenia | M. Butala | EF679348 | EF679423 | EF679500 | ||
| CPC 12139 | Hordeum vulgare | Netherlands | P.W. Crous | EF679349 | EF679424 | EF679501 | ||
| CPC 12212 | Hordeum vulgare | Belgium | J.Z. Groenewald | EF679351 | EF679426 | EF679503 | ||
| CPC 12921 | Eucalyptus stellulata, leaves | Australia | B.A. Summerell | EF679352 | EF679427 | EF679504 | ||
| CPC 22268; EMSL 1726 | Indoor air sample | USA: MN | Ž. Jurjević | MF472900 | MF473327 | MF473750 | ||
| CPC 22312; EMSL 1808 | Indoor air sample | USA: NJ | Ž. Jurjević | MF472901 | MF473328 | MF473751 | ||
| CPC 22313; EMSL 1809 | Indoor air sample | USA: NJ | Ž. Jurjević | MF472902 | MF473329 | MF473752 | ||
| CPC 22343; EMSL 1856 | Indoor air sample, bedroom | USA: NY | Ž. Jurjević | MF472903 | MF473330 | MF473753 | ||
| CPC 22349; EMSL 1862 | Indoor air sample, bedroom | USA: CA | Ž. Jurjević | MF472904 | MF473331 | MF473754 | ||
| CPC 22358; EMSL 1871 | Indoor air sample | UK: England | Ž. Jurjević | MF472905 | MF473332 | MF473755 | ||
| CPC 22377; EMSL 1890 | Indoor air sample, bedroom | USA: NY | Ž. Jurjević | MF472906 | MF473333 | MF473756 | ||
| DTO 005-E8 | Indoor environment | Germany | G. Fischer | MF472907 | MF473334 | MF473757 | ||
| DTO 084-F3 | Indoor environment | Germany | LGA | KP701883 | KP701760 | KP702006 | ||
| DTO 086-D5 | Swab sample, archive | Netherlands | M. Meijer | KP701888 | KP701765 | KP702011 | ||
| DTO 089-B9 | Air sample, kitchen | Netherlands | M. Meijer | KP701891 | KP701768 | KP702014 | ||
| DTO 089-G4 | Air sample, bedroom | Netherlands | J. Houbraken | KP701894 | KP701771 | KP702017 | ||
| DTO 089-G6 | Air sample, bedroom | Netherlands | J. Houbraken | KP701895 | KP701772 | KP702018 | ||
| DTO 089-H3 | Air sample, bathroom | Netherlands | J. Houbraken | KP701896 | KP701773 | KP702019 | ||
| DTO 090-D3 | Swab sample, archive | Netherlands | M. Meijer | KP701900 | KP701777 | KP702023 | ||
| DTO 090-H4 | Swab sample, archive | Netherlands | M. Meijer | MF472908 | MF473335 | MF473758 | ||
| DTO 101-A1 | Indoor environment, wet wall | Netherlands | J. Houbraken | KP701903 | KP701780 | KP702026 | ||
| DTO 101-I8 | Floor under curtain | Hungary | — | MF472909 | MF473336 | MF473759 | ||
| DTO 106-C2 | Indoor air, crocodile area of zoo | Netherlands | B. Dictus | KP701906 | KP701783 | KP702029 | ||
| DTO 108-F9 | Indoor environment | France | J. Dijksterhuis | MF472910 | MF473337 | MF473760 | ||
| DTO 109-E5; BA 1905 | Indoor environment | Denmark | B. Andersen | MF472911 | MF473338 | MF473761 | ||
| DTO 109-E6; BA 1906 | Indoor environment | Denmark | B. Andersen | KP701912 | KP701789 | KP702035 | ||
| DTO 109-F3; BA 1918 | Indoor environment | Denmark | B. Andersen | KP701916 | KP701793 | KP702039 | ||
| DTO 109-F5; BA 1920 | Indoor environment | Denmark | B. Andersen | KP701918 | KP701795 | KP702041 | ||
| DTO 109-I3; BA 1897 | Indoor environment | Denmark | B. Andersen | MF472912 | MF473339 | MF473762 | ||
| DTO 110-B7 | Wall of basement | Denmark | B. Andersen | KP701923 | KP701800 | KP702046 | ||
| DTO 111-A5 | Air sample, bedroom | Denmark | U. Thrane | KP701924 | KP701801 | KP702047 | ||
| DTO 127-E4; AR377 | Air sample, bakery | USA: GA | — | MF472913 | MF473340 | MF473763 | ||
| DTO 147-I6 | Indoor environment | Hungary | — | MF472914 | MF473341 | MF473764 | ||
| DTO 323-C3 | Indoor air | China | — | MF472915 | MF473342 | MF473765 | ||
| DTO 323-E1 | Indoor air | China | — | MF472916 | MF473343 | MF473766 | ||
| DTO 323-G5 | Indoor air | China | — | MF472917 | MF473344 | MF473767 | ||
| C. allii | herbarum | CBS 101.81; ATCC 200948; PD 80/165 | Allium porrum, velvet spots | Netherlands | — | JN906977 | JN906983 | JN906996 |
| C. angulosum | cladosporioides | CBS 140692*; UTHSC DI-13-235; FMR 13348 | Man, bronchoalveolar lavage fluid | USA: TX | D.A. Sutton | LN834425 | LN834521 | LN834609 |
| CPC 11526 | Acacia mangium | Thailand | W. Himaman | HM148127 | HM148371 | HM148616 | ||
| CPC 14566 | Corymbia foelscheana | Australia | B.A. Summerell | HM148147 | HM148391 | HM148636 | ||
| CPC 22271; EMSL 1741 | Indoor air sample | USA: SC | Ž. Jurjević | MF472918 | MF473345 | MF473768 | ||
| C. angustiherbarum | herbarum | CBS 140479*; CPC 17814 | Pinus ponderosa | USA: UT | W. Quaedvlieg | KT600378 | KT600475 | KT600574 |
| C. angustisporum | cladosporioides | CBS 125983*; CPC 12437 | Alloxylon wickhamii | Australia | B.A. Summerell | HM147995 | HM148236 | HM148482 |
| CPC 22345; EMSL 1858 | Outside air sample | USA: AL | Ž. Jurjević | MF472919 | MF473346 | MF473769 | ||
| CPC 22371; EMSL 1884 | Indoor air sample, office | USA: FL | Ž. Jurjević | MF472920 | MF473347 | MF473770 | ||
| DTO 127-E6; AR387 | Air sample, bakery | USA: WI | — | KP701935 | KP701812 | KP702057 | ||
| C. angustiterminale | cladosporioides | CBS 140480*; CPC 15564 | Banksia grandis | Australia | A.R. Wood | KT600379 | KT600476 | KT600575 |
| C. antarcticum | herbarum | CBS 690.92* | Caloplaca regalis | Antarctica | C. Moller | EF679334 | EF679405 | EF679484 |
| C. anthropophilum | cladosporioides | CBS 117483; CPC 11684 | — | USA | M. Blackwell | HM148007 | HM148248 | HM148494 |
| CBS 122130; ATCC 38012; IFO 6539; JCM 10684; NBRC 6539 | Bamboo slats | Japan | — | HM148008 | HM148249 | HM148495 | ||
| CBS 140685*; FMR 13382; UTHSC DI-13-269 | Man, bronchoalveolar lavage fluid | USA: MN | D.A. Sutton | LN834437 | LN834533 | LN834621 | ||
| CBS 132.29 | — | — | — | HM148010 | HM148251 | HM148497 | ||
| CBS 674.82; ATCC 200936; ATCC 38026; CBS 320.87; IMI 126640 | Gossypium sp., seed | Israel | — | HM148014 | HM148255 | HM148501 | ||
| CPC 10142 | Chenopodium ficifolium | South Korea | H.D. Shin | HM148015 | HM148256 | HM148502 | ||
| CPC 11119 | Ricinus communis | South Korea | H.D. Shin | HM148016 | HM148257 | HM148503 | ||
| CPC 11122 | Phytolacca americana | South Korea | H.D. Shin | HM148019 | HM148260 | HM148506 | ||
| CPC 11123 | Vigna unguiculata (= V. sinensis) | South Korea | H.D. Shin | HM148020 | HM148261 | HM148507 | ||
| CPC 11131 | Dalbergia sp. | India | W. Gams | HM148021 | HM148262 | HM148508 | ||
| CPC 11406 | Plectranthus sp. | South Korea | H.D. Shin | HM148026 | HM148267 | HM148513 | ||
| CPC 12852 | Pruned wood | USA: LA | K. Seifert | HM148032 | HM148273 | HM148519 | ||
| CPC 13235 | Eucalyptus sp. | Australia | P.W. Crous | HM148033 | HM148274 | HM148520 | ||
| CPC 13734 | Areca sp. | Thailand | I. Hidayat | HM148036 | HM148277 | HM148523 | ||
| CPC 14009; MRC 10150 | Triticum aestivum | South Africa | — | HM148037 | HM148278 | HM148524 | ||
| CPC 14356; BA 1676 | Food, coffee leaf | Uganda | J.L. Sørensen | HM148049 | HM148290 | HM148536 | ||
| CPC 14705 | Fraxinus chinensis subsp. rhynchophylla | South Korea | H.D. Shin | HM148050 | HM148291 | HM148537 | ||
| CPC 15038 | Eucalyptus sp., endophyte spots | Indonesia | M.J. Wingfield | HM148051 | HM148292 | HM148538 | ||
| CPC 22272; EMSL 1722 | Indoor air sample, ship | USA: CA | Ž. Jurjević | MF574171 | MF574173 | MF574175 | ||
| CPC 22315; EMSL 1818 | Indoor air sample, living room | USA: GA | Ž. Jurjević | MF472921 | MF473348 | MF473771 | ||
| CPC 22393; EMSL 1908 | Indoor air sample, hospital | USA: AZ | Ž. Jurjević | MF472922 | MF473349 | MF473772 | ||
| DTO 127-E9; AR409 | Air sample, bakery | USA: GA | — | MF472923 | MF473350 | MF473773 | ||
| DTO 317-I7 | Indoor air | China | — | MF472924 | MF473351 | MF473774 | ||
| DTO 318-E3 | Indoor air | China | — | MF472925 | MF473352 | MF473775 | ||
| DTO 323-C2 | Indoor air | China | — | MF472926 | MF473353 | MF473776 | ||
| DTO 323-C6 | Indoor air | China | — | MF472927 | MF473354 | MF473777 | ||
| DTO 323-C7 | Indoor air | China | — | MF472928 | MF473355 | MF473778 | ||
| DTO 323-D2 | Indoor air | China | — | MF472929 | MF473356 | MF473779 | ||
| DTO 323-D8 | Indoor air | China | — | MF472930 | MF473357 | MF473780 | ||
| DTO 323-D9 | Indoor air | China | — | MF472931 | MF473358 | MF473781 | ||
| DTO 324-C4 | Indoor air | China | — | MF472932 | MF473359 | MF473782 | ||
| DTO 324-D3 | Indoor air | China | — | MF472933 | MF473360 | MF473783 | ||
| UTHSC DI-13-207; FMR 13320 | Man, cerebrospinal fluid | USA: TX | D.A. Sutton | LN834413 | LN834509 | LN834597 | ||
| UTHSC DI-13-226; FMR 13339 | Man, bronchoalveolar lavage fluid | USA: TX | D.A. Sutton | LN834421 | LN834517 | LN834605 | ||
| C. aphidis | sphaerospermum | CBS 132182**; CPC 13204 | Unidentified aphid | Germany | N. Ale-Agha | JN906978 | JN906984 | JN906997 |
| C. arthropodii | herbarum | CBS 124043**; CPC 16160 | Arthropodium cirratum | New Zealand | C.F. Hill | JN906979 | JN906985 | JN906998 |
| C. asperulatum | cladosporioides | CBS 126339; CPC 11158 | Eucalyptus leaf litter | India | W. Gams | HM147997 | HM148238 | HM148484 |
| CBS 126340*; CPC 14040 | Protea susannae | Portugal | — | HM147998 | HM148239 | HM148485 | ||
| CPC 22364; EMSL 1877 | Indoor air sample, bathroom | USA: CA | Ž. Jurjević | MF472934 | MF473361 | MF473784 | ||
| C. australiense | cladosporioides | CBS 125984*; CPC 13226 | Eucalyptus moluccana | Australia | B.A. Summerell | HM147999 | HM148240 | HM148486 |
| C. austroafricanum | cladosporioides | CBS 140481*; CPC 16763 | Leaf litter | South Africa | M. Gryzenhout | KT600381 | KT600478 | KT600577 |
| C. austrohemisphaericum | sphaerospermum | CBS 140482*; CPC 12068 | Lagunaria patersonia, black mould on fruit surface | New Zealand | C.F. Hill | KT600382 | KT600479 | KT600578 |
| CPC 16250 | Cussonia thyrsiflora | South Africa | P.W. Crous | KT600383 | KT600480 | — | ||
| CPC 17029 | Musa sp. | Australia | P.W. Crous | KT600384 | KT600481 | KT600579 | ||
| DTO 305-E8; TA05NZ-351A | House dust | New Zealand | T. Atkinson | MF472935 | MF473362 | MF473785 | ||
| C. basiinflatum | herbarum | CBS 822.84* | Hordeum vulgare | Germany | — | HM148000 | HM148241 | HM148487 |
| C. chalastosporoides | cladosporioides | CBS 125985*; CPC 13864 | Fruiting bodies of Teratosphaeria proteae-arboreae on leaves of Protea nitida | South Africa | P.W. Crous | HM148001 | HM148242 | HM148488 |
| C. chubutense | cladosporioides | CBS 124457*; CPC 13979; CIEFAP 321 | Pinus ponderosa | Argentina | A. Greslebin | FJ936158 | FJ936161 | FJ936165 |
| C. cladosporioides | cladosporioides | CBS 101367; IMI 379759 | Soil | Brazil | — | HM148002 | HM148243 | HM148489 |
| CBS 112388*; DTO 039-G6 | Air, indoor environment | Germany | Ch. Trautmann | HM148003 | HM148244 | HM148490 | ||
| CBS 113738 | Grape bud | USA: WA | F.M. Dugan lab | HM148004 | HM148245 | HM148491 | ||
| CBS 113739 | Culm node of crested wheat grass | USA: WA | F.M. Dugan lab | HM148005 | HM148246 | HM148492 | ||
| CBS 113740 | Grape berry | USA: WA | F.M. Dugan lab | HM148006 | HM148247 | HM148493 | ||
| CBS 126341; CPC 12763 | Spinacia oleracea, seed | USA: WA | L. du Toit | HM148009 | HM148250 | HM148496 | ||
| CBS 143.35; MUCL 10090 | Pisum sativum | South Africa | B.J. Dippenaar | HM148011 | HM148252 | HM148498 | ||
| CBS 144.35; ATCC 11284; IFO 6371; IMI 049627 | Pisum sativum | USA: CA | — | HM148012 | HM148253 | HM148499 | ||
| CBS 145.35; MUCL 926 | Pisum sativum | Germany | — | HM148013 | HM148254 | HM148500 | ||
| CPC 11120 | Viola mandshurica | South Korea | H.D. Shin | HM148017 | HM148258 | HM148504 | ||
| CPC 11121 | Celosia cristata | South Korea | H.D. Shin | HM148018 | HM148259 | HM148505 | ||
| CPC 11161 | Eucalyptus sp. | India | W. Gams | HM148022 | HM148263 | HM148509 | ||
| CPC 11393 | Valeriana officinalis | South Korea | H.D. Shin | HM148023 | HM148264 | HM148510 | ||
| CPC 11398 | Phragmidium griseum on Rubus crataegifolius | South Korea | H.D. Shin | HM148024 | HM148265 | HM148511 | ||
| CPC 11404 | Rubus coreanus | South Korea | H.D. Shin | HM148025 | HM148266 | HM148512 | ||
| CPC 12187 | Stellaria aquatica, leaves | South Korea | H.D. Shin | HM148027 | HM148268 | HM148514 | ||
| CPC 12214 | Morus rubra, leaves | Germany | N. Ale-Agha | HM148028 | HM148269 | HM148515 | ||
| CPC 12760 | Spinacia oleracea, seed | USA: WA | L. du Toit | HM148029 | HM148270 | HM148516 | ||
| CPC 12762 | Spinacia oleracea, seed | USA: WA | L. du Toit | HM148030 | HM148271 | HM148517 | ||
| CPC 12764 | Spinacia oleracea, seed | USA: WA | L. du Toit | HM148031 | HM148272 | HM148518 | ||
| CPC 13667 | Eucalyptus robertsonii subsp. hemisphaerica | Australia | B.A. Summerell | HM148034 | HM148275 | HM148521 | ||
| CPC 13669 | Eucalyptus robertsonii subsp. hemisphaerica | Australia | B.A. Summerell | HM148035 | HM148276 | HM148522 | ||
| CPC 14015; MRC 10260 | Triticum aestivum | South Africa | — | HM148038 | HM148279 | HM148525 | ||
| CPC 14017; MRC 10809 | Triticum aestivum | South Africa | — | HM148039 | HM148280 | HM148526 | ||
| CPC 14018; MRC 10810 | Triticum aestivum | South Africa | — | HM148040 | HM148281 | HM148527 | ||
| CPC 14019; MRC 10813 | Triticum aestivum | South Africa | — | HM148041 | HM148282 | HM148528 | ||
| CPC 14021; MRC 10827 | Triticum aestivum | South Africa | — | HM148042 | HM148283 | HM148529 | ||
| CPC 14024; MRC 11280 | Asimina sp. | South Africa | — | HM148043 | HM148284 | HM148530 | ||
| CPC 14244 | Magnolia sp. | USA: LA | P.W. Crous | HM148044 | HM148285 | HM148531 | ||
| CPC 14271 | Twigs of an unidentified tree | France | P.W. Crous | HM148045 | HM148286 | HM148532 | ||
| CPC 14292; BA 1691 | Soil, pea field | Denmark | B. Andersen | HM148046 | HM148287 | HM148533 | ||
| CPC 14293; BA 1692 | Cellulose powder, paint manufacturer | Denmark | B. Andersen | HM148047 | HM148288 | HM148534 | ||
| CPC 14355; BA 1676 | Food, mouldy pea | USA: WY | J.L. Sørensen | HM148048 | HM148289 | HM148535 | ||
| CPC 15167; HJS 1069 | Living mite inhabiting a strawberry leaf | Slovenia | — | HM148052 | HM148293 | HM148539 | ||
| CPC 18230 | Phaenocoma prolifera, leaf bracts | South Africa | K.L. Crous & P.W. Crous | JF499834 | JF499872 | JF499878 | ||
| CPC 22264; EMSL 1722 | Indoor air sample | USA: GA | Ž. Jurjević | MF472936 | MF473363 | MF473786 | ||
| CPC 22265; EMSL 1723 | Indoor air sample | USA: MN | Ž. Jurjević | MF472937 | MF473364 | MF473787 | ||
| CPC 22347; EMSL 1860 | Indoor air sample, bedroom | USA: MI | Ž. Jurjević | MF472938 | MF473365 | MF473788 | ||
| CPC 22348; EMSL 1861 | Indoor air sample, kitchen | USA: FL | Ž. Jurjević | MF472939 | MF473366 | MF473789 | ||
| CPC 22365; EMSL 1878 | Indoor air sample, bedroom | USA: VT | Ž. Jurjević | MF472940 | MF473367 | MF473790 | ||
| CPC 22367; EMSL 1880 | Indoor air sample, living room | USA: VA | Ž. Jurjević | MF472941 | MF473368 | MF473791 | ||
| CPC 22380; EMSL 1893 | Indoor air sample, bedroom | USA: AZ | Ž. Jurjević | MF472942 | MF473369 | MF473792 | ||
| DTO 082-F1 | Indoor air sample, living room | Netherlands | B. Favié | KP701879 | KP701756 | KP702002 | ||
| DTO 090-C6 | Swab sample, archive | Netherlands | M. Meijer | KP701898 | KP701775 | KP702021 | ||
| DTO 101-G2 | Indoor environment, table | Hungary | — | MF472943 | MF473370 | MF473793 | ||
| DTO 101-H7 | Floor under curtain | Hungary | — | MF472944 | MF473371 | MF473794 | ||
| DTO 102-A4 | Bathroom | Hungary | van Mil | KP701905 | KP701782 | KP702028 | ||
| DTO 109-I4; BA 1898 | Indoor environment | Denmark | B. Andersen | KP701920 | KP701797 | KP702043 | ||
| DTO 109-I6; BA 1900 | Indoor environment | Denmark | B. Andersen | KP701922 | KP701799 | KP702045 | ||
| DTO 127-D8; AR362 | Air sample, bakery | Netherlands | — | KP701933 | KP701810 | KP702055 | ||
| DTO 147-A9 | Indoor environment | Hungary | — | KP701941 | KP701818 | KP702063 | ||
| C. colocasiae | cladosporioides | CBS 115191; CPC 4323; Lynfield 436 | Colocasia esculenta (=C. antiquorum) | Fiji | C.F. Hill | AY251075 | HM148308 | HM148553 |
| CBS 119542; CPC 12726; JCM 13264 | Colocasia esculenta (=C. antiquorum) | Japan | — | HM148066 | HM148309 | HM148554 | ||
| CBS 386.64*; ATCC 200944; MUCL 10084 | Colocasia esculenta (=C. antiquorum) | Taiwan | K. Sawada | HM148067 | HM148310 | HM148555 | ||
| CPC 5124 | Apium graveolens | New Zealand | C.F. Hill | AY251076 | HM148311 | HM148556 | ||
| C. colombiae | cladosporioides | CBS 274.80B* | Cortaderia sp. | Colombia | W. Gams | FJ936159 | FJ936163 | FJ936166 |
| C. coloradense sp. nov. | sphaerospermum | CBS 143357*; CPC 22238; EMSL 1685 | Air sample, bedroom | USA: CO | Ž. Jurjević | MF472945 | MF473372 | MF473795 |
| C. crousii | cladosporioides | CBS 140686*; UTHSC DI-13-247; FMR 13360 | Man, bronchoalveolar lavage fluid | USA: SC | D.A. Sutton | LN834431 | LN834527 | LN834615 |
| C. cucumerinum | cladosporioides | CBS 158.51; ATCC 11279; IFO 6370; IMI 049628; VKM F-817 | Cucumis sativus | Netherlands | — | HM148071 | HM148315 | HM148560 |
| CBS 171.52*; MUCL 10092 | Cucumis sativus | Netherlands | — | HM148072 | HM148316 | HM148561 | ||
| CBS 172.54 | Cucumis sativus | Netherlands | G.W. van der Helm | HM148073 | HM148317 | HM148562 | ||
| C. cycadicola | sphaerospermum | CBS 137970*; CPC 17251 | Cycas media, leaves | Australia | P.W. Crous & R.G. Shivas | KJ869122 | KJ869236 | KJ869227 |
| C. delicatulum | cladosporioides | CBS 126342; CPC 14287; BA 1681 | Indoor air | Denmark | B. Andersen | HM148079 | HM148323 | HM148568 |
| CBS 126343; CPC 14299; BA 1698 | Building material | Denmark | B. Andersen | HM148080 | HM148324 | HM148569 | ||
| CBS 126344*; CPC 11389 | Tilia cordata, leaves | Germany | K. Schubert | HM148081 | HM148325 | HM148570 | ||
| CBS 139574; DTO 082-F3 | Indoor air, living room | Netherlands | B. Favié | KP701880 | KP701757 | KP702003 | ||
| CPC 14285; BA 1679 | Indoor air | Denmark | B. Andersen | HM148083 | HM148327 | HM148572 | ||
| CPC 14286; BA 1680 | Indoor air | Denmark | B. Andersen | HM148084 | HM148328 | HM148573 | ||
| CPC 14289; BA 1683 | Door frame | Denmark | B. Andersen | HM148085 | HM148329 | HM148574 | ||
| CPC 14360; BA 1718 | Indoor air | Denmark | B. Andersen | HM148087 | HM148331 | HM148576 | ||
| CPC 14363; BA 1724 | Indoor air | Denmark | B. Andersen | HM148088 | HM148332 | HM148577 | ||
| CPC 14372; BA 1740 | Dust, school | Denmark | B. Andersen | HM148089 | HM148333 | HM148578 | ||
| DTO 090-F4 | Swab sample, archive | Netherlands | M. Meijer | MF472946 | MF473373 | MF473796 | ||
| DTO 134-D3; DR22 | Indoor environment | Algeria | L. Belhoucine | KP701939 | KP701816 | KP702061 | ||
| DTO 134-D4 | Indoor environment, apartment building | Algeria | L. Belhoucine | MF472947 | MF473374 | MF473797 | ||
| DTO 134-D5; O200 | Indoor environment, apartment building | Algeria | L. Belhoucine | MF472948 | MF473375 | MF473798 | ||
| DTO 134-D6; BT27 | Indoor environment | Algeria | L. Belhoucine | MF472949 | MF473376 | MF473799 | ||
| DTO 134-D7; BT91 | Indoor environment | Algeria | L. Belhoucine | MF472950 | MF473377 | MF473800 | ||
| DTO 134-D8; BT92 | Indoor environment | Algeria | L. Belhoucine | MF472951 | MF473378 | MF473801 | ||
| DTO 145-C4 | Indoor environment | Germany | — | KP701940 | KP701817 | KP702062 | ||
| DTO 167-H5 | Indoor air, poultry houses | Poland | K. Plewa | KP701964 | KP701841 | KP702086 | ||
| DTO 168-F8 | Indoor air, poultry houses | Poland | K. Plewa | MF472952 | MF473379 | MF473802 | ||
| DTO 305-H7; TA05NZ-346 | House dust | New Zealand | T. Atkinson | MF472953 | MF473380 | MF473803 | ||
| DTO 305-I9; TA05NZ-340 | House dust | New Zealand | T. Atkinson | MF472954 | MF473381 | MF473804 | ||
| C. domesticum sp. nov. | sphaerospermum | CBS 143358*; CPC 22307; EMSL 1803 | Indoor air sample | USA: NJ | Ž. Jurjević | MF472955 | MF473382 | MF473805 |
| CPC 22225; EMSL 1658 | Indoor air sample, air conditioner | USA: PA | Ž. Jurjević | MF472956 | MF473383 | MF473806 | ||
| CPC 22226; EMSL 1659 | Indoor air sample, living room | USA: PA | Ž. Jurjević | MF472957 | MF473384 | MF473807 | ||
| CPC 22318; EMSL 1821 | Indoor air sample | USA: FL | Ž. Jurjević | MF472958 | MF473385 | MF473808 | ||
| CPC 22402; EMSL 1930 | Indoor air sample, classroom | USA: TX | Ž. Jurjević | MF472959 | MF473386 | MF473809 | ||
| CPC 22408; EMSL 1936 | Indoor air sample | USA: NJ | Ž. Jurjević | MF472960 | MF473387 | MF473810 | ||
| CPC 22413; EMSL 1962 | Attic, wood roofing sample | USA: PA | Ž. Jurjević | MF472961 | MF473388 | MF473811 | ||
| DTO 305-H2; AA03US-480 | House dust, basement HVAC room | USA: CA | A. Amend | MF472962 | MF473389 | MF473812 | ||
| DTO 306-B6; AA03US-525 | House dust, basement HVAC room | USA: CA | A. Amend | MF472963 | MF473390 | MF473813 | ||
| DTO 307-E8; AA03US-368 | House dust, basement HVAC room | USA: CA | A. Amend | MF472964 | MF473391 | MF473814 | ||
| DTO 307-H3; AA03US-402 | House dust, basement HVAC room | USA: CA | A. Amend | MF472965 | MF473392 | MF473815 | ||
| DTO 308-B1; AA03US-387 | House dust, basement HVAC room | USA: CA | A. Amend | MF472966 | MF473393 | MF473816 | ||
| C. dominicanum | sphaerospermum | CBS 119415*; EXF-732; dH 16386 | Hypersaline water, salt lake | Dominican Republic | N. Gunde-Cimerman | DQ780353 | JN906986 | KJ596641 |
| CPC 11683 | Citrus sp., fruit | Iran | — | DQ780357 | — | EF101369 | ||
| CPC 15932 | Dracaena fragrans | Philippines | C.J.R. Cumagun | KT600390 | KT600487 | KT600585 | ||
| CPC 20109 | Unknown vine | Taiwan | P.W. Crous | KT600391 | KT600488 | KT600586 | ||
| CPC 22240; EMSL 1687 | Outside air sample | USA: CO | Ž. Jurjević | MF472967 | MF473394 | MF473817 | ||
| CPC 22241; EMSL 1688 | Outside air sample | USA: CO | Ž. Jurjević | MF472968 | MF473395 | MF473818 | ||
| CPC 22244; EMSL 1697 | Air sample, hospital | Aruba | Ž. Jurjević | MF472969 | MF473396 | MF473819 | ||
| CPC 22319; EMSL 1822 | Indoor air sample | Bermuda | Ž. Jurjević | MF472970 | MF473397 | MF473820 | ||
| EXF-696 | Hypersaline water, saltern | Dominican Republic | N. Gunde-Cimerman | EF101367 | — | EF101367 | ||
| EXF-718 | Hypersaline water, salt lake | Dominican Republic | N. Gunde-Cimerman | DQ780356 | KJ596581 | EF101370 | ||
| EXF-720 | Hypersaline water, saltern | Dominican Republic | N. Gunde-Cimerman | DQ780355 | KJ596579 | KJ596643 | ||
| EXF-727 | Hypersaline water, saltern | Dominican Republic | N. Gunde-Cimerman | DQ780354 | KJ596580 | — | ||
| C. echinulatum | herbarum | CBS 123191; CPC 15386; reference | Dianthus barbatus | New Zealand | C.F. Hill | JN906980 | JN906987 | JN906999 |
| C. europaeum sp. nov. | cladosporioides | CBS 116744; dH 14053 | Acer pseudoplatanus, leaves | Germany | L. Pehl | HM148053 | HM148294 | HM148540 |
| CBS 134914*; CPC 14296; BA1695 | Indoor building material, school | Denmark | B. Andersen | HM148056 | HM148298 | HM148543 | ||
| CBS 125.80 | Cirsium vulgare, seadcoat | Netherlands | — | DQ780941 | HM148295 | EF101351 | ||
| CPC 13220 | Lichens on leaves of Acer platanoides | Germany | B. Heuchert | HM148054 | HM148296 | HM148541 | ||
| CPC 14238 | Sambucus nigra, fruit | Netherlands | P.W. Crous | HM148055 | HM148297 | HM148542 | ||
| DTO 056-H7 | Swab sample, house | Netherlands | M. Meijer | KP701871 | KP701748 | KP701994 | ||
| DTO 072-E4 | Indoor air, archive | Netherlands | M. Meijer | KP701875 | KP701752 | KP701998 | ||
| DTO 086-B3 | Swab sample, archive | Netherlands | M. Meijer | KP701886 | KP701763 | KP702009 | ||
| DTO 109-E7; BA 1907 | Indoor environment | Denmark | B. Andersen | KP701913 | KP701790 | KP702036 | ||
| DTO 151-H5 | Indoor environment | Portugal | — | MF472971 | MF473398 | MF473821 | ||
| C. exasperatum | cladosporioides | CBS 125986*; CPC 14638 | Eucalyptus tintinnans | Australia | B.A. Summerell | HM148090 | HM148334 | HM148579 |
| C. exile | cladosporioides | CBS 125987*; CPC 11828 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | USA: WA | D. Glawe | HM148091 | HM148335 | HM148580 |
| C. flabelliforme | cladosporioides | CBS 126345*; CPC 14523 | Melaleuca cajuputi | Australia | B.A. Summerell | HM148092 | HM148336 | HM148581 |
| C. flavovirens | cladosporioides | CBS 140462*; FMR 13386; UTHSC DI-13-273 | Man, toenail | USA: FL | D.A. Sutton | LN834440 | LN834536 | LN834624 |
| C. floccosum | herbarum | CBS 140463*; FMR 13325; UTHSC DI-13-212 | Man, ethmoid sinus | USA: MN | D.A. Sutton | LN834416 | LN834512 | LN834600 |
| CPC 15522 | Allium sativum | Ukraine | A. Akulov | MF472972 | MF473399 | MF473822 | ||
| CPC 17802 | Pine needles | Mexico | M. de Jesús Yáñez-Morales | MF472973 | MF473400 | MF473823 | ||
| CPC 22260; EMSL 1715 | Indoor air sample | USA: MN | Ž. Jurjević | MF472974 | MF473401 | MF473824 | ||
| CPC 22309; EMSL 1805 | Indoor air sample | USA: TN | Ž. Jurjević | MF472975 | MF473402 | MF473825 | ||
| CPC 22354; EMSL 1867 | Indoor air sample, living room | USA: CO | Ž. Jurjević | MF472976 | MF473403 | MF473826 | ||
| CPC 22399; EMSL 1927 | Indoor air sample, bedroom | USA: MO | Ž. Jurjević | MF472977 | MF473404 | MF473827 | ||
| CPC 22968; EMSL 2033 | Indoor air sample, basement | USA: UT | Ž. Jurjević | MF472978 | MF473405 | MF473828 | ||
| DTO 323-H6 | Indoor air | China | — | MF472979 | MF473406 | MF473829 | ||
| C. funiculosum | cladosporioides | CBS 122128; ATCC 16160; IFO 6536; JCM 10682; NBRC 6536 | Ficus carica | Japan | — | HM148093 | HM148337 | HM148582 |
| CBS 122129*; ATCC 38010; IFO 6537; JCM 10683; NBRC 6537 | Vigna umbellata | Japan | — | HM148094 | HM148338 | HM148583 | ||
| CPC 22247; EMSL 1705 | Air sample, hospital | USA: AL | Ž. Jurjević | MF472980 | MF473407 | MF473830 | ||
| CPC 22282; EMSL 1756 | Indoor air sample | USA: NJ | Ž. Jurjević | MF472981 | MF473408 | — | ||
| CPC 22298; EMSL 1782 | Indoor air sample, office | USA: MA | Ž. Jurjević | MF472982 | MF473409 | MF473831 | ||
| CPC 22391; EMSL 1906 | Indoor air sample, bedroom | USA: NJ | Ž. Jurjević | MF472983 | MF473410 | MF473832 | ||
| DTO 127-E7; AR405 | Air sample, bakery | USA | — | MF472984 | MF473411 | MF473833 | ||
| C. fusiforme | sphaerospermum | CBS 119414*; EXF-449 | Hypersaline water, saltern | Slovenia | L. Butinar | DQ780388 | JN906988 | KJ596640 |
| CBS 452.71 | Chicken food | Canada | — | DQ780390 | MF473412 | EF101371 | ||
| EXF-397 | Hypersaline water, saltern | Slovenia | — | DQ780389 | KJ596595 | EF101373 | ||
| C. gamsianum | cladosporioides | CBS 125989*; CPC 11807 | Strelitzia sp. | South Africa | W. Gams | HM148095 | HM148339 | HM148584 |
| C. globisporum | cladosporioides | CBS 812.96* | Meat stamp | Sweden | M. Olsen | HM148096 | HM148340 | HM148585 |
| CPC 19124; BA 2038 | Indoor environment, window frame | Denmark | B. Andersen | MF472985 | MF473413 | MF473834 | ||
| C. grevilleae | cladosporioides | CBS 114271*; CPC 2913; JT 974 | Grevillea sp., leaves | Australia | P.W. Crous & B.A. Summerell | JF770450 | JF770472 | JF770473 |
| C. halotolerans | sphaerospermum | CBS 114065; DTO 036-G3 | Air | Germany | U. Weidner | MF472986 | MF473414 | MF473835 |
| CBS 119416*; EXF-572; FMR 13493 | Hypersaline water, salterns | Namibia | N. Gunde-Cimerman | DQ780364 | JN906989 | KJ596633 | ||
| CBS 139583; DTO 147-B9 | Indoor environment | Hungary | — | KP701942 | KP701819 | KP702064 | ||
| CPC 22275; EMSL 1745 | Indoor air sample | USA: SC | Ž. Jurjević | MF472987 | MF473415 | MF473836 | ||
| CPC 22278; EMSL 1749 | Indoor air sample, pineapple storage room | USA: DE | Ž. Jurjević | MF472988 | MF473416 | MF473837 | ||
| CPC 22281; EMSL 1755 | Indoor air sample, pineapple storage room | USA: DE | Ž. Jurjević | MF472989 | MF473417 | MF473838 | ||
| CPC 22293; EMSL 1774 | Indoor air sample, living room | USA: NJ | Ž. Jurjević | MF472990 | MF473418 | MF473839 | ||
| CPC 22308; EMSL 1804 | Indoor air sample | USA: NJ | Ž. Jurjević | MF472991 | MF473419 | MF473840 | ||
| CPC 22335; EMSL 1848 | Indoor air sample, bedroom | USA: NJ | Ž. Jurjević | MF472992 | MF473420 | MF473841 | ||
| CPC 22337; EMSL 1850 | Indoor air sample, 11th floor | USA: NY | Ž. Jurjević | MF472993 | MF473421 | MF473842 | ||
| CPC 22360; EMSL 1873 | Indoor air sample, 19th floor | USA: NY | Ž. Jurjević | MF472994 | — | MF473843 | ||
| CPC 22366; EMSL 1879 | Indoor air sample, living room | USA: NJ | Ž. Jurjević | MF472995 | MF473422 | MF473844 | ||
| CPC 22372; EMSL 1885 | Indoor air sample, hospital | USA: NY | Ž. Jurjević | MF472996 | MF473423 | MF473845 | ||
| CPC 22381; EMSL 1894 | Indoor air sample, bathroom | USA: WI | Ž. Jurjević | MF472997 | MF473424 | MF473846 | ||
| CPC 22390; EMSL 1905 | Indoor air sample, bedroom | USA: NJ | Ž. Jurjević | MF472998 | MF473425 | MF473847 | ||
| CPC 22397; EMSL 1925 | Indoor air sample, classroom | USA: TX | Ž. Jurjević | MF472999 | MF473426 | MF473848 | ||
| CPC 22401; EMSL 1929 | Indoor air sample, living room | USA: NJ | Ž. Jurjević | MF473000 | MF473427 | MF473849 | ||
| CPC 22411; EMSL 1960 | Attic, wood roofing sample | USA: PA | Ž. Jurjević | MF473001 | MF473428 | MF473850 | ||
| CPC 22412; EMSL 1961 | Attic, wood roofing sample | USA: PA | Ž. Jurjević | MF473002 | MF473429 | MF473851 | ||
| CPC 22414; EMSL 1963 | Attic, wood roofing sample | USA: PA | Ž. Jurjević | MF473003 | MF473430 | MF473852 | ||
| dH12862 | Culture contaminant | Brazil | — | DQ780371 | EF101400 | — | ||
| DTO 049-E7 | Swab sample, house | Netherlands | J. Houbraken | MF473004 | MF473431 | MF473853 | ||
| DTO 049-E8 | Swab sample, house | Netherlands | J. Houbraken | MF473005 | MF473432 | MF473854 | ||
| DTO 102-A1 | Bathroom | Hungary | van Mil | KP701904 | KP701781 | KP702027 | ||
| DTO 102-A3 | Bathroom | Hungary | van Mil | MF473006 | MF473433 | MF473855 | ||
| DTO 108-F7 | Indoor environment | France | J. Dijksterhuis | MF473007 | MF473434 | MF473856 | ||
| DTO 109-D1 | Bathroom wall | Thailand | P. Noonim | MF473008 | MF473435 | MF473857 | ||
| DTO 109-D3 | Indoor air, open Petri-dish | Thailand | P. Noonim | KP701911 | KP701788 | KP702034 | ||
| DTO 114-H7 | Swab sample, indoor environment | Netherlands | P. Noonim | KP701925 | KP701802 | KP702048 | ||
| DTO 114-I3 | Swab sample, indoor environment | Netherlands | P. Noonim | KP701926 | KP701803 | KP702049 | ||
| DTO 117-H3; HM2 RS5 | Indoor environment of house | Netherlands | M. Meijer & O. Terhoeven | KP701929 | KP701806 | KP702052 | ||
| DTO 127-E3; AR373 | Air sample, bakery | USA: GA | — | MF473009 | MF473436 | MF473858 | ||
| DTO 127-E8; AR407 | Air sample, bakery | USA: GA | — | KP701936 | KP701813 | KP702058 | ||
| DTO 130-C9 | Swab sample, food plant | Netherlands | M. Meijer | MF473010 | MF473437 | MF473859 | ||
| DTO 147-B3 | Indoor environment | Hungary | — | MF473011 | MF473438 | MF473860 | ||
| DTO 147-B8 | Indoor environment | Hungary | — | MF473012 | MF473439 | MF473861 | ||
| DTO 153-C3 | Bathroom | Netherlands | F. Hagen | KP701952 | KP701829 | KP702074 | ||
| DTO 153-C5 | Bathroom | Netherlands | F. Hagen | MF473013 | MF473440 | MF473862 | ||
| DTO 160-I2 | Fungal growth in living room | Netherlands | J. Najafzadeh | MF473014 | MF473441 | MF473863 | ||
| DTO 160-I3 | Fungal growth in living room | Netherlands | J. Najafzadeh | MF473015 | MF473442 | MF473864 | ||
| DTO 160-I5 | Black spots in bathroom | Netherlands | J. Najafzadeh | MF473016 | MF473443 | MF473865 | ||
| DTO 161-D5 | Swab sample, wooden window frame in apartment | Netherlands | J. Houbraken | KP701957 | KP701834 | KP702079 | ||
| DTO 305-E4; AA03US-390 | House dust, basement HVAC room | USA: CA | A. Amend | MF473017 | MF473444 | MF473866 | ||
| DTO 305-E5; AA03US-412 | House dust, basement HVAC room | USA: CA | A. Amend | MF473018 | MF473445 | MF473867 | ||
| DTO 305-E6; KJ03SA-372 | House dust, small apartment | South Africa | K. Jacobs | MF473019 | MF473446 | MF473868 | ||
| DTO 305-E7; KJ03SA-381 | House dust, small apartment | South Africa | K. Jacobs | MF473020 | MF473447 | MF473869 | ||
| DTO 305-E9; AA01MX-246 | House dust, rental studio | Mexico | A. Amend | MF473021 | MF473448 | MF473870 | ||
| DTO 305-F1; AA03US-378 | House dust, basement HVAC room | USA: CA | A. Amend | MF473022 | MF473449 | MF473871 | ||
| DTO 305-F2; PN08TH-553 | House dust from four rooms | Thailand | P. Noonim | MF473023 | MF473450 | MF473872 | ||
| DTO 305-F3; AA03US-528 | House dust, basement HVAC room | USA: CA | A. Amend | MF473024 | MF473451 | MF473873 | ||
| DTO 305-F4; AA03US-385 | House dust, basement HVAC room | USA: CA | A. Amend | MF473025 | MF473452 | MF473874 | ||
| DTO 305-F6; AA07MX-882 | House dust, in a hotel | Mexico | A. Amend | MF473026 | MF473453 | MF473875 | ||
| DTO 305-F9; MB02UK-43 | House dust, living room, bedroom | UK: England | M. Bidartondo | MF473027 | MF473454 | MF473876 | ||
| DTO 305-G1; MB02UK-62 | House dust, living room, bedroom | UK: England | M. Bidartondo | MF473028 | MF473455 | MF473877 | ||
| DTO 305-G2; MB02UK-41 | House dust, living room, bedroom | UK: England | M. Bidartondo | MF473029 | MF473456 | MF473878 | ||
| DTO 305-G5; PN09TH-863 | House dust, in meeting hall | Thailand | P. Noonim | MF473030 | MF473457 | MF473879 | ||
| DTO 305-G6; AA03US-493 | House dust, basement HVAC room | USA: CA | A. Amend | MF473031 | MF473458 | MF473880 | ||
| DTO 305-G7; AA03US-498 | House dust, basement HVAC room | USA: CA | A. Amend | MF473032 | MF473459 | MF473881 | ||
| DTO 305-G8; KJ03SA-398 | House dust, small apartment | South Africa | K. Jacobs | MF473033 | MF473460 | MF473882 | ||
| DTO 305-G9; AA07MX-872 | House dust, in a hotel | Mexico | A. Amend | MF473034 | MF473461 | MF473883 | ||
| DTO 305-H3; AA03US-410 | House dust, basement HVAC room | USA: CA | A. Amend | MF473035 | MF473462 | MF473884 | ||
| DTO 305-H6; AA03US-437 | House dust, basement HVAC room | USA: CA | A. Amend | MF473036 | MF473463 | MF473885 | ||
| DTO 305-I3; MB02UK-55 | House dust, living room, bedroom | UK: England | M. Bidartondo | MF473037 | MF473464 | MF473886 | ||
| DTO 305-I4; AA03US-442 | House dust, basement HVAC room | USA: CA | A. Amend | MF473038 | MF473465 | MF473887 | ||
| DTO 305-I6; AA07MX-944 | House dust, in a hotel | Mexico | A. Amend | MF473039 | MF473466 | MF473888 | ||
| DTO 305-I8; KJ10SA-43 | House dust | South Africa | K. Jacobs | MF473040 | MF473467 | MF473889 | ||
| DTO 306-A2; AA03US-441 | House dust, basement HVAC room | USA: CA | A. Amend | MF473041 | MF473468 | MF473890 | ||
| DTO 306-A4; AA03US-523 | House dust, basement HVAC room | USA: CA | A. Amend | MF473042 | MF473469 | MF473891 | ||
| DTO 306-A9; AA03US-499 | House dust, basement HVAC room | USA: CA | A. Amend | MF473043 | MF473470 | MF473892 | ||
| DTO 306-B1; AA03US-501 | House dust, basement HVAC room | USA: CA | A. Amend | MF473044 | MF473471 | MF473893 | ||
| DTO 306-B3; AA03US-471 | House dust, basement HVAC room | USA: CA | A. Amend | MF473045 | MF473472 | MF473894 | ||
| DTO 306-B4; AA03US-508 | House dust, basement HVAC room | USA: CA | A. Amend | MF473046 | MF473473 | MF473895 | ||
| DTO 306-B5; AA03US-452 | House dust, basement HVAC room | USA: CA | A. Amend | MF473047 | MF473474 | MF473896 | ||
| DTO 306-B8; AA03US-558 | House dust, basement HVAC room | USA: CA | A. Amend | MF473048 | MF473475 | MF473897 | ||
| DTO 306-B9; AA03US-416 | House dust, basement HVAC room | USA: CA | A. Amend | MF473049 | MF473476 | MF473898 | ||
| DTO 306-C2; AA07MX-817 | House dust, in a hotel | Mexico | A. Amend | MF473050 | MF473477 | MF473899 | ||
| DTO 306-C5; AA03US-370 | House dust, basement HVAC room | USA: CA | A. Amend | MF473051 | MF473478 | MF473900 | ||
| DTO 306-C6; AA03US-369 | House dust, basement HVAC room | USA: CA | A. Amend | MF473052 | MF473479 | MF473901 | ||
| DTO 306-C7; AA03US-383 | House dust, basement HVAC room | USA: CA | A. Amend | MF473053 | MF473480 | MF473902 | ||
| DTO 306-C8; AA03US-552 | House dust, basement HVAC room | USA: CA | A. Amend | MF473054 | MF473481 | MF473903 | ||
| DTO 306-C9; MB02UK-63 | House dust, living room, bedroom | UK: England | M. Bidartondo | MF473055 | MF473482 | MF473904 | ||
| DTO 306-D3; AA03US-463 | House dust, basement HVAC room | USA: CA | A. Amend | MF473056 | MF473483 | MF473905 | ||
| DTO 306-D4; AA03US-377 | House dust, basement HVAC room | USA: CA | A. Amend | MF473057 | MF473484 | MF473906 | ||
| DTO 306-D5; 7050035.81-631 | House dust | Canada | Health Canada | MF473058 | MF473485 | MF473907 | ||
| DTO 306-D6; AA03US-538 | House dust, basement HVAC room | USA: CA | A. Amend | MF473059 | MF473486 | MF473908 | ||
| DTO 306-D7; KJ03SA-370 | House dust, small apartment | South Africa | K. Jacobs | MF473060 | MF473487 | MF473909 | ||
| DTO 306-D9; KJ10SA-8 | House dust | South Africa | K. Jacobs | MF473061 | MF473488 | MF473910 | ||
| DTO 306-E1; AA03US-425 | House dust, basement HVAC room | USA: CA | A. Amend | MF473062 | MF473489 | MF473911 | ||
| DTO 306-E2; AA03US-519 | House dust, basement HVAC room | USA: CA | A. Amend | MF473063 | MF473490 | MF473912 | ||
| DTO 306-E5; KJ03SA-382 | House dust, small apartment | South Africa | K. Jacobs | MF473064 | MF473491 | MF473913 | ||
| DTO 306-E6; AA03US-564 | House dust, basement HVAC room | USA: CA | A. Amend | MF473065 | MF473492 | MF473914 | ||
| DTO 306-E8; AA03US-554 | House dust, basement HVAC room | USA: CA | A. Amend | MF473066 | MF473493 | MF473915 | ||
| DTO 306-E9; KJ03SA-364 | House dust, small apartment | South Africa | K. Jacobs | MF473067 | MF473494 | MF473916 | ||
| DTO 306-F1; MB02UK-39 | House dust, living room, bedroom | UK: England | M. Bidartondo | MF473068 | MF473495 | MF473917 | ||
| DTO 306-F2; KJ09SA-132 | House dust | South Africa | K. Jacobs | MF473069 | MF473496 | MF473918 | ||
| DTO 306-F3; AA03US-510 | House dust, basement HVAC room | USA: CA | A. Amend | MF473070 | MF473497 | MF473919 | ||
| DTO 306-F4; Arg-26 | House dust | Argentina | G. Reppchen | MF473071 | MF473498 | MF473920 | ||
| DTO 307-E9; KJ03SA-393 | House dust, small apartment | South Africa | K. Jacobs | MF473072 | MF473499 | MF473921 | ||
| DTO 307-F4; MB02UK-66 | House dust, living room, bedroom | UK: England | M. Bidartondo | MF473073 | MF473500 | MF473922 | ||
| DTO 307-F6; KJ10SA-48 | House dust | South Africa | K. Jacobs | MF473074 | MF473501 | MF473923 | ||
| DTO 307-F7; AA03US-430 | House dust, basement HVAC room | USA: CA | A. Amend | MF473075 | MF473502 | MF473924 | ||
| DTO 307-F8; AA03US-454 | House dust, basement HVAC room | USA: CA | A. Amend | MF473076 | MF473503 | MF473925 | ||
| DTO 307-F9; KJ10SA-37 | House dust | South Africa | K. Jacobs | MF473077 | MF473504 | MF473926 | ||
| DTO 307-G1; AA03US-426 | House dust, basement HVAC room | USA: CA | A. Amend | MF473078 | MF473505 | MF473927 | ||
| DTO 307-G2; TA10NZ-207A | House dust | New Zealand | T. Atkinson | MF473079 | MF473506 | MF473928 | ||
| DTO 307-G3; AA03US-448 | House dust, basement HVAC room | USA: CA | A. Amend | MF473080 | MF473507 | MF473929 | ||
| DTO 307-G4; MB02UK-49 | House dust, living room, bedroom | UK: England | M. Bidartondo | MF473081 | MF473508 | MF473930 | ||
| DTO 307-G5; AA03US-429 | House dust, basement HVAC room | USA: CA | A. Amend | MF473082 | MF473509 | MF473931 | ||
| DTO 307-G7; AA03US-420 | House dust, basement HVAC room | USA: CA | A. Amend | MF473083 | MF473510 | MF473932 | ||
| DTO 307-G8; AA03US-515 | House dust, basement HVAC room | USA: CA | A. Amend | MF473084 | MF473511 | MF473933 | ||
| DTO 307-H5; AA03US-431 | House dust, basement HVAC room | USA: CA | A. Amend | MF473085 | MF473512 | MF473934 | ||
| DTO 307-H6; AA03US-428 | House dust, basement HVAC room | USA: CA | A. Amend | MF473086 | MF473513 | MF473935 | ||
| DTO 307-H7; AA03US-421 | House dust, basement HVAC room | USA: CA | A. Amend | MF473087 | MF473514 | MF473936 | ||
| DTO 307-H8; AA03US-460 | House dust, basement HVAC room | USA: CA | A. Amend | MF473088 | MF473515 | MF473937 | ||
| DTO 307-H9; AA03US-484 | House dust, basement HVAC room | USA: CA | A. Amend | MF473089 | MF473516 | MF473938 | ||
| DTO 307-I1; AA03US-423 | House dust, basement HVAC room | USA: CA | A. Amend | MF473090 | MF473517 | MF473939 | ||
| DTO 307-I4; AA03US-440 | House dust, basement HVAC room | USA: CA | A. Amend | MF473091 | MF473518 | MF473940 | ||
| DTO 307-I7; AA03US-511 | House dust, basement HVAC room | USA: CA | A. Amend | MF473092 | MF473519 | MF473941 | ||
| DTO 307-I8; AA03US-381 | House dust, basement HVAC room | USA: CA | A. Amend | MF473093 | MF473520 | MF473942 | ||
| DTO 308-A1; AA03US-401 | House dust, basement HVAC room | USA: CA | A. Amend | MF473094 | MF473521 | MF473943 | ||
| DTO 308-A3; AA03US-422 | House dust, basement HVAC room | USA: CA | A. Amend | MF473095 | MF473522 | MF473944 | ||
| DTO 308-A4; AA03US-467 | House dust, basement HVAC room | USA: CA | A. Amend | MF473096 | MF473523 | MF473945 | ||
| DTO 308-A5; AA03US-432 | House dust, basement HVAC room | USA: CA | A. Amend | MF473097 | MF473524 | MF473946 | ||
| DTO 308-A6; AA03US-411 | House dust, basement HVAC room | USA: CA | A. Amend | MF473098 | MF473525 | MF473947 | ||
| DTO 308-A7; AA03US-391 | House dust, basement HVAC room | USA: CA | A. Amend | MF473099 | MF473526 | MF473948 | ||
| DTO 308-A8; AA03US-507 | House dust, basement HVAC room | USA: CA | A. Amend | MF473100 | MF473527 | MF473949 | ||
| DTO 308-A9; AA03US-400 | House dust, basement HVAC room | USA: CA | A. Amend | MF473101 | MF473528 | MF473950 | ||
| DTO 308-B3; AA03US-520 | House dust, basement HVAC room | USA: CA | A. Amend | MF473102 | MF473529 | MF473951 | ||
| DTO 308-B4; AA03US-464 | House dust, basement HVAC room | USA: CA | A. Amend | MF473103 | MF473530 | MF473952 | ||
| DTO 308-B6; AA03US-408 | House dust, basement HVAC room | USA: CA | A. Amend | MF473104 | MF473531 | MF473953 | ||
| DTO 308-B7; AA01MX-245 | House dust, rental studio | Mexico | A. Amend | MF473105 | MF473532 | MF473954 | ||
| DTO 323-C4 | Indoor air | China | — | MF473106 | MF473533 | MF473955 | ||
| DTO 323-F3 | Indoor air | China | — | MF473107 | MF473534 | MF473956 | ||
| DTO 323-F6 | Indoor air | China | — | MF473108 | MF473535 | MF473957 | ||
| DTO 323-F8 | Indoor air | China | — | MF473109 | MF473536 | MF473958 | ||
| DTO 323-F9 | Indoor air | China | — | MF473110 | MF473537 | MF473959 | ||
| DTO 323-G9 | Indoor air | China | — | MF473111 | MF473538 | MF473960 | ||
| DTO 323-H4 | Indoor air | China | — | MF473112 | MF473539 | MF473961 | ||
| DTO 323-I2 | Indoor air | China | — | MF473113 | MF473540 | MF473962 | ||
| DTO 323-I3 | Indoor air | China | — | MF574172 | MF574174 | MF574176 | ||
| DTO 323-I7 | Indoor air | China | — | MF473114 | MF473541 | MF473963 | ||
| DTO 324-B3 | Indoor air | China | — | MF473115 | MF473542 | MF473964 | ||
| DTO 324-B4 | Indoor air | China | — | MF473116 | MF473543 | MF473965 | ||
| DTO 324-B5 | Indoor air | China | — | MF473117 | MF473544 | MF473966 | ||
| DTO 324-B6 | Indoor air | China | — | MF473118 | MF473545 | MF473967 | ||
| DTO 324-C1 | Indoor air | China | — | MF473119 | MF473546 | MF473968 | ||
| C. herbaroides | herbarum | CBS 121626*; CPC 12052; EXF-1733 | Hypersaline water, salterns | Israel | P. Zalar | EF679357 | EF679432 | EF679509 |
| C. herbarum | herbarum | CBS 121621**; ATCC MYA-4682; CPC 12177 | Hordeum vulgare | Netherlands | P.W. Crous | EF679363 | EF679440 | EF679516 |
| CBS 121622; CPC 11600 | Delphinium barbeyi, stems | USA: CO | A. Ramaley | DQ289800 | EF679435 | DQ289867 | ||
| CBS 111.82; JCM 11532 | Arctostaphylos uva-ursi | Switzerland | — | AJ238469 | EF679433 | EF679510 | ||
| CBS 300.49 | Biscutella laevigata | Switzerland | — | EF679358 | EF679434 | EF679511 | ||
| CPC 11601 | Delphinium barbeyi, stems | USA: CO | A. Ramaley | EF679359 | EF679436 | EF679512 | ||
| CPC 11602 | Delphinium barbeyi, stems | USA: CO | A. Ramaley | EF679360 | EF679437 | EF679513 | ||
| CPC 11603 | Delphinium barbeyi, stems | USA: CO | A. Ramaley | EF679361 | EF679438 | EF679514 | ||
| CPC 11604 | Delphinium barbeyi, stems | USA: CO | A. Ramaley | EF679362 | EF679439 | EF679515 | ||
| CPC 12178 | Hordeum vulgare | Netherlands | P.W. Crous | EF679364 | EF679441 | EF679517 | ||
| CPC 12179 | Hordeum vulgare | Netherlands | P.W. Crous | EF679365 | EF679442 | EF679518 | ||
| CPC 12180 | Hordeum vulgare | Netherlands | P.W. Crous | EF679366 | EF679443 | EF679519 | ||
| CPC 12181 | Hordeum vulgare | Netherlands | P.W. Crous | EF679367 | EF679444 | EF679520 | ||
| CPC 12183 | Hordeum vulgare | Netherlands | P.W. Crous | EF679368 | EF679445 | EF679521 | ||
| C. hillianum | cladosporioides | CBS 125988*; CPC 15459; C92 | Typha orientalis, leaf mold | New Zealand | R. Beever | HM148097 | HM148341 | HM148586 |
| CPC 15458 | Typha orientalis, leaf mold | New Zealand | R. Beever | HM148098 | HM148342 | HM148587 | ||
| C. inversicolor | cladosporioides | CBS 139573; DTO 072-C9 | Indoor air, archive | Netherlands | M. Meijer | KP701874 | KP701751 | KP701997 |
| CBS 401.80*; ATCC 200941 | Triticum aestivum, leaf | Netherlands | — | HM148101 | HM148345 | HM148590 | ||
| CBS 484.80 | Cortaderia sp. | Colombia | — | HM148103 | HM148347 | HM148592 | ||
| CPC 11818 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | USA: WA | D. Glawe | HM148104 | HM148348 | HM148593 | ||
| CPC 14190 | Outside air | Netherlands | M. Meijer | HM148106 | HM148350 | HM148595 | ||
| CPC 14191 | Outside air | Netherlands | M. Meijer | HM148107 | HM148351 | HM148596 | ||
| CPC 14241 | Sambucus nigra, fruit | Netherlands | P.W. Crous | HM148108 | HM148352 | HM148597 | ||
| CPC 14368; BA 1735 | School dust | Denmark | B. Andersen | HM148109 | HM148353 | HM148598 | ||
| CPC 19108; BA 2015 | Indoor air | Denmark | B. Andersen | MF473120 | MF473547 | MF473969 | ||
| CPC 22287; EMSL 1763 | Indoor air sample, bedroom | USA: OR | Ž. Jurjević | MF473121 | MF473548 | MF473970 | ||
| CPC 22289; EMSL 1765 | Indoor air sample, living room | USA: AK | Ž. Jurjević | MF473122 | — | MF473971 | ||
| CPC 22300; EMSL 1788 | Indoor air sample, living room | USA: OR | Ž. Jurjević | MF473123 | MF473549 | MF473972 | ||
| CPC 22385; EMSL 1900 | Indoor air sample, bedroom | USA: WA | Ž. Jurjević | MF473124 | MF473550 | MF473973 | ||
| DTO 108-F8 | Indoor environment | France | J. Dijksterhuis | KP701908 | KP701785 | KP702031 | ||
| DTO 109-E9; BA 1909 | Indoor environment | Denmark | B. Andersen | MF473125 | MF473551 | MF473974 | ||
| C. ipereniae | cladosporioides | CBS 140483*; CPC 16238 | Puya sp. | Chile | A. van Iperen | KT600394 | KT600491 | KT600589 |
| CPC 16855 | Arctostaphylos pallida | USA: CA | P.W. Crous | KT600395 | KT600492 | KT600590 | ||
| C. iranicum | cladosporioides | CBS 126346*; CPC 11554 | Citrus sinensis, leaf | Iran | W. Gams | HM148110 | HM148354 | HM148599 |
| C. iridis | herbarum | CBS 107.20 | — | — | — | EF679369 | EF679446 | EF679522 |
| CBS 138.40** | Iris sp., leaves | Netherlands | — | EF679370 | EF679447 | EF679523 | ||
| C. langeronii | sphaerospermum | CBS 101880 | Moist aluminium school window frame | Belgium | E.S. Hoekstra | DQ780380 | MF473552 | EF101359 |
| CBS 139581; DTO 124-D5 | Air sample, food plant | Netherlands | M. Meijer | KP701931 | KP701808 | KP702053 | ||
| CBS 189.54* | Man, mycosis | Brazil | Fonseca | DQ780379 | JN906990 | EF101357 | ||
| CBS 601.84 | Picea abies, wood | Germany | — | DQ780382 | MF473553 | EF101360 | ||
| CPC 19121; BA 2035 | Indoor air | Denmark | — | MF473126 | MF473554 | MF473975 | ||
| CPC 22235; EMSL 1681 | Indoor air sample, storage room | USA: DE | Ž. Jurjević | MF473127 | MF473555 | MF473976 | ||
| CPC 22261; EMSL 1716 | Indoor air sample | USA: MN | Ž. Jurjević | MF473128 | MF473556 | MF473977 | ||
| CPC 22299; EMSL 1783 | Indoor air sample | USA: PA | Ž. Jurjević | MF473129 | MF473557 | MF473978 | ||
| CPC 22325; EMSL 1831 | Indoor air sample, washroom | Ireland | Ž. Jurjević | MF473130 | MF473558 | MF473979 | ||
| CPC 22326; EMSL 1832 | Indoor air sample, washroom | Ireland | Ž. Jurjević | MF473131 | MF473559 | MF473980 | ||
| DTO 004-C3 | Swab sample, house | Netherlands | J. Houbraken | MF473132 | MF473560 | MF473981 | ||
| DTO 085-H6 | Indoor air, archive | Netherlands | M. Meijer | KP701885 | KP701762 | KP702008 | ||
| DTO 124-D2 | Air sample, food plant | Netherlands | M. Meijer | MF473133 | MF473561 | MF473982 | ||
| C. lebrasiae | sphaerospermum | CBS 138283*; UBOCC-A-112063 | Milk bread | France | M. Le Bras | KJ596568 | KJ596583 | KJ596631 |
| C. licheniphilum | cladosporioides | CBS 125990*; CPC 13224 | Lichen Phaeophysica orbicularis and Physcia sp. on stems and bark of Acer platanoides | Germany | W. von Brackel | HM148111 | HM148355 | HM148600 |
| C. limoniforme | herbarum | CBS 113737 | Grape berry | USA: WA | F.M. Dugan lab | KT600396 | KT600493 | KT600591 |
| CBS 140484*; CPC 12039 | Musa acuminata | Egypt | R.S. Summerbell | KT600397 | KT600494 | KT600592 | ||
| CGMCC 3.18037 | Populus euphratica, rhizosphere | China | Y. Hao | KX938396 | KX938413 | KX938379 | ||
| CGMCC 3.18038 | Populus euphratica, rhizosphere | China | — | KX938397 | KX938414 | KX938380 | ||
| CPC 12048; EXF-1060 | Hypersaline water | Israel | P. Zalar | KT600398 | KT600495 | KT600593 | ||
| CPC 12049; EXF-1062 | Hypersaline water | Israel | P. Zalar | KT600399 | KT600496 | KT600594 | ||
| CPC 12050; EXF-1081 | Hypersaline water | Israel | P. Zalar | KT600400 | KT600497 | KT600595 | ||
| CPC 13923 | Eucalyptus sp. | Cyprus | A. van Iperen | KT600401 | KT600498 | KT600596 | ||
| CPC 18086; KSU C1 | Tomato | — | — | KT600402 | KT600499 | KT600597 | ||
| CPC 22350; EMSL 1863 | Indoor air sample, bedroom | USA: CA | Ž. Jurjević | MF473134 | MF473562 | MF473983 | ||
| CPC 22384; EMSL 1899 | Sample from under kitchen sink | USA: CA | Ž. Jurjević | MF473135 | MF473563 | MF473984 | ||
| CPC 22394; EMSL 1909 | Indoor air sample, hospital | USA: AZ | Ž. Jurjević | MF473136 | MF473564 | MF473985 | ||
| CPC 22395; EMSL 1910 | Indoor air sample, hospital | USA: AZ | Ž. Jurjević | MF473137 | MF473565 | MF473986 | ||
| DTO 082-F2 | Indoor air, living room | Netherlands | B. Favié | MF473138 | MF473566 | MF473987 | ||
| DTO 090-H8 | Swab sample, archive | Netherlands | M. Meijer | KP701901 | KP701778 | KP702024 | ||
| DTO 305-G4; BH02AU-115 | House dust | Australia: Tasmania | B. Horton | MF473139 | MF473567 | MF473988 | ||
| C. longicatenatum | cladosporioides | CBS 140485*; CPC 17189 | Unknown plant | Australia | P.W. Crous | KT600403 | KT600500 | KT600598 |
| C. longissimum | sphaerospermum | CBS 300.96* | Soil along coral reef coast | Papua New Guinea | A. Aptroot | DQ780352 | EU570259 | EF101385 |
| C. lycoperdinum | cladosporioides | CBS 126347; CPC 12102 | Galls of Apiosporina morbosa on Prunus sp. | Canada | K.A. Seifert | HM148112 | HM148356 | HM148601 |
| CBS 126348; CPC 11833 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | USA: WA | D. Glawe | HM148113 | HM148357 | HM148602 | ||
| CBS 274.80C | Puya sp. | Colombia | W. Gams | HM148114 | HM148358 | HM148603 | ||
| CBS 574.78C; VKM F-2759 | Aureobasidium caulivorum | Russia | — | HM148115 | HM148359 | HM148604 | ||
| CPC 22256; EMSL 1711.b | Outside air sample | USA: MN | Ž. Jurjević | MF473140 | MF473568 | MF473989 | ||
| C. macrocarpum | herbarum | CBS 121623*; CPC 12752 | Spinacia oleracea | USA: WA | L. du Toit | EF679375 | EF679453 | EF679529 |
| CBS 121811; CPC 12755 | Spinacia oleracea | USA: WA | L. du Toit | EF679376 | EF679454 | EF679530 | ||
| CBS 175.82 | Water | Romania | — | EF679371 | EF679448 | EF679524 | ||
| CBS 223.31; ATCC 11287; IFO 6379; IMI 049635; JCM 11501 | Mycosphaerella tulasnei | — | — | AF222830 | EF679449 | EF679525 | ||
| CBS 299.67 | Triticum aestivum | Turkey | — | EF679372 | EF679450 | EF679526 | ||
| CPC 11817 | Cleistothecia of Phyllactinia guttata on leaves of Corylus sp. | USA: WA | D. Glawe | EF679373 | EF679451 | EF679527 | ||
| CPC 12054; EXF-2287 | Hypersaline water, salterns (precrystalisation pond) | Slovenia | P. Zalar | EF679374 | EF679452 | EF679528 | ||
| CPC 12756 | Spinacia oleracea | USA: WA | L. du Toit | EF679377 | EF679455 | EF679531 | ||
| CPC 12757 | Spinacia oleracea | USA: WA | L. du Toit | EF679378 | EF679456 | EF679532 | ||
| CPC 12758 | Spinacia oleracea | USA: WA | L. du Toit | EF679379 | EF679457 | EF679533 | ||
| CPC 12759 | Spinacia oleracea | USA: WA | L. du Toit | EF679380 | EF679458 | EF679534 | ||
| CPC 14305; BA 1704 | Indoor environment, dust, school | Denmark | B. Andersen | MF473141 | MF473569 | MF473990 | ||
| C. montecillanum | cladosporioides | CBS 140486*; CPC 17953 | Pine needles | Mexico | M. de Jesús Yáñez-Morales | KT600406 | KT600504 | KT600602 |
| CPC 15605 | Taraxacum sp. | Mexico | M. de Jesús Yáñez-Morales | KT600407 | KT600505 | KT600603 | ||
| CPC 17804 | Pine needles | Mexico | M. de Jesús Yáñez-Morales | KT600408 | KT600506 | KT600604 | ||
| C. myrtacearum | cladosporioides | CBS 126349; CPC 13689; NSM 734672 | Eucalyptus placita | Australia | B.A. Summerell | HM148116 | HM148360 | HM148605 |
| CBS 126350*; CPC 14567 | Corymbia foelscheana | Australia | B.A. Summerell | HM148117 | HM148361 | HM148606 | ||
| C. needhamense sp. nov. | cladosporioides | CBS 143359*; CPC 22353; EMSL 1866 | Indoor air sample, office | USA: MA | Ž. Jurjević | MF473142 | MF473570 | MF473991 |
| C. neerlandicum sp. nov. | cladosporioides | CBS 143360*; DTO 086-C5 | Swab sample, archive | Netherlands | M. Meijer | KP701887 | KP701764 | KP702010 |
| C. neolangeronii sp. nov. | sphaerospermum | CBS 109868 | Mortar of Muro Farnesiano | Italy | C. Urzi | DQ780377 | MF473571 | EF101362 |
| CBS 797.97* | Indoor environment | Netherlands | O. Adan | MF473143 | — | MF473992 | ||
| CPC 22236; EMSL 1682 | Indoor air sample, pineapple storage room | USA: DE | Ž. Jurjević | MF473144 | MF473572 | MF473993 | ||
| CPC 22262; EMSL 1717 | Outside air sample | USA: MN | Ž. Jurjević | MF473145 | MF473573 | MF473994 | ||
| CPC 22263; EMSL 1718 | Indoor air sample | USA: MN | Ž. Jurjević | MF473146 | MF473574 | — | ||
| CPC 22266; EMSL 1724 | Indoor air sample | USA: MN | Ž. Jurjević | MF473147 | MF473575 | MF473995 | ||
| CPC 22267; EMSL 1725 | Indoor air sample | USA: MN | Ž. Jurjević | MF473148 | MF473576 | MF473996 | ||
| CPC 22314; EMSL 1810 | Indoor air sample | USA: NJ | Ž. Jurjević | MF473149 | — | MF473997 | ||
| DTO 162-A4 | Wall in a storage room of antiquities with mold growth | Netherlands | J. Houbraken | KP701962 | KP701839 | KP702084 | ||
| C. neopsychrotolerans | cladosporioides | CGMCC 3.18031* | Saussurea involucrata, rhizosphere soil | China | G. Wang | KX938383 | KX938400 | KX938366 |
| CGMCC 3.18032 | Saussurea involucrata, rhizosphere soil | China | G. Wang | KX938384 | KX938401 | KX938367 | ||
| C. ossifragi | herbarum | CBS 842.91*; ATCC 200946 | Narthecium ossifragum, green leaf | Norway | M. di Menna | EF679381 | EF679459 | EF679535 |
| CBS 843.91 | Narthecium ossifragum, green leaf | Norway | M. di Menna | EF679382 | EF679460 | EF679536 | ||
| C. oxysporum | cladosporioides | CBS 125991; CPC 14371; IBT 14868 | Soil, near the terracotta army | China | S. Gravesen | HM148118 | HM148362 | HM148607 |
| CBS 126351; CPC 14308; IBT 25029 | Indoor air | Venezuela | B. Andersen | HM148119 | HM148363 | HM148608 | ||
| C. paracladosporioides | cladosporioides | CBS 171.54*; ATCC 11278, 200943; IFO 6369; IMI 049626; MUCL 917; NCTC 4097 | — | — | — | HM148120 | HM148364 | HM148609 |
| C. parahalotolerans sp. nov. | sphaerospermum | CBS 139585*; DTO 161-D3 | Swab sample, apartment | Netherlands | J. Houbraken | KP701955 | KP701832 | KP702077 |
| CPC 22280; EMSL 1754 | Indoor air sample, hotel room | USA: ME | Ž. Jurjević | MF473150 | MF473577 | MF473998 | ||
| CPC 22330; EMSL 1843 | Indoor air sample, family room | USA: NH | Ž. Jurjević | MF473151 | — | MF473999 | ||
| CPC 22336; EMSL 1849 | Indoor air sample | USA: NJ | Ž. Jurjević | MF473152 | MF473578 | MF474000 | ||
| CPC 22342; EMSL 1855 | Indoor air sample, 18th floor | USA: NY | Ž. Jurjević | MF473153 | — | MF474001 | ||
| CPC 22373; EMSL 1886 | Indoor air sample, hospital | USA: NY | Ž. Jurjević | MF473154 | — | MF474002 | ||
| CPC 22376; EMSL 1889 | Indoor air sample, hospital | USA: NY | Ž. Jurjević | MF473155 | — | MF474003 | ||
| DTO 161-D6 | Swab sample, apartment | Netherlands | J. Houbraken | KP701958 | KP701835 | KP702080 | ||
| DTO 305-F7; AA07MX-953 | House dust, in a hotel | Mexico | A. Amend | MF473156 | MF473579 | MF474004 | ||
| DTO 305-F8; AA07MX-935 | House dust, in a hotel | Mexico | A. Amend | MF473157 | MF473580 | MF474005 | ||
| DTO 305-I5; AA03MX-750 | House dust, in a hardware store | Mexico | A. Amend | MF473158 | MF473581 | MF474006 | ||
| DTO 306-C1; AA07MX-836 | House dust, in a hotel | Mexico | A. Amend | MF473159 | MF473582 | MF474007 | ||
| DTO 306-E4; AA02MX-573 | House dust, in a church | Mexico | A. Amend | MF473160 | MF473583 | MF474008 | ||
| DTO 307-H4; AA03MX-612 | House dust, in a hardware store | Mexico | A. Amend | MF473161 | MF473584 | MF474009 | ||
| DTO 323-B8 | Indoor air | China | — | MF473162 | MF473585 | MF474010 | ||
| DTO 323-C1 | Indoor air | China | — | MF473163 | MF473586 | MF474011 | ||
| DTO 323-C8 | Indoor air | China | — | MF473164 | MF473587 | MF474012 | ||
| DTO 323-F4 | Indoor air | China | — | MF473165 | MF473588 | MF474013 | ||
| DTO 323-H2 | Indoor air | China | — | MF473166 | MF473589 | MF474014 | ||
| DTO 323-H3 | Indoor air | China | — | MF473167 | MF473590 | MF474015 | ||
| DTO 324-A7 | Indoor air | China | — | MF473168 | MF473591 | MF474016 | ||
| DTO 324-B7 | Indoor air | China | — | MF473169 | MF473592 | MF474017 | ||
| C. paralimoniforme | herbarum | CGMCC 3.18103* | Meadow soil | China | J. Zhuang | KX938392 | KX938409 | KX938375 |
| CGMCC 3.18104 | Thododendron sp., rhizosphere soil | China | Y. Hao | KX938393 | KX938410 | KX938376 | ||
| C. parapenidielloides | cladosporioides | CBS 140487*; CPC 17193 | Eucalyptus sp. | Australia | P.W. Crous | KT600410 | KT600508 | KT600606 |
| C. parasubtilissimum sp. nov. | herbarum | CBS 143361*; CPC 22332; EMSL 1845 | Indoor air sample, bathroom | USA: NM | Ž. Jurjević | MF473170 | MF473593 | MF474018 |
| CPC 22396; EMSL 1924 | Indoor air sample, recreational vehicle | USA: CA | Ž. Jurjević | MF473171 | MF473594 | MF474019 | ||
| C. penidielloides | sphaerospermum | CBS 140489*; CPC 17674 | Acacia verticillata | Australia | P.W. Crous | KT600412 | KT600510 | KT600608 |
| C. perangustum | cladosporioides | CBS 125996*; CPC 13815 | Cussonia sp. | South Africa | P.W. Crous | HM148121 | HM148365 | HM148610 |
| CBS 126365; CPC 11820 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | USA: WA | D. Glawe | HM148123 | HM148367 | HM148612 | ||
| CPC 11663 | Oncoba spinosa | New Zealand | C.F. Hill | HM148128 | HM148372 | HM148617 | ||
| CPC 11815 | Chasmothecia of Phyllactinia guttata on leaves of Corylus sp. | USA: WA | D. Glawe | HM148130 | HM148374 | HM148619 | ||
| CPC 11819 | Chasmothecia of Phyllactinia guttata on leaves of Corylus sp. | USA: WA | D. Glawe | HM148131 | HM148375 | HM148620 | ||
| CPC 11821 | Chasmothecia of Phyllactinia guttata on leaves of Corylus sp. | USA: WA | D. Glawe | HM148132 | HM148376 | HM148621 | ||
| CPC 11831 | Chasmothecia of Phyllactinia guttata on leaves of Corylus sp. | USA: WA | D. Glawe | HM148133 | HM148377 | HM148622 | ||
| CPC 12216 | Morus rubra | Germany | N. Ale-Agha | HM148135 | HM148379 | HM148624 | ||
| CPC 13727 | Teratosphaeria maculiformis | South Africa | P.W. Crous | HM148139 | HM148383 | HM148628 | ||
| CPC 13730 | Protea caffra | South Africa | P.W. Crous | HM148140 | HM148384 | HM148629 | ||
| CPC 13774 | Protea caffra | South Africa | P.W. Crous | HM148141 | HM148385 | HM148630 | ||
| CPC 13870 | Teratosphaeria fibrillosa | South Africa | P.W. Crous | HM148142 | HM148386 | HM148631 | ||
| CPC 14247 | Magnolia sp. | USA: LA | P.W. Crous | HM148145 | HM148389 | HM148634 | ||
| CPC 15192 | Protea cynaroides | South Africa | L. Mostert | HM148149 | HM148393 | HM148638 | ||
| CPC 22297; EMSL 1781 | Indoor air sample | USA: PA | Ž. Jurjević | MF473172 | MF473595 | MF474020 | ||
| CPC 22327; EMSL 1833 | Indoor air sample | USA: ME | Ž. Jurjević | MF473173 | — | MF474021 | ||
| CPC 22328; EMSL 1834 | Indoor air sample | USA: ME | Ž. Jurjević | MF473174 | MF473596 | MF474022 | ||
| CPC 22329; EMSL 1835 | Indoor air sample, library | USA: CT | Ž. Jurjević | MF473175 | MF473597 | MF474023 | ||
| CPC 22331; EMSL 1844 | Indoor air sample, bedroom closet | USA: CA | Ž. Jurjević | MF473176 | MF473598 | MF474024 | ||
| CPC 22375; EMSL 1888 | Indoor air sample, hospital | USA: NY | Ž. Jurjević | MF473177 | MF473599 | MF474025 | ||
| CPC 22378; EMSL 1891 | Indoor air sample, bedroom | USA: CA | Ž. Jurjević | MF473178 | MF473600 | MF474026 | ||
| DTO 127-E1; AR368 | Air sample, bakery | USA: GA | — | KP701934 | KP701811 | KP702056 | ||
| DTO 127-E2; AR371 | Air sample, bakery | USA: GA | — | MF473179 | MF473601 | MF474027 | ||
| DTO 323-E4 | Indoor air | China | — | MF473180 | MF473602 | MF474028 | ||
| DTO 323-E8 | Indoor air | China | — | MF473181 | MF473603 | MF474029 | ||
| DTO 323-E9 | Indoor air | China | — | MF473182 | MF473604 | MF474030 | ||
| DTO 324-A2 | Indoor air | China | — | MF473183 | MF473605 | MF474031 | ||
| DTO 324-A6 | Indoor air | China | — | MF473184 | MF473606 | MF474032 | ||
| DTO 324-D1 | Indoor air | China | — | MF473185 | MF473607 | MF474033 | ||
| C. phaenocomae | cladosporioides | CBS 128769*; CPC 18223 | Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499837 | JF499875 | JF499881 |
| C. phlei | herbarum | CBS 358.69** | Phleum pratense | Germany | — | JN906981 | JN906991 | JN907000 |
| C. phyllactiniicola | cladosporioides | CBS 126352*; CPC 11836 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | USA: WA | D. Glawe | HM148150 | HM148394 | HM148639 |
| CBS 126353; CPC 11823 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | USA: WA | D. Glawe | HM148151 | HM148395 | HM148640 | ||
| C. phyllophilum | cladosporioides | CBS 125992*; CPC 11333 | Taphrina sp. on Prunus cerasus | Germany | K. Schubert | HM148154 | HM148398 | HM148643 |
| CPC 13873 | Teratosphaeria proteae-arboreae on Protea arborea | South Africa | P.W. Crous | HM148155 | HM148399 | HM148644 | ||
| C. pini-ponderosae | cladosporioides | CBS 124456*; CPC 13980; CIEFAP 322 | Pinus ponderosa | Argentina | A. Greslebin | FJ936160 | FJ936164 | FJ936167 |
| C. prolongatum | herbarum | CGMCC 3.18035 | Populus euphratica, rhizosphere | China | Y. Hao | KX938395 | KX938412 | KX938378 |
| CGMCC 3.18036* | Populus euphratica, rhizosphere | China | Y. Hao | KX938394 | KX938411 | KX938377 | ||
| C. pseudiridis | herbarum | CBS 116463*; LYN 1065 | Iris sp., large leaf lesions | New Zealand | C.F. Hill | EF679383 | EF679461 | EF679537 |
| C. pseudochalastosporoides | cladosporioides | CBS 140490*; CPC 17823 | Pine needles | Mexico | M. de Jesús Yáñez-Morales | KT600415 | KT600513 | KT600611 |
| C. pseudocladosporioides | cladosporioides | CBS 117134 | Cloud water | — | M. Sancelme | HM148156 | HM148400 | HM148645 |
| CBS 117153 | Paeonia sp., living leaves | Germany | R. Kirschner | HM148157 | HM148401 | HM148646 | ||
| CBS 125993*; CPC 14189 | Outside air | Netherlands | M. Meijer | HM148158 | HM148402 | HM148647 | ||
| CBS 139575; DTO 084-F1 | Indoor environment | Germany | — | KP701881 | KP701758 | KP702004 | ||
| CBS 139580; DTO 121-H1 | Bakery | Germany | — | KP701930 | KP701807 | MF474034 | ||
| CBS 149.66; NRRL A-14110 | Triticum aestivum | USA: IL | — | HM148161 | HM148405 | HM148650 | ||
| CBS 176.82 | Pteridium aquilinum | Romania | — | HM148162 | HM148406 | HM148651 | ||
| CBS 574.78A; VKM F-422 | Mycophilic on unidentified substrate | Russia | — | HM148163 | HM148407 | HM148652 | ||
| CBS 667.80; IHEM 3705 | Malus sylvestris, leaf | Italy | — | HM148165 | HM148409 | HM148654 | ||
| CBS 673.69 | Air | Netherlands | — | EF679353 | EF679428 | EF679505 | ||
| CPC 11605 | Agrimonia pilosa | South Korea | H.D. Shin | HM148167 | HM148411 | HM148656 | ||
| CPC 12850 | Pruned wood | USA: LA | K. Seifert | HM148169 | HM148413 | HM148658 | ||
| CPC 13488 | Vernonia sp. | Brazil | O. Pereira | HM148171 | HM148415 | HM148660 | ||
| CPC 13992 | Kentucky coffee tree, pods | USA: VA | P.W. Crous | HM148174 | HM148418 | HM148663 | ||
| CPC 13998; CAMS 001160 | Aloe dichotoma | South Africa | — | HM148175 | HM148419 | HM148664 | ||
| CPC 14001; MRC 03240 | Oats | South Africa | — | HM148176 | HM148420 | HM148665 | ||
| CPC 14010; MRC 10183 | Sorghum sp. | South Africa | — | HM148182 | HM148426 | HM148671 | ||
| CPC 14013; MRC 10221 | Triticum aestivum | South Africa | — | HM148183 | HM148427 | HM148672 | ||
| CPC 14020; MRC 10814 | Triticum aestivum | South Africa | — | HM148185 | HM148429 | HM148674 | ||
| CPC 14193 | Outside air | Netherlands | M. Meijer | HM148186 | HM148430 | HM148675 | ||
| CPC 22237; EMSL 1683 | Air sample, car air conditioner | USA: FL | Ž. Jurjević | MF473186 | MF473608 | MF474035 | ||
| CPC 22283; EMSL 1759 | Indoor air sample, hotel room | USA: NJ | Ž. Jurjević | MF473187 | MF473609 | MF474036 | ||
| CPC 22284; EMSL 1760 | Indoor air sample, hotel room | USA: NJ | Ž. Jurjević | MF473188 | MF473610 | MF474037 | ||
| CPC 22285; EMSL 1761 | Indoor air sample, airport - control tower | USA: MA | Ž. Jurjević | MF473189 | MF473611 | MF474038 | ||
| CPC 22292; EMSL 1773 | Indoor air sample, living room | USA: NJ | Ž. Jurjević | MF473190 | MF473612 | MF474039 | ||
| CPC 22311; EMSL 1807 | Indoor air sample | USA: NJ | Ž. Jurjević | MF473191 | MF473613 | MF474040 | ||
| CPC 22334; EMSL 1847 | Indoor air sample, bedroom | USA: OH | Ž. Jurjević | MF473192 | MF473614 | MF474041 | ||
| CPC 22338; EMSL 1851 | Indoor air sample | USA: NY | Ž. Jurjević | MF473193 | MF473615 | MF474042 | ||
| CPC 22340; EMSL 1853 | Indoor air sample, 27th floor | USA: NY | Ž. Jurjević | MF473194 | MF473616 | MF474043 | ||
| CPC 22341; EMSL 1854 | Indoor air sample | USA: NY | Ž. Jurjević | MF473195 | MF473617 | MF474044 | ||
| CPC 22351; EMSL 1864 | Indoor air sample, bedroom, 2nd floor | USA: NJ | Ž. Jurjević | MF473196 | MF473618 | MF474045 | ||
| CPC 22356; EMSL 1869 | Indoor air sample, bedroom closet | USA: TN | Ž. Jurjević | MF473197 | MF473619 | MF474046 | ||
| CPC 22362; EMSL 1875 | Indoor air sample, living room | USA: PA | Ž. Jurjević | MF473198 | MF473620 | MF474047 | ||
| CPC 22368; EMSL 1881 | Indoor air sample, office | USA: GA | Ž. Jurjević | MF473199 | MF473621 | MF474048 | ||
| CPC 22369; EMSL 1882 | Sumatra dragonfruit sample | USA: NJ | Ž. Jurjević | MF473200 | MF473622 | MF474049 | ||
| CPC 22382; EMSL 1895 | Indoor air sample, bathroom | USA: TX | Ž. Jurjević | MF473201 | MF473623 | MF474050 | ||
| CPC 22386; EMSL 1901 | Indoor air sample, classroom | USA: RI | Ž. Jurjević | MF473202 | MF473624 | MF474051 | ||
| CPC 22389; EMSL 1904 | Indoor air sample, living room | USA: NJ | Ž. Jurjević | MF473203 | MF473625 | MF474052 | ||
| CPC 22392; EMSL 1907 | Indoor air sample, hospital | USA: AZ | Ž. Jurjević | MF473204 | MF473626 | MF474053 | ||
| CPC 22966; EMSL 2014 | Indoor air sample, office | USA: AZ | Ž. Jurjević | MF473205 | MF473627 | MF474054 | ||
| DTO 079-F4 | Wallpaper from a house | Netherlands | J. Hooiveld | KP701877 | KP701754 | KP702000 | ||
| DTO 150-A7 | Indoor environment | Portugal | — | MF473206 | MF473628 | MF474055 | ||
| DTO 150-C1 | Indoor environment | Portugal | — | KP701943 | KP701820 | KP702065 | ||
| DTO 150-C7 | Indoor environment | Portugal | — | MF473207 | MF473629 | MF474056 | ||
| DTO 150-D1 | Indoor environment | Portugal | — | MF473208 | MF473630 | MF474057 | ||
| DTO 151-A4 | Indoor environment | Portugal | — | MF473209 | MF473631 | MF474058 | ||
| DTO 151-A8 | Indoor environment | Portugal | — | MF473210 | MF473632 | MF474059 | ||
| DTO 151-B7 | Indoor environment | Portugal | — | MF473211 | MF473633 | MF474060 | ||
| DTO 151-D1 | Indoor environment | Portugal | — | KP701946 | KP701823 | KP702068 | ||
| DTO 151-E7 | Indoor environment | Portugal | — | MF473212 | MF473634 | MF474061 | ||
| DTO 151-G7 | Indoor environment | Portugal | — | KP701949 | KP701826 | KP702071 | ||
| DTO 152-A5 | Indoor environment | Portugal | — | MF473213 | MF473635 | MF474062 | ||
| DTO 152-A6 | Indoor environment | Portugal | — | MF473214 | MF473636 | MF474063 | ||
| DTO 152-D6 | Indoor environment | Portugal | — | MF473215 | MF473637 | MF474064 | ||
| DTO 152-H5 | Indoor environment | Portugal | — | MF473216 | MF473638 | MF474065 | ||
| DTO 152-H6 | Indoor environment | Portugal | — | MF473217 | MF473639 | MF474066 | ||
| DTO 152-H7 | Indoor environment | Portugal | — | MF473218 | MF473640 | MF474067 | ||
| DTO 307-F3; 7330009-34-883 | House dust | Canada | Health Canada | MF473219 | MF473641 | MF474068 | ||
| DTO 307-G9; 7050035.81-622 | House dust | Canada | Health Canada | MF473220 | MF473642 | MF474069 | ||
| DTO 308-A2; 7330009.24-784 | House dust | Canada | Health Canada | MF473221 | MF473643 | MF474070 | ||
| DTO 323-D3 | Indoor air | China | — | MF473222 | MF473644 | MF474071 | ||
| C. psychrotolerans | sphaerospermum | CBS 119412*; dH 16390; EXF-391 | Hypersaline water | Slovenia | S. Sonjak | DQ780386 | JN906992 | KJ596632 |
| DTO 305-G3; BH10AU-180 | House dust | Australia: Tasmania | L. Agustini | MF473223 | MF473645 | MF474072 | ||
| DTO 307-H2; TA05NZ-343 | House dust | New Zealand | T. Atkinson | MF473224 | MF473646 | MF474073 | ||
| EXF-332 | Hypersaline water, saltern | Slovenia | — | DQ780385 | KJ596591 | EF101364 | ||
| EXF-714 | Hypersaline water | Dominican Republic | — | DQ780384 | KJ596592 | EF101366 | ||
| C. pulvericola sp. nov. | sphaerospermum | CBS 109788; DAOM 226470 | Indoor air, residence | Canada | — | MF473225 | MF473647 | MF474074 |
| CBS 143362*; DTO 305-H8; TA05NZ-345 | House dust | New Zealand | T.J. Atkinson | MF473226 | MF473648 | MF474075 | ||
| CPC 22403; EMSL 1931 | Indoor air sample, living room | USA: ME | Ž. Jurjević | MF473227 | MF473649 | MF474076 | ||
| DTO 130-D6 | Swab sample, food plant | Netherlands | M. Meijer | MF473228 | MF473650 | MF474077 | ||
| DTO 249-F4 | Indoor environment, wooden window frame | Netherlands | F. Segers | KP701971 | KP701848 | KP702093 | ||
| DTO 255-F7 | Indoor environment, swab sample | Netherlands | G. Piccolo Maitan-Alfenas | KP701979 | KP701856 | KP702101 | ||
| DTO 255-H5 | Indoor environment, swab sample | Netherlands | G. Piccolo Maitan-Alfenas | KP701987 | KP701864 | KP702109 | ||
| DTO 307-E7; BH10AU-183 | House dust | Australia: Tasmania | L. Agustini | MF473229 | MF473651 | MF474078 | ||
| C. puyae | herbarum | CBS 274.80A* | Puya goudotiana | Colombia | W. Gams | KT600418 | KT600516 | KT600614 |
| C. ramotenellum | herbarum | CBS 109031; IBT 13731 | Cheese | Denmark | J. Frisvad | KT600419 | KT600517 | KT600615 |
| CBS 109501; dH 12343 | Man, deep mycosis | Turkey | — | KT600420 | KT600518 | KT600616 | ||
| CBS 121627; CPC 12047; EXF-967 | Air conditioning system, bathroom | Slovenia | M. Butala | EF679385 | EF679463 | EF679539 | ||
| CBS 121628*; CPC 12043; EXF-454 | Hypersaline water | Slovenia | P. Zalar | EF679384 | EF679462 | EF679538 | ||
| CBS 139577; DTO 089-C1 | Air sample, kitchen | Netherlands | M. Meijer | KP701892 | KP701769 | KP702015 | ||
| CBS 118.24; ATCC 36972; MUCL 10098 | Paeonia sp. | Italy | — | KT600421 | KT600519 | KT600617 | ||
| CBS 133.29; ATCC 36970 | Populus tremuloides, leaf spot | — | — | KT600422 | KT600520 | KT600618 | ||
| CBS 169.54; CBS 170.54; IMI 025324; NCTC 6740 | Arundo sp., leaf | UK: England | — | AJ300335 | MF473652 | MF474079 | ||
| CBS 170.54; CBS 169.54; IMI 025324; NCTC 6740 | Arundo sp., leaf | UK: England | — | AY213640 | FJ936162 | EF101352 | ||
| CBS 261.80 | Margarine | Spain | — | KT600423 | KT600522 | KT600620 | ||
| CPC 11395 | Dioscorea tenuipes | South Korea | H.D. Shin | KT600424 | KT600523 | KT600621 | ||
| CPC 11401 | Weigela subsessilis | South Korea | H.D. Shin | KT600425 | KT600524 | KT600622 | ||
| CPC 11826 | Chasmothecia of Phyllactinia guttata on leaves of Corylus sp. | USA: WA | D. Glawe | KT600426 | KT600525 | KT600623 | ||
| CPC 11832 | Chasmothecia of Phyllactinia guttata on leaves of Corylus sp. | USA: WA | D. Glawe | KT600427 | KT600526 | KT600624 | ||
| CPC 12126; Hill 1192 | Yucca elephantipes, leaf spot | New Zealand | C.F. Hill | KT600428 | KT600527 | KT600625 | ||
| CPC 12313 | Rosa sp. | Germany | N. Ale-Agha | KT600429 | KT600528 | KT600626 | ||
| CPC 12385 | Eucalyptus sp. | Australia | P.W. Crous | KT600430 | KT600529 | KT600627 | ||
| CPC 13407 | Ginkgo biloba | Portugal | P.W. Crous | KT600431 | KT600530 | KT600628 | ||
| CPC 13789 | Protea sp. | Spain: Tenerife | P.W. Crous | KT600432 | KT600531 | KT600629 | ||
| CPC 13792 | Unknown plant | Spain: Tenerife | P.W. Crous | KT600433 | KT600532 | KT600630 | ||
| CPC 13795 | Leucospermum sp. | Spain: Tenerife | P.W. Crous | KT600434 | KT600533 | KT600631 | ||
| CPC 13798 | Leucadendron sp. | Spain: Tenerife | P.W. Crous | KT600435 | KT600534 | KT600632 | ||
| CPC 13801 | Leucadendron sp. | Spain: Tenerife | P.W. Crous | KT600436 | KT600535 | KT600633 | ||
| CPC 13943 | Quercus infectoria | Cyprus | A. van Iperen | KT600437 | KT600536 | KT600634 | ||
| CPC 14300; BA 1699 | Indoor building material | Denmark | B. Andersen | KT600438 | KT600537 | KT600635 | ||
| CPC 14306; BA1705 | Food, garfish gill | Denmark | B. Andersen | KT600439 | KT600538 | KT600636 | ||
| CPC 18224 | Phaenocoma prolifera, leaf bracts | South Africa | K.L. Crous & P.W. Crous | JF499839 | JF499877 | JF499883 | ||
| CPC 19119; BA 2033 | Indoor air | Denmark | B. Andersen | MF473230 | MF473653 | MF474080 | ||
| CPC 22370; EMSL 1883 | Indoor air sample, hallway | USA: CA | Ž. Jurjević | MF473231 | MF473654 | MF474081 | ||
| DTO 084-F5 | Indoor environment | Germany | LGA | MF473232 | MF473655 | MF474082 | ||
| DTO 097-H3 | Swab sample, indoor environment | Netherlands | G.J. Dolphyn | MF473233 | MF473656 | MF474083 | ||
| DTO 109-F4; BA 1919 | Indoor environment | Denmark | B. Andersen | KP701917 | KP701794 | KP702040 | ||
| DTO 150-F5 | Indoor environment | Portugal | — | MF473234 | MF473657 | MF474084 | ||
| DTO 151-G3 | Indoor environment | Portugal | — | KP701947 | KP701824 | KP702069 | ||
| DTO 151-G6 | Indoor environment | Portugal | — | KP701948 | KP701825 | KP702070 | ||
| DTO 152-B3 | Indoor environment | Portugal | — | MF473235 | MF473658 | MF474085 | ||
| DTO 152-D9 | Indoor environment | Portugal | — | KP701950 | KP701827 | KP702072 | ||
| DTO 305-H1; TA10NZ-295 | House dust | New Zealand | T. Atkinson | MF473236 | MF473659 | MF474086 | ||
| DTO 305-I1; TA10NZ-240 | House dust | New Zealand | T. Atkinson | MF473237 | MF473660 | MF474087 | ||
| DTO 306-A3; TA10NZ-322 | House dust | New Zealand | T. Atkinson | MF473238 | MF473661 | MF474088 | ||
| DTO 306-B2; TA10NZ-324 | House dust | New Zealand | T. Atkinson | MF473239 | MF473662 | MF474089 | ||
| DTO 306-C4; KJ09SA-88 | House dust | South Africa | K. Jacobs | MF473240 | MF473663 | MF474090 | ||
| DTO 306-D1; TA10NZ-215B | House dust | New Zealand | T. Atkinson | MF473241 | MF473664 | MF474091 | ||
| DTO 306-D2; TA10NZ-289A | House dust | New Zealand | T. Atkinson | MF473242 | MF473665 | MF474092 | ||
| DTO 306-E7; TA10NZ-232 | House dust | New Zealand | T. Atkinson | MF473243 | MF473666 | MF474093 | ||
| DTO 306-F5; TA10NZ-308 | House dust | New Zealand | T. Atkinson | MF473244 | MF473667 | MF474094 | ||
| DTO 307-F2; TA10NZ-297A | House dust | New Zealand | T. Atkinson | MF473245 | MF473668 | MF474095 | ||
| DTO 307-I2; TA10NZ-286 | House dust | New Zealand | T. Atkinson | MF473246 | MF473669 | MF474096 | ||
| DTO 323-B7 | Indoor air | China | — | MF473247 | MF473670 | MF474097 | ||
| DTO 323-D4 | Indoor air | China | — | MF473248 | MF473671 | MF474098 | ||
| DTO 323-D5 | Indoor air | China | — | MF473249 | MF473672 | MF474099 | ||
| DTO 323-D6 | Indoor air | China | — | MF473250 | MF473673 | MF474100 | ||
| C. rectoides | cladosporioides | CBS 125994*; CPC 11624 | Vitis flexuosa | South Korea | H.D. Shin | HM148193 | HM148438 | HM148683 |
| CBS 126357; CPC 11405 | Plectranthus sp. | South Korea | H.D. Shin | HM148194 | HM148439 | HM148684 | ||
| C. rhusicola | herbarum | CBS 140492*; CPC 15219 | Rhus sp. | South Africa | F. Roets | KT600440 | KT600539 | KT600637 |
| C. ruguloflabelliforme | sphaerospermum | CBS 140494*; CPC 19707 | Diatrapaceae sp. on Aloe sp. | South Africa | P.W. Crous | KT600458 | KT600557 | KT600655 |
| C. rugulovarians | cladosporioides | CBS 140495*; CPC 18444 | Unidentified Poaceae, leaf sheaths | Brazil | P.W. Crous | KT600459 | KT600558 | KT600656 |
| C. salinae | sphaerospermum | CBS 102047; MZKI B-1069 | Hypersaline water, crystalisation pond | Slovenia | S. Sonjak | MF473251 | MF473674 | MF474101 |
| CBS 119413*; dH 16389; EXF-335 | Hypersaline water, saltern | Slovenia | S. Sonjak | DQ780374 | JN906993 | EF101390 | ||
| C. scabrellum | cladosporioides | CBS 126358*; CPC 14976; HJS 1031 | Ruscus hypoglossum | Slovenia | H.J. Schroers | HM148195 | HM148440 | HM148685 |
| C. silenes | cladosporioides | CBS 109082* | Silene maritima | UK: Wales | A. Aptroot | EF679354 | EF679429 | EF679506 |
| C. sinense sp. nov. | herbarum | CBS 143363*; DTO 324-D2 | Indoor air | China | — | MF473252 | MF473675 | MF474102 |
| C. sinuatum | cladosporioides | CGMCC 3.18096* | Soil | China | T. Liu | KX938385 | KX938402 | KX938368 |
| CGMCC 3.18097 | Soil | China | T. Liu | KX938386 | KX938403 | KX938369 | ||
| CGMCC 3.18098 | Soil | China | T. Liu | KX938387 | KX938404 | KX938370 | ||
| C. sinuosum | herbarum | ATCC 11285; CBS 164.48 | Musci | France | — | KT600441 | KT600540 | KT600638 |
| CBS 121629*; CPC 11839; Hill 1134A; ICMP 15819 | Fuchsia excorticata | New Zealand | A. Blouin | EF679386 | EF679464 | EF679540 | ||
| CBS 393.68 | Air | Netherlands | — | KT600442 | KT600541 | KT600639 | ||
| CPC 14000; MRC 02998 | Triticum aestivum | South Africa | — | KT600443 | KT600542 | KT600640 | ||
| CPC 15454 | Crocus sativus | New Zealand | J. Rennie | KT600444 | KT600543 | KT600641 | ||
| CPC 17632 | Eryngium maritimum | Germany | U. Damm | KT600445 | KT600544 | KT600642 | ||
| CPC 18365 | Iris pseudacorus | Netherlands | P.W. Crous | KT600446 | KT600545 | KT600643 | ||
| DTO 109-I2; BA 1896 | Indoor environment | Denmark | B. Andersen | KP701919 | KP701796 | KP702042 | ||
| C. sloanii sp. nov. | sphaerospermum | CBS 143364*; DTO 130-D5 | Swab sample, food plant | Netherlands | M. Meyer | MF473253 | MF473676 | MF474103 |
| C. soldanellae | herbarum | CBS 132186*; CPC 13153 | Soldanella alpina | Germany | K. Bensch | JN906982 | JN906994 | JN907001 |
| C. sp. | herbarum | CPC 12485 | Pinus ponderosa | Argentina | A. Greslebin | EF679395 | EF679473 | EF679549 |
| C. sphaerospermum | sphaerospermum | CBS 102045; EXF-2524; MZKI B-1066 | Hypersaline water | Spain | P. Zalar | DQ780351 | EU570262 | EF101378 |
| CBS 117728; ATCC 38493; CPC 12098; NRRL 8131 | Wood | USA | — | AF393709 | EU570268 | EU570275 | ||
| CBS 139576; DTO 084-F4 | Indoor environment | Germany | — | KP701884 | KP701761 | KP702007 | ||
| CBS 139584; DTO 150-H8 | Indoor environment | Portugal | — | KP701944 | KP701821 | KP702066 | ||
| CBS 109.14; ATCC 36950; MUCL 10093 | Carya illinoensis, leaf scale | USA | — | DQ780350 | EU570260 | EF101384 | ||
| CBS 193.54*; ATCC 11289; IMI 49637 | Man, nails | Netherlands | G.A. de Vries | DQ780343 | EU570261 | EU570269 | ||
| CPC 11822 | Phyllactinia guttata on Corylus avellana | USA | D. Glawe | EU570254 | EU570263 | EU570270 | ||
| CPC 12476 | Ambrosia artemisiifolia | Germany | J. Nitzsche | EU570255 | EU570264 | EU570271 | ||
| CPC 13368 | Phaseolus lunatus | Germany | N. Ale-Agha | EU570256 | EU570265 | EU570272 | ||
| CPC 13995 | Thatch | South Africa | G. Marais | EU570257 | EU570266 | EU570273 | ||
| CPC 14016; MRC 10263 | Triticum aestivum | South Africa | — | EU570258 | EU570267 | EU570274 | ||
| CPC 22270; EMSL 1728 | Indoor air sample | USA: MN | Ž. Jurjević | MF473254 | MF473677 | MF474104 | ||
| CPC 22301; EMSL 1789 | Indoor air sample, bathroom | USA: CA | Ž. Jurjević | MF473255 | MF473678 | MF474105 | ||
| CPC 22302; EMSL 1790 | Indoor air sample, bathroom | USA: CA | Ž. Jurjević | MF473256 | MF473679 | MF474106 | ||
| CPC 22317; EMSL 1820 | Indoor air sample | USA: MS | Ž. Jurjević | MF473257 | MF473680 | MF474107 | ||
| CPC 22339; EMSL 1852 | Indoor air sample, warehouse | USA: NY | Ž. Jurjević | MF473258 | MF473681 | MF474108 | ||
| CPC 22357; EMSL 1870 | Indoor air sample | UK: England | Ž. Jurjević | MF473259 | MF473682 | MF474109 | ||
| CPC 22361; EMSL 1874 | Indoor air sample, bedroom | USA: VT | Ž. Jurjević | MF473260 | MF473683 | MF474110 | ||
| CPC 22379; EMSL 1892 | Indoor air sample, family room | USA: CA | Ž. Jurjević | MF473261 | MF473684 | MF474111 | ||
| DTO 017-C7 | Swab sample, bathroom | Netherlands | J. Houbraken | KP701867 | KP701744 | KP701990 | ||
| DTO 049-H5 | Indoor environment | Netherlands | J. Houbraken & M. Meijer | KP701870 | KP701747 | KP701993 | ||
| DTO 086-E7 | Air filter | Netherlands | I.J. Vlug | KP701889 | KP701766 | KP702012 | ||
| DTO 086-E8 | Air filter | Netherlands | I.J. Vlug | KP701890 | KP701767 | KP702013 | ||
| DTO 089-E9 | Indoor air, living room | Netherlands | J. Houbraken | KP701893 | KP701770 | KP702016 | ||
| DTO 090-A1 | Indoor air sample, kitchen | Netherlands | J. Houbraken | KP701897 | KP701774 | KP702020 | ||
| DTO 090-H9 | Swab sample, archive | Netherlands | M. Meijer | MF473262 | MF473685 | MF474112 | ||
| DTO 090-I1 | Swab sample, archive | Netherlands | M. Meijer | KP701902 | KP701779 | KP702025 | ||
| DTO 106-D4 | Indoor air, butterfly area of zoo | Netherlands | B. Dictus | KP701907 | KP701784 | KP702030 | ||
| DTO 117-G5; HM1 RS3 | Indoor environment of house | Netherlands | M. Meijer & O. Terhoeven | KP701927 | KP701804 | KP702050 | ||
| DTO 117-H2; HM2 RS4 | Indoor environment of house | Netherlands | M. Meijer & O. Terhoeven | KP701928 | KP701805 | KP702051 | ||
| DTO 127-E5; AR385 | Air sample, bakery | USA: WI | — | MF473263 | MF473686 | MF474113 | ||
| DTO 150-I3 | Indoor environment | Portugal | — | MF473264 | MF473687 | MF474114 | ||
| DTO 150-I8 | Indoor environment | Portugal | — | KP701945 | KP701822 | KP702067 | ||
| DTO 153-B7 | Indoor air sample, bathroom | Netherlands | F. Hagen | KP701951 | KP701828 | KP702073 | ||
| DTO 153-C1 | Indoor air sample, bathroom | Netherlands | F. Hagen | MF473265 | MF473688 | MF474115 | ||
| DTO 160-I4 | Black spots in bathroom | Netherlands | J. Najafzadeh | KP701954 | KP701831 | KP702076 | ||
| DTO 161-D4 | Swab sample, wall in apartment | Netherlands | J. Houbraken | KP701956 | KP701833 | KP702078 | ||
| DTO 161-D7 | Swab sample, apartment | Netherlands | J. Houbraken | KP701959 | KP701836 | KP702081 | ||
| DTO 161-D8 | Swab sample, wall near window in apartment | Netherlands | J. Houbraken | KP701960 | KP701837 | KP702082 | ||
| DTO 161-D9 | Swab sample, wall near window in apartment | Netherlands | J. Houbraken | KP701961 | KP701838 | KP702083 | ||
| DTO 161-E1 | Swab sample, wall near window in apartment | Netherlands | J. Houbraken | MF473266 | MF473689 | MF474116 | ||
| DTO 194-A4 | Indoor environment, hospital | Netherlands | V. Zaat | KP701965 | KP701842 | KP702087 | ||
| DTO 244-C6 | HA-coated hay pin | Germany | R. Raltenbacher | KP701970 | KP701847 | KP702092 | ||
| DTO 305-F5; KJ03SA-383B | House dust, small apartment | South Africa | K. Jacobs | MF473267 | MF473690 | MF474117 | ||
| DTO 306-D8; AA03US-373 | House dust, basement HVAC room | USA: CA | A. Amend | MF473268 | MF473691 | MF474118 | ||
| DTO 306-E3; AA03US-478 | House dust, basement HVAC room | USA: CA | A. Amend | MF473269 | MF473692 | MF474119 | ||
| DTO 307-G6; KJ08SA-151 | House dust | South Africa | K. Jacobs | MF473270 | MF473693 | MF474120 | ||
| DTO 307-H1; BH02AU-119 | House dust | Australia: Tasmania | B. Horton | MF473271 | MF473694 | MF474121 | ||
| DTO 307-I3; AA03US-549 | House dust, basement HVAC room | USA: CA | A. Amend | MF473272 | MF473695 | MF474122 | ||
| EXF-1061 | Hypersaline water, Dead Sea | Israel | — | DQ780346 | — | EF101379 | ||
| EXF-455 | Hypersaline water, saltern | Slovenia | — | DQ780349 | KJ596600 | EF101375 | ||
| EXF-458 | Hypersaline water, saltern | Slovenia | — | DQ780345 | — | EF101374 | ||
| EXF-738 | Bathroom | Slovenia | — | DQ780348 | — | EF101383 | ||
| EXF-739 | Bathroom | Slovenia | — | DQ780344 | KJ596601 | EF101381 | ||
| EXF-962 | Bathroom | Slovenia | — | DQ780347 | — | EF101382 | ||
| C. spinulosum | herbarum | CBS 119907*; EXF-334; MZKI B-1067 | Hypersaline water | Slovenia | S. Sonjak | EF679388 | EF679466 | EF679542 |
| EXF-382 | Hypersaline water, saltern | Slovenia | — | DQ780407 | — | EF101356 | ||
| C. subcinereum | herbarum | CBS 140465*; FMR 13370; UTHSC DI-13-257 | Man, sputum | USA: MT | D.A. Sutton | LN834433 | LN834529 | LN834617 |
| C. subinflatum | herbarum | CBS 121630*; CPC 12041; EXF-343 | Hypersaline water, saltern | Slovenia | S. Sonjak | EF679389 | EF679467 | EF679543 |
| CPC 22303; EMSL 1791 | Indoor air sample | USA: MN | Ž. Jurjević | MF473273 | MF473696 | MF474123 | ||
| CPC 22400; EMSL 1928 | Indoor air sample, bathroom | USA: MO | Ž. Jurjević | MF473274 | MF473697 | MF474124 | ||
| C. subtilissimum | herbarum | CBS 113753 | Bing cherry fruits | USA | F.M. Dugan lab | EF679396 | EF679474 | EF679550 |
| CBS 113754* | Grape berry | USA | F.M. Dugan lab | EF679397 | EF679475 | EF679551 | ||
| CPC 12044; EXF-462 | Hypersaline water, saltern (reserve pond) | Slovenia | P. Zalar | EF679398 | EF679476 | EF679552 | ||
| C. subuliforme | cladosporioides | CBS 126500*; CPC 13735; FIH 401 | Chamaedorea metallica | Thailand | I. Hidayat & J. Meeboon | HM148196 | HM148441 | HM148686 |
| DTO 130-H8 | Indoor air, open Petri-dish | Thailand | P. Noonim | KP701938 | KP701815 | KP702060 | ||
| DTO 323-D1 | Indoor air | China | — | MF473275 | MF473698 | MF474125 | ||
| DTO 324-B8 | Indoor air | China | — | MF473276 | MF473699 | MF474126 | ||
| DTO 324-C7 | Indoor air | China | — | MF473277 | MF473700 | MF474127 | ||
| C. succulentum | sphaerospermum | CBS 140466*; FMR 13375; UTHSC DI-13-262 | Dolphin, bronchus | USA: FL | D.A. Sutton | LN834434 | LN834530 | LN834618 |
| C. tenellum | herbarum | CBS 121633; CPC 12051; EXF-1083 | Hypersaline water, saltern | Israel | N. Gunde-Cimerman | EF679400 | EF679478 | EF679554 |
| CBS 121634*; CPC 12053; EXF-1735 | Hypersaline water, Dead Sea | Israel | P. Zalar | EF679401 | EF679479 | EF679555 | ||
| CBS 139582; DTO 127-D7; AR295 | Air sample, bakery | USA | — | KP701932 | KP701809 | KP702054 | ||
| CPC 11813 | Phyllactinia sp. on leaves of Corylus sp. | USA: WA | D. Glawe | EF679399 | EF679477 | EF679553 | ||
| CPC 22290; EMSL 1771 | Indoor air sample, bathroom | USA: MI | Ž. Jurjević | MF473278 | MF473701 | MF474128 | ||
| CPC 22291; EMSL 1772 | Indoor air sample, bedroom | USA: OR | Ž. Jurjević | MF473279 | MF473702 | MF474129 | ||
| CPC 22410; EMSL 1941 | Indoor air sample, classroom | USA: MI | Ž. Jurjević | MF473280 | MF473703 | MF474130 | ||
| C. tenuissimum | cladosporioides | CBS 125995*; CPC 14253 | Lagerstroemia sp. | USA: LA | P.W. Crous | HM148197 | HM148442 | HM148687 |
| CBS 126359; CPC 12794 | Musa sp. | USA: HI | I. Budenhagen | HM148198 | HM148443 | HM148688 | ||
| CBS 126501; CPC 14410 | Musa sp. | Ivory Coast | K. Daouda | HM148199 | HM148444 | HM148689 | ||
| CBS 117.79 | Fruit | Burundi | J. Rammelo | HM148200 | HM148445 | HM148690 | ||
| CBS 262.80 | Fruit | Nigeria | — | HM148201 | HM148446 | HM148691 | ||
| CPC 10538 | Musa sp. | Mozambique | A. Viljoen | HM148202 | HM148447 | HM148692 | ||
| CPC 10882 | Gnaphalium affine | South Korea | H.D. Shin | HM148204 | HM148449 | HM148694 | ||
| CPC 11521 | Acacia mangium | Thailand | W. Himaman | HM148214 | HM148459 | HM148704 | ||
| CPC 11612 | Musa sp. | Indonesia | M. Arzanlou | HM148206 | HM148451 | HM148696 | ||
| CPC 11929 | Acacia mangium | Thailand | W. Himaman | HM148215 | HM148460 | HM148705 | ||
| CPC 12223 | Unidentified rust fungus | Brazil | U. Braun | HM148208 | HM148453 | HM148698 | ||
| CPC 12795 | Musa sp. | Polynesia | I. Budenhagen | HM148209 | HM148454 | HM148699 | ||
| CPC 13252 | Rock | Australia | P.W. Crous | HM148216 | HM148461 | HM148706 | ||
| CPC 13732 | Shorea siamensis | Laos | P. Phengsintham | HM148217 | HM148462 | HM148707 | ||
| CPC 14196 | Basella alba (=B. rubra), leaves | Laos | P. Phengsintham | HM148218 | HM148463 | HM148708 | ||
| CPC 14311; BA 1710 | Decayed branch under water | Venezuela | K. Lyhne | HM148219 | HM148464 | HM148709 | ||
| CPC 14370; BA 1737 | Soil, bat cave | Bali | J.C. Frisvad | HM148221 | HM148466 | HM148711 | ||
| CPC 22277; EMSL 1748 | Chili papper sample | Mexico | Ž. Jurjević | MF473281 | MF473704 | MF474131 | ||
| CPC 22320; EMSL 1823 | Indoor air sample | Bermuda | Ž. Jurjević | MF473282 | MF473705 | MF474132 | ||
| CPC 22344; EMSL 1857 | Indoor air sample, bedroom | USA: AZ | Ž. Jurjević | MF473283 | MF473706 | MF474133 | ||
| CPC 22383; EMSL 1896 | Indoor air sample, bathroom | USA: TX | Ž. Jurjević | MF473284 | MF473707 | MF474134 | ||
| CPC 22398; EMSL 1926 | Indoor air sample, classroom | USA: TX | Ž. Jurjević | MF473285 | MF473708 | MF474135 | ||
| DTO 109-A1 | Bathroom ceiling | Thailand | P. Noonim | KP701910 | KP701787 | KP702033 | ||
| DTO 109-C4 | Mycolab door | Thailand | P. Noonim | MF473286 | MF473709 | MF474136 | ||
| DTO 109-C7 | Indoor air, open Petri-dish | Thailand | P. Noonim | MF473287 | MF473710 | MF474137 | ||
| DTO 131-A4 | Indoor air, open Petri-dish | Thailand | P. Noonim | MF473288 | MF473711 | MF474138 | ||
| DTO 323-C5 | Indoor air | China | — | MF473289 | MF473712 | MF474139 | ||
| DTO 323-C9 | Indoor air | China | — | MF473290 | MF473713 | MF474140 | ||
| DTO 323-G2 | Indoor air | China | — | MF473291 | MF473714 | MF474141 | ||
| DTO 323-G3 | Indoor air | China | — | MF473292 | MF473715 | MF474142 | ||
| DTO 323-G4 | Indoor air | China | — | MF473293 | MF473716 | MF474143 | ||
| DTO 323-G8 | Indoor air | China | — | MF473294 | MF473717 | MF474144 | ||
| DTO 323-I4 | Indoor air | China | — | MF473295 | MF473718 | MF474145 | ||
| DTO 323-I6 | Indoor air | China | — | MF473296 | MF473719 | MF474146 | ||
| DTO 323-I8 | Indoor air | China | — | MF473297 | MF473720 | MF474147 | ||
| DTO 323-I9 | Indoor air | China | — | MF473298 | MF473721 | MF474148 | ||
| DTO 324-A1 | Indoor air | China | — | MF473299 | MF473722 | MF474149 | ||
| DTO 324-A3 | Indoor air | China | — | MF473300 | MF473723 | MF474150 | ||
| DTO 324-C2 | Indoor air | China | — | MF473301 | MF473724 | MF474151 | ||
| DTO 324-C3 | Indoor air | China | — | MF473302 | MF473725 | MF474152 | ||
| DTO 324-C5 | Indoor air | China | — | MF473303 | MF473726 | MF474153 | ||
| DTO 324-C6 | Indoor air | China | — | MF473304 | MF473727 | MF474154 | ||
| DTO 324-C9 | Indoor air | China | — | MF473305 | MF473728 | MF474155 | ||
| C. tianshanense | cladosporioides | CGMCC 3.18033* | Saussurea involucrata, rhizosphere soil | China | G. Wang | KX938381 | KX938398 | KX938364 |
| CGMCC 3.18034 | Saussurea involucrata, rhizosphere soil | China | G. Wang | KX938382 | KX938399 | KX938365 | ||
| C. tuberosum | herbarum | CBS 140693*; UTHSC DI-13-217; FMR 13330 | Man, nasal biopsy | USA: FL | D.A. Sutton | LN834417 | LN834513 | LN834601 |
| FMR 13332; UTHSC DI-13-219 | Man, foot | USA: WA | D.A. Sutton | LN834419 | LN834515 | LN834603 | ||
| C. uredinicola | cladosporioides | CPC 5390; ATCC 46649 | Hyperparasite on Cronartium fusiforme f. sp. quercum on Quercus nigra leaves | USA: AL | W.D. Kelley | AY251071 | HM148467 | HM148712 |
| C. uwebraunianum sp. nov. | cladosporioides | CBS 139572; DTO 072-C8 | Indoor air, archive | Netherlands | M. Meijer | KP701873 | KP701750 | KP701996 |
| CBS 143365*; DTO 072-D8 | Indoor air, archive | Netherlands | M. Meijer | MF473306 | MF473729 | MF474156 | ||
| DTO 082-E3 | Indoor air, archive | Netherlands | M. Meijer | KP701878 | KP701755 | KP702001 | ||
| DTO 090-D2 | Swab sample, archive | Netherlands | M. Meijer | KP701899 | KP701776 | KP702022 | ||
| DTO 109-E8; BA 1908 | Indoor environment | Denmark | B. Andersen | KP701914 | KP701791 | KP702037 | ||
| DTO 305-H9; TA10NZ-294A | House dust | New Zealand | T. Atkinson | MF473307 | MF473730 | MF474157 | ||
| C. variabile | herbarum | CBS 121635**; CPC 12753 | Spinacia oleracea | USA: WA | L. du Toit | EF679403 | EF679481 | EF679557 |
| CBS 121636**; CPC 12751 | Spinacia oleracea | USA: WA | L. du Toit | EF679402 | EF679480 | EF679556 | ||
| C. varians | cladosporioides | CBS 126360; CPC 11327 | Ulmus sp. | Germany | K. Schubert | HM148222 | HM148468 | HM148713 |
| CBS 126361; CPC 11134 | Leaf debris | India | W. Gams | HM148223 | HM148469 | HM148714 | ||
| CBS 126362*; CPC 13658; HAL 2061 F | Catalpa bungei | Russia | V.A. Melnik | HM148224 | HM148470 | HM148715 | ||
| C. velox | sphaerospermum | CBS 119417*; CPC 11224 | Bambusa sp. | India | W. Gams | DQ780361 | JN906995 | EF101388 |
| CPC 18450 | Zea mays | Brazil | P.W. Crous | KT600457 | KT600556 | KT600654 | ||
| CPC 22359; EMSL 1872 | Indoor air sample | USA: MA | Ž. Jurjević | MF473308 | MF473731 | MF474158 | ||
| DTO 317-H1 | Indoor air | China | — | MF473309 | MF473732 | MF474159 | ||
| DTO 323-H8 | Indoor air | China | — | MF473310 | MF473733 | MF474160 | ||
| EXF-466 | Hypersaline water, saltern | Slovenia | — | DQ780359 | KJ596597 | EF101386 | ||
| EXF-471 | Hypersaline water, saltern | Slovenia | — | DQ780360 | KJ596599 | EF101387 | ||
| C. verrucocladosporioides | cladosporioides | CBS 126363*; CPC 12300 | Rhus chinensis | South Korea | H.D. Shin | HM148226 | HM148472 | HM148717 |
| C. verruculosum | herbarum | CGMCC 3.18099* | Soil | China | T. Liu | KX938388 | KX938405 | KX938371 |
| CGMCC 3.18100 | Soil | China | T. Liu | KX938389 | KX938406 | KX938372 | ||
| C. versiforme | herbarum | CBS 140491*; CPC 19053 | Hordeum sp. | Iran | P.W. Crous | KT600417 | KT600515 | KT600613 |
| C. vicinum sp. nov. | cladosporioides | CBS 143366*; CPC 22316; EMSL 1819 | Indoor air sample | USA: WI | Ž. Jurjević | MF473311 | MF473734 | MF474161 |
| CBS 306.84 | Urediniospores of Puccinia allii | UK: England | G.S. Taylor | HM148057 | HM148299 | HM148544 | ||
| CPC 11664; Hill 1076-2 | Oncoba spinosa | New Zealand | C.F. Hill | HM148058 | HM148300 | HM148545 | ||
| CPC 13867 | Leptosphaeria sp. | South Africa | P.W. Crous | HM148059 | HM148301 | HM148546 | ||
| CPC 15457 | Imported buds of Prunus avium | New Zealand | J. Rennie | HM148060 | HM148302 | HM148547 | ||
| DTO 305-H5; TA10NZ-280B | House dust | New Zealand | T. Atkinson | MF473312 | MF473735 | MF474162 | ||
| C. vignae | cladosporioides | CBS 121.25; ATCC 200933; MUCL 10110 | Vigna unguiculata (= V. sinensis), living stems | USA: IN | M.W. Gardner | HM148227 | HM148473 | HM148718 |
| C. westerdijkiae sp. nov. | cladosporioides | CBS 113746* | Bing cherry fruits | USA: WA | R.G. Roberts | HM148061 | HM148303 | HM148548 |
| CPC 10150 | Fatoua villosa | South Korea | H.D. Shin | HM148062 | HM148304 | HM148549 | ||
| CPC 13362 | Paeonia obovata | Germany | P.W. Crous | HM148063 | HM148305 | HM148550 | ||
| CPC 13978 | Pinus ponderosa, needles | Argentina | A. Greslebin | HM148064 | HM148306 | HM148551 | ||
| CPC 14284; BA 1674 | Triticum sp., grain | Germany | B. Andersen | HM148065 | HM148307 | HM148552 | ||
| DTO 084-F2 | Indoor environment | Germany | LGA | KP701882 | KP701759 | KP702005 | ||
| DTO 109-F2; BA 1911 | Indoor environment | Denmark | B. Andersen | KP701915 | KP701792 | KP702038 | ||
| DTO 152-A9 | Indoor environment | Portugal | — | MF473313 | MF473736 | MF474163 | ||
| DTO 152-H9 | Indoor environment | Portugal | — | MF473314 | MF473737 | MF474164 | ||
| C. wyomingense sp. nov. | herbarum | CBS 143367*; CPC 22310; EMSL 1806 | Indoor air sample, living room | USA: WY | Ž. Jurjević | MF473315 | MF473738 | MF474165 |
| C. xanthochromaticum | cladosporioides | CBS 126364; CPC 14532 | Erythrophleum chlorostachys | Australia | B.A. Summerell | HM148122 | HM148366 | HM148611 |
| CBS 140691*; UTHSC DI-13-211; FMR 13324 | Man, bronchoalveolar lavage fluid | USA: TX | D.A. Sutton | LN834415 | LN834511 | LN834599 | ||
| CBS 167.54; ATCC 11276; IMI 049624 | — | — | — | HM148124 | HM148368 | HM148613 | ||
| CGMCC 3.18101 | Alpine soil | China | T. Liu | KX938390 | KX938407 | KX938373 | ||
| CGMCC 3.18102 | Alpine soil | China | Y. Hao | KX938391 | KX938408 | KX938374 | ||
| CPC 11046 | Margarine | Australia | N. Charley | HM148125 | HM148369 | HM148614 | ||
| CPC 11133 | Eucalyptus sp. | India | W. Gams | HM148126 | HM148370 | HM148615 | ||
| CPC 11609 | Musa sp. | India | M. Arzanlou | EF679356 | EF679431 | EF679508 | ||
| CPC 11806 | Strelitzia sp. | South Africa | W. Gams | HM148129 | HM148373 | HM148618 | ||
| CPC 11856 | Acacia mangium | Thailand | W. Himaman | HM148134 | HM148378 | HM148623 | ||
| CPC 12792 | Musa sp. | Polynesia | I. Budenhagen | HM148136 | HM148380 | HM148625 | ||
| CPC 12793 | Musa sp. | Polynesia | I. Budenhagen | HM148137 | HM148381 | HM148626 | ||
| CPC 14004; MRC 03367 | Oats | South Africa | — | HM148143 | HM148387 | HM148632 | ||
| CPC 14008; MRC 10135 | Triticum aestivum | South Africa | — | HM148144 | HM148388 | HM148633 | ||
| CPC 14256 | Pecan tree, leaves | USA | P.W. Crous | HM148146 | HM148390 | HM148635 | ||
| CPC 14911 | Strelitzia sp. | South Africa | P.W. Crous | HM148148 | HM148392 | HM148637 | ||
| CPC 22239; EMSL 1686 | Indoor air sample, bedroom | USA: CO | Ž. Jurjević | MF473316 | MF473739 | MF474166 | ||
| CPC 22321; EMSL 1824 | Indoor air sample | Bermuda | Ž. Jurjević | MF473317 | MF473740 | MF474167 | ||
| DTO 108-G8 | Indoor air, open Petri-dish | Thailand | P. Noonim | KP701909 | KP701786 | KP702032 | ||
| DTO 317-I2 | Indoor air | China | — | MF473318 | MF473741 | MF474168 | ||
| DTO 323-E2 | Indoor air | China | — | MF473319 | MF473742 | MF474169 | ||
| DTO 323-E3 | Indoor air | China | — | MF473320 | MF473743 | MF474170 | ||
| DTO 323-E5 | Indoor air | China | — | MF473321 | MF473744 | MF474171 | ||
| DTO 323-E6 | Indoor air | China | — | MF473322 | MF473745 | MF474172 | ||
| DTO 323-E7 | Indoor air | China | — | MF473323 | MF473746 | MF474173 | ||
| C. xylophilum | cladosporioides | CBS 113749 | Bing cherry fruits | USA: WA | — | HM148228 | HM148474 | HM148719 |
| CBS 113756 | Bing cherry fruits | USA: WA | — | HM148229 | HM148475 | HM148720 | ||
| CBS 125997*; CPC 12403 | Picea abies, dead wood | Russia | D.A. Shabunin | HM148230 | HM148476 | HM148721 | ||
ATCC: American Type Culture Collection, Virginia, USA; BA: Personal culture collection of Birgitte Andersen, Denmark; CAMS: SERA’s Centre for Applied Mycological Studies, Forestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria, South Africa; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CGMCC: China General Microbiological Culture Collection Center, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China; CIEFAP: Centro de Investigación y Extensión Forestal Andino Patagónico, Argentina; CPC: Culture collection of Pedro Crous, housed at CBS; DAOM: Plant Research Institute, Department of Agriculture (Mycology), Ottawa, Canada; dH: de Hoog Culture Collection, housed at CBS; DTO: Working collection of Jos Houbraken housed at CBS; EMSL: Working collection of Ž. Jurjević, EMSL Analytical, Inc., Cinnaminson, New Jersey, USA; EXF: Culture Collection of Extremophilic Fungi, Biotechnical Faculty, Ljubljana, Slovenia; FMR: Facultat de Medicina, Universitat Rovira i Virgili, Reus, Spain; Hill: Personal culture collection of Frank Hill, New Zealand; HJS: Personal culture collection of Hans-Josef Schroers, Agricultural institute of Slovenia, Ljubljana, Slovenia; IBT: IBT Culture Collection of Fungi, DTU Bioengineering, Technical University of Denmark, Denmark; ICMP: International Collection of Micro-organisms from Plants, Landcare Research, Private Bag 92170, Auckland, New Zealand; IFO: Institute for Fermentation, Osaka, Japan; IHEM: Collection of the Laboratorium voor Microbiologie en Microbiele Genetica, Gent, Belgium; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, UK; JCM: Japan Collection of Microorganism, RIKEN BioResource Center, Japan; Lynfield: Private culture collection and herbarium of Frank Hill, New Zealand; MRC: Medical Research Council, Cape Town, South Africa; MUCL: Mycotheque de l'Universite catholique de Louvain, Laboratoire de Mycologie Systematique et Appliquee, Universite catholique de Louvain, Louvain-la-Neuve, Belgium; MZKI: Microbiological Culture Collection of the National Institute of Chemistry, Ljubljana, Slovenia; NBRC: NITE Biological Resource Center, Department of Biotechnology, National Institute of Technology and Evaluation, Kisarazu, Chiba, Japan; NCPF: The National Collection of Pathogenic Fungi, Holborn, London, UK; NCTC: National Collection of Type Cultures, PHLS Central Public Health Laboratory, London, UK; NRRL: National Center for Agricultural Utilization Research, Peoria, Illinois, USA; PD: Plant Protection Service, nVWA, Division Plant, Wageningen, The Netherlands; UTHSC: Fungus Testing Laboratory at the University of Texas Health Science Center, San Antonio, TX, USA; VKM: All-Russian Collection of Microorganisms, Russian Academy of Sciences, Institute of Biochemistry and Physiology of Microorganisms, 142292 Pushchino, Moscow Region, Russia.
*: ex-type culture.; **: ex-epitype culture.
Abbreviations for USA according to ISO 3166.
act: partial actin gene, tef1: partial translation elongation factor 1-alpha gene, ITS: internal transcribed spacer region including intervening 5.8S rRNA gene.
DNA isolation, amplification and sequence analysis
Fungal colonies were established on agar plates, and genomic DNA was isolated as described in Groenewald et al. (2013). DNA amplification of the internal transcribed spacer regions and intervening 5.8S rRNA gene (ITS) of the nrDNA cistron, partial actin (act) and translation elongation factor 1-alpha (tef1) genes followed Groenewald et al., 2005, Groenewald et al., 2013. The ITS was not included in the multigene phylogenetic analyses as this locus has limited resolution below genus level.
Novel sequences generated in this study were added to draft alignments representing the C. cladosporioides, C. herbarum and C. sphaerospermum species complexes and containing sequences from several studies (Zalar et al., 2007, Schubert et al., 2007b, Schubert et al., 2009, Bensch et al., 2010, Bensch et al., 2012, Bensch et al., 2015, Segers et al., 2015, Sandoval-Denis et al., 2016, Ma et al., 2017). Based on draft phylogenetic trees, these alignments were subsequently trimmed back to include representatives of previously published sequences and species rather than all available sequences. Preference was also given to the inclusion of sequences from indoor environments where possible.
Phylogenetic analyses consisted of maximum parsimony (MP), maximum likelihood (ML) and Bayesian (BI) analyses of the trimmed combined act/tef1 alignments representing the C. cladosporioides, C. herbarum and C. sphaerospermum species complexes. In addition, a phylogenetic analysis was performed using only the available ITS sequences. The phylogenetic analyses were performed as described by Wang et al. (2016) with the following modifications: for the MP analyses 100 random taxon additions were used and for the BI analyses trees were sampled every 100 generations and the heating parameter was set to 0.15 for the C. cladosporioides and C. herbarum and C. sphaerospermum species complexes. Novel sequences were deposited in NCBI's GenBank nucleotide database (Table 1) and the alignments and trees in TreeBASE (study accession number 21415).
Morphology
Light microscopy (LM): Microscopic observations of isolates were made from colonies cultivated for 7 d under continuous near ultraviolet light at 25 °C on SNA. Preparations were mounted in Shear’s solution (Crous et al. 2009). To study conidial development and branching patterns of conidial chains, squares of transparent adhesive tape (Titan Ultra Clear Tape, Conglom Inc., Toronto, Canada) were placed on conidiophores growing in the zone between the colony margin and 2 cm inwards, and mounted between two drops of Shear’s solution under a glass cover slip. Conidial terminology follows Schubert et al. (2007b). Wherever possible, 50 measurements (×1 000 magnification, differential interference contrast microscopy, Zeiss Axioscope 2 PLUS) were made of conidia with outliers given in parentheses. For culture characteristics colonies were cultivated on PDA, OA and MEA for 14 d at 25 °C in the dark, after which surface and reverse colours were rated using the charts of Rayner (1970). Photographs of characteristic structures were captured with a Zeiss Axio Imager A2 microscope equipped with a Nikon DS-Ri2 high-definition colour camera head using differential interference contrast (DIC) optics and the Nikon software NIS-elements D v. 4.50.
Low-temperature scanning electron microscopy (SEM): Isolates of Cladosporium spp. were grown on SNA with 30 g agar/L for 3–7 d at room temperature under black light. Relevant parts of the small colonies with conidiophores and conidia were selected carefully under a dissecting microscope, excised with a surgical blade as small agar (3 × 3 mm) blocks, and transferred into a copper cup for snap-freezing in nitrogen slush. Agar blocks were glued to the copper surface with frozen tissue medium (KP-Cryoblock, Klinipath, Duiven, The Netherlands). To ensure preservation of the very delicate spatial structure of the conidiophore Scotch tape was placed loosely on the cup. This prevented that the liquid nitrogen damaged the conidiophores. During freezing the tape was disconnected from the cup. Samples were examined in a JEOL 5600LV scanning electron microscope (JEOL, Tokyo, Japan) equipped with an Oxford CT1500 Cryostation for cryo-scanning electron microscopy (cryoSEM). Electron micrographs were acquired from uncoated frozen samples, or after sputter-coating by means of a goldtarget for several (typically 3, but dependent on the density of the gold layer) times during 30 s. Micrographs of uncoated samples were taken at an acceleration voltage of 2,5 kV, and consisted out of 30 averaged fast scans (SCAN 2 mode), and at 5 kV in case of the coated samples (SCAN 4 mode).
Results
DNA phylogeny
Three phylogenetic analyses were performed on each of the combined act/tef1 alignments, representing the C. cladosporioides, C. herbarum and C. sphaerospermum species complexes. Core statistics for the different analyses are shown in Table 2. Additional details on the phylogenetic trees are provided in the species notes where necessary. Overall, the phylogenies presented in Fig. 1, Fig. 2, Fig. 3 are highly similar in terms of the terminal clades irrespective of whether the phylogenetic trees were obtained from the maximum parsimony, Bayesian or maximum-likelihood analyses (data not shown, trees deposited in TreeBASE).
Table 2.
Statistical information of the different multilocus analyses performed in this study. act: partial actin gene; tef1: partial translation elongation factor 1-alpha gene.
| Dataset | Statistics for Bayesian analyses |
||||
|---|---|---|---|---|---|
| Substitution models |
Unique site patterns |
Number of trees sampled | |||
| act | tef1 | act | tef1 | ||
| C. cladosporioides complex | HKY+G | HKY+I+G | 145 | 235 | 963 978 |
| C. herbarum complex | HKY+G | HKY+I+G | 124 | 186 | 286 952 |
|
C. sphaerospermum complex |
HKY+G |
HKY+I+G |
155 |
296 |
137 928 |
| Statistics for the parsimony analyses | |||||
|
Number of strains (incl. outgroup(s)) |
Number of included characters |
Number of parsimony-informative characters |
Number of parsimony-uninformative characters |
Number of constant characters |
|
| C. cladosporioides complex | 412 | 548 | 326 | 43 | 89 |
| C. herbarum complex | 220 | 403 | 253 | 59 | 91 |
| C. sphaerospermum complex | 309 | 505 | 365 | 78 | 62 |
| Tree length | Consistency index (CI) | Retention index (RI) | Rescaled CI (RC) | Number of saved trees | |
| C. cladosporioides complex | 3 053 | 0.294 | 0.894 | 0.263 | 1 000 |
| C. herbarum complex | 1 591 | 0.407 | 0.893 | 0.363 | 1 000 |
|
C. sphaerospermum complex |
1 968 |
0.518 |
0.955 |
0.494 |
1 000 |
| Statistics for the maximum-likelihood analyses | |||||
|
Tree length |
Alpha parameter value |
Invar parameter value |
Final ML Optimisation Likelihood |
||
| C. cladosporioides complex | 14.177192 | 1.200382 | 0.194342 | −12952.10072 | |
| C. herbarum complex | 7.591637 | 1.015297 | 0.163303 | −6775.467992 | |
| C. sphaerospermum complex | 6.896787 | 1.904976 | 0.151042 | −7017.365135 | |
Fig. 1.
The first of 1 000 equally most parsimonious trees obtained from a heuristic search of the C. cladosporioides species complex alignment. Bayesian posterior probabilities (BPP; >0.74), maximum-likelihood bootstrap support values (MLBS; >74 %) and maximum parsimony bootstrap support values (PBS; >74 %)) are shown at the nodes (BPP/MLBS/PBS). Thickened lines with an asterisk (*) represent nodes with PP = 1.00, MLBS = 100 % and PBS = 100 % and a hash (#) represents nodes with PP = >0.94, MLBS = >94 % and PBS = >94 %. The scale bar represents the number of changes. Species names are indicated to the right of the tree and clades/lineages are numbered to facilitate easier reference in the text. Species boundaries are indicated with coloured blocks. Names of novel species and culture numbers with type status are printed in bold face. Species from indoor environments are indicated with a blue star symbol in front of the species name. Isolation source and country of origin information are provided where known. The tree was rooted to Cercospora beticola (strain CBS 116456).
Fig. 2.
Bayesian consensus phylogram (50 % majority rule) of the C. herbarum species complex alignment. Bayesian posterior probabilities (BPP; >0.74), maximum-likelihood bootstrap support values (MLBS; >74 %) and maximum parsimony bootstrap support values (PBS; >74 %)) are shown at the nodes (BPP/MLBS/PBS). Thickened lines with an asterisk (*) represent nodes with PP = 1.00, MLBS = 100 % and PBS = 100 % and a hash (#) represents nodes with PP = >0.94, MLBS = >94 % and PBS = >94 %. The scale bar represents the expected changes per site. Species names are indicated to the right of the tree and clades/lineages are numbered to facilitate easier reference in the text. Species boundaries are indicated with coloured blocks. Names of novel species and culture numbers with type status are printed in bold face. Species from indoor environments are indicated with a blue star symbol in front of the species name. Isolation source and country of origin information are provided where known. The tree was rooted to Cercospora beticola (strain CBS 116456).
Fig. 3.
Bayesian consensus phylogram (50 % majority rule) of the C. sphaerospermum species complex alignment. Bayesian posterior probabilities (BPP; >0.74), maximum-likelihood bootstrap support values (MLBS; >74 %) and maximum parsimony bootstrap support values (PBS; >74 %)) are shown at the nodes (BPP/MLBS/PBS). Thickened lines with an asterisk (*) represent nodes with PP = 1.00, MLBS = 100 % and PBS = 100 % and a hash (#) represents nodes with PP = >0.94, MLBS = >94 % and PBS = >94 %. The scale bar represents the expected changes per site. Species names are indicated to the right of the tree and clades/lineages are numbered to facilitate easier reference in the text. Species boundaries are indicated with coloured blocks. Names of novel species and culture numbers with type status are printed in bold face. Species from indoor environments are indicated with a blue star symbol in front of the species name. Isolation source and country of origin information are provided where known. The tree was rooted to Cercospora beticola (strain CBS 116456).
The C. cladosporioides species complex phylogeny presented in Fig. 1 delimits 66 species clades. The position of clades changes between the different analyses, as can be observed by the low or absent support values on the backbone of the tree. In general, the BI phylogeny contained more polytomies for species clades and therefore the MP phylogeny is presented in Fig. 1. In some cases, differences are also observed for the terminal nodes. For example, in the BI phylogeny, C. cf. tenuissimum (Clades 61) and C. oxysporum (Clade 62) are collapsed to a basal polytomy with clades 63 and 64 (C. colocasiae and C. tenuissimum), whereas C. perangustum (Clade 4) becomes unresolved lineages at the base of the ML tree and C. tianshanense (Clade 1) moves into the ML tree as a sister clade to C. paracladosporioides (Lineage 13). The ML phylogeny also failed to resolve C. pseudocladosporioides (Clade 56) completely and included both C. crousii (Clade 56) and C. funiculosum (Clade 55) as lineages inbetween isolates of C. pseudocladosporioides. Cladosporium crousii was included within the C. pseudocladosporioides clade (Clade 56) in all three phylogenetic analyses, but always on a longer branch; the act sequence of this species is identical to sequences of C. pseudocladosporioides while the tef1 is clearly distinct from all known sequences.
The C. herbarum species complex phylogeny presented in Fig. 2 delimits 37 species clades. The position of clades changes between the different analyses, as can be observed by the low or absent support values on the backbone of the tree. For example, the position of C. arthropodii (lineage 32) is basal in the MP phylogeny, but identical to the position in Fig. 2 in the ML phylogeny. In some cases, differences are also observed for the terminal nodes. In the MP and ML phylogenies, C. tuberosum (Clade 3) clusters inbetween the two subclades of C. floccosum (Clade 4). Cladosporium basiinflatum was included within the C. ramotenellum clade (Clade 37) in all three analyses, but always on a long branch.
The C. sphaerospermum species complex phylogeny presented in Fig. 3 delimits 23 species clades. The position of clades changes between the different analyses, as can be observed by the low or absent support values on the backbone of the tree. In a few cases, differences are also observed for the terminal nodes. For example, the position of C. lebrasiae (lineage 5) is sister to C. dominicanum (Clade 4) in the MP phylogeny, but not well-resolved in the ML and BI phylogenies.
Maximum parsimony and Bayesian ITS phylogenies were also generated from sequences representing all Cladosporium species currently known from ITS sequence data (Supplementary Fig. S1). For the maximum parsimony analysis, 507 characters were included, 88 of which were parsimony-informative, 318 which were constant and 101 which were variable and parsimony-uninformative. The maximum of 1 000 equally most parsimonious trees were saved (Tree length = 429; CI = 0.681; RI = 0.845; RC = 0.575). The Bayesian analysis lasted 19 980 000 generations and yielded 299 702 trees which were used to calculate the best tree and the posterior probability values after discarding the burn-in trees; a SYM+I+G model was used and there were 150 unique site patterns in the alignment. These phylogenies show that ITS lacks the resolution to distinguish many species of Cladosporium, especially in the C. cladosporioides and C. herbarum species complexes. Although the three species complexes can be recognised in broad lines in the phylogenetic tree, there are some overlap among the species complexes. For example, C. ruguloflabelliforme is found inbetween sequences of the C. cladosporioides species complex while it belongs to the C. sphaerospermum species complex and C. basiinflatum is found inbetween sequences of the C. cladosporioides species complex while it belongs to the C. herbarum species complex. Assignment of an unknown isolate to a species complex should therefore be done based on high association to several species from the species complex and not based on a high association with only one species from the species complex. Overall, the topology of the resulting trees was poorly supported, both in the Bayesian and maximum parsimony analyses.
Taxonomy
The status of numerous indeterminate strains isolated from indoor environments included in this study have been subjected to polyphasic analyses, which revealed 16 novel species. The circumscriptions and delimitations of these species are mainly based on quantitative as well as qualitative morphological features and on molecular data. Features that proved to be diagnostic at species rank were discussed in Bensch et al., 2012, Bensch et al., 2015 and are also applied here. Together with previously described species which proved to occur in indoor environments, the new taxa are treated in alphabetical order below. Detailed descriptions (on SNA if not indicated differently), supplementing literature (listed under Lit.), illustrations (listed under Ill.) and comments are provided.
Cladosporium aerium Bensch & Samson, sp. nov. MycoBank MB822217. Fig. 4.
Fig. 4.
Cladosporium aerium (CBS 143356). A–C. Colonies on PDA, MEA and OA. D–I. Conidiophores and conidia. J. Microcyclic conidiogenesis with a secondary ramoconidium forming a conidiophore with a conidium attached. K–L. Conidial chains. Scale bars = 10 μm.
Etymology: Name refers to the substrate from which it was isolated, indoor air.
Holotype: China, isol. from indoor air, CBS H-23248. Ex-type culture: CBS 143356 = DTO 323-B4.
Diagnosis: Differs from C. allii in having narrower conidiophores as well as shorter and narrower, 0–2-septate conidia.
In vitro (on PDA): Mycelium abundantly formed, hyphae narrowly cylindrical-oblong or irregular in outline due to swellings, lateral outgrowth and constrictions, loosely branched, (1–)1.5–5 μm wide, septate, not constricted at septa, subhyaline, pale brown or pale olivaceous brown, almost smooth, asperulate to irregularly verruculose or verrucose, walls unthickened, occasionally anastomosing. Conidiophores macronematous, solitary, formed laterally or terminally from hyphae, straight or often somewhat flexuous, cylindrical-oblong or irregular in outline due to swellings and constrictions, often subnodulose or with unilateral swellings both terminally and intercalary, sometimes once slightly to distinctly geniculate-sinuous, rarely once branched, 17–190(–210) × (3–)4–5 μm, swellings 5–6.5(–8) μm diam, 0–4-septate, not constricted at septa, pale to medium olivaceous brown, smooth or almost so, walls slightly thickened; sometimes a few micro- and semimacronematous conidiophores formed. Conidiogenous cells integrated, mostly terminal, occasionally also intercalary, often quite long, 20–78 μm, cylindrical, subnodulose, with a single or rarely two unilateral swellings and occasionally an additional swollen shoulder at a lower level with 1–3(–4) conspicuous loci restricted to these swellings or shoulders, sometimes once geniculate-sinuous, with up to five loci per cell, loci protuberant, 1.5–2 μm diam, thickened and darkened-refractive. Ramoconidia absent. Conidia solitary or formed in short, unbranched or branched chains, chains with only up to five conidia, solitary, terminal and intercalary conidia ellipsoid, broadly ovoid or subcylindrical, (8–)9.5–17(–19) × (4.5–)5–6.5(–7) μm (av. ± SD: 12.5 ± 2.8 × 5.7 ± 0.9), 0(–1)-septate, hila 1–2 μm diam, basally formed conidia ellipsoid or subcylindrical, 13–24 × (5–)6–7(–8) μm (av. ± SD: 18.0 ± 3.1 × 6.4 ± 0.7), 0–1-septate, septum median or in the upper half, becoming curved or sinuous with age, occasionally slightly constricted, pale olivaceous to medium olivaceous brown, verruculose to distinctly verrucose, verrucae up to 1 μm high, densely aggregated, walls unthickened or slightly thick-walled, slightly or distinctly attenuated towards apex and base, with 1–2(–3) distal hila, hila 1–2 μm diam, thickened and darkened-refractive. Microcyclic conidiogenesis giving rise to secondary conidiophores occasionally occurring.
Culture characteristics: Colonies on PDA attaining 29–44 mm diam after 14 d at 25 °C, smoke-grey and olivaceous due to abundant and dense aerial mycelium, olivaceous grey and grey olivaceous towards margins, reverse leaden-grey, fluffy, margins narrow, white, somewhat feathery, regular or slightly undulate, growth flat, sporulation loose, mainly at colony margins. Colonies on MEA reaching 30–49 mm diam after 14 d at 25 °C, smoke-grey due to abundant aerial mycelium, whitish or glaucous-grey towards margins, reverse olivaceous grey, velvety or fluffy, margins narrow, white, regular to undulate, growth flat to low convex, often radially furrowed, several small exudates formed, sporulation mainly at colony margins. Colonies on OA 21–42 mm diam after 14 d at 25 °C, smoke-grey, pale olivaceous grey with patches of iron-grey, reverse olivaceous to iron-grey, fluffy-felty, margins somewhat undulate, aerial mycelium abundant, dense, fluffy, covering almost the entire colony, growth flat, numerous very small exudates formed giving the surface a glittering appearance, sporulation at colony margins.
Substrate and distribution: Indoor air, Asia (China).
Additional materials examined: China, isol. from indoor air, DTO 323-G6; DTO 323-G7.
Notes: The description given above is from PDA; on SNA only very few conidiophores and conidia were formed after 7 d. Cladosporium aerium (Fig. 1, clade 20) is morphologically similar to C. phlei (Fig. 1, clade 12) and C. sinuosum (Fig. 1, clade 2); all three species have distinctly geniculate, subnodulose conidiophores and distinctly ornamented conidia. However, C. phlei forms ramoconidia and has longer and wider conidia and C. sinuosum possesses much longer conidiophores with swellings reaching up to 10 μm diam and shorter but wider conidia (Bensch et al., 2012, Bensch et al., 2015). Cladosporium allii (Fig. 1, clade 19) which is the closest phylogenetic relative of C. aerium, differs in having wider conidiophores as well as longer and wider, 0–2(–4)-septate conidia (Bensch et al. 2012).
Cladosporium allicinum (Fr. : Fr.) Bensch et al., Stud. Mycol. 72: 50. 2012. MycoBank MB800304. Fig. 5.
Fig. 5.
Cladosporium allicinum (DTO 109-E5). A–C. Colonies on PDA, MEA and OA. D–G. Macronematous conidiophores with conidial chains. H–I. Micronematous conidiophores. J. Conidia. Scale bars = 10 μm.
Holotype: Sweden, Skåne, on tip blight of living leaves of Allium sp. (Amaryllidaceae), Fr. no. F-09810, UPS-FRIES. Neotype of Cladosporium bruhnei (designated in Schubert et al. 2007b): Belgium, Kampenhout, isol. from Hordeum vulgare (Poaceae), 26 Jun. 2005, J.Z. Groenewald, CBS H-19856. Isoneotype: HAL 2023 F. Ex-neotype cultures: CBS 121624 = CPC 12211, CPC 12212.
Lit.: Schubert et al. (2007b: 118–120).
Ill.: Schubert et al. (2007b: 118–120, figs 9–12), Bensch et al. (2012: 50–51, figs 14–17).
Mycelium superficial, hyphae branched, 1.5–8 μm wide, pluriseptate, broader hyphae usually slightly constricted at the septa and somewhat swollen, hyaline to subhyaline, almost smooth to somewhat verruculose or irregularly rough-walled, sometimes appearing to have a slime coat, walls unthickened. Conidiophores macronematous, sometimes also micronematous, arising as lateral or terminal branches from plagiotropous or ascending hyphae, erect, straight to more or less flexuous, sometimes geniculate, nodulose, usually with small headlike swellings, sometimes also with intercalary nodules, sometimes swellings protruding and elongated to one side, unbranched, occasionally branched, (7–)20–330 μm, sometimes even longer, (2–)3–5 μm wide, swellings (4–)5–8 μm wide, pluriseptate, not constricted at the septa, septa sometimes not very conspicuous, subhyaline to pale brown or pale olivaceous, smooth or somewhat verruculose, walls unthickened or almost so, more thickened with age. Conidiogenous cells integrated, usually terminal, cylindrical with a terminal head-like swelling, sometimes with a second swelling, 15–40 μm long, proliferation sympodial, with few conidiogenous loci confined to swellings, up to six loci per swelling, loci protuberant, conspicuous, 1–2 μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed, up to 34(–40) μm long, 3–4 μm wide, 0–2-septate. Conidia catenate, formed in branched chains, straight to slightly curved, small terminal conidia subglobose, ovoid to obovoid or somewhat limoniform, (3–)4–7(–9) × (2–)2.5–3.5 μm (av. ± SD: 5.3 ± 1.3 × 2.8 ± 0.4), aseptate; intercalary conidia ovoid, ellipsoid, 6–11(–12.5) × (2.5–)3–4 μm (av. ± SD: 8.6 ± 1.7 × 3.4 ± 0.5), 0(–1)-septate, secondary ramoconidia ellipsoid to subcylindrical or cylindrical, (8–)10–24(–31) × (3–)3.5–5(–7) μm (av. ± SD: 14.4 ± 4.1 × 4.2 ± 0.6), 0–1(–3)-septate, very rarely 5-septate, with up to 5 distal hila, subhyaline to pale brown or pale olivaceous, minutely verruculose to verrucose (mostly granulate with some muricate projections under SEM), walls unthickened or almost so, apex rounded or slightly attenuated towards apex and base, hila protuberant, conspicuous, 1–2 μm wide, up to 1 μm high, thickened and darkened-refractive; microcyclic conidiogenesis occurring.
Culture characteristics: Colonies on PDA reaching 22–32 mm diam after 14 d at 25 ºC, olivaceous grey to iron grey, sometimes whitish, smoke grey to pale olivaceous due to abundant aerial mycelium covering almost the whole colony, with age collapsing becoming olivaceous grey, occasionally zonate, velvety to floccose, margin narrow, entire edge, white, glabrous to somewhat feathery, aerial mycelium sparse to abundant, white, fluffy, growth regular, flat to low convex, sometimes forming few exudates in the colony centre, sporulating. Colonies on MEA reaching 21–32 mm diam after 14 d at 25 ºC, grey olivaceous, olivaceous grey to dull green or iron grey, sometimes whitish to pale smoke grey due to abundant aerial mycelium, olivaceous grey to iron grey reverse, velvety, margin narrow, entire edge to slightly undulate, white, radially furrowed, glabrous to slightly feathery, aerial mycelium sparse to abundant, mainly in the centre, white, fluffy, growth convex to raised, radially furrowed, distinctly wrinkled in the colony centre, without prominent exudates, sporulating. Colonies on OA reaching 20–32 mm diam after 14 d at 25 °C, smoke grey, grey olivaceous to olivaceous grey, greenish black or iron grey reverse, margin narrow, entire edge, colourless to white, glabrous, aerial mycelium sparse to abundant, dark smoke grey, diffuse, high, later collapsed, felty, growth flat, without prominent exudates, sporulation profuse.
Substrates and distribution: On living and decaying plant and fungal material, human, air, hypersaline and industrial water; worldwide.
Additional materials examined: China, isol. from indoor air, DTO 323-C3, DTO 323-E1, DTO 323-G5. Denmark, isol. from indoor environment, B. Andersen, CBS 139578 = DTO 109-I5, DTO 109-E5 = BA 1905, DTO 109-E6 = BA 1906, DTO 109-F3 = BA 1918, DTO 109-F5 = BA 1920, DTO 109-I3; BA 1897; Lyngby, isol. from an air sample, bedroom, U. Thrane, DTO 111-A5; isol. from wall basement, B. Andersen, DTO 110-B7. France, isol. from indoor environment, J. Dijksterhuis, DTO 108-F9. Germany, isol. from indoor environment, DTO 084-F3; G. Fischer, DTO 005-E8. Hungary, isol. from floor under curtain, DTO 101-I8; isol. from indoor environment, DTO 147-I6. The Netherlands, isol. from indoor air, area crocodiles, Zoo, DTO 106-C2; isol. from a wet wall, indoor, J. Houbraken, DTO 101-A1; Eindhoven, isol. from air sample, bedroom, J. Houbraken, DTO 089-G4, DTO 089-G6, DTO 089-H3; 's Hertogenbosch, from swab sample archive, M. Meijer, DTO 086-D5; Rijssen, isol. from an air sample, kitchen, M. Meijer, DTO 089-B9; Rijswijk, from swab sample archive, M. Meijer, DTO 090-D3; Utrecht, from swab sample archive, M. Meijer, DTO 090-H4. UK, Ditherington, isol. from indoor air sample, Dec. 2012, Ž. Jurjević, EMSL 1871 = CPC 22358. USA, California, Modesto, isol. from an indoor air sample, bedroom, Dec. 2012, Ž. Jurjević, EMSL 1862 = CPC 22349; Georgia, Tucker, isol. from an air sample, bakery, DTO 127-E4 = AR377; Minnesota, isol. from indoor air sample, Ž. Jurjević, EMSL 1726 = CPC 22268; New Jersey, Chatman, isol. from indoor air sample, Oct. 2012, Ž. Jurjević, EMSL 1808 = CPC 22312; Ž. Jurjević, EMSL 1809 = CPC 22313; New York, isol. from indoor air sample, bedroom, Dec. 2012, Ž. Jurjević, EMSL 1856 = CPC 22343; isol. from indoor air sample, bedroom, 15th floor, Jan. 2013, Ž. Jurjević, EMSL 1890 = CPC 22377.
Notes: Cladosporium allicinum (Fig. 2, clade 27) proved to be one of the most common Cladosporium species occurring in indoor environments after C. halotolerans (Fig. 3, clade 23), C. sphaerospermum (Fig. 3, clade 20) and C. pseudocladosporioides (Fig. 1, clade 56) (see also Segers et al. 2015). Surprisingly, none of the isolates included in the study of Segers et al. (2015) nor in this study turned out to be C. herbarum. This is of interest as C. herbarum is the most-studied species in allergy research (Breitenbach, 2008, Poll et al., 2009). Segers et al. (2015) therefore recommended that specifically the common indoor fungi, C. sphaerospermum, C. halotolerans and C. allicinum, should be evaluated to assess whether the allergy screening panels of these fungi have to be adapted.
Cladosporium angulosum Sandoval-Denis et al., Persoonia 36: 289. 2016. MycoBank MB815333.
Holotype: USA, Texas, from human bronchoalveolar lavage fluid, Sep. 2008, D.A. Sutton, CBS H-22380. Ex-type culture: CBS 140692 = UTHSC DI-13-235 = FMR 13348.
Ill.: Sandoval-Denis et al. (2016: 289, fig. 3).
Mycelium superficial and immersed, hyphae unbranched or loosely branched, 1–3 μm wide, septate, subhyaline or pale olivaceous brown, smooth or minutely verruculose, thin-walled, often forming loose to dense ropes. Conidiophores macro- and micronematous, arising terminally or laterally from hyphae or hyphal ropes, erect, straight to slightly flexuous, narrowly cylindrical-oblong, non-nodulose, usually not geniculate, unbranched or branched, frequently branching near the base in a 90° angle, branches short, often only as short lateral prolongations just below a septum, 9–150(–190) × (1.5–)2–4 μm, sometimes slightly attenuated towards the apex, septate, septa darkened, pale to medium olivaceous brown, smooth or minutely verruculose, especially towards the apex, thin-walled or slightly thickened. Conidiogenous cells terminal or intercalary, cylindrical, 8–46 × 2–3.5 μm, bearing up to four conidiogenous loci of 1–1.5 μm diam, darkened and refractive. Ramoconidia subcylindrical, straight, 24.5–46 × 2–3.5 μm, 0–1-septate, pale olivaceous brown, smooth or finely roughened, with protuberant, thickened and darkened scars, base broadly truncate, 2–2.5 μm wide, unthickened or slightly thickened, somewhat refractive. Conidia catenate, numerous conidia formed in densely branched chains, 1–4 conidia in the terminal unbranched part, small terminal conidia subglobose or obovoid, 2.5–4.5(–5) × (1.5–)2–2.5(–3) μm (av. ± SD: 3.6 ± 0.7 × 2.2 ± 0.4), aseptate; intercalary conidia ovoid, limoniform or ellipsoid, 4–10(–14.5) × 2–3 μm (av. ± SD: 7.2 ± 2.7 × 2.6 ± 0.3), 0(–1)-septate, with 1–4 hila at the apex, attenuated towards apex and base; secondary ramoconidia ellipsoid or subcylindrical to cylindrical, (7–)9–21.5(–30) × 2–3(–3.5) μm (av. ± SD: 15.9 ± 6.6 × 2.8 ± 0.5), 0–1(–2)-septate, often constricted at septum, with (2–)3–4(–5) distal hila, pale to medium olivaceous brown, smooth or loosely minutely verruculose, thin-walled, with protuberant 0.5–1.5 μm diam conidial hila; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on PDA attaining 50–56 mm diam after 14 d at 25 °C, olivaceous grey, olivaceous or iron-grey, reverse dull green to olivaceous black velvety to floccose, with regular white margin and a raised or umbonate centre and radially folded towards the periphery. Colonies on MEA reaching up to 75 mm diam after 14 d at 25 °C, white to pale olivaceous grey or rosy buff, reverse olivaceous grey or ochraceous, floccose or fluffy, margins narrow, radially furrowed, aerial mycelium abundantly formed, loose to dense. Colonies on OA reaching 52–55 mm diam after 14 d at 25 °C, grey olivaceous or pale olivaceous grey, reverse olivaceous grey, velvety to floccose or fluffy-felty, with regular margin, flat. Without prominent exudates on all media.
Cardinal temperature for growth: Optimum 25 °C, maximum 35 °C, minimum 5 °C (from Sandoval-Denis et al. 2016).
Substrates and distribution: Isolated from plant, human bronchoalveolar lavage fluid and indoor air; Asia (Thailand), Australasia (Australia), Central America (Panama), North America (USA).
Additional materials examined: Australia, Emerald Spring, isol. from Corymbia foelscheana, 22 Sep. 2007, B. Summerell, CPC 14566. USA, South Carolina, Charleston, isol. from indoor air sample, Aug. 2012, Ž. Jurjević, EMSL 1741 = CPC 22271.
Notes: Cladosporium angulosum (Fig. 1, clade 2) was introduced by Sandoval-Denis et al. (2016) as a closely related but phylogenetically distinct species of C. perangustum (Fig. 1, clade 4) showing sufficient genetic distance with respect to the ex-type strain of C. perangustum. Morphologically it differs from the latter species by forming smaller intercalary conidia and secondary ramoconidia. Conidia forming long branched chains with up to 14 conidia in the terminal unbranched part as described in Sandoval-Denis et al. (2016) could not be observed in the material examined. The strain CPC 14566 released some sulphur-yellow pigment into the PDA agar and some amber-coloured pigment into the OA agar. This has not been reported for the ex-type strain of C. angulosum. Cladosporium xanthochromaticum (Fig. 1, clade 3), another element of the C. perangustum s. lat. complex, was named for the production of a yellow diffusable pigment released into PDA agar and also some strains belonging to C. perangustum s. str. are able to produce an olivaceous buff or orange pigment in PDA agar and an amber coloured or orange pigment in OA agar. Cladosporium xanthochromaticum differs from C. angulosum in having longer conidia and in not growing at 35 °C (Sandoval-Denis et al. 2016).
The two isolates from Ananas comosus collected in Panama and reported in Bensch et al. (2015) as first records of C. perangustum in Central America proved to belong to C. angulosum (Sandoval-Denis et al. 2016).
Cladosporium angustisporum Bensch et al., Stud. Mycol. 67: 17. 2010. MycoBank MB517071. Fig. 6.
Fig. 6.
Cladosporium angustisporum (CPC 22345). A–C. Colonies on PDA, MEA and OA. D–H. Conidiophores and conidial chains. I. Ramoconidium and conidia. J–L. Conidial chains. Scale bars = 10 μm.
Holotype: Australia, North Queensland, Daintree National Park, isol. from Alloxylon wickhamii (Proteaceae), coll. B.A. Summerell, isol. P.W. Crous, CBS H-20423. Ex-type culture: CBS 125983 = CPC 12437.
Ill.: Bensch et al. (2010: 21, figs 5−6).
Mycelium immersed and superficial; hyphae branched, 1−3 μm wide, septate, mostly not constricted at septa, subhyaline to olivaceous brown, smooth to verruculose or irregularly rough-walled, walls unthickened, sometimes irregular in outline due to swellings and constrictions, forming expanded hyphal ropes. Conidiophores solitary, macro- and micronematous, erect or ascending, arising terminally or laterally from hyphae, straight or flexuous, filiform to cylindrical-oblong, non-nodulose, usually not geniculate, unbranched or once branched, sometimes two types of conidiophores, short and long ones, 22−280 × (1.5−)2−4 μm, pluriseptate, not constricted at septa, but sometimes irregular in outline due to wider or narrower parts within the stalk, pale to medium olivaceous brown or pale olivaceous, smooth or verruculose at the base, walls unthickened or slightly thickened. Conidiogenous cells integrated, mainly terminal, sometimes also intercalary, neither nodulose nor geniculate, narrowly cylindrical-oblong, 10−27 μm long, with several loci crowded at the apex, in intercalary conidiogenous cells loci mainly situated on small lateral denticles just below a septum, subdenticulate, conspicuous, 1−1.5(−2) μm diam, thickened and darkened-refractive. Ramoconidia cylindrical, 18−42(−55) μm long, 0−1(−3)-septate, concolouress with tips of conidiophores, base broadly truncate, 2.5−3 μm wide, unthickened but sometimes slightly refractive. Conidia catenate, in branched chains, with 1−5 conidia in the terminal unbranched part of the chain, branching in all directions, small terminal conidia obovoid to narrowly ellipsoid, 3−6.5 × 1.5−2 μm (av. ± SD: 4.9 ± 1.0 × 1.8 ± 0.3), aseptate, intercalary conidia narrowly ellipsoid, fusiform, (4−)5.5−11.5(−13) × (1.5−)2−2.5(−3) μm (av. ± SD: 8.1 ± 2.4 × 2.4 ± 0.4), 0(−1)-septate, with 1−3 distal hila, secondary ramoconidia ellipsoid to subcylindrical or cylindrical, (6−)7.5−26 × 2−3 μm (av. ± SD: 14.9 ± 6.1 × 2.7 ± 0.4), 0−1-septate, pale olivaceous or pale olivaceous brown, smooth or almost so, appearing to be reticulate, walls unthickened, somewhat attenuated towards apex and base, with 2−4(−5) distal hila, hila conspicuous, subdenticulate, 0.5−2 μm diam, thickened and darkened-refractive.
Culture characteristics: Colonies on PDA attaining 55−65 mm diam after 14 d at 25 °C, olivaceous or mouse-grey due to abundant sporulation with pale olivaceous grey or smoke-grey patches of aerial mycelium, reverse leaden-grey and iron-grey, velvety or fluffy, margin whitish, feathery, broad, aerial mycelium abundant, woolly to fluffy, loose diffuse or dense, growth low or high, without prominent exudates. Colonies on MEA reaching 45−62 mm diam after 14 d at 25 °C, smoke-grey, whitish to pale olivaceous grey due to abundant aerial mycelium, reverse iron-grey to pale greenish-grey, velvety to woolly-fluffy, margin narrow, whitish, regular or undulate, aerial mycelium abundant, loose diffuse or dense, fluffy, growth low convex, radially furrowed, sometimes with few prominent exudates, sporulation profuse. Colonies on OA attaining 60−65 mm diam after 14 d at 25 °C, olivaceous grey with patches of white and smoke-grey due to aerial mycelium, reverse leaden-grey and iron-grey, velvety or fluffy, margin regular, glabrous, growth flat, without exudates, sporulation profuse.
Substrate and distribution: On plant material as well as isolated from indoor and outside air, also reported from clinical samples; Australia, North America (USA).
Additional materials examined: USA, Alabama, Mobile, isol. from outside air sample, Dec. 2012, Ž. Jurjević, EMSL 1858 = CPC 22345; Florida, Miami, isol. from indoor air sample, office, Jan. 2013, Ž. Jurjević, EMSL 1884 = CPC 22371; Wisconsin, Oak Creek, isol. from air sample, bakery, DTO 127-E6 = AR387.
Notes: Cladosporium angustisporum (Fig. 1, clade 58) belongs to the C. cladosporioides species complex (Fig. 1) and is morphologically very close to C. cladosporioides s. str. but differs in having distinctly narrower conidia, 1.5−3 μm wide. Phylogenetically, C. angustisporum is allied to C. subuliforme (Fig. 1, clade 59) but the latter species is morphologically distinguishable in having slightly wider terminal and intercalary conidia and often awl-shaped conidiophores with a wider base and an attenuated apex (Bensch et al. 2010).
Until now C. angustisporum was only know from the type collected in Australia, but probably has an even wider distribution. It was recently reported from a clinical sample in the USA (Sandoval-Denis et al. 2015) and has been isolated several times from indoor and outside air (this study).
Cladosporium anthropophilum Sandoval-Denis et al., Persoonia 36: 290. 2016. MycoBank MB815334.
Holotype: USA, Minnesota, from human bronchoalveolar lavage fluid, Sep. 2012, D.A. Sutton, CBS H-22381. Ex-type culture: CBS 140685 = UTHSC DI-13-269 = FMR 13382.
Ill.: Sandoval-Denis et al. (2016: 290, fig. 4).
Mycelium superficial and immersed, hyphae unbranched or branched, (1–)2–4 μm wide, septate, subhyaline to pale olivaceous, smooth or minutely verruculose at or towards the base of conidiophores, thick-walled, anastomosing. Conidiophores macro- and semimacronematous, erect, cylindrical, non-nodulose, sometimes geniculate, usually branched, up to 550 μm long, 2–5 μm wide, often slightly attenuated towards the apex, septate, pale to medium olivaceous brown, slightly roughened to verruculose toward the base, with a thickened and refractive wall; occasionally micronematous conidiophores formed, 1.5–2 μm wide. Conidiogenous cells terminal and intercalary, cylindrical or subcylindrical, 15–54 × 3–5 μm, often with a swollen apex, bearing 3–8(–10) conidiogenous loci, protuberant, subdenticulate, crowded, 1–2.5 μm diam, thickened and somewhat darkened. Ramoconidia cylindrical, 20–51 × 2–5 μm, 0(–2)-septate, pale olivaceous, smooth, with conidial scars protuberant, thickened and darkened. Conidia forming short, branched chains with up to four conidia in the terminal unbranched part of the chain, small terminal conidia ovoid or ellipsoid, 3.5–9 × 2–3 μm (av. ± SD: 5.1 ± 1.3 × 2.5 ± 0.5), intercalary conidia limoniform to ellipsoid, 4.5–12(–19) × 2–3(–4) μm (av. ± SD: 9.3 ± 2.3 × 3.0 ± 0.5), aseptate; secondary ramoconidia ellipsoid to subcylindrical, 7–28(–30) × (2–)3–4(–5) μm (av. ± SD: 18.7 ± 6.3 × 3.4 ± 0.6), 0–1(–2)-septate, often attenuated at the centre, subhyaline or pale olivaceous brown, smooth or finely roughened, reticulate under SEM, with 2–5 protuberant hila forming dense clusters at the distal end, 0.5–2 μm diam; microcyclic conidiogenesis sometimes occurring.
Culture characteristics: On PDA attaining 17–80 mm diam after 14 d at 25 °C, grey olivaceous, olivaceous or greenish olivaceous, reverse leaden-grey or olivaceous black, velvety or powdery, margin white, regular, flat or folded, aerial mycelium sparse, diffuse, sometimes showing cottony to floccose white to grey cushions. Colonies on MEA reaching 50–72 mm diam after 14 d at 25 °C, grey olivaceous, glaucous-grey towards margins, reverse iron-grey, powdery or fluffy-felty, margin regular, radially furrowed or wrinkled, aerial mycelium diffuse or more abundant in colony centre, fluffy-felty. Colonies on OA attaining 27–74 mm diam after 14 d at 25 °C, smoke-grey, grey olivaceous or olivaceous, greenish olivaceous towards margins, reverse leaden-grey, iron-grey or leaden-black, flat, velvety or fluffy-felty, margin fimbriate, aerial mycelium sparse or more abundant. Sporulation profuse on all media, without prominent exudates and diffusible pigment.
Cardinal temperature for growth: Optimum 25 °C, maximum 35 °C, minimum 5 °C (from Sandoval-Denis et al. 2016).
Substrates and distribution: Isolated from human clinical samples, indoor air, food and plant material; Africa (South Africa, Uganda), Asia (China, India, Indonesia, Israel, Japan, South Korea, Thailand), Australasia (Australia), North America (USA).
Additional materials examined: China, isol. from indoor air, DTO 317-I7, DTO 318-E3, DTO 323-C2, DTO 323-C6, DTO 323-C7, DTO 323-D2, DTO 323-D8, DTO 323-D9, DTO 324-C4, DTO 324-D3. India, isol. from Dalbergia sp., W. Gams, CPC 11131. Israel, isol. from seeds of Gossypium sp., CBS 674.82 = ATCC 200936. Japan, isol. from bamboo slats, CBS 122130 = ATCC 38012. South Africa, Baberton, Laeveld Coop, isol. from Triticum aestivum, CPC 14009. South Korea, isol. from Phytolacca americana, H.D. Shin, CPC 11122; isol. from Ricinus communis, 2003, H.D. Shin, CPC 11119. Uganda, Mubende, isol. from food, coffee leaf, B. Andersen, CPC 14356 = BA 1676. USA, Arizona, Tuscon, isol. from indoor air sample, hospital, Jan. 2013, Ž. Jurjević, EMSL 1908 = CPC 22393; Georgia, isol. from air sample, bakery, DTO 127-E9 = AR409; McDonough, isol. from indoor air sample, living room, Nov. 2012, Ž. Jurjević, EMSL 1818 = CPC 22315.
Notes: Cladosporium anthropophilum was recently introduced by Sandoval-Denis et al. (2016) and proved to be a common saprobic fungus (see Table 1). It also represents a clinically relevant fungus, being the second most prevalent species identified in a set of clinical isolates from the USA after C. halotolerans (Sandoval-Denis et al. 2015), and has been isolated quite frequently from indoor environments. Although discussed as phylogenetically distant (Sandoval-Denis et al. 2016), C. anthropophilum (Fig. 1, clade 65) is shown to be morphologically and phylogenetically closely related to C. cladosporioides (Fig. 1, part 66). It mainly differs by its longer conidiophores, up to 550 μm long, with numerous loci crowded at or towards the often subnodulose apex and ovoid to ellipsoid terminal conidia, 3.5–9 μm long, showing a fine, dense reticulation under SEM, whereas C. cladosporioides forms shorter conidiophores (10–250 μm) with usually (1−)2−4 conidiogenous loci at the apex and subglobose to limoniform, 3−6(−7) μm long terminal conidia with an irregularly reticulate or striped wall. Cladosporium anthropophilum also resembles C. pseudocladosporioides and C. tenuissimum, but they are genetically well differentiated (Fig. 1, clades 65, 56 and 64, respectively) and morphologically, C. anthropophilum shows longer terminal conidia, [3.5–9 μm long (av. ± SD: 5.1 ± 1.3) vs 3−5.5 (av. ± SD: 4.1 ± 0.7) in C. pseudocladosporioides and (2−)2.5−5(−6) (av. ± SD: 3.7 ± 1.0) in C. tenuissimum] and forms longer conidiophores than C. pseudocladosporioides (15–155 μm long) (Bensch et al., 2012, Sandoval-Denis et al., 2016).
Cladosporium asperulatum Bensch et al., Stud. Mycol. 67: 21. 2010. MycoBank MB517072. Fig. 7.
Fig. 7.
Cladosporium asperulatum (CPC 22364). A–C. Colonies on PDA, MEA and OA. D–H. Conidiophores and conidial chains. I. Ramoconidium with conidial chains. Scale bars = 10 μm.
Holotype: Portugal, isol. from Protea susannae (Proteaceae), 1 May 2007, P.W. Crous, CBS H-20424. Ex-type culture: CBS 126340 = CPC 14040.
Lit.: Bensch et al. (2012: 70−72; 2015: 41).
Ill.: Bensch et al. (2010: 22−24, figs 7−9; 2012: 70−72, figs 42−44).
Mycelium immersed, sparingly superficial; hyphae unbranched or very sparingly branched, 2−4.5 μm wide, septate, not constricted at septa, subhyaline to pale or medium olivaceous brown, smooth to minutely verruculose or irregularly verrucose, walls unthickened or almost so, sometimes forming loose to dense ropes of a few or several hyphae. Conidiophores macro- and micronematous, solitary, arising terminally or laterally from hyphae, erect, straight to slightly flexuous, cylindrical-oblong, sometimes slightly geniculate towards the apex, non-nodulose, (15−)45−210(−360) × (2−)3−4(−5) μm, sometimes up to 5 μm wide at the base, unbranched, occasionally branched, branches below the apex or at a lower level, usually below a septum, sometimes up to 105 μm long, pluriseptate with 0−12 septa, not constricted, pale to medium olivaceous brown, paler towards the apex and sometimes attenuated, smooth to asperulate or minutely verruculose, walls slightly thickened; micronematous conidiophores filiform or narrowly cylindrical-oblong, about 2 μm wide, paler and narrower, subhyaline or pale olivaceous brown, mostly with a single apical scar. Conidiogenous cells integrated, mainly terminal, cylindrical-oblong, sometimes slightly geniculate-sinuous towards the apex, 22−38 μm long, smooth or almost so, with 2−4 apical loci, protuberant, subdenticulate, sometimes situated on peg-like prolongations, 1−2 μm diam, thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 15−50 × 3−4 μm, 0(−1)-septate, concolouress with tips of conidiophores, smooth or almost so, base broadly truncate, (2.2−)2.5−3(−3.2) μm wide, unthickened. Conidia catenate, in branched chains, up to 8(−10) conidia in the terminal unbranched part of the chain, small terminal conidia obovoid, 4.5−7(−8) × 2−3(−3.5) μm (av. ± SD: 5.4 ± 1.0 × 2.6 ± 0.4), intercalary conidia ovoid, fusiform to ellipsoid, 5−11(−13) × 2.5−3(−4) μm (av. ± SD: 8.0 ± 2.2 × 2.9 ± 0.4), aseptate, secondary ramoconidia ellipsoid, fusiform, subcylindrical, (7.5−)9−26(−37) × (2.5−)3−4(−5) μm (av. ± SD: 17.9 ± 6.5 × 3.4 ± 0.6), 0(−1)-septate, very rarely with a second septum, not constricted at septa, subhyaline to pale olivaceous brown, smooth to minutely verruculose or irregularly rough-walled (LM), under SEM loosely verruculose or surface with irregularly reticulate structure or embossed stripes probably caused by diminishing turgor and shrivelling of tender conidia, walls slightly thickened, attenuated towards apex and base, hila protuberant, subdenticulate, 0.8−2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 48−53 mm diam after 14 d at 25 °C, olivaceous grey, iron-grey or grey olivaceous at margins, sometimes zonate, reverse leaden-grey, greyish blue to iron-grey, powdery to fluffy or hairy, margin white, narrow, glabrous, aerial mycelium abundantly formed, dense, fluffy and high in colony centre, growth flat to low convex with somewhat elevated colony centre, sometimes with prominent exudates, sporulation profuse. Colonies on MEA reaching 45−64 mm diam after 14 d at 25 °C, olivaceous grey to pale greenish grey, reverse olivaceous grey to iron-grey, powdery to fluffy, margin white to smoke-grey, narrow, regular, glabrous to feathery, radially furrowed, aerial mycelium abundant, sometimes several prominent exudates formed appearing blackish, sporulation profuse. Colonies on OA attaining 45−55 mm diam after 14 d at 25 °C, grey olivaceous or olivaceous, smoke-grey due to abundant fluffy-felty aerial mycelium, margin regular, without exudates, sporulation profuse.
Substrates and distribution: Isolated from plant material and indoor environment; Asia (India), Europe (Portugal), North America (Mexico, USA).
Additional materials examined: India, isol. from Eucalyptus leaf litter (Myrtaceae), 1 Mar. 2004, coll. W. Gams, isol. P.W. Crous, CBS 126339 = CPC 11158. USA, California, Frazier Park, isol. from indoor air sample, bathroom, Dec. 2012, Ž. Jurjević, EMSL 1877 = CPC 22364.
Notes: Cladosporium asperulatum (Fig. 1, clade 28) is phylogenetically close to but distinct from C. myrtacearum (Fig. 1, clade 26; see Bensch et al. 2010) and C. angustiterminale (Fig. 1, clade 27; see Bensch et al. 2015). Morphologically this species is comparable with C. subtilissimum (Fig. 2, clade 25), which belongs to the C. herbarum species complex, but differs in having 0−12-septate, somewhat longer conidiophores and narrower conidia (Schubert et al. 2007b). It has recently been reported from Mexico (Bensch et al. 2015) and now proves to be also occurring in indoor environments.
Cladosporium austrohemisphaericum Bensch et al., Stud. Mycol. 82: 42. 2015. MycoBank MB814626. Fig. 8.
Fig. 8.
Cladosporium austrohemisphaericum (DTO 305-E8). A–C. Colonies on PDA, MEA and OA. D–I. Unbranched or branched conidiophores with conidial chains. J. Ramoconidium with conidial chains. Scale bars = 10 μm.
Holotype: New Zealand, Auckland, Morrin Reserve, −37.00, 175.00, isolated from black mould on the surface of a fruit of Lagunaria patersonia (Malvaceae), 18 Apr. 2005, C.F. Hill, Hill 1163, CBS H-22350. Ex-type culture: CBS 140482 = CPC 12068.
Ill.: Bensch et al. (2015: 46, fig. 10).
Mycelium immersed, branched, 1–4 μm wide, septate, subhyaline to very pale olivaceous brown, asperulate, minutely verruculose, verruculose or even verrucose, walls unthickened, without any swellings and constrictions. Conidiophores micro- to semimacronematous or macronematous, arising terminally and laterally from erect or ascending hyphae, erect, solitary or in pairs or loose groups, straight to flexuous, filiform to narrowly cylindrical-oblong, sometimes once geniculate at or towards the apex, unbranched or once branched, branches often only as short lateral peg-like prolongations just below a septum, 20–135(–180) × (2–)2.5–3.5 μm, at the base up to 4.5 μm wide, septate, often only with up to four not very conspicuous septa, sometimes disarticulating at septa and forming ramoconidia and fragments, subhyaline to pale or medium olivaceous brown, minutely verruculose, asperulate, sometimes verrucose or irregularly rough-walled especially towards the base and almost smooth at or towards the apex, walls unthickened or slightly thick-walled, slightly attenuating towards the apex, sometimes conidiophores reduced to conidiogenous cells. Conidiogenous cells integrated, mostly terminal, sometimes intercalary, filiform to narrowly cylindrical-oblong, sometimes once geniculate, non-nodulose, (6–)13–45(–60) μm long, with 1–3(–4) apical loci, conspicuous, subdenticulate to denticulate, 1–2 μm diam, thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 12–45 × 2–3(–3.5) μm, 0–1(–2)-septate, subhyaline to pale olivaceous brown, almost smooth to asperulate or minutely verruculose, base broadly truncate, 2–3 μm wide, neither thickened nor darkened. Conidia numerous, catenate, formed in branched chains, branching in all directions, in younger chains often dichotomously branched, 1–3 conidia in the terminal unbranched part of the chain, small terminal conidia globose, subglobose to obovoid or ovoid, 2–5(–7) × (1–)1.5–3 μm (av. ± SD: 3.3 ± 1.0 × 2.1 ± 0.5), aseptate, subhyaline to pale or medium olivaceous brown, minutely verruculose to verruculose or verrucose, hila 0.5–0.8 μm diam or narrower, intercalary conidia ovoid to ellipsoid-ovoid, 4–11 × 2–3.5 μm (av. ± SD: 7.1 ± 2.1 × 2.6 ± 0.4), 0(–1)-septate, septa sometimes not very conspicuous, surface ornamentation as in small terminal conidia, rounded or only very slightly attenuated towards the ends, with 2–4 distal hila, 0.5–1 μm diam, secondary ramoconidia ellipsoid to subcylindrical, (8–)10–27(–30) × 2–3.5(–4) μm (av. ± SD: 18.5 ± 6.2 × 2.9 ± 0.4), 0–1(–3)-septate, with age constricted at septa, septum median or in the upper half, 1–3(–4) distal hila, subhyaline to pale olivaceous brown, almost smooth to loosely verruculose or irregularly rough-walled, not or only slightly attenuated towards apex and base, hila conspicuous, subdenticulate, 1–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on PDA attaining 35–45 mm diam after 14 d at 25 °C, grey olivaceous to dull green or iron-grey, reverse greyish blue to olivaceous black, velvety to powdery, margin white, narrow, glabrous to feathery, regular, aerial mycelium absent or sparse, loose, diffuse, growth flat or low convex, without prominent exudates, sporulation profuse. Colonies on MEA reaching 26–44 mm diam after 14 d at 25 °C, grey olivaceous to greenish grey or glaucous-grey at margins, paler in the centre, reverse olivaceous to olivaceous grey or iron-grey, velvety to powdery, margin white, very narrow, feathery, radially furrowed, growth flat to low convex with slightly elevated colony centre, wrinkled and folded, few prominent exudates formed, sporulation profuse. Colonies on OA attaining 26–34 mm diam after 14 d at 25 °C, grey olivaceous or iron-grey, smoke-grey due to abundant sporulation, reverse leaden-grey to leaden-black, powdery, margin white, very narrow, glabrous, slightly undulate, aerial mycelium absent or diffuse, without prominent exudates.
Substrates and distribution: On plant material and fruits of different hosts as well as indoor environments (house dust); Australasia (Australia, New Zealand), South Africa.
Additional material examined: New Zealand, isol. from house dust, DTO 305-E8 = TA05NZ-351A.
Notes: A single isolate from house dust collected in New Zealand morphologically fits the concept of the recently described species C. austrohemisphaericum which was isolated from black mould on the surface of a fruit in New Zealand. Therefore, it is herein treated as an additional isolate of that species although all four known isolates sit on quite long branches in a well-supported clade (Fig. 3, clade 9) and may each represent a cryptic species. For now we refrain from introducing further novel species for these morphologically similar isolates until additional isolates are available to formalise species concepts for these lineages.
Cladosporium cladosporioides (Fresen.) G.A. de Vries, Contr. Knowl. Genus Cladosporium: 57. 1952. MycoBank MB294915. Fig. 9
Fig. 9.
Cladosporium cladosporioides (CBS 112388, adapted from Bensch et al. 2012). A–C. Colonies on PDA, MEA and OA. D–I. Macronematous conidiophores and conidial chains. Scale bar = 10 μm.
Type: Germany, on overwintered leaves of Hydrangea sp. (Hydrangeaceae) (not preserved). Neotype (designated in Bensch et al. 2010): Germany, isol. from indoor air, Ch. Trautmann, CBS H-20428. Ex-type culture: CBS 112388.
Lit.: Ellis (1971: 319), Domsch et al. (1980: 202), Ho et al. (1999: 121), Samson et al. (2000: 108), de Hoog et al. (2000: 583), Samson et al. (2001: 340), Park et al., 2004, Heuchert et al., 2005: 46–47), Bensch et al. (2010: 29–34), Bensch et al. (2012: 90–93).
Ill.: Fresenius (1850: Taf. 3, Figs 23–28), de Vries (1952: 58–59, Figs 10–11), Ellis (1971: 318, fig. 219 C), Domsch et al. (1980: 203, fig. 82), Ho et al. (1999: 122, figs 8–9), de Hoog et al. (2000: 583–584, figs), Samson et al. (2000: 108, fig. 48; 109, pl. 46), Bensch et al. (2010: 30–32, figs 17–19).
Mycelium immersed, rarely superficial; hyphae sparse, unbranched or sparingly branched, (1−)2−4(−5) μm wide, septate, septa occasionally darkened, without any swellings and constrictions, subhyaline, pale olivaceous brown or pale brown, smooth to minutely verruculose or rough-walled, walls unthickened. Conidiophores solitary, macro- or semimacronematous, sometimes micronematous, arising terminally from ascending hyphae or laterally from plagiotropous hyphae, straight to somewhat flexuous, narrowly cylindrical to cylindrical-oblong, sometimes filiform, non-nodulose, usually not geniculate-sinuous, occasionally once geniculate, 40−300(−350) × (2.5−)3−4(−5.5) μm, unbranched or occasionally branched, branches usually short, only as peg-like lateral outgrowth just below a septum, occasionally up to 60 μm, mostly in the upper third, pluriseptate, usually not constricted at septa, sometimes slightly constricted and one of the upper septa slightly darkened where ramoconidia are formed, pale to medium olivaceous brown or brown, smooth to minutely verruculose or verruculose especially towards the base, walls unthickened or slightly thickened, occasionally slightly attenuated towards the apex, base sometimes swollen, up to 7 μm wide; micronematous conidiophores shorter, narrower, paler, unbranched, 9−150 × (1−)1.5−2.5(−3) μm wide. Conidiogenous cells integrated, usually terminal, sometimes intercalary with conidiogenous loci situated on small peg-like or denticle-like lateral outgrowths just below a septum, cylindrical-oblong, not geniculate, non-nodulose, (7−)16−38 μm long, with up to four loci crowded at the apex, subdenticulate to denticulate, protuberant, 1−2(−2.5) μm diam, central dome often not very conspicuous, flat, somewhat thickened and darkened-refractive. Ramoconidia seceding at one of the upper, somewhat darkened septa, straight to slightly curved, cylindrical-oblong, 15−50 × (2.5−)3−5 μm, with up to three septa, pale olivaceous brown, concolourous with tips of conidiophores, smooth, base not cladosporioid, 2.5−4 μm wide, unthickened or slightly thickened, sometimes slightly refractive. Conidia numerous, catenate, in long branched chains, up to 10 conidia in the upper unbranched part, branching in all directions, small terminal conidia subglobose, obovoid, ovoid to limoniform, 3−6(−7) × (1.5−)2−2.5(−3) μm (av. ± SD: 4.7 ± 0.9 × 2.4 ± 0.3), aseptate, intercalary conidia limoniform, ellipsoid-ovoid, sometimes fusiform or subcylindrical, 5−12(−14.5) × (2−)2.5−3(−4) μm (av. ± SD: 8.1 ± 2.2 × 2.9 ± 0.3), aseptate, with up to 3(−4) distal hila, secondary ramoconidia ellipsoid, subcylindrical to cylindrical-oblong, (7−)10−33(−38) × (2−)2.5−4(−6) μm (av. ± SD: 19.4 ± 6.6 × 3.2 ± 0.5), 0(−1)-septate, rarely with two septa, not constricted at septa, with up to four distal hila, subhyaline, pale brown or pale olivaceous brown, smooth, under SEM smooth or surface with somewhat irregularly reticulate structure or embossed stripes probably caused by diminishing turgor and shrivelling of tender young conidia, thin-walled, sometimes cell structure unusual, with a small cavity in the cells, hila conspicuous, subdenticulate to denticulate, 0.5−2(−2.5) μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA up to 80 mm diam after 14 d at 25 °C, grey olivaceous to dull green or olivaceous grey, reverse iron-grey, leaden grey or olivaceous black, velvety to floccose, margins grey olivaceous to white, feathery, regular, aerial mycelium sparse, diffuse, or sometimes abundantly formed, dense, floccose-felty, low, forming mats, growth flat to low convex, usually without prominent exudates, occasionally with several small prominent exudates. Colonies on MEA 54−72 mm diam after 14 d at 25 °C, grey olivaceous to olivaceous or olivaceous grey, pale olivaceous grey or whitish due to aerial mycelium, olivaceous black or olivaceous buff at margins, reverse olivaceous black or iron-grey, velvety to floccose, margins white to grey olivaceous, glabrous to feathery, aerial mycelium sparse, scattered, diffuse to floccose, sometimes abundantly formed, covering almost the whole colony, floccose-felty, whitish, growth flat to effuse, somewhat radially furrowed, without prominent exudates. Colonies on OA 65−70 mm diam after 14 d at 25 °C, grey olivaceous, towards margins at first greenish olivaceous, then dull-green and again grey olivaceous, sometimes white, reverse olivaceous grey to leaden-grey, sometimes pale mouse-grey, velvety to floccose, margins narrow, glabrous, regular, aerial mycelium scattered to sometimes abundant, floccose or felty, loose to somewhat dense, growth flat, no prominent exudates; sporulation usually profuse on all media.
Substrates and distribution: On fading and decaying plant material, on living leaves as secondary invader, isolated from air, soil, foodstuffs, water-damaged building materials and numerous other materials; cosmopolitan.
Additional materials examined: Denmark, isol. from indoor environment, B. Andersen, DTO 109-I4 = BA 1898, DTO 109-I6 = BA 1900. Hungary, isol. from indoor environment, DTO 147-A9; DTO 101-G2; isol. from floor under curtain, DTO 101-H7; isol. from a bathroom, DTO 102-A4. The Netherlands, air sample, bakery, DTO 127-D8 = AR362; Rijswijk, from swab sample archive, M. Meijer, DTO 090-C6; Weert, isol. from indoor air sample, living room, B. Favié, DTO 082-F1. USA, Arizona, Peoria, isol. from indoor air sample, bedroom, Jan. 2013, Ž. Jurjević, EMSL 1893 = CPC 22380; Florida, St. Augustine, isol. from indoor air sample, kitchen, Dec. 2012, Ž. Jurjević, EMSL 1861 = CPC 22348; Georgia, isol. from indoor air sample, Aug. 2012, Ž. Jurjević, EMSL 1722 = CPC 22264; Michigan, Dryden, isol. from indoor air sample, bedroom, Dec. 2012, Ž. Jurjević, EMSL 1860 = CPC 22347; Minnesota, isol. from indoor air sample, Aug. 2012, Ž. Jurjević, EMSL 1723 = CPC 22265; Vermont, Williston, isol. from indoor air sample, bedroom, Dec. 2012, Ž. Jurjević, EMSL 1878 = CPC 22365; Virginia, Arlington, isol. from indoor air sample, living room, Jan. 2013, Ž. Jurjević, EMSL 1880 = CPC 22367.
Notes: Cladosporium cladosporioides (Fig. 1, clade 66) as previously circumscribed on the basis of morphology represents a heterogeneous complex of numerous phylogenetically and more or less also morphologically distinct species (Bensch et al. 2010). Cladosporium cladosporioides s. lat. is one of the most common, saprobic Cladosporium species with worldwide distribution, frequently occurring as secondary invader on necrotic parts of many different host plants, isolated from air, soil, textiles and numerous other substrates (Ellis 1971) and found as a common endophytic fungus (Riesen and Sieber, 1985, El-Morsy, 2000, Kumaresan and Suryanarayanan, 2002). Furthermore, the conidia of this species are among the most ubiquitous bioaerosols found in indoor and outdoor samples (Domsch et al., 1980, Mullins, 2001, Park et al., 2004).
Yamamoto, 1959, Ellis, 1971, De Hoog et al., 2000 and Samson et al. (2000) discussed strains of “C. cladosporioides” with asperulate or finely verruculose conidia, which proved to represent different, phylogenetically clearly distinct species, as for instance C. asperulatum (Fig. 1, clade 28) and C. perangustum (Fig. 1, clade 4). Sandoval-Denis et al. (2016) introduced C. anthropophilum (Fig. 1, clade 65), a common saprobic fungus which can also represent a clinically relevant fungus (Sandoval-Denis et al. 2015), and discussed it to be phylogenetically distant from C. cladosporioides but in our analysis it now clusters close to it (Fig. 1, clades 65, 66). However, the association between the two clades is only supported by the Bayesian analysis (BPP = 0.97). Although difficult to separate morphologically, C. anthropophilum mainly differs in forming longer (up to 550 μm) conidiophores with numerous conidiogenous loci crowded at or towards the apex and ovoid to ellipsoid terminal conidia (3.5–9 μm long) which show a fine, dense reticulation under SEM (Sandoval-Denis et al. 2016).
Three morphologically almost indistinguishable but phylogenetically distinct lineages, indicated in Bensch et al. (2010) as C. cladosporioides s. lat. Lineages 1, 2 and 4 which cluster apart from C. cladosporioides s. str. (Fig. 1, clade 66) are introduced as new species in this paper, namely C. europaeum (Fig. 1, clade 35), C. vicinum (Fig. 1, clade 34) and C. westerdijkiae (Fig. 1, clade 43). Given their high morphological similarity the use of a molecular approach for the correct identification of all these species is highly recommended.
Cladosporium coloradense Bensch & Samson, sp. nov. MycoBank MB822218. Fig. 10
Fig. 10.
Cladosporium coloradense (CBS 143357). A–C. Colonies on PDA, MEA and OA. D–K. Conidiophores and conidial chains. L–M. Ramoconidia and conidial chains. Scale bars = 10 μm.
Etymology: Name refers to the place where it was collected, Colorado.
Holotype: USA, Colorado, Denver, isol. from air sample, bedroom, June 2012, Ž. Jurjević, CBS H-23249. Ex-type culture: CPC 22238 = CBS 143357 = EMSL 1685.
Diagnosis: Differs from C. succulentum by its narrowly ellipsoid terminal conidia and its longer conidiophores and conidia.
Superficial mycelium sparingly formed, unfertile hyphae filiform, narrowly cylindrical-oblong, 1−2.5 μm wide, septate, neither constricted nor swollen, subhyaline, walls unthickened, fertile hyphae forming conidiophores, darker and wider, often somewhat swollen at the base of conidiophores, 3−5(−6) μm wide, pale to medium olivaceous brown, somewhat constricted at septa, smooth, walls somewhat thickened, sometimes forming loose aggregations. Conidiophores macro- and micronematous, arising laterally or terminally from hyphae, solitary or in pairs, sometimes arising in loose groups of four from hyphal aggregations, straight or slightly flexuous, often very long, narrowly cylindrical-oblong, neither geniculate nor nodulose, unbranched, occasionally branched, (18−)30−510 μm long or even longer, (2.5−)3−4 μm wide, up to 5.5 μm wide at the base, pluriseptate, 1−18-septa, pale to medium olivaceous brown, often paler towards the apex, smooth or almost so, walls thickened, 0.5−1 μm thick. Conidiogenous cells integrated, terminal and intercalary, cylindrical or subcylindrical, neither geniculate nor nodulose, (13−)21−36 μm long, in terminal cells 2−4 loci crowded at the uppermost apex, in intercalary ones 1−3 loci situated on small lateral outgrowths just below or above a septum, loci 1−2 μm diam. Ramoconidia subcylindrical or cylindrical, 25−43 × 3−4.5 μm, 0(−2)-septate, base 2(−3) μm wide, neither thickened nor darkened. Conidia catenate, numerously formed, paler than conidiophores and ramoconidia, up to five conidia in the terminal unbranched part of the chain, branching in all directions, small terminal conidia narrowly ellipsoid, 3−5.5 × 1.5−2 μm (av. ± SD: 4.1 ± 0.7 × 1.7 ± 0.2), apex rounded, attenuated towards the base, subhyaline, pale olivaceous or pale olivaceous brown, almost smooth or asperulate, intercalary conidia narrowly ellipsoid, 4.5−10 2−3 μm (av. ± SD: 7.7 ± 2.7 × 2.5 ± 0.4), aseptate, with 1−3(−4) distal scars, almost smooth, asperulate or loosely minutely verruculose, secondary ramoconidia narrowly ellipsoid or subcylindrical, 9.5−19(−25) × 3−3.5(−4.5) μm (av. ± SD: 15.6 ± 3.9 × 3.3 ± 0.4), aseptate, almost smooth or asperulate, pale olivaceous brown or pale medium olivaceous brown, walls unthickened or very slightly thick-walled, with 2−4 distal scars, hila conspicuous, 0.5−2 μm diam; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on PDA reaching 43−58 mm diam after 14 d at 25 °C, olivaceous, iron-grey, reverse iron-grey, greyish blue towards margins, velvety or fluffy, margins glabrous, aerial mycelium diffuse, fluffy, without prominent exudates, sporulation profuse. Colonies on MEA attaining 41−49 mm diam after 14 d at 25 °C, olivaceous grey, olivaceous due to abundant sporulation mainly in colony centre, reverse olivaceous grey to iron-grey, powdery to velvety, margin narrow, white, glabrous or slightly feathery, aerial mycelium loose, diffuse to more densely and fluffy, high, growth low convex with somewhat elevated colony centre, radially furrowed, without exudates. Colonies on OA reaching 35−40 mm diam after 14 d at 25 °C, iron-grey, olivaceous due to abundant sporulation, reverse olivaceous grey to iron-grey, powdery or fluffy, margin regular, glabrous, aerial mycelium loose diffuse, high, growth flat, without exudates.
Substrates and distribution: Indoor air; North America (USA).
Notes: With its narrowly ellipsoid conidia C. coloradense (Fig. 3, clade 14) is not a very typical member of the C. sphaerospermum species complex, but reminds one of species belonging to the C. cladosporioides species complex. Similar as in C. aciculare (Fig. 3, clade 16) and C. fusiforme (Fig. 3, clade 17) the conidial shape departs from the globose to subglobose shape of typical members of this species complex. Both species are phylogenetically allied but C. aciculare can be distinguished by its narrower conidiophores, secondary ramoconidia and conidiogenous loci and hila (Bensch et al. 2015); and C. fusiforme possesses shorter conidiophores and wider, fusiform apical conidia (Zalar et al., 2007, Bensch et al., 2012). Its closest phylogenetic relative is C. succulentum (Fig. 3, clade 15), isolated from a dolphin bronchus, which can be differentiated from the new species by its oval to short clavate terminal conidia and its shorter conidiophores and conidia (Sandoval-Denis et al. 2016). Until now the species is known only from a single isolate.
Cladosporium delicatulum Cooke, Grevillea 5(33): 17. 1876. MycoBank MB164571. Fig. 11.
Fig. 11.
Cladosporium delicatulum (DTO 167-H5). A–C. Colonies on PDA, MEA and OA. D–I. Conidiophores and conidial chains. Scale bars = 10 μm.
Holotype: India, on dead leaves (litter), Colonel Hobsen, No. 23 (K [M] 121551). Isotypes: Vize, Micro-Fungi Exot. 24 (e.g., B 700006230).
Lit.: Bensch et al. (2010: 37–40; 2012: 102–106; 2015: 45).
Ill.: Bensch et al. (2010: 38–40, figs 22–25; 2012: 103–105, figs 87–92).
Mycelium immersed, rarely superficial; hyphae unbranched or sparingly branched, (0.5−)1−3(−4) μm wide, septate, without swellings and constrictions, subhyaline to pale olivaceous or pale olivaceous brown, smooth to minutely verruculose, sometimes loosely verrucose, sometimes forming ropes. Conidiophores macro- and micronematous, solitary, arising terminally and laterally from hyphae, erect, straight to somewhat flexuous, cylindrical-oblong, non-nodulose, sometimes slightly geniculate towards the apex, unbranched, occasionally branched, once or several times, often as short peg-like prolongations, 50−165(−200) × 3−4.5(−5) μm, 2−4(–7)-septate, sometimes attenuated at septa, pale olivaceous to pale medium olivaceous brown, smooth, sometimes loosely minutely verruculose at the base, walls unthickened or almost so, about 0.5 μm wide, sometimes slightly attenuated towards the apex, up to 5.5 μm wide at the base; micronematous conidiophores narrower and pale olivaceous, 19−75(−100) × (1.5−)2−2.5 μm. Conidiogenous cells integrated, terminal, sometimes intercalary, situated on small peg-like prolongations, cylindrical-oblong, sometimes geniculate at or towards the apex, non-nodulose, occasionally the whole cell inflated in shape like a secondary ramoconidium, 11−37 μm long, with (1−)2−3(−4) apical loci, crowded at the apex, conspicuous, subdenticulate to denticulate, sometimes situated on small lateral outgrowths, quite broad, truncate, rim and dome not distinctly visible, 1.5−2.2 μm diam, thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 13−46 × 2.5−4(−5) μm, 0−1(−2)-septate, sometimes distinctly constricted at the median septum, base broadly truncate, 2−3 μm wide, neither thickened nor darkened-refractive. Conidia numerous, in densely branched chains, branching in all directions, up to four conidia in the terminal unbranched part of the chain, small terminal conidia obovoid, subglobose or globose, 2.5−4.5(−6) × (1.5−)2−2.5(−3.5) μm (av. ± SD: 3.7 ± 0.8 × 2.4 ± 0.4), aseptate, apex rounded, sometimes irregular due to additional lateral hila, intercalary conidia limoniform to ellipsoid-ovoid or sometimes irregular in outline due to lateral hila, 4−13(−17.5) × 2.5−3.5(−4) μm (av. ± SD: 7.8 ± 3.0 × 3.0 ± 0.4), 0−1-septate, attenuated towards apex and base, with 1−4(−6) distal hila, secondary ramoconidia ellipsoid-ovoid to subcylindrical or cylindrical, (6−)8−23.5(−31) × (2.5−)3−4.5(−5) μm (av. ± SD: 15.6 ± 5.4 × 3.6 ± 0.5), 0−1(−2)-septate, very rarely 3-septate, not constricted at septa, pale olivaceous to pale olivaceous brown, smooth or almost so, walls unthickened, often only slightly attenuated towards apex and base, with (1−)2−4(−5) distal hila, hila conspicuous, subdenticulate or denticulate, 0.5−2.2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 60−78 mm diam after 14 d at 25 °C, olivaceous grey, grey olivaceous to olivaceous and olivaceous black, reverse olivaceous black, floccose to villose, margins grey olivaceous, feathery, regular, aerial mycelium scattered to abundant, covering almost the whole colony surface, floccose to villose, low to rarely high, growth flat, without prominent exudates, sporulation sparse. Colonies on MEA reaching 67−76 mm diam after 14 d at 25 °C, smoke-grey to pale olivaceous grey, olivaceous grey or glaucous grey at margins, reverse olivaceous grey, floccose, fluffy, margins white, glabrous to feathery, regular, aerial mycelium abundant, covering the whole colony surface, floccose to fluffy, growth flat, radially furrowed and wrinkled in colony centre, without prominent exudates, sporulation sparse or absent. Colonies on OA reaching 55−74 mm diam after 14 d at 25 °C, smoke-grey to pale olivaceous grey, grey olivaceous or olivaceous due to abundant sporulation, reverse pale greenish grey to olivaceous grey, velvety to floccose, margins regular, glabrous, narrow, colourless, aerial mycelium sparse to abundant, covering the whole surface, floccose, loose to dense, low, growth flat, without prominent exudates, sporulation sparse to profuse.
Substrates and distribution: Isolated from air, building material and dust, saprobic on dead leaves, fruits, stems, tubers, or occurring as secondary invader on necrotic lesions caused by other fungi in vivo; widely distributed, Africa (Algeria), Asia (China, India, Taiwan), Australasia (New Zealand), Europe (Denmark, France, Germany, Poland, The Netherlands), North America (Mexico, USA), South America (Uruguay).
Additional materials examined: Algeria, isol. from indoor environment, L. Belhoucine, DTO 134-D3 = DR22, DTO 134-D4, DTO 134-D5 = O200, DTO 134-D6 = BT27, DTO 134-D7 = BT91, DTO 134-D8 = BT92. Denmark, isol. from indoor air, 2007, B. Andersen, BA 1679 = CPC 14285, BA 1680 = CPC 14286, BA 1681= CBS 126342 = CPC 14287; isol. from building material, school, 2007, B. Andersen, BA 1698 = CBS 126343 = CPC 14299; isol. from building material, 2007, B. Andersen, BA 1683 = CPC 14289; Asperen, swap sample archive, M. Meijer, DTO 090-F4; Broenshoej, isol. from indoor air, control room, 2000, B. Andersen, BA 1724 = CPC 14363; indoor air sample, in cup board, water damaged room, 2000, B. Andersen, BA 1718 = CPC 14360; Valleroed, isol. from dust, school, 2000, B. Andersen, BA 1740 = CPC 14372; Weert, isol. from indoor air, living room, B. Favié, DTO 082-F3 = CBS 139574. Germany, isol. from indoor environment, DTO 145-C4; Sachsen-Anhalt, Halle (Saale), Robert-Franz-Ring, isol. from leaves of Tilia cordata (Tiliaceae), 2 Aug. 2004, K. Schubert, CBS H-20430, CBS 126344 = CPC 11389, reference strain of C. delicatulum. New Zealand, isol. from house dust, DTO 305-H7, DTO 305-I9 = TA05NZ-340. Poland, isol. from indoor air in poultry houses, K. Plewa, DTO 167-H5, DTO 168-F8.
Notes: This species is undoubtedly a widespread saprobic hyphomycete commonly isolated from indoor environments. Morphologically it is comparable with C. cladosporioides (Fig. 1, clade 66) but C. delicatulum (Fig. 1, clade 44) differs from the latter species in having 0−1-septate intercalary conidia and secondary ramoconidia, only a few conidia in the terminal unbranched part of conidial chains, shorter often slightly geniculate conidiophores and shorter secondary ramoconidia. Cladosporium westerdijkiae (Fig. 1, clade 43) is the closest relative in the tree but can be distinguished from C. delicatulum by usually aseptate and somewhat longer ramoconidia and secondary ramoconidia. Cladosporium inversicolor (Fig. 1, clade 42) is distinct by its longer conidial chains, longer small terminal and intercalary conidia, wider intercalary conidia and secondary ramoconidia, longer ramoconidia with a broader base, with conidia being smooth to loosely verruculose or irregularly rugose. The old, sparse type material of C. delicatulum is from India. New Indian collections and cultures are not available. Therefore, a formal epitypification of this species has not yet been proposed, but the German strain from Tilia cordata can serve as reference strain to fix the application of C. delicatulum and agrees well with the Indian type material (Bensch et al. 2010).
Cladosporium domesticum Bensch & Samson, sp. nov. MycoBank MB822219. Fig. 12, Fig. 13.
Fig. 12.
Cladosporium domesticum (CBS 143358). A–C. Colonies on PDA, MEA and OA. D–H. Macronematous conidiophores with conidial chains. I–J. Micronematous conidiophores with conidial chains. K–L. Conidial chains. Scale bars = 10 μm.
Fig. 13.
Cladosporium domesticum (DTO 305-H2). A, B. Shows rows of rounded cells present at agar level that can form aerial hyphae and/or conidiophores. C–H. Details of conidia next to aerial or substrate fungal structures. Note the less distinct ornamentation of the C. sphaerospermum type containing out of ridges and warts. Scars on conidia (D, H) and ramoconidia (with differences in size, G) are visible. Note the very long “neck” area between conidia in D, F–H. Scale bars = 2 (C, E–H), 5 (D), 10 (A, B) μm.
Etymology: domesticum - Latin for house, all isolates from indoor environments.
Holotype: USA, New Jersey, Trenton, isol. from indoor air sample, Oct. 2012, Ž. Jurjević, CBS H-23250. Ex-type culture: CBS 143358 = CPC 22307 = EMSL 1803.
Diagnosis: Differs from C. halotolerans by its 0–2-septate ramoconidia (0–5-septate in C. halotolerans), its less densely septate conidiophores and its slightly narrower conidia. The small terminal and intercalary conidia are not globose and not distinctly darker than ramoconidia and conidiophores as it is typical for C. halotolerans.
Mycelium unbranched or branched, 0.5−2.5(−4) μm wide, filiform or narrowly cylindrical-oblong, septate, mostly without any constrictions or swellings, if swollen then swellings up to 6 μm diam, subhyaline or pale olivaceous, smooth or almost so or minutely verruculose especially those giving rise to conidiophores, often forming ropes of several hyphae, occasionally swollen hyphal cells or dense hyphal aggregations, swollen cells globose, doliiform or irregular in outline. Conidiophores macro-, semimacro- or micronematous, arising from hyphae, occasionally also from swollen hyphal cells or hyphal aggregations, erect, straight, filiform or narrowly cylindrical-oblong, neither nodulose nor geniculate, unbranched or branched, often with one or several denticles or peg-like short lateral prolongations just below a septum, (3−)30−125(−200) × 1.5−3 μm, septa appear to be darkened, sometimes somewhat constricted and thickened where ramoconidia will be seceded, subhyaline or very pale olivaceous, smooth or almost so, sometimes irregularly rough-walled, sometimes attenuated towards the apex, sometimes conidiophores very short, reduced to conidiogenous cells, formed as short denticle-like outgrowth of hyphae. Conidiogenous cells integrated, terminal and intercalary, (5−)10−39 μm long, with 1−3 conidiogenous loci at the apex or situated on short lateral prolongations, loci conspicuous, 1−1.5 μm diam, thickened and darkened-refractive. Ramoconidia formed but transition between ramoconidia and secondary ramoconidia difficult, 16−43 × 1.5−2.5 μm, 0−2-septate, base about 2 μm wide. Conidia catenate, numerous conidia formed in branched chains with branching in all directions, 1−5 conidia in the terminal unbranched part of the chain, small terminal conidia subglobose or obovoid, (2−)2.5−3.5(−4.5) × (1.5−)2−2.5(−3) μm (av. ± SD: 3.3 ± 0.8 × 2.2 ± 0.3), subhyaline or pale olivaceous brown, almost smooth to mostly irregularly verruculose, intercalary conidia limoniform, ovoid or ellipsoid, 4−11(−13) × 2−2.5(−3) μm (av. ± SD: 6.7 ± 2.2 × 2.4 ± 0.4), 0(−1)-septate, surface ornamentation and colour as in small terminal conidia, with 1−3 distal hila, secondary ramoconidia ellipsoid or subcylindrical, (6−)9−24(−31) × (1.5−)2−3(−3.5) μm (av. ± SD: 16.5 ± 6.0 × 2.4 ± 0.4), 0−1(−3)-septate, pale olivaceous brown, smooth or almost so or irregularly verruculose as in smaller conidia, with (1−)2−4 distal hila, hila 0.5–1.5 μm diam; microcyclic conidiogenesis occurring.
Culture characteristics: Colonies on PDA reaching 35−50 mm diam after 14 d at 25 °C, pale olivaceous grey or olivaceous grey mainly in colony centre due to dense and abundant aerial mycelium, towards margins large patches of grey olivaceous or olivaceous where profusely sporulating, reverse leaden-grey and olivaceous grey, powdery or fluffy-felty, margins white, regular, glabrous or somewhat feathery, aerial mycelium diffuse to mostly dense, sometimes very high in a few spots, growth flat or low convex with elevated and wrinkled colony centre, sometimes forming several prominent exudates, up to 2 mm diam. Colonies on MEA attaining 30−46 mm diam after 14 d at 25 °C, grey olivaceous where profusely sporulating, whitish or smoke-grey due to aerial mycelium, glaucous-grey, olivaceous grey or iron-grey at margins, reverse olivaceous grey and greyish sepia, velvety or felty, margins white, narrow, glabrous or somewhat feathery, radially furrowed, colony centre elevated, wrinkled and folded, aerial mycelium forming dense mats, low or high in a few spots, sometimes numerous small exudates starting to be formed. Colonies on OA reaching 35−50 mm diam after 14 d at 25 °C, grey olivaceous or olivaceous where sporulating, pale olivaceous grey to iron-grey due to aerial mycelium or where sterile, reverse smoke-grey, leaden-grey and olivaceous grey, velvety or fluffy-felty, margins glabrous, regular, aerial mycelium loose diffuse or mostly dense, low to very high, fluffy, without prominent exudates.
Substrates and distribution: Indoor environments (air, house dust); North America (USA).
Additional materials examined: USA, isol. from house dust, DTO 305-H2 = AA03US-480, DTO 306-B6 = AA03US-525, DTO 307-E8 = AA03US-368, DTO 307-H3 = AA03US-402, DTO 308-B1; AA03US-387; Florida, Oldsmar, isol. from indoor air sample, Nov. 2012, Ž. Jurjević, EMSL 1821 = CPC 22318; New Jersey, Trenton, isol. from indoor air sample, Oct. 2012, Ž. Jurjević, EMSL 1803 = CPC 22307; isol. from indoor air sample, 1st floor, Jan. 2013, Ž. Jurjević, EMSL 1936 = CPC 22408; Pennsylvania, isol. from attic wood roofing sample, Jan. 2012, Ž. Jurjević, EMSL 1962 = CPC 22413; Huntingdon Valley, isol. from indoor air sample, air conditioner, May 2012, Ž. Jurjević, EMSL 1658 = CPC 22225; Texas, Georgetown, isol. from indoor air sample, classroom, Jan. 2013, Ž. Jurjević, EMSL 1930 = CPC 22402.
Notes: Cladosporium domesticum (Fig. 3, clade 21) is phylogenetically and morphologically closely allied to C. halotolerans (Fig. 3, clade 23) from which it can be differentiated by its 0–2-septate ramoconidia (0–5-septate in C. halotolerans), its less densely septate conidiophores and its slightly narrower conidia which are not arranged like a string of pearls. The small terminal and intercalary conidia are not globose and not distinctly darker than ramoconidia and conidiophores as is typical for C. halotolerans. On OA ramoconidia of C. domesticum are commonly formed and the conidiophores are much longer, up to 375 μm long or even longer.
Cladosporium parahalotolerans (Fig. 3, clade 22), also newly described and phylogenetically close to both C. halotolerans and C. domesticum, forms wider conidia and ramoconidia.
Cladosporium dominicanum Zalar et al., Stud. Mycol. 58: 169. 2007. MycoBank MB510995. Fig. 14.
Fig. 14.
Cladosporium dominicanum (CPC 22244). A–C. Colonies on PDA, MEA and OA. D–I. Conidiophores with conidial chains. J–K. Conidial chains. Scale bars = 10 μm.
Holotype: Dominican Republic, salt lake Enriquillo, isol. from hypersaline water, Jan. 2001, N. Gunde-Cimerman, isol. P. Zalar, CBS H-19733. Ex-type culture: EXF-732 = CBS 119415.
Lit.: Bensch et al. (2012: 108–110; 2015: 45).
Ill.: Zalar et al. (2007: 170, fig. 6), Bensch et al. (2012: 109, fig. 97).
Mycelium unbranched to sparingly branched, septate, not constricted at septa, pale olivaceous brown, minutely verruculose to irregularly rough-walled, walls unthickened or almost so, protoplasm somewhat aggregated in the centre of the cells, granular, without extracellular polysaccharide-like material. Conidiophores micro- and semimacronematous, hardly distinguishable from hyphae, arising laterally and terminally on erect or ascending hyphae, erect, somewhat flexuous, filiform to cylindrical-oblong, usually neither geniculate nor nodulose, unbranched or branched, once or several times, branches as short lateral prolongations below a septum, (5–)10–100(–200) × (1–)2–2.5(–3.5) μm, aseptate or with few septa, pale olivaceous brown, smooth to minutely verruculose, walls thin-walled to slightly thickened; micronematous conidiophores often only as short denticle- or peg-like lateral outgrowths of hyphae. Conidiogenous cells integrated, terminal, sometimes intercalary or conidiophores reduced to conidiogenous cell, cylindrical, with a single or few apical loci, protuberant, denticulate, 0.8–1.5 μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed, up to 40 μm long, base about 2 μm wide. Conidia catenate, in branched chains, branching in all directions, up to eight conidia in the unbranched parts, small terminal conidia globose or subglobose to usually short-ovoid, narrower at both ends, (2–)3–3.5(–4.5) × 2–2.5 μm (av. ± SD: 3.0 ± 0.5 × 2.0 ± 0.2), aseptate, smooth to minutely verruculose, intercalary conidia ovoid, limoniform to ellipsoid, (3.5–)4–8.5(–12) × 2–3 μm (av. ± SD: 6.0 ± 2.1 × 2.6 ± 0.3), 0(–1)-septate, smooth to minutely verruculose, with 1–3(–4) distal hila, secondary ramoconidia cylindrical to almost spherical, attenuated towards apex and base, (6.5–)9–23(–28) × (2–)2.5–3(–4) μm, (av. ± SD: 15.4 ± 5.0 × 2.8 ± 0.4), 0–1(–2)-septate, not constricted at the median septum, with up to four distal scars, subhyaline to pale olivaceous or light brown, smooth or almost so, walls unthickened to slightly thickened, hila protuberant, conspicuous, denticulate, 0.5–1.5 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on PDA reaching 18–36 mm diam after 14 d at 25 °C, grey olivaceous in colony centre due to abundant sporulation, glaucous grey to greenish grey, reverse greenish grey, velvety to hairy or felty, margin regular, white, somewhat feathery, aerial mycelium abundant, high, fluffy to felty, covering most of the surface, flat or slightly furrowed, with at margin, numerous small droplets of light reseda-green (2E6) exudates sometimes present. Colonies on MEA reaching 30–32 mm diam after 14 d at 25 °C, reseda green (2E6), reverse dark green-brown, velvety, furrowed, with undulate margin. Colonies on MEA + 5 % NaCl reaching 37–41 mm diam after 14 d at 25 °C, reseda-green (2E6), reverse brownish green, radially furrowed, velvety, sporulating in the central part or all over the colony, margin white and regular. Colonies on OA reaching 19–34 mm diam after 14 d at 25 °C, dark mouse-grey, reverse black, velvety to loosely powdery with raised central part due to fasciculate bundles of conidiophores, aerial mycelium sparse, whitish to smoke-grey, without exudates, sporulating.
Maximum tolerated salt concentration: 75 % of tested strains develop colonies at 20 % NaCl after 7 d, while after 14 d all strains grow and sporulate.
Cardinal temperatures: No growth at 4 and 10 °C, optimum 25 °C (30–32 mm diam), maximum 30 °C (2–15 mm diam), no growth at 37 °C.
Differential parameters: No growth at 10 °C, oval conidia, large amounts of sterile mycelium (from Zalar et al. 2007).
Substrates and distribution: Saprobic on fruit surfaces, hypersaline waters in (sub)tropical climates, indoor environments; Asia (Iran, Philippines, Taiwan), North America (Bermuda, USA), Central America (Dominican Republic), South America (Aruba, Venezuela).
Additional materials examined: Aruba, Oranjestad, isol. from air sample, hospital, Jul. 2012, Ž. Jurjević, EMSL 1697 = CPC 22244. Bermuda, Samerset, isol. from indoor air sample, Nov. 2012, Ž. Jurjević, EMSL 1822 = CPC 22319. USA, Colorado, Denver, isol. from outside air sample, Jun. 2012, Ž. Jurjević, EMSL 1687, 1688 = CPC 22240, 22241.
Notes: Cultures of C. dominicanum (Fig. 3, clade 4) sporulate less abundantly than C. sphaerospermum (Fig. 3, clade 20) and C. halotolerans (Fig. 3, clade 23) and tend to lose their ability to sporulate with subculturing (Zalar et al. 2007). The species proved to have a wider host range and distribution than known before (Zalar et al., 2007, Bensch et al., 2012, Bensch et al., 2015). It is not only known from fruit surfaces and hypersaline water but was also isolated both from indoor and outside air. The strains reported by Segers et al. (2015) as C. dominicanum proved to belong to the newly described species C. pulvericola (Fig. 3, clade 1). For a comparison with C. pulvericola please consult the notes under the latter species.
The included ex-type isolate of Cladosporium lebrasiae (Fig. 3, clade 5), a species recently described from milk bread rolls in France (Razafinariovo et al. 2016), clusters on a long branch among isolates of C. dominicanum (Fig. 3, clade 4). On the loci used in the present phylogeny, it is 93–98 % similar to C. dominicanum. In the parsimony analysis, this isolate clusters as a sister lineage to C. dominicanum (data not shown). Additional isolates are necessary to prove whether C. lebrasiae is a distinct species.
Cladosporium europaeum Bensch & Samson, sp. nov. MycoBank MB822220.
Etymology: Refers to the continent of origin, Europe.
Holotype: Denmark, isol. from indoor building material, school, 2007, B. Andersen, CBS H-23251. Ex-type culture: CBS 134914 = BA 1695 = CPC 14296.
Diagnosis: Differs from C. vicinum, its closest phylogenetic neighbour in having shorter conidiogenous cells, secondary ramoconidia and ramoconidia.
Mycelium immersed and superficial; hyphae sparingly branched, 2–4 μm wide, septate, without swellings and constrictions, pale olivaceous or pale olivaceous brown, smooth, minutely verruculose or rough-walled. Conidiophores macronematous, sometimes micronematous, arising terminally and laterally from hyphae, solitary, erect, straight or flexuous, cylindrical-oblong, neither geniculate nor nodulose, unbranched or once branched, 35–150(–290) × (2.5–)3–4.5 μm, septate, pale olivaceous or pale olivaceous brown, smooth, often minutely verruculose or rough-walled at the base; micronematous conidiophores about 2 μm wide. Conidiogenous cells integrated, terminal and intercalary, cylindrical-oblong, 6–36 μm long, with (1–)2–4 loci at the apex or on small lateral outgrowths in intercalary cells or situated on lateral shoulders, 1–2 μm diam. Ramoconidia cylindrical-oblong, 18–39 × 3–4 μm, 0–2-septate, smooth, base broadly truncate, 2–3 μm wide. Conidia numerously formed in branched chains, branching in all directions, with up to six conidia in the terminal unbranched part of the chain, small terminal conidia subglobose or obovoid, 2.5–4.5(–5.5) × 2–2.5(–3) μm (av. ± SD: 3.8 ± 0.7 × 2.3 ± 0.3), intercalary conidia ovoid, limoniform or ellipsoid, 4–14 × (2–)2.5–3.5(–4) μm (av. ± SD: 7.7 ± 2.6 × 3.0 ± 0.4), 0(–1)-septate, with 1–3(–4) distal hila, secondary ramoconidia ellipsoid or subcylindrical (7–)10–25(–28) × (2.5–)3–4 μm (av. ± SD: 16.4 ± 5.3 × 3.2 ± 0.4), 0–1-septate, pale olivaceous or pale olivaceous brown, smooth, walls unthickened, attenuated towards apex and base, with up to four distal hila, hila conspicuous, subdenticulate or denticulate, 0.5–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on PDA attaining 73–82 mm diam after 14 d at 25 °C, grey olivaceous, olivaceous grey to olivaceous black with patches of smoke-grey or white due to aerial mycelium, reverse iron-grey, velvety or powdery, margin feathery, aerial mycelium sparse, more abundantly only in a few spots, growth flat, no exudates. Colonies on MEA reaching 50–76 mm diam after 14 d at 25 °C, grey olivaceous, reverse iron-grey, powdery or velvety, margin feathery, radially furrowed, wrinkled and with elevated colony centre, aerial mycelium forming large whitish or smoke-grey patches, fluffy-woolly, dense, no exudates. Colonies on OA attaining about 55 mm diam after 14 d at 25 °C, pale olivaceous or brownish, white and smoke-grey due to patches of fluffy-felty aerial mycelium, reverse iron-grey or leaden-grey, powdery or fluffy-felty, margin glabrous, growth flat, sometimes few prominent olivaceous buff exudates formed. Sporulation profuse on all media.
Substrates and distribution: Isolated from plant material, lichens and indoor environments; Europe (Denmark, Germany, Portugal, The Netherlands).
Additional materials examined: Denmark, isol. from indoor environment, B. Andersen, DTO 109-E7 = BA 1907. Germany, isol. from leaves of Acer pseudoplatanus (Aceraceae), L. Pehl, CBS 116744 = dH 14053; Bavaria, isol. from a lichen on leaves of Acer platanoides (Aceraceae), 2006, W. von Brackel, CPC 13220. Portugal, isol. from indoor environment, DTO 151-H5. The Netherlands, Amsterdam, indoor air archive, M. Meijer, DTO 072-E4; 's Hertogenbosch, swab sample archive, Meijer, DTO 086-B3; Leiden, isol. from seed coat of Cirsium vulgare (Aceraceae), CBS 125.80; Millingerwaards, isol. from fruits of Sambucus nigra (Caprifoliaceae), 29 Aug. 2007, P.W. Crous, CPC 14238; Utrecht, swab sample, house, M. Meijer, DTO 056-H7.
Notes: Cladosporium europaeum (Fig. 1, clade 35), formerly treated as C. cladosporioides Lineage 1 (Bensch et al. 2010) differs from C. cladosporioides s. str. (Fig. 1, clade 66) in producing shorter, 0−1-septate conidia and ramoconidia and is phylogenetically distant with 538/538 (100 %), 410/436 (94 %) and 214/222 (96 %) sequence similarity for ITS, tef1 and act, respectively when the ex-type sequences are compared. Cladosporium vicinum (Fig. 1, clade 34), its closest phylogenetic neighbour shows longer conidiogenous cells, secondary ramoconidia and ramoconidia.
Cladosporium floccosum Sandoval-Denis et al., Persoonia 36: 293. 2016. MycoBank MB814509. Fig. 15.
Fig. 15.
Cladosporium floccosum (CPC 22399). A–C. Colonies on PDA, MEA and OA. D–I. Conidiophores and conidia. J. Ramoconidium. K–L. Microcyclic conidiogenesis with conidia forming secondary conidiophores. M. Conidia. Scale bars = 10 μm.
Holotype: USA, Minnesota, from human ethmoid sinus, Sep. 2010, D.A. Sutton, CBS H-22327. Ex-type culture: CBS 140463 = UTHSC DI-13-212 = FMR 13325.
Ill.: Sandoval-Denis et al. (2016: 292, fig. 7).
Mycelium unbranched or loosely branched, filiform to cylindrical-oblong, fertile hyphae occasionally somewhat swollen and slightly constricted at septa, 1−4(−4.5) μm wide, septate, septa not very conspicuous, hyaline, subhyaline or pale olivaceous brown, smooth or almost so to verruculose or somewhat irregularly rough-walled especially in fertile hyphae at or near the base of conidiophores, sometimes forming small ropes of few hyphae, cell lumen often appearing granulose. Conidiophores macronematous, arising terminally or laterally from plagiotropous or ascending hyphae, erect, straight or curved, cylindrical or usually irregularly in outline in being often nodulose and once or few times distinctly geniculate-sinuous, rectangular, after a nodule has been formed growth often continues in a 45−90° angle at or somewhat below the nodule, shape very characteristic, swellings up to 8 μm diam, mostly unbranched, 10−150 μm long, but mostly shorter, up to 80 μm long, (2.5−)3−5 μm wide, 0−3(−6)-septate, pale to medium olivaceous brown, smooth, verruculose or somewhat irregularly rough-walled at or towards the base, walls refractive, slightly thickened or thickened. Occasionally micronematous conidiophores formed being short, non-nodulose and paler. Conidiogenous cells integrated, terminal and intercalary, usually nodulose and often distinctly geniculate-sinuous, 1−2 nodules per cell, 6−35 μm long, conidiogenous loci mainly confined to nodules, 1−5 loci per nodule, conspicuous, protuberant, 1−2(−2.5) μm diam, somewhat thickened and darkened-refractive. Ramoconidia occasionally formed, 0−1-septate, base 3−3.5 μm wide. Conidia solitary or formed in short unbranched chains with up to four conidia, very rarely in branched chains with few conidia possessing two distal hila, solitary and terminal conidia ellipsoid-ovoid, obovoid, rarely subglobose, sometimes subcylindrical, 6−15(−21.5) × (4−)5−7(−8) μm (av. ± SD: 11.7 ± 3.3 × 6.0 ± 0.9), 0−1-septate, apex rounded, often attenuated towards the base, lumen appearing to be granular, intercalary and basal conidia ellipsoid or subcylindrical, more or less attenuated towards apex and base, (8.5−)10−21(−27) × (4.5−)5.5−8(−10) μm (av. ± SD: 16.3 ± 4.0 × 7.0 ± 1.0), 0−1-septate, septum median or in the lower half, septum becoming sinuous with age, pale to medium olivaceous brown, densely verruculose, verrucose or echinulate, walls unthickened or only very slightly thickened, conidiogenous hila conspicuous, 1−2 μm diam, sometimes situated on small stalk-like prolongations, somewhat thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining 50−68 mm diam after 14 d at 25 °C, olivaceous grey with patches of pale olivaceous grey aerial mycelium, reverse leaden-grey or iron-grey, fluffy. Colonies on MEA reaching 43−63 mm diam after 14 d at 25 °C, pale olivaceous grey and pale greenish grey with white or smoke-grey patches, reverse olivaceous grey, fluffy-felty, aerial mycelium abundant, dense, colony centre somewhat elevated, radially furrowed and folded. Colonies on OA reaching 47−61 mm diam after 14 d at 25 °C, olivaceous grey or grey olivaceous, reverse leaden-grey or iron-grey, fluffy-felty, margins regular, aerial mycelium abundant, diffuse or dense, white. Without prominent exudates, sporulation profuse on all media.
Substrate and distribution: Isolated from plant material, indoor air and a clinical sample; Asia (China), Europe (Ukraine), North America (Mexico, USA).
Additional materials examined: China, isol. from indoor air, DTO 323-H6. Mexico, Montecillo, Texcoco, isol. from pine needles (Pinaceae), 12 Oct. 2009, M. de Jesús Yáñez-Morales, as “Penidiella”, CPC 17802. Ukraine, Kharkov district, Zolochev area, Chepelino village, isol. from Allium sativum (Alliaceae), 5 Jul. 2008, A. Akulov, stored as “Stemphyllium vesicarium”, CPC 15522. USA, Colorado, Fort Collins, isol. from indoor air sample, living room, Dec. 2012, Ž. Jurjević, EMSL 1867 = CPC 22354; Minnesota, isol. from indoor air sample, Aug. 2012, Ž. Jurjević, EMSL 1715 = CPC 22260; Missouri, Fort Leonard Wood, isol. from indoor air sample bedroom, Jan. 2013, Ž. Jurjević, EMSL 1927 = CPC 22399; Tennessee, isol. from indoor air sample, Oct. 2012, Ž. Jurjević, EMSL 1805 = CPC 22309; Utah Draper, isol. from indoor air sample, basement, Feb. 2013, Ž. Jurjević, EMSL 2033 = CPC 22968.
Notes: Cladosporium floccosum (Fig. 2, clade 4), recently described from a clinical sample in the USA (Sandoval-Denis et al. 2016) proves to occur also in indoor environments and on plant material. The shape of its conidiophores is very characteristic in being nodulose and once or several times distinctly geniculate, sometimes being rectangular and its conidia are 0−1-septate, densely verruculose, verrucose or echinulate formed solitary or in short unbranched chains. It resembles C. sinuosum (Fig. 2, clade 2) and the newly introduced species C. aerium (Fig. 2, clade 20). However, C. sinuosum produces longer and slightly wider conidiophores (up to 380 μm long, 4−6(−7) μm wide) and slightly wider conidia, (4–)5–8(–9) wide; and C. aerium forms slightly longer and narrower conidia (8–)9.5–24 × (4.5–)6–7(–8) μm (av. ± SD: 18.0 ± 3.1 × 6.4 ± 0.7). Both species are phylogenetically distant from C. floccosum (C. sinuosum and C. aerium in clades 2 and 20, respectively, vs clade 4 in Fig. 2).
Cladosporium funiculosum W. Yamam., Sci. Rep. Hyogo Univ. Agric., Ser. Agric. 4(1): 5. 1959. emend. MycoBank MB102888. Fig. 16.
Fig. 16.
Cladosporium funiculosum (DTO 127-E7). A–C. Colonies on PDA, MEA and OA. D–H. Conidiophores and conidia. I–J. Long conidial chains. Scale bars = 10 μm.
Holotype: Japan, isol. from leaves of Vigna umbellata [=Phaseolus chrysanthos] (Fabaceae), probably authentic strain of C. funiculosum. Ex-type culture: CBS 122129 = ATCC 38010 = IFO 6537 = JCM 10683.
Lit.: Bensch et al. (2010: 47−49; 2012: 128−129).
Ill.: Bensch et al. (2010: 48, figs 34−35; 2012: 128−129, figs 128−129).
Mycelium immersed and superficial, hyphae loosely branched, filiform to cylindrical-oblong or irregular in outline due to swellings, 1–3 μm wide, septate, smooth or loosely verruculose to densely verruculose, walls unthickened, sometimes forming ropes. Conidiophores micro-, semimacro- and macronematous, solitary, arising terminally and laterally from plagiotropous or ascending hyphae or hyphal strains, filiform to narrowly cylindrical-oblong, neither geniculate nor nodulose, unbranched, occasionally once branched, 10−120 × (2−)2.5−3.5(−4) μm, usually rather short, 0−2(−5)-septate, not constricted at septa, subhyaline to pale olivaceous brown, smooth or almost so, asperulate or minutely verruculose, walls unthickened. Conidiogenous cells integrated, terminal, sometimes intercalary, proliferation often distinctly sympodial, but neither geniculate nor nodulose, 10−45 μm long, with (1−)2−3(−4) loci crowded at the apex, sometimes few additional loci at a lower level, subdenticulate, 1−2 μm diam, somewhat thickened and darkened-refractive. Ramoconidia occasionally formed. Conidia catenate, in long unbranched or basely, often dichotomously branched chains, up to 8(−14) conidia in the unbranched terminal part, straight, small terminal conidia obovoid, narrowly ovoid, ellipsoid, sometimes narrowly obclavate, (2.5−)4−9 × (1.5−)2−2.5(−3) μm (av. ± SD: 5.3 ± 1.6 × 2.3 ± 0.3), aseptate, intercalary conidia narrowly ellipsoid, fusiform to subcylindrical, 5−13(−16) × 2−3 μm (av. ± SD: 9.6 ± 3.0 × 2.7 ± 0.3), 0−1-septate, with 1−3 distal hila, secondary ramoconidia ellipsoid to subcylindrical or cylindrical, (7−)11−23(−27) × 2.5−4.5(−5) μm (av. ± SD: 16.2 ± 5.1 × 3.3 ± 0.7), 0−1(−2)-septate, not constricted at septa, septum often somewhat in the upper half, with (1−)2−3(−4) distal hila, often with a second hilum near the base forming additional conidia “backwards”, subhyaline to pale olivaceous, smooth or almost so, sometimes reticulate, walls unthickened, slightly to distinctly attenuated towards apex and base, hila conspicuous, subdenticulate, 0.5−2 μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 57−78 mm diam after 14 d at 25 °C, glaucous-grey or olivaceous with tufts of pale olivaceous grey, reverse greenish grey, grey olivaceous or greyish blue, floccose, fluffy-felty, margin white to olivaceous, regular, aerial mycelium abundant, floccose to villose, low to high, growth effuse to low convex, somewhat wrinkled, sometimes with numerous small to large prominent exudates. Colonies on MEA 58−80 mm diam after 14 d at 25 °C, greenish or pale olivaceous grey to buff or rosy-buff, reverse olivaceous grey and iron-grey, velvety or floccose to felty, margin white, glabrous to feathery, aerial mycelium abundant, covering most of the colony surface, floccose to felty, smoke-grey or pale olivaceous grey, dense, low, growth effuse, radially furrowed and wrinkled, without prominent exudates. Colonies on OA attaining 47−67 mm diam after 14 d at 25 °C, white to smoke-grey, pale olivaceous grey or olivaceous grey, colony centre buff or rosy-buff, at margins faun, reverse leaden-grey, olivaceous grey to fawn, floccose to fluffy, margins glabrous, aerial mycelium abundant, covering almost the whole surface, floccose to felty, growth flat, with numerous small prominent exudates.
Substrate and distribution: Isolated from plant material and indoor air; Asia (Japan), North America (USA).
Additional materials examined: USA, Alabama, Birmingham, isol. from air sample, hospital, Jul. 2012, Ž. Jurjević, EMSL 1705 = CPC 22247; Massachusetts, Lekvile, isol. from indoor air sample, office, Oct. 2012, Ž. Jurjević, EMSL 1782 = CPC 22298; New Jersey, isol. from indoor air sample, Ž. Jurjević, EMSL 1756 = CPC 22282; Manasquan, isol. from indoor air sample, bedroom, Jan. 2013, Ž. Jurjević, EMSL 1906 = CPC 22391; Georgia, Tucker, isol. from indoor air sample, bakery, DTO 127-E7 = AR405.
Notes: The history of description, typification and deposited cultures of this species was discussed in Bensch et al. (2012). Conidiophore measurements and the species epithet “funiculosum” introduced in Yamamoto (1959) probably refer to hyphal strands and not conidiophores since these are often hardly distinguishable from hyphae or hyphal strands in the authentic strain. Cladosporium funiculosum was previously only known from two Japanese collections isolated from plant material (Bensch et al. 2010). Its species concept is herein emended to encompass several isolates from indoor environments collected in North America. It is characterised by its quite undifferentiated conidiophores and its smooth or somewhat reticulate conidia formed in long branched chains which is typical for species belonging to the C. cladosporioides species complex. Furthermore, it was reported from clinical samples in the USA (Sandoval-Denis et al. 2015). Cladosporium funiculosum (Fig. 1, clade 55) is phylogenetically distinct from other Cladosporium species.
Cladosporium globisporum Bensch et al., Stud. Mycol. 67: 51. 2010. MycoBank MB517080. Fig. 17.
Fig. 17.
Cladosporium globisporum (CPC 19124). A–C. Colonies on PDA, MEA and OA. D–H. Conidiophores and conidial chains. I–J. Micronematous conidiophores. K. Conidial chain. Scale bars = 10 μm.
Holotype: Sweden, isol. from meat stamp, 1986, M. Olsen, No. M291, CBS H-20435. Ex-type culture: CBS 812.96.
Lit.: Bensch et al. (2012: 139−141).
Ill.: Bensch et al. (2010: 51−53, figs 38−40), Bensch et al. (2012: 141, figs 146−148).
Mycelium mainly immersed, sparingly branched, 2−5 μm wide, septate, not constricted at septa, pale brown, smooth to minutely verruculose, walls unthickened. Conidiophores macro- and micronematous, solitary, arising terminally and laterally from ascending or plagiotropous hyphae, erect, straight to slightly flexuous, cylindrical-oblong to filiform, non-nodulose, sometimes geniculate, unbranched to once branched, branches as short denticle-like lateral outgrowths, later becoming longer, 17−165 × 3−5 μm, micronematous conidiophores (1−)2−2.5(−3) μm wide, 0−4-septate, cells quite long, not constricted at septa, septa often darkened, pale to pale medium brown, slightly paler towards the apex, minutely verruculose, asperulate, walls unthickened or slightly thickened, up to 1 μm wide. Conidiogenous cells integrated, often distinctly sympodially proliferating, terminal, usually non-nodulose, sometimes slightly geniculate, filiform to cylindrical-oblong, somewhat flexuous, 17−55 μm long, with up to three apical loci, sitting close together at the apex, conspicuous, subdenticulate to denticulate, (1.2−)1.5−2(−2.2) μm diam, thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 19−41(−56) × 3−4(−5) μm, 0(−2)-septate, base broadly truncate. Conidia catenate, in densely branched chains, straight to slightly curved, with 1−3 conidia in the terminal unbranched part of the chain, small terminal conidia globose, subglobose to obovoid, 2.5−6(−8) × (2.5−)3−4 μm (av. ± SD: 4.1 ± 1.3 × 3.1 ± 0.4), broadly rounded at the apex, intercalary conidia subglobose, broadly ellipsoid-ovoid, (4−)5−9(−14) × 3−4(−5) μm (av. ± SD: 6.9 ± 2.4 × 3.7 ± 0.5), aseptate, with up to 3(−5) distal hila, often distinctly denticulate, secondary ramoconidia ellipsoid to subcylindrical, 9−27(−30) × (3−)3.5−5(−6) μm (av. ± SD: 16.7 ± 5.7 × 4.2 ± 0.5), 0(−1)-septate, with 3−4 distal hila, sometimes hila not only distal but also lateral in the middle of the cell, pale brown, smooth or almost so, under SEM surface reticulate or with somewhat embossed stripes caused by diminishing turgor and shrivelling of tender young conidia, walls unthickened or only slightly so, attenuated towards apex and base, hila conspicuous, often distinctly denticulate, 0.5−2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA grey olivaceous to olivaceous, reverse leaden-grey or olivaceous black, velvety to powdery or floccose, margin colourless to white, feathery, aerial mycelium sparse, loose, fluffy, only few areas covered, growth flat, without exudates, sporulation profuse. Colonies on MEA grey olivaceous, pale olivaceous grey towards margins, reverse olivaceous grey, velvety, due to aerial mycelium several white patches, fluffy, loose to dense, without exudates, sporulation profuse. Colonies on OA grey olivaceous to pale olivaceous due to profuse sporulation or olivaceous buff, reverse leaden-grey to iron-grey, velvety to powdery, glittering due to numerous small, not very prominent exudates (like little water drops), margin colourless, feathery, aerial mycelium absent or sparse, growth flat.
Substrate and distribution: Isolated from indoor environments (Denmark) and meat stamp (Sweden).
Additional material examined: Denmark, isol. from indoor environments, window frame, 7 Feb. 2011, B. Andersen, BA 2038 = CPC 19124.
Notes: Cladosporium globisporum (Fig. 1, clade 17) is morphologically somewhat intermediate between the C. cladosporioides and C. sphaerospermum species complexes. The conidiophores are C. cladosporioides-like, whereas the terminal and intercalary globose or subglobose conidia are reminiscent of C. sphaerospermum, although they are smooth and not verruculose as in the latter species (Bensch et al., 2010, Bensch et al., 2012). It has so far only been known from the type specimen (Sweden, meat stamp), but the examined strain isolated from a window frame fits the species concept very well.
Cladosporium halotolerans Zalar et al., Stud. Mycol. 58: 172. 2007. MycoBank MB492439. Fig. 18.
Fig. 18.
Cladosporium halotolerans (DTO 161-D3). A–F. Conidiophores and conidial chains. G–I. Conidial chains. Scale bars = 10 μm.
Holotype: Namibia, isolated from hypersaline water of salterns, 1 Sep. 2000, coll. N. Gunde-Cimerman, isol. P. Zalar, 1 Oct. 2000, CBS H-19734. Ex-type culture: EXF-572 = CBS 119416.
Lit.: Haubold et al. (1998), Buzina et al., 2003, Meklin et al., 2004, Sandoval-Denis et al., 2015, Segers et al., 2016.
Ill.: Zalar et al. (2007: 172, fig. 8).
Mycelium party submerged, partly superficial; hyphae sparingly branched, (1–)2–4 μm wide, pluriseptate, septa often appearing somewhat darkened, usually not constricted, pale brown or pale olivaceous brown, almost smooth or minutely verruculose, walls unthickened, occasionally forming ropes. Conidiophores micro- to semimacronematous, arising laterally and terminally from hyphae, erect, straight to somewhat flexuous, narrowly cylindrical-oblong, occasionally slightly geniculate, non-nodulose, micronematous conidiophores filiform or only as short peg-like or denticle-like lateral outgrowths of hyphae, usually unbranched, sometimes intercalary with short lateral denticulate outgrowths just below a septum, 4–150(–300) × 2–3.5(–5.5) μm, micronematous conidiophores 1–1.5(–2) μm wide, mostly 0–3-septate, septa often appearing darkened, sometimes pluriseptate with up to 10 septa in short succession, especially towards the apex, septa not constricted, pale olivaceous brown, smooth to minutely verruculose, walls unthickened or almost so, sometimes forming ramoconidia and fragments. Conidiogenous cells integrated, terminal or sometimes intercalary, or conidiophores reduced to conidiogenous cells, cylindrical, 4–38 μm long, usually neither geniculate nor nodulose, with a single or up to four protuberant, subdenticulate or denticulate conidiogenous loci, 0.7–1.5(–2) μm diam, thickened and darkened. Ramoconidia 15–37(–46) × 2–3.5(–4) μm, 0–3(–5)-septate, base broadly truncate, about 2 μm wide, slightly thickened and somewhat darkened-refractive. Conidia catenate, in branched chains, conidial chains branching in all directions, terminal chains with up to 6(–9) conidia, small terminal conidia very numerously formed, globose or subglobose, 2–4(–6) × 2–3.5(–5) μm (av. ± SD: 3.5 ± 0.6 × 2.6 ± 0.5), aseptate, intercalary conidia subglobose, ovoid or ellipsoid, 3.5–9(–11) × (2–)2.5–3(–4) μm (av. ± SD: 6.2 ± 1.6 × 3.1 ± 0.5), 0(–1)-septate, pale to medium brown, often appear to be darker than conidiophores and secondary ramoconidia, minutely verruculose or verruculose, secondary ramoconidia ellipsoid, fusiform or cylindrical, 7–25(–31) × 2–3.5(–6.5) μm (av. ± SD: 16.2 ± 6.0 × 2.9 ± 2.0), 0–3(–4)-septate, mostly 1-septate, not constricted at septa, septa often somewhat darkened, pale to medium brown, almost smooth to minutely verruculose, walls unthickened, slightly attenuated towards apex and base, with up to four distal hila, hila protuberant, subdenticulate or denticulate, 0.5–1.5(–2) μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on PDA attaining 27–43 mm diam after 14 d at 25 °C, olivaceous, grey olivaceous or olivaceous grey, reverse olivaceous grey to leaden-grey or olivaceous black, velvety, powdery to felty-woolly, margins white, regular, glabrous or feathery, aerial mycelium absent or sparse, growth flat with a somewhat elevated colony centre, without prominent exudates, sporulation profuse. Colonies on MEA attaining 18–44 mm diam after 14 d at 25 °C, smoke-grey, pale olivaceous grey or olivaceous grey, sometimes glaucous grey at margin, reverse olivaceous grey, powdery to felty-woolly, margin colourless to white, glabrous or feathery, colony centre furrowed, aerial mycelium felty, abundant, covering most of the colony surface, sporulating. Colonies on MEA + 5 % NaCl 24–48 mm diam after 14 d at 25 °C, olive, furrowed, velvety, with more pale, undulate margins, reverse dark green to black. Colonies on OA reaching 29–40 mm diam after 14 d at 25 °C, smoke-grey to grey olivaceous or dark mouse-grey, reverse olivaceous or olivaceous grey, velvety to felty, fluffy, margin white, somewhat feathery, aerial mycelium sparse, diffuse or abundantly formed, high, dense, whitish, growth flat with papillate surface, sporulation profuse.
Maximum tolerated salt concentration: Only 15 % of tested strains develop colonies at 20 % NaCl after 7 d, whereas after 14 d all cultures grow and sporulate.
Cardinal temperatures: No growth at 4 °C, optimum at 25 °C, maximum at 30 °C. No growth at 37 °C (from Zalar et al. 2007).
Substrates and distribution: Saprobic, frequently isolated from indoor environments but also from hypersaline water in subtropical climates, Arctic ice and biomats, contaminant in lesions of humans and animals, plants, rock, soil, conifer wood and mycorrhizal roots; probably circumglobal, Africa (Namibia, South Africa), Arctics, Asia (China, India, Israel, Thailand, Turkey), Australasia (New Zealand), Europe (Belgium, Bosnia and Herzegovina, Denmark, Germany, France, Hungary, Italy, Russia, Slovenia, Spain, Sweden, Switzerland, The Netherlands, UK), North America (Canada, Mexico, USA), Central and South America (Argentina, Brazil, Dominican Republic).
Additional materials examined: China, isol. from indoor air, DTO 323-F3. UK, isol. from house dust, DTO 306-C9. USA, California, isol. from house dust, basement HVAC room, A. Amend, DTO 305-H6; DTO 306-B3 = AA03US-471, DTO 306-B8. Additional isolates are listed in Table 1.
Notes: Cladosporium halotolerans (Fig. 3, clade 23) proved to be a common species with a worldwide distribution occurring on a wide range of different substrates. Sandoval-Denis et al. (2015) reported C. halotolerans as the most frequent Cladosporium species recovered from clinical samples in the USA and it proved to be the most common species isolated from indoor environments (this study) representing about a third of all new indoor isolates.
Cladosporium sphaerospermum (Fig. 3, clade 20) is morphologically close but differs in producing somewhat wider, 2.5–4.5(–6) μm, often branched, pluri- and densely septate conidiophores, slightly longer terminal conidia, (2–)3–5(–7) μm, longer ramoconidia, up to 50(–67) μm long and with up to five septa being commonly beaked (alternarioid) on MEA and PDA. Cladosporium domesticum (Fig. 3, clade 21) and C. parahaloterans (Fig. 3, clade 22) are introduced in the present study as two new species occurring in indoor environments; they proved to be closely related but are both phylogenetically as well as morphologically distinguishable from C. halotolerans. Cladosporium parahalotolerans forms wider conidia and ramoconidia; and C. domesticum produces narrower conidia and ramoconidia.
Cladosporium inversicolor Bensch et al., Stud. Mycol. 67: 55. 2010. MycoBank MB517082. Fig. 19.
Fig. 19.
Cladosporium inversicolor (CPC 22300). A–C. Colonies on PDA, MEA and OA. D–H. Conidiophores and conidial chains. J. Ramoconidium and conidia. K–L. Conidia. Scale bars = 10 μm.
Holotype: The Netherlands, isol. from a leaf of Triticum aestivum (Poaceae), deposited Jul. 1980 as C. cladosporioides, isol. by N.J. Fokkema, ident. by G.A. de Vries, CBS H-20437. Ex-type culture: CBS 401.80 = ATCC 200941.
Lit.: Bensch et al. (2012: 163−165; 2015: 45).
Ill.: Bensch et al. (2010: 55−56, figs 43−44), Bensch et al. (2012: 164, figs 175−176).
Mycelium immersed and sparingly superficial; hyphae mainly unbranched, 1.5−3(−4.5) μm wide, septate, not constricted at septa, without swellings, pale olivaceous to pale olivaceous brown, smooth to often minutely verruculose, walls unthickened. Conidiophores macronematous, solitary, arising terminally and laterally from hyphae, erect, straight to somewhat flexuous, cladosporioides-like, cylindrical-oblong, somewhat geniculate-sinuous towards or at the apex, non-nodulose, unbranched or once branched, 15−225 × 2.5−4(−5) μm, aseptate or with few septa, not constricted at septa, subhyaline to very pale olivaceous brown, smooth, sometimes rough-walled at the base; occasionally also micronematous, about 1.5 μm wide. Conidiogenous cells integrated, mainly terminal, cylindrical-oblong, non-nodulose, sometimes geniculate at or towards the apex due to sympodial proliferation, 15−66 μm long, with (1−)2−3 loci, conspicuous, subdenticulate, 1−2 μm diam, somewhat thickened and darkened-refractive. Ramoconidia occasionally formed, cylindrical-oblong, 17−42 × 3−3.5 μm, 0−1(−3)-septate, occasionally with up to three septa, base (1.8−)2−3 μm wide, unthickened. Conidia numerous, catenate, in often dichotomously branched chains, sometimes branching in more directions, terminal unbranched parts of the chains often very long, up to eight conidia, sometimes even up to 17 conidia, small terminal conidia obovoid to ellipsoid, sometimes subglobose, (3−)5−7(−8.5) × 2−3(−3.5) μm (av. ± SD: 5.4 ± 1.5 × 2.6 ± 0.4), aseptate, apex rounded, attenuated towards the base, intercalary conidia ovoid, fusiform to ellipsoid, (5−)7−13(−20) × (2−)2.5−3.5(−4) μm (av. ± SD: 9.8 ± 3.4 × 2.9 ± 0.4), aseptate, attenuated towards apex and base, with 1−3(−4) distal hila, secondary ramoconidia subcylindrical, 10.5−24(−29) × (2.2−)2.8−4(−4.2) μm (av. ± SD: 16.6 ± 3.9 × 3.3 ± 0.5), 0−1(−2)-septate, but mainly aseptate, not constricted at septa, pale to olivaceous brown, small terminal conidia and intercalary conidia slightly darker than ramoconidia, secondary ramoconidia and conidiophores, smooth to loosely minutely verruculose or irregularly rough-walled, rugose, verruculose-rugose surface ornamentation especially in small terminal and intercalary conidia, conidia slightly attenuated towards apex and base, with (1−)2−4(−6) distal hila, walls unthickened or almost so, hila conspicuous, subdenticulate, 0.5−2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 42−70 mm diam after 14 d at 25 °C, olivaceous grey or olivaceous, grey olivaceous towards margins, leaden-grey to olivaceous black reverse with grey olivaceous margins, floccose, margins regular, white or colourless, aerial mycelium sparse to abundant, diffuse to floccose, loose to dense, growth effuse. Colonies on MEA 39−60 mm diam after 14 d at 25 °C, grey olivaceous to olivaceous grey or olivaceous, reverse iron-grey to black, velvety or powdery to floccose, margins colourless or white, regular or somewhat undulate, radially furrowed and somewhat wrinkled, aerial mycelium whitish to smoke-grey, felty-floccose, growth effuse. Colonies on OA 43−60 mm diam after 14 d at 25 °C, grey olivaceous to greenish olivaceous, olivaceous, olivaceous grey or olivaceous buff, reverse pale greenish grey to olivaceous grey, leaden-grey or iron-grey, velvety to floccose, margins glabrous, olivaceous grey, narrow, aerial mycelium smoke-grey to pale olivaceous grey, felty, growth flat. Sporulation profuse and without prominent exudates on all media.
Substrates and distribution: On plant material, isol. from air, indoor environments and food, also mycophilic; Africa (South Africa), Europe (Denmark, France, Germany, The Netherlands), North America (USA), South America (Colombia).
Additional materials examined: Denmark, isol. from indoor air, 2 Feb. 2011, B. Andersen, CPC 19108; isol. from indoor environment, B. Andersen, DTO 109-E9 = BA 1909. France, isol. from indoor environment, J. Dijksterhuis, DTO 108-F8. The Netherlands, Amsterdam, indoor air archive, M. Meijer, CBS 139573 = DTO 072-C9. USA, Oregon, Portland, isol. from indoor air sample, living room, October 2012, Ž. Jurjević, EMSL 1806 = CPC 22300; Salem, isol. from indoor air sample, bedroom, Sep. 2012, Ž. Jurjević, EMSL 1763 = CPC 22287; Washington, Tacoma, isol. from indoor air sample, bedroom, Jan. 2013, Ž. Jurjević, EMSL 1900 = CPC 22385.
Notes: Cladosporium inversicolor (Fig. 1, clade 42) belongs to the C. cladosporioides species complex. The name of this species is derived from the unusual pigmentation of conidia with small and intercalary conidia being usually darker than ramoconidia, secondary ramoconidia and conidiophores, which is unique and distinctive among Cladosporium species of this complex.
Cladosporium langeronii (Fonseca et al.) Vuillemin, Champ. Paras. Myc. Homme: 78. 1931. MycoBank MB328341. Fig. 20, Fig. 21.
Fig. 20.
Cladosporium langeronii (DTO 124-D5). A–C. Colonies on PDA, MEA and OA. D–F. Conidiophores and conidia. G. Superficial mycelium. H. Ramoconidium and conidial chains. I–J. Conidial chains. Scale bars = 10 μm.
Fig. 21.
Cladosporium langeronii (DTO 124-D5). A. Survey of colony structure of conidia on conidiophores. B. Young conidiophores formed on series of rounded cells, in one case with a transverse septum. C. As B, Here the distinct ornamentation of conidia is visible. D. Conidial chains, showing markedly less ornamentation at the apical end of the ramoconidia. E. Young conidiophore, with conidial chain, showing smooth apical zones and smooth necks between spores. F. Conidial chains showing the more distinct ornamentation in terminal conidia. Ornamentation exists out of distinct ridges that are more or less parallel. G–J. Details of conidial ornamentation with smooth apical zones and necks except in terminal conidia. Figure J shows a conidium initial. Scale bars = 2 (I, J), 5 (E–H), 10 (B–D), 20 (A) μm.
Basionym: Hormodendrum langeronii Fonseca et al., Sciencia Med. 5: 563. 1927.
Neotype: Brazil, isolated from human ulcero-nodular mycosis of hand and arm, 1927, coll. & isol. by da Fonseca, CBS H-19737. Ex-type culture: CBS 189.54.
Lit.: Zalar et al. (2007: 173–174), Bensch et al. (2012: 171–172).
Ill.: Zalar et al. (2007: 174, fig. 9), Bensch et al. (2012: 171: fig. 184).
Mycelium partly immersed, partly superficial; hyphae branched, 1–3 μm wide, septate, without swellings and constrictions, subhyaline to pale brown, smooth or almost so, sometimes enveloped in polysaccharide-like material, sometimes forming few swollen hyphal cells, up to 7 μm diam, arranged like a starting stroma giving rise to several conidiophores appearing loosely fasciculate. Conidiophores macro- and micronematous, arising terminally and laterally from submerged and superficial hyphae, erect or ascending, straight to slightly flexuous. Macronematous conidiophores cylindrical-oblong, sometimes geniculate-sinuous, non-nodulose, (20–)50–235(–470) × 2.5–4.5(–6) μm, unbranched or often branched, once or several times, branches not only as short peg-like prolongations but longer, distinct, one branching often below the apex, pluriseptate, not constricted at septa, medium to dark brown, somewhat paler at the apex, smooth to verruculose or irregularly rough-walled, walls slightly thickened, about 0.5 μm wide. Conidiogenous cells integrated, terminal, sometimes also intercalary, cylindrical, 9–25 μm long, slightly attenuated at the apex, sometimes seceding and forming ramoconidia, usually with a single apical scar, protuberant, 0.8–1.5(–2) μm diam, thickened and darkened-refractive. Micronematous conidiophores filiform, mostly unbranched, rarely branched, 6–120 μm long or longer, 1–2 μm wide, pale brown, septate, smooth or almost so, walls unthickened. Conidiogenous cells integrated, terminal or sometimes discrete, with a single apical scar, protuberant, 0.5–1 μm diam, thickened and darkened-refractive. Ramoconidia cylindrical, 0–1-septate, (10–)11–22(–42) × (3–)3.5–4.5(–5) μm, base broadly truncate, 2–3.5 μm wide, slightly thickened and somewhat darkened. Conidia catenate, in dichotomously branched chains, with up to 7(–8) conidia in the terminal, unbranched parts, straight, small terminal conidia subglobose or ovoid, (2.5–)4–5.5(–8) × (2–)3–4(–5) μm (av. ± SD: 3.7 ± 0.6 × 3.2 ± 0.4 μm), aseptate, rarely 1-septate, hila 0.5–0.8 μm diam, apex rounded, intercalary conidia broadly ovoid to ellipsoid, 5–8(–11) × 3–4 μm (av. ± SD: 6.7 ± 2.0 × 3.7 ± 0.5 μm), 0(–1)-septate, not constricted, attenuated towards apex and base, with a single apical hilum, 0.5–1 μm diam, secondary ramoconidia ellipsoid to cylindrical, (5.5–)9–20(–26) × (2.5–)3–4.5(–5.5) μm (av. 14.4 ± 4.3 × 3.5 ± 0.5 μm), 0–1(–2)-septate, not constricted at septa, pale to medium or dark brown, irregularly verruculose to sometimes loosely verrucose, walls slightly or more distinctly thickened, with 1–2(–3) distal hila, hila protuberant, peg-like, denticulate, 0.8–1.5(–2) μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring. Conidia formed by micronematous conidiophores paler, narrower, usually only in unbranched chains, filiform, ellipsoid to obclavate, 3–12 × 1.5–2.5 μm, 0(–1)-septate.
Culture characteristics: Colonies on PDA, OA and MEA with restricted growth, attaining 2.5–4.5, 1.5–7 and 1–5.5 mm diam after 14 d at 25 °C, respectively. Colonies flat or heaped (up to 3 mm), dark green, with black reverse and slightly undulate margin with immersed mycelium. Sporulating on all media. On MEA + 5 % NaCl growth is faster, colonies attaining 8.5–12 mm diam after 14 d at 25 °C, sporulating and growing deeply into the agar.
Maximum tolerated salt concentration: All strains develop colonies at 17 % NaCl after 14 d at 25 °C.
Cardinal temperatures: No growth at 4 °C, optimum/maximum at 25 °C (1–5.5 mm diam), no growth at 30 °C (from Zalar et al. 2007).
Substrate and distribution: Indoor environments, air, conifer wood, humans; Europe (Belgium, Denmark, Ireland, The Netherlands), North America (USA), South America (Brazil).
Additional materials examined: Belgium, isol. from a moist aluminium school window frame, CBS 101880. Denmark, isol. from indoor air, 2 Feb 2011, BA 2035 = CPC 19121. Ireland, Dublin, isol. from indoor air sample, washroom, Nov. 2012, Ž. Jurjević, EMSL 1831, 1832 = CPC 22325, 22326. The Netherlands, Eindhoven, isol. from a swab sample, house, J. Houbraken, DTO 004-C3; ‘s Hertogenbosch, indoor air archive, M. Meijer, DTO 085-H6; Ospel, air sample food plant, DTO 124-D2, DTO 124-D5 = CBS 139581. USA, Delaware, isol. from indoor air storage sample, Pineapple room, June 2012, Ž. Jurjević, EMSL 1681 = CPC 22235; Minnesota, isol. from indoor air sample, Aug. 2012, Ž. Jurjević, EMSL 1716 = CPC 22261; Pennsylvania, Kutztown, isol. from indoor air sample, Oct. 2012, Ž. Jurjević, EMSL 1783 = CPC 22299.
Notes: Cladosporium langeronii (Fig. 3, clade 13) is a saprobic species belonging to the C. sphaerospermum species complex. It has been repeatedly isolated from indoor environments. The strain CBS 109868, which was previously identified and treated as C. langeronii (Zalar et al. 2007), proved to belong to the newly described species C. neolangeronii (Fig. 3, clade 10). The latter species which is both morphologically as well as phylogenetically closely allied differs from C. langeronii in having longer ramoconidia and secondary ramoconidia as well as faster growth rates. Zalar et al. (2007) stated already that C. langeronii most likely represents a complex of at least two species with strains from the Arctic and the Antarctic probably being distinct from C. langeronii on species level. These isolates from polar ice and biomats from the Arctic and Antarctic clustered with CBS 109868 in the phylogenetic analyses carried out by Zalar et al. (2007) and are, therefore, conspecific with C. neolangeronii.
Cladosporium limoniforme Bensch et al., Stud. Mycol. 82: 47. 2015. MycoBank MB814628. Fig. 22.
Fig. 22.
Cladosporium limoniforme (CPC 22395). A–C. Colonies on PDA, MEA and OA. D–K. Conidiophores and conidial chains. L–M. Conidia. Scale bars = 10 μm.
Holotype: Egypt, isolated from Musa acuminata (Musaceae), 2005, coll. R.S. Summerbell, isol. P.W. Crous, CBS H-22354. Ex-type culture: CBS 140484 = CPC 12039.
Ill.: Bensch et al. (2015: 49–50, figs 13–14).
Mycelium sparingly formed, usually unbranched, 1.5–3 μm wide, pale olivaceous brown or subhyaline, asperulate to minutely verruculose, walls unthickened, sometimes forming small ropes of a few hyphae. Conidiophores micro- to semimacronematous, sometimes macronematous, short, sometimes only as very short lateral branches of hyphae, not very prominent, sometimes hardly distinguishable from hyphae, usually reduced to conidiogenous cells or 1(–2)-septate, terminally arising from hyphae, occasionally laterally arising from plagiotropous hyphae, unbranched, rarely branched, usually neither geniculate nor nodulose, rarely once geniculate, 5–90(–130) × (1–) 2–3(–4) μm, mostly only up to 60 μm long, subhyaline, pale brown to pale olivaceous brown, concolourous with hyphae, smooth or almost so to asperulate or somewhat irregularly rough-walled. Conidiogenous cells integrated, terminal, occasionally intercalary, narrowly cylindrical, neither geniculate nor nodulose, 15–34(–50) μm long, with 1–3 pronounced scars at the apex or situated on short lateral outgrowths at the apex in terminal cells, in intercalary cells a single or two loci situated on small lateral prolongations just below a septum, conidiogenous loci 1–1.5 μm diam, somewhat thickened and darkened-refractive. Ramoconidia 15–40(–50) μm long, 0(–1)-septate, base 2–2.5(–3) μm wide, somewhat refractive. Conidia catenate, very numerous, usually 3–7(–8) conidia in the terminal unbranched part of the chain, occasionally up to 13, pale olivaceous brown or pale brown, ornamentation variable, loosely verruculose, sometimes somewhat spiny or irregularly rough-walled, walls unthickened, small terminal conidia obovoid to subglobose, apex rounded, attenuated towards the base, 3–4.5(–6.5) × (2–)2.5–3 μm (av. ± SD: 4.1 ± 0.8 × 2.6 ± 0.4), aseptate, intercalary conidia limoniform, ovoid to ellipsoid, sometimes fusiform, sometimes rostrate, (4–)5–10(–12) × 2.5–3.5(–4) μm (av. ± SD: 7.0 ± 1.9 × 3.1 ± 0.5), aseptate, very rarely 1-septate, attenuated towards apex and base, with 1–2(–3) distal hila, secondary ramoconidia ellipsoid, fusiform to subcylindrical, (8–)9.5–23(–30) × (2.5–)3–4 μm (av. ± SD: 16.2 ± 5.0 × 3.4 ± 0.4), 0–1-septate, septum sometimes becoming sinuous with age, pale olivaceous brown or pale brown, surface ornamentation variable, loosely verruculose, sometimes somewhat spiny or irregularly rough-walled, walls unthickened, with 2–3(–4) distal hila, hila protuberant, 0.5–1.5 μm diam, slightly thickened and somewhat darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining 34–65 mm diam after 14 d at 25 °C, smoke-grey, iron-grey to dark grey olivaceous, sometimes dull green due to abundant sporulation, reverse iron-grey to olivaceous black, velvety to granular or floccose; margins regular, broad, white, glabrous to feathery; aerial mycelium sparse, diffuse, sometimes more abundantly formed in colony centre and then villose to densely tufted; growth flat, regular, sometimes with numerous small to large prominent exudates. Colonies on MEA reaching 39–57 mm diam after 14 d at 25 °C, grey olivaceous, greenish olivaceous to smoke-grey or glaucous-grey towards margins, sometimes large parts smoke-grey to glaucous-grey or whitish due to aerial mycelium, reverse olivaceous grey, iron-grey to black, granular, velvety to floccose; margins regular, narrow to broad, white, feathery to glabrous; aerial mycelium sparse or covering large parts of the colony; growth flat with somewhat elevated colony centre, radially furrowed, sporulation profuse. Colonies on OA attaining up to 69 mm diam after 14 d at 25 °C, grey olivaceous to olivaceous due to abundant sporulation forming concentric zones, reverse pale olivaceous grey to olivaceous grey or leaden-grey, velvety, floccose to felty; margins regular, narrow to broad, glabrous to feathery, greenish olivaceous; aerial mycelium absent, sparse or more abundantly formed covering large parts of the colony, smoke-grey; growth flat, without prominent exudates, sporulation profuse.
Substrate and distribution: Isolated from plant material, indoor environments and hypersaline water; Africa (Egypt), Asia (Israel), Australia, Europe (Cyprus, The Netherlands) and North America (USA).
Strains examined: Australia, isol. from house dust, DTO 305-G4 = BH02AU-115. Cyprus, Polis, isol. from Eucalyptus sp. (Myrtaceae), 18 Mar. 2007, coll. A. van Iperen, isol. P.W. Crous, CPC 13923. Israel, Dead Sea, Ein Bokek, isol. from hypersaline water, 2004, P. Zalar, EXF-1062 = CPC 12049; Ein Gedi, 31.45, 35.3833, isol. from hypersaline water, 2004, P. Zalar, EXF-1060 = CPC 12048, EXF-1081 = CPC 12050. The Netherlands, Utrecht, swab sample, archive, M. Meijer, DTO 090-H8; Weert, isol. from indoor air living room, B. Favié, DTO 082-F2. USA, isolated from grape berry, F.M. Dugan lab, CBS 113737; Arizona, Tuscon, isol. from indoor air sample, hospital, Jan 2013, Ž. Jurjević, EMSL 1909, 1910 = CPC 22394, 22395; California, Indio, isol. from under kitchen sink sample, Jan 2013, Ž. Jurjević, EMSL 1899 = CPC 22384; La Mesa, isol. from indoor air sample, bedroom, Dec. 2012, Ž. Jurjević, EMSL 1863 = CPC 22350. Unknown, from tomato, CPC 18086 = KSU C1.
Notes: Cladosporium limoniforme (Fig. 2, clade 36) is well characterised by its few micronematous conidiophores forming large numbers of conidia and its limoniform intercalary conidia. Conidial surface ornamentation is typical for species belonging to the C. herbarum species complex. It is phylogenetically but not morphologically allied to C. aggregatocicatricatum (Fig. 2, clade 34). The latter species clearly differs in having much longer macronematous conidiophores being once or several times slightly to distinctly geniculate-sinuous or subnodulose with clusters of pronounced scars at apices or intercalary. The closest phylogenetic relative of C. limoniforme proved to be C. prolongatum (Fig. 2, clade 35) which was recently described from soil in China but differs in having shorter secondary ramoconidia and a densely verruculose conidial surface ornamentation (Ma et al. 2017). Cladosporium paralimoniforme (Fig. 2, clade 1), an additional species described from soil in China, resembles C. limoniforme but forms a distinct clade distant from C. limoniforme in the C. herbarum species complex and is distinguishable in having shorter conidiophores, ramoconidia and secondary ramoconidia (Ma et al. 2017).
Cladosporium lycoperdinum Cooke, Grevillea 12(61): 32. 1883. MycoBank MB217533.
Lectotype (designated in Heuchert et al. 2005): USA, South Carolina, Aiken, on Lycoperdon sp. (Agaricales), Ravenel & Cooke, Fungi Amer. Exs. 595 (K 121561). Isolectotypes: Ravenel & Cooke, Fungi Amer. Exs. 595 (e.g., BPI 427244, NY).
Lit.: Heuchert et al. (2005: 33–36), Bensch et al. (2010: 58–60; 2012: 178–180).
Ill.: Heuchert et al. (2005: 34–35, figs 11–12), Bensch et al. (2010: 59, fig. 48; 2012: 194–195).
Mycelium unbranched or loosely branched, filiform to cylindrical-oblong, (0.5−)1−5 μm wide, not constricted at septa, subhyaline to pale or medium olivaceous brown, smooth or almost so to often minutely verruculose or loosely verrucose, walls unthickened or almost so, occasionally forming ropes. Conidiophores macro- and micronematous, solitary, arising terminally and laterally from hyphae, erect, straight or slightly flexuous, macronematous conidiophores cylindrical-oblong or filiform, non-nodulose, usually not geniculate, occasionally slightly geniculate at or towards the apex due to sympodial proliferation, unbranched or once, rarely twice branched, branches often only as short lateral peg-like prolongations just below a septum, 20−250 × (2.5−)3−6(−6.5) μm, pluriseptate, with septa occasionally in short succession, not constricted at septa, few septa sometimes darkened just below potential ramoconidia or where conidiophores disarticulate into shorter pieces, pale olivaceous to medium olivaceous brown, smooth to somewhat irregularly rough-walled or minutely verruculose, especially at or towards the base, walls unthickened or almost so, about 0.5 μm wide, sometimes slightly attenuated towards the apex or intercalary somewhat wider; micronematous conidiophores narrower, shorter and paler, 9−105 × 1.5−2.5 μm, filiform, not geniculate, unbranched or once branched, 0−5-septate, subhyaline to pale olivaceous, conidiogenous cells 6.5−50 μm long, loci 0.5−1.2 μm diam. Conidiogenous cells integrated, terminal, intercalary or sometimes pleurogenous, often seceding and forming ramoconidia, cylindrical-oblong, sometimes slightly geniculate due to sympodial proliferation, 10−57 μm long, with (1−)2−4 loci at or towards the apex, sometimes with additional loci situated on a lower level, in intercalary conidiogenous cells loci usually situated on small peg-like lateral outgrowths, loci conspicuous, subdenticulate to denticulate, 1−2 μm diam, thickened and darkened-refractive. Ramoconidia often formed, cylindrical-oblong, 13.5−55 × 3−5(−5.5) μm, 0−3(−6)-septate, not constricted at septa, with 2−4 distal hila, base broadly truncate, 2.2−3(−3.5) μm wide, unthickened or slightly thickened, often somewhat darkened or refractive, without dome and rim. Conidia catenate, in branched chains branching in all directions, up to 5(−7) conidia in the terminal unbranched part of the conidial chains, straight, small terminal conidia subglobose to obovoid or narrowly ellipsoid, (2−)3.5−5 × (1.5−)2−2.5(−3) μm (av. ± SD: 4.2 ± 0.7 × 2.0 ± 0.3), aseptate, intercalary conidia limoniform, ovoid to ellipsoid, 4−14(−16.5) × (2−)2.5−3(−4) μm (av. ± SD: 8.6 ± 3.0 × 2.8 ± 0.5), 0(−1)-septate, with 1−3(−4) distal hila, secondary ramoconidia ellipsoid to cylindrical, sometimes almost doliiform, 8−32(−38) × (2.5−)3−4(−5) μm (av. ± SD: 15.6 ± 6.3 × 3.5 ± 0.5), 0−1(−3)-septate, not constricted at septa, pale olivaceous to pale olivaceous brown, smooth or almost so, walls unthickened or almost so, with 2−5 distal hila, intercalary conidia and secondary ramoconidia sometimes formed in dense whirls at the conidiogenous cells or secondary ramoconidia, hila conspicuous, subdenticulate, 0.5−2(−2.5) μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining 50−68 mm diam after 14 d at 25 °C, olivaceous grey, grey olivaceous towards margins, reverse leaden-grey to olivaceous black, floccose to fluffy, margins white to grey olivaceous, feathery, regular, aerial mycelium abundant, covering the whole colony surface, floccose to fluffy, growth flat to low convex, without prominent exudates, sporulation profuse. Colonies on MEA reaching 50−62 mm diam after 14 d at 25 °C, olivaceous grey to pale olivaceous grey, sometimes smoke-grey or white, reverse olivaceous grey to iron-grey, floccose to felty, margins white, narrow, feathery, regular, aerial mycelium abundant, covering the whole colony surface, growth flat to low convex, sometimes radially furrowed, without prominent exudates, sporulation profuse. Colonies on OA attaining 58−70 mm diam after 14 d at 25 °C, olivaceous to greenish olivaceous, olivaceous grey at margins, reverse leaden-grey to olivaceous grey, floccose to felty, margins glabrous, aerial mycelium abundant covering almost the whole colony surface, loose to dense, low to rarely high, growth at, without prominent exudates, sporulation profuse.
Substrate and distribution: On ascomycetes and fruiting bodies of different basidiomycetous fungi, as well as isolated from plant material and outside air; Europe (Germany, Russia), North America (Canada, USA) and South America (Colombia, Uruguay).
Additional material examined: USA, Minnesota, isol. from outside air sample, Jul. 2012, Ž. Jurjević, EMSL 1711b = CPC 22256.
Notes: The outside air sample from Minnesota proved to cluster with isolates that have been identified as C. lycoperdinum (Fig. 1, clade 33). An epitype for that species has not yet been designated since type material was collected on a basidiomycete, but the available cultures, which morphologically coincide with C. lycoperdinum (Heuchert et al. 2005), were isolated from ascomycetes or plant material (Bensch et al. 2010).
Cladosporium macrocarpum Preuss, in Sturm, Deutsch. Fl. 3(26): 27. 1848. MycoBank MB217783.
Neotype (designated by Schubert et al. 2007b): USA, Washington, isolated from Spinacia oleracea (Chenopodiaceae), 1 Jan. 2003, L. du Toit, CBS H-19855. Isoneotype: HAL 2020 F. Ex-neotype culture: CBS 121623 = CPC 12755.
Lit.: Bensch et al. (2012: 180−185).
Ill.: Schubert et al. (2007b: 129−132, figs 22−25), Bensch et al. (2012: 180−183, figs 196−199).
Mycelium unbranched or loosely branched, 1–4.5(–5) μm wide, septate, sometimes slightly constricted at septa, hyaline to pale brown, smooth to minutely verruculose, walls unthickened or slightly thickened. Conidiophores micronematous and macronematous, solitary, arising terminally from plagiotropous hyphae or terminally from ascending hyphae. Macronematous conidiophores erect, straight to somewhat flexuous, cylindrical-oblong, nodulose to nodose, with a single apical or usually several swellings either somewhat distinct from each other or often in short succession giving conidiophores a knotty appearance, swellings sometimes laterally elongated or formed at the top of a branch-like outgrowth below the apical swelling, sometimes distinctly geniculate, unbranched, sometimes branched, 12–260 × (3–)4–6 μm, swellings 5–10 μm wide, pluriseptate, sometimes slightly constricted at septa, pale to medium brown or olivaceous brown, somewhat paler at apices, smooth to minutely verruculose or verruculose, walls somewhat thickened, sometimes even two- layered. Conidiogenous cells integrated, terminal or intercalary, cylindrical, nodulose with lateral shoulders or nodose with swellings round about the stalk, with conidiogenous loci confined to swellings, 12–37 μm long, with up to 12 loci per cell, usually with up to six, loci conspicuous, protuberant, (1–)1.5–2 μm diam, somewhat thickened and darkened-refractive. Micronematus conidiophores almost indistinguishable from hyphae, straight, narrowly filiform, non-nodulose or with a single or few swellings, mostly with small head-like swollen apices, usually only few micrometer long, 1.5–3 μm wide, aseptate or with only few septa, subhyaline, smooth or almost so, walls unthickened, with a single or only few conidiogenous loci, narrow, 0.8–1.2 μm diam, thickened and somewhat darkened-refractive. Conidia catenate, in branched chains, small terminal conidia subglobose, obovoid, oval, limoniform, 4–11 × (3–)4–6 μm [av. ± SD, 7.6 (± 1.9) × 5.0 (± 0.8) μm], aseptate, intercalary conidia broadly ovoid-ellipsoid, 10–17 × (4.5–)5–9 μm [av. ± SD, 12.7 (± 2.1) × 6.8 (± 0.8) μm], 0–1-septate; secondary ramoconidia broadly ellipsoid to subcylindrical, 14–25(–30) × (5–)6–9(–10) μm [av. ± SD, 19.4 (± 3.5) × 7.6 (± 1.0) μm], 0–2(–3)-septate, sometimes slightly constricted at the septa, septa somewhat sinuous with age, pale brown to medium olivaceous brown or brown, sometimes even dark brown, verruculose to echinulate (muricate under SEM), walls thickened, up to 1 μm thick, mostly broadly rounded at apex and base, sometimes attenuated, sometimes guttulate by oil drops, with up to three apical hila, mostly 1–2, hila sessile (apparently somewhat immersed) to somewhat protuberant, 1–2(–2.5) μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occurring with conidia forming secondary micro- and macronematous conidiophores, conidia often germinating with long hyphae. Conidia formed by micronematous conidiophores usually smaller, narrower and paler, catenate, in short unbranched or branched chains, subglobose, obovoid to limoniform, ellipsoid or fusiform, 2.5–16 × 1.5–5 μm, 0(–1)-septate, few longer conidia subcylindrical to clavate, up to 37(–43) μm long, 0–2(–3)-septate, occasionally with up to four septa, sometimes slightly constricted at the septa, subhyaline to pale brown, almost smooth to minutely verruculose, walls unthickened, hila 0.8–1.2 μm diam, thickened and darkened-refractive.
Culture characteristics: Colonies on PDA reaching 30–43 mm in diam after 14 d at 25 °C, dark dull green to olivaceous grey, olivaceous grey, dark olivaceous to iron-grey reverse, pulvinate, velvety, sometimes somewhat zonate, paler zones towards the margin, margin regular, entire edge, almost colourless to white, glabrous to feathery, aerial mycelium sparse to more abundant in the colony centre or covering large areas of the colony, hairy, fluffy or felty, whitish to smoke-grey, sometimes becoming reddish, livid red to vinaceous, growth flat, regular, sometimes forming few prominent exudates, exudates sometimes slightly purplish, sporulation profuse with two kinds of conidiophores, low and high. Colonies on MEA reaching 31–50 mm in diam after 14 d at 25 °C, grey olivaceous to olivaceous grey or iron-grey, sometimes pale olivaceous grey to whitish due to abundant aerial mycelium, olivaceous grey or iron-grey reverse, velvety or powdery, margin narrow, entire edge, colourless to white, glabrous, aerial mycelium sparse to abundant, hairy or felty, growth regular, flat to low convex, radially furrowed, without prominent exudates, sporulation profuse. Colonies on OA reaching 29–40 mm in diam after 14 d at 25 °C, grey olivaceous, olivaceous grey to dark smoke-grey, olivaceous black or iron-grey reverse, margin entire edge, narrow, colourless or white, glabrous, aerial mycelium sparse, mainly in the colony centre, felty, white to smoke-grey or grey-olivaceous, felty, growth flat, regular, without exudates, sporulating.
Substrate and distribution: Decaying plant material, on dead fruiting bodies of other fungi, occasionally as secondary invader on lesions caused by other fungi, isolated from dust, human, water, incl. hypersaline water; widespread, almost cosmopolitan.
Additional material examined: Denmark, isol. from dust, school, 2007, B. Andersen, BA 1704 = CPC 14305.
Notes: This isolate from dust agrees well with the species concept of C. macrocarpum (Fig. 2, clade 16).
Cladosporium needhamense Bensch & Samson, sp. nov. MycoBank MB822221. Fig. 23.
Fig. 23.
Cladosporium needhamense (CBS 143359). A–C. Colonies on PDA, MEA and OA. D–G. Macronematous conidiophores and conidia. H, J. Micronematous conidiophores and conidia. I. Ramoconidium and conidial chains. K. Conidial chains. Scale bars = 10 μm.
Etymology: Name refers to the place where the type specimen was collected, Needham.
Holotype: USA, Massachusetts, Needham, isol. from indoor air sample, office, Dec. 2012, Ž. Jurjević, CBS H-23252. Ex-type culture: CBS 143359 = CPC 22353 = EMSL 1866.
Diagnosis: Differs from C. uwebraunianum in having shorter conidiogenous cells (3–22 μm vs 17–50(–65) μm) and in forming densely branched chains, with 1–6(–8) conidia in the terminal unbranched part of the chains.
Superficial mycelium commonly formed, filiform or narrowly cylindrical-oblong, loosely branched, (0.5–)1–3.5 μm wide, sometimes up to 6 μm wide and then constricted at septa, pluriseptate, subhyaline or pale olivaceous or olivaceous brown, smooth or almost so, minutely verruculose or irregularly rough-walled, sometimes forming ropes of a few hyphae. Conidiophores micro-, semimacro- and macronematous, numerously formed both laterally and terminally, arising from hyphae as short peg-like lateral outgrowths or longer, filiform to cylindrical-oblong, straight or flexuous, sometimes geniculate due to sympodial proliferation, once or several times, variable with regard to shape and size, unbranched or branched, 3–120 μm long, micronematous conidiophores 0.5–2 μm wide, macro- and semimacronematous conidiophores 2.5–3.5(–4) μm wide, septate, sometimes distinctly constricted at one of the septa, subhyaline or olivaceous brown, almost smooth, verruculose or irregularly rough-walled. Conidiogenous cells 3–22 μm long, terminal with dense clusters of pronounced scars at or towards the apex, up to seven loci closely aggregated, or reduced to conidiogenous cells, formed as short peg-like lateral outgrowth of hyphae, loci conspicuous, 0.5–2 μm diam, thickened and darkened-refractive. Ramoconidia commonly formed, cylindrical-oblong, up to 52 μm long, 3–4 μm wide, base about 2.5 μm wide. Conidia numerously formed in densely branched chains, with 1–6(–8) conidia in the terminal unbranched part of the conidial chain, small terminal conidia obovoid, ovoid or ellipsoid, 4–6 × 1.5–2(–3) μm (av. ± SD: 4.6 ± 0.9 × 2.1 ± 0.5), intercalary conidia ellipsoid, limoniform or fusiform, (5–)6.5–12(–14) × 2.5–3 μm (av. ± SD: 9.1 ± 2.8 × 2.8 ± 0.2), with (1–)2–4 distal hila, secondary ramoconidia ellipsoid to cylindrical, 8–33(–37) × 2–4(–4.5) μm (av. ± SD: 20.7 ± 9.9 × 3.4 ± 0.7), 0–2-septate, septum median or in the upper half, with dense clusters of pronounced scars (2–6 hila) at the distal end, sometimes with additional hila near the basal hilum, smooth or irregularly rugulose, subhyaline or pale olivaceous, conidia formed by micronematous conidiophores shorter, narrower and paler, hila conspicuous, 0.5–2 μm diam; microcyclic conidiogenesis sometimes occurring.
Culture characteristics: Colonies on PDA attaining 65–72 mm diam after 14 d at 25 °C, grey olivaceous, smoke-grey and pale olivaceous grey, reverse iron-grey, fluffy-felty, margin regular, white, growth low convex, without prominent exudates. Colonies on MEA 68–76 mm diam after 14 d at 25 °C, whitish, smoke-grey and pale olivaceous grey, reverse olivaceous grey and iron-grey, velvety or fluffy, margins glabrous, radially furrowed, aerial mycelium abundant, dense, fluffy, several small but prominent exudates formed. Colonies on OA 55–65 mm diam after 14 d at 25 °C, grey olivaceous, pale olivaceous grey or smoke-grey, reverse leaden-grey and olivaceous grey, velvety or fluffy-felty. Sporulating on all media.
Substrate and distribution: Indoor environment; North America (USA).
Notes: Cladosporium needhamense (Fig. 1, clade 49), a morphologically very variable species, is phylogenetically inbetween C. verrucocladosporioides (Fig. 1, clade 48), C. phaenocomae (Fig. 1, clade 50) and C. australiense (Fig. 1, clade 51). It differs from C. australiense in that the latter species has macronematous, often seta-like and very long conidiophores (48–285 μm), only occasionally forming ramoconidia and smooth conidia (Bensch et al. 2012). Cladosporium verrucocladosporioides forms 0–1-septate, wider terminal and intercalary conidia showing a more prominent surface ornamentation (Bensch et al. 2010); and C. phaenocomae produces finely verruculose conidia and narrower conidiogenous loci and conidial hila (Crous & Groenewald 2011).
Cladosporium uwebraunianum (Fig. 1, clade 52), newly described from indoor environments, is also closely related but is distinct in having longer conidiogenous cells (17–50(–65) μm long), and conidia formed in long branched chains with up to 10(–13) conidia in the terminal unbranched part of the chain. Until now C. needhamense is known only from a single isolate.
Cladosporium neerlandicum Bensch & Samson, sp. nov. MycoBank MB822222. Fig. 24.
Fig. 24.
Cladosporium neerlandicum (CBS 143360). A–C. Colonies on PDA, MEA and OA. D–I. Conidiophores and conidia. J. Conidial chains. Scale bars = 10 μm.
Etymology: Name refers to the country, where the type specimen was isolated, The Netherlands.
Holotype: The Netherlands, 's Hertogenbosch, swab sample archive, M. Meijer, CBS H-23253. Ex-type culture: CBS 143360 = DTO 086-C5.
Diagnosis: Differs from C. acalyphae in having shorter, 0−3-septate conidiophores and shorter as well as narrower, smooth conidia.
Mycelium immersed, sparsely superficial, hyphae unbranched or loosely branched, 1.5−5 μm wide, septate, often slightly or distinctly constricted at the somewhat darkened and thickened septa, pale to medium olivaceous brown, verruculose. Conidiophores solitary or in pairs, macronematous, occasionally micronematous, straight or sometimes slightly flexuous, subcylindrical or conical being attenuated towards the apex, usually not geniculate, unbranched or once branched, (8−)12−60 μm long, 3−5(−6) μm wide at the base, 2.5−3.5 μm wide at the apex, 0−3-septate, septa somewhat darkened, pale to medium olivaceous brown, smooth, walls slightly thickened; micronematous conidiophores filiform, about 2 μm wide. Conidiogenous cells terminal, subcylindrical or cylindrical, neither geniculate nor nodulose, 7.5−20 μm long, with 2−5 loci crowded at the apex, loci 1−1.5(−1.8 μm) diam. Ramoconidia not occurring. Conidia catenate with conidial chains branching in all directions, with 1−5 conidia in the terminal unbranched part of the chains, small terminal conidia obovoid or ellipsoid, 4−8 × (2−)2.5−3 μm (av. ± SD: 5.8 ± 1.4 × 2.7 ± 0.4), apex rounded or with a single hilum, intercalary conidia ellipsoid, 5.5−11 × (2.5−)3−3.5 μm (av. ± SD: 7.4 ± 2.0 × 3.1 ± 0.3), aseptate, with 1−4 distal hila crowded at the apex, secondary ramoconidia ellipsoid or subcylindrical, (8−)9.5−18(−23) × 3−3.5(−4) μm (av. ± SD: 13.6 ± 3.8 × 3.4 ± 0.3), 0−1-septate, with (2−)3−6 distal hila forming dense cluster of pronounced scars, sometimes hila also situated on lateral prolongations or with one or few additional hila the lower end, pale olivaceous or pale olivaceous brown, smooth or almost so, hila protuberant, subdenticulate, 0.5−1.5(−1.8) μm diam, somewhat darkened, conidia often germinating, germ tubes up to 80 μm long or even longer, septate, about 1 μm wide.
Culture characteristics: Colonies on PDA attaining 33−37 mm diam after 14 d at 25 °C, olivaceous or olivaceous grey, reverse leaden-grey and iron-grey, velvety or floccose, margins narrow, undulate, white, growth flat, sometimes radially furrowed with slightly elevated and folded colony centre, aerial mycelium loose, diffuse. Colonies on MEA reaching 30−35 mm diam after 14 d at 25 °C, smoke-grey, glaucous grey towards margin, reverse olivaceous grey, velvety or powdery, margins white, undulate, glabrous, radially furrowed or wrinkled. Colonies on OA 24−34 mm diam after 14 d at 25 °C, olivaceous, iron-grey or olivaceous black towards margins, reverse olivaceous grey or iron-grey, powdery or fluffy, margins narrow, regular or slightly undulate. Sporulation profuse on all media, without prominent exudates.
Substrate and distribution: Indoor environment; Europe (The Netherlands).
Notes: Phylogenetically C. neerlandicum (Fig. 1, clade 40) is closely allied to C. acalyphae (Fig. 1, clade 39), a species described from South Korea on Acalypha australis. The latter species differs however in having very long, pluriseptate conidiophores (up to 430 μm long), ramoconidia and longer and wider, finely verruculose (reticulate under SEM) conidia (Bensch et al., 2010, Bensch et al., 2012). On act, the two species are 167/171 (98 %) similar and on tef1 they are 254/256 (99 %) similar; they are identical on ITS. Until now C. neerlandicum is known only from a single isolate.
Cladosporium neolangeronii Bensch & Samson, sp. nov. MycoBank MB822223. Fig. 25.
Fig. 25.
Cladosporium neolangeronii (CBS 797.97). A–C. Colonies on PDA, MEA and OA. D–H. Macronematous conidiophores and conidia. I, K. Micronematous conidiophores and conidia. J. Ramoconidium and conidia. Scale bars = 10 μm.
Etymology: Name refers to its morphological and phylogenetic similarity with C. langeronii.
Holotype: The Netherlands, ‘s-Hertogenbosch’ and Breda, isol. from indoor environment, 1996, O. Adan (until now stored as “C. sphaerospermum” in the CBS collection), CBS H-23254. Ex-type culture: CBS 797.97.
Diagnosis: Differs from C. langeronii in having faster growth rates and longer ramoconidia.
Mycelium loosely branched, filiform or narrowly cylindrical, hyphae 1.5–5(−6) μm wide, septate, sometimes constricted and swollen, subhyaline, pale to medium olivaceous brown, smooth or almost so or minutely verruculose, walls unthickened or only slightly thickened, occasionally forming ropes or stromatic hyphal aggregations composed of swollen hyphal cells. Conidiophores mainly macronematous and micronematous, arising terminally or laterally from hyphae, solitary, in pairs of two or in small groups of 3–4, filiform to subcylindrical or cylindrical-oblong, 20–440(−640) × (2–)2.5–4(−5) μm, sometimes wider at the base and attenuated and paler towards the apex, neither geniculate nor nodulose, unbranched or branched, once or several times, branchlets sometimes quite long, up to 100 μm or even longer, pluriseptate, not constricted, pale olivaceous to medium olivaceous brown, smooth or almost so or minutely verruculose especially towards the apex, walls unthickened or slightly to distinctly thick-walled, sometimes up to 1 μm thick. Conidiogenous cells integrated, terminal and intercalary, cylindrical-oblong, 10–60 μm long, with 1–5 loci at the apex, in intercalary cells mostly a single locus situated on small lateral prolongations or subdenticulate just below a septum, loci 1–2(−2.5) μm diam, somewhat thickened and darkened; often seceding at septa and forming ramoconidia. Ramoconidia frequently formed, cylindrical, 35–52 × (2−)3−4 μm, 0–1-septate, smooth or almost so or irregularly minutely verruculose, base truncate, 2–3 μm wide, slightly darkened. Conidia catenate, numerous, in branched chains, branching in all directions or dichotomously, with 1−5(−6) conidia in the terminal unbranched part of the chain; small terminal conidia globose, subglobose, obovoid, occasionally subrostrate or rostrate at the base, 2.5–5 × (2–)3−4(−4.5) μm (av. ± SD: 4.0 ± 0.6 × 3.3 ± 0.5), aseptate, intercalary conidia subglobose, ovoid, limoniform or ellipsoid, 4.5–11(–15) × (2−)3–4 μm (av. ± SD: 7.7 ± 2.9 × 3.6 ± 0.5), usually aseptate, sometimes irregular in shape due to lateral hila, 1–3 distal hila, sometimes subrostrate or rostrate towards hila, small terminal and intercalary conidia medium olivaceous brown, loosely and irregularly verruculose or verrucose, young conidia paler; secondary ramoconidia ellipsoid to subcylindrical or cylindrical, (6–)11–25(–35) × (2.5–)3−4(−5) μm (av. ± SD: 19.7 ± 6.6 × 3.4 ± 0.6), 0–1(−3)-septate, pale or medium olivaceous brown, surface ornamentation often not as prominent as in terminal and intercalary conidia, almost smooth, loosely minutely verruculose or irregularly rough-walled, walls somewhat thickened, slightly attenuated toward the base, with (1–)2–4(–5) distal hila, hila conspicuous, subdenticulate, 0.5–2(−2.5) μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining 12–23 mm diam after 14 d at 25 °C, iron-grey or olivaceous black, pale olivaceous grey or olivaceous grey due to aerial mycelium, reverse olivaceous black, velvety or powdery, margin narrow, white, aerial mycelium loose, diffuse to denser, floccose, growth low convex to convex with elevated colony centre, radially furrowed. Colonies on MEA reaching 7–19 mm diam after 14 d at 25 °C, grey olivaceous, olivaceous grey and olivaceous due to abundant sporulation, in colony centre smoke-grey due to dense aerial mycelium, glaucous-grey at margins, reverse iron-grey, floccose or fluffy, margins narrow, white, growth low convex or convex, radially furrowed and folded in colony centre. Colonies on OA attaining 10–20 mm diam after 14 d at 25 °C, olivaceous grey and iron-grey, reverse leaden-grey, velvety-floccose, aerial mycelium loose to dense, especially in colony centre, growth flat. Sporulation profuse on all media, on PDA and MEA sometimes prominent exudates formed.
Substrate and distribution: Isolated from indoor environments and from a mortar of Muro Farnesiano; Europe (Italy, The Netherlands), North America (USA).
Additional materials examined: Italy, Parma, isol. from mortar of Muro Farnesiano, coll. by C. Urzi, Dept. Sci. Microbiol. Gen. Mol., Univ. of Messina, Italy, No. MC 783, CBS 109868. The Netherlands, wall in a storage room of antiquities with mold growth, J. Houbraken, DTO 162-A4. USA, Delaware, isol. from indoor air storage sample, pineapple room, Jun. 2012, Ž. Jurjević, EMSL 1682 = CPC 22236; Minnesota, isol. from indoor air sample, Aug. 2012, Ž. Jurjević, EMSL 1724, 1725 = CPC 22266, 22267; isol. from outside air sample, Aug. 2012, Ž. Jurjević, EMSL 1717 = CPC 22262, 22263; New Jersey, Chatman, isol. from indoor air sample, Oct. 2012, Ž. Jurjević, EMSL 1810 = CPC 22314.
Notes: Cladosporium neolangeronii (Fig. 3, clade 10) is both morphologically as well as phylogenetically closely related to C. langeronii (Fig. 3, clade 13) and C. psychrotolerans (Fig. 3, clade 12). Cladosporium psychrotolerans differs in having paler and narrower, smooth or minutely verruculose conidia; and C. langeronii has lower growth rates (2.5–4.5, 1.5–7 and 1–5.5 mm on PDA, OA and MEA) and shorter ramoconidia (10–22(–42) μm long) (Zalar et al. 2007).
Cladosporium parahalotolerans Bensch & Samson, sp. nov. MycoBank MB822224. Fig. 26.
Fig. 26.
Cladosporium parahalotolerans (CBS 139585). A–C. Colonies on PDA, MEA and OA. D–I. Conidiophores and conidial chains. J–K. Ramoconidium and conidial chains. L–M. Micronematous conidiophores and conidia. Scale bars = 10 μm.
Etymology: Name refers to its morphological and phylogenetic similarity with C. halotolerans.
Holotype: The Netherlands, Gilze, swab sample in an apartment, J. Houbraken, CBS H-23255. Ex-type culture: CBS 139585 = DTO 161-D3.
Diagnosis: Differs from C. halotolerans in having distinctly wider conidia and less densely septate conidiophores.
Mycelium internal and superficial, hyphae sparingly branched, filiform or narrowly cylindrical-oblong, 1−4 μm wide, septate, subhyaline or pale olivaceous brown, almost smooth or minutely verruculose, sometimes forming ropes. Conidiophores macro-, semimacro- and micronematous, arising terminally or laterally from hyphae, filiform or narrowly cylindrical-oblong, unbranched or branched, 5−130 × 2−3.5(−4) μm, 1−7-septate, septa often darkened where ramoconidia secede, but not constricted, subhyaline, pale olivaceous up to pale medium olivaceous brown, smooth or almost so. Conidiogenous cells integrated, terminal and intercalary, in micronematous conidiophores usually reduced to conidiogenous cell, 5−35 μm long, with 2−4 loci at the uppermost apex or in intercalary cells 1−2 loci situated on a short peg-like lateral outgrowth just below a septum, loci subdenticulate, 1−1.5 μm diam. Ramoconidia subcylindrical or cylindrical, 24−37 × 2.5−3.5(−4) μm, 0−1(−3)-septate, with 2−4 distal scars, non-cladosporioid base about (2−)2.5−3 μm wide. Conidia catenate, in branched chains, 1−3(−6) conidia in the terminal unbranched part of the conidial chain, small terminal conidia sphaerical, 3−5 × 3.5−4 μm (av. ± SD: 3.8 ± 0.4 × 3.7 ± 0.3), intercalary conidia sphaerical or ovoid 4.5−9(−11) × (2.5−)3.5−4.5(−5) μm (av. ± SD: 6.4 ± 1.6 × 4.0 ± 0.4), pale olivaceous to often medium olivaceous brown, spore masses appear even darker, often distinctly darker than secondary ramoconidia, ramoconidia and conidiophores, minutely verruculose or verruculose, not attenuated towards apex and base, secondary ramoconidia ellipsoid or subcylindrical, (7−)8.5−23(−30) × (2.5−)3−4(−4.5) μm (av. ± SD: 16.9 ± 7.0 × 3.4 ± 0.5), 0−1(−3)-septate, septa often appear somewhat darkened, pale olivaceous or pale medium olivaceous brown, smooth or almost so, hila protuberant, subdenticulate, 0.5−1.5 μm diam; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on PDA attaining 27–40 mm diam after 14 d at 25 °C, olivaceous or olivaceous grey, reverse olivaceous grey to leaden-grey or olivaceous black, velvety, powdery to felty-woolly, margins white, aerial mycelium diffuse or floccose. Colonies on MEA attaining 18–40 mm diam after 14 d at 25 °C, smoke-grey, pale olivaceous grey or olivaceous grey, sometimes glaucous-grey at margin, reverse olivaceous grey, powdery to felty-woolly, margin colourless to white, glabrous or feathery, radially furrowed, aerial mycelium felty, abundant. Colonies on OA reaching 29–40 mm diam after 14 d at 25 °C, grey olivaceous, olivaceous or olivaceous black, reverse olivaceous or olivaceous grey, velvety or floccose, margin narrow, somewhat feathery, aerial mycelium sparse, diffuse or abundantly formed, high, dense. Without prominent exudates but sporulation profuse on all media.
Substrate and distribution: Indoor environments; Asia (China), Europe (The Netherlands), North America (Mexico, USA).
Additional materials examined: China, isol. from indoor air, DTO 323-B8, DTO 323-C1, DTO 323-C8, DTO 323-F4, DTO 323-H2, DTO 323-H3, DTO 324-A7, DTO 324-B7. Mexico, isol. from house dust, DTO 305-F7 = AA07MX-953, DTO 305-F8 = AA07MX-935, DTO 305-I5 = AA03MX-750, DTO 306-C1 = AA07MX-836, DTO 306-E4 = AA02MX-573, DTO 307-H4; AA03MX-612. The Netherlands, Gilze, swab sample in apartment, J. Houbraken, DTO 161-D6. USA, Maine, isol. from indoor air sample, hotel room, Sep. 2012, Ž. Jurjević, EMSL 1784 = CPC 22280; New Hamshire, Alstead, isol. from indoor air sample, family room, Dec. 2012, Ž. Jurjević, EMSL 1843 = CPC 22330; New Jersey, Rockaway, isol. from indoor air sample, Dec. 2012, Ž. Jurjević, EMSL 1849 = CPC 22336; New York, New York, isol. from indoor air sample, 18th floor, Dec. 2012, Ž. Jurjević, EMSL 1855 = CPC 22342; isol. from indoor air sample, hospital, Jan. 2013, Ž. Jurjević, EMSL 1886, 1889 = CPC 22373, 22376.
Notes: Cladosporium parahalotolerans (Fig. 3, clade 22) is morphologically and phylogenetically related to C. halotolerans (Fig. 3, clade 23) and C. domesticum (Fig. 3, clade 21). However, the new species is genetically well differentiated (478/478 (100 %), 256/291 (88 %) and 163/165 (99 %) sequence similarity for ITS, tef1 and act to C. halotolerans, 545/556 (98 %), 245/295 (83 %) and 143/168 (85 %) sequence similarity for ITS, tef1 and act to C. domesticum respectively when ex-type sequences are compared) and produces distinctly wider conidia and less densely septate conidiophores.
Cladosporium parasubtilissimum Bensch & Samson, sp. nov. MycoBank MB822225. Fig. 27.
Fig. 27.
Cladosporium parasubtilissimum (CBS 143361). A–C. Colonies on PDA, MEA and OA. D–L. Macro- and micronematous conidiophores and conidial chains. Scale bars = 10 μm.
Etymology: Name refers to the morphological similarity with C. subtilissimum.
Holotype: USA, New Mexico, Albuquerque, isol. from indoor air sample, bathroom, Nov. 2012, Ž. Jurjević, CBS H-23256. Ex-type culture: CBS 143361 = CPC 22332 = EMSL 1845.
Diagnosis: Differs from C. subtilissimum by having shorter and slightly narrower conidia formed in shorter chains with 1−4(−5) conidia in the unbranched terminal part of the chain.
Mycelium internal and superficial, hyphae usually unbranched, filiform or narrowly cylindrical-oblong, 1.5−4 μm wide, without swellings and constrictions, septate, septa sometimes darkened, subhyaline or pale olivaceous, verruculose, verrucose or irregularly rough-walled, walls unthickened. Conidiophores macro- and micronematous, filiform or narrowly cylindrical-oblong, unbranched or once branched, non-nodulose, sometimes once geniculate, macronematous conidiophores 15−200 × 2.5−4 μm, 0−6-septate, micronematous conidiophores 9−60 × 2−2.5 μm, 0−4-septate, pale or medium olivaceous brown, smooth or almost so, sometimes asperulate, walls unthickened or slightly thick-walled. Conidiogenous cells terminally and intercalary, cylindrical-oblong, occasionally with a single geniculation, 9−25 μm long, with 2−4(−5) loci crowded at the uppermost apex, sometimes with 1−2(−3) additional loci at a lower level, sometimes situated on lateral prolongations at the apex, loci conspicuous, subdenticulate, 1−2 μm diam, thickened and darkened. Ramoconidia rarely formed, up to 34 μm long, base about 2.5 μm wide. Conidia numerous, catenate, formed in branched chains, branching in all directions, 1−4(−5) conidia in the unbranched terminal part of the conidial chain, small terminal conidia obovoid or ellipsoid, sometimes subglobose, 3−4.5(−5.5) × (2−)2.5−3 μm (av. ± SD: 4.0 ± 0.7 × 2.5 ± 0.3), apex rounded or attenuated towards apex and base, intercalary conidia ellipsoid-ovoid, limoniform, 5.5−12(−13.5) × (2.5−)3−4 μm (av. ± SD: 7.8 ± 2.4 × 3.2 ± 0.4), aseptate, with (1−)2−3(−4) distal hila, about 0.5−1 μm diam, secondary ramoconidia ellipsoid or subcylindrical, (6.5−)9−26 × 3−4(−5) μm (av. ± SD: 15.4 ± 5.2 × 3.7 ± 0.5), 0−1(−2)-septate, with (1−)2−4 distal hila, sometimes even up to eight distal hila crowded at the distal end and then conidia somewhat irregular in shape due to these clusters of scars, intercalary conidia then formed in dense whirls, hila 1−2 μm diam, pale to medium olivaceous brown, minutely verruculose or verruculose, walls unthickened, hila conspicuous, microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 48−57 mm diam after 14 d at 25 °C, olivaceous grey or pale olivaceous grey, reverse leaden-grey and iron-grey, velvety or fluffy-felty, margin regular to undulate, somewhat feathery, radially furrowed, aerial mycelium loose, diffuse to dense, low to high, fluffy-felty, forming pale olivaceous grey patches, sporulation profuse. Colonies on MEA reaching up to 50 mm diam after 14 d at 25 °C, olivaceous grey, whitish, smoke grey or pale olivaceous grey due to the fluffy-felty aerial mycelium mainly formed in colony centre, reverse iron-grey or black, margin narrow, white, feathery, radially furrowed, growth low convex with slightly elevated colony centre, sporulation profuse. Colonies on OA attaining 45−65 mm diam after 14 d at 25 °C, olivaceous grey or olivaceous due to abundant sporulation, reverse leaden-grey and olivaceous grey, velvety or fluffy, margin regular, white, aerial mycelium loose, diffuse or forming a few smoke-grey high and fluffy spots. Sporulation profuse on all media but no prominent exudates formed.
Substrate and distribution: Indoor air; North America (USA).
Additional material examined: USA, California, Gerber, isol. from indoor air sample, recreational vehicle, Jan. 2013, Ž. Jurjević, EMSL 1924 = CPC 22396.
Notes: Both phylogenetically and morphologically this new species (Fig. 2, clade 26) is closely related to C. subtilissimum (Fig. 2, clade 25) but the latter species can be distinguished by its longer and slightly wider conidia formed in long chains with up to 12 or even more conidia (Bensch et al. 2012).
Cladosporium perangustum Bensch et al., Stud. Mycol. 67: 65. 2010. MycoBank MB517085. Fig. 28.
Fig. 28.
Cladosporium perangustum (DTO 127-E1). A–C. Colonies on PDA, MEA and OA. D–H. Macronematous conidiophores and conidial chains. I–J. Micronematous conidiophores and conidia. Scale bars = 10 μm.
Holotype: South Africa, Pretoria, Walter Sisulu park, isol. from Cussonia sp. (Araliaceae), 20 Feb. 2007, P.W. Crous, CBS H-20451. Ex-type culture: CBS 125996 = CPC 13815.
Lit.: Bensch et al. (2012: 208−210; 2015: 57), Jang et al., 2013, Sandoval-Denis et al., 2016.
Ill.: Bensch et al. (2010: 66−67, figs 54−56; 2012: 209−210, figs 233−235), Jang et al. (2013: 23, figs 1−2).
Mycelium immersed and superficial; hyphae filiform to narrowly cylindrical-oblong, loosely branched, (0.5−)1−4 μm wide, septate, sometimes irregular due to intercalary swellings and constrictions, subhyaline to pale olivaceous or pale olivaceous brown, smooth to usually verruculose or irregularly rough-walled, walls unthickened or almost so, sometimes swollen at the base of conidiophores, sometimes forming dense ropes. Conidiophores solitary, sometimes in pairs, macro-, semimacro- or micronematous, arising terminally and laterally from hyphae or from swollen hyphal cells, erect, straight or slightly flexuous, filiform to narrowly cylindrical-oblong, usually neither geniculate nor nodulose, sometimes geniculate-sinuous or unilaterally slightly swollen at the apex, unbranched, occasionally branched, once or several times, branches short, peg-like or up to 30 μm long, conidiophores (8−)12−130(−150) × (1.5−)2−3.5(−4) μm, 0−6-septate, usually not constricted at septa, occasionally septa darkened, subhyaline, pale olivaceous or pale olivaceous brown, more or less rough-walled, especially towards the base of conidiophores, asperulate-verruculose, at the apex smooth or almost so, walls unthickened or slightly thickened, about 0.5 μm wide, sometimes slightly attenuated towards the apex, at the base sometimes up to 4.5 μm wide. Conidiogenous cells integrated, mainly terminal, sometimes also intercalary, narrowly cylindrical-oblong, sometimes geniculate-sinuous, in intercalary cells loci situated on small peg-like lateral prolongations or just below the septum, 7−40 μm long, with 1−4(−5) apically crowded loci, forming clusters of pronounced scars, conspicuous, subdenticulate to denticulate, 0.8−1.5 μm diam, thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 25−45 × 2.5−3(−4) μm, 0−1(−2)-septate, base truncate, 2−2.5(−4) μm wide, sometimes slightly darkened or refractive. Conidia numerous, catenate, in branched chains, branching in all directions, 1−4 conidia in the terminal unbranched part of the chain, small terminal conidia globose, subglobose or ovoid to obovoid, 2−4(−5) × (1.5−)2−2.5 μm (av. ± SD: 3.2 ± 0.7 × 2.1 ± 0.2), apex broadly rounded or slightly attenuated, intercalary conidia ovoid, limoniform to ellipsoid, somewhat fusiform or subcylindrical, 4−15.5(−18) × 2−3(−3.5) μm (av. ± SD: 8.6 ± 3.8 × 2.5 ± 0.4), 0(−1)-septate, attenuated towards apex and base, with 1−3(−5) distal hila, secondary ramoconidia narrowly ellipsoid to cylindrical- oblong, 6−33(−40) × 2−3(−3.5) μm (av. ± SD: 17.3 ± 7.3 × 2.5 ± 0.4), 0−1(−3)-septate, septum median or often somewhat in the upper half, with 2−4(−7) distal hila, pale olivaceous brown, smooth or almost so to finely verruculose (LM), under SEM smooth or surface with somewhat irregularly reticulate structure or embossed stripes probably caused by diminishing turgor and shrivelling of tender conidia, thin-walled, hila conspicuous, subdenticulate to denticulate, (0.8−)1−1.5 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining 33−76 mm diam after 14 d at 25 °C, grey olivaceous to olivaceous, olivaceous grey or iron-grey, sometimes with patches of smoke-grey or pale greenish grey, reverse olivaceous grey, iron-grey or olivaceous black, sometimes releasing an olivaceous buff or orange to luteous soluble pigment into the agar, velvety, fluffy, floccose or powdery, margins glabrous to feathery, whitish, olivaceous buff or pale luteous due to the pigment, broad, regular or somewhat undulate, aerial mycelium diffuse to loosely floccose or felty, growth effuse, usually without prominent exudates, occasionally numerous small to large prominent exudates formed, sporulation profuse. Colonies on MEA reaching 40−72 mm diam after 14 d at 25 °C, pale olivaceous grey to glaucous grey or grey olivaceous, whitish to smoke-grey due to aerial mycelium, reverse olivaceous grey to iron-grey, occasionally releasing an orange soluble pigment into the agar, velvety to floccose, margins white, narrow, regular to undulate, glabrous to somewhat feathery, aerial mycelium abundantly formed, covering most parts of colony surface, loosely to densely floccose or felty, white to pale olivaceous grey or smoke-grey, growth effuse with sometimes elevated colony centre, radially furrowed, sometimes few small prominent exudates formed, sporulation profuse. Colonies on OA 40−75 mm diam after 14 d at 25 °C, whitish to smoke-grey and pale olivaceous grey or grey olivaceous, reverse pale olivaceous grey, pale greenish grey to olivaceous grey, leaden-grey or sometimes amber-coloured due to the pigment released into the agar, velvety or fluffy to felty-floccose, margins white or greenish olivaceous, glabrous, regular, aerial mycelium abundant, covering large parts of the colony surface, dense, low to high, white, growth effuse, sometimes few prominent exudates formed, sporulating.
Substrate and distribution: On plant material, ascomycetes and isolated from indoor environments; Africa (South Africa), Asia (China, Korea), Australasia (New Zealand), Europe (Germany), North America (USA).
Additional materials examined: China, isol. from indoor air, DTO 323-E4, DTO 323-E8, DTO 323-E9, DTO 324-A2, DTO 324-A6, DTO 324-D1. Germany, Essen, botanical garden, 51.45, 7.0167, isol. from Morus rubra (Moraceae), 2005, N. Ale-Agha, CPC 12216. New Zealand, Auckland, Auckland University campus, isol. from leaves of Oncoba spinosa (Salicaceae), Sep. 2004, C.F. Hill, Hill 1076-1 = CPC 11663. South Africa, Pretoria, Walter Sisulu park, isol. from Protea caffra (ascospore isolate) (Proteaceae), 2 Jan. 2007, P.W. Crous, CPC 13730, 13774; isol. from Teratosphaeria maculiformis (Teratosphaeriaceae) on Protea caffra, 2 Jan. 2007, P.W. Crous, CPC 13727; Western Cape Province, Jonkershoek Nature Reserve, isol. from Teratosphaeria fibrillosa (Teratosphaeriaceae), 30 Mar. 2007, P.W. Crous, CPC 13870; Western Cape, Betties Bay, Harold Porter National Park, isol. from Protea cynaroides (Proteaceae), 4 Dec. 2008, L. Mostert, CPC 15192. USA, California, San Diego, isol. from indoor air sample, bedroom closet, Dec. 2012, Ž. Jurjević, EMSL 1844 = CPC 22331; Thousand Oaks, isol. from indoor air sample, bedroom, Jan. 2013, Ž. Jurjević, EMSL 1891 = CPC 22378; Connecticut, Mancester, isol. from indoor air, library, Nov. 2012, Ž. Jurjević, EMSL 1835 = CPC 22329; Georgia, Tucker, isol. from air sample, bakery, DTO 127-E1 = AR368, DTO 127-E2 = AR371; Louisiana, Baton Rouge, isol. from Magnolia sp. (Magnoliaceae), 8 Sep. 2007, P.W. Crous, CPC 14247; Maine, Westbrook, isol. from indoor air sample, Nov. 2012, Ž. Jurjević, EMSL 1833 = CPC 22327, CPC 22328; New York, New York, isol. from indoor air sample, hospital, Jan. 2013, Ž. Jurjević, EMSL 1888 = CPC 22375; Pennsylvania, Chaddes Ford, isol. from indoor air sample, Oct. 2012, Ž. Jurjević, EMSL 1781 = CPC 22297; Washington, Seattle, University of Washington campus, isol. from chasmothecia of Phyllactinia guttata (Erysiphales) on leaves of Corylus avellana (Betulaceae), 16 Sep. 2004, D. Glawe (CBS 126365 = CPC 11820, CPC 11815, 11819, 11821, 11831).
Notes: Bensch et al., 2010, Bensch et al., 2012 already discussed the phylogenetic variability within the subclades of C. perangustum (Fig. 1, clade 4, previously also including clades 2 and 3) but based on the quite conserved morphology refrained from splitting this species based on the sampling available at that stage. However, Sandoval-Denis et al. (2016) introduced two additional species, C. angulosum (Fig. 1, clade 2) and C. xanthochromaticum (Fig. 1, clade 3) for two of the subclades of C. perangustum. Cladosporium angulosum differs in having slightly shorter intercalary conidia and secondary ramoconidia. Conidiophores described as typical for C. angulosum in being frequently branched in a 90° angle (Sandoval-Denis et al. 2016) are sometimes also formed in strains of C. perangustum (see Fig. 28). Cladosporium xanthochromaticum has slightly longer and wider secondary ramoconidia and usually smooth conidiophores; its ramoconidia are slightly wider but not shorter as in C. perangustum. Due to high similarity and overlapping characters within these three species an identification based on morphology alone will be difficult. Therefore, a molecular approach is highly recommended for a correct identification.
Cladosporium pseudocladosporioides Bensch et al., Stud. Mycol. 67: 71. 2010. MycoBank MB517087. Fig. 29.
Fig. 29.
Cladosporium pseudocladosporioides (DTO 151-A4). A–C. Colonies on PDA, MEA and OA. D–J. Conidiophores and conidial chains. Scale bars = 10 μm.
Holotype: The Netherlands, Zwolle, isol. from outside air, 7 Jan. 2007, M. Meijer, CBS H-20445. Ex-type cultures: CBS 125993 = CPC 14189, CPC 14193.
Lit.: Bensch et al. (2012: 226−228).
Ill.: Bensch et al. (2010: 71−72, figs 60−61; 2012: 226−227, figs 257−258).
Mycelium immersed and superficial; hyphae unbranched or sparingly branched, (0.5−)1−4 μm wide, septate, sometimes constricted at septa, especially in wider ones, subhyaline to pale olivaceous or pale olivaceous brown, smooth or almost so, walls sometimes slightly thickened, about 0.5 μm wide, sometimes irregular in outline due to swellings and constrictions, sometimes forming small ropes of few hyphae, sometimes cells swollen, up to 6.5 μm wide, fertile hyphae minutely verruculose, mainly at the base of conidiophores. Conidiophores macronematous, sometimes also micronematous, solitary or in small loose groups, arising terminally and laterally from hyphae or swollen hyphal cells, erect, straight to slightly flexuous, cylindrical-oblong, non-nodulose, sometimes once geniculate-sinuous or slightly swollen at the apex, unbranched or branched once or twice, occasionally three times, branches often only as short denticle-like lateral outgrowths just below a septum, 15−155 μm long, 2−4 μm, sometimes attenuated towards apex, 0−5-septate, sometimes slightly constricted at septa, pale to pale medium olivaceous brown, sometimes paler towards the apex, smooth or almost so, at the base asperulate or finely verruculose like fertile hyphae, walls slightly thickened, about 0.5 μm wide or unthickened; micronematous conidiophores filiform, narrower, not attenuated, about 1.8 μm wide. Conidiogenous cells integrated, terminal, sometimes intercalary, slightly attenuated, narrowly cylindrical-oblong, sometimes once geniculate, non-nodulose, (6.5−)9−33 μm long, with 1−4 loci at the apex, occasionally with up to seven loci crowded at or towards the apex, in intercalary cells loci situated on small lateral peg-like outgrowths, 1−2(−3) loci, conspicuous, subdenticulate, 1−1.5(−1.8) μm diam, somewhat thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 19−48 × 3−4 μm, 0−2(−3)-septate, pale olivaceous brown, smooth, base broadly truncate, 2−3 μm wide, unthickened or slightly thickened, sometimes slightly refractive. Conidia very numerous, catenate, in branched chains, branching in all directions with 3−6(−9) conidia in the terminal unbranched part of the chain, small terminal conidia obovoid, ovoid to limoniform or ellipsoid, sometimes subglobose, 3−5.5 × (1−)1.5−2.5 μm (av. ± SD: 4.1 ± 0.7 × 2.1 ± 0.3), apex rounded or attenuated towards apex and base, intercalary conidia ovoid, limoniform to ellipsoid or subcylindrical, 4.5−13(−19) × (1.8−)2−3 μm (av. ± SD: 8.8 ± 3.9 × 2.6 ± 0.3), 0(−1)-septate, slightly attenuated towards apex and base, with 1−4(−5) distal hila, secondary ramoconidia ellipsoid-ovoid to subcylindrical or cylindrical-oblong, (6.5−)8−23(−29) × (2−)2.5−3.5(−4) μm (av. ± SD: 16.1 ± 5.1 Χ 2.9 ± 0.3), 0−1(−2)-septate, septum median or often somewhat in the lower half, pale olivaceous to pale olivaceous brown, smooth or almost so, sometimes slightly rough-walled, walls unthickened, with (1−)2−4(−6) distal hila, conspicuous, subdenticulate, 0.5−1.5(−1.8) μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 65−78 mm diam after 14 d at 25 °C, olivaceous grey to grey olivaceous, reverse leaden-grey to olivaceous black, felty-floccose, margins regular, glabrous to feathery, grey olivaceous, aerial mycelium felty-floccose, growth effuse to low convex, few small prominent exudates formed, sporulation profuse. Colonies on MEA attaining 52−75 mm diam after 14 d at 25 °C, smoke-grey to dark smoke-grey or grey olivaceous, reverse iron-grey, floccose, margins white, narrow, glabrous to somewhat feathery, aerial mycelium white, floccose, abundant, dense, growth effuse and somewhat radially furrowed, mostly without prominent exudates, sporulation profuse. Colonies on OA reaching 55−73 mm diam after 14 d at 25 °C, olivaceous to grey olivaceous or olivaceous buff, pale olivaceous grey to greenish grey towards margins, reverse pale greenish grey, leaden-grey to iron-grey, floccose, margins colourless, glabrous, regular, aerial mycelium floccose to felty, sometimes covering large parts of colony surface, growth effuse with few prominent exudates, sporulation profuse.
Substrates and distribution: On plant material and fungal fruiting bodies, isolated from air, indoor environments, clinical samples, soil, water and food; widely distributed, Africa (South Africa, Uganda), Asia (China, Indonesia, South Korea), Australasia (Australia, New Zealand), Europe (France, Germany, Italy, Portugal, Romania, Russia, Slovenia, The Netherlands), North America (Canada, USA), South America (Brazil, Chile).
Additional materials examined: Canada, isol. from house dust, Health Canada, DTO 307-F3, DTO 307-G9. China, isol. from indoor air, DTO 323-D3. Germany, isol. from indoor environment, CBS 139575 = DTO 084-F1. Portugal, isol. from indoor environment, DTO 151-A4, The Netherlands, isol. from outside air, M. Meijer, CBS 125993 = CPC 14189; isol. from a wallpaper from a house, J. Hooiveld, DTO 079-F4. USA, Arizona, Tuscon, isol. from indoor air sample, office, Feb. 2013, Ž. Jurjević, EMSL 2014 = CPC 22966; isol. from indoor air sample, hospital, Jan. 2013, Ž. Jurjević, EMSL 1907 = CPC 22392; Florida, Coral Springs, isol. from air sample, car air conditioner, Jun. 2012, Ž. Jurjević, EMSL 1683 = CPC 22237; Georgia, Carrollton, isol. from indoor air sample, office, Jan. 2013, Ž. Jurjević, EMSL 1881 = CPC 22368; New Jersey, Bridgeport, isol. from indoor air sample, bedroom, 2nd floor, Dec. 2012, Ž. Jurjević, EMSL 1864 = CPC 22351; Manasquan, isol. from indoor air sample, living room, Jan. 2013, Ž. Jurjević, EMSL 1904 = CPC 22389; New York, New York, isol. from indoor air sample, 27th floor, Dec. 2012, Ž. Jurjević, EMSL 1853, 1854 = CPC 22340, 22341; Ohio, Columbus, isol. from indoor air sample, bedroom, Dec. 2012, Ž. Jurjević, EMSL 1847 = CPC 22334; Pennsylvania, Chalfont, isol. from indoor air sample, living room, Dec. 2012, Ž. Jurjević, EMSL 1875 = CPC 22362; Rhode Island, North Providence, isol. from indoor air sample, classroom, Jan. 2013, Ž. Jurjević, EMSL 1901 = CPC 22386; Texas, Haltom City, isol. from indoor air sample, bathroom, Jan. 2013, Ž. Jurjević, EMSL 1895 = CPC 22382. Additional isolates are listed in Table 1.
Notes: Cladosporium pseudocladosporioides (Fig. 1, clade 56) is a common, widespread saprobic hyphomycete phylogenetically and morphologically very close to C. cladosporioides (Fig. 1, clade 66) but clearly distinct by forming a separate lineage in phylogenetic analyses (also see Bensch et al. 2010) and by having shorter and somewhat narrower, 0−1(−2)-septate secondary ramoconidia, narrower conidiogenous loci and hila, and hyphae sometimes forming ropes. However, the distinction between the two species only based on morphology is difficult and not always possible with certainty, which is additionally complicated by the internal genetic structure of the C. pseudocladosporioides clade, suggesting that it possibly represents a complex containing cryptic species (observed in both the act and tef1 alignments in Bensch et al. 2010). Uncertain strains should simply be referred to as C. cladosporioides s. lat. (complex). Cladosporium paracladosporioides (Fig. 1, lineage 13) is also similar but differs in having wider, 0−3-septate secondary ramoconidia, wider conidiogenous loci and hila and is phylogenetically distinct (see Bensch et al. 2010).
Sandoval-Denis et al. (2015) reported C. pseudocladosporioides as one of the more frequently isolated species from clinical samples in the USA. Within the C. cladosporioides complex it proved to be the most common species occurring in indoor environments (this study).
In the present analysis, Cladosporium crousii recently described from human bronchoalveolar lavage fluid in the USA (Sandoval-Denis et al. 2016), clusters on a long branch within the larger C. pseudocladosporioides clade (Fig. 1, clade 56) and is therefore probably conspecific with the latter species. The given description in Sandoval-Denis et al. (2016) is very close to that of C. pseudocladosporioides but in their analysis the ex-type strain clustered close to but outside that species. This could be an artefact of the phylogenetic analysis due to the much larger sampling of C. pseudocladosporioides strains in the present study, as C. crousii is 206/238 (87 %) similar on tef1 and up to 100 % identical on act to the closest C. pseudocladosporioides sequences included in our phylogeny.
Cladosporium psychrotolerans Zalar et al., Stud. Mycol. 58: 175. 2007. MycoBank MB492428. Fig. 30.
Fig. 30.
Cladosporium psychrotolerans (DTO 307-H2). A–C. Colonies on PDA, MEA and OA. D–H. Conidiophores and conidia. I. Micronematous conidiophores. J–L. Ramoconidia and conidia. M. Conidia. Scale bars = 10 μm.
Holotype: Slovenia, Sečovlje salterns, isolated from hypersaline water, May 1999, S. Sonjak, CBS H-19730. Ex-type culture: EXF-391 = CBS 119412.
Lit.: Bensch et al. (2012: 229–230).
Ill.: Zalar et al. (2007: 166, fig. 5 e, 176, fig. 11), Bensch et al. (2012: 230, fig. 261).
Mycelium partly superficial and partly submerged, with numerous lateral pegs, consistently enveloped in polysaccharide-like material; hyphae unbranched or sparingly branched, 1–3(–5) μm wide, septate, not constricted at septa, pale brown or pale olivaceous brown, almost smooth to verruculose, thin-walled. Conidiophores macro- and micronematous, arising terminally and laterally from hyphae, erect or ascending, straight or somewhat flexuous, neither geniculate nor nodulose, cylindrical-oblong, unbranched or branched, once or few times, 20–220 × (2–)3–4(–5) μm, micronematous 1–2 μm wide, septate, not constricted at septa, pale olivaceous brown or brown, smooth or almost so, sometimes verruculose at the base, walls slightly thickened, about 0.5 μm wide. Conidiogenous cells integrated, terminal and intercalary, cylindrical, 12–65 μm long, producing sympodial clusters of pronounced, conspicuous denticles (1–4 loci) at their distal ends, loci 1.5–2 μm diam, often seceding at a septum and behaving like conidia. Ramoconidia cylindrical with a broadly truncate base, 16–43(–47) × (2–)3–4(–4.5) μm, aseptate, rarely 1(–2)-septate, not or only very slightly attenuated towards the base, base 2–2.5(–3) μm wide, somewhat darkened-refractive. Conidia catenate, in branched chains, branching in all directions, terminal chains with up to six conidia, small terminal conidia subglobose to ovoid, globose, (2–)3–5 × 2–2.5(–3) μm (av. ± SD: 3.9 ± 0.8 × 2.7 ± 0.4), aseptate, pale brown, smooth to minutely verruculose, rounded at the apex, attenuated towards the base, hila 0.5–0.8 μm diam, intercalary conidia ovoid, limoniform to ellipsoid, 5–9(–13) × 2.5–3(–3.5) μm (av. ± SD: 7.2 ± 1.9 Χ 3.2 ± 0.5), 0(–1)-septate, pale brown, smooth to minutely verruculose, with up to three distal hila, 0.5–1 μm diam, secondary ramoconidia ellipsoid to cylindrical, (7.5–)12–25(–31) × 2.5–3.5(–4.5) μm (av. ± SD: 17.8 ± 5.6 × 3.3 ± 0.4), 0–1(–2)-septate, not constricted at septa, pale brown or olivaceous brown, smooth, somewhat attenuated towards apex and base, with 3(–5) distal hila, protuberant, denticulate, 1–2 ìm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA reaching 10–27 μm diam after 14 d at 25 °C, grey olivaceous to olivaceous, becoming pale olivaceous grey or smoke grey due to abundant aerial mycelium, reverse olivaceous grey to iron-grey and leaden-grey, velvety to felty-woolly; margin narrow to wide, white, regular to undulate, glabrous to feathery; aerial mycelium at first absent, later abundantly formed, felty, high; growth flat to later convex, sometimes either heaped or radially furrowed; few prominent exudates formed; sporulation profuse. Colonies on MEA reaching 8–19 mm diam after 14 d at 25 °C, grey olivaceous, glaucous-grey at margin, smoke-grey to pale mouse-grey or whitish due to aerial mycelium, reverse olivaceous grey to iron-grey, velvety to woolly-felty, margin white, narrow, glabrous to feathery, radially furrowed; aerial mycelium abundant, fluffy; few prominent exudates formed; sporulation profuse. Colonies on MEA with 5 % NaCl growing much faster than on other media, reaching 25–38 mm diam after 14 d at 25 °C, of different colours, mostly reseda-green and granulate due to profuse sporulation, margin olive-yellow, reverse yellow to dark green. Colonies on OA reaching 7–20 mm diam after 14 d at 25 °C, at first grey olivaceous to olivaceous, reverse leaden-grey to leaden-black, later pale mouse-grey to pale olivaceous due to aerial mycelium, reverse black, velvety to felty; margin white, glabrous, regular or either undulate or arachnoid, deeply furrowed; aerial mycelium sparse to felty, dense, pale mouse-grey, covering only parts of the colony, mainly the colony centre; growth flat with papillate surface; without prominent exudates; sporulation profuse.
Maximum tolerated salt concentration: MEA + 17 % NaCl after 14 d.
Cardinal temperatures: No growth at 4 °C, optimum and maximum temperature at 25 °C (8–19 mm diam), no growth at 30 °C (from Zalar et al. 2007).
Substrates and distribution: Isolated from hypersaline water, indoor environments and plant material; Australasia (Australia, New Zealand), Europe (Germany, Slovenia), North America (USA), West Indies (Dominican Republic).
Additional materials examined: Australia, isol. from house dust, DTO 305-G3 = BH10AU-180. New Zealand, isol. from house dust, DTO 307-H2 = TA05NZ-343.
Notes: Cladosporium psychrotolerans (Fig. 3, clade 12), which belongs to the C. sphaerospermum species complex, differs from C. halotolerans (Fig. 3, clade 23) in having 0–1(–2)-septate secondary ramoconidia with septa neither darkened nor thickened and globose, subglobose or ovoid small terminal conidia. It has been repeatedly isolated from indoor environments and is now also reported from Australasia. Phylogenetically, it is closely related to C. sloanii (Fig. 3, clade 11), C. langeronii (Fig. 3, clade 13) and C. neolangeronii (Fig. 3, clade 10). However, C. langeronii is particularly well distinguishable from all other Cladosporium species by its slow growing colonies and its larger apical conidia (4–5.5 × 3–4 μm vs 3–4 × 2.5–3 μm in Cladosporium psychrotolerans) (Zalar et al. 2007); and C. neolangeronii exhibits longer conidiophores and has somewhat darker and wider apical conidia. Cladosporium sloanii is a xerophilic species growing on MA+ 20 % sucrose and DG 18 but usually not on the typical media used for Cladosporium and differs by having usually shorter conidiophores and wider conidia. Cladosporium neopsychrotolerans, recently described from soil in China, is also a psychrotolerant species and shares similar cultural characters but is both morphologically and phylogenetically distant from C. psychrotolerans in clustering in the C. cladosporioides species complex (Ma et al. 2017).
Cladosporium pulvericola Bensch & Samson, sp. nov. MycoBank MB822226. Fig. 31.
Fig. 31.
Cladosporium pulvericola (CBS 143362). A–C. Colonies on PDA, MEA and OA. D–F, J. Macronematous conidiophores and conidial chains. G–I, L–N. Micronematous conidiophores and conidial chains. K. Conidia. Scale bars = 10 μm.
Etymology: From the Latin pulveris, of dust, -cola, living in, named for the substrate from which the type specimen was isolated, house dust.
Holotype: New Zealand, Otago, Dunedin, Warrington, 284 Coast Road, isol. from house dust, Duststream collection tube on vacuum cleaner, 1 May 2009, T.J. Atkinson, CBS H-23257. Ex-type culture: CBS 143362 = DTO 305-H8 = TA05NZ-345.
Diagnosis: Differs from C. dominicanum in having shorter conidiophores, slightly longer secondary ramoconidia and a significantly lower growth rate.
Mycelium filiform or narrowly cylindrical, sparsely branched, (0.5–)2–4 μm wide, pluriseptate, subhyaline, pale olivaceous or pale to medium olivaceous brown, smooth or almost so to minutely or irregularly rough-walled, sometimes forming ropes of a few hyphae. Conidiophores macro- and micronematous, cylindrical-oblong, occasionally once geniculate, non-nodulose, mostly unbranched, (3–)12–80(–100) × 2.5–4 μm, micronematous starting as small lateral outgrowth of hyphae, 1–2 μm wide, septate, subhyaline, pale to medium olivaceous brown, smooth or minutely verruculose, walls thickened in macronematous conidiophores. Conidiogenous cells integrated, usually terminal, cylindrical, 6–18 μm long, with 2–4 loci crowded at the apex and sometimes 1–2 additional loci at a lower level, in micronematous conidiophores often only a single locus at the apex, loci conspicuous, 1–1.5 μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed, up to 35 μm long, often 1-septate, base about 2.5 μm wide. Conidia very numerous, catenate, formed in branched chains, 1–7 conidia in the terminal unbranched part of the chains, small terminal conidia very small, subglobose, obovoid or limoniform, (1.5–)2.5–4(–5.5) × (1–)1.5–2.5(–3) μm (av. ± SD: 3.3 ± 0.8 × 2.3 ± 0.5 μm), aseptate, apex rounded or with a single distal hilum, subhyaline or very pale olivaceous, hila about 0.5 μm diam or even narrower, smooth or almost so, with age somewhat darker and with a more prominent verruculose surface ornamentation, intercalary conidia ovoid or ellipsoid, 4–12 × 2–3(–3.5) μm (av. ± SD: 7.2 ± 2.5 × 2.6 ± 0.4 μm), 0–1-septate, very pale olivaceous or pale olivaceous brown, smooth or almost so to somewhat irregularly rough-walled, (1–)2–3 distal hila, hila (0.5–)0.8–1 μm diam, secondary ramoconidia ellipsoid or subcylindrical, (7–)10–25(–33) × (2–)2.5–3(–4) μm (av. ± SD: 17.6 ± 6.5 × 2.9 ± 0.4 μm), 0–1(–3)-septate, pale olivaceous brown, almost smooth or irregularly rough-walled, walls unthickened or almost so, with 2–3(–5) distal hila, hila 1–1.5 μm diam, conspicuous, darkened-refractive; microcyclic conidiogenesis occurring, sometimes germinating.
Culture characteristics: Colonies on PDA attaining 9–32 mm diam after 14 d at 25 °C, greenish olivaceous, olivaceous grey to dull-green, zonate, reverse leaden-grey to leaden-black, with a narrow, regular, white margin, aerial mycelium loose, diffuse, smoke-grey, growth convex with slightly elevated colony centre, wrinkled at margins, without exudates, sporulation profuse. Colonies on MEA reaching 10–28 mm diam after 14 d at 25 °C, smoke-grey, grey olivaceous, greenish glaucous towards margin, reverse olivaceous grey or iron-grey, powdery or velvety, margins narrow, white, radially furrowed, aerial mycelium sparse, diffuse, wrinkled and folded in colony centre, a few prominent exudates formed, sporulation profuse. Colonies on OA attaining 10–18 mm diam after 14 d at 25 °C, grey olivaceous or olivaceous grey, olivaceous when sporulating profusely, sometimes glaucous-grey at margin, reverse iron-grey or leaden-grey, velvety or powdery, margins narrow, white, regular, aerial mycelium loose or fluffy and high, smoke-grey, growth flat, without exudates.
Substrate and distribution: Indoor air, dust and indoor surfaces; Australasia (Australia, New Zealand), Europe (The Netherlands), North America (Canada, USA).
Additional materials examined: Australia, Tasmania, isol. from house dust, L. Agustini, DTO 307-E7 = BH10AU-183. Canada, isol. from air in a residence, 2001, isol. by J. Bissett, deposited as C. sphaerospermum, CBS 109788 = DAOM 226470. The Netherlands, Born, swab sample, food plant, M. Meijer, DTO 130-D6; The Hague, swab sample, DTO 249-F4; Utrecht, swab sample, DTO 255-F7; DTO 255-H5 = CBS139591. USA, Maine, Falmouth, isol. from indoor air sample, living room, Jan. 2013, Ž. Jurjević, EMSL 1931 = CPC 22403.
Notes: Cladosporium pulvericola (Fig. 3, clade 1) is a typical taxon of the C. sphaerospermum species complex. It is morphologically and phylogenetically closely allied to C. dominicanum (Fig. 3, clade 4) but differs in having shorter conidiophores, slightly longer secondary ramoconidia and a significantly lower growth rate. Cladosporium sphaerospermum (Fig. 3, clade 20) is distinguishable by its slightly wider conidiophores with often several darkened and somewhat thickened septa, 0–3-septate, slightly wider secondary ramoconidia and often verrucose small terminal conidia.
Cladosporium ramotenellum K. Schub. et al., Stud. Mycol. 58: 137. 2007, emended in Bensch et al. 2015. MycoBank MB504577. Fig. 32.
Fig. 32.
Cladosporium ramotenellum (DTO 097-H3). A–C. Colonies on PDA, MEA and OA. D–H. Macronematous conidiophores and conidial chains. I. Micronematous conidiophores. J–K. Conidial chains. Scale bars = 10 μm.
Holotype: Slovenia, Sečovlje, isolated from hypersaline water from reverse ponds, salterns, 2005, P. Zalar, CBS H-19862. Isotype: HAL 2026 F. Ex-type culture: CBS 121628 = CPC 12043 = EXF-454.
Lit.: Bensch et al. (2012: 230–232; 2015: 60–62), Lee et al., 2011, Jang et al., 2013.
Ill.: Schubert et al. (2007b: 138–139, figs 31–33), Bensch et al. (2012: 231–232, figs 262–264), Jang et al. (2013: 25, figs 3−4).
Mycelium unbranched or only sparingly branched, 1.5–4 μm wide, septate, without swellings and constrictions, hyaline or subhyaline, smooth, sometimes irregularly rough-walled, walls unthickened. Conidiophores solitary, macro- and micronematous, arising as lateral branches of plagiotropous hyphae or terminally from ascending hyphae, erect, straight or slightly flexuous, cylindrical, neither geniculate nor nodulose, without capitate apices or intercalary swellings, unbranched, sometimes branched, branches often only as short lateral prolongations, mainly formed below a septum, 14–120(–230) × (1–)2–4(–5) μm, septate, not constricted at septa, subhyaline to pale olivaceous or brown, smooth to minutely verruculose, walls unthickened, sometimes guttulate. Conidiogenous cells integrated, terminal, sometimes also intercalary, cylindrical, 10–28(–50) μm long, proliferation sympodial, sometimes swollen, up to 7 μm wide, with few conidiogenous loci, mostly 1–3, loci sometimes situated on small lateral prolongations, protuberant, 0.5–1.5(–2) μm diam, thickened and somewhat darkened-refractive. Ramoconidia cylindrical-oblong, 15–55 × 2–4(–5) μm, 0–1(–3)-septate, rarely up to 4-septate, subhyaline to very pale olivaceous, smooth or almost so, with a broadly truncate base lacking a dome and raised rim, 2–3 μm wide, not thickened but somewhat refractive. Conidia numerous, polymorphous, catenate, in branched chains with 2–5(–6) conidia in the terminal unbranched part of the chain, straight, sometimes slightly curved, small terminal conidia numerous, globose, subglobose or ovoid, obovoid or limoniform, 2.5–6(–7) × 2–4(–4.5) μm (av. ± SD: 4.5 ± 1.1 × 2.8 ± 0.6 μm), aseptate, without distal hilum or with a single apical hilum, intercalary conidia ellipsoid, limoniform to subcylindrical, 5–12( 15) × (2.5–)3–4(–5) μm (av. ± SD: 8.7 ± 2.6 × 3.6 ± 0.5 μm), 0–1-septate; secondary ramoconidia ellipsoid, subcylindrical to cylindrical-oblong, (6–)9–30(–39) × (2.5–)3–4(–5) μm (av. ± SD: 17.9 ± 6.2 × 3.9 ± 0.6 μm], sometimes swollen up to 7 μm, 0–1(–3)-septate, usually not constricted at septa, sometimes distinctly constricted at the median septum, subhyaline to very pale olivaceous, minutely verruculose (granulate under SEM), walls unthickened or almost so, apex broadly rounded or slightly attenuated towards apex and base, sometimes guttulate, hila protuberant, conspicuous, 0.8–1.5( 2) μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis occurring.
Culture characteristics: Colonies on PDA reaching 46–49 mm diam after 14 d at 25 °C, olivaceous to grey olivaceous due to abundant sporulation, appearing zonate in forming concentric zones, margin entire edge to slightly undulate, white, glabrous, aerial mycelium absent or sparse, growth flat with a somewhat folded and wrinkled colony centre, without prominent exudates, sporulation profuse. Colonies on MEA reaching 48–49 mm diam after 14 d at 25 °C, grey olivaceous to olivaceous grey, velvety, olivaceous grey to iron-grey reverse, margin entire edge to undulate, radially furrowed, glabrous to feathery, aerial mycelium sparse, diffuse, growth flat with slightly elevated colony centre, distinctly wrinkled, prominent exudates not formed, abundantly sporulating. Colonies on OA attaining 40 mm diam after 14 d at 25 °C, grey olivaceous, margin entire edge, hyaline or white, glabrous, aerial mycelium absent or sparse, growth flat, without exudates, sporulation profuse.
Substrate and distribution: Hypersaline water, air, indoor environments, food and plant material; Africa (South Africa), Australasia (Australia, New Zealand), Asia (China, South Korea), Europe (Cyprus, Denmark, Germany, Italy, Portugal, Slovenia, Spain, The Netherlands, Turkey, UK), North America (USA).
Additional materials examined: China, isol. from indoor air, DTO 323-B7, DTO 323-D4, DTO 323-D5, DTO 323-D6. Denmark, isol. from indoor environment, B. Andersen, BA 1919 = DTO 109-F4; isol. from indoor air, 2 Feb. 2011, B. Andersen, BA 2033 = CPC 19119. Germany, isol. from indoor environment, LGA, DTO 084-F5. New Zealand, isol. from house dust, T. Atkinson, DTO 305-H1 = TA10NZ-295, DTO 305-I1 = TA10NZ-240, DTO 306-A3 = TA10NZ-322, DTO 306-B2 = TA10NZ-324, DTO 306-D1 = TA10NZ-215B, DTO 306-D2 = TA10NZ-289A, DTO 306-E7 = TA10NZ-232, DTO 306-F5; TA10NZ-308, DTO 307-F2 = TA10NZ-297A, DTO 307-I2 = TA10NZ-286. Portugal, indoor environment, DTO 150-F5, DTO 151-G3, DTO 151-G6, DTO 152-B3, DTO 152-D9. South Africa, isol. from house dust, K. Jacobs, DTO 306-C4 = KJ09SA-88. The Netherlands, swab sample indoor environment, G.J. Dolphyn, DTO 097-H3; Rijssen, air sample kitchen, M. Meijer, CBS 139577 = DTO 089-C1. USA, California, isol. from indoor air sample, hallway, Jan. 2013, Ž. Jurjević, EMSL 1883 = CPC 22370.
Notes: Cladosporium ramotenellum (Fig. 2, clade 37) was originally described from two Slovenian isolates (Schubert et al. 2007b), one being the type isolated from hypersaline water and an additional strain isolated from an air conditioning system. Recent molecular and morphological studies showed this species to be a common saprobic species occurring on various substrates with a wider geographic distribution. Based on these studies its species description was emended in Bensch et al. (2015). Samson (2014) showed that C. ramotenellum is also quite common in indoor environments which can be confirmed in the present study. Furthermore, it has been reported from clinical samples in the United States in Sandoval-Denis et al. (2015). Cladosporium basiinflatum was included within the C. ramotenellum clade in all three analyses, but always on a long branch; this isolate is up to 100 % identical on tef1 and 180/219 (82 %) similar on act to the closest C. ramotenellum sequences included in our phylogeny.
Cladosporium sinense Bensch & Samson, sp. nov. MycoBank MB822227. Fig. 33, Fig. 34.
Fig. 33.
Cladosporium sinense (CBS 143363). A–C. Colonies on PDA, MEA and OA. D–G, J. Conidiophores and conidia. H. Surface ornamentation of conidiophores and conidia shown in an air bubble. I, K–L. Conidial chains. Scale bars = 10 μm.
Fig. 34.
Cladosporium sinense (CBS 143363). A. Overview of bundles of aerial hyphae, conidiophores and conidia. B. Bundles of hyphae also end as conidiophores. The conspicuous ornamentation of the C. herbarum type is already visible at the ends of the conidiophores. C. Detail of A. showing a smooth conidiophore stipe with short branch, ramoconidia, terminal conidia and conidium initial. D. Detail of B showing the transition of a branch of an aerial hyphae (see B) into a ramoconidium. Note the scars on the ramoconidium and the round terminal ornamented conidia. E. Nearly intact top end of a conidiophore containing ramoconidium and most derived structures. F. Details of conidia and scars. Note the distinct ornamentation of conidia consisting out of single regular extensions. G–J. Details of conidiophores and conidia. The different sizes of the scars on conidiophore stipes are well visible (G and I). These flattened scars are different from the scars on conidia that exhibit a core surrounded by a rim structure. Scale bars = 2 (F–G, I–J), 5 (C–E, H), 10 (A, B) μm.
Etymology: Refers to the country of origin, China.
Holotype: China, Beijing, office building, isol. from indoor air, Sep. 2010, CBS H-23258. Ex-type culture: CBS 143363 = DTO 324-D2.
Diagnosis: Differs from C. aggregatocicatricatum in having shorter, neither nodulose nor geniculate-sinuous conidiophores as well as shorter and narrower conidia.
Mycelium abundantly formed, hyphae filiform or narrowly cylindrical, sparsely branched, 0.5–3(–4) μm wide, subhyaline or very pale olivaceous, septate, neither constricted nor swollen, smooth or almost so, asperulate, minutely verruculose or somewhat irregularly ornamented, especially where conidiophores are formed, sometimes anastomosing, often forming ropes of two or few hyphae. Conidiophores macronematous, solitary, erect or ascending, straight or curved, arising mostly laterally but also terminally from hyphae, narrowly cylindrical-oblong, often slightly attenuated towards the apex, neither nodulose nor geniculate, unbranched, 13–90(–110) × 2–3.5 μm, at the base up to 4.5 μm wide, pale to medium olivaceous or olivaceous brown, often slightly paler towards the apex, 0–4(–5)-septate, not constricted but septa sometimes darkened, smooth or almost so to asperulate with LM, walls unthickened or slightly thickened. Conidiogenous cells integrated, usually terminal, very rarely intercalary, short cylindrical-oblong, 13–30 μm long, with (1–)2–4 distal loci crowded at the apex and forming dense clusters of pronounced scars, loci conspicuous, subdenticulate, 1–1.5 μm diam, somewhat thickened and darkened. Ramoconidia formed, 18–40 × 2.5–3(–3.5) μm, 0–2-septate, with 2–4 distal scars, base about 2(–2.5) μm wide, non-cladosporioid but slightly thickened and somewhat darkened. Conidia catenate, formed in branched chains, branching in all directions, with 1–3 conidia in the terminal unbranched part of the chain, small terminal conidia subglobose or obovoid, 3–4 × 2–2.5 (–3) μm (av. ± SD: 3.5 ± 0.5 × 2.3 ± 0.4 μm), apex broadly rounded; intercalary conidia limoniform or ellipsoid, 3.5–8.5(–10) × 2.5–3.5 μm (av. ± SD: 6.2 ± 2.0 × 2.9 ± 0.3 μm], aseptate, very rarely 1-septate, with 1–3 distal hila; secondary ramoconidia ellipsoid, subcylindrical or cylindrical, (5.5–)8–23 × (2.5–)3–3.5(–4) μm (av. ± SD: 14.3 ± 5.0 × 3.2 ± 0.4 μm), 0(–1)-septate, with 2–4 distal hila densely crowded at the uppermost apex, pale olivaceous or olivaceous brown, almost smooth, often asperulate or loosely to densely minutely verruculose (LM), walls unthickened or almost so, hila conspicuous, subdenticulate, 0.5–1.5 μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 43–50 mm diam after 14 d at 25 °C, olivaceous to grey olivaceous, reverse greyish-blue to olivaceous grey, fluffy, margin glabrous, aerial mycelium abundantly formed, fluffy, loose to dense, growth low convex, with few prominent exudates, sporulation profuse. Colonies on MEA reaching 38–44 mm diam after 14 d at 25 °C, pale olivaceous grey, glaucous-grey to white at colony margins, reverse olivaceous grey, fluffy, margin white, glabrous, somewhat undulate, radially furrowed, somewhat folded in colony centre, several large exudates formed, sporulation profuse. Colonies on OA attaining 42–50 mm diam after 14 d at 25 °C, olivaceous, pale olivaceous grey towards margins, reverse greenish grey to olivaceous grey, fluffy-felty, margins regular, glabrous, aerial mycelium abundantly formed, dense, high, growth low convex, sporulation profuse, without prominent exudates.
Substrate and distribution: Indoor air; Asia (China).
Notes: This new species (Fig. 2, lineage 33) is phylogenetically allied to C. aggregatocicatricatum (Fig. 2, clade 34) but the latter species differs in having longer, once or several times slightly to distinctly, loosely to densely geniculate-sinuous or subnodulose conidiophores as well as longer and wider conidia (Bensch et al. 2015). Until now C. sinense is known only from a single isolate.
Cladosporium sinuosum K. Schub. et al., Stud. Mycol. 58: 141. 2007, emended in Bensch et al. 2015. MycoBank MB504578. Fig. 35.
Fig. 35.
Cladosporium sinuosum (DTO 109-I2). A–C. Colonies on PDA, MEA and OA. D–G. Conidiophores and conidia. H. Superficial mycelium. I. Ramoconidium and conidia. J. Conidia. Scale bars = 10 μm.
Holotype: New Zealand, Te Anau, isolated from leaves of Fuchsia excorticata (Onagraceae), 31 Jan. 2005, A. Blouin, C.F. Hill 1134A, CBS H-19863. Ex-type culture: CBS 121629 = CPC 11839 = ICMP 15819.
Lit.: Bensch et al. (2012: 245–246; 2015: 67–68).
Ill.: Schubert et al. (2007b: 140–141, figs 34–35), Bensch et al. (2012: 245–246, figs 281–282; 2015: 69–71, figs 34–36).
Mycelium filiform or narrowly cylindrical-oblong, loosely branched, 1–5(–7) μm wide, irregular in outline due to swellings and constrictions, sometimes swollen up to 7 μm, subhyaline to pale or medium olivaceous brown, smooth, minutely verruculose or irregularly rough-walled, walls unthickened, sometimes forming loose stromatic hyphal aggregations of swollen hyphal cells, hyphal cells up to 15 μm diam, medium brown or olivaceous brown, walls somewhat thickened; sterile hyphae sometimes forming ropes. Conidiophores macronematous, erect, solitary or on loose groups, straight to often flexuous, arising terminally and laterally from hyphae or from swollen bulbous hyphal cells, long, subnodulose or nodulose, with uni- or multilateral swellings, several times slightly to distinctly geniculate-sinuous due to sympodial proliferation, sometimes even zig-zag-like (see Bensch et al. 2012, fig. 282B), unbranched or branched, up to 380 μm long, (3.5–)4–6(–7) μm wide, swellings up to 10 μm wide, pluriseptate, septa often in short succession and somewhat darkened-refractive, medium olivaceous brown, smooth or minutely verruculose, walls thickened, sometimes even distinctly two-layered, 1(–1.5) μm thick. Conidiogenous cells integrated, terminal and intercalary, cylindrical-oblong, with 1–2 uni- or multilateral swellings per cell, rarely more, geniculate-sinuous, 8–35(–49) μm long, loci confined to swellings, up to four loci per nodule, loci conspicuous, prominent, 1–2(–2.2) μm diam, thickened and darkened-refractive. Ramoconidia not observed. Conidia solitary or in short unbranched or branched chains, up to four conidia in a chain, conidia without a distal hilum ovoid, obovoid to broadly ellipsoid or doliiform, (5–)8–15(–17) × (4–)5–8(–9) μm (av. ± SD: 11.3 ± 2.8 × 7.0 ± 1.2 μm), 0–1-septate, basal and intercalary conidia ellipsoid-ovoid to subcylindrical, 11–19(–24) × (5–)6–9(–11) μm (av. ± SD: 15.9 ± 2.7 × 7.7 ± 1.0 μm), 0–1(–2)- septate, septa median or somewhat in the upper half, becoming curved or sinuous with age, pale olivaceous to medium olivaceous brown or pale greyish brown, densely verrucose to echinulate, walls appearing to be thick-walled due to surface ornamentation, 1–2 μm wide, with 1–2(–3) distal hila, hila protuberant, more or less conspicuous, sometimes immersed in surface ornamentation and therefore not very prominent, 1–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed on SNA but occurring while growing on PDA, MEA and OA.
Culture characteristics: Colonies on PDA attaining 16–47 mm diam after 14 d at 25 °C, smoke-grey to pale olivaceous grey due to aerial mycelium, grey olivaceous towards margins, reverse leaden-grey or olivaceous black, fluffy-felty, margins somewhat feathery, aerial mycelium high, loose to dense, fluffy, growth low convex, without prominent exudates. Colonies on MEA reaching 18–55 mm diam after 14 d at 25 °C, greenish grey to grey olivaceous, white or smoke-grey due to abundant aerial mycelium, reverse olivaceous grey, woolly-felty, margins white, narrow, glabrous to somewhat feathery, radially furrowed and folded, aerial mycelium loose to dense, fluffy to woolly or diffuse, growth flat or effuse, sporulation profuse. Colonies on OA attaining 15–37 mm diam after 14 d at 25 °C, white, smoke-grey to pale olivaceous grey, olivaceous grey at margins, reverse iron-grey or leaden-grey, woolly-felty, margins crenate, aerial mycelium abundant, covering almost the whole colony, woolly-felty, dense, low to high, growth flat, sporulation profuse.
Substrate and distribution: Isolated from various plants and mosses, air and indoor environments; Africa (South Africa), Australasia (New Zealand), Europe (Denmark, France, Germany, The Netherlands).
Additional material examined: Denmark, isol. from indoor environment, B. Andersen, DTO 109-I2 = BA 1896.
Notes: Cladosporium sinuosum (Fig. 2, clade 2), introduced by Schubert et al. (2007b) as a member of the C. herbarum species complex, was described from a single collection on living leaves of Fuchsia excorticata from New Zealand. In Bensch et al. (2015) the species concept was emended since several isolates from different substrates from Europe and South Africa were shown to belong to this species in that phylogenetic study. The isolate from indoor environments in Denmark agrees well with the emended species concept.
Cladosporium floccosum (Fig. 2, clade 4), introduced by Sandoval-Denis et al. (2016) as a new species associated with human infections, is morphologically very similar to C. sinuosum but differs in having shorter, rarely branched conidiophores and slightly shorter terminal conidia (up to 12.5 μm long). It proved to occur also in indoor environments, although there appears to be some intraspecific variation in this species.
Cladosporium sloanii Bensch & Samson, sp. nov. MycoBank MB822228. Fig. 36.
Fig. 36.
Cladosporium sloanii (CBS 143364). A–C. Colonies on DG18 and MA + 20 % sucrose. C–G, I. Conidiophores and conidia. H, J–L. Ramoconidia and conidia. M. Conidial chains. Scale bars = 10 μm.
Etymology: Latin, sloanii, named in honour of Alfred P. Sloan.
Holotype: The Netherlands, Born, isol. from swab sample food plant, M. Meijer, CBS H-23259. Ex-type culture: CBS 143364 = DTO 130-D5.
Diagnosis: Xerophilic species that does not grow on general media, but well on DG18 and MA + 20 % sucrose.
Mycelium sparingly formed, hyphae cylindrical-oblong, (2–)3–5 μm wide, septate, often with swellings and constriction, pale olivaceous, smooth or almost so to minutely verruculose, forming swollen hyphal cells or stromatic hyphal aggregations, hyphal cells up to 9(–12) μm diam, medium to dark olivaceous brown. Conidiophores macronematous, arising solitary from hyphae, mainly laterally, or in small groups from swollen hyphal cells or stromatic hyphal aggregations, cylindrical-oblong, sometimes geniculate towards the apex, unbranched or branched, 40–90(–235) × 2.5–4 μm, up to 5 μm wide at the base, often slightly attenuated towards the apex, 1–4(–7)-septate, septa sometimes in short succession, often somewhat darkened, sometimes slightly constricted, pale to medium olivaceous brown, smooth or almost so. Conidiogenous cells integrated, mainly terminal, cylindrical-oblong, 12–31 μm long, with 1–3 conidiogenous loci at the apex, loci conspicuous, 1–2 μm diam, thickened and darkened-refractive. Ramoconidia frequently formed, cylindrical, 12–36(–42) × (2.5–)3–4 μm, 0(–3)-septate, smooth or minutely verruculose, not attenuated towards the base, base broadly truncate, 2.5–3.5(–4) μm wide, somewhat refractive. Conidia catenate, often formed in dichotomously branched chains, with 1–2(–3) conidia in the terminal unbranched part, small terminal conidia globose, subglobose, obovoid or ellipsoid, 3–7(–11) × (2.5–)3–4(–5) μm (av. ± SD: 5.9 ± 2.5 × 3.5 ± 1.0 μm), intercalary conidia ovoid, ellipsoid, 4.5–11(–13) × 3–4.5 μm (av. ± SD: 7.6 ± 2.6 × 3.6 ± 0.7 μm), 0(–1)-septate, with 1–2(–3) distal hila, secondary ramoconidia ellipsoid or subcylindrical, slightly attenuated towards apex and base, 9.5–21(–28) × 3–4(–4.5) μm (av. ± SD: 16.4 ± 4.4 × 3.7 ± 0.4 μm), 0–1(–2)-septate, septa sometimes refractive or distinctly constricted, pale to medium olivaceous brown, becoming dark brown and more swollen with age, smooth or almost so to often minutely verruculose, sometimes irregularly verruculose, hila conspicuous, 1–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on DG18 reaching 8−9 mm diam after 14 d at 25 °C, olivaceous grey, reverse olivaceous black, velvety, margin narrow, whitish, feathery, crenate, aerial mycelium loose to dense, growth high, up to 2 mm, several very small exudates formed, sporulating. Colonies on MA + 20 % sucrose attaining 4−14 mm diam after 14 d at 25 °C, olivaceous grey to iron-grey, reverse leaden-black, velvety to powdery, margin glabrous to somewhat feathery, narrow, crenate, aerial mycelium loose diffuse to more densely, several exudates formed especially at colony margins, sporulating. Sporulating on SNA, only very sparsely sporulating on OA, only few spores formed.
Substrates and distribution: Swab sample food plant; Europe (The Netherlands).
Notes: Visagie et al. (2014) described Aspergillus sloanii among interesting new species isolated from dust; this species is not able to grow on any of the media generally used for Aspergillus identifications, which was a remarkable finding. Cladosporium sloanii (Fig. 3, clade 11), known from a single isolate, is also not able to grow on most of the generally used media for Cladosporium identification. It is an obligate xerophilic species only growing on low water activity media such as DG18 and MA + 20 % sucrose, which is so far unique for species belonging to the genus Cladosporium. Cladosporium halotolerans and C. sphaerospermum also proved to be able to grow at lower water activity (Segers et al., 2015, Segers et al., 2016) but are not restricted in their growth abilities to these media. Cladosporium psychrotolerans, the closest relative of C. sloanii, differs in forming longer conidiophores and narrower conidia.
Cladosporium sphaerospermum Penzig, Michelia 2(8): 473. 1882. MycoBank MB119529. Fig. 37, Fig. 38.
Fig. 37.
Cladosporium sphaerospermum (DTO 160-I2). A–C. Colonies on PDA, MEA and OA. D–H. Conidiophores and conidial chains. I–J. Conidial chains. Scale bars = 10 μm.
Fig. 38.
Cladosporium sphaerospermum (DTO 160-I2). A. Conidiophores, ramoconidia and terminal conidia showing characteristic ornamentation. B. Scars on ramoconidia and conidial chains. Note the smooth apical zones on the spores. C. Conidial chains and scars. Note that terminal conidia do not have smooth regions. D. Conidiophore with primary and secondary ramoconidia and conidial chains. Note the smooth cell wall of conidiophore stipe and primary ramoconidium. E. Ramoconidia and chains. F. Branching points on ramoconidium with smooth apical zones and scars. G–J. Details of ramoconidia, intercalary conidia and terminal conidia. Note the ornamentation consisting out of ridges, which are often twisted (see I, J); the smooth cells wall next to the scars (H) and between conidia (G). Scale bars = 2 (G–J), 5 (A–F) μm.
Neotype: (designated by Zalar et al. 2007): Sine loco, isolated from a human nail, 1949, R.W. Zappey, CBS H-19738. Ex-neotype culture: CBS 193.54 = ATCC 11289 = IMI 049637.
[Type: Italy, Padova, on faded leaves and stems of Citrus sp. (Rutaceae), Feb. 1882, O. Penzig (not preserved)].
Lit.: de Hoog et al. (2000: 591), Samson et al. (2000: 114, 2001: 340), Zalar et al. (2007: 177–179). Dugan et al. (2008: 9–16), Bensch et al. (2012: 250–254), Segers et al. (2015).
Ill.: de Hoog et al. (2000: 591–592, figs), Samson et al. (2000: 114, fig. 51; 115, pl. 49), Zalar et al. (2007: 166, fig. 5 g, 178, fig. 12), Dugan et al. (2008: 13–14, figs 2–3), Bensch et al. (2012: 251–253, figs 287–289).
Mycelium partly submerged, partly superficial; hyphae sparingly branched, 1–3 μm wide, septate, pale to pale medium olivaceous brown, smooth to sometimes minutely verruculose, walls slightly thickened, not enveloped in polysaccharide-like material. Conidiophores micro- and macronematous, arising terminally and laterally from hyphae, erect or ascending, straight to slightly flexuous. Macronematous conidiophores cylindrical-oblong, neither geniculate nor nodulose, unbranched or branched, (10–)45–130(–300) × 2.5–4.5(–6) μm, pluriseptate, with relatively dense septation (cells mostly 4.5–23 μm long), septa darkened and somewhat thickened, pale medium to medium olivaceous brown, smooth to minutely verruculose, walls thickened. Conidiogenous cells integrated, terminal, sometimes intercalary, cylindrical, usually short, 6–18 μm long, proliferation sympodial, with a single or few apical scars, loci protuberant, denticulate, 0.8–1.5 μm diam, thickened and darkened-refractive. Micronematous conidiophores filiform to narrowly cylindrical-oblong, up to 80 μm long or even longer, 1–2 μm wide, pluriseptate, not that densely septate as macronematous conidiophores, septa also somewhat darkened and thickened, pale to medium olivaceous brown, walls almost unthickened. Conidiogenous cells integrated, terminal and intercalary, short cylindrical, 9–27 μm long, with a few subdenticulate loci, 0.5–0.8 μm diam, thickened and darkened-refractive. Ramoconidia often formed, cylindrical, (11.5–)20.5–50(–67) × (2.5–)3(–3.5) μm, with up to five septa, base broadly truncate, 2–3 μm wide, slightly thickened and somewhat darkened-refractive, but not coronate. Conidia catenate, in branched chains, branching in all directions, with up to six conidia in the unbranched parts, straight, small terminal conidia globose to subglobose, sometimes ovoid, (2–)3–5(–7) × (2–)3–3.5 μm (av. ± SD: 4.1 ± 0.7 × 3.2 ± 0.3 μm), aseptate, minutely verruculose to verrucose, narrower at both ends, intercalary conidia with 1–2 apical hila subglobose, ovoid to ellipsoid, 4.5–10(–12) × 2.5–3.5(–4.5) μm (av. ± SD: 6.5 ± 1.6 × 3.6 ± 0.3 μm), aseptate, attenuated towards apex and base, secondary ramoconidia ellipsoid to cylindrical, 8–24(–38) × (2–)2.5–3.5(–4) μm (av. ± SD: 15.4 ± 5.1 × 3.6 ± 0.5 μm), 0–3(–4)-septate, not constricted at septa, but septa somewhat darkened and thickened, pale to usually medium olivaceous brown, sometimes dark brown, smooth to minutely verruculose, walls thickened, with up to six pronounced, denticulate distal hila, 0.8–1.5 μm diam, sometimes loci situated at the end of protuberant, short, terminal projections, 1–2 μm long or even longer in secondary ramoconidia with beak-like ends, sometimes alternarioid, obclavate, subrostrate (not observed when cultivated on SNA after 7 d, but on PDA and MEA), thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA reaching 21–50 mm diam in 14 d at 25 °C, grey olivaceous or greenish olivaceous, reverse dark grey olivaceous, iron-grey or greyish blue, velvety, margin white, regular, narrow, somewhat feathery, aerial mycelium absent or sparse, growth flat with an elevated colony centre, numerous prominent exudates formed, sporulating, some strains release green soluble pigment into the agar. Colonies on MEA attaining 15–45 mm diam after 14 d at 25 °C, grey olivaceous to olivaceous grey, reverse olivaceous grey to iron-grey, powdery, velvety, margin colourless or white, feathery, regular, radially furrowed, aerial mycelium almost absent, growth low convex with elevated colony centre, centre often wrinkled forming a crater-like structure, without prominent exudates, sporulation profuse. Colonies on OA reaching 21–38 mm diam after 14 d at 25 °C, dark grey olivaceous, olivaceous or olivaceous grey due to profuse sporulation, reverse greenish grey, velvety, aerial mycelium absent, growth flat with papillate surface, without prominent exudates. Colonies on MEA with 5 % NaCl growing faster than on other media, reaching 31–60 mm diam after 14 d at 25 °C, mainly olive, either being almost flat or radially furrowed, with margin of superficial mycelium, sporulation dense, reverse ochraceous or dark green.
Maximum tolerated salt concentration: On MEA + 20 % NaCl 89 % of all strains tested develop colonies after 7 d, 96 % after 14 d.
Cardinal temperatures: No growth at 4 °C, optimum at 25 °C, maximum at 30 °C, no growth at 37 °C. (from Zalar et al. 2007).
Substrates and distribution: Occurring as secondary invader on numerous plants, saprobic on dead leaves, stems, wood and other plant organs, isolated from outdoor and indoor air, soil, hypersaline water, indoor wet cells, foodstuffs and other organic matter, paint, silicon, textiles and occasionally isolated from human and animals (nails, nasal mucus, etc.); cosmopolitan.
Additional materials examined: Australia, Tasmania, isol. from house dust, B. Horton, DTO 307-H1; BH02AU-119. Portugal, isol. from indoor environment, DTO 150-I3; DTO 150-I8. South Africa, isol. from house dust, K. Jacobs, DTO 305-F5 = KJ03SA-383B, DTO 307-G6 = KJ08SA-151. The Netherlands, Gilze, swab sample of wall near window in apartment, DTO 161-E1, J. Houbraken; Utrecht, swab sample archive, M. Meijer, DTO 090-H9. UK, Ditherington, isol. from indoor air sample, Dec. 2012, Ž. Jurjević, EMSL 1870 = CPC 22357. USA, isol. from house dust, A. Amend, DTO 306-D8 = AA03US-373, DTO 306-E3 = AA03US-478, DTO 307-I3 = AA03US-549; California, Newport Beach, isol. from indoor air sample, bathroom, Oct. 2012, Ž. Jurjević, EMSL 1789, 1790 = CPC 22301, 22302; San Francisco, isol. from indoor air sample, family room, Jan. 2013, Ž. Jurjević, EMSL 1892 = CPC 22379; Minnesota, isol. from indoor air sample, Aug. 2012, Ž. Jurjević, EMSL 1728 = CPC 22270; Mississippi, Ridgeland, isol. from indoor air sample, Nov. 2012, Ž. Jurjević, EMSL 1820 = CPC 22317; New York, Hamlet, isol. from indoor air sample, warehouse, Dec. 2012, Ž. Jurjević, EMSL 1852 = CPC 22339; Vermont, Williston, isol. from indoor air sample, bedroom, Dec. 2012, Ž. Jurjević, EMSL 1874 = CPC 22361; Wisconsin, Oak Creek, isol. from air sample, bakery, DTO 127-E5 = AR385. Additional isolates are listed in Table 1.
Notes: Cladosporium sphaerospermum (Fig. 3, clade 20) was described by Penzig (1882) from decaying Citrus leaves and branches in Italy. Penzig’s original material is not known to be preserved. Later, a culture derived from CBS 193.54, originating from a human nail, was accepted as typical for C. sphaerospermum. However, de Vries (1952), incorrectly cited it as “lectotype”, and thus the same specimen was designated as neotype in Zalar et al. (2007), with the derived culture (CBS 193.54) used as ex-neotype strain. Zalar et al. (2007) considered C. sphaerospermum as halo- or osmotolerant. Although C. sphaerospermum has commonly been isolated from osmotically stressed environments, it is also known from non-stressed niches. It is a cosmopolitan species that has been studied from the perspectives of phylogeny, halotolerance and general ecology (summarised in Zalar et al. 2007), biodegradative capacities (e.g., Weber et al., 1995, Prenafeta-Boldú et al., 2001, Potin et al., 2004, Nieves-Rivera et al., 2006, Kim et al., 2007), and clinical aspects (summarised in De Hoog et al., 2000, Zalar et al., 2007, Sandoval-Denis et al., 2015). In the study of Sandoval-Denis et al. (2015) most of the clinical isolates morphologically identified as C. sphaerospermum were genetically reidentified as belonging to the phenotypically similar species C. halotolerans, which according to their data, emerged as the most common species from clinical origin.
Furthermore, Cladosporium sphaerospermum proved to be a common species isolated from indoor environments (Segers et al. 2015; this study, see Table 1). It is a phylogenetically well-delineated species (see Fig. 3, clade 20 and Zalar et al. 2007) which differs from C. halotolerans in forming often branched and densely septate, somewhat wider conidiophores, 2.5–4.5(–6) μm, and producing slightly longer small terminal conidia, (2–)3–5(–7) and with up to 5-septate ramoconidia being up to 50(–67) μm long, commonly beaked (alternarioid) on MEA and PDA.
Cladosporium subinflatum K. Schub. et al., Stud. Mycol. 58: 143. 2007. MycoBank MB504579. Fig. 39.
Fig. 39.
Cladosporium subinflatum (CPC 22303). A–C. Colonies on PDA, MEA and OA. D–G. Conidiophores and conidial chains. H–I. Micronematous conidiophores. J. Conidial chains with conidia showing the densely verruculose to echinulate surface ornamentation. Scale bars = 10 μm.
Holotype: Slovenia, Sečovlje, crystallisation ponds, salterns, isolated from hypersaline water, 2005, S. Sonjak, CBS H-19864. Isotype: HAL 2027 F. Ex-type culture: CBS 121630 = CPC 12041 = EXF-343.
Lit.: Bensch et al. (2012: 258–260), Bensch et al. (2015: 68).
Ill.: Schubert et al. (2007b: 143–144, figs 37–39), Bensch et al. (2012: 258–259, figs 296–298).
Mycelium unbranched or occasionally branched, 1.5–4 μm wide, later more frequently branched and wider, up to 7 μm wide, sometimes anastomosing, septate, not constricted at the septa, but sometimes single septa darkened, subhyaline or pale olivaceous brown, almost smooth to somewhat verruculose or irregularly rough-walled in fertile hyphae, walls unthickened. Conidiophores mainly macronematous, sometimes also micronematous, arising terminally from ascending hyphae or laterally from plagiotropous hyphae, erect or subdecumbent, straight or flexuous, sometimes bent, cylindrical, nodulose, usually with small head-like swellings, sometimes swellings also on a lower level or intercalary, occasionally geniculate, unbranched, occasionally branched, (5–)10–100(–270) × (1.5–)2.5–4.5(–5.5) μm, swellings 3–6.5 μm wide, aseptate or with few septa, not constricted at the septa, pale brown, pale or medium olivaceous brown, smooth, usually verruculose or irregularly rough-walled and paler, subhyaline towards the base, walls thickened, sometimes appearing even two-layered, up to 1 μm thick; micronematous conidiophores narrower, paler and shorter, mostly without capitate apex, short narrowly cylindrical, up to 35 μm long, 2–3 μm wide. Conidiogenous cells integrated, usually terminal or conidiophores reduced to conidiogenous cells, cylindrical, nodulose, usually with small head-like swellings with loci confined to swellings, sometimes geniculate, 5–42 μm long, proliferation sympodial, with several loci, up to four situated at nodules or on lateral swellings, protuberant, conspicuous, denticulate, (0.8–)1–2 μm diam, thickened and darkened-refractive. Ramoconidia rarely formed. Conidia catenate, in short branched chains, 1–4 conidia in the terminal unbranched part of the chain, more or less straight, numerous globose and subglobose conidia, ovoid, obovoid, broadly ellipsoid to cylindrical, small terminal conidia subglobose, obovoid or ellipsoid, (3–)4–7(–9) × (2.5–)3–4 μm (av. ± SD: 5.4 ± 1.4 × 3.3 ± 0.5 μm), intercalary conidia ovoid, ellipsoid, 5.5–9(–12.5) × (3–)3.5–4(–4.5) μm (av. ± SD: 8.5 ± 2.1 × 3.8 ± 0.4 μm), aseptate, with 1(–2) distal hila, secondary conidia ellipsoid or subcylindrical, (7–)8.5–20(–25) × (3–)4–5.5(–7) μm (av. ± SD: 13.5 ± 4.2 × 4.6 ± 0.5 μm), 0–1(–2)-septate, with (1–)2–3(–4) distal hila, pale to medium olivaceous brown, ornamentation variable, mainly densely verruculose to echinulate (loosely muricate under SEM), spines up to 0.8 μm high, sometimes irregularly verrucose with few scattered tubercles or irregularly echinulate, walls unthickened or slightly thickened, apex rounded or slightly attenuated towards apex and base, hila conspicuous, protuberant, denticulate, 0.5–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis observed.
Culture characteristics: Colonies on PDA attaining 26–60 mm diam after 14 d at 25 ºC, pale olivaceous grey to olivaceous grey, or dull-green, reverse iron-grey or olivaceous black, margin regular, entire edge, narrow, colourless to white, glabrous, aerial mycelium abundantly formed, fluffy, dense, growth flat, somewhat folded in the colony centre, deep into the agar, few prominent exudates formed with age, sporulation profuse. Colonies on MEA attaining 25–60 mm diam after 14 d at 25 °C, olivaceous grey to olivaceous due to abundant sporulation in the colony centre, pale greenish grey towards margin, iron-grey or olivaceous grey on reverse, velvety to powdery, margin narrow, white, glabrous, radially furrowed, aerial mycelium diffuse, growth convex with papillate surface, wrinkled colony centre, without prominent exudates, sporulation profuse. Colonies on OA attaining 26–58 mm diam after 14 d at 25 °C, olivaceous, dull-green towards margins, reverse iron-grey, leaden-grey to greenish black, velvety to fluffy, margin regular, aerial mycelium loose, diffuse or denser in colony centre, growth flat, deep into the agar, with a single exudate, abundantly sporulating.
Substrate and distribution: Hypersaline water, indoor air and plant material; Europe (Slovenia, Ukraine), North America (USA).
Additional materials examined: USA, Minnesota, Fergus Falls, isol. from indoor air sample, Oct. 2012, Ž. Jurjević, EMSL 1791 = CPC 22303; Missouri, Fort Leonard Wood, isol. from indoor air sample bathroom, Jan. 2013, Ž. Jurjević, EMSL 1928 = CPC 22400.
Notes: Cladosporium subinflatum (Fig. 2, clade 21) is a saprobic hyphomycete well characterised by the formation of numerous globose or subglobose conidia, resembling members of the C. sphaerospermum species complex (Fig. 3), with its coarse surface ornamentation ranging from verruculose to distinctly spiny. Cladosporium spinulosum (Fig. 2, clade 28), also isolated from hypersaline water, is morphologically close to C. subinflatum, but differs from the latter species in having somewhat narrower macronematous conidiophores, narrower conidiogenous loci and hila, and conidia with longer spines, up to 1.3 μm. Cladosporium allicinum (Fig. 2, clade 27) may superficially also be confusable, but its conidia are minutely verruculose to verrucose but never spiny.
The species was previously known only from hypersaline environments and plant material but is now also reported from indoor environments and known from clinical samples (Sandoval-Denis et al. 2015).
Cladosporium subuliforme Bensch et al., Stud. Mycol. 67: 77. 2010. MycoBank MB517090. Fig. 40.
Fig. 40.
Cladosporium subuliforme (DTO 324-C7). A–C. Colonies on PDA, MEA and OA. D–H. Macronematous conidiophores and conidial chains. I, K. Micronematous conidiophores. J. Ramoconidium seceded at a conidiophore. L. Conidial chains. Scale bars = 10 μm.
Holotype: Thailand, Chiang Mai, Sansai, Mai Jo, palm nursery, isol. from Chamaedorea metallica (Arecaceae), 26 Dec. 2006, coll. I. Hidayat & J. Meeboon, FIH 401, isol. P.W. Crous, CBS H-20448. Ex-type culture: CBS 126500 = CPC 13735.
Lit.: Bensch et al. (2012: 264−265; 2015: 68), Ramos-García et al. (2016).
Ill.: Bensch et al. (2010: 78, figs 67−68; 2012: 264−265, figs 305−306).
Mycelium internal and superficial, abundantly formed; hyphae sparingly branched, 1−4 μm wide, septate, sometimes slightly constricted at the base of conidiophores, subhyaline to pale olivaceous brown, smooth to minutely verruculose or verruculose, often somewhat swollen at the base of conidiophores, up to 6 μm wide, sometimes forming ropes. Conidiophores macro-, semimacro- or micronematous, solitary or in pairs, arising terminally and laterally from hyphae, erect, straight to mostly flexuous, filiform to narrowly cylindrical-oblong, often slightly to distinctly attenuated towards the apex and wider at the base, not nodulose or geniculate, unbranched or branched, branches often only as short peg-like lateral outgrowths just below a septum bearing conidiogenous loci, branches occasionally longer, up to 20 μm, 9−330 × (1.5−)2−4 μm, often wider towards the base, pluriseptate, usually not constricted at septa, pale to medium olivaceous brown, smooth to sometimes minutely verruculose, parts of the stalk occasionally verrucose or irregularly rough-walled, basal cell sometimes swollen up to 8(−10) μm, walls unthickened or only slightly thickened, about 0.5 μm wide. Conidiogenous cells integrated, mainly terminal but also intercalary, narrowly cylindrical-oblong, neither nodulose nor geniculate, 9−40 μm long, with up to five loci crowded at the uppermost apex, in intercalary cells loci often situated on small denticle- or peg-like lateral outgrowths just below a septum, loci conspicuous, subdenticulate, (0.8−)1−1.5(−2) μm diam, thickened and darkened-refractive. Ramoconidia commonly formed, cylindrical-oblong, differentiation between ramoconidia and secondary ramoconidia often quite difficult, (14−)17−39 × (1.5−)2−3 μm, 0(−1)-septate, pale olivaceous brown, smooth, walls unthickened, not attenuated towards the base, base broadly truncate, 2−2.5 μm wide, unthickened, but often somewhat darkened or refractive. Conidia numerous, catenate, in branched chains, up to 2−6 conidia in the unbranched terminal part of the chain, branching in all directions, straight, small terminal conidia obovoid, subglobose, ovoid to limoniform or ellipsoid, 2.5−4.5(−5.5) × 2−2.5 μm (av. ± SD: 4.1 ± 0.7 × 2.2 ± 0.3), aseptate, rounded at the apex, attenuated towards the base, intercalary conidia ellipsoid to subcylindrical, 5−13 × 2−3(−3.5) μm (av. ± SD: 8.3 ± 2.6 × 2.8 ± 0.4), aseptate, with up to four distal hila, attenuated towards apex and base, secondary ramoconidia ellipsoid to subcylindrical, sometimes cylindrical-oblong, (6−)8−27(−34) × 2−3.5 μm (av. ± SD: 17.6 ± 7.3 × 2.9 ± 0.4), 0−1-septate, not constricted at septa, median or somewhat in the lower half, usually somewhat attenuated towards the base, (2−)3−4(−5) distal hila, pale olivaceous brown, smooth or almost so (LM), walls unthickened, hila conspicuous, subdenticulate to denticulate, (0.2−)0.5−1.5(−2) μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining up to 80 mm diam after 14 d at 25 °C, grey olivaceous to mainly olivaceous grey, reverse olivaceous grey, velvety to floccose, fluffy, margins grey olivaceous to white, feathery, regular or slightly undulate, aerial mycelium abundant, loose, fluffy, growth effuse to low convex, without exudates, sporulation profuse. Colonies on MEA reaching 60−80 mm diam after 14 d at 25 °C, greenish olivaceous to pale olivaceous grey and olivaceous buff, glaucous-grey at margins, reverse olivaceous grey, floccose to fluffy, margins white, glabrous, regular to somewhat undulate, radially furrowed and wrinkled, effuse, aerial mycelium abundant, fluffy, mainly in colony centre, without exudates, sporulation profuse. Colonies on OA attaining up to 80 mm diam after 14 d at 25 °C, whitish to smoke-grey and pale olivaceous grey, olivaceous buff and dull green towards margins, somewhat zonate, grey olivaceous due to sporulation, reverse leaden-grey, floccose to felty, margins dull green or colourless, regular, glabrous, aerial mycelium abundant, floccose to fluffy-felty, covering large parts of colony surface, growth effuse, without exudates, sporulating.
Substrate and distribution: Isolated from plant material and indoor environments; Africa (South Africa), Asia (China, Thailand), Central and South America (Brazil, Cuba), North America (Mexico, USA).
Additional materials examined: China, isol. from indoor air, DTO 323-D1, DTO 324-B8, DTO 324-C7. Thailand, Surat Thani, isol. from indoor air (open Petri-dish), P. Noonim, DTO 130-H8.
Notes: Cladosporium subuliforme (Fig. 1, clade 59) belongs to the C. cladosporioides species complex, but deviates from allied species, specifically C. cladosporioides (Fig. 1, clade 66) and C. tenuissimum (Fig. 1, clade 64), by its long narrow subulate conidiophores with several loci crowded at the apex and its numerous ramoconidia with narrow loci and hila. Cladosporium angustisporum (Fig. 1, clade 58) is phylogenetically close to this species (also see Bensch et al., 2010, Bensch et al., 2012, Bensch et al., 2015) but morphologically easily separable. The conidiophores are not subuliform and the terminal conidia are somewhat longer and narrower.
Sandoval-Denis et al. (2015) reported C. subuliforme for the first time from clinical samples in the United States. In the present study it is now also reported to occur in indoor environments.
Cladosporium tenellum K. Schub. et al., Stud. Mycol. 58: 149. 2007. MycoBank MB504581. Fig. 41.
Fig. 41.
Cladosporium tenellum (CPC 22290). A–C. Colonies on PDA, MEA and OA. D–H. Conidiophores and conidial chains. I–J. Micronematous conidiophores. K. Ramoconidium and conidia. Scale bars = 10 μm.
Holotype: Israel, Ein Bokek, Dead Sea, isolated from hypersaline water, 2004, M. Ota, CBS H-19866. Isotype: HAL 2029 F. Ex-type culture: CBS 121634 = CPC 12053 = EXF-1735.
Lit.: Bensch et al. (2012: 268–269).
Ill.: Schubert et al. (2007b: 148–149, figs 43–45), Bensch et al. (2012: 268–269, figs 311–313).
Mycelium sparingly branched, 1–3 μm wide, septate, septa often not very conspicuous, not constricted at the septa, sometimes slightly swollen, subhyaline, smooth, walls unthickened. Conidiophores macro- and micronematous, solitary, arising terminally or laterally from plagiotropous or ascending hyphae, erect or subdecumbent, almost straight to more or less flexuous, cylindrical, sometimes geniculate towards the apex, but not nodulose, sometimes with short lateral prolongations at the apex, unbranched to once or twice branched (angle usually 30–45° degree, sometimes up to 90°), branches usually below a septum, 6–200 × (1–)2–4(–5) μm, septate, septa often not very conspicuous, occasionally appearing somewhat darkened, not constricted at the septa, sometimes septa in short succession, subhyaline to pale brown, almost smooth to usually asperulate, walls unthickened or almost so. Conidiogenous cells integrated, terminal or intercalary, sometimes conidiophores reduced to conidiogenous cells, cylindrical, sometimes geniculate, non-nodulose, 6–40 μm long, proliferation sympodial, with several conidiogenous loci often crowded at the apex and sometimes also at a lower level, situated on small lateral shoulders, unilateral swellings or prolongations, with up to 6(–10) denticulate loci, forming sympodial clusters of pronounced scars, intercalary conidiogenous cells with short or somewhat long lateral outgrowths, short denticle-like or long branches with several scars at the apex, usually below a septum, loci protuberant, 1–1.5(–2) μm diam, thickened and darkened-refractive. Ramoconidia sometimes occurring, cylindrical, up to 32 μm long, 2.5–4(–4.5) μm wide, with a broadly truncate, unthickened base, about 2(–2.5) μm wide. Conidia catenate, formed in branched chains, straight, small terminal conidia globose, subglobose, ovoid, (2.5–)3–5(–6) × (2–)2.5–3.5(–4) μm (av. ± SD: 4.0 ± 0.7 × 2.9 ± 0.5 μm), aseptate, asperulate, with 0–1 distal hila, intercalary conidia ovoid or ellipsoid, 5–11(–13) × 3 4.5( 5) μm (av. ± SD: 7.4 ± 1.9 × 3.8 ± 0.6 μm), aseptate, with 1–4 distal hila, secondary ramoconidia ellipsoid-ovoid, ellipsoid to subcylindrical, (6–)8–21(–28) × (2.5–)3–5(–6) μm (av. ± SD: 14.4 ± 4.7 × 4.6 ± 3.8 μm), 0–1-septate, rarely with up to three septa, sometimes slightly constricted at septa, subhyaline, pale brown to medium olivaceous brown, asperulate or verruculose (muricate, granulate or colliculate under SEM), walls unthickened or slightly thickened, apex rounded or slightly to distinctly attenuated towards apex and base, often forming several apical hila, up to 7(–9), crowded, situated on small lateral outgrowths giving them a somewhat irregular appearance, hila protuberant, 0.5–1.5 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis sometimes occurring.
Culture characteristics: Colonies on PDA reaching 27–34 mm diam after 14 d at 25 ºC, smoke-grey, grey olivaceous to olivaceous grey, olivaceous grey to iron-grey reverse, velvety to powdery, margin regular, entire edge, narrow, colourless to white, aerial mycelium absent or sparingly formed, felty, whitish, growth regular, flat, radially furrowed, with folded and elevated colony centre, deep into the agar, with age forming few to numerous prominent exudates, sporulation profuse, few high conidiophores formed. Colonies on MEA reaching 25–44 mm diam after 14 d at 25 ºC, olivaceous grey to olivaceous or iron-grey due to abundant sporulation in the colony centre, velvety, margin regular, entire edge, narrow, colourless, white to pale olivaceous grey, aerial mycelium loose, diffuse, growth convex with papillate surface, radially furrowed, wrinkled, without prominent exudates, sporulating. Colonies on OA reaching 23–32 mm diam after 14 d at 25 ºC, grey olivaceous, olivaceous grey to olivaceous due to abundant sporulation in the colony centre, olivaceous or iron-grey reverse, velvety, margin regular, entire edge, narrow, colourless or white, aerial mycelium sparse, diffuse, floccose, growth flat to low convex, radially furrowed, wrinkled, without prominent exudates, sporulation profuse.
Substrate and distribution: Hypersaline water, indoor environments and plant material; Middle East (Israel), North America (USA).
Additional materials examined: USA, isol. from air sample, bakery, CBS 139582 = DTO 127-D7 = AR295; Michigan, Big rapids, isol. from indoor air sample, classroom, Jan. 2013, Ž. Jurjević, EMSL 1941 = CPC 22410; Okemos, isol. from indoor air sample, bathroom, Sep. 2012, Ž. Jurjević, EMSL 1771 = CPC 22290; Oregon, Salem, isol. from indoor air sample, bedroom, Sep. 2012, Ž. Jurjević, EMSL 1772 = CPC 22291.
Notes: Cladosporium tenellum (Fig. 2, clade 22) comprises characters of various species complexes of the genus Cladosporium. The formation of globose or subglobose terminal conidia is reminiscent of members of the C. sphaerospermum species complex (Fig. 3). Based on the general morphology and size of conidiophores and conidia C. tenellum is rather comparable with species of the C. cladosporioides species complex (Fig. 1), e.g. C. cladosporioides s. str. characterised by smooth conidiophores and conidia with only few conidiogenous loci and conidial hila crowded at the apex and somewhat wider conidiophores, 3–5(–6) μm. However, it belongs to the C. herbarum species complex (Fig. 2) where it resembles C. subtilissimum (Fig. 2, clade 25) and C. ramotenellum (Fig. 2, clade 37; Schubert et al. 2007b). In C. subtilissimum the small terminal conidia are not globose but rather narrowly obovoid to limoniform, the conidiogenous loci and conidial hila are somewhat wider, (0.5–)0.8–2(–2.2) μm, and at the apices of conidiophores and conidia only few scars are formed. Cladosporium ramotenellum possesses longer and narrower, 0–3-septate conidia, 2.5–35 × 2–4(–5) μm, but forms only few conidiogenous loci and conidial hila at the apices of conidiophores and conidia (Bensch et al. 2012). It has not only been isolated from hypersaline water and plant material but also from indoor environments.
Cladosporium tenuissimum Cooke, Grevillea 6(40): 140. 1878. MycoBank MB145672. Fig. 42.
Fig. 42.
Cladosporium tenuissimum (DTO 323-G3). A–C. Colonies on PDA, MEA and OA. D–H. Macronematous conidiophores and conidial chains. I–J. Micronematous conidiophores and conidia. Scale bars = 10 μm.
Lectotype (designated by Heuchert et al. 2005): USA, South Carolina, Aiken, on leaf sheets of Zea mays (Poaceae), H.W. Ravenel, Ravenel, Fungi Amer. Exs. 160 (NY). Isolectotypes: Ravenel, Fungi Amer. Exs. 160 (e.g., K, PH 01020427). Topotype material: Roumeguère, Fungi Sel. Gall. Exs. 5295 (e.g., NY). Epitype (designated by Bensch et al. 2010): USA, Louisiana, Baton Rouge, isol. from fruits of Lagerstroemia sp. (Lythraceae), 8 Sep. 2007, P.W. Crous, CBS H-20449. Ex-epitype culture: CBS 125995 = CPC 14253.
Lit.: Ellis (1976: 326), Ho et al. (1999: 140), Heuchert et al. (2005: 50–52), Bensch et al. (2010: 78–81; 2012: 269–272).
Ill.: Ellis (1976: 327, fig. 245 A), Ho et al. (1999: 143, figs 46–47), Heuchert et al. (2005: 51, fig. 20), Bensch et al. (2010: 80–81, figs 69–70; 2012: 270–271, figs 314–316).
Mycelium immersed and superficial, hyphae branched, (0.5−)1−5 μm wide, septate, sometimes constricted at septa, subhyaline to pale or medium brown, with swellings and constrictions, often irregular in outline, smooth to sometimes minutely verruculose, sometimes appearing rough-walled, walls unthickened or very slightly thickened, sometimes forming ropes. Conidiophores solitary, macro- and micronematous, arising terminally and laterally from hyphae; macronematous conidiophores solitary, sometimes in groups of 2−3, erect, straight or slightly flexuous, cylindrical-oblong to almost filiform, sometimes slightly to distinctly geniculate towards the apex, often subnodulose or nodulose with an apical and sometimes a few additional swellings on a lower level, swellings quite distant from the apex and from each other, most conidiophores neither geniculate nor nodulose, unbranched or branched, branching often at an angle of 45−90°, just below the apex or at a lower level, branches sometimes only as short denticle-like prolongations just below a septum, occasionally long, conidiophores 30−310(−460) × 2.5−4 μm (on OA up to 900 μm long), septate, sometimes distinctly constricted at septa, pale to medium brown or olivaceous brown, smooth, sometimes slightly rough-walled at the base, walls somewhat thickened, sometimes slightly attenuated towards the apex and distinctly swollen at the base, with age conidiophores becoming darker and more thick-walled; micro- to semimacronematous conidiophores narrower, paler, filiform to narrowly cylindrical-oblong, non-nodulose or only slightly swollen at the apex, unbranched, 17−85 × (1−)2−2.5 μm, with few septa or reduced to conidiogenous cells, pale brown or subhyaline, smooth, walls unthickened or almost so, with a single or up to seven subdenticulate, pronounced loci crowded at the apex. Conidiogenous cells integrated, terminal and intercalary, cylindrical-oblong, sometimes short geniculate at the apex, often nodulose, swellings up to 5 μm wide, cells (4−)10−44 μm long, loci often situated on swellings but not restricted to them, mostly only a single swelling per cell, in terminal cells apex usually head-like, uni- or multilaterally swollen with up to eight pronounced, subdenticulate to denticulate loci crowded at the tip, in intercalary conidiogenous cells loci often sitting at about the same level (arranged like a garland round about the stalk) or situated on small lateral shoulders, loci 1−1.5(−2) μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed, subcylindrical or cylindrical-oblong, 22−41 × 3−4(−5) μm, 0(−1)-septate, base broadly truncate, 2−3.5 μm wide. Conidia catenate, in densely branched chains, 1−4(−6) conidia in the terminal unbranched part of the chain, branching in all directions, straight, small terminal conidia subglobose, obovoid, limoniform, sometimes globose, (2−)2.5−5(−6) × (1.5−)2−3 μm (av. ± SD: 3.7 ± 1.0 × 2.2 ± 0.4), aseptate, apex broadly rounded, intercalary conidia ovoid, ellipsoid or subcylindrical, 4−12(−17) × (1−)2−3(−4.5) μm (av. ± SD: 8.1 ± 2.7 × 2.8 ± 0.6), aseptate, occasionally 1-septate, with up to 5(−7) distal hila, sometimes cell lumen distinct, secondary ramoconidia ellipsoid, fusiform to subcylindrical or cylindrical, (6−)7−25(−31) × (2−)2.5−4(−5) μm (av. ± SD: 15.0 ± 5.8 × 3.2 ± 0.5), with (1−)2−6(−7) distal hila, sometimes with 1−2 hila at the basal end, 0−1(−2)-septate, sometimes distinctly constricted at septa, with age more frequently septate, pale brown or pale olivaceous brown, smooth, occasionally irregularly rough-walled, walls unthickened or almost so, attenuated towards apex and base, hila conspicuous, subdenticulate to denticulate, 0.5−1.8(−2) μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring with conidia forming secondary conidiophores.
Culture characteristics: Colonies on PDA attaining up to 84 mm diam after 14 d at 25 °C, smoke-grey to grey olivaceous or olivaceous grey, reverse leaden-grey to olivaceous black, woolly to fluffy, margin glabrous to feathery, grey olivaceous to white, aerial mycelium abundant, high, fluffy, smoke-grey, dense, without prominent exudates, sporulating. Colonies on MEA reaching 70−80 mm diam after 14 d at 25 °C, smoke-grey to pale olivaceous grey, pale olivaceous due to abundant sporulation, reverse olivaceous grey, woolly, fluffy, margins narrow, glabrous to feathery, colourless to white, sometimes radially furrowed and wrinkled, aerial mycelium abundant, fluffy, dense, high, pale olivaceous grey, covering large parts of the colony surface, growth low convex, few prominent exudates formed, sporulating. Colonies on OA attaining 65−73 mm diam after 14 d at 25 °C, smoke-grey, pale olivaceous grey to whitish due to aerial mycelium, greenish grey towards margin, reverse olivaceous grey to iron-grey or leaden-grey, woolly-fluffy to felty, margin colourless to white, narrow, glabrous, aerial mycelium high, abundantly formed, fluffy to felty, whitish, growth at to low convex, mostly without prominent exudates, sporulating.
Substrate and distribution: On different host plants isolated from dead leaves, twigs, stems, wood and other organic matter, also isolated from air, bread, clinical samples, soil and water; cosmopolitan but especially common in the tropics.
Additional materials examined: Bermuda, Samerset, isol. from indoor air sample, Nov. 2012, Ž. Jurjević, EMSL 1823 = CPC 22320. China, isol. from indoor air, DTO 323-C5, DTO 323-C9, DTO 323-G2, DTO 323-G3, DTO 323-G4, DTO 323-G8, DTO 323-I4, DTO 323-I6, DTO 323-I8, DTO 323-I9, DTO 324-A1, DTO 324-A3, DTO 324-C2, DTO 324-C3, DTO 324-C5, DTO 324-C6, DTO 324-C9. Mexico, isol. from chili pepper sample, Aug. 2012, Ž. Jurjević, EMSL 1748 = CPC 22277. Thailand, Surat Thani, isol. from bathroom ceiling, P. Noonim, DTO 109-A1; from indoor environments (mycolab door), P. Noonim, DTO 109-C4; isol. from indoor air (open Petri-dish), P. Noonim, DTO 109-C7; Trang, isol. from indoor air (open Petri-dish), P. Noonim, DTO 131-A4. USA, Arizona, Casa Grande, isol. from indoor air sample, bedroom, Dec. 2012, Ž. Jurjević, EMSL 1857 = CPC 22344; Texas, Georgetown, isol. from indoor air sample, classroom, Jan. 2013, Ž. Jurjević, EMSL 1926 = CPC 22398.
Notes: Cladosporium tenuissimum (Fig. 1, clade 64) is a common saprobic hyphomycete comparable and confusable with C. cladosporioides (Fig. 1, clade 66), but genetically as well as morphologically distinct as demonstrated and discussed in Bensch et al., 2010, Bensch et al., 2012. Cladosporium stanhopeae, a species described on Stanhopea (Orchidaceae) from Germany (Schubert, 2004, Schubert, 2005), resembles C. tenuissimum but is tentatively maintained as a separate species until isolates from that host can be included in molecular studies.
Cladosporium tenuissimum has been reported from several clinical samples in the USA (Sandoval-Denis et al. 2015) as the second most frequently isolated species after C. halotolerans and proved to be also commonly occurring in indoor environments.
Cladosporium uwebraunianum Bensch & Samson, sp. nov. MycoBank MB822229. Fig. 43, Fig. 44.
Fig. 43.
Cladosporium uwebraunianum (CBS 143365). A–C. Colonies on PDA, MEA and OA. D–H, J. Conidiophores and conidial chains. I. Ramoconidium and conidial chains. K. Conidial chains. Scale bars = 10 μm.
Fig. 44.
Cladosporium uwebraunianum (CBS 143365). A. Survey of conidiophores sprouting from a common base, consisting out of a tissue of broadened connected cells, partially located under the agar surface. B. Free-standing conidiophore with intact stipes, ramoconidia, intercalary and terminal conidia. C. Conidia on conidiophore. Conidia are very smooth; some bear a subtle net-like ornamentation (typical for the C. cladosporioides complex). Some initials are visible; other chains are broken as judged by the scars on the conidia. D. Two intact conidiophores bearing numerous spores. This micrograph shows the compactness of the spore mass and also illustrates that conidial chains support each other throughout formation. E. Conidia on conidiophore showing some initials. F. Chains of conidia, two of the ending in terminally conidia. Scars are visible on a secondary ramoconidium. G. Details of the conidiophore. Note the very smooth surface of the conidia and conidiophore. Fine breaks delineate several spores. H, J, K. Details of scars of intercalary and also terminal conidia (H, J) and initial (J). I. Details of scars on a conidiophore. Note the difference in size of the scars, compare with the lines in Figure G. Scale bars = 2 (H–K), 5 (F, G), 10 (B–E), 50 (A) μm.
Etymology: In honour of Uwe Braun for his valuable and extensive work on Cladosporium and other cladosporium-like genera.
Holotype: The Netherlands, Amsterdam, indoor air, archive, M. Meijer, CBS H-23260. Ex-type culture: CBS 143365 = DTO 072-D8.
Diagnosis: Differs from the phylogenetically closely related C. australiense in producing shorter conidiophores (up to 95(–135) μm), longer conidiogenous cells (17–50(–65) μm) and conidia formed in long branched chains with up to 10(–13) conidia in the terminal unbranched part of the chain.
Mycelium unbranched or loosely branched, hyphae (1–)2–5(–6.5) μm wide, septate, pale or medium olivaceous brown, smooth or almost so, minutely verruculose or irregularly rough-walled, walls slightly thickened. Conidiophores macro- and micronematous, formed solitary or in small groups of three laterally or terminally from hyphae, straight or somewhat flexuous, neither geniculate nor nodulose, cylindrical-oblong, quite short, 15–95(–135) μm long, 2–2.5 μm wide in micronematous conidiophores, 2.5–4 μm wide in macronematous conidiophores, unbranched or branched, branchlets as small lateral outgrowths just below or above a septum, 0–2(–4)-septate, pale to medium sometimes even dark olivaceous brown, smooth, walls slightly thickened. Conidiogenous cells usually terminally or conidiophores reduced to conidiogenous cells, rarely intercalary in branched conidiophores, 17–50(–65) μm long, with 2–3(–4) distal scars situated at the apex, loci more or less truncate, 1–2 μm diam. Ramoconidia occasionally formed, 23–42 × 3–4 μm, base (2.5–)3(–3.5) μm wide. Conidia numerously formed in branched chains, branching in all directions, with up to 10(–13) conidia in the terminal unbranched part of the conidial chains, small terminal conidia obovoid, limoniform or ellipsoid, (3–)4–7(–10) × 2–3 μm (av. ± SD: 5.9 ± 1.5 × 2.5 ± 0.4), intercalary conidia ellipsoid or subcylindrical, (6–)7–12(–15) × 2.5–3(–3.5) μm (av. ± SD: 9.1 ± 2.4 × 2.8 ± 0.3), 0(–1)-septate, with (1–)2–3(–4) distal hila, secondary ramoconidia subcylindrical or cylindrical, 8.5–27(–35) × (2.5–)3–4 μm (av. ± SD: 17.2 ± 5.8 × 3.5 ± 0.5), 0(–2)-septate, with 2–3 distal hila, pale or medium olivaceous brown, sometimes pale olivaceous, smooth or almost so, small terminal and intercalary conidia appear to be reticulate, walls unthickened, hila 0.5–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA reaching 49–58 mm diam after 14 d at 25 °C, grey olivaceous, olivaceous or olivaceous black, reverse olivaceous grey and leaden-grey, velvety or powdery, margins glabrous, white, aerial mycelium loose diffuse, low or higher, growth flat, sometimes radially furrowed, without prominent exudates, profusely sporulating. Colonies on MEA attaining 51–58 mm diam after 14 d at 25 °C, olivaceous, grey olivaceous or olivaceous grey, reverse iron-grey and leaden-grey, velvety or powdery, margins white, somewhat feathery, aerial mycelium sparse, loose diffuse, growth flat to low convex, radially furrowed, colony centre somewhat elevated, without prominent exudates, densely sporulating. Colonies on OA reaching 47–57 mm diam after 14 d at 25 °C, greenish olivaceous or olivaceous due to dense sporulation, dull-green towards margins, reverse iron-grey or leaden-grey, velvety or powdery, margins narrow, glabrous, regular, aerial mycelium sparse, loose diffuse, growth flat, with numerous very small exudates giving the surface a glittering appearance.
Substrates and distribution: Isolated from indoor environments (air, house dust); Australasia (New Zealand), Europe (Denmark, The Netherlands).
Additional materials examined: Denmark, isol. from indoor environments, B.A. Andersen, DTO 109-E8 = BA 1908. New Zealand, isol. from house dust, DTO 305-H9 = TA10NZ-294A. The Netherlands, Amsterdam, indoor air, archive, M. Meijer, DTO 072-C8, DTO 082-E3; Rijswijk, swap sample, archive, M. Meijer, DTO 090-D2.
Notes: Cladosporium uwebraunianum (Fig. 1, clade 52) is closely related to C. australiense (Fig. 1, clade 51), but morphologically they are clearly differentiated. The former species is characterised by shorter conidiophores (up to 95(–135) μm), longer conidiogenous cells (17–50(–65) μm) and conidia formed in long branched chains with up to 10(–13) conidia in the terminal unbranched part of the chain. In contrast, C. australiense exhibits very long, seta-like conidiophores (48–285 μm long) with shorter conidiogenous cells (6–15(–40) μm) and conidial chains with only 2–4(–5) conidia in the terminal part of the chain (Bensch et al. 2010). Cladosporium funiculosum (Fig. 1, clade 55) is morphologically very similar in also forming quite long conidial chains with 8(–14) conidia in the unbranched terminal part, but the chains are often dichotomously branched and the conidiophores narrower (2–3 μm).
Cladosporium velox Zalar et al., Stud. Mycol. 58: 181. 2007. MycoBank MB492435. Fig. 45.
Fig. 45.
Cladosporium velox (DTO 317-H1). A–C. Colonies on PDA, MEA and OA. D–H. Macronematous conidiophores and conidial chains. I–J. Micronematous conidiophores and conidia. Scale bars = 10 μm.
Holotype: India, Charidij, isolated from Bambusa sp. (Poaceae), W. Gams, CBS H-19735. Ex-type culture: CBS 119417.
Lit.: Bensch et al. (2012: 284–286; 2015: 68).
Ill.: Zalar et al. (2007: 166, fig. 5 i, 180, fig. 14), Bensch et al. (2012: 285, fig. 334).
Mycelium partly superficial partly submerged; hyphae branched, 2–4 μm wide, septate, often with swellings and constrictions, therefore appearing irregular in outline, pale brown to pale olivaceous brown, smooth, walls unthickened to slightly thickened, often somewhat swollen at the base of conidiophores, without extracellular polysaccharide-like material. Conidiophores arising laterally or terminally from plagiotropous or ascending hyphae, erect, straight to slightly flexuous, filiform to narrowly cylindrical-oblong, sometimes slightly geniculate, due to this geniculation slightly subnodulose, occasionally nodulose, (10–)25–150(–250) × (2–)2.5–4(–4.5) μm, unbranched or branched, branches often only as short denticle-like prolongations below a septum, later branches longer, dichotomously branched in an angle of 30–45°, 0–7-septate, not constricted, septa often somewhat darkened, especially where ramoconidia are seceding, pale to medium olivaceous brown, smooth, walls somewhat thickened, often slightly attenuated towards the apex. Conidiogenous cells integrated, mainly terminal but also intercalary, sometimes conidiophores reduced to conidiogenous cells, filiform to narrowly cylindrical-oblong, 20–42 μm long, proliferation sympodial, with a single or several conidiogenous loci, often somewhat crowded at the apex, subdenticulate, protuberant, 0.8–1.5 μm diam, thickened and darkened-refractive. Ramoconidia subcylindrical or cylindrical, 20–50(–63) × 2.5–3 μm, 0–1-septate, base truncate, 2–3 μm wide, somewhat darkened-refractive. Conidia catenate, in branched chains, branching in all directions, terminal chains with up to five conidia, straight, small terminal conidia globose, subglobose, ovoid, 2.5–4 × (1.5–)2–2.5 μm (av. ± SD: 3.2 ± 0.4 × 2.1 ± 0.3), aseptate, apex rounded, intercalary conidia limoniform to narrowly ellipsoid, 3.5–10(–13) × 2–3 μm (av. ± SD: 6.7 ± 2.5 × 2.5 ± 0.4), aseptate, with up to 3(–4) distal hila, attenuated towards apex and base, secondary ramoconidia narrowly ellipsoid to cylindrical-oblong, straight to slightly curved, (6–)10–30(–42) × 2–3.5(–4.5) μm (av. ± SD: 20.0 ± 8.6 × 2.9 ± 0.6), 0–1-septate, not constricted at septa, with up to 4(–5) distal hila, pale brown, smooth or almost so to very finely verruculose, walls unthickened or almost so, slightly attenuated towards apex and base, hila conspicuous, subdenticulate to denticulate, 0.8–1.5 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA reaching 35–65 mm diam after 14 d at 25 °C, grey olivaceous to olivaceous, reverse leaden-grey, iron-grey or olivaceous black, velvety to powdery, margin broad, white, regular, glabrous to feathery, aerial mycelium absent or sparse, growth regular, low convex, sometimes with numerous prominent exudates, sporulation profuse. Colonies on MEA reaching 30–55 mm diam after 14 d at 25 °C, olivaceous, grey olivaceous and pale olivaceous grey towards margins, radially furrowed, with raised, crater-shaped colony centre, with white, undulate, submerged margin, sporulation profuse. Colonies on OA reaching 30–52 mm diam after 14 d at 25 °C, olivaceous, reverse iron-grey and leaden-grey, velvety to powdery, margin regular, aerial mycelium sparse, without prominent exudates, sporulation profuse. Colonies on MEA with 5 % NaCl reaching 35–45 mm diam after 14 d at 25 °C, pale green, reverse pale green, velvety, flat with regular margin, sporulation poor.
Cardinal temperatures: Minimum at 10 °C (9 mm diam), optimum at 25 °C (30–42 mm diam) and maximum at 30 °C (5–18 mm diam) (from Zalar et al. 2007).
Substrates and distribution: Hypersaline water, indoor air and plant material (bamboo and Zea mays); Asia (China, India), Europe (Slovenia), North America (USA), South America (Brazil).
Additional materials examined: China, isol. from indoor air sample, DTO 317-H1, DTO 323-H8. USA, Massachusetts, Needham, isol. from indoor air sample, office, Dec. 2012, Ž. Jurjević, EMSL 1872 = CPC 22359.
Notes: Cladosporium velox (Fig. 3, clade 18) is a species of the C. sphaerospermum species complex. The small terminal conidia are, however, more ovoid and almost smooth (light microscopy). It was first described from bamboo collected in India and a few additional isolates from hypersaline water from salterns in Slovenia (Zalar et al. 2007). Bensch et al. (2015) recorded it also from Brazil isolated from Zea mays. The three additional isolates from indoor air samples collected in North America and China indicate that the species is probably much wider distributed than previously assumed.
Cladosporium vicinum Bensch & Samson, sp. nov. MycoBank MB822230.
Etymology: Latin vicinus in the meaning of next to, neighbouring refers to the close phylogenetic and morphological relationship with C. europaeum.
Holotype: USA, Wisconsin, Racine, isol. from indoor air sample, Nov. 2012, Ž. Jurjević, CBS H-23261. Ex-type culture: CBS 143366 = CPC 22316 = EMSL 1819.
Diagnosis: Differs from C. cladosporioides in forming more frequently septate conidia (usually aseptate in C. cladosporioides s. str. vs 0–1(–3) septate in C. vicinum).
Mycelium internal and superficial; hyphae sparingly branched, (1–)2–5.5 μm wide, septate, subhyaline or pale olivaceous, smooth or minutely verruculose, walls unthickened or slightly thickened. Conidiophores macro- and micronematous, arising terminally and laterally from hyphae, erect, solitary, occasionally in pairs of two, straight or slightly flexuous. Macronematous conidiophores cylindrical-oblong, non-nodulose, rarely once geniculate unbranched or branched, branches only as short peg-like lateral outgrowths just below a septum, 80–190(–235) × 3–5(–6) μm, septate, sometimes slightly attenuated or constricted at septa, pale olivaceous or pale olivaceous brown, smooth, walls unthickened or almost so. Conidiogenous cells integrated, terminal and intercalary, cylindrical-oblong, (5–)23–60 μm long, terminal cells with 1–5(–7) loci crowded at or towards the apex and occasionally 1–2 additional loci at a lower level, often seceded as ramoconidia, in intercalary cells loci situated on small denticle-like lateral outgrowth just below a septum, loci conspicuous, subdenticulate or denticulate, 1–2(–2.5) μm diam, thickened and darkened-refractive. Micronematous conidiophores narrower and paler, filiform or narrowly cylindrical-oblong, 23–75(–125) × (1–)2–2.8 μm, septate, subhyaline or pale olivaceous, often with only a single locus at the apex, loci 1–1.5 μm diam, conidia formed by micronematous conidiophores narrower, about 2.5 μm wide. Ramoconidia cylindrical-oblong, 20–60(–70) × 3–4(–4.5) μm, 0–1(–3)-septate, base broadly truncate, (2.2–)2.5–3.5 μm wide, somewhat refractive. Conidia catenate, in branched chains, branching in all directions, with up to 6(–9) conidia in the unbranched terminal part of the chains, small terminal conidia subglobose or obovoid, 2–5 × 2–2.5(–3) μm (av. ± SD: 3.5 ± 0.8 × 2.2 ± 0.3), apex rounded, intercalary conidia limoniform, ellipsoid or subcylindrical, 4–16(–19) × (2–)2.5–3.5(–4) μm (av. ± SD: 8.5 ± 3.6 × 3.0 ± 0.5), 0(–1)-septate, with 1–4(–6) distal hila, secondary ramoconidia ellipsoid, subcylindrical or cylindrical, (7–)9–31.5(–40) × (2.5–)3–4(–5) μm (av. ± SD: 20.2 ± 8.4 × 3.6 ± 0.5), 0–1(–3)-septate, median or often in the upper half, with (1–)2–4(–5) distal hila, pale olivaceous or pale to medium olivaceous brown, smooth, occasionally slightly rough-walled, walls unthickened or almost so, hila conspicuous, subdenticulate or denticulate, 0.5–2(–2.5) μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on PDA reaching 55–79 mm diam after 14 d at 25 °C, olivaceous grey or iron-grey, reverse olivaceous black, floccose or felty, margins regular, glabrous or feathery, aerial mycelium abundantly formed, loose to dense, smoke-grey, growth flat to low convex. Colonies on MEA reaching 58–82 mm diam after 14 d at 25 °C, grey olivaceous or olivaceous grey, reverse iron-grey, floccose or fluffy-felty, margin regular, feathery, aerial mycelium whitish, smoke-grey or pale olivaceous grey, abundant, growth effuse, flat or low convex, radially furrowed, somewhat wrinkled in colony centre. Colonies on OA attaining 60–65 mm diam after 14 d at 25 °C, grey olivaceous or smoke-grey, dull-green at margins, reverse pale greenish-grey or olivaceous grey, floccose or felty, margins regular, glabrous, aerial mycelium covering large parts, smoke-grey, growth effuse. Without prominent exudates, sporulation profuse on all media.
Substrates and distribution: Isolated from indoor environments and plant material; Africa (South Africa), Australasia (New Zealand) Europe (UK), North America (USA).
Additional materials examined: New Zealand, isol. from house dust, DTO 305-H5 = TA10NZ-280B; isol. from imported buds of Prunus avium, J. Rennie, CPC 15457; Auckland, Auckland University campus, isol. from leaves of Oncoba spinosa, Sep. 2004, C.F. Hill 1076-2, CPC 11664. South Africa, isol. from Leptosphaeria sp., P.W. Crous, CPC 13867. UK, Manchester, isol. from uredospores of Puccinia allii, May 1984, G.S. Taylor, CBS 306.84.
Notes: This new species (Fig. 1, clade 34) is formerly known as C. cladosporioides Lineage 2 sensu Bensch et al. (2010). Bensch et al. (2010) hesitated in naming this phylogenetically distinct lineage since it is morphologically almost indistinguishable from C. cladosporioides s. str. Morphologically, C. vicinum is the closest of the three phylogenetically distinct lineages to C. cladosporioides s. str. (Fig. 1, clade 66) but differs in more frequently forming septate conidia (usually aseptate in C. cladosporioides s. str. vs 0–1(–3)-septate in C. vicinum). Cladosporium europaeum (formerly C. cladosporioides Lineage 1 sensu Bensch et al. (2010); Fig. 1, clade 35) is the closest phylogenetic relative of C. vicinum (see species notes under C. europaeum for sequence similarities) but produces somewhat shorter conidiogenous cells, secondary conidia and ramoconidia. Cladosporium westerdijkiae (formerly C. cladosporioides Lineage 4 sensu Bensch et al. (2010); Fig. 1, clade 43) introduced below differs from C. vicinum in having shorter intercalary conidia and secondary ramoconidia which are usually aseptate.
Cladosporium westerdijkiae Bensch & Samson, sp. nov. MycoBank MB822231.
Etymology: Named for Johanna Westerdijk, the first director of the Centraalbureau voor Schimmelcultures (now renamed as Westerdijk Fungal Biodiversity Institute) and the first female professor in the Netherlands.
Holotype: USA, Washington State, isol. from bing cherry fruits, R.G. Roberts, CBS H-23262. Ex-type culture: CBS 113746.
Diagnosis: Differs from C. cladosporioides in producing slightly shorter and narrower conidia formed in shorter conidial chains (only up to four in the terminal unbranched part of the chain vs up to 10 in C. cladosporioides).
Mycelium immersed, sparingly superficial; hyphae unbranched or sparingly branched, 1–5 μm wide, septate, sometimes slightly constricted at septa, subhyaline or pale olivaceous brown, smooth or minutely verruculose or irregularly rough-walled, walls unthickened or slightly so, sometimes forming ropes. Conidiophores marco- and micronematous, solitary, arising terminally and laterally from hyphae, erect, straight, flexuous or sometimes once bent at the apex, cylindrical-oblong or filiform, neither nodulose nor geniculate, unbranched, occasionally branched, 23–125(–185) × 3–5 μm, 0–3(–4)-septate, subhyaline or pale to medium olivaceous brown, smooth, sometimes minutely verruculose or irregularly rough-walled towards the base, walls unthickened or almost so, sometimes slightly attenuated towards the apex; micronematous conidiophores shorter, narrower and paler, filiform or narrowly cylindrical-oblong, 17–78 × 2–3 μm, subhyaline or pale olivaceous brown. Conidiogenous cells integrated, usually terminal, very rarely intercalary, cylindrical, (12–)23–54 μm long, in micronematous conidiophores 16–36 μm, with a single or two apical loci, sometimes up to four loci, conspicuous, denticle-like, sometimes situated on peg-like lateral prolongations, 1–2 μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed, 22–52 × 3.5–4.5 μm, aseptate, base 3–3.5 μm wide, unthickened but somewhat refractive. Conidia numerous, catenate, with up to 4(–6) conidia in the terminal unbranched part of the conidial chains, small terminal conidia oval, 4–5(–5.5) × 2–2.5 μm (av. ± SD: 4.6 ± 0.6 × 2.1 ± 0.2), intercalary conidia oval or ellipsoid, 5–8.5(–12) × 2–3 μm (av. ± SD: 6.5 ± 1.7 × 2.6 ± 0.4), aseptate, with 1–2(–3) distal hila, very pale olivaceous, secondary ramoconidia ellipsoid, subcylindrical or cylindrical, (6–)9–27(–35) × 3–4(–5) μm (av. ± SD: 17.4 ± 6.8 × 3.6 ± 0.5), 0(–1)-septate, with up to 3 distal hila, pale olivaceous brown, smooth, walls unthickened, slightly attenuated towards apex and base, hila subdenticulate or denticulate, protuberant, 0.8–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on PDA reaching up to 61–75 mm diam after 14 d at 25 °C, grey olivaceous, olivaceous grey or dull-green, reverse greyish blue or iron-grey, powdery or floccose, margin colourless or white, narrow, feathery, aerial mycelium loose, diffuse, whitish, growth flat, without prominent exudates. Colonies on MEA attaining 46–75 mm diam after 14 d at 25 °C, grey olivaceous or olivaceous grey, sometimes greenish glaucous at margins, reverse leaden-grey or iron-grey, velvety, margins narrow, glabrous or feathery, radially furrowed, folded and wrinkled in colony centre, aerial mycelium sparse, diffuse, no prominent exudates formed. Colonies on OA reaching 53–75 mm diam after 14 d at 25 °C, olivaceous grey or grey olivaceous, greenish grey towards margins, reverse leaden-grey or iron-grey, powdery to felty-floccose, margins very narrow, aerial mycelium mainly on colony centre, growth flat, sometimes numerous small, not very prominent exudates formed giving the colony a glittering appearance. Sporulation profuse on all media.
Substrates and distribution: Isolated from plant material and indoor environments; Asia (South Korea), Europe (Denmark, Germany, Portugal), North America (USA), South America (Argentina).
Additional materials examined: Denmark, isol. from indoor environment, DTO 109-F2 = BA 1911. Germany, isol. from indoor environment, DTO 084-F2. Portugal, isol. from indoor environment, DTO 152-A9, DTO 152-H9. South Korea, Pochon, National Arboretum, isol. from Fatona villosa, 18 Oct. 2002, H.D. Shin, CPC 10150.
Notes: Cladosporium westerdijkiae (Fig. 1, clade 43) was formerly treated as C. cladosporioides Lineage 4 sensu Bensch et al. (2010) as it was phylogenetically distinct but morphologically almost indistinguishable from C. cladosporioides s. str. (Fig. 1, clade 66). As more isolates could be included it is herein named and described as a new species. It is genetically distant to C. cladosporioides (clade 43 vs clade 66 in Fig. 1). Furthermore, the conidia are slightly shorter and narrower and form shorter conidial chains (only up to four in the terminal unbranched part of the chain vs up to 10 in C. cladosporioides). Its closest phylogenetic neighbour proved to be C. delicatulum (Fig. 1). This species differs in forming shorter conidiogenous cells (11−37 μm long), 0−1(−2)-septate ramoconidia and slightly shorter, 0−1(−2)-septate secondary ramoconidia.
Cladosporium wyomingense Bensch & Samson, sp. nov. MycoBank MB822233. Fig. 46.
Fig. 46.
Cladosporium wyomingense (CBS 143367). A–C. Colonies on PDA, MEA and OA. D–F, H–J. Macronematous conidiophores and conidial chains. G, K–L. Micronematous conidiophores and conidia. M. Ramoconidium and conidia. N–O. Conidial chains. Scale bars = 10 μm.
Etymology: Named after the place of origin, Wyoming, where the type specimen was collected.
Holotype: USA, Wyoming, isol. from indoor air sample, living room, Oct. 2012, Ž. Jurjević, CBS H-23263. Ex-type culture: CBS 143367 = CPC 22310 = EMSL 1806.
Diagnosis: Differs from C. herbarum and C. macrocarpum in having shorter and narrower conidiophores and slightly shorter and narrower conidia.
Mycelium abundantly formed, filiform or narrowly cylindrical, branched, 1−4 μm wide, septate, neither swollen nor constricted, subhyaline or pale olivaceous, almost smooth, asperulate or loosely verruculose, especially those hyphae forming conidiophores with surface ornamentation. Conidiophores macro- and micronematous, arising terminally or laterally from plagiotropous or ascending hyphae, macronematous conidiophores narrowly cylindrical-oblong, often distinctly geniculate, sometimes growth proceeding at an angle of 45−90°, subnodulose, sometimes forming lateral shoulders at or towards the apex, mostly unbranched, 10−70(−120) × 2.5−3.5(−4) μm, 0−3(−4)-septate, pale olivaceous or pale olivaceous brown, smooth or almost so, asperulate or minutely verruculose, walls slightly thickened; micronematous conidiophores shorter, narrower, 1.5−2 μm wide, and paler, subhyaline. Conidiogenous cells integrated, mainly terminal, occasionally also intercalary, 8−21(−43) μm long, geniculate and subnodulose, with loci often situated on lateral shoulders or short lateral prolongations, up to six loci per cell, conspicuous, 1−2 μm diam, thickened and darkened-refractive; in micronematous conidiophores cells usually without swellings and geniculations, with 1−2 loci at the apex, about 1 μm diam. Ramoconidia occasionally formed. Conidia catenate, formed in unbranched or basely branched chains, 3−7(−10) conidia in the unbranched part of the chain, verruculose or echinulate, small terminal conidia subglobose, obovoid or ellipsoid, occasionally globose, 3.5−10(−12.5) × 3−5(−5.5) μm (av. ± SD: 6.8 ± 2.9 × 4.0 ± 0.9), often with a broadly rounded apex; intercalary conidia ovoid and ellipsoid, 6.5−11.5 × 4−5 μm (av. ± SD: 9.1 ± 1.7 × 4.4 ± 0.4), 0(−1)-septate, slightly attenuated towards apex and base, with 1(−2) distal hila; secondary ramoconidia ellipsoid, fusiform or subcylindrical, (7−)10−22(−28) × (3−)4−6(−7) μm (av. ± SD: 16.4 ± 5.2 × 4.9 ± 0.7), 0−1-septate, slightly attenuated towards apex and base, with 1−2(−3) distal hila, pale olivaceous or medium olivaceous brown, hila conspicuous, (0.5−)0.8−2 μm diam, thickened and darkened; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA reaching up to 60 mm diam after 14 d at 25 °C, olivaceous grey and pale olivaceous grey, dull-green towards margins, reverse leaden-grey, dull green towards margins, fluffy-felty, margin broad, white, feathery, somewhat undulate, aerial mycelium abundant, loose to dense, low to high, without prominent exudates, sporulating. Colonies om MEA attaining up to 60 mm diam after 14 d at 25 °C, smoke-grey, pale olivaceous grey, olivaceous grey at margins where sporulation is profuse, reverse olivaceous grey, fluffy-felt, margin white, feathery, aerial mycelium abundant, loose to high, colony centre folded and wrinkled, radially furrowed, without prominent exudates. Colonies on OA reaching up to 45 mm diam after 14 d at 25 °C, smoke-grey, pale greenish grey, dull-green towards margins, reverse smoke-grey and olivaceous grey, fluffy-felt, margin slightly undulate, aerial mycelium low to high, often felted, dense, with numerous very small exudates, sporulation sparse.
Substrates and distribution: Indoor air; North America (USA).
Notes: With its subnodulose conidiophores and ornamented conidia, C. wyomingense (Fig. 2, lineage 14) is a typical member of the C. herbarum species complex. It is allied to C. angustiherbarum (Fig. 2, lineage 13), C. phlei (Fig. 2, clade 12), C. herbarum (Fig. 2, clade 15) and C. macrocarpum (Fig. 2, clade 16) but differs in having shorter and narrower conidiophores and slightly shorter and narrower conidia (Bensch et al. 2012). Morphologically it resembles C. angustiherbarum (Fig. 2, lineage 13) but the latter species possesses narrower conidiogenous loci and conidial hila and the conidiophores do not grow in an up to 90° angle (Bensch et al. 2015). Until now it is known only from a single isolate.
Cladosporium xanthochromaticum Sandoval-Denis et al., Persoonia 36: 295. 2016. MycoBank MB817340.
Holotype: USA, Texas, from human bronchoalveolar lavage fluid, Sep. 2010, D.A. Sutton, CBS H-22388. Ex-type culture: CBS 140691 = UTHSC DI-13-211 = FMR 13324.
Ill.: Sandoval-Denis et al. (2016: 296, fig. 11).
Mycelium superficial and immersed, hyphae branched, 1–3 μm wide, septate, subhyaline, pale olivaceous or pale olivaceous brown, smooth or slightly rough-walled, thin-walled, sometimes forming ropes, occasionally swollen at the base of conidiophores. Conidiophores erect, solitary, macro- or micronematous, arising terminally or laterally from hyphae as short peg-like lateral outgrowths or longer, filiform or narrowly cylindrical-oblong, non-nodulose, occasionally once geniculate, unbranched or branched typically immediately before a septum, up to 210 μm long, (1.5–)2–4 μm wide, septate, pale brown, pale olivaceous or olivaceous brown, usually smooth and thin-walled. Conidiogenous cells terminal, sometimes also intercalary, cylindrical, sometimes geniculate, 12–37 × 3–4 μm, bearing up to three conidiogenous loci of 1–1.5 μm diam, darkened and refractive. Ramoconidia subcylindrical to cylindrical, 17–42(–50) × 2–3.5(–4) μm, 0–1-septate, smooth or finely roughened, base about 2–2.5(–3.5) μm wide. Conidia forming branched chains, with 2–6(–7) conidia in the terminal unbranched part, small terminal conidia obovoid, limoniform or short ellipsoid (2.5–)3–5(–9) × (1.5–)2–2.5(–3) μm (av. ± SD: 4.1 ± 1.2 × 2.1 ± 0.4), aseptate; intercalary conidia ovoid, limoniform or ellipsoid, (4.5–)5–14(–18) × 2–3.5(–4) μm (av. ± SD: 8.2 ± 3.3 × 2.6 ± 0.5), 0(–1)-septate, with 1–4 distal hila; secondary ramoconidia ellipsoid to cylindrical, (7–)10–30(–38) × (2–)2.5–4 μm (av. ± SD: 20.5 ± 7.3 × 2.9 ± 0.5), 0–1(–3)-septate, sometimes slightly constricted at the median septum, pale olivaceous brown, smooth- and thin-walled, with protuberant, somewhat darkened, 0.5–1.5 μm diam conidial hila; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining 60–75 mm diam after 14 d at 25 °C, grey olivaceous or olivaceous, reverse grey olivaceous, olivaceous grey or olivaceous, olivaceous buff towards margins, sometimes with a light yellow, grey-yellow or citrine-green diffusible pigment released into the agar, velvety, floccose or felty, margin regular, white to yellow, flat or folded at centre, with abundant submerged mycelium. Colonies on MEA reaching 62–70 mm diam after 14 d at 25 °C, olivaceous, reverse iron-grey, velvety or floccose, margins white, narrow, radially furrowed, sometimes a few small but prominent exudates formed. Colonies on OA attaining 40–65 mm diam after 14 d at 25 °C, olivaceous or grey olivaceous, whitish and smoke grey due to aerial mycelium, reverse olivaceous grey, leaden-grey or leaden-black, floccose or fluffy-felty, radiate, margin regular, white, narrow, growth flat, and with abundant submerged mycelium; sometimes releasing an amber-coloured pigment into the agar. Sporulation profuse on all media. Cardinal temperature for growth – Optimum 20 °C, maximum 30 °C, minimum 5 °C.
Substrate and distribution: Isolated from plant material, food, indoor environments and human bronchoalveolar lavage fluid; Africa (South Africa), Asia (China, India, Polynesia, Thailand), Australasia (Australia), North America (Bermuda, USA).
Additional materials examined: Sine loco, sine dato, isol. by C.H. Hassall, No. 4-1949, ident. by G.A. de Vries as C. cladosporioides, CBS 167.54 = ATCC 11276 = IMI 049624. Australia, isol. from margarine, N. Charley, CPC 11046; isol. from Erythrophleum chlorostachys (Fabaceae), 9 Jan. 2007, B.A. Summerell, CBS 126364 = CPC 14532. Bermuda, Samerset, isol. from indoor air sample, Nov. 2012, Ž. Jurjević, EMSL 1824 = CPC 22321. China, isol from indoor air sample, DTO 317-I2, 323-E2 – 323-E7. India, isol. from Eucalyptus sp. (Myrtaceae), 3 Jan. 2004, coll. W. Gams, isol. P.W. Crous, CPC 11133; isol. from Musa sp. (Musaceae), 25 Oct. 2004, M. Arzanlou, CPC 11609. Polynesia, reserve Pun Kukui in forest, isol. from banana “Eka ulu”, 2006, coll. I. Budenhagen, isol. P.W. Crous, CPC 12792, 12793. South Africa, Alkmar, Laeveld Coop, isol. from wheat, 1988, CPC 14008 = MRC 10135; Durban, botanical garden Durban near Reunion, -29.85, 31.0167, isol. from Strelitzia sp. (Strelitziaceae), 2005, coll. W. Gams, isol. P.W. Crous, CPC 11806; Free State, Danielsrus, isol. from oats, 1983, CPC 14004 = MRC 03367; Transkei, Mazeppa Bay, isol. from Strelitzia sp., growing on fruiting structures, 1 June 2008, P.W. Crous, CPC 14911. Thailand, isol. from Acacia mangium (Fabaceae), 2005, coll. W. Himaman, isol. P.W. Crous, CPC 11526, 11856; Surat Thani, isol from indoor air (open Petri-dish), P. Noomin, DTO 108-G8. USA, Colorado, Denver, isol. from air sample, bedroom, June 2012, Ž. Jurjević, EMSL 1686 = CPC 22239; Louisiana, Baton Rouge, isol. from leaves of pecan tree, 8 Sep. 2007, P.W. Crous, CPC 14256.
Notes: Sandoval-Denis et al. (2016) splitted C. perangustum, a phylogenetically diverse but morphologically quite uniform species, into three species, C. perangustum s. str. (Fig. 1, clade 4), C. angulosum (Fig. 1, clade 2) and C. xanthochromaticum (Fig. 1, clade 3). Forming a basal lineage in the C. cladosporioides species complex they are characterised by narrow conidia and slightly roughened conidiophores and conidia. The ramoconidia in C. xanthochromaticum proved to be not significantly shorter than in C. perangustum (Sandoval-Denis et al. 2016) but often slightly wider, but the conidiophores are usually smooth compared to the asperulate or verruculose ones in C. perangustum. Furthermore, the secondary ramoconidia are also slightly wider [(2–)2.5–4 μm vs 2–3(–3.5) μm in C. perangustum]. Cladosporium angulosum differs from C. xanthochromaticum in having shorter conidia and in growing at 35 °C (Sandoval-Denis et al. 2016). All three species proved to occur in indoor environments.
Key to the most frequently occurring Cladosporium species in indoor environments
-
1
Conidial surface ornamentation usually smooth, occasionally finely roughened; faster growth rates (up to 75 mm diam on MEA after 14 d)……………………………………2
-
1
Conidial surface ornamentation usually minutely verruculose to verrucose; slower growth rates (up to 45 mm diam on MEA after 14 d)……………………………………………3
-
2
Conidiophores longer, up to 310(–460) µm long, often with a head-like swollen apex, sometimes with few nodules on a lower level…………………………………..C. tenuissimum
-
2
Conidiophores shorter, up to 155 µm long, usually neither nodulose nor geniculate……..C. pseudocladosporioides
-
3
Conidiophores nodulose, usually with small terminal head-like swellings, sometimes with additional intercalary swellings, secondary ramoconidia 3–5(–7) μm wide…………………………………………………………….C. allicinum
-
3
Conidiophores non-nodulose, secondary ramoconidia narrower, 2–4(–5) μm wide…………………………………4
-
4
Conidia minutely verruculose, small terminal conidia subglobose or obovoid, conidial septa not darkened…………………………………………………………C. ramotenellum
-
4
Small terminal and intercalary conidia usually globose, minutely verruculose to distinctly verrucose, but secondary ramoconidia almost smooth, septa usually darkened……5
-
5
Conidiophores in vitro 2–3.5(–5.5) μm wide, usually unbranched, 0–3-septate; small terminal conidia 2–4(–6) μm long; ramoconidia up to 37(–46) μm long, usually 0–3-septate……………………………………….C. halotolerans
-
5
Conidiophores somewhat wider, 2.5–4.5(–6) μm, often branched, pluriseptate, with often dense septation; small terminal conidia slightly longer, (2–)3–5(–7); ramoconidia up to 50(–67) μm long, with up to five septa…………………………………………………………C. sphaerospermum
Discussion
The genus Cladosporium has been extensively reviewed in recent years in efforts to clarify the phylogeny and taxonomic structure of its species and allied fungi, and has resulted in a modern redefinition of the genus (Crous et al., 2007a, Crous et al., 2007b, Schubert et al., 2007b, Zalar et al., 2007, Bensch et al., 2010, Bensch et al., 2012, Bensch et al., 2015). However, until recently, no attempt had been made to study the impact of these new approaches in the diversity of Cladosporium species occurring in indoor environments. This study presents a molecular phylogenetic study of species in this genus known from culture, with the intention to identify the common indoor species. Since fungi present in indoor environments can produce toxins or carry allergens which cause health hazards, it is important to know which fungal species are present indoors. Cladosporium species are found on plant material, in soil and air and are isolated from food and building material. Several species are known from clinical samples (Sandoval-Denis et al. 2016).
Of the 46 species found indoors 14 species are found in relation with human-derived samples. Sixteen species are described as new of which six species belonged to the C. cladosporioides species complex, four to the C. herbarum species complex and six to the C. sphaerospermum species complexes, respectively. Cladosporium halotolerans proved to be the most common species in indoor environments in this study (144 isolates), followed by C. sphaerospermum (46 isolates) and C. pseudocladosporioides (46 isolates) as well as C. allicinum (36 isolates).
Based on the studies of Fradkin et al. (1987) and Horner et al. (2004) one would expect to find C. cladosporioides as a dominant indoor fungus. This fungus is dominant in outdoor air and as the composition of indoor species reflects the composition of outdoor species one would expect to find C. cladosporioides as dominant indoors. However, a pilot study of indoor samples suggest (Segers et al. 2015) that members of the C. sphaerospermum species complex are also important and in the selection used in this study predominant in indoor environments. This was the case in indoor air samples, but even more so when samples were taken from indoor surfaces. As these fungi could grow at a lower water activity compared to the other Cladosporium species complexes, this habit might help the fungi to survive on indoor surfaces. Even more important was the ability of C. halotolerans, a member of the C. sphaerospermum species complex, to deal with transient changes in relative humidity during growth (Segers et al. 2016). Colonies of the fungus resumed growth better compared to the indoor fungi Aspergillus niger and Penicillium rubens and hardly showed cell damage after the changes. This occurred despite the fact that the latter fungi grow on media with a static water availability that was similar or lower compared to C. halotolerans. Under these conditions this fungus exhibits a very condensed growth pattern existing by the formation of rounded, pigmented cells in the central colony, the occurrence of bundles of hyphae and very quick spore formation. Cladosporium halotolerans and P. rubens were able to grow on phosphogypsum without added nutrients (Segers et al. 2017). Thus C. sphaerospermum and the related taxa develop under low nutrient conditions and deal with humidity changes, both so characteristic for indoor situations. As C. herbarum is the most studied species in allergy research (Breitenbach, 2008, Poll et al., 2009) the indoor dominance of C. halotolerans and other taxa is interesting. From our studies it is evident that C. herbarum does not belong to the common indoor Cladosporia and therefore, evaluation if allergens produced by C. herbarum are the same as produced by the other Cladosporia is important. If there are differences, we could gain insight how important indoor Cladosporia are in evoking titers of antibodies and allergic reactions compared to outdoor Cladosporia. The ability of C. halotolerans to deal with dynamic water availability is probably related to the ecological niche of this fungus (Segers et al. 2016). Cladosporium species grow on leaves and are therefore called phylloplane fungi (Park, 1982, Moody et al., 1999). The available water for fungi growing on leaves is highly dynamic and is influenced by changing temperature, dew formation, sunlight, and rain. It is interesting that the indoor environment is also characterized by changes in humidity during the day. Park (1982) reports that phylloplane fungi can restore growth after minutes to hours of rehydration after drying for 2–3 wk.
This study and the study of Sandoval-Denis et al. (2016) show that pure morphological identification of Cladosporium species are no longer unequivocally possible without the aid of molecular data. One example of this is the four C. cladosporioides lineages sensu Bensch et al. (2010) which were morphologically indistinguishable from C. cladosporioides s. str. and at that time not formally named by the authors due to the lack of diagnostic morphological characters. In the present study, three of these lineages are introduced as new species, namely C. europaeum (“Lineage 1”), C. vicinum (“Lineage 2”) and C. westerdijkiae (“Lineage 4”). The third lineage was published as C. silenes by Crous et al. (2011). Likewise, Sandoval-Denis et al. (2016) introduced two additional species, C. angulosum and C. xanthochromaticum, for the two lineages sister to the clade containing the type strain in the phylogenetically variable species C. perangustum. Although ITS is a suitable locus to identify an isolate as belonging to the genus Cladosporium, and to some extent even a specific species complex, additional loci are required to reach a conclusive species, or even species complex, identification. Therefore, the use of a molecular approach for the correct identification of all these species is highly recommended.
Acknowledgements
This research was supported by a grant from the Alfred P. Sloan Foundation Program on the Microbiology of the Built Environment (Grant No. G-2014-14529). The authors thank the technical staff, Mieke Starink-Willemse and Patrick Arensman (DNA isolation and sequencing), Arien van Iperen (cultures), Trix Merkx (deposit of strains) and Marjan Vermaas (photo plates) for their invaluable assistance. We are grateful to A. Amend, T. Atkinson, M. Bidartondo, K. Jacobs, P. Noonim and all others who assisted with collections from indoor environments. Shaun Pennycook is thanked for giving nomenclatural advice.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.simyco.2018.03.002
Appendix A. Supplementary data
The following are the supplementary data related to this article:
The first of 1 000 equally most parsimonious trees obtained from a heuristic search of an ITS alignment (representing the Cladosporium species known from ITS data. The Bayesian posterior probabilities (BPP; >0.74) and maximum parsimony bootstrap support values (PBS; >74 %) are shown at the nodes (BPP/PBS) and thickened lines represent those branches which are present in the strict consensus tree. The scale bar represents the number of changes. Species names are indicated to the right of the tree, followed by the culture accession number (all species) and the GenBank accession number (for the species not described in this paper). Species from indoor environments are indicated with a brown box. The species complexes are coloured according to the legend. The tree was rooted to Cercospora beticola (strain CBS 116456).
References
- Bensch K., Braun U., Groenewald J.Z. The genus Cladosporium. Studies in Mycology. 2012;72:1–401. doi: 10.3114/sim0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K., Groenewald J.Z., Braun U. Common but different: The expanding realm of Cladosporium. Studies in Mycology. 2015;82:23–74. doi: 10.1016/j.simyco.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K., Groenewald J.Z., Dijksterhuis J. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales) Studies in Mycology. 2010;67:1–94. doi: 10.3114/sim.2010.67.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra J.D.P., Sandoval-Denis M., Paiva L.M. New endophytic Toxicocladosporium species from cacti in Brazil, and description of Neocladosporium gen. nov. IMA Fungus. 2017;8(1):77–97. doi: 10.5598/imafungus.2017.08.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U., Crous P.W., Dugan F.M. Phylogeny and taxonomy of cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s.str. Mycological Progress. 2003;2(1):3–18. [Google Scholar]
- Braun U., Crous P.W., Nakashima C. Cercosporoid fungi (Mycosphaerellaceae) 3. Species on monocots (Poaceae, true grasses) IMA Fungus. 2015;6:25–97. doi: 10.5598/imafungus.2015.06.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U., Crous P.W., Schubert K. Taxonomic revision of the genus Cladosporium s. lat. 8. Reintroduction of Graphiopsis (= Dichocladosporium) with further reassessments of cladosporioid hyphomycetes. Mycotaxon. 2008;103:207–216. [Google Scholar]
- Breitenbach M. The spectrum of fungal allergy. International Archives of Allergy and Immunology. 2008;145(1):58–86. doi: 10.1159/000107578. [DOI] [PubMed] [Google Scholar]
- Buzina W., Braun H., Freudenschuss K. Fungal biodiversity as found in nasal mucus. Medical Mycology. 2003;41:149–161. doi: 10.1080/mmy.41.2.149.161. [DOI] [PubMed] [Google Scholar]
- Crous P.W., Braun U., Groenewald J.Z. Mycosphaerella is polyphyletic. Studies in Mycology. 2007;58:1–32. doi: 10.3114/sim.2007.58.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Braun U., Schubert K. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology. 2007;58:33–56. doi: 10.3114/sim.2007.58.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Braun U., Wingfield M.J. Phylogeny and taxonomy of obscure genera of microfungi. Persoonia. 2009;22:139–161. doi: 10.3767/003158509X461701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Groenewald J.Z. Why everlastings don't last. Persoonia. 2011;26:70–84. doi: 10.3767/003158511X574532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Schroers H.-J., Groenewald J.Z. Metulocladosporiella gen. nov. for the causal organism of Cladosporium speckle disease of banana. Mycological Research. 2006;110:264–275. doi: 10.1016/j.mycres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Crous P.W., Shivas R.G., Quaedvlieg W. Fungal Planet description sheets: 214–280. Persoonia. 2014;32:184–306. doi: 10.3767/003158514X682395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Tanaka K., Summerell B.A. Additions to the Mycosphaerella complex. IMA Fungus. 2011;2(1):49–64. doi: 10.5598/imafungus.2011.02.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Burgess T.I. Fungal Planet description sheets: 558–624. Persoonia. 2017;38:240–384. doi: 10.3767/003158517X698941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J.C. A contribution to the systematics of Cladosporium. Revision of the fungi previously referred to Heterosporium. Mycological Papers. 1997;172:1–157. [Google Scholar]
- De Hoog G.S., Guarro J., Gené J. 2nd ed. CBS, Utrecht, The Netherlands and Universitat rovira I virgili; Reus, Spain: 2000. Atlas of clinical fungi. [Google Scholar]
- De Vries G.A. CBS; Baarn: 1952. Contribution to the knowledge of the genus Cladosporium Link ex Fr. [Google Scholar]
- Domsch K.H., Gams W., Anderson T.H. Vols. 1 & 2. Academic Press; London, UK: 1980. (Compendium of soil fungi). [Google Scholar]
- Dugan F.M., Braun U., Groenewald J.Z. Morphological plasticity in Cladosporium sphaerospermum. Persoonia. 2008;21:9–16. doi: 10.3767/003158508X334389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan F.M., Schubert K., Braun U. Check-list of Cladosporium names. Schlechtendalia. 2004;11:1–103. [Google Scholar]
- Ellis M.B. CMI; Kew, UK: 1971. Dematiaceous hyphomycetes. [Google Scholar]
- Ellis M.B. CMI; Kew, UK: 1976. More dematiaceous hyphomycetes. [Google Scholar]
- El-Morsy E.M. Fungi isolated from the endorhizosphere of halophytic plants from the Red Sea Coast of Egypt. Fungal Diversity. 2000;5:43–54. [Google Scholar]
- Flannigan B. Microorganisms in indoor air. In: Flannigan B., Samson R., Miller D., editors. Microorganisms in Home and Indoor Work Environments: Diversity, Health Impacts, Investigation and Control. 2nd ed. CRC Press; USA: 2001. pp. 17–31. [Google Scholar]
- Fradkin A., Tarlo S.M., Tobin R.S. Species identification of airborne molds and its significance for the detection of indoor pollution. Japca - The International Journal of Air Pollution Control and Hazardous Waste Management. 1987;37(1):51–53. doi: 10.1080/08940630.1987.10466201. [DOI] [PubMed] [Google Scholar]
- Fresenius J.B.G.W. Heinrich Ludwig Brömmer Verlag; Frankfurt, Germany: 1850. Beiträge zur Mykologie 1. [Google Scholar]
- Groenewald M., Groenewald J.Z., Crous P.W. Distinct species exist within the Cercospora apii morphotype. Phytopathology. 2005;95:951–959. doi: 10.1094/PHYTO-95-0951. [DOI] [PubMed] [Google Scholar]
- Groenewald J.Z., Nakashima C., Nishikawa J. Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology. 2013;75:115–170. doi: 10.3114/sim0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubold E.M., Aronson J.F., Cowan D.F. Isolation of fungal rDNA from bottlenose dolphin skin infected with Loboa loboi. Medical Mycology. 1998;36:263–267. [PubMed] [Google Scholar]
- Heuchert B., Braun U., Schubert K. Morphotaxonomic revision of fungicolous Cladosporium species (hyphomycetes) Schlechtendalia. 2005;13:1–78. [Google Scholar]
- Ho M.H.-M., Castañeda R.F., Dugan F.M. Cladosporium and Cladophialophora in culture: descriptions and an expanded key. Mycotaxon. 1999;72:115–157. [Google Scholar]
- Horner W.E., Worthan A.G., Morey P.R. Air- and dustborne mycoflora in houses free of water damage and fungal growth. Applied Environmental Microbiology. 2004;70(11):6394–6400. doi: 10.1128/AEM.70.11.6394-6400.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y., Lee Y.M., Kim G.H. Two species of Cladosporium associated with wood discoloration in Korea. Mycotaxon. 2013;124:21–29. [Google Scholar]
- Kim J.J., Kang S.-M., Choi Y.-S., Kim G.-H. Microfungi potentially disfiguring CCA-treated wood. International Biodeterioration & Biodegradation. 2007;60:197–201. [Google Scholar]
- Kumaresan V., Suryanarayanan T.S. Endophyte assemblage in young, mature and senescent leaves of Rhizophora apiculata: evidence for the role of endophytes in mangrove litter degeneration. Fungal Diversity. 2002;9:81–91. [Google Scholar]
- Lee Y.M., Jang Y., Kim G.H. Phylogenetic analysis and discoloration characteristics of major molds inhabiting woods. Part 3. Genus Cladosporium. Holzforschung. 2011;66(4):537–541. [Google Scholar]
- Ma R., Chen Q., Fan Y.L. Six new soil-inhabiting Cladosporium species from plateaus in China. Mycologia. 2017;109(2):244–260. doi: 10.1080/00275514.2017.1302254. [DOI] [PubMed] [Google Scholar]
- Marin-Felix Y., Groenewald J.Z., Cai L. Genera of phytopathogenic fungi: GOPHY 1. Studies in Mycology. 2017;86:99–216. doi: 10.1016/j.simyco.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meklin T., Haugland R.A., Reponen T. Quantitative PCR analysis of house dust can reveal abnormal mold conditions. Journal of Environmental Monitoring. 2004;6:615–620. doi: 10.1039/b400250d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody S.A., Newsham K.K., Ayres P.G. Variation in the responses of litter and phylloplane fungi to UV-B radiation (290–315 nm) Mycological Research. 1999;103:1469–1477. [Google Scholar]
- Mullins J. Microorganisms in outdoor air. In: Flannigan B., Samson R.A., Miller J.D., editors. Microorganisms in Home and Indoor Work Environments: Diversity, Health Impacts, Investigation and Control. CRC Press; USA: 2001. pp. 3–16. [Google Scholar]
- Nieves-Rivera Á.M., Rodríguez N.J., Dugan F.M., Zaidi B.R., Williams E.H., Jr. Characterization of Cladosporium oxysporum and C. sphaerospermum using polyaromatic hydrocarbons (PAHs) as their sole carbon source in tropical coastal seawater. In: Mendez-Vilas A., editor. Modern multidisciplinary applied microbiology: Exploiting microbes and their interactions. Wiley-VCH; Weinheim, Germany: 2006. pp. 483–487. [Google Scholar]
- Park D. Phylloplane fungi: tolerance of hyphal tips to drying. Transactions of the British Mycological Society. 1982;79:174–178. [Google Scholar]
- Park H.G., Managbanag J.R., Stamenova E.K. Comparative analysis of common indoor Cladosporium species based on molecular data and conidial characters. Mycotaxon. 2004;89(2):441–451. [Google Scholar]
- Penzig A.J.O. Funghi agrumicoli. Michelia. 1882;2(8):385–508. [Google Scholar]
- Poll V., Denk U., Shen H.D. The vacuolar serine protease, a cross-reactive allergen from Cladosporium herbarum. Molecular Immunology. 2009;46(7):1360–1373. doi: 10.1016/j.molimm.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Potin O., Veignie E., Rafin C. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by Cladosporium sphaerospermum isolated from an aged PAH contaminated soil. FEMS Microbiology Ecology. 2004;51:71–78. doi: 10.1016/j.femsec.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Prenafeta-Boldú F.X., Kuhn A., Luykx D.M.A.M. Isolation and characterization of fungi growing on volatile aromatic hydrocarbons as their sole carbon and energy source. Mycological Research. 2001;4:477–484. [Google Scholar]
- Ramos-García B., Shagarodsky T., Sandoval-Denis M. Morphology and phylogeny of Cladosporium subuliforme, causing yellow leaf spot of pepper in Cuba. Mycotaxon. 2016;131(3):693–702. [Google Scholar]
- Rayner R.W. CMI and British Mycological Society; Kew, Surrey, England, UK: 1970. A mycological colour chart. [Google Scholar]
- Razafinarivo J., Jany J.L., Crous P.W. Cladosporium lebrasiae, a new fungal species isolated from milk bread rolls in France. Fungal Biology. 2016;120:1017–1029. doi: 10.1016/j.funbio.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Riesen T., Sieber T. Swiss Federal Institute of Technology; Zürich, Switzerland: 1985. Endophytic fungi in winter wheat (Triticum aestivum L.) [Google Scholar]
- Samson R.A. 2014. New insights into the biodiversity and ecology of the indoor mycobiota.http://microbe.net/wp-content/uploads/2014/10/Samson-Sloan-Berkeley-Rob-PC3_v2.pdf [Google Scholar]
- Samson R.A., Houbraken J., Summerbell R.C. Common and important species of Actinomycetes and fungi in indoor environments. In: Flannigan B., Samson R.A., Miller J.D., editors. Microorganisms in Home and Indoor Work Environments: Diversity, Health Impacts, Investigation and Control. CRC Press; USA: 2001. pp. 287–473. [Google Scholar]
- Samson R.A., Houbraken J., Thrane U. Food and indoor fungi. CBS Laboratory Manual Series 2. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2010. [Google Scholar]
- Samson R.A., van Reenen-Hoekstra E.S., Frisvad J.C. 6th ed. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2000. Introduction to food- and airborne fungi. [Google Scholar]
- Sandoval-Denis M.P., Sutton D.A., Martin-Vicente A. Cladosporium species recovered from clinical samples in the United States. Journal of Clinical Microbiology. 2015;53:2990–3000. doi: 10.1128/JCM.01482-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Denis M., Gené J., Sutton D.A. New species of Cladosporium associated with human and animal infections. Persoonia. 2016;36:281–298. doi: 10.3767/003158516X691951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. Martin-Luther-University Halle-Wittenberg; Germany: 2005. Morphotaxonomic revision of foliicolous Cladosporium species (hyphomycetes) Ph.D. dissertation. http://sundoc.bibliothek.uni-halle.de/diss-online/05/05H208/index.htm. [Google Scholar]
- Schubert K., Braun U. Taxonomic revision of the genus Cladosporium s. lat. 2. Cladosporium species occurring on hosts of the families Bignoniaceae and Orchidaceae. Sydowia. 2004;56(2):296–317. [Google Scholar]
- Schubert K., Braun U., Groenewald J.Z. Cladosporium leaf-blotch and stem rot of Paeonia spp. caused by Dichocladosporium gen. nov. Studies in Mycology. 2007;58:95–104. doi: 10.3114/sim.2007.58.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K., Greslebin A., Groenewald J.Z. New foliicolous species of Cladosporium from South America. Persoonia. 2009;22:111–122. doi: 10.3767/003158509X449381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K., Groenewald J.Z., Braun U. Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology. 2007;58:105–156. doi: 10.3114/sim.2007.58.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers F.J.J., Meijer M., Houbraken Xerotolerant Cladosporium sphaerospermum are predominant on indoor surfaces compared to other Cladosporium species. PLoS One. 2015;10(12):1–15. doi: 10.1371/journal.pone.0145415. e0145415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers F.J.J., van Laarhoven K.A., Huinink H.P. The indoor fungus Cladosporium halotolerans survives humidity dynamics markedly better than Aspergillus niger and Penicillium rubens despite less growth at lowered steady-state water activity. Applied and Environmental Microbiology. 2016;82:5089–5098. doi: 10.1128/AEM.00510-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers F.J.J., van Laarhoven K.A., Wösten H.A.B. Growth of indoor fungi on gypsum. Journal of Applied Microbiology. 2017;123(2):429–435. doi: 10.1111/jam.13487. [DOI] [PubMed] [Google Scholar]
- Seifert K.A., Nickerson N.L., Corlett M. Devriesia, a new hyphomycete genus to accommodate heat-resistant, cladosporium-like fungi. Canadian Journal of Botany. 2004;82:914–926. [Google Scholar]
- Visagie C.M., Hirooka Y., Tanney J.B. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Studies in Mycology. 2014;78:63–139. doi: 10.1016/j.simyco.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Lombard L., Groenewald J.Z. Phylogenetic reassessment of the Chaetomium globosum species complex. Persoonia. 2016;36:83–133. doi: 10.3767/003158516X689657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F.J., Hage K.C., de Bont J.A. Growth of the fungus Cladosporium sphaerospermum with toluene as the sole carbon and energy source. Applied and Environmental Microbiology. 1995;61:3562–3566. doi: 10.1128/aem.61.10.3562-3566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto W. Some species of Cladosporium from Japan. Science Reports of the Hyogo University of Agriculture, Series, Agriculture. 1959;4(1):1–6. [Google Scholar]
- Zalar P., de Hoog G.S., Schroers H.-J. Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments. Studies in Mycology. 2007;58:157–183. doi: 10.3114/sim.2007.58.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The first of 1 000 equally most parsimonious trees obtained from a heuristic search of an ITS alignment (representing the Cladosporium species known from ITS data. The Bayesian posterior probabilities (BPP; >0.74) and maximum parsimony bootstrap support values (PBS; >74 %) are shown at the nodes (BPP/PBS) and thickened lines represent those branches which are present in the strict consensus tree. The scale bar represents the number of changes. Species names are indicated to the right of the tree, followed by the culture accession number (all species) and the GenBank accession number (for the species not described in this paper). Species from indoor environments are indicated with a brown box. The species complexes are coloured according to the legend. The tree was rooted to Cercospora beticola (strain CBS 116456).